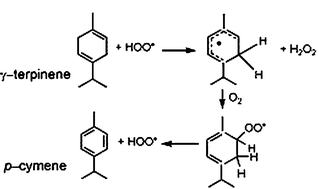
The peroxidation of γ-terpinene (TH) initiated by tert-butoxyl radicals has been investigated by means of nanosecond laser flash photolysis techniques in acetonitrile at room temperature. The absolute rate constants for the reactions implicated in the peroxidation process have been determined and the main short-lived intermediates, terpenyl and terpenylperoxyl radicals, have been detected. The rate constant for H-atom abstraction from TH by tert-butoxyl radicals, k3, has been found to be 1.4 × 108 M−1 s−1 while the values for the self-quenching of T˙ radicals, 2k4, and the addition of O2 to T˙, k9, are, respectively, 1.2 × 1010 M−1 s−1 and 1.3 × 109 M−1 s−1. The overall results strongly substantiate the peroxidation mechanism recently proposed by Foti and Ingold, J. Agric. Food Chem., 2003, 51, 2758, and provide a solid basis for a better understanding of the unusual antioxidant activity exhibited by this hydrocarbon.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...