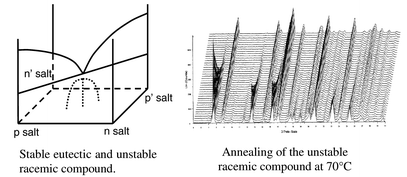
New studies on the reciprocal quaternary system (±)-2-phenylpropionic acid [(±)-1]–(±)-α-methylbenzylamine [(±)-2]–ethanol confirm the presence of a stable conglomerate (p and p′ salts), but polymorphism of the n salt as well as an unstable racemic compound have been detected. The kinetic parameters of the irreversible transformation of the unstable racemic compound into the stable conglomerate have been determined from experimental X-ray powder diffraction data. The simultaneous resolution of (±)-1 and (±)-2 by means of preferential crystallisation (auto-seeded process) of the stable pair of enantiomorphous salts, was achieved. However, the entrainment effect (given by the maximum enantiomeric excess of the counter enantiomer in the mother solution, reached at the end of the stereoselective crystallisation (eefmax = 5.2%)) is limited. This is consistent with the existence of the unstable racemic compound, as accounted for in a recent model of molecular interactions occurring at the crystal–mother solution interface in the course of preferential crystallisation. An extended version of this model rationalises a kinetic advantage of the crystal growth rate of racemic compounds over the conglomerates as well as a large supersaturation capacity of the mother solution in such a reciprocal quaternary system (±)-acid, (±)-base and solvent.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...