High-sensitivity NO2 detection using Se-doped In2O3 thin films synthesized via RF magnetron sputtering
Lijuan
Xie
abc,
Ziyao
Wang
bd,
Jiabei
Li
bc,
Shuo
Li
b,
Xiaohong
Li
 *ac,
Xi
Yang
*ac,
Xi
Yang
 *b and
Guoqiang
Li
*a
*b and
Guoqiang
Li
*a
aSchool of Manufacture Science and Engineering, Key Laboratory of Testing Technology for Manufacturing Process, Ministry of Education, Southwest University of Science and Technology, Mianyang 621010, PR China. E-mail: lixiaohong@swust.edu.cn; guoqli@swust.edu.cn
bInstitute of Chemical Materials, China Academy of Engineering Physics, Mianyang 621900, PR China. E-mail: xyang@caep.cn
cJoint Laboratory for Extreme Conditions Matter Properties, School of Mathematics and Physics, Southwest University of Science and Technology, Mianyang 621010, PR China
dSchool of Chemical Engineering and Technology, Tianjin Polytechnic University, Tianjin 300387, PR China
First published on 31st October 2025
Abstract
NO2 is a key atmospheric pollutant, and its efficient detection is crucial for environmental protection and human health. Indium oxide (In2O3) exhibits promising gas-sensing properties owing to its wide band gap (∼3.7 eV) and abundant oxygen vacancies arising from non-stoichiometry. However, poor selectivity, limited sensitivity, and high operating temperatures still hinder practical use. Here, we propose a two-step strategy: an In2Se3 precursor film is first deposited by RF magnetron sputtering and then oxidized at high temperature to achieve Se doping of In2O3. This approach introduces In–Se coordination, increases the oxygen-vacancy concentration, and generates a porous surface microstructure, thereby facilitating gas adsorption and charge transfer. The optimized Se–In2O3 film (1.55 at% Se) shows a 7.8-fold enhancement in NO2 response with a detection limit of 3.3 ppb, together with excellent selectivity and 28-day stability. Spectroscopic analyses (XPS, EPR, and TEM) confirm that Se doping reshapes the electronic structure and defect chemistry of In2O3, enabling synergistic improvements in sensitivity and response kinetics. This work highlights a scalable defect-engineering route for high-performance In2O3-based NO2 sensors.
1. Introduction
With the rapid development of industrialization, toxic and hazardous gases in the air, represented by VOCs, NO2, SO2, NH3, and H2S, seriously threaten the living environment and health conditions of human beings.1–3 Among the many gaseous pollutants, NO2 has attracted much attention because of its key position in environmental monitoring.4 NO2 is one of the major by-products produced during the combustion of fossil fuels, which can trigger acid rain, photochemical smog and ozone depletion, and poses a serious environmental risk.5 More seriously, prolonged or high exposure to NO2 may cause respiratory inflammation, aggravate asthma symptoms, and even jeopardize life.6,7 Therefore, it is of great practical significance to develop NO2 gas sensors with high response, high selectivity, low detection limit and long-term stability.8Metal oxide semiconductor (MOS) gas sensors represented by In2O3,9 SnO2,10 ZnO,11 and WO312 have been widely studied for their advantages of high sensitivity, low cost, and easy fabrication. Among them, In2O3, as a typical n-type semiconductor material, is widely used in the fields of gas sensors, solar cells, and ultraviolet detection by virtue of its high conductivity and wide band gap (∼3.7 eV).13,14 In particular, the non-stoichiometric properties and abundant oxygen defects of In2O3 endow it with excellent surface activity, making it an ideal sensitive material for detecting oxidizing gases such as NO2.15,16 It has been shown that the modulation of In2O3 morphology (e.g., brick-like, hollow microcubic, flower-like structures, nanorods and nanoballs)9,17 can result in high response values for ppm-level NO2 detection, with detection limits as low as tens of ppb.18 Despite significant progress in NO2 detection using In2O3, MOS-based gas sensors still face practical challenges in real-world applications, including high operating temperatures, cross-sensitivity, and humidity interference.19,20 To address these challenges, researchers have proposed various strategies to enhance their sensing performance, such as elemental doping, nanostructure design, and composite material construction.21,22 These approaches aim to achieve efficient, low-power, and stable detection of harmful gases like NO2.23–25
Among numerous strategies to enhance gas-sensing performance, doping modification has become a key approach in gas-sensing material research. This technique effectively promotes gas adsorption and desorption processes by introducing catalytic active sites, reducing grain size, and increasing surface defects.26 Currently, various doping methods are applied to modify In2O3-based films: Lu et al. used the metal–organic framework (MOF) MIL-68 (In) as a precursor, using a two-step “hydrothermal synthesis-calcination” method to prepare Ce-doped In2O3 microtubes. The response value for 10 ppm formaldehyde reached 563.07, 4.59 times higher than that of the undoped In2O3 sensor (response value of 122.59 at 230 °C), while reducing the operating temperature by 20 °C, effectively lowering energy consumption.27 Salunke et al. synthesized Zn-doped In2O3 nanoparticles via a two-step process of “sol–gel precursor preparation-calcination crystallization.” At a low operating temperature of 150 °C, the sensor exhibited a sensitivity of 98.74% toward 100 ppm H2S and demonstrated excellent selectivity.28 However, these methods generally suffer from limitations such as complex processes, difficulty in controlling doping uniformity, or poor compatibility with microelectronic processes. In contrast, magnetron sputtering offers advantages including low-temperature film deposition, controllable composition, dense and uniform films, excellent compatibility with semiconductor processes, and ease of scaling for mass production, making it a key technology for achieving high-performance gas-sensitive film doping modification. For instance, Wang et al. achieved a response value of 1054 at 325 °C for 100 ppm n-butanol using Au-doped In2O3 films prepared via magnetron sputtering.29 However, the high cost of precious metal doping limits practical applications. Consequently, exploring alternative element doping systems that balance cost-effectiveness with performance enhancement has become a current research priority. Moreover, conventional magnetron sputtering technology still faces bottlenecks in controlling doping within gas-sensitive thin films, particularly in achieving high uniformity and controllable in situ doping. Further optimization and breakthroughs in both fabrication processes and material design are urgently needed.
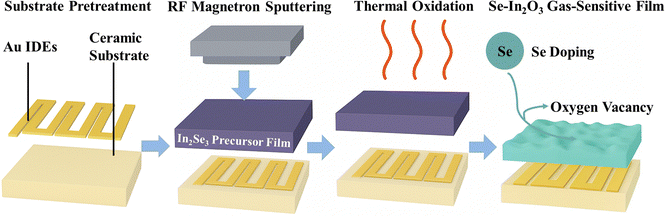
In this study, the defect chemistry of In2O3 films was modulated and their gas sensing performance enhanced by doping with selenium. Due to Se's larger atomic radius and lower electronegativity compared to O, it effectively substitutes O sites, forming In–Se coordination bonds and modulating the electronic structure.30 To achieve uniform and controllable Se doping, a two-step synthesis strategy was employed: first depositing the In2Se3 precursor via RF magnetron sputtering, followed by in situ oxidation annealing to achieve controlled defect formation and a porous surface structure (Scheme 1). The fabricated 1.55 at% Se-doped In2O3 film exhibited significantly enhanced NO2 response (7.8-fold increase), with a detection limit of 3.3 ppb and excellent stability over 28 days. These results demonstrate that appropriate Se doping provides an effective defect engineering approach for constructing highly sensitive and stable oxide semiconductor gas sensors.
2. Experimental section
2.1. Preparation of gas-sensitive films
Ceramic substrates with interdigitated Au electrodes (10 × 5 × 0.6 mm) were used. Substrates were ultrasonically cleaned in acetone for 3–5 min, followed by anhydrous ethanol for 3–5 min, and dried at 60 °C. An In2Se3 precursor film was then deposited by RF magnetron sputtering under high-purity Ar (see Table 1 for parameters). The as-deposited film was oxidized in air in a muffle furnace at 400–800 °C (3 h) with a ramp rate of 10 °C min−1, yielding Se-doped In2O3. The samples are denoted S-1 (400 °C), S-2 (500 °C), S-3 (600 °C), S-4 (700 °C), and S-5 (800 °C).| Sputtering parameters | Specific values/conditions |
|---|---|
| Sputtering temperature | RT |
| Sputtering power | 200 W |
| Sputtering time | 30 min |
| Background vacuum level | 1.9 × 10−3 Pa |
| Sputtering working pressure | 0.55 Pa |
| Substrate rotation speed | 15 rpm |
| Deposition atmosphere (Ar flow rate) | 9 sccm |
| Target material type | 99.99% In2Se3 |
2.2. Characterization
In order to systematically investigate the modulation mechanism of Se doping on In2O3 thin films, a comprehensive analysis system covering macroscopic morphology, microstructure, chemical composition and electronic states was constructed in this study using multidimensional characterization techniques. A detailed description of the adopted characterization techniques is given in the SI.2.3. Testing of gas sensors
The prepared gas-sensitive element was placed in a sealed intelligent temperature-controlled test probe platform for performance testing, and good contact with the device electrodes was achieved by a gold wire probe inside the platform. A Keithley 2400 source meter was used as the electrical test equipment in the experiment to obtain the response signal of the sensor by recording the resistance change of the sensor before and after exposure to the target gas. As shown in Fig. 1, dry air was used as the background gas during the testing process, and was also used to dilute the target gas in order to construct the gas exposure environment at different concentrations. The flow ratio of dry air to target gas was precisely controlled by a gas mass flow controller to keep the total gas flow at 200 sccm. The performance evaluation of the gas sensors was based on the following key parameters: response value, response/recovery time, selectivity, repeatability, operating temperature, detection limit, and long-term stability.31 For n-type semiconductors, the response value is defined as S = Rg/Ra for oxidizing gases and S = Ra/Rg for reducing gases (see eqn (S2) and (S3) in the SI).32 The time required for the sensor to reach 90% steady state is defined as the response time (Tres) and recovery time (Trecov). The theoretical detection limit (LOD) is calculated using LOD = 3σ/S (eqn (S4)) in the SI for details.333. Results and discussion
3.1. Material characterization
In order to investigate the effect of Se doping on the crystal structure and grain growth of In2O3, the experiments were carried out using an XRD technique to analyze the material structure. Due to the thin thickness of the fabricated film, it is difficult to perform XRD testing directly, so the In2Se3 powder was calcined under the same heat treatment conditions to obtain the 1.55 at% Se-doped In2O3 powder material for XRD testing. The experimental results are shown in Fig. S3a. As can be seen from the figure, except for the difference in the intensity of the diffraction peaks, the diffraction peaks of the Se-doped sample and the pure In2O3 sample are basically in the same position, which match the standard pattern of cubic In2O3 (JCPDS no. 71-2494),34 and the diffraction peaks of SeO2 or other impurity phases have not been detected, which indicates that Se has been successfully doped into the crystal lattice of In2O3 without altering the crystal structure. The average grain sizes of pure In2O3 and 1.55 at% Se-doped In2O3 were calculated using the Scherrer's formula (eqn (S5)) to be 40.18 nm and 26.68 nm,35 indicating that Se doping significantly inhibited the growth of the grains, a phenomenon which may be related to the lattice distortion induced by doping. In addition, Fig. S3b shows that the diffraction peaks are shifted to a low angle after Se doping. The increase of its crystal plane spacing d can be deduced from Bragg's law (eqn (S6)),36 which further suggests that Se doping leads to the expansion of the lattice constant. Considering the ionic-radius differences among Se4+, O2− and In3+ and charge compensation effects, Se4+ may enter the lattice by replacing In3+ or occupying the position of O2−, which causes local lattice distortion, and the system maintains electroneutrality through the formation of oxygen vacancies.37 This characterization analysis revealed that Se doping did not change the lattice structure of In2O3 and inhibited the grain growth through lattice distortion, revealing a possible doping mechanism.In order to investigate the effects of Se doping on the microscopic morphology, elemental distribution and surface structure of In2O3 thin films, SEM combined with energy spectrometer (EDS) surface scanning and atomic force microscopy (AFM) was used to systematically characterize pure In2O3, pure In2Se3 and Se-doped In2O3 thin films (Fig. 2). As seen from the SEM images, the surfaces of both pure In2O3 and pure In2Se3 films consisted of dense nanoparticles, which showed a high degree of overall homogeneity. In contrast, the Se-doped In2O3 films (Fig. 2c–g) exhibit porous structural features, and the pore size gradually increases with the increase in thermal oxidation temperature. This phenomenon suggests that Se doping induces a rise in defect density along with lattice expansion, thus promoting the formation of pores. Compared with the pure In2O3 thin films, Se doping significantly increases the porosity of the materials, which contributes to the enhancement of the adsorption/desorption kinetic process of gas molecules on the surface of the materials, and thus enhances the sensing performance.38,39 Further combined with the EDS surface scanning results of the S-2 samples (Fig. 2i–k), a uniform distribution of O, Se, and In elements can be observed, which verifies that the stable and uniform doping of Se in the In2O3 lattice was achieved by magnetron sputtering combined with a thermal oxidation process. Finally, the average thickness of the resulting films is about 150 nm, as shown by AFM (Fig. 2h), which provides a constitutive basis for subsequent device performance tests.
In order to obtain higher resolution structural information and to deeply analyze the microstructure and lattice arrangement characteristics of the films, TEM was used to analyze the materials on a nanoscale. Since the pristine films could not be peeled off from the substrate, the In2Se3 powder was calcined under the same heat treatment conditions to obtain 1.55 at% Se-doped In2O3 powder material for TEM testing. The experimental results are shown in Fig. S4. In the high-resolution transmission electron microscopy (HRTEM) image of pure In2O3 (Fig. S4a), the (211) crystal surface shows clear lattice stripes with a measured crystal spacing of 0.4135 nm,34 and its selected area electron diffraction (SAED) pattern shows a series of concentric diffraction rings corresponding to the crystal surface indices of (211), (222), (332), (400), (440), (622) and (833), indicating that the sample has a polycrystalline structure and the grain orientation is randomly distributed. In contrast, the HRTEM image of the Se-doped sample (Fig. S4b) shows a slight increase in the (211) crystal plane stripe spacing to 0.4155 nm, indicating that Se doping has led to a minor expansion of the crystal plane spacing. This result is consistent with the aforementioned trend of increasing lattice constant obtained based on XRD analysis, which further verifies that Se may enter the In2O3 lattice in the form of substituting O atoms. The SAED patterns of Se-doped samples still show polycrystalline diffraction ring features and the crystallographic indices are consistent with those of pure In2O3, but the intensities of some of the higher-order diffraction rings (e.g., (622) and (833)) are relatively weakened, reflecting the effect of doping on the distribution of the crystallographic diffraction intensities. TEM results show successful Se incorporation into the In2O3 lattice, leading to a slight lattice expansion, and the crystal structure may be fine-tuned by changing the grain size or modulating the local crystallinity, which provides an important microstructural basis for the subsequent study of the performance mechanism.
In order to systematically analyze the effects of the chemical states of the elements in Se-doped In2O3 and the variation of the oxygen vacancy concentration on the gas-sensitive performance, the surface elemental compositions and chemical states of the pure In2O3 films and In2O3 films with different Se doping amounts (S-1 to S-5) were characterized in detail by XPS. The C 1s peak at 284.8 eV was used as the energy calibration basis for all XPS measurements.
From the full XPS spectra (Fig. 3a and Fig. S5), it can be seen that only the characteristic signal peaks of In and O elements are detected in the pure In2O3 film, whereas the signals of Se elements are detected in the samples from S-1 to S-5 in addition to the elements of In and O, which indicates that Se is functionally doped into the In2O3 lattice. The Se doping concentrations of S-1 to S-5 were 2.15 at%, 1.55 at%, 0.92 at%, 0.33 at%, and 0.08 at%. Due to the low Se content, the Se 3d signal in the full spectrum is weak, which needs to be further analyzed in combination with the high-resolution fine spectrum (Fig. 3b). The Se 3d fine spectrum shows a weak signal near 59 eV, which corresponds to the oxidation state of Se4+, suggesting that Se exists in the samples in the high valence state.
The In 3d fine spectrum of pure In2O3 (Fig. 3c) shows that the In 3d5/2 peak and the In 3d3/2 peak are located at 444.37 eV and 451.91 eV, respectively, which is consistent with the typical electronic structure of the oxidation state of In3+.40 In contrast, the In 3d peaks for S-2 shift slightly to higher binding energy (Δ ≈ 0.03 eV), indicating that Se doping induced a local charge redistribution, but the magnitude of the peak shifts is small, suggesting that Se did not significantly change the chemical environment of In.41 To further directly verify the process of Se element incorporation into the In2O3 lattice and the formation of In–Se bonds, Raman spectroscopy analysis was performed on the annealed powder samples (Fig. S6). Compared to pristine In2O3, the Se-doped samples exhibited slight peak broadening and shift, indicating increased lattice distortion and defect formation following Se substitution for O atoms. However, no new peaks corresponding to independent In–Se bonds were observed, confirming that Se exists as a dopant within the In2O3 lattice rather than forming a secondary phase.42 This phenomenon supports that Se enters the lattice by substituting O sites to form In–Se bonds rather than directly replacing In3+.
High-resolution O 1s spectra (Fig. 3d) show components at 529.77 eV, 531.39 eV, and 532.77 eV, assigned to lattice oxygen (Olat), defect-related oxygen (Odef), and adsorbed oxygen (Oads), respectively.43,44 Analyzed by peak area integration, the relative content of Olat and Odef decreased after Se doping, while the proportion of Oads slightly increased (Fig. 3d). This phenomenon suggests that Se doping activates and promotes the dissociation of ambient O2 molecules, resulting in the generation of more surface adsorbed oxygen species. The abundance of adsorbed oxygen contributes to the enhancement of the oxidative reactivity of the gas molecules on the solid active surface, which has a positive significance for the improvement of the response performance of the gas sensor.45 In addition, the abundant Odef can serve as an active site anchor target gas, which further enhances the gas adsorption and recognition ability of the material.46
To further investigate the enhancement mechanism of Se doping on the gas-sensitive properties of In2O3, an EPR technique was used to analyze the oxygen vacancy concentration changes of pure In2O3 and Se-doped In2O3 (Fig. 4 and Fig. S6). Pure In2O3 exhibits a characteristic paramagnetic signal peak near 3520 G with a g-factor value of 2.003, which is attributed to the center of oxygen vacancy-related defects.47 In contrast, the Se-doped samples S-1 to S-5 showed a gradual enhancement of the signal intensity at the same location, reflecting some differences in the doping-induced defect microenvironments. Among them, the peak intensity of the S-2 sample at 3520 G is increased by about 30% compared with that of pure In2O3, which directly proves that Se doping effectively promotes the formation of oxygen vacancies, and further corroborates the reliability of the XPS results. The increase of oxygen vacancies not only helps to enhance the adsorption ability of the material to gas molecules, but also improves its surface reactivity, which significantly enhances the gas-sensitive response properties. In summary, the EPR test results provide a strong experimental basis for the mechanism of Se doping to modulate the concentration of oxygen vacancies in In2O3 and to enhance the gas-sensitive properties.
 | ||
| Fig. 4 EPR spectra of (a) pure In2O3 and (b) Se-doped In2O3 (S-2). Both samples exhibit a signal centered at g ≈ 2.003, characteristic of oxygen vacancies. | ||
3.2. Gas-sensing performance
The response process of a typical metal oxide gas-sensitive element consists of two key steps: first, the adsorption of gas molecules on the surface of the sensitive material, and second, the charge transfer between the gas molecules and the sensitive material, both of which are affected by the operating temperature.48 Appropriately increasing the working temperature helps to overcome the activation energy barrier required in the chemisorption process, thus enhancing the response properties of the gas.49Fig. 5a shows that the response to 5 ppm NO2 increases with temperature and peaks at 200 °C, reflecting a balance between surface-oxygen mobility and NO2 adsorption energy; at higher temperatures, desorption dominates and the response decreases.50 At 200 °C (5 ppm), the responses for In2O3 and S-1 to S-5 were 15, 2, 124, 28, 9, and 4, respectively (Fig. 5b), indicating that moderate Se doping (S-2, 1.55 at% Se) maximizes performance (7.8 × relative to pristine). Consistently, XPS and EPR reveal increased O-vacancy content for S-2, supporting a synergistic role of Se-induced defects in enhancing sensitivity. Table 2 summarizes the gas-sensitive performances of NO2 gas sensors based on In2O3 materials, in which the 1.55 at% Se-doped In2O3 sensor exhibits the optimal sensing performance.
| Materials | T (°C) | NO2 (ppm) | Response | Ref. |
|---|---|---|---|---|
| Au-decorated In2O3 microcubes | RT | 10 | 7.5 | 51 |
| Au-decorated In2O3 nanoparticles | RT | 5 | 95 | 52 |
| Zn-doped In2O3 nanotubes | RT | 10 | 15 | 53 |
| Ni-doped In2O3 nanoparticles | 200 | 10 | 70 | 54 |
| RGO@In2O3 composite nanofibers | RT | 1 | 14.18 | 55 |
| In2O3@GO composites | 225 | 40 | 78 | 56 |
| In2O3/TiO2 nanofibers | RT | 5 | 49.29 | 57 |
| In2O3/SnS2 nanorods | 70 | 0.5 | 67 | 58 |
| In2O3/PANI nanosheets | 250 | 3 | 12.8 | 59 |
| In2O3/Ti3C2 composites | 150 | 10 | 33.8 | 60 |
| γ-In2Se3/In2O3 nanoflowers | 170 | 8 | 9.68 | 61 |
| Rb-Doped ZnO/In2O3 | RT | 5 | 42.9 | 62 |
| Se–In2O3 | 200 | 5 | 124 | This work |
In addition, the response/recovery time is an important parameter for evaluating the performance of gas sensors, reflecting the detection efficiency of the device.63Fig. 5c compares the response/recovery times of pure In2O3 and S-1 to S-5 devices for 5 ppm NO2 at 200 °C, which are 11.46/7.76, 13.54/10.48, 8.27/4.73, 8.99/6.43, 9.79/8.07, and 14.39/10.81, respectively. Despite the outstanding sensitivity of the S-2 device, its response/recovery times are relatively longer than those of some oxide-based gas sensors. This phenomenon can be primarily attributed to two factors: First, the thin film surface exhibits porosity yet local compactness. While this characteristic facilitates gas adsorption, it restricts molecular diffusion and desorption, resulting in sluggish dynamic response.64 Second, Se doping increases oxygen vacancy density and induces localized In–Se coordination. While this enhances surface reactivity, it also strengthens the chemical adsorption of NO2 molecules, prolonging the desorption process.65 Future performance improvements can be achieved by accelerating surface reaction kinetics and enhancing recovery performance through approaches such as morphology control, oxygen vacancy modulation, or catalytic surface modification.
It is noteworthy that the gas-sensitive performance of the S-5 device is poor, although the SEM image shows that the device has the largest pore structure and the EPR analysis indicates that it has the highest concentration of oxygen vacancies. This is mainly due to the significant volatilization of Se element during the 800 °C high-temperature annealing process, and the quantitative XPS analysis shows that the actual doping of Se in the S-5 device is only 0.8 at%. The results further indicate that the optimization of the electronic structure induced by Se doping is more crucial for the improvement of gas-sensing performance than the specific surface area effect due to the pore structure. In other words, Se doping is the core factor for improving the performance of In2O3-based NO2 gas sensors in this study.
In summary, the S-2 device exhibits optimal sensing performance. In order to further analyze its sensing characteristics, the conditions of the S-2 device under different experiments were studied in depth. As shown in Fig. 6a, its baseline resistance rises rapidly and stabilizes when NO2 gas is fed, and then drops rapidly and returns to the initial baseline level when the gas supply is stopped, indicating that the device has good response/recovery characteristics. In addition, the baseline resistance of S-2 shows a gradual decrease as the operating temperature increases, verifying its n-type semiconductor behavior.66
Fig. 6b-c show the dynamic response curves of the S-2 device and its linear fitting relationship for different concentrations of NO2 gas at the optimal operating temperature, respectively. As the NO2 concentration increased from 0.5 ppm to 20 ppm, the sensor response values increased steadily, which were 10, 22, 116, 276, and 484, respectively. The slope of the linear fit curves was 1.04, and the coefficient of determination (R2) was as high as 0.996, which indicated that there was an excellent linear correlation between the response values of the devices and the gas concentration. Based on the linear fitting results, the theoretical detection limit of the S-2 device for NO2 was calculated to be as low as 3.3 ppb using eqn (S4),33 indicating that the Se-doped In2O3 gas sensor has the potential for ultra-low concentration detection of NO2.
Repeatability is one of the important performance indicators for gas sensors. Fig. 6d shows that the fluctuation of the response value of the device is controlled within ±1.2% and the fluctuation of the recovery time is not more than ±8% in seven consecutive response/recovery cycles of exposure to 5 ppm NO2 gas at the optimal operating temperature, indicating that the device has an excellent reproducibility and operational stability.
In addition to repeatability, long-term stability and humidity tolerance are critical performance metrics for gas-sensing devices, directly determining the service life and reliability of sensors in practical applications. As shown in Fig. 7a (Fig. S8), in a 28 days continuous test of the S-2 device at 5 ppm NO2, the response value of the device was 104 in the first test, and the response and recovery performance was stable in the subsequent tests (7–28 days), with the response value maintained above 110. The poor performance in the first test stems from the fact that the surface state of the material has not been fully stabilized, e.g., surface defects, adsorbed contaminants, etc., which may affect the electron transport and gas reactivity. With the aging process and the extension of the test time, the surface state of the material is gradually stabilized, and the gas-sensitive performance is also stabilized.67
Selectivity is a key performance parameter for gas sensors applied in complex working conditions, especially in real environments where target gases often coexist with multiple interfering gases. To evaluate the selectivity and anti-interference capability of the S-2 device, we tested its response to six typical gases (including NO2, acetone, CO, H2, etc., all with a concentration of 5 ppm) at an operating temperature of 200 °C, and the results are shown in Fig. 7b. The response value of the S-2 device to NO2 is as high as 124, which is much higher than that to other interfering gases (e.g., the response value of Acetone is less than 1.63, the response value of CO is 1.55, and the response value of H2 is 1.44), showing extremely high NO2 selectivity. In summary, the Se–In2O3 device demonstrates exceptional sensitivity, selectivity, and stability, highlighting its significant application potential in environmental monitoring and air quality assessment. Its ultra-low detection limit (3.3 ppb) enables early warning of trace nitrogen dioxide pollution, which has great value for urban air pollution monitoring, industrial emission control, and indoor air safety evaluation. Furthermore, the sensor maintains outstanding stability over 28 days and operates at a relatively low temperature of 200 °C, indicating compatibility with low-power portable sensing systems and integrated smart environmental platforms.
3.3. Gas-sensing mechanism
As a typical n-type metal oxide semiconductor, the gas-sensitive response mechanism of In2O3 is mainly based on the change of carrier concentration caused by gas adsorption. When exposed to air, O2 is adsorbed on the surface of In2O3 in the form of molecules or atoms, forms an electron-depletion layer, lowering the free-carrier concentration and increasing the device resistance.18,68 When exposed to reducing gases (e.g., CO, H2, etc.), the gas molecules provide electrons to the adsorbed oxygen to reduce it to gaseous oxygen molecules or low valent oxygen species, and the electron-withdrawal layer becomes thinner and the device resistance decreases. When exposed to oxidizing gases such as NO2, the NO2 molecules, as strong electron acceptors, compete for binding sites with the surface adsorbed oxygen molecules and preferentially adsorb on the surface of In2O3, which further obtains electrons from the In2O3 lattice, exacerbating the surface electron depletion and leading to an increase in the device resistance.66,69,70Combined with the aforementioned material structure characterization results and gas-sensitive performance test data, the response mechanism of Se-doped In2O3 to NO2 can be explained by three synergistic mechanisms: first, low-coordinated In and unsaturated O are the preferred adsorption sites for NO2 (Fig. 8a).71 Second, oxygen vacancies dominate physical adsorption and charge-transfer processes (Fig. 8b).72 Se doping induced the generation of oxygen vacancies through the charge compensation effect. XPS high-resolution O 1s spectra (Fig. 3d) showed that the percentage of Odef was increased after doping, and the EPR results further indicated that the signal intensity of oxygen vacancies of the S-2 device was enhanced by about 30% compared with that of the pure In2O3. As highly active adsorption sites, the oxygen vacancies quickly capture NO2 molecules through physical adsorption and promote the transfer of electrons from In2O3 to NO2, thus enhancing the sensing performance. Third, In–Se bonds mediate enhanced chemisorption (Fig. 8c). Se4+ forms In–Se bonds by replacing O2− sites in the In2O3 lattice. The electron-rich nature of the In–Se bond results in a localized electron aggregation state on the surface, forming a strong electrostatic interaction with the electron-deficient NO2 molecule.73,74 In addition, the polarity of In–Se bonds is lower than that of In-O bonds, which weakens the adsorption tendency of the surface with oxygen species, prompting the surface active sites to favor the adsorption of NO2 molecules rather than oxygen molecules.75
In summary, the oxygen vacancy-induced physical adsorption and charge transfer mechanism, the In–Se bond-mediated chemical binding enhancement mechanism, and the surface low-coordination structure in the Se-doped In2O3 device work together to enhance the adsorption of NO2 gas. The synergy of the three forms the basis of the microscopic mechanism of the excellent gas-sensitized performance of the device.
4. Conclusions
This study established a controllable route for preparing Se-doped In2O3 films by RF magnetron sputtering of In2Se3 precursors followed by in situ oxidation annealing. Uniform incorporation of Se into the In2O3 lattice induced localized In–Se coordination interactions and enriched oxygen vacancies, thereby enhancing surface reactivity and interfacial charge transfer. For 1.55 at% Se-doped In2O3, the response to 5ppm NO2 reached 124 at 200 °C with a detection limit of 3.3 ppb, demonstrating excellent 28-day stability and strong selectivity. Performance enhancement stems from the synergistic effect of Se-induced In–Se coordination and increased oxygen vacancy density, while the porous surface microstructure enhances NO2 adsorption and facilitates charge transport. Collectively, the combination of appropriate Se doping and microstructural porosity provides a simple and scalable defect engineering strategy for high-performance oxide semiconductor gas sensors.Author contributions
All authors have given approval to the final version of the manuscript. Lijuan Xie: designed the study, carried out gas-sensing measurements, data curation, formal analysis, investigation, and writing – original draft. Ziyao Wang: gas-sensing measurements and investigation. Jiabei Li: formal analysis. Shuo Li: investigation and formal analysis. Xiaohong Li: investigation and writing review. Xi Yang: designed the study, carried out the investigation, formal analysis, writing review, and funding acquisition. Guoqiang Li: writing review.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this work are available upon reasonable request from the corresponding author.The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: thermal analysis data of materials, preparation details of gas-sensitive films, material characterization (XRD, TEM, XPS, Raman, EPR), and analysis of device moisture resistance. See DOI: https://doi.org/10.1039/d5tc03395k.
Acknowledgements
This research was supported by the Sichuan Science and Technology program (2024YFHZ0075) and the Postgraduate Innovation Fund Project by Southwest University of Science and Technology (25ycx2045).References
- Z. Chen, J.-N. Wang, G.-X. Ma and Y.-S. Zhang, Lancet, 2013, 382, 1959–1960 CrossRef PubMed.
- R. Naidu, B. Biswas, I. R. Willett, J. Cribb, B. Kumar Singh, C. Paul Nathanail, F. Coulon, K. T. Semple, K. C. Jones, A. Barclay and R. J. Aitken, Environ. Int., 2021, 156, 106616 CrossRef CAS PubMed.
- X. Li, P. Liu, Z. Wang, P. Liu, X. Wei, Y. Wu and T. Lei, Renewable Energy, 2025, 241 Search PubMed.
- H. Pakdel, M. Borsi, M. Ponzoni and E. Comini, Chemosensors, 2024, 12, 54 CrossRef CAS.
- A. Tamvakos, K. Korir, D. Tamvakos, D. Calestani, G. Cicero and D. Pullini, ACS Sens., 2016, 1, 406–412 CrossRef CAS.
- M. W. Frampton, J. Boscia, N. J. Roberts, M. Azadniv, A. Torres, C. Cox, P. E. Morrow, J. Nichols, D. Chalupa, L. M. Frasier, F. R. Gibb, D. M. Speers, Y. Tsai and M. J. Utell, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2002, 282, L155–L165 CrossRef CAS PubMed.
- N. Greenberg, R. S. Carel, E. Derazne, H. Bibi, M. Shpriz, D. Tzur and B. A. Portnov, J. Toxicol. Environ. Health, Part A, 2016, 79, 342–351 CrossRef CAS PubMed.
- X. Meng, Y. Lin, K. Li, Z. Pang, L. Cheng and C. Zheng, IEEE Trans. Dielectr. Electr. Insul., 2025 DOI:10.1109/tdei.2025.3572332.
- Z. Jin, Y. Mou, J. Zhao, F. Liu, L. Liu, D. Zhao, S. Li, J. Liu and L. Wu, Chem. Eng. J., 2024, 499, 156531 CrossRef CAS.
- L. Zhou, X. Chang, W. Zheng, X. Liu and J. Zhang, Chem. Eng. J., 2023, 475, 146300 CrossRef CAS.
- Y. Yao, H. Sun, C. Cao, R. Yang, Z. Yan, Y. Deng, S. Liu, Q. Xu and Y. Qin, Nano Energy, 2025, 133, 110512 CrossRef CAS.
- A. Verma and B. C. Yadav, J. Hazard. Mater., 2024, 463, 132872 CrossRef CAS PubMed.
- A. Schleife, M. D. Neumann, N. Esser, Z. Galazka, A. Gottwald, J. Nixdorf, R. Goldhahn and M. Feneberg, New J. Phys., 2018, 20, 053016 CrossRef.
- M. Wang, G. Wang, W. Gong, S. Cheng, L. Zhao, X. Xu, D. Gong, F. Ye, L. Mo, H. Diao and W. Wang, Sol. Energy Mater. Sol. Cells, 2023, 253, 112229 CrossRef CAS.
- S. P. Patil, V. L. Patil, S. A. Vanalakar, S. S. Shendage, S. A. Pawar, J. H. Kim, J. Ryu, D. R. Patil and P. S. Patil, Mater. Lett., 2022, 306, 130916 CrossRef CAS.
- J. Ri, X. Li, C. Shao, Y. Liu, C. Han, X. Li and Y. Liu, Sens. Actuators, B, 2020, 317, 128194 CrossRef CAS.
- W. Niu, K. Kang, J. Hao, X. Chen, Y. Dong, H. Ren, Y. Guo, Y. Wang, P. Zhang, W. Hu, Y. Wu, Y. He and Y. Guo, ACS Sens., 2024, 9, 6103–6112 CrossRef CAS PubMed.
- N. Wang, J.-X. Ye, J.-B. Sun, X.-F. Zhang, Z.-P. Deng, Y.-M. Xu, L.-H. Huo and S. Gao, Sens. Actuators, B, 2022, 361, 131692 CrossRef CAS.
- H. Mei, J. Peng, T. Wang, T. Zhou, H. Zhao, T. Zhang and Z. Yang, Nano-Micro Lett., 2024, 16, 269 CrossRef CAS PubMed.
- J. Li, Y. Deng, L. Liu, Y. Chen, F. Jiang, M. Chen, J. Xia and Y. Deng, Small, 2025, 5, e05870 CrossRef PubMed.
- X. Chen, S. Lv, Y. Liu, H. Gu, X. Sun, Q. Hu, Y. Zhao, Z. Li, T. Guo, J. Kang, L.-M. Liu and L. Guo, Angew. Chem., Int. Ed., 2025, e202515222, DOI:10.1002/ange.202515222.
- Y. Guan, Y. Ding, Y. Fang, J. Li, Y. Liu, R. Wang, J. Hao, H. Xie, C. Xu, L. Zhen, Y. Li and L. Yang, Nat. Commun., 2025, 16, 4149 CrossRef CAS PubMed.
- Z. Jin, Y. Mou, J. Zhao, F. Liu, L. Liu, J. Liu, S. Li, F. Wang, Z. Wang and L. Wu, Chem. Eng. J., 2025, 512, 162338 CrossRef CAS.
- N. Du, H. Zhang, B. D. Chen, X. Y. Ma, Z. H. Liu, J. B. Wu and D. R. Yang, Adv. Mater., 2007, 19, 1641–1645 CrossRef CAS.
- Y. Yang, D. Zhang, D. Wang, Z. Xu and J. Zhang, J. Mater. Chem. A, 2021, 9, 14495–14506 RSC.
- J. Xie, Y. Cao, D. Jia, Y. Li and Y. Wang, Ceram. Int., 2016, 42, 90–96 CrossRef CAS.
- Z. J. Lu, J. C. Xu, B. Hong, J. Li, Y. X. Zeng, X. L. Peng, H. W. Chen and X. Q. Wang, Mater. Chem. Phys., 2024, 323, 129661 CrossRef CAS.
- S. C. Kulkarni, V. T. Salunke, S. Naeem and A. V. Patil, Ceram. Int., 2025, 51, 12253–12261 CrossRef CAS.
- G. Wang, Y. Wang, L. Guo, T. Chen, W. Zhao, X. Liu, J. Wang, X. Wang and Y. Yang, Sens. Actuators, B, 2025, 422, 136579 CrossRef CAS.
- J.-Y. Zhao and J.-M. Zhang, J. Phys. Chem. C, 2017, 121, 19334–19340 CrossRef CAS.
- A. Dey, Mater. Sci. Eng. B, 2018, 229, 206–217 CrossRef CAS.
- X. Zhou, X. Cheng, Y. Zhu, A. A. Elzatahry, A. Alghamdi, Y. Deng and D. Zhao, Chin. Chem. Lett., 2018, 29, 405–416 CrossRef CAS.
- J. Burgués, J. M. Jiménez-Soto and S. Marco, Anal. Chim. Acta, 2018, 1013, 13–25 CrossRef PubMed.
- X. Huang, C. Li, L. Qian, M. Li, H. Li, X. Niu and B. Yang, Mater. Today Commun., 2019, 21, 100680 CrossRef CAS.
- P. Bindu and S. Thomas, J. Theor. Appl. Phys., 2014, 8, 123–134 CrossRef.
- W. L. Bragg, Nat. Phys., 2014, 23, 153 Search PubMed.
- H. Zhu, Z. Yuan, Y. Shen, C. Han, H. Ji, Z. Mu and F. Meng, Sens. Actuators, B, 2022, 373, 132726 CrossRef CAS.
- A. Sanger, S. B. Kang, M. H. Jeong, M. J. Im, I. Y. Choi, C. U. Kim, H. Lee, Y. M. Kwon, J. M. Baik, H. W. Jang and K. J. Choi, Adv. Sci., 2018, 5, 100816 Search PubMed.
- A. M. Ritzmann, J. M. Dieterich and E. A. Carter, Phys. Chem. Chem. Phys., 2016, 18, 12260–12269 RSC.
- Z. Wang, C. Hou, Q. De, F. Gu and D. Han, ACS Sens., 2018, 3, 468–475 CrossRef CAS PubMed.
- Z. Sun, X. Yan, L. Huang, Y. Zhang, Z. Hu, C. Sun, X. Yang, G. Pan and Y. Cheng, Sens. Actuators, B, 2023, 381, 133355 CrossRef CAS.
- N. Zhao, X. Chang, X. Liu, J. Li, W. Zheng and J. Zhang, Angew. Chem., Int. Ed., 2025, e202512808, DOI:10.1002/anie.202512808.
- J. Bai, Y. Kong, Z. Liu, H. Yang, M. Li, D. Xu and Q. Zhang, Appl. Surf. Sci., 2022, 583, 152521 CrossRef CAS.
- G. Cheng, S. Xie, B. Lan, X. Zheng, F. Ye, M. Sun, X. Lu and L. Yu, J. Mater. Chem. A, 2016, 4, 16462–16468 RSC.
- H. Wang, P. Duan, J. Tian, Q. Duan, K. Jin and J. Sun, Sens. Actuators, B, 2024, 426, 137082 CrossRef.
- Y. Zhu, J. Wu, Z. Zhang, W. Wu, X. Wang, Y. Zhao and C. Zhao, ACS Sens., 2025, 10, 4348–4360 CrossRef CAS PubMed.
- J. Miao, Y. Fan, S. Zhu, X. Li, L. Lei, K. Yan, S. Zheng, W. Wang and H. Fan, Sens. Actuators, B, 2024, 402, 135109 CrossRef CAS.
- H. Geistlinger, Sens. Actuators, B, 1993, 17, 47–60 CrossRef CAS.
- Z. Hua, Y. Li, Y. Zeng and Y. Wu, Sens. Actuators, B, 2018, 255, 1911–1919 CrossRef CAS.
- S. Roso, D. Degler, E. Llobet, N. Barsan and A. Urakawa, ACS Sens., 2017, 2, 1272–1277 CrossRef CAS PubMed.
- X. Zhang, Y. Zhang, J. Wang, S. Liu, Z. Liang, S. Hussain, M. Wang, G. Liu and G. Qiao, Sens. Actuators, A, 2023, 361, 114590 CrossRef CAS.
- Z. Cai, J. Park and S. Park, J. Alloys Compd., 2023, 965, 171352 CrossRef CAS.
- F. Meng, Y. Li, C. Han, Z. Zhang, X. Yan and M. Yang, Chem. Eng. J., 2025, 510, 161674 CrossRef CAS.
- Z. Jin, C. Wang, L. Wu, H. Song, X. Yao, J. Liu, J. Zhao, Z. Zeng and F. Wang, Sens. Actuators, B, 2023, 377, 133058 CrossRef CAS.
- W. Yang, Y. Huo, T. Wang, X. Liu, D. Li, H. Yu, X. Dong and Y. Yang, Sens. Actuators, B, 2025, 430, 137359 CrossRef CAS.
- S. Shah, S. Han, S. Hussain, G. Liu, T. Shi, A. Shaheen, Z. Xu, M. Wang and G. Qiao, Ceram. Int., 2022, 48, 12291–12298 CrossRef CAS.
- Z. Cai, J. Park, D. Jun and S. Park, CrystEngComm, 2023, 25, 2546–2556 RSC.
- D. Sun, W. Wang, Y. Fan, Y. Chen and S. Ruan, J. Alloys Compd., 2025, 1014, 178738 CrossRef CAS.
- J. N. O. Amu-Darko, S. Hussain, Q. Gong, X. Zhang, Z. Xu, M. Wang, G. Liu and G. Qiao, J. Environ. Chem. Eng., 2023, 11, 109211 CrossRef CAS.
- J. N. O. Amu-Darko, S. Hussain, M. Wang, S. Lei, A. A. Alothman, S. Mohammad, G. Qiao and G. Liu, Sens. Actuators, B, 2024, 407, 135464 CrossRef CAS.
- H. Xing, X. Li, S. Sun, B. Huang and X. Li, Sens. Actuators, B, 2024, 415, 136034 CrossRef CAS.
- Y. Yang, J. Cui, Z. Luo, Z. Luo and Y. Sun, Sensors (Basel), 2024, 24, 5311 CrossRef CAS PubMed.
- C. Fan, J. Shi, Y. Zhang, W. Quan, X. Chen, J. Yang, M. Zeng, Z. Zhou, Y. Su, H. Wei and Z. Yang, Nanoscale, 2022, 14, 3441–3451 RSC.
- A. Sharma, S. B. Eadi, H. Noothalapati, M. Otyepka, H. D. Lee and K. Jayaramulu, Chem. Soc. Rev., 2024, 53, 2530–2577 RSC.
- R. Bhusari, J.-S. Thomann, J. Guillot and R. Leturcq, ACS Appl. Nano Mater., 2022, 5, 10248–10257 CrossRef CAS.
- D. A. Mirabella and C. M. Aldao, ACS Sens., 2024, 9, 1938–1944 CrossRef CAS PubMed.
- Y. M. Kwon, B. Oh, R. Purbia, H. Y. Chae, G. H. Han, S.-W. Kim, K.-J. Choi, Y. Lee, J. J. Kim and J. M. Baik, Sens. Actuators, B, 2023, 375, 132939 CrossRef CAS.
- S. Yang, H. Yin, Z. Wang, G. Lei, H. Xu, Z. Lan and H. Gu, Front. Chem., 2023, 11, 1174207 CrossRef CAS PubMed.
- G. Lu, J. Xu, J. Sun, Y. Yu, Y. Zhang and F. Liu, Sens. Actuators, B, 2012, 162, 82–88 CrossRef CAS.
- Y.-K. Lv, Y.-Y. Li, R.-H. Zhou, Y.-P. Pan, H.-C. Yao and Z.-J. Li, ACS Appl. Mater. Interfaces, 2020, 12, 34245–34253 CrossRef CAS PubMed.
- P. M. Bulemo, D. H. Kim, H. Shin, H. J. Cho, W. T. Koo, S. J. Choi, C. Park, J. Ahn, A. T. Guntner, R. M. Penner and I. D. Kim, Chem. Rev., 2025, 125, 4111–4183 CrossRef CAS PubMed.
- Y. J. Kwon, S. Y. Kang, P. Wu, Y. Peng, S. S. Kim and H. W. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 13646–13658 CrossRef CAS PubMed.
- K. J. Xiao, A. Carvalho and A. H. Castro Neto, Phys. Rev. B: Condens. Matter Mater. Phys., 2017, 96, 054412 CrossRef.
- X. Tang, J. Shang, Y. Gu, A. Du and L. Kou, J. Mater. Chem. A, 2020, 8, 7331–7338 RSC.
- Y. Cai, G. Zhang and Y.-W. Zhang, J. Phys. Chem. C, 2017, 121, 10182–10193 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |