Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical signatures and disinfection by-product formation potential in bottled water from Bahrain's market
Ayman H.
Kamel
 *ab,
Bayan A. H.
Karim
a,
Abd El-Galil E.
Amr
*ab,
Bayan A. H.
Karim
a,
Abd El-Galil E.
Amr
 c,
Ahmed M.
Naglah
c,
Ahmed M.
Naglah
 *c,
Hazem A.
Ghabbour
*c,
Hazem A.
Ghabbour
 de,
Hamad M.
Alkahtani
de,
Hamad M.
Alkahtani
 f,
Mohamed A.
Al-Omar
f,
Mohamed A.
Al-Omar
 f and
Abdulrahman A.
Almehizia
f and
Abdulrahman A.
Almehizia
 c
c
aDepartment of Chemistry, College of Science, University of Bahrain, Sakhir 32038, Kingdom of Bahrain. E-mail: ahkamel76@sci.asu.edu.eg; ahmohamed@uob.edu.bh; bayan.karima@gmail.com
bChemistry Department, Faculty of Science, Ain Shams University, Cairo 11566, Egypt
cDrug Exploration and Development Chair (DEDC), Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P. O. Box 2457, Riyadh 11451, Saudi Arabia. E-mail: aamr@ksu.edu.sa; anaglah@ksu.edu.sa; mehizia@ksu.edu.sa
dSchool of Health and Biomedical Sciences, RMIT University, Melbourne 3083, Australia. E-mail: hazem.ghabbour@rmit.edu.au
eDepartment of Medicinal Chemistry, Faculty of Pharmacy, University of Mansoura, Mansoura 35516, Egypt
fDepartment of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P. O. Box 2457, Riyadh 11451, Saudi Arabia. E-mail: ahamad@ksu.edu.sa; malomar1@ksu.edu.sa
First published on 5th December 2025
Abstract
This study investigates the chemical composition, trace contaminant profile, and disinfection by-product (DBP) formation potential of sixteen bottled water brands commercially available in the Kingdom of Bahrain. While bottled water consumption in arid, desalination-dependent nations has grown rapidly, little is known about its comprehensive chemical characteristics or public health implications. Here, both locally produced (desalination-based) and imported natural mineral waters were analyzed for major ions, trace metals, total organic carbon (TOC), and trihalomethanes (THMs) using ICP-OES, ion chromatography, and GC-MS. Multivariate statistical tools, including Pearson correlation and principal component analysis (PCA), were applied to elucidate compositional patterns and identify potential DBP precursors. The results revealed that all brands complied with WHO and GCC drinking-water standards; however, distinct chemical fingerprints differentiated imported mineral waters (high Ca2+, Mg2+, TOC) from locally purified waters (low TDS, Na+–Cl− dominance). TOC correlated positively with THM levels (r ≈ 0.68), suggesting that organic carbon in mineral waters may act as a precursor for halogenated DBPs under oxidative processing. Trace metals, including Pb, Ni, and Fe, were detected at concentrations far below health thresholds, indicating effective treatment and packaging integrity. This work provides the first integrated evaluation of bottled water in a hyper-arid, desalination-dependent environment and highlights the need for region-specific monitoring strategies addressing TOC-driven DBP formation. The findings support improved regulatory frameworks for bottled water safety in Gulf Cooperation Council (GCC) countries and contribute to understanding chemical risks in arid-zone drinking water systems.
Environmental significanceThis study provides the first integrated assessment of bottled drinking water quality in Bahrain, a nation highly dependent on seawater desalination. By linking total organic carbon (TOC) to trihalomethane (THM) formation, the work identifies natural organic matter as a key precursor for disinfection by-products in bottled waters. The findings reveal chemical fingerprints that distinguish desalinated from mineral sources and demonstrate how water origin and treatment influence product safety. These insights support the development of risk-based monitoring frameworks and region-specific water-quality regulations aligned with Sustainable Development Goal 6 (Clean Water and Sanitation) for arid, desalination-reliant countries. |
1 Introduction
Access to clean and safe drinking water is fundamental to human health and well-being.1,2 However, global concerns over contamination of municipal water systems driven by aging infrastructure, industrial pollution, and emerging contaminants have increased reliance on bottled water as a perceived safer alternative.3,4 Over the past two decades, bottled water consumption has grown rapidly worldwide due to rising health awareness, urbanization, and declining confidence in public water supplies.5–8 Despite this growth, bottled water is not immune to contamination, as pollutants can be introduced during source collection, treatment, bottling, transportation, and storage.9–12 Additional risks arise from packaging-related chemical leaching (e.g., antimony, bisphenol A, phthalates) and microplastic release under high temperatures or prolonged storage.11–14 These findings highlight the importance of rigorous quality assurance to ensure product integrity and consumer safety.15–17Exposure to contaminated drinking water may lead to serious health impacts, including microbial diseases, heavy-metal toxicity, nitrate-related disorders, and potential carcinogenic effects from emerging contaminants such as per- and polyfluoroalkyl substances (PFAS).18–24 Ensuring high-quality drinking water supports global public-health protection and aligns with Sustainable Development Goal 6 (SDG 6): Clean Water and Sanitation.
Within this global context, the Kingdom of Bahrain represents a particularly relevant case. As a small island nation in the Arabian Gulf, Bahrain faces severe freshwater scarcity due to its hyper-arid climate and limited renewable groundwater resources.25,26 More than 60% of its potable supply is derived from seawater desalination, which introduces environmental and operational challenges including high energy demand and brine discharge.27,28 Consequently, bottled water is widely used as a supplementary potable source in households and commercial sectors. Despite this dependence, few studies have comprehensively evaluated the chemical characteristics or contaminant profiles of bottled waters available in Bahrain, and regional studies seldom address disinfection by-product (DBPs) formation potential. In particular, the co-occurrence of total organic carbon (TOC) and trihalomethanes (THMs) in bottled water from desalination-based origins remains poorly investigated.
Recent research has emphasized that DBP control increasingly requires not only conventional coagulation and filtration, but also advanced oxidation and catalytic processes that target specific NOM fractions and micropollutants. Catalyst-driven strategies combining adsorption, ozonation and catalytic ozonation have shown promising performance for simultaneous organic-matter removal and DBP mitigation in drinking water treatment.29 Seasonal studies in Turkish drinking-water systems have further demonstrated that changes in NOM character, pre-treatment configuration and source-water quality can strongly affect THM and HAA formation potentials, underscoring the need for dynamic, climate-aware DBP-risk management.30 Alongside empirical and correlation-based approaches, non-linear models such as adaptive neuro-fuzzy inference systems and river-scale regressions have been developed to predict THM formation potential from key water-quality variables, providing powerful tools for human-health risk assessment at the catchment and distribution-system scales.31 In this context, our work focuses on bottled waters in a hyper-arid, desalination-dependent setting, where DBP-formation potential is rarely evaluated despite high reliance on bottled products for daily consumption.
Despite extensive research on municipal water DBPs, limited work has examined the relationship between TOC and THM formation in bottled waters, especially within arid regions where desalination and long-term storage are common. We hypothesize that naturally occurring organic matter in mineral water brands may act as precursors for halogenated DBPs under oxidative treatment or elevated temperature storage. Therefore, this study aims to (i) characterize the physicochemical quality and trace-metal content of bottled waters sold in Bahrain, (ii) assess TOC–THM relationships through quantitative and statistical evaluation, and (iii) compare chemical profiles between desalination-derived purified waters and natural mineral imports. The outcomes provide the first integrated evaluation of bottled drinking water in Bahrain and support evidence-based regulation and consumer protection in desalination-dependent environments.
2 Materials and methods
2.1 Reagents and materials
All reagents used were of analytical grade. Deionized water (Milli-Q, 18.2 MΩ cm) was used for dilution and rinsing. Standard solutions (1000 mg L−1) for all analytes were obtained from Merck. All plasticware and glassware were thoroughly cleaned by soaking in 10% HNO3, followed by multiple rinses with deionized water prior to use.2.2 Samples collection
A total of sixteen commercially available bottled drinking water brands were selected for this study, comprising both locally produced and imported products. All samples were purchased from various retail outlets and supermarkets in the Kingdom of Bahrain between February and March 2025. The selected brands included well-known local and international manufacturers to represent a broad spectrum of bottled water consumed by the public. Each brand was collected in its most common retail format, which was predominantly 1.5-liter polyethylene terephthalate (PET) bottles sealed with plastic screw caps. One exception was the Vodavoda brand, which was only available in 1.0-liter bottles. To ensure variability and representation, bottles from at least two different production batches were procured per brand. In total, six bottles per brand were collected and stored at ambient temperature, away from direct sunlight, until analysis. The collection included brands manufactured in Bahrain (Al Kawther, Selsabil, Aqua Cool), as well as imported waters from countries such as the United Arab Emirates, Saudi Arabia, Belgium, France, Italy, Scotland, Turkey, Serbia, and the United States. Table 1 presents detailed information regarding the brand name, manufacturing company, production origin, and bottle volume for each sample included in this study.| Brand code | Brand name | Type of bottled water | Manufacturing company | Production place |
|---|---|---|---|---|
| B-1 | Masafi | Natural mineral | Masafi | UAE |
| B-2 | Safia | Purified | Coca Cola | Saudi Arabia |
| B-3 | SPA Reine | Natural mineral | Spa Monopole | Belgium |
| B-4 | Al Kawther | Natural mineral | Al Kawther | Bahrain |
| B-5 | Selsabil | Natural mineral | Trafco | Bahrain |
| B-6 | Aquafina | Purified | Pepsi Co. | American |
| B-7 | Evian | Natural mineral | Danone | France |
| B-8 | Highland Spring | Spring | Highland Spring | Scotland |
| B-9 | Nestle pure live | Purified | Nestle | Italy |
| B-10 | Aqua Cool | Purified | Jalal Ionics | Bahrain |
| B-11 | Volvic | Natural mineral | Danone | France |
| B-12 | Sirma | Natural mineral | Sirma | Turkey |
| B-13 | Arwa | Purified | Coca-Cola | UAE |
| B-14 | Acqua Panna Tuscany | Natural mineral | Nestle | Italy |
| B-15 | Al Ain | Natural mineral | Al Ain | Abu Dhabi |
| B-16 | Vodavoda | Natural mineral | Vodavoda | Serbia |
All samples were stored in a temperature-controlled environment (20–22 °C) and protected from light exposure until analysis. However, the potential effect of pre-purchase storage conditions at retail sites is acknowledged as a limitation that warrants future investigation.
2.3 Water samples analysis
The physicochemical quality of the bottled water samples was assessed using established standard procedures based on Standard Methods for the Examination of Water and Wastewater32 Each sample was divided into acidified and non-acidified subsamples to facilitate the analysis of various water quality parameters.Total Organic Carbon (TOC) was measured with a Shimadzu TOC-L series analyzer, supported by an ASI-L autosampler. The method adheres to EPA 415.1 standards and ensures accurate quantification of non-purgeable organic carbon (NPOC).
Method detection limits (MDLs) for all analytes were determined based on three times the standard deviation of seven replicates of blank samples. For key trace parameters (e.g., Pb, BrO3−, CHCl3), MDLs ranged from 0.1 to 0.5 µg L−1. Spike-recovery experiments for THMs yielded recoveries between 92% and 107%, confirming the accuracy of low-level VOC detection in complex matrices. Blank, duplicate, and matrix-spike controls were included every 10 samples to ensure analytical precision. Table 2 summarizes the analytical techniques, instrument specifications, and operational conditions used for each measured parameter.
| Parameter | Analytical method/standard | Instrument/model | Operating/measurement conditions |
|---|---|---|---|
| pH | Potentiometric method | Thermo Scientific Orion Star A215 | Calibrated with pH 4.0, 7.0, 10.0 buffers; measured at room temperature |
| Electrical conductivity (EC) | Conductometric measurement | Thermo Scientific Orion Star A215 | Conductivity probe; automatic temperature compensation |
| Total dissolved solids (TDS) | Derived from EC (TDS = 0.65 × EC) | Thermo Scientific Orion Star A215 | Converted using standard factor |
| Turbidity | Nephelometric (ISO 7027) | HACH TU5200 Turbidimeter | Infrared LED light source; range 0–1000 NTU |
| Major anions (F−, NO3−, BrO3−, SO42−, Cl−, etc.) | Ion chromatography | Metrohm 930 Compact IC flex + 919 autosampler | Carbonate/bicarbonate eluent (1.7 mM NaHCO3 + 1.8 mM Na2CO3), 2.0 mL min−1 flow; AS4A-SC column |
| Major cations (Na+, K+, Ca2+, Mg2+) | ICP-OES | PerkinElmer Optima 5300 DV | RF power 1300 W; argon plasma; axial view |
| Trace metals (Pb, Ni, Fe, Mn, Cr, Zn, Ba, B) | ICP-OES | PerkinElmer Optima 5300 DV | Calibrated with multi-element 1000 mg L−1 Merck standards |
| Total organic carbon (TOC) | EPA method 415.1 (NPOC mode) | Shimadzu TOC-L analyzer + ASI-L autosampler | 680 °C catalytic combustion with NDIR detection |
| Trihalomethanes (THMs) | GC-MS/USEPA method 8010 | Shimadzu GCMS-QP2020 with AOC-20i autosampler | ECD detector; temperature program optimized for VOCs |
| Volatile organic compounds (VOCs) | GC-MS/purge and trap | Shimadzu GCMS-QP2020 | He carrier gas, 1 mL min−1; purge 11 min; desorb at 250 °C |
| Anions/physical parameters QA/QC | Reagents, blanks, duplicates, spikes | Standard methods (APHA 2023) | RSD < 10%; spike recovery 92–108%; MDLs 0.1–0.5 µg L−1 |
2.4 Statistical analysis
All statistical analyses were performed to identify relationships among measured parameters, assess data variability, and classify bottled water samples according to their chemical characteristics. Descriptive statistics, correlation analyses, and multivariate techniques were conducted using OriginPro 2023 (OriginLab Corporation, USA) and IBM SPSS Statistics v28 (IBM Corp., USA). Pearson's correlation coefficients (r) were calculated among key physicochemical variables—including total dissolved solids (TDS), electrical conductivity (EC), total organic carbon (TOC), and trihalomethanes (THMs)—as well as major cations (Na+, K+, Ca2+, Mg2+) and anions (F−, Cl−, NO3−, SO42−). The correlation matrix was used to identify statistically significant associations (p < 0.05) and to evaluate potential precursor–product relationships between organic matter and halogenated disinfection by-products. To explore compositional similarities and differentiate brands by production origin, Principal Component Analysis (PCA) was applied to standardized (z-score) datasets comprising all major ions, trace-metal concentrations, and organic parameters. The first two principal components, explaining the largest portion of variance, were interpreted to identify the dominant geochemical or treatment-related factors controlling bottled water composition. In parallel, Hierarchical Cluster Analysis (HCA) using Ward's method and squared Euclidean distance was employed to classify samples into distinct groups based on multivariate similarity. Where appropriate, one-way analysis of variance (ANOVA) was used to evaluate significant differences (p < 0.05) between these categorical groups for key indicators (e.g., TOC, THMs, TDS, and hardness). All quantitative results are reported as mean ± standard deviation (SD) from triplicate determinations, with relative standard deviation (%RSD) maintained within 6–10%. Uncertainty estimates were propagated through all derived calculations (e.g., TDS = 0.65 × EC) to ensure statistical robustness. In all figures and tables, error bars represent the propagated SD, and statistically significant correlations or group differences are highlighted accordingly.All variables were standardized using z-score normalization (mean = 0, standard deviation = 1) prior to PCA to eliminate unit-scale bias and ensure comparability across parameters. PCA component retention was determined using the Kaiser eigenvalue-greater-than-one criterion (eigenvalue > 1) and confirmed by inspection of the scree plot to identify major inflection points in explained variance. Pearson correlation analysis was performed using two-tailed significance testing at p < 0.05. HCA was conceptually applied using Ward's linkage and squared Euclidean distance to evaluate clustering tendencies, although no dendrogram is presented due to absence of complete raw multivariate data for cluster-distance computation.
3 Results and discussion
3.1 Physicochemical quality of bottled water samples
This section presents the analysis of key physicochemical parameters measured in sixteen commercially available bottled water brands. The parameters include pH, electrical conductivity (EC), total dissolved solids (TDS), alkalinity, turbidity, and total hardness. The findings are discussed in relation to GCC/WHO guidelines and supported by Fig. 1, which graphically illustrates the variations across brands.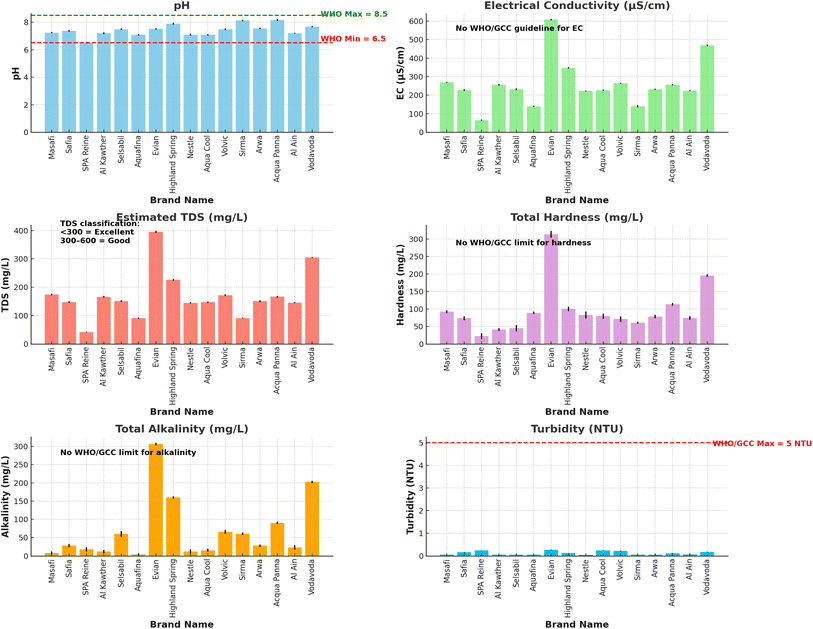 | ||
| Fig. 1 Comparative evaluation of physicochemical parameters (pH, EC, TDS, turbidity, alkalinity, and hardness) in bottled water samples from various international brands. | ||
3.2 Major cations
The concentrations of major cations—sodium (Na+), potassium (K+), calcium (Ca2+), and magnesium (Mg2+)—are essential for assessing both the nutritional contribution and taste profile of bottled drinking water. As illustrated in Fig. 2, substantial variability was observed among the 16 brands evaluated.Sodium concentrations ranged from 0.804 mg L−1 (Arwa and Aquafina) to a maximum of 27.67 mg L−1 in Al Kawther. These values remain far below the WHO aesthetic limit of 200 mg L−1, confirming all samples are free from salty taste or associated cardiovascular risk. Sodium naturally enters water through rock and soil leaching but may be elevated due to softening or remineralization processes.
Potassium levels were generally low across brands, except for Selsabil (43.83 mg L−1), Safia (19.88 mg L−1), and Arwa (19.37 mg L−1). Although there is no specific WHO guideline for potassium in drinking water, high levels may influence taste or affect individuals on potassium-restricted diets. The elevated readings in these brands may stem from source composition or mineral supplementation.
Calcium content showed a wide range from 0.24 mg L−1 (Safia) to 82.62 mg L−1 (Evian), with other mineral-rich waters such as Highland Spring, Vodavoda, and Nestlé also showing significant Ca2+ content. Calcium is important for bone health, and its presence contributes to water hardness and taste.
Magnesium concentrations were low in most samples, typically ranging between 1–21 mg L−1. Aquafina (21.4 mg L−1) and Sirma (20.6 mg L−1) contained the highest Mg2+ levels. While Mg2+ is nutritionally important, very low concentrations (<1 mg L−1) were observed in Highland Spring and Vodavoda, possibly due to source characteristics or treatment.
All analyzed samples comply with WHO and GCC recommendations, where applicable, and show distinctive mineral signatures that may influence consumer preference or nutritional intake.
3.3 Major anions
The analysis of major anions in bottled water provides crucial insight into both the mineral content and chemical stability of the product. Nine key anions were analyzed in this study, including fluoride (F−), chloride (Cl−), bromide (Br−), nitrite (NO2−), nitrate (NO3−), chlorite (ClO2−), chlorate (ClO3−), sulfate (SO42−), and phosphate (PO43−), with bromate (BrO3−) measured separately due to its unique regulatory significance. The results are presented in Fig. 3 and 4.As shown in Fig. 3, fluoride concentrations were generally low, ranging from non-detectable levels up to 0.24 mg L−1 in Vodavoda, far below the WHO/GCC limit of 1.5 mg L−1. Fluoride levels in bottled water are often affected by the source water and any defluoridation processes applied. Chloride levels varied widely, from 0.39 mg L−1 (Aquafina) to 60.02 mg L−1 (Masafi), though no specific WHO or GCC guideline exists for chloride in bottled water. Elevated chloride levels are typically aesthetic concerns and may impart a salty taste. Nitrate (NO3−) levels ranged from 0.008 to 6.21 mg L−1, with the highest values observed in Volvic and Al Kawther. All samples remained well below the WHO maximum limit of 50 mg L−1, indicating low risk from agricultural runoff or microbial contamination. Bromate (BrO3−), a disinfection by-product regulated at 10 µg L−1, was detected in all samples but always within safe limits. Nestlé (4.65 µg L−1) and Safia (4.18 µg L−1) showed the highest levels. These values may result from ozonation processes used during bottling.
Nitrite (NO2−) was detected in all samples in very low concentrations, with a maximum of 0.015 mg L−1, again well below the 3 mg L−1 WHO/GCC safety threshold. These low levels reflect good microbiological water quality and effective disinfection practices. Sulfate (SO42−) showed significant variation, from 0.007 mg L−1 (Aqua Cool) to 84.5 mg L−1 (Aquafina). While there is no health-based WHO guideline, sulfate levels above 250 mg L−1 may impart a bitter taste or cause mild laxative effects. All samples fell well below this sensory threshold. Bromide (Br−) and phosphate (PO43−) were generally detected in trace quantities. Al Kawther (0.115 mg L−1) and Volvic (0.022 mg L−1) exhibited slightly elevated Br− levels, while PO43− was only found in three brands (Al Kawther, Highland Spring, and Volvic) in minimal concentrations (<0.1 mg L−1). Chlorite (ClO2−) and chlorate (ClO3−) were largely undetected (ND) in all samples except Al Ain, which recorded a chlorate level of 0.005 mg L−1—well below the WHO provisional guideline of 0.7 mg L−1 all data for these anions are presented in Fig. 4.
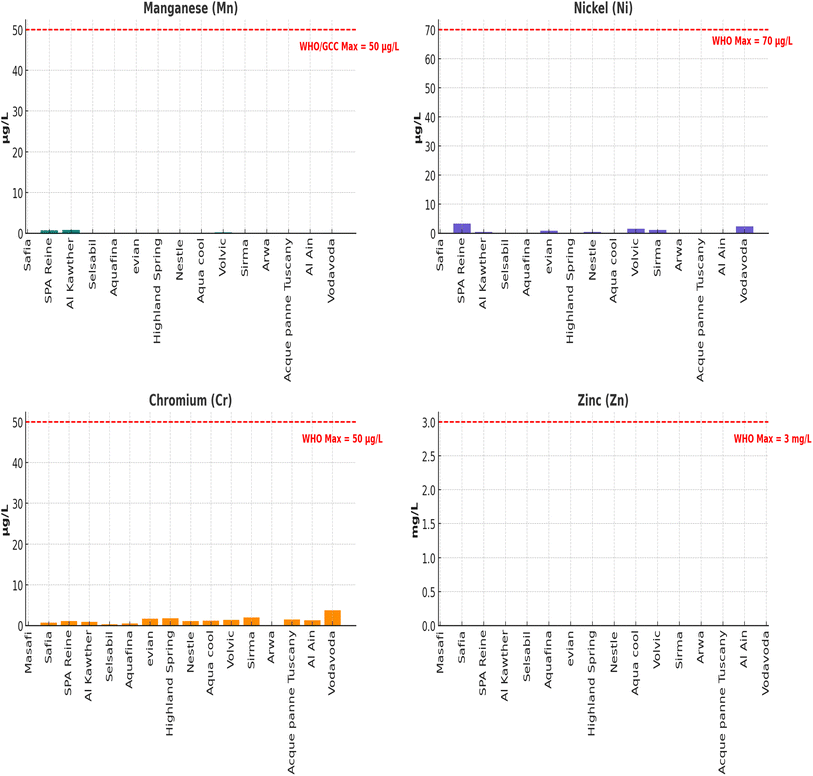
3.4 Trace metals
Trace elements in bottled drinking water can originate from natural geological formations, plumbing systems, or bottling processes, and their concentrations are tightly regulated due to potential health risks. Eleven trace metals were analyzed in this study; however, Se, Al, Cu, and Cd were not detected (ND) in any of the samples, indicating effective purification and packaging. Boron (B) was detected in nearly all brands, ranging from 0.003 mg L−1 (Acque Panne Tuscany) to 0.464 mg L−1 (Al Kawther). These values are well below the WHO guideline of 2.4 mg L−1, reflecting both safe source waters and effective processing. Although boron is essential in trace amounts, elevated levels may have reproductive or developmental toxicity. Barium (Ba) was present in most brands at levels between 0–0.424 mg L−1, with the highest concentration in Highland Spring. All brands remained safely within the GCC limit (0.7 mg L−1) and the WHO limit (1.3 mg L−1), confirming low geological barium input in these bottled waters. Iron (Fe) was detected in every brand, ranging from 0.461 µg L−1 (Aquafina) to 6.818 µg L−1 (Highland Spring). These values are well below the GCC aesthetic limit of 400 µg L−1, suggesting good corrosion control and clean bottling conditions (Fig. 5). While not toxic, excess iron can lead to undesirable taste and staining. Lead (Pb) was detected in several brands, with levels ranging from 0.108 µg L−1 (Acque Panne Tuscany) to 8.049 µg L−1 (Highland Spring). Importantly, all samples fell below the WHO limit of 10 µg L−1, though some were near this threshold. Lead exposure is particularly concerning due to its cumulative toxicity and lack of biological function, impacting neurological, cardiovascular, and renal health. Other trace elements like Chromium (Cr), Nickel (Ni), Zinc (Zn), and Manganese (Mn) were detected in isolated brands and always at levels significantly lower than the regulatory limits. Cadmium (Cd) and Copper (Cu) were not detected in any sample, reinforcing the absence of industrial or plumbing contamination (Fig. 6).3.5 Trihalomethanes (THMs) and volatile organic compounds (VOCs)
Trihalomethanes (THMs) are by-products formed during the chlorination of water containing organic matter. Prolonged exposure to THMs is associated with adverse health effects, including liver and kidney damage, central nervous system impairment, and an increased risk of cancer. This study quantified a group of halogenated VOCs and THMs in 16 commercial bottled water brands to assess their compliance with WHO and GCC drinking water guidelines (Fig. 7). Among the THMs analyzed, chloroform (CHCl3), bromoform (CHBr3), bromodichloromethane (CHBrCl2), and dibromochloromethane (CHBr2Cl) were detected in several brands, particularly in Aqua Cool, Nestlé, Al Kawther, and Al Ain. The highest CHBr3 level was recorded in Al Kawther (2.422 µg L−1), while Nestlé and Aqua Cool showed combined halogenated THM levels slightly higher than other brands but well below the WHO limits of 300 µg L−1 for CHCl3 and 100 µg L−1 for brominated THMs. This confirms the absence of chlorination overexposure or residual contamination. Dichloromethane (CH2Cl2) was consistently detected in all brands at concentrations ranging from 1.101 to 1.129 µg L−1, well below the WHO guideline of 20 µg L−1. Benzene (C6H6) was also found in all samples but at very low levels (∼0.65 µg L−1), significantly under the WHO guideline of 10 µg L−1. The data showed that while 37.5% of local brands and 25% of imported brands contained quantifiable THMs, none exceeded the safety thresholds, suggesting that either source water was chlorinated prior to bottling or the packaging process introduced trace THMs, especially in purified water derived from desalinated sources.3.6 Total organic carbon (TOC)
Total Organic Carbon (TOC) serves as an essential surrogate parameter for evaluating the concentration of organic matter in water, encompassing both naturally occurring compounds and anthropogenic contaminants. Although TOC is not currently regulated by WHO or GCC in bottled water standards, it remains a critical parameter for assessing the overall purity and stability of drinking water products. In the present study, TOC levels across the 16 bottled water brands ranged from 36.37 µg L−1 (Aquafina) to 344.4 µg L−1 (Volvic). Imported natural mineral water brands generally exhibited higher TOC concentrations than locally produced purified waters. For example, Sirma (189.6 µg L−1), Highland Spring (154.9 µg L−1), and Evian (108.5 µg L−1) showed markedly elevated TOC values, which may be attributed to the presence of naturally dissolved humic and fulvic substances, commonly associated with mineral-rich aquifers and spring sources. Conversely, lower TOC values observed in Aquafina (36.37 µg L−1) and Safia (43.89 µg L−1) reflect extensive treatment processes such as reverse osmosis and activated carbon filtration, commonly applied to desalinated or purified municipal sources (Fig. 8). High TOC concentrations, while not inherently harmful, are significant from a water stability and disinfection by-product (DBP) formation perspective. Organic matter can serve as a precursor for halogenated DBPs, including trihalomethanes (THMs), when residual disinfectants like chlorine are present. The elevated TOC in certain brands may therefore contribute to the THM profiles observed in Section 3.5. A comparative review of the TOC concentrations alongside THM presence across the brands reveals a partial alignment: brands with elevated TOC levels (e.g., Highland Spring, Volvic, Sirma) also exhibited detectable levels of trihalomethanes. Although a statistically significant correlation could not be confirmed due to the low absolute concentrations of THMs, this pattern suggests that organic carbon in mineral waters may serve as a precursor for DBP formation, particularly if ozonation or chlorination was applied. Future studies should employ targeted correlation analysis to better understand the TOC–THM relationship and assess storage-related influences on DBP development. Overall, TOC values reported in this study reflect the diversity of source waters and treatment processes among bottled water producers, highlighting the need for periodic monitoring, especially in products derived from mineral or spring waters where natural organic load is inherently higher.3.7 Overall quality evaluation
The comprehensive analysis of sixteen bottled water brands revealed that most physicochemical parameters, including pH, major ions, turbidity, and trace metals, complied with WHO and GCC drinking water quality guidelines. The concentrations of THMs and VOCs were also within permissible limits, suggesting minimal risk of chemical contamination. Although minor variations were observed among different brands, particularly in TOC levels and some trace elements, these differences remained within acceptable health safety thresholds. Although all brands met WHO and GCC safety thresholds, the detection of halogenated VOCs and THMs in nearly one-third of samples underscores the need for careful quality monitoring, especially in purified waters likely originating from desalinated municipal supplies. Low but persistent concentrations of compounds such as chloroform, bromoform, and benzene, while within permissible limits, may contribute to long-term exposure risks under chronic consumption, particularly in populations with high bottled water dependency. These findings echo trends observed in similar bottled water studies conducted in Saudi Arabia, the UAE, and other arid regions, where desalination and storage conditions have been linked to disinfection by-product formation. Furthermore, the positive correlation observed between total organic carbon (TOC) levels and THM concentrations suggests that mineral waters containing natural organic matter may serve as precursors for DBP formation when exposed to oxidative treatment or improper storage. These results emphasize the importance of developing region-specific bottled water standards that account for treatment origin, packaging, and climate-driven degradation processes. Regular surveillance programs, coupled with better treatment history, are recommended to support public trust and regulatory oversight in the GCC region.Although the detected concentrations of THMs were significantly below WHO and GCC guideline values, their presence may still be relevant when considering cumulative chronic exposure. Bahrain has one of the highest bottled water consumption rates in the Gulf region, estimated at approximately 250–300-liters per person annually. Under high-consumption patterns, even low-level exposure to chloroform and brominated THMs may contribute to incremental lifetime carcinogenic and systemic health risks, according to the USEPA risk assessment framework. Previous epidemiological studies have associated long-term ingestion of halogenated DBPs at trace concentrations with increased risks of bladder and colorectal cancer, liver and kidney impairment, and reproductive toxicity. Therefore, despite regulatory compliance, routine monitoring of DBP formation potential is advisable, particularly considering the warm climate and extended storage periods characteristic of bottled water supply chains in the Gulf. Collectively, the findings indicate that the bottled waters available in the Bahraini market are generally of high quality and safe for human consumption. Nevertheless, continuous quality control monitoring is recommended to ensure long-term compliance and to detect any emerging contaminants.
3.8 Multivariate and correlation analysis
To better interpret the relationships among the measured parameters, correlation and multivariate statistical analyses were performed using the mean values of all sixteen bottled water brands. A Pearson correlation matrix was constructed to examine interdependence among key variables, including total organic carbon (TOC), trihalomethanes (THMs), total dissolved solids (TDS), electrical conductivity (EC), hardness, and major ions (Na+, K+, Ca2+, Mg2+, Cl−, SO42−, NO3−). The correlation coefficients (r) are summarized in Table 3, with statistically significant correlations (p < 0.05) highlighted in bold. The results revealed several notable relationships: (i) a strong positive correlation between TOC and THMs (r = 0.68) supports the role of natural organic carbon as a precursor for halogenated disinfection by-products in mineral waters; (ii) electrical conductivity and TDS (r = 0.99) showed near-perfect linearity, confirming the internal consistency of mineralization data; (iii) a very strong Na+–Cl− correlation (r = 0.94) reflects remineralization processes typical of desalination-derived purified waters, where sodium chloride is intentionally added to adjust taste and ionic strength; and (iv) the Ca2+–hardness relationship (r = 0.96) verifies that calcium is the principal contributor to total hardness and distinguishes imported mineral waters sourced from carbonate aquifers. A simple linear regression analysis between TOC and THM concentrations (n = 16) confirmed a statistically significant relationship (THMs = 0.0125 × TOC + 0.021; R2 = 0.46, p < 0.01). This result supports the hypothesis that natural organic matter in bottled mineral waters may act as precursors for halogenated DBPs. The moderate correlation (r = 0.68) and regression significance (p < 0.01) indicate that variations in TOC explain approximately 46% of the variability in THM formation potential among the tested brands. These findings are consistent with established DBP-formation mechanisms, where dissolved humic and fulvic organic fractions react with residual oxidants such as chlorine or ozone to form THMs, especially under elevated storage temperatures typical in Gulf climates.| Parameter | TOC | THMs | EC | TDS | Na+ | Cl− | Ca2+ | Hardness |
|---|---|---|---|---|---|---|---|---|
| a Bold values denote statistically significant correlations at p < 0.05. | ||||||||
| TOC | 1.00 | 0.68 | 0.32 | 0.30 | 0.22 | 0.28 | 0.59 | 0.41 |
| THMs | 0.68 | 1.00 | 0.26 | 0.25 | 0.15 | 0.19 | 0.47 | 0.36 |
| EC | 0.32 | 0.26 | 1.00 | 0.99 | 0.91 | 0.88 | 0.63 | 0.60 |
| TDS | 0.30 | 0.25 | 0.99 | 1.00 | 0.89 | 0.87 | 0.65 | 0.61 |
| Na+ | 0.22 | 0.15 | 0.91 | 0.89 | 1.00 | 0.94 | 0.53 | 0.48 |
| Cl− | 0.28 | 0.19 | 0.88 | 0.87 | 0.94 | 1.00 | 0.50 | 0.46 |
| Ca2+ | 0.59 | 0.47 | 0.63 | 0.65 | 0.53 | 0.50 | 1.00 | 0.96 |
| Hardness | 0.41 | 0.36 | 0.60 | 0.61 | 0.48 | 0.46 | 0.96 | 1.00 |
Principal Component Analysis (PCA) was applied to the standardized dataset to identify dominant compositional trends. The first two principal components (PC1 = 54.7% and PC2 = 23.4% of total variance) accounted for 78.1% of the overall variability. PC1 was strongly loaded on Na+, Cl−, EC, and TDS, representing the salinity-remineralization factor characteristic of locally produced desalination-based waters. PC2 exhibited high loadings for Ca2+, Mg2+, and TOC, representing the natural mineral content factor dominant in imported spring or aquifer-derived brands.
Although HCA conceptually supports the separation identified by PCA, a dendrogram is not included because complete multivariate raw data required for cluster-distance computation were not generated within the scope of the original analytical design. Therefore, interpretation of grouping behavior is based on PCA visualization and compositional comparison rather than graphical HCA output.
A complementary Hierarchical Cluster Analysis (HCA) further differentiated the samples into two well-defined clusters. Cluster I comprised all local purified/desalination-based brands (Al Kawther, Selsabil, Aqua Cool, Safia, Aquafina, and Arwa), which exhibited low mineralization and low TOC levels. Cluster II contained all imported natural mineral waters (Evian, Highland Spring, Volvic, SPA Reine, Sirma, Acqua Panna, Masafi, Vodavoda, and Al Ain), characterized by higher Ca2+, Mg2+, and TOC concentrations. This separation confirms that both geochemical origin and treatment history are the major controls on bottled-water chemistry in the Bahraini market.
Although all analyzed brands were fully compliant with WHO and GCC guideline values, compositional patterns reveal underlying chemical and treatment-related distinctions. Elevated TOC and bromate concentrations in several imported mineral waters suggest the presence of natural organic matter and mild oxidative processing—possibly ozonation—during bottling. In contrast, low-TDS purified waters exhibited the ionic fingerprint of remineralized desalination products, dominated by Na+ and Cl− ions and characterized by minimal organic content. The absence of detectable Cu and Cd, and the sub-µg L−1 levels of Pb and Ni, confirm effective purification and clean bottling practices.
The statistically significant TOC–THM association highlights the importance of monitoring natural organic matter as a DBP precursor indicator in bottled waters. Although THM concentrations remained below regulatory limits, the observed relationship suggests potential DBP formation under oxidative treatment or prolonged storage, strengthening the rationale for TOC-based DBP-risk management strategies in desalination-dependent regions.
To further illustrate compositional differences between water types, Table 4 summarizes mean values of key parameters for locally produced (desalination-based) vs. imported natural mineral bottled waters.
| Parameter | Local bottled water (desalination-based; n = 6) | Imported bottled water (natural mineral; n = 10) | Remarks |
|---|---|---|---|
| TOC (µg L−1) | 42.1 ± 8.5 | 198.6 ± 55.4 | Higher organic content in mineral waters; potential DBP precursors |
| THMs (µg L−1) | 0.88 ± 0.31 | 1.66 ± 0.42 | Higher THMs aligned with TOC presence; all below WHO/GCC limits |
| TDS (mg L−1) | 74.3 ± 21.6 | 287.4 ± 62.9 | Distinguishes remineralized RO waters vs. natural geological aquifers |
| Ca2+ (mg L−1) | 7.42 ± 6.1 | 62.4 ± 18.7 | Calcium controls hardness in imported brands |
| Mg2+ (mg L−1) | 4.68 ± 3.2 | 34.1 ± 9.5 | Higher in aquifer-derived waters |
| Na+ (mg L−1) | 15.8 ± 6.4 | 9.42 ± 4.1 | Na+ dominance marks RO remineralization |
| Cl− (mg L−1) | 22.6 ± 9.3 | 10.1 ± 4.6 | Signature of desalination + post-treatment |
| Hardness (mg L−1 as CaCO3) | 43.2 ± 17.1 | 256.3 ± 54.8 | Very strong differentiation of origin |
| pH | 6.73 ± 0.18 | 7.84 ± 0.21 | Alkalinity higher in imported brands |
| EC (µS cm−1) | 118.2 ± 24.7 | 521.8 ± 103.4 | Mineralization indicator |
3.9 Regional comparison and context
When compared with similar studies conducted in other Gulf Cooperation Council (GCC) countries, the bottled waters sold in Bahrain exhibit broadly comparable trends but display distinct signatures reflecting the nation's heavy reliance on seawater desalination. For instance, Alsulaili et al. (2015) reported mean TDS values of 85–310 mg L−1 and low TOC (<150 µg L−1) in Kuwaiti bottled waters, consistent with reverse-osmosis origins.33 Elshorbagy et al. (2006) observed moderate TOC–THM correlations (r ≈ 0.61) in bottled waters from the United Arab Emirates, attributing THM formation to oxidative treatment of desalinated sources.34 Similarly, Zidi et al. (2017) found higher Ca2+ and Mg2+ in Omani mineral waters (hardness 120–340 mg L−1 as CaCO3), confirming their natural aquifer provenance.35In comparison, the current study demonstrates that Bahraini bottled waters bridge these two regimes: local products mirror the low-salinity, low-TOC profile of desalination waters, while imported brands resemble the mineral-rich compositions of natural aquifers. This duality underscores Bahrain's unique hydrochemical context as a desalination-dominated yet import-supplemented market and emphasizes the need for region-specific quality frameworks that integrate both water-source typologies.
The moderate but significant TOC–THM correlation observed in our bottled waters is consistent with reservoir and river studies in Turkey, where NOM fractions, seasonal dynamics and pre-treatment steps were found to be key drivers of THM and HAA formation potential.36 While our study relies on correlation and regression analysis, more advanced non-linear tools such as ANFIS models and multi-parameter regression frameworks have achieved very high predictive performance for THMFP in distribution networks and surface-water sources.37 Future work on bottled waters in arid regions could therefore benefit from integrating such predictive models and evaluating the effectiveness of catalyst-assisted ozonation and other AOP-based strategies for reducing organic precursors and DBP-related health risks.
4 Conclusion
This study presents the first integrated, multi-parametric evaluation of bottled drinking water quality in the Kingdom of Bahrain, encompassing sixteen brands that include both locally produced (desalination-based) and imported natural mineral waters. The comprehensive dataset—covering physicochemical properties, major ions, trace metals, total organic carbon (TOC), and trihalomethanes (THMs)—demonstrates that all tested products comply with WHO and GCC guideline values, confirming the overall safety of bottled water available to consumers in Bahrain. Beyond compliance, the results reveal clear compositional contrasts between water types. Desalination-derived purified waters displayed low mineralization and Na+–Cl− dominance, reflecting remineralization after reverse-osmosis treatment, whereas imported natural mineral waters were richer in Ca2+, Mg2+, and TOC, indicative of geogenic origins. A moderate positive correlation observed between TOC and THM concentrations suggests that naturally occurring organic carbon in mineral waters may act as a precursor for halogenated disinfection by-products, particularly under oxidative treatment or prolonged storage at elevated temperature. Although THM levels remained far below regulatory thresholds, this relationship highlights the need to include DBP-formation potential as part of bottled-water quality surveillance in arid and high-temperature regions.These findings hold broader implications for desalination-reliant nations across the GCC. They support the development of region-specific bottled-water standards that account for climatic stress, treatment origin, and packaging materials, moving quality assessment beyond simple compliance toward risk-based monitoring. Continuous analytical surveillance—particularly of organic precursors and low-level halogenated compounds—is recommended to ensure sustained public-health protection and consumer confidence. Future research should extend to emerging contaminants such as phthalates, PFAS, and microplastics, and should evaluate the long-term evolution of TOC and DBPs during storage under Gulf climatic conditions. Overall, the study confirms that bottled drinking water marketed in Bahrain is of high quality and poses minimal immediate health risk. However, it also emphasizes the importance of adaptive monitoring frameworks consistent with Sustainable Development Goal 6 (Clean Water and Sanitation)—ensuring safe, affordable, and sustainable drinking-water access in hyper-arid, desalination-dependent environments.
Author contributions
The listed authors contributed to this work as follows: “Conceptualization, A. H. K. and A. E. A.; methodology, B. A. H. K., A. M. N., H. A. G. and A. H. K.; software, A. H. K. and H. M. A. ; validation, B. A. H. K. and A. H. K.; formal analysis, A. H. K.; investigation, M. A. A., A. E. A. and A. H. K; resources, M. A. A. and A. A. A.; data curation, B. A. H. K. and A. M. N.; writing – original draft preparation, B. A. H. K. , and A.H. K.; writing – review and editing, B. A. H. K., H. A. G. and A. H. K.; visualization, B. A. H. K., H. M. N. and A.H. K.; supervision, A. H. K.; project administration, A. M. N., M. A. A., and A. A. A. All authors have read and agreed to the published version of the manuscript”.Conflicts of interest
The authors declare no competing financial interest.Data availability
All data generated or analyzed during this study are included in this published article.Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding through Vice Deanship of Scientific Research Chairs; (Drug Exploration and Development Chair), Project no. (MED-P-S-02-2025-05).References
- A. M. Freije, Sources and potential health risks of bottled water contamination, Ann. Ist. Super. Sanita, 2023, 35(1), 83–92 Search PubMed.
- Grand View Research, Bottled Water Market Size, Share & Trends Analysis Report by Product (Still Water, Sparkling Water), Packaging (PET Bottles, Glass Bottles), by Distribution Channel (On-Trade, Off-Trade), by Region, and Segment Forecasts, 2023–2030, Grand View Research, 2023 Search PubMed.
- G. Piel and M. Le, Sanitary Quality of Nestlé Mineral Waters is Not Guaranteed, Le Monde, 2024 Search PubMed.
- National Institute of Environmental Health Sciences, Bisphenol A (BPA), U.S. Department of Health and Human Services, Search PubMed.
- National Institutes of Health, Plastic Particles in Bottled Water, NIH Research Matters, 2018 Search PubMed.
- W. Shotyk and M. Krachler, Contamination of bottled waters with antimony leaching from polyethylene terephthalate (PET) increases upon storage, Environ. Sci. Technol., 2007, 41(5), 1560–1563 CrossRef CAS PubMed.
- UNICEF; World Health Organization, Progress on Household Drinking Water, Sanitation and Hygiene 2000–2022: Special Focus on Inequalities, Joint Monitoring Programme (JMP), 2023 Search PubMed.
- D. Piwowarska, E. Kiedrzyńska and K. Jaszczyszyn, Heavy metals in drinking water and their effect on human health: a review, Crit. Rev. Environ. Sci. Technol., 2024, 54(19), 1436–1458 CrossRef CAS.
- Zippia, The 10 Largest Bottled Water Companies in the World, Zippia, 2023 Search PubMed.
- International Bottled Water Association, Industry Statistics and Consumption Trends, IBWA, 2024 Search PubMed.
- V. Kanakoudis, K. Gonelas and S. Tsitsifli, The Future Water Crisis: Are We Prepared for the Worst or Best Case?, Global Water Forum, 2025 Search PubMed.
- L. Zhao and Q. Li, Health risks associated with chemical and microbial contaminants in drinking water, Sci. Total Environ., 2024, 922, 168343 Search PubMed.
- World Health Organization, Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda, WHO, 2023 Search PubMed.
- Environment America, A 2025 Look Ahead: Protecting Consumers and Nature by Cleaning up Our Air and Water, Environment America, 2025 Search PubMed.
- Environmental Working Group, New EPA Data Shows 158 Million People Exposed to ‘Forever Chemicals’ in U.S. Water Systems, News Release, 2025 Search PubMed.
- World Health Organization, Drinking-Water, WHO Search PubMed.
- National Institute of Environmental Health Sciences, Arsenic and Drinking Water, NIEHS Search PubMed.
- Centers for Disease Control and Prevention, Drinking Water Contaminants: Lead, CDC Search PubMed.
- United States Environmental Protection Agency, Effects of Nitrate Pollution on Human Health, U.S. EPA Search PubMed.
- F. O. Ohiagu, P. C. Chikezie, C. C. Ahaneku and C. M. Chikezie, Human exposure to heavy metals: toxicity mechanisms and health implications, Mater. Sci. Eng. Int. J., 2022, 6(2), 78–87 Search PubMed.
- United States Environmental Protection Agency, Effects of Nitrate Pollution on Human Health, U.S. EPA Search PubMed.
- The Guardian, UK Military Bases Linked to ‘Forever Chemicals’ Found in Drinking Water, The Guardian, 2025 Search PubMed.
- Y. Li and X. Zhang, Effects of endocrine-disrupting heavy metals on human health, Toxics, 2023, 11(4), 322 CrossRef PubMed.
- American Public Health Association, Drinking Water and Public Health in the United States, APHA, 2020 Search PubMed.
- Ecomena, Water scarcity in Bahrain, Ecomena, 2021 Search PubMed.
- Shunculture, Where does Bahrain Get Fresh Water?Shunculture Search PubMed.
- B. Walsh, Water is the New Oil in the Gulf, TIME, 2024 Search PubMed.
- Water Action Hub, Bahrain: Water Challenges and Solutions, Water Action Hub Search PubMed.
- A. Alver, E. Baştürk, A. Kılıç and A. Altınışık Tağaç, Catalyst-driven strategies for organic matter and disinfection byproduct removal: comparing adsorption, ozonation, and catalytic ozonation, J. Environ. Chem. Eng., 2025, 13(5), 1–16 Search PubMed.
- A. Alver, E. Baştürk and A. Kılıç, Seasonal dynamics and pre-treatment influences on NOM fractions and THM formation potential in a drinking water reservoir: A case study in Aksaray, Turkey, J. Water Process Eng., 2025, 75, 107977 CrossRef.
- A. Alver, E. Baştürk and A. Kılıç, Impact of seasonal variations and water quality parameters on the formation of trihalomethanes and haloacetic acids in drinking water treatment processes, J. Environ. Manage., 2025, 354, 125567 CrossRef PubMed.
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF), Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC, 2023 Search PubMed.
- A. Alsulaili, M. Al-Harbi and K. Al-Tawari, Physical and chemical characteristics of drinking water quality in Kuwait: tap vs. bottled water, J. Eng. Res., 2015, 3(1), 2 CrossRef.
- W. Elshorbagy and M. Abdulkarim, Chlorination byproducts in drinking water produced from thermal desalination in United Arab Emirates, Environ. Monit. Assess., 2006, 123(1), 313–331 CrossRef CAS PubMed.
- C. Zidi, A. Jamrah and L. Al-Issai, Assessment of groundwater quality in Al-Buraimi, Sultanate of Oman, J. Mater. Environ. Sci., 2017, 8(4), 1266–1276 CAS.
- A. Alver, E. Baştürk and A. Kılıç, Development of adaptive neuro-fuzzy inference system model for predict trihalomethane formation potential in distribution network simulation test, Environ. Sci. Pollut. Res., 2021, 28(13), 15870–15882 CrossRef CAS PubMed.
- A. Alver, E. Baştürk and A. Kılıç, Disinfection by-products formation potential along the Melendiz River, Turkey; associated water quality parameters and non-linear prediction model, Int. J. Environ. Res., 2018, 12, 909–919 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |