Synthesis of N3- and 2-NH2-substituted 6,7-diphenylpterins and their use as intermediates for the preparation of oligonucleotide conjugates designed to target photooxidative damage on single-stranded DNA representing the bcr–abl chimeric gene
Abstract
Two 17-mer oligodeoxynucleotide-5′-linked-(6,7-diphenylpterin) conjugates, 2 and 3, were prepared as photosensitisers for targeting photooxidative damage to a 34-mer DNA oligodeoxynucleotide (ODN) fragment 1 representing the chimeric bcr–abl gene that is implicated in the pathogenesis of chronic myeloid leukaemia (CML). The base sequence in the 17-mer was 3′ G G T A G T T A T T C C T T C T T 5′. In the first of these ODN conjugates (2) the pterin was attached at its N3 atom, via a –(CH2)3OPO(OH)– linker, to the 5′-OH group of the ODN. Conjugate 2 was prepared from 2-amino-3-(3-hydroxypropyl)-6,7-diphenyl-4(3H)-pteridinone 10, using phosphoramidite methodology. Starting material 10 was prepared from 5-amino-7-methylthiofurazano[3,4-d]pyrimidine 4via an unusual highly resonance stabilised cation 8, incorporating the rare 2H,6H-pyrimido[6,1-b][1,3]oxazine ring system. In the characterisation of 10 two pteridine phosphazenes, 15 and 29, were obtained, as well as new products containing two uncommon tricyclic ring systems, namely pyrimido[2,1-b]pteridine (20 and 24) and pyrimido[1,2-c]pteridine (27). In the second ODN conjugate 3 the linker was –(CH2)5CONH(CH2)6OPO(OH)– and was attached to the 2-amino group of the pterin. In the preparation of 3, the N-hydroxysuccinimide ester 37 of 2-(5-carboxypentylamino)-6,7-diphenyl-4(3H)-pteridinone 36 was condensed with the hexylamino-modified 17-mer. Excitation of 36 with near UV light in the presence of the single-stranded target 34-mer, 5′T G A C C A T C A A T A A G14 G A A G18 A A G21 C C C T T C A G C G G C C 3′1 caused oxidative damage at guanine bases, leading to alkali-labile sites which were monitored by polyacrylamide gel electrophoresis. Cleavage was observed at all guanine sites with a marked preference for cleavage at G14. In contrast, excitation of ODN–pteridine conjugate 2 in the presence of 1 caused oxidation of the latter predominantly at G18, with a smaller extent of cleavage at G15 and G14 (in the double-stranded portion) and G21. These results contrast with our previous observation of specific cleavage at G21 with ruthenium polypyridyl sensitisers, and suggest that a different mechanism, probably one involving Type 1 photochemical electron transfer, is operative. Much lower yields were found with the ODN–pteridine conjugate 3, perhaps as a consequence of the longer linker between the ODN and the pteridine in this case.
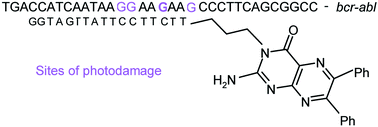

 Please wait while we load your content...
Please wait while we load your content...