Practical and efficient enantioselective synthesis of α-amino acids in aqueous media
Abstract
Enantiomerically pure natural and unnatural α-amino acids have been synthesized from a chiral methyleneoxazolidinone by means of a highly diastereoselective 1,4-conjugate addition of alkyl iodides in aqueous media. The zinc–copper conjugate addition reaction exhibits high chemoselectivity, with the possibility of using functionalized iodides, to afford a single diastereomer in short reaction times and with good yields.
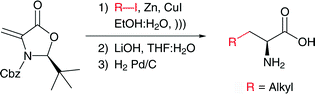

 Please wait while we load your content...
Please wait while we load your content...