 Open Access Article
Open Access ArticleRate of weathering of Cold Lake Blend diluted bitumen at different water temperatures taking into consideration uncontrolled environmental factors
Thomas
King
 *a,
Brian
Robinson
a,
Jennifer
Mason
a and
Michel
Boufadel
b
*a,
Brian
Robinson
a,
Jennifer
Mason
a and
Michel
Boufadel
b
aDepartment of Fisheries and Ocean Canada, Bedford Institute of Oceanography, Dartmouth, Nova Scotia B2Y 4A2, Canada
bCenter for Natural Resources Development and Protection, Department of Civil and Environmental Engineering, New Jersey Institute of Technology, Newark, NJ 07102, USA. E-mail: thomas.king@dfo-mpo.gc.ca
First published on 6th January 2026
Abstract
The rate of weathering of Cold Lake Blend diluted bitumen in floating microcosms in an experimental flume tank was monitored over time (0, 6, 24, 48, 72, 96, and 168 hours) under controlled temperature conditions (4, 10, 15 and 25 °C). At all temperatures, the most rapid change in the physical properties (density and viscosity) and chemical composition (saturates and aromatics) of the oil occurred within the first 48 hours. Correlation analyses showed that seawater temperature and the uncontrolled factors (wind speed and air temperature) were significantly correlated on the rate of change in the physical properties of the oil. Gas chromatography mass/spectrometry analyses of the composite samples of weathered oil showed similar results for changes in its chemical composition. At 168 hours of weathering, the relative decrease of the saturates (C10 to C17 normalized to 17α, 21β-hopane) increased with the temperature. The percent decrease was 16, 24, 42, and 57 at 4, 10, 15 and 25 °C respectively. A similar trend was observed for the aromatics. From these, the percent decrease of naphthalene and its alkylated homologues was largest at 15 °C and above. These changes in the chemical composition and physical properties of the oil most likely resulted from the loss of the diluent. The data from this study on the weathering of diluted bitumen over a range of seawater temperatures, is the first of its kind, and thus may be used in predictive models to improve recommendations for responding and/or to improve contingency planning to oil spills.
Environmental significanceThe weathering of Cold Lake Blend diluted bitumen results in changes in the physicochemical properties of this Canadian oil, which can affect decisions on the type of oil spill response options to deploy. To date no studies have been produced to show such changes during the weathering of this oil over a range of seawater temperature conditions for Canadian territorial waters. The data, from this study, is needed to advance trajectory models to better predict the fate and behaviour of the diluted bitumen to support the development of future oil spill response plans. |
1. Introduction
Canada's Alberta oil sands produce approximately 2 million barrels-per-day (bpd) of upgraded and diluted bitumen products of which >80% is exported to the US, and the rest is consumed locally. Currently, oil sands products are primarily transported by pipeline (58%) and rail (31%) from production sites in Alberta Canada to inland oil refineries in the US.1 The remaining is transported by rail and pipeline to refineries in Canada.2 However, this is changing with the completion of the Trans Mountain pipeline expansion. The pipeline will transport an additional 0.6 Mbpd of diluted bitumen to the Port of Vancouver in Burnaby, BC, where it will be transferred to tankers to ship to Asian-Pacific markets or to the US.3 As Canadian crude bitumen is too viscous to transport by pipelines and rail cars to refineries in Canada and the United States (US), it is diluted with a thinning agent (diluent) to meet transport specifications for viscosity and density.Cold Lake diluted bitumen is a mixture of crude bitumen with 30% condensate specifically formulated for transport by pipeline. Recent studies have shown rapid changes in the C5–C15 portion of condensate within 24 to 120 hours of a spill through natural processes, such as evaporation and dissolution.4–6 Weathering and its associated changes in the physicochemical properties of diluted bitumen may influence decisions on the type of oil spill response options to deploy.4,6 Factors such as temperature, sunlight exposure, and wind on the weathering of diluted bitumen on water have been investigated.5,6 However, these previous studies have not fully covered the range of temperature conditions for Canada's territorial waters. This additional data is needed to advance trajectory models to better predict the fate and behaviour of the diluted bitumen to support the development of future oil spill response plans. To address this issue, studies on the weathering of Cold Lake diluted bitumen (a common product shipped throughout Canada), were conducted using replicate microcosms within a temperature-controlled circular flume tank located at the Bedford Institute of Oceanography, Dartmouth, NS, Canada in 2019 and 2020.
2. Experimental methodology
2.1. Oil product
The oil product used for the study was Cold Lake Blend diluted bitumen (winter blend). It contains 30% condensate and 70% bitumen from the Alberta oil sands.2.2. Circular flume tank
King et al.4,5 designed a circular flume tank that is located at the Bedford Institute of Oceanography, Dartmouth, Nova Scotia, Canada. It is fabricated from fiberglass and the dimensions are found in Fig. 1. Briefly from King et al.4,5 seawater was pumped directly from the Bedford Basin, Nova Scotia (NS) and filtered (5 µm filter sock; Atlantic Purifications Systems Inc., NS, Canada) into the circular tank. The tank was filled with water to a final volume of 1.31 m3 (1310 litres) at a depth of 0.76 m. The inlet of the circulating pump (¾ HP ReeFlo single phase, 1725 rpm, 115 VAC) was connected to a submerged vertical and horizontal injection system (manifold) constructed of 0.038 m polyvinylchloride (PVC) pipe with evenly spaced 0.013 m spigots and ball valves to control water flow. The flow system was set up to produce rotational water currents, within the tank, in a clockwise direction. The velocity was uniform with depth to reduce flow short-circuiting, especially at the tank bottom; thus, producing water currents at an average speed of 20.7 ± 0.4 cm s−1 (i.e., around 0.4 knots). Water temperature, in the tank, was controlled using a chiller (Payne refrigeration unit (Model PA13NAO42-B) and a Johnson temperature controller (Model A419).2.3. Floating microcosm
In order to keep replicate oil film thickness floating on the water surface, a microcosm system was adopted from King et al.4,5 (Fig. 2) with modifications. A circular disk of (2.5 cm thick) was cut from a sheet of chemically resistant polyoxymethylene that does not absorb sunlight. Thus, oil temperature does not increase noticeably relative to that of the water. The oil slicks were retained in cylindrical inserts (13 hold the were cut in the disk with dimensions 10.2 cm i.d. and 7.6 cm tall) to allow for easy application and retrieval of oil during the studies. The disk was made buoyant by fixing a Styrofoam sheet (2.5 cm thick) to the bottom of the disk (with a similar design to align the holes). Two floating microcosms were placed into the circular flume tank under static (i.e. no flow) conditions and combined provided 26 separate holds (rings) to contain oil slicks. | ||
| Fig. 2 Schematic of the floating microcosm (not drawn to scale with a diameter of 54.5 cm and inner rings of 10.2 cm diameter). | ||
2.4. Environmental conditions
Environmental conditions (water and air temperatures, light energy, rainfall amounts and wind speeds) were recorded during the flume tank studies. The wind speeds and air temperatures were taken from a weather station, ID 8200573 (operated by Environment and Climate Change Canada) located near the Bedford Basin. The salinity measurements were made during sampling times (see below) using a hand held meter (YSI model #30-1-FT; Yellow Springs, USA). Water temperature and light intensity (wavelength range from 150 to 1200 nm) was measured (every 15 minutes up to 192 h) using a in situ HOBO Pendant Data Logger (ITM instruments Inc. Model UA-002-08; Montreal, Canada).2.5. Oil application
Twelve grams of oil were placed into each of the numbered containment rings (of the floating microcosm) to achieve a 1.5 mm slick thickness. Uniform oil slicks in separate oil containment rings provided a convenient way to compare the rate of weathering of the oil at a range of temperatures (4, 10, 15 and 25 °C) that were selected for the study and to sample the oil at various points in time.2.6. Sample collection
Three rings were assigned for each time point and triplicate oil samples (time/temperature) were collected during oil weathering experiments. The duration was selected to be greater than the typical time of response after an oil spill, which ranges from 6 to 72 hours depending on the size and location of the spill.7 As per King et al.4,5 samples of oil were removed from the water surface, of the rings, using a stainless steel spatula and stored in a 20 mL vial for further analysis. The final sample mass was not recorded, since it contained some water that would affect the total mass of oil recovered.2.7. Analyses of oil samples and data analysis
The viscosity and the density of each sample of unweathered and weathered oil were measured in triplicate using an Anton Paar SVM 3000 Viscometer following ASTM D7042 (2014) and D5002 (2010), respectively.8,9 Note, all physical measurements for the experiments were taken at 15 °C for comparison purposes.The chemical composition of the collected oil was obtained by gas chromatograph (GC, Agilent 7890B) using mass spectrometer (MS, Agilent 5977A) detection in selected ion monitoring (SIM, electron ionization, mass transfer line held at 280 °C) mode based on method 8270D.10 All oil samples were dissolved in dichloromethane and prepared volumetrically at a concentration of 5.0 mg L−1 to allow direct comparison. One ml of each of the oils at 5.0 mg L−1 was spiked with internal standards and surrogates (deuterated alkanes and aromatics). These were purified using a silica gel column and solvent transferred to isooctane. The extracts of oil were chromatographed on a Supelco column (SLB™-5ms, 30 m × 0.25 mm × 0.25 µm film thickness) using the following GC conditions: cool on-column injection with oven track mode (track 3 °C higher than the oven temperature program) 85 °C hold 2 min, ramp at 4 °C min−1 to 280 °C, hold 10 min. Concentrations of the individual alkanes and aromatics were quantified using a 7 point calibrations curve.
Statistical testing (Microsoft Excel, correlation analysis) was used to determine if changes in water temperature and other uncontrolled environmental factors (wind speed, light intensity, air temperature and salinity) had an influence on the physical properties (density and viscosity) of the test oil weathered over time on seawater.
2.8. Weathering models
Previously developed models4,5 were used to capture the time-based changes in the density and viscosity of oils during weathering. The concept was to use modified Monod-type models that captures both the rapid initial changes and the slower changes thereafter. The formulae4 are as follows: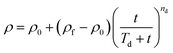 | (1) |
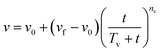 | (2) |
3 Results and discussion
3.1 Test conditions
The averaged measurements for climatic parameters (wind speed, air and water temperatures, and light intensity (solar radiation) recorded during the experiments conducted in Atlantic Canada in 2019 and 2020 are presented in Table S1. During the experimental runs while water temperatures were controlled (4, 10, 15, and 25 °C) salinity remained within a narrow range (29 to 30 ppt). With the exception of the experiment conducted at 10 °C (where the average air temperature was double the water temperature) the average air temperature was relatively close to the fixed water temperature settings. Wind speeds were relatively comparable (Table S1) for all the experiments. The natural light intensity varied between the four experiments due to seasonal changes in day length and daily differences in cloud-cover. Although this could influence the weathering of the oil, the variability (see Table S1 for averages and standard deviations and Tables S4 and S5 for correlation results) among the experiments indicates that the light intensity was a minimal contributing factor to the final results. Air and water temperature played a more important role in the weathering of the oil.3.2 Physical properties of oils and model results
The unweathered Cold Lake Blend diluted bitumen has an initial density and viscosity of 0.9281 ± 0.0064 g cm−3 and 300 ± 30 centistokes (cSt.) respectively. The averaged data based on triplicates (Table S2) are reported in Fig. 3 for density and Fig. 4 for viscosity.The models for density and viscosity, eqn (1) and (2), respectively, were fitted to the density and viscosity data obtained from the weathering experiments at different seawater temperatures. Initial, final and parameter (‘T’ and ‘n’) values of the density and viscosity models are reported in Table S3. The results reported in Fig. 3 and 4 show that the fits were generally good. The fit was also generally acceptable as evidenced by the coefficients of determination, which ranged from R2 > 0.85 to 0.99 (Table S3).
In Fig. 3, one notes that the density is largest for the higher water temperature, which reflects the increased evaporation at the higher temperature, leading to a more rapid depletion of the light oil fractions compared with the lower temperatures evaluated. Fig. 4 (note that plot is logarithm of the viscosity to achieve the best fit) shows that the viscosity was largest (for the same time) when the water temperature increased. Noting that oil viscosity decreases with water temperature (for this study all measurements were taken at 15 °C for comparison purposes) the only reason for the increase is the loss of low molecular weight components, and the remaining larger ones whose abundance increase the viscosity. The least amount of change in viscosity was observed at 4 °C and the most at 25 °C. This reaffirms that the oil is more resistant to weathering processes in cold marine waters. The information (changes in the physical properties over time and at different temperatures) may be used to advance oil spill models to better predict the fate and behaviour of the oil as it weathers over time at a range of water temperatures to improve oil spill response planning.
The results show that the models (eqn (1) and (2)) were appropriate for capturing the different rates of changes in the densities and viscosities of oils after the initial release over a range of water temperatures. The final values of density and viscosity of oil weathered at 25 °C were relatively much higher than those at 4 °C, and thus the larger ‘T’ value at 25 °C reflects a more strongly weathered oil. The ‘n’ value was less than 1.0 for the density and the viscosity of the oil weathered over the range of water temperatures.
3.3 Statistical analyses
The results for correlation analyses (Microsoft Excel) are presented in Tables S4 and S5. The highlighted columns in the tables represent significant correlations (p < 0.05) among the changes in the density and viscosity with the controlled and uncontrolled test conditions.Statistical analyses showed that varying water temperature (ranging from 4 to 25 °C) were significantly correlated on changes in the physical properties of the oil. Also, the uncontrolled factors (wind speed and air temperature) were directly correlated to the varied controlled water temperature. Fingas11,12 found through experiments that the evaporation of oils did not increase markedly with wind speed, and that the evaporation of oil is not limited by the air boundary layer, but by the diffusion of oil components to the interface between the oil and the atmosphere. Nevertheless, wind speed were not that variable, so was not expected to impact oil evaporation directly. However, there was a fairly significant correlation as presented in Tables S4 and S5. It should be noted that the experimental design does not permit us to separate the effects of water temperature from the co-varying air temperature and wind.
3.4 Chemical analyses of the unweathered and weathered oil product
Gas chromatography coupled with mass spectrometry analysis detected the presence of C10–C35 saturates and 2 to 6 ring aromatics and their alkylated homologues for the oil (T = 0, Fig. 5 and 6). A few composite samples of oil (prepared using 25 mg from each replica) were selected at time points 0, 48 and 168 hours for the 4, 10, 15 and 25 °C seawater temperatures to evaluate the loss of saturates and aromatics during the weathering studies. These time points were selected, because the most rapid changes in the physical properties (Fig. 3 and 4) of the oil occurred during the first 48 hours and thereafter plateauing up to 168 hours (the termination point) of weathering. In addition, composite samples of oil at the selected time points were used to minimize the number and cost associated with GC-MS analyses. The saturates normalized to the conservative marker 17α, 21β-hopane, stable up to 160 °C,13 showed that major losses were observed in the C10 (n-decane) to C19 (nonadecane) range (Fig. 5), increasing with increasing temperature for the weathered compared to the unweathered oil. The averaged percentage decreases for the saturates (C10 to C19) normalized to hopane were 16, 34, 42, and 57 at 4, 10, 15 and 25 °C respectively (Table 1). It important to note that these findings are complement to the long-term weathering of diluted bitumen in freshwater mesocosms that identified the major processes influencing hydrocarbon loss.14| Compound | Unw | Weathered | % Decrease of weathered | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 °C | 10 °C | 15 °C | 25 °C | 4 °C | 10 °C | 15 °C | 25 °C | ||
| a Unw-unweathered, naph-naphthalene, % decrease of weathered = normalized ((unw-weathered)/unw) × 100%. | |||||||||
| n-decane | 4.1 | 2.1 | 1.1 | 0.9 | 0.2 | 49 | 73 | 78 | 95 |
| Undecane | 3.6 | 2.2 | 1.5 | 1.2 | 0.3 | 39 | 58 | 67 | 92 |
| Dodecane | 2.3 | 1.9 | 1.2 | 1.1 | 0.4 | 17 | 48 | 52 | 83 |
| Tridecane | 2.6 | 1.9 | 1.5 | 1.3 | 0.8 | 27 | 42 | 50 | 69 |
| Tetradecane | 2.0 | 1.9 | 1.4 | 1.4 | 1.0 | 5 | 30 | 30 | 50 |
| Pentadecane | 1.8 | 1.7 | 1.3 | 1.3 | 1.1 | 6 | 28 | 28 | 39 |
| Hexadecane | 2.0 | 1.9 | 1.3 | 1.2 | 1.2 | 5 | 35 | 40 | 40 |
| Heptadecane | 1.6 | 1.5 | 1.4 | 1.1 | 0.9 | 6 | 12 | 31 | 44 |
| Octadecane | 1.3 | 1.2 | 1.2 | 1.0 | 0.9 | 8 | 8 | 23 | 31 |
| Nonadecane | 1.2 | 1.2 | 1.1 | 1.0 | 0.9 | 0 | 8 | 17 | 25 |
| Averaged % loss (C 10 to C 19 ) | 16 | 34 | 42 | 57 | |||||
| Naph | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 50 | 50 | 100 | 100 |
| Methyl-naph | 0.7 | 0.3 | 0.3 | 0.2 | 0.1 | 57 | 57 | 71 | 86 |
| Dimethyl-naph | 1.4 | 1.0 | 0.9 | 0.8 | 0.7 | 29 | 36 | 43 | 50 |
| Trimethyl-naph | 2.5 | 2.1 | 1.9 | 1.7 | 1.7 | 16 | 24 | 32 | 32 |
| Tetramethy-naph | 2.7 | 2.1 | 2.1 | 1.9 | 1.9 | 22 | 22 | 30 | 30 |
| Averaged % loss (naphthalene and alkylated homologues) | 35 | 38 | 55 | 60 | |||||
The changes in the aromatics normalized to hopane (Fig. 6) were detected in the 2-ring polycyclic aromatic hydrocarbons (naphthalene) and its alkylated homologues for the oil weathered up to 168 hours. These showed a similar trend to the saturates with a greater percentage loss occurring at 15 and 25 °C (Table 1). The greatest losses in the aromatics occurred with naphthalene and methyl-naphthalenes (Fig. 6) with percentage decreases of 100% and 86% respectively at 25 °C. It has been reported in the literature that the rate of biodegradation, for less volatile chemicals, decreases in cold water15 and the dissolution of low molecular weight hydrophobic compounds decreased compared to temperate water.16 This decrease in solubility helps to explain the observed recalcitrance of hydrophobic compounds and the potential for aquatic species to have longer exposure times to them in cold water environments during a spill of Cold Lake Blend diluted bitumen. Further to these findings, we attempted to provide time series plots (ln[hopane normalized concentrations] versus time; however composite samples were used for the hydrocarbon analyses and only three time points were selected. At 4 °C water temperature no trendlines were determined, because in all cases the ln[hopane normalized concentrations] were similar at 48 and 168 h (Table S6). As the water temperature increases the number of linear trendlines were more apparent mostly for the low molecular weight saturates, naphthalene and methylnapthalenes, acenaphthene, and fluorene with coefficient of determination varying from 0.88 to 0.99 (Table S6).
The Cold Lake Blend (CLB) diluted bitumen used in the study is a mixture of crude bitumen diluted with 30% condensate, a liquid product of natural gas extraction. The chemical composition of the condensate is primarily of low molecular weight aromatics and saturates in the range of C5 to C15, which are susceptible to evaporation.17,18 This has been substantiated in the literature by others who have reported that evaporation is the most important of the various physical processes (evaporation, photo-oxidation, biodegradation, dispersion, etc.) affecting oil spills on the water surface.19 The majority of the oil evaporation occurs in the first few days after a spill and accounts for a 5 to 75% loss of the mass of the spilled oil depending on the oil type and environmental conditions.17 As the water temperature increases, the intermolecular forces of attraction in the oil become weaker, increasing the rate of evaporation of the condensate portion of the oil. The evaporation of these low molecular weight chemicals (Fig. 5 and 6) associated with the condensate portion (diluent) of the oil resulted in an increase in the physical properties measurements as showed in Fig. 3 and 4. The rate of evaporation was greatest at 25 °C resulting in a greater increase in the physical properties measurements compared to the weathering of the oil at the lower temperatures (4, 10 and 15 °C). The typical response time after an oil spill, which ranges from 6 to 72 hours depending on the location and size of the spill relies heavily on science to support decisions on the type of response options to deploy. In particular the physical properties can greatly affect the effectiveness or choice of response options to combat the spill.12,20–23
Although emphasis is placed on evaporation of the saturates and aromatics present in the condensate portion of Cold Lake Blend, these chemicals are also water soluble so dissolution would take place as well. However, dissolution of an oil slick is a slower process than evaporation.24 In addition, one particular study found that UV light increased the sensitivity of the coral reef larvae to the water accommodated fractions of condensate (primarily of low molecular weight aromatics and aliphatics in the range of n-C5 to C10) by ca. 43%, which suggests the presence of oxidized (C–OH) chemicals produced during photo-chemical weathering of condensate.25 However, light intensity varied among the four experiments due to seasonal changes in day length, which complicates the rate of photo-chemical weathering of the Cold Lake Blend diluted bitumen in this study. Another study showed an increase in pericardial edema in fish embryos exposed (irrespective of exposure duration) to the LMW fractions associated with condensate,26 which is the diluent used in Cold Lake Blend diluted bitumen.
The information obtained from this study is critical to improve response plans to advance the decision-making process during a spill and to better assess the impacts to aquatic ecosystems.
4 Conclusions
Seawater temperature had an effect on the rate of change of the physical properties (density and viscosity) of Cold Lake Blend diluted bitumen weathered over 168 hours. In addition, correlation analyses confirmed that air temperature also influenced the weathering of the oil during the study. As expected, the rate of change in the physical properties of the oil were the least at 4 °C and the most at 25 °C. At all temperatures, the rate of change was greatest in the first 48 hours. However, this study was conducted under controlled realistic conditions and the information can used to assist spill responders specifically in spill areas where the environmental conditions, in this study, are relevant. The density values measured over time for all four experiments never exceed that of the seawater it was weathered in, so no sinking of the weathered oil occurred. The previously developed modified monod-type models (density and viscosity) fitted to the data with coefficients of determination ranging from R2 > 0.85 to 0.99. This demonstrates that the models can be used to evaluate changes in the physical properties of the oil weathered over a range of temperatures.The chemistry data reveals that the changes in the chemical composition of the oil, in particular the saturates (C10 to C19) and the aromatics (2-ring and their alkylated homologues) resulted most likely from the evaporation of the diluent portion of the diluted bitumen product. The rate of evaporation of the chemicals increased with increasing temperature with the greatest changes in the chemical composition occurring within the first 48 hours of the study. The evaporation for the saturates and aromatics was slower under cold water (4 and 10 °C) conditions indicating a greater risk to marine species exposed to these low molecular weight chemicals, associated with the condensate (diluent) portion of the oil. In other words, a slower release would suggest longer exposure time for aquatic species under cold water conditions.
The information, in particular, the modeling results for the physical properties could be implemented into oil spill trajectory models to enhance future predictions on the fate and transport of the oil product as it weathers under different water temperature conditions and the chemistry data for use in risk assessments to improve oil spill preparedness and response plans.
Author contributions
Dr Thomas King designed the experiments, conducted the study, interpreted the data and wrote the manuscript. Mr Brian Robinson managed the tank experiments, sample collection, validation of GC-MS and physical properties data and editing and reviewing the manuscript. Mrs Jennifer Mason conducted the GC-MS analyses, provided the concentration of analytes and graphical portions of it. Dr Michel Boudfadel reviewed all formulations and provided edits and review of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
All Raw data for this article are available from the repository at Harvard Dataverse https://doi.org/10.7910/DVN/C1MYVN.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5va00400d.
Acknowledgements
The authors would like to acknowledge the assistance of Melissa Faulkner and Andrew Davis for the preparation and extraction of the oil samples for chemical analyses and Graeme Soper and Patrick Toole for the measuring of the physical properties of the oil samples.References
- B. Nimana, A. Verma, G. DI Lullo, M. Rahman, C. Canter, B. Olateju and H. Z. A. Kumar, Life cycle analysis of bitumen transportation to refineries by rail and pipeline, Environ. Sci. Technol., 2016, 51, 680–691 CrossRef PubMed.
- Canada Energy Regulator, CER – Canada's Pipeline System 2021 – Crude Oil Pipeline Transportation System, cer-rec.gc.ca accessed July 23rd, 2025 Search PubMed.
- Oil Sands Magazine. Products from the Oil Sands: dilbit, synbit and synthetic crude explained, Products from the Oil Sands: Dilbit, Synbit and Synthetic Crude Explained, Oil Sands Magazine accessed July 21st, 2014 Search PubMed.
- T. King, B. Robinson, F. Cui, M. Boufadel, K. Lee and J. Clyburne, An oil spill decision matrix in response to surface spills of various bitumen blends, Environ. Sci.: Processes Impacts, 2017, 19(1), 929–939 Search PubMed.
- T. King, P. Toole, B. Robinson, S. Ryan, K. Lee, M. Boufadel, H. Li and J. Clyburne, Influence of climatic parameters on changes in the density and viscosity of diluted bitumen after a spill, J. Environ. Sci. Pollut. Res., 2019, 5(3), 373–382 Search PubMed.
- T. King, B. Robinson, S. Ryan and J. Clyburne, Canadian bitumen is engineered for transport, but the type of product produced can affect spill contingency planning, Environ. Sci.: Processes Impacts, 2022, 22, 863–872, 10.1039/c9em00493a.
- Transport Canada, Response Organizations Standards, TP12401E.DOC, (canada.ca) accessed July 20th, 2025 Search PubMed.
- ASTM D7042, Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (And the Calculation of Kinematic Viscosity), ASTM International, PA, USA, 2014 Search PubMed.
- ASTM D 5002, Standard Test Method for Density and Relative Density of Crude Oils by Digital Density Analyzer, ASTM International, PA, USA, 2010 Search PubMed.
- Environmental Protection Agency (EPA), Method 8270D, “Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC-MS), in Test Methods for Evaluating Solid Waste – Physical/Chemical Methods, Update III (US EPA No. SW-846), Washington, DC, Office of Solid Waste, 2007 Search PubMed.
- M. Fingas, Evaporation modeling. Chapter 9, in Oil Spill Science and Technology: Prevention, Response, and Cleanup, ed. Fingas, M. F., Elsevier/Gulf Professional Publication, 2011, pp. 201–242 Search PubMed.
- M. Fingas, Modeling oil and petroleum evaporation, J. Pet. Sci. Res., 2013, 2(3), 104–115 Search PubMed.
- Y. Han, G. John and T. Clement, Understanding the thermal degradation patterns of hopane biomarker compounds present in crude oil, Sci. Total Environ., 2019, 667, 792–798 CrossRef CAS PubMed.
- S. Stoyanovich, L. Saunders, Y. Z, M. Hanson, B. Hollebone, D. Orihel, V. Palace, J. Rodriguez-Gil, F. Mirnaghi, K. Shah and J. Blais, Chemical weathering patterns of diluted bitumen spilled into freshwater limnocorrals, Environ. Sci. Technol., 2023, 57, 9266–9276 CrossRef CAS PubMed.
- R. Prince, Bioremediation of marine oil spills, in Handbook of Hydrocarbon and Lipid Microbiology, ed. K. M. Timmins, Springer-Verlag, Berlin, Heidelberg, 2010, pp. 2617–2630 Search PubMed.
- O. G. Brakstad and L. G. Faksness, Biodegradation of water-accommodated fractions and dispersed oil in the seawater column, in Proceedings of the SPE International Conference on Health Safety and Environment in Oil and Gas Exploration and Production, 26–28 June, 2000, Stavanger, Norway, 2026, Society of Petroleum Engineers, Richardson, TX Search PubMed.
- M. Fingas, Review of the properties and behaviours of diluted bitumen. Proceeding of the 39th Arctic and Marine Oil Spill Program Technical Seminar, Environment and Climate Change, Canada, 2015, pp. 470–494 Search PubMed.
- Government of Canada, Order Adding Toxic Substances to Schedule 1 to the Canadian Environmental Protection Act, 1999, amendment 139, Canada Gazette, accessed July 23rd, 2025 Search PubMed.
- S. Ross and . Buist, Preliminary lab study to determine the effects of emulsification on oil spill evaporation, In Proceeding of the Eighteen Arctic and Marine Oil Spill Program (AMOP), Technical Seminar, Environment and Climate Change, Canada, 1995, pp. 91–110 Search PubMed.
- T. King, B. Robinson, M. Boufadel and K. Lee, Flume tank studies to elucidate the fate and behavior of diluted bitumen spilled at sea, Mar. Pollut. Bull., 2014, 83, 32–37 CrossRef CAS.
- Z. Li, K. Lee, T. King, M. Boufadel and A. Venosa, Effects of temperature and wave conditions on chemical dispersion efficacy of heavy fuel oil in an experimental flowthrough wave tank, Mar. Pollut. Bull., 2010, 60(9), 1550–1559 CrossRef CAS PubMed.
- T. King, B. Robinson, C. McIntyre, P. Toole, S. Ryan, F. Saleh, M. Boufadel and K. Lee, Fate of surface spills of Cold Lake Blend Diluted Bitumen treated with dispersant and mineral fines in a wave tank, Environ. Eng. Sci., 2015, 32(3), 250–261 CrossRef CAS.
- International Tanker Owners Pollution Federation, Use of skimmers in oil pollution, Documents & Guides - ITOPF Accessed July 20th, 2012 Search PubMed.
- M. Riazi and Y. Roomi, A model to predict rate of dissolution of toxic compounds into seawater from an oil spill, Int. J. Toxicol., 2008, 27(5), 379–386, DOI:10.1080/10915810802503578.
- A. Negri, D. Brinkman, F. Flores, E. Botté, J. Jones and N. Webster, Acute ecotoxicology of natural oil and gas condensate to coral reef larvae, Sci. Rep., 2016, 6, 21153, DOI:10.1038/srep21153.
- F. Gissi, J. Strzelecki, M. Binet, L. Golding, M. Adams, T. Elsdon, T. Robertson and S. Hook, A comparison of short-term and continuous exposure toxicity tests of produced waters, condensate, and crude oil in marine invertebrates and fish, Environ. Toxicol. Chem., 2021, 40(9), 2587–2600 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |





