The synthesis and structural characterisation of some azo-containing phosphine chalcogenides and comparison to non-phosphorus-containing analogues
Abstract
p-HO-Ph(Ph2)P(E) (E = S, 1b, Se, 1c) reacts with the diazonium salts [4-R-PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N][BF4] (R = H, Me, Et, iPr, tBu, NMe2, NO2) to afford the new compounds [1-HO-2-(4-R-PhN
N][BF4] (R = H, Me, Et, iPr, tBu, NMe2, NO2) to afford the new compounds [1-HO-2-(4-R-PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)-4-Ph2P(E)C6H3] (E = S, R = H, 2a; Me, 2b; Et, 2c; iPr, 2d; tBu, 2e; NMe2, 2f; NO2, 2g; E = Se, R = H, 2h; Me, 2i) in acceptable yield. Similarly m-HO-Ph(Ph2)P(S) 3 reacts with two molar equivalents of the diazonium salts [4-R-PhN
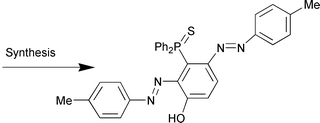
N)-4-Ph2P(E)C6H3] (E = S, R = H, 2a; Me, 2b; Et, 2c; iPr, 2d; tBu, 2e; NMe2, 2f; NO2, 2g; E = Se, R = H, 2h; Me, 2i) in acceptable yield. Similarly m-HO-Ph(Ph2)P(S) 3 reacts with two molar equivalents of the diazonium salts [4-R-PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N][BF4] (R = H, Me, NMe2, NO2) to give the new compounds 1-HO-2,4-(4-R-PhN
N][BF4] (R = H, Me, NMe2, NO2) to give the new compounds 1-HO-2,4-(4-R-PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)-3-Ph2P(S)-C6H24a–d (R = H, 4a; Me, 4b; NMe2, 4c; NO2, 4d). All of the new compounds have been characterised by
N)-3-Ph2P(S)-C6H24a–d (R = H, 4a; Me, 4b; NMe2, 4c; NO2, 4d). All of the new compounds have been characterised by ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N][BF4] which afford 1-HO-3-CH3-4-(4-Me-PhN
N][BF4] which afford 1-HO-3-CH3-4-(4-Me-PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)C6H35 and 1-HO-3-CH3-2,4-(4-Me-PhN
N)C6H35 and 1-HO-3-CH3-2,4-(4-Me-PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)C6H26 respectively. The compounds 2b·0.55CH2Cl2·0.2C6H14, 4b·CH2Cl2, 5 and 6b have been further characterised by
N)C6H26 respectively. The compounds 2b·0.55CH2Cl2·0.2C6H14, 4b·CH2Cl2, 5 and 6b have been further characterised by

 Please wait while we load your content...
Please wait while we load your content...