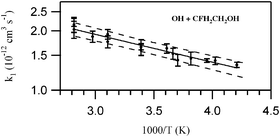
The rate coefficient for the reaction OH + CFH2CH2OH → products (k1) between 238 and 355 K was measured using the pulsed laser photolysis-laser induced fluorescence (PLP-LIF) technique to be (5.15 ± 0.88)
× 10−12 exp[−(330 ± 45)/T] cm3 molecule−1 s−1; k1(298 K)
= 1.70 × 10−12 cm3 molecule−1 s−1. The quoted uncertainties are 2σ
(95% confidence level) and include estimated systematic errors. The present results are discussed in relation to the measured rate coefficients for the reaction of OH with other fluorinated alcohols and those calculated using recently reported structure additivity relationships for fluorinated compounds (K. Tokuhashi, H. Nagai, A. Takahashi, M. Kaise, S. Kondo, A. Sekiya, M. Takahashi, Y. Gotoh and A. Suga, J. Phys. Chem. A, 1999, 103, 2664–2672, ). Infrared absorption cross sections for CFH2CH2OH are reported and they are used to calculate the global warming potentials (GWP) for CFH2CH2OH of ∼8, ∼2, and ∼1, respectively, for the 20, 100 and 500 year horizons. A brief discussion of the atmospheric degradation of CFH2CH2OH is provided. It is concluded that CFH2CH2OH is an acceptable substitute for CFCs in terms of its impact on Earth’s climate and the composition of the atmosphere. The room temperature rate coefficient for the reaction OH + CH3CH2OH → products (k10) was measured to be 3.26 × 10−12 cm3 molecule−1 s−1, in good agreement with recent measurements from this laboratory.

 Please wait while we load your content...
Please wait while we load your content...