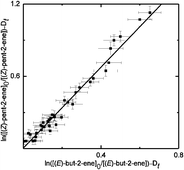
Rate coefficients for reactions of nitrate radicals (NO3) with (Z)-pent-2-ene, (E)-pent-2-ene, (Z)-hex-2-ene, (E)-hex-2-ene, (Z)-hex-3-ene, (E)-hex-3-ene and (E)-3-methylpent-2-ene were determined to be (6.55 ± 0.78)
× 10−13 cm3 molecule−1 s−1, (3.78 ± 0.45)
× 10−13 cm3 molecule−1 s−1, (5.30 ± 0.73)
× 10−13 cm3 molecule−1 s−1, (3.83 ± 0.47)
× 10−13 cm3 molecule−1 s−1, (4.37 ± 0.49)
× 10−13 cm3 molecule−1 s−1, (3.61 ± 0.40)
× 10−13 cm3 molecule−1 s−1 and (8.9 ± 1.5)
× 10−12 cm3 molecule−1 s−1, respectively. We performed kinetic experiments at room temperature and atmospheric pressure using a relative-rate technique with GC–FID analysis. The experimental results demonstrate a surprisingly large cis–trans
(Z–E) effect, particularly in the case of the pent-2-enes, where the ratio of rate coefficients is ca. 1.7. Rate coefficients are discussed in terms of electronic and steric influences, and our results give some insight into the effects of chain length and position of the double bond on the reaction of NO3 with unsaturated hydrocarbons. Atmospheric lifetimes were calculated with respect to important oxidants in the troposphere for the alkenes studied, and NO3-initiated oxidation is found to be the dominant degradation route for (Z)-pent-2-ene, (Z)-hex-3-ene and (E)-3-methylpent-2-ene.

 Please wait while we load your content...
Please wait while we load your content...