Multiple hydrogen bonds and tautomerism in naphthyridine derivatives
Abstract
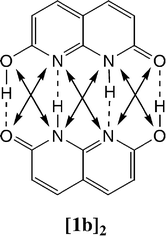
The behaviour of three 2,7-disubstituted 1,8-naphthyridines able to exhibit tautomerism has been studied by NMR in solution and in two cases in the solid state. The three derivatives studied are 2,7-dihydroxy- (1), 2-acetamido-7-amino- (3) and 2,7-diacetamido-1,8-naphthyridine (4). To explore the problem of secondary interactions, a series of complexes, with up to four simultaneous hydrogen bonds, where the monomers are generated using pyridine and 4-pyridone as building blocks, have been theoretically studied. The calculated interaction energies have been correlated with the number of hydrogen bonds and with attractive and repulsive secondary interactions. Further analysis of the electron density and orbital interactions shows that the secondary interactions, both attractive and repulsive, have a purely electrostatic origin. The X-ray structure of compounds 3 and 4 have been determined. In the solid state these compounds exist in the “diamino” tautomers with the N–H proton of the amido groups pointing towards the naphthyridine nitrogen. DFT and GIAO calculations have been essential to disentangle the problem of the structure of these compounds.

 Please wait while we load your content...
Please wait while we load your content...