Methoxy-substituted centrohexaindanes through the fenestrane route†
Abstract
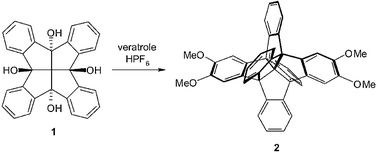
Tetrahydroxyfenestrindane undergoes HPF6-catalyzed condensation with anisole, 2-methylanisole and the three dimethoxybenzenes to furnish several centrohexaindanes bearing oxyfunctionalization at the opposite ends of one of the three [2,2′]-spirobiindane axes.

 Please wait while we load your content...
Please wait while we load your content...