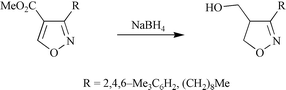
Crystallographic and theoretical studies have been used to investigate substituent effects, which are manifest in electrochemical and yeast-catalysed reactions of 4- and 5-acyl-, alkoxycarbonyl-, cyano- and phenyl-substituted isoxazoles. The results show that isoxazoles substituted at the 4-position with π-electron-withdrawing substituents have enhanced C4–C5 bond polarity and are structurally similar to Michael acceptors. As a consequence there is elongation and weakening of their N–O bonds. By contrast, their 5-substituted regioisomers and isoxazoles substituted at C4 with conjugating, but not π-electron-withdrawing, substituents have diminished C4–C5 bond polarity. This results in the selective electrochemical and yeast-catalysed reduction of 4-substituted isoxazoles, as well as their hydrogenolytic ring cleavage and conjugate reduction with sodium borohydride.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...