Synthesis and mesomorphic properties of tetra- and octa-substituted phthalocyanines†
Abstract
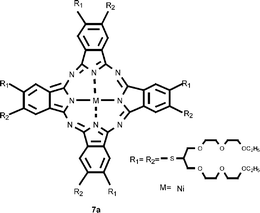
Thia-bridged tetra- and octa-poly(oxyethylene)-substituted metal free- and Ni(II) phthalocyanines have been synthesized from the corresponding phthalonitrile derivatives in the presence of the anhydrous metal salt (NiCl2) or a strong organic base. The new compounds have been characterized by elemental analyses, UV/vis,

 Please wait while we load your content...
Please wait while we load your content...