Synthesis of meso-substituted porphyrins carrying carboranes and oligo(ethylene glycol) units for potential applications in boron neutron capture therapy†
Abstract
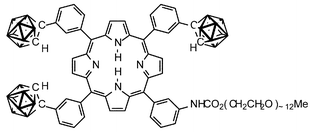
Selective delivery of 10B to tumours is one of the major remaining problems in boron neutron capture therapy (BNCT) of cancer. Porphyrins are selectively accumulated in tumours. Thus two series of carborane-carrying porphyrins were constructed, with additional functionality for attachment of uncharged potentially water-solubilising polyethers. 3-(1,2-Dicarbaclosododecaboran(12)-1-ylmethoxy)benzaldehyde was prepared by protection of the aldehyde of 3-(prop-2-ynyloxy)benzaldehyde as a dithioacetal, treatment with decaborane(14) and deprotection. Condensation with a 3-nitrophenyldipyrromethane gave a separable mixture of meso-(3-nitrophenyl)-meso-(3-carboranylmethoxyphenyl)porphyrins, resulting from extensive scrambling at the porphyrinogen stage. Similarly, condensation of 3-(1,2-dicarbaclosododecaboran(12)-1-yl)benzaldehyde with this dipyrromethane gave an analogous mixture of meso-(3-nitrophenyl)-meso-(3-carboranylphenyl)porphyrins. In this second series, the two regioisomeric bis(nitrophenyl)bis(carboranylphenyl)porphyrins could only be distinguished by X-ray crystallography, their NMR spectra being identical. The nitro groups of the mono(nitrophenyl)porphyrins and the bis(nitrophenyl)porphyrins were reduced to the corresponding amines with tin(II) chloride and the monoamines were coupled with a ω-methoxy poly(ethylene glycol) chloroformate of mean MW 600 to give the MeOPEGylated tricarboranyl porphyrins.

 Please wait while we load your content...
Please wait while we load your content...