A cyclic (H2O)4 cluster characterized in the solid state disappears on heating and regenerates from water vapor: A supramolecular reversible gas–solid reaction†
Abstract
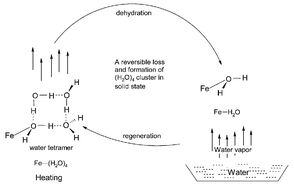
An unusual trinuclear iron(III) cluster described herein, [Fe3(μ3-O)(μ2-CH3COO)6(C5H5NO)2(H2O)] ClO4·4H2O (1) (C5H5NO = 2-pyridone), features a cyclic hydrogen bonded supramolecular water tetramer at one of the iron centres, as characterized by X-ray crystallography and thermogravimetry. The water cluster is formed by one iron-coordinated water and three lattice/solvent water molecules. The molecular environment of the water tetramer in the crystal structure consists of a Fe3+ ion (which is covalently bonded to one water of the (H2O)4 cluster), a perchlorate anion and the fourth lattice water. The facile removal of the lattice water molecules was anticipated from the knowledge of the type of interaction of these water molecules with their surroundings. Indeed, this hydrogen bonded water tetramer disappears with the formation of dehydrated solid [Fe3(μ3-O)(μ2-CH3COO)6(C5H5NO)2(H2O)] ClO4 (2), when the compound 1 is heated at ∼135 °C. Interestingly, when the dehydrated solid 2 is exposed to water vapor, it regenerates to 1 in a gas–solid reaction. The exposure of 2 to D2O vapor yields partially deuterated complex [Fe3(μ3-O)(μ2-CH3COO)6(C5H5NO)2(H2O)] ClO4·4D2O (3). As expected, the material 3 changes to 2 on heating at ∼135 °C, which again, on exposure to water vapor, returns to 1. The reversible loss/formation of (H2O)4 cluster in a gas–solid reaction has been established by elemental analyses, IR and X-ray powder diffraction studies including the single crystal X-ray structural analysis of 1.

 Please wait while we load your content...
Please wait while we load your content...