 Open Access Article
Open Access ArticleZinc-induced ordering in L10-type platinum-based nanoalloys for the electrocatalytic oxygen reduction reaction
Wu
Tian
a,
Ryota
Sato
 *b,
Tomoki
Uchiyama†
c,
Yasutomi
Tatetsu
d,
Kenshi
Matsumoto
b,
Yoshiharu
Uchimoto
*b,
Tomoki
Uchiyama†
c,
Yasutomi
Tatetsu
d,
Kenshi
Matsumoto
b,
Yoshiharu
Uchimoto
 c and
Toshiharu
Teranishi
c and
Toshiharu
Teranishi
 *ab
*ab
aDepartment of Chemistry, Kyoto University, Uji, Kyoto 611-0011, Japan
bInstitute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011, Japan. E-mail: r-sato@scl.kyoto-u.ac.jp; teranisi@scl.kyoto-u.ac.jp
cGraduate School of Human and Environmental Studies, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan
dDepartment of Health Informatics, Meio University, Biimata, Nago, Okinawa 905-8585, Japan
First published on 14th January 2026
Abstract
Platinum (Pt)-based intermetallic compound (IMC) nanoparticles (NPs) show high activity and durability in the oxygen reduction reaction (ORR) even in the highly acidic environment of polymer electrolyte fuel cells. Although Pt-based IMCs with L10 crystal structure have been thoroughly studied, further improvement of ordering degree is required to enhance their ORR catalytic performance. Typical syntheses of L10-ordered PtM (M is usually a 3d transition metal) IMC NPs require high-temperature annealing to induce the disorder–order transformation, which leads to unfavorable NP aggregation. Given that L10-PtZn has a much lower phase transformation temperature than other L10-PtM, Zn was included to allow the phase transformation of L10-Pt(M,Zn) NPs to occur at relatively low temperature, thus suppressing aggregation and increasing the average ordering degree of NPs. We fabricated sub-6-nm L10-Pt(M,Zn) NPs with a high ordering degree (>80%) through a facile wet chemical route followed by low-temperature annealing. Lattice constant tuning achieved by precise composition control revealed a positive correlation between the strain effect and corresponding ORR activity. Highly ordered L10-Pt5Co4Zn1 and L10-Pt5Ni4Zn1 NPs showed high specific activities of 1.71 and 1.40 mA cmPt−2, respectively, which are almost comparable to that of L10-PtCo NPs.
1. Introduction
A proton-exchange membrane fuel cell (PEMFC) can be defined as a galvanic cell that converts chemical energy directly into electrical energy. PEMFC s show promise as a type of next-generation energy source because of their advantages including fast fueling, high energy density, and zero pollution.1 However, the high cost, low efficiency, and short life time of the catalysts for the cathodic oxygen reduction reaction (ORR) are major technical obstacles for the production of commercially feasible PEMFCs. After decades of research developing highly efficient ORR catalysts, scarce and expensive platinum (Pt) is still an indispensable core component of cathodic ORR catalysts. Recent studies have shown that incorporation of transition metals (M) with smaller atomic radius (such as Fe, Co, Ni, Cu, etc.) into Pt nanoparticles (NPs) introduces beneficial compressive strain into the Pt-rich atomic layers that are formed on the outermost surface by acid treatment. A theoretical study suggested that compressive surface strain in the Pt layer weakens its binding with oxygen intermediates, thus increasing the cathodic ORR performance of PtM-Pt core–shell (PtM@Pt) catalysts.2 As highlighted by Pt3Ni@Pt nanoframes,3 PtNi@Pt bunched nanocages,4 and Pt3Co@Pt NPs,5 both alloying Pt with M, especially Co and Ni with quite small atomic radii, and constructing core–shell structures with Pt-rich surfaces can induce a compressive strain effect in the Pt outermost layer, which can effectively accelerate ORR kinetics as well as lower the Pt content of the catalysts. Although some catalysts achieved high ORR performance, most current Pt-based catalysts suffer from poor durability because of the leaching of M in the strongly acidic electrolyte at high potential, which leads to structural changes that decrease activity.To prevent M leaching from PtM NPs, highly ordered PtM intermetallic compounds (IMCs) with well-defined stoichiometry have attracted much attention as stable core materials for surface strain-engineered PtM@Pt NPs recently. PtM IMCs possess higher acid corrosion resistance than that of disordered PtM NPs because of their larger formation enthalpy.6,7 There are already multiple reports on the synthesis of binary L10-PtM and ternary L10-type Pt (M1,M2) NPs as high-performance ORR catalysts including L10-PtZn,8 L10-PtNi,9,10 L10-PtCo,6,11,12 L10-PtFe,7 and L10-PtCoNi.13,14 Low-temperature synthesis of Pt-based IMC NPs containing M with high melting points (Fe: 1535 °C; Co: 1495 °C; Ni: 1453 °C) is challenging even though Pt IMCs are thermodynamically more favourable than their solid-solution counterparts at room temperature.15 High-temperature thermal annealing is usually required to induce phase transformation from disordered PtM alloys into ordered PtM IMCs in the solid state by overcoming the high energy barrier for atom diffusion to achieve ordering.16,17 High-temperature annealing causes severe sintering of NPs, especially when at high NP loading on supports, resulting in a large decrease of the specific surface area (surface-to-volume ratio) of the NPs. In addition, the long-range order parameter (S) that quantifies the ordering degree is compromised when the annealing temperature exceeds the relatively lower phase-transition temperature (TPT) from an L10-type IMC to A1-type solid solution in the corresponding bulk Pt–M binary phase diagram (TPT: PtFe ∼1300 °C, PtCo ∼820 °C, PtNi ∼630 °C).9 For NPs with high specific surface area, TPT decreases because of the increased contribution of entropy as a function of temperature in the Gibbs free energy equation resulting from the increased exposure of the disordered surface. Thus, as a problem unique to NPs with a size distribution, a situation may arise where smaller particles with A1 structure and particles with L10 structure that are larger than a critical particle size coexist depending on the heat treatment temperature. As a result, the average S for ensemble NPs tends to decrease with particle size. The S achieved after thermal annealing, which is the key parameter to improve the electrocatalytic performance of L10-PtM, has seldom been discussed in previous studies. A feasible way to improve the ordering degree of small IMC NPs and thus increase the activity and durability of ORR catalysts for fuel-cell applications still needs to be demonstrated.
The introduction of low-melting-point metals into IMC nanoparticles (NPs) is a promising strategy for lowering the activation energy of atomic diffusion.16–18 Elements from groups 12–15 are suitable candidates due to their low melting points, such as Zn (420 °C), Cd (321 °C), Ga (30 °C), In (157 °C), Sn (232 °C), Pb (328 °C), and Bi (∼271 °C). However, incorporating such metals may reduce the compressive strain in the surface Pt atomic layers, thereby decreasing the specific activity for the oxygen reduction reaction (ORR), because most of these metals have larger atomic radii than 3d transition metals such as Co and Ni (atomic radius: Co ∼125 pm, Ni ∼125 pm). Among these low-melting-point metals, we selected Zn because it has a relatively small atomic radius (134 pm) and forms a thermodynamically stable L10 structure with Pt.
Herein, we use an organometallic zero-valance Zn complex to incorporate hard-to-reduce Zn into Pt–M NPs by a facile wet method. The incorporation of Zn lowers the ordering temperature of Pt–M–Zn NPs in the formation of ordered IMC NPs, which suppresses sintering and leads to a high ordering degree. To improve ORR activity, i.e., to optimize the strain effect on the Pt outermost layer formed by acid treatment, compositionally controlled L10-type Pt(M,Zn) (M = Co or Ni) NPs with high ordering degree are synthesized through low-temperature annealing. The activity of the highly ordered L10-Pt5Co4Zn1 and L10-Pt5Ni4Zn1 NP catalysts is investigated under ORR conditions relevant to PEMFCs.
2. Experimental
2.1 Chemicals
Platinum(II) acetylacetonate (Pt(acac)2, 97%), cobalt(III) acetylacetonate (Co(acac)3, 98%), nickel(II) acetylacetonate (Ni(acac)2, 95%), oleylamine (OAm, 80–90%), borane-tert-butylamine complex (BBA, 97%), and Nafion (5% in a mixture of 2-propanol and water) were purchased from Sigma-Aldrich. Diphenylzinc (Ph2Zn, 99%), n-hexane (98.5%), and ethanol (>99%) were purchased from Fujifilm Wako Pure Chemical. Ph2Zn was dissolved in OAm to form a 10 mM solution and stored in an argon (Ar)-filled glovebox. Other chemicals were used without further purification.2.2 Synthesis of A1-type Pt–M–Zn (M = Co, Ni) NPs
As a typical synthesis of A1-type Pt5Co4Zn1 NPs, 0.05 mmol of Pt(acac)2, 0.048 mmol of Co(acac)3, 1.6 mL of 10 mM Ph2Zn OAm solution, and 5.0 mL of OAm were mixed in a glass pressure tube in a glovebox under an inert reaction atmosphere. The reaction solution was heated with an oil bath at 80 °C for 30 min and then at 230 °C for 120 min, followed by cooling to room temperature. Next, 10 mL of n-hexane and 30 mL of ethanol were added to the reaction solution to precipitate the product. After centrifugation at 9000 rpm (9510 rcf) for 15 min and washing three times with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 volume ratio mixture of n-hexane and ethanol, A1-type Pt5Co4Zn1 NPs were collected and dispersed in n-hexane for further use. By adjusting the feed ratio of Co(acac)3, Ni(acac)2, and Ph2Zn, the compositions of A1-type Pt–M–Zn NPs were changed as desired. For the preparation of Pt5Ni5 NPs, 10 mg of BBA was added as a stronger reductant to decrease the NP size.19
4 volume ratio mixture of n-hexane and ethanol, A1-type Pt5Co4Zn1 NPs were collected and dispersed in n-hexane for further use. By adjusting the feed ratio of Co(acac)3, Ni(acac)2, and Ph2Zn, the compositions of A1-type Pt–M–Zn NPs were changed as desired. For the preparation of Pt5Ni5 NPs, 10 mg of BBA was added as a stronger reductant to decrease the NP size.19
2.3 Preparation of carbon-supported L10-Pt(M,Zn)@Pt NPs (L10-Pt(M,Zn)@Pt/C)
Carbon black (60 mg, Vulcan XC-72) was suspended in 60 mL n-hexane by sonication for 30 min and then an n-hexane dispersion of A1-type Pt–M–Zn NPs was added to this suspension. The loading amount of Pt was maintained at around 11 to 14 wt%. After sonication for 1 h, the carbon-supported Pt–M–Zn NPs (Pt–M–Zn/C) were dried and annealed under 4% hydrogen (H2) in Ar at a designated temperature (550 or 600 °C) to convert the A1 structure into L10 structure. L10-Pt(M,Zn)/C was then treated in 0.1 M HClO4 aqueous solution at 60 °C for 12 h to give carbon-supported catalysts with L10-Pt(M,Zn)-Pt core–shell NPs (L10-Pt(M,Zn)@Pt/C) for further study. Disordered A1-PtCo@Pt/C and A1-PtNi@Pt/C catalysts for ORR testing were synthesized using identical procedures, followed by annealing at 400 °C to ensure uniform elemental distribution.2.4 Thermogravimetric analysis (TGA) to measure Pt loading amount
TGA was conducted to measure the Pt loading amount of L10-Pt(M,Zn)@Pt/C. Before sample measurement, a blank TGA measurement was conducted using an empty pan under the same conditions as the sample measurement for background correction. The sample measurement was then conducted under 20% oxygen (O2) in nitrogen (N2) flowing at 300 mL min−1. Temperature programming was set at 5 °C min−1 from 50 to 1000 °C to fully oxidize the carbon support. The residue consisted of Pt alloys. Background correction was performed by subtracting the thermogravimetric curve of the blank measurement from that of the sample. The Pt mass loading of each sample was determined by combining TGA results with the sample composition determined by scanning electron microscopy-energy-dispersive X-ray spectroscopy (SEM-EDX).2.5 Estimation of surface strain in L10-Pt(M,Zn)@Pt NPs
The surface strain in the Pt shell on the L10-Pt(M,Zn) core of the L10-Pt(M,Zn)@Pt NPs was estimated by comparison of the area of a triangle consisting of three neighboring atoms on {111} planes of L10-Pt(M,Zn) and that of pure Pt using the following equation to calculate the lattice mismatch strain. | (1) |
2.6 Calculation of the long-range order parameter of L10-Pt(M,Zn) NPs
The average degree of chemical (atomic) order for ensemble L10-Pt(M,Zn) NPs can be evaluated as the long-range order (LRO) parameter (S) extracted from the XRD pattern by comparing the integrated intensity ratio of the 110 superlattice reflection peak to the principal 111 reflection peak, I110/I111. The experimental I110/I111 derived from the refined XRD pattern can be defined as:20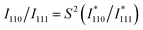 | (2) |
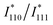 is the simulated intensity ratio for the perfectly ordered L10-type intermetallic compound (IMC) with the ideal stoichiometric compositions of Pt50M50−xZnx. Thus, S values for perfectly ordered L10-Pt(M,Zn) and perfectly disordered A1-Pt(M,Zn) alloys are 1 and 0, respectively. To experimentally determine I110 and I111 with a high degree of accuracy, Rietveld refinement was performed in the 2θ range of 30° to 90° to avoid interference from the broad peak around 25° derived from the carbon support. The
is the simulated intensity ratio for the perfectly ordered L10-type intermetallic compound (IMC) with the ideal stoichiometric compositions of Pt50M50−xZnx. Thus, S values for perfectly ordered L10-Pt(M,Zn) and perfectly disordered A1-Pt(M,Zn) alloys are 1 and 0, respectively. To experimentally determine I110 and I111 with a high degree of accuracy, Rietveld refinement was performed in the 2θ range of 30° to 90° to avoid interference from the broad peak around 25° derived from the carbon support. The 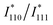 ratio depends on the composition of Pt50M50−xZnx to a non-negligible degree. Therefore, the
ratio depends on the composition of Pt50M50−xZnx to a non-negligible degree. Therefore, the 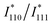 ratios of fully ordered alloys with corresponding compositions were simulated, as listed in Table S2.
ratios of fully ordered alloys with corresponding compositions were simulated, as listed in Table S2.
2.7 Catalyst ink preparation and electrochemical measurements
Catalyst ink for electrochemical measurements was prepared by mixing carbon-supported catalyst (1 mg) with ultrapure water (800 µL), 2-propanol (200 µL), and Nafion solution (5 wt%, 10 µL) and sonicating the mixture for 1 h. Catalyst ink (10 µL) was then deposited on a glassy carbon rotating disk electrode (5 mm in diameter) and dried via a spin-assisted method at 700 rpm. The experiments were conducted in 0.1 M HClO4 electrolyte using a three-electrode system with Pt wire as a counter electrode and a reversible hydrogen electrode (RHE, 5% H2) as a reference electrode. The measured potential was corrected by adding 0.0385 V according to the Nernst equation. Cyclic voltammetry (CV) and linear sweep voltammetry (LSV) were used to evaluate catalytic performance. All potentials are referenced to RHE. Cleaning CV measurements were conducted by scanning the electrode in the range of 0.02–1.20 V at 100 mV s−1 for 50 cycles under N2 saturation. The final CV was collected in the range of 0.02–1.10 V at 50 mV s−1 to calculate the electrochemical surface area (ECSA) from the Hupd adsorption peak area of the final CV. LSV of the ORR was conducted by positively scanning from 0.2 to 1.2 V at 10 mV s−1 during rotation at 2500 to 100 rpm in an O2-saturated atmosphere. The final ORR linear scanning current was corrected by subtracting the current obtained in N2-saturated atmosphere using the same method and conditions. The specific activity (is) and mass activity (im) at 0.9 V (vs. RHE) were calculated based on the Koutecky–Levich equation. Accelerated durability tests (ADTs) were conducted in a half-cell configuration using constant-potential polarization at 0.60 and 0.95 V (3 s at each potential). After every 2500 cycles, cyclic voltammograms (CVs) were recorded to evaluate the ECSA of the catalysts.2.8 Material characterization
The SEM-EDX measurements were performed using an Edax Apollo XF attachment on a scanning electron microscope (SEM, Hitachi S-4800) at 20 kV, which detected Co-K, Ni-K, Zn-K, and Pt-L peaks. XRD patterns were collected at room temperature using a powder diffractometer in Bragg–Brentano configuration (Malvern Panalytical Aeris) with Cu Kα radiation (λ = 1.542 Å) at 40 kV and 15 mA. Rietveld refinements were performed using HighScore Plus, which is the commercial powder diffraction analysis software from Malvern Panalytical.21 Magnetic hysteresis M–H loops were measured on a superconducting quantum interface device (Quantum Design MPMS3) with a field of up to 7 T. Transmission electron microscopy (TEM) images were obtained on a transmission electron microscope (HT7820, Hitachi) at an accelerating voltage of 120 kV. High-resolution transmission electron microscopy (HRTEM), high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM), EDX spectroscopy, and electron energy loss spectroscopy (EELS) were conducted with a transmission electron microscope (JEM-ARM200F, JEOL) at an accelerating voltage of 200 kV.2.9 Computational calculations
Density functional theory (DFT) calculations were performed on L10-Pt1M1 (M = Fe, Co, Ni, Zn) and Zn-doped L10-Pt1M0.5Zn0.5 (M = Fe, Co, Ni) systems using the OpenMX code.22 The generalized gradient approximation of Perdew–Burke–Ernzerhof was employed for the exchange–correlation functional, and pseudo-atomic orbital basis sets (s3p2d1 for Fe, Co, Ni, and s3p2d2f1 for Pt) were used. Cutoff radii were set to 6.0 a.u. for Fe, Co, Ni, and 9.0 a.u. for Pt, with a cutoff energy of 500 Ry. A k-point mesh of 23 × 23 × 17 was used for Zn-doped L10-Pt1M0.5Zn0.5 systems and one of 16 × 16 × 17 was used for the other systems. Conventional cells were chosen as 1 × 1 × 1 for L10-Pt1M1 systems and √2 × √2 × 1 for Zn-doped L10-Pt1M0.5Zn0.5 systems to accommodate for different atomic positions. The formation energies Eform of L10-Pt1M1 and L10-Pt2M1Zn1 were calculated using the following equation.| Eform = Ealloy − (α × µ[Pt] + β × µ[M]) | (3) |
To investigate the effects of Zn addition on L10-PtM (M = Fe, Co, Ni) and its structural stability, Eform calculations were performed for systems where Zn was added to L10-PtM and A1-PtM. The generation of structures with different Zn concentrations was accomplished using special quasirandom structures (SQS), which can consider randomness with high accuracy.23 The generated structures were constructed using a 2 × 2 × 2 supercell of the L10 structure, which contained a total of 32 atoms: 16 Pt atoms and either M or Zn atoms (16 in total). In the A1 structure, the randomness of all elements was considered, whereas in the L10 structure, only the positions of the Pt atoms were fixed, allowing for substitutions of M atoms and Zn. For each Zn concentration x, all structures generated by SQS were selected, with a constant Pt concentration. Eform calculations were conducted using Matlantis v.7.0.0, a versatile atomistic simulator that implements neural network potentials.24 By performing structural optimizations on all model structures generated by SQS, the total energy of the systems was obtained. Subsequently, Eform for each system was calculated.
3. Results and discussion
3.1 Synthesis of highly ordered alloy L10-Pt(M,Zn)@Pt/C
Considering that L10-PtZn possesses a much lower TPT than those of other L10-PtM alloy NPs, we envisioned that Zn would promote ordering in the transformation of A1 to L10 structure at relatively low temperature to achieve both a high degree of ordering and sintering suppression. However, incorporation of hard-to-reduce Zn into nanosized PtM alloy with ideal composition and monodispersity is challenging because of the large negative standard electrode potential of Zn2+/Zn (E° at 25 °C = −0.76 V). Synthesis of PtZn IMC NPs usually involves the reduction of Zn2+ in Pt/Zn2+ nanocomposites, such as Pt NPs encapsulated in a ZnO matrix,8 Pt NPs surrounded by zeolitic imidazolate frameworks containing Zn2+,18 Pt NPs coated with a thin ZnO shell,25 and Zn-covered Pt NPs obtained by Zn vapor deposition.26 The high reaction temperature and complicated procedures in these strategies make it difficult to properly manipulate the composition and morphology of PtZn IMC NPs.Here, we used an organometallic zero-valance Zn complex to incorporate difficult-to-reduce Zn into Pt–M NPs by a facile wet chemical process to synthesize monodisperse Pt–M–Zn NPs. Zero-valent organozinc enables mild and well-controlled incorporation of Zn, suppressing independent Zn nucleation and promoting homogeneous alloying, which leads to uniform nanoparticle morphology and composition. The overall synthesis of carbon-supported L10-type Pt(M,Zn)-Pt core–shell NPs (denoted as L10-Pt(M,Zn)@Pt/C) is schematically represented in Fig. 1. First, platinum(II) bis(acetylacetonate) (Pt(acac)2), cobalt(III) tris(acetylacetonato) (Co(acac)3), nickle(II) bis(acetylacetonate) (Ni(acac)2), diphenylzinc (Ph2Zn) with specified molar ratios were co-reduced in oleylamine (OAm). Ph2Zn with zero-valance state Zn was used to directly incorporate Zn into the PtM alloys to avoid the Zn2+ reduction procedure. The synthesized A1-type (i.e., face-centered cubic (fcc)-based solid solution) Pt–M–Zn NPs were supported on carbon (denoted as A1-Pt–M–Zn/C) and then transformed into highly ordered L10 structure by low-temperature reductive annealing (denoted as L10-Pt(M,Zn)/C), followed by acid post-treatment to generate a Pt shell on the NP surface (L10-Pt(M,Zn)@Pt/C).
3.2 Precise composition control and phase transformation of A1-Pt–M–Zn NPs
Fig. 2A–F and S4A, B show transmission electron microscopy (TEM) images of the as-synthesized Pt–M–Zn NPs and corresponding size distributions. The average sizes of the monodisperse Pt–M–Zn NPs determined by measuring 200 randomly selected NPs for each sample were 3.5–6.5 nm, which matched well with X-ray diffraction (XRD) results (Table S3). The particle size decreased with increasing Zn content in both M cases because of highly active Ph2Zn.27 Based on the theory that too large or too small particle size is not suitable in terms of electrochemically active surface area (ECSA) or ordering from A1 to L10 structure during annealing, respectively, the optimal NP size is empirically considered to be in the 4–6-nm range. For the specific stoichiometry required for L10 structure formation, we controlled the atomic ratio of Pt/nonprecious metal in the NPs to be around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table S4). The XRD patterns of a series of as-synthesized Pt–M–Zn/C are presented in Fig. 2G and H. The diffraction peaks indicate that their crystal structures are fcc, meaning that the Pt–M–Zn NPs possess A1 structure with disordered distributions of all elements. The diffraction peaks shift to lower angle with increasing Zn/M ratio.
1 (Table S4). The XRD patterns of a series of as-synthesized Pt–M–Zn/C are presented in Fig. 2G and H. The diffraction peaks indicate that their crystal structures are fcc, meaning that the Pt–M–Zn NPs possess A1 structure with disordered distributions of all elements. The diffraction peaks shift to lower angle with increasing Zn/M ratio.
The as-synthesized A1-type NPs uniformly distributed on carbon supports were subjected to reductive annealing to induce phase transformation. The loading amount of Pt was controlled in the range of 11–15 wt%, as confirmed by thermogravimetric analysis (see Table S4). Although ordering of PtM NPs at high temperature is usually accompanied by sintering,28 severe sintering was not observed for A1-Pt–M–Zn/C annealed at 600 and 550 °C (see Fig. S5 and S6, respectively). XRD patterns of annealed samples (Fig. 3A, B, D and E) contained symmetric and broad diffraction peaks, indicating no severe aggregation, and new clear superlattice peaks at 33° (assignable to 110 peaks) demonstrating the formation of the L10 structure. As expected from calculations, we observed splitting of the 200 peaks of fcc structure into 200 and 002 peaks of L10 structure with shifts of the 200 peak to lower angle and 002 peak to higher angle with increasing Zn content. These changes indicated longer lattice constants along 〈100〉 directions (a and b) and shorter lattice constants along 〈001〉 directions (c) after annealing; that is, tetragonality (a/c) increased, consistent with the formation of L10 structure. Element distributions in L10-Pt(Co,Zn) and L10-Pt(Ni,Zn) were further analyzed by high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) and energy-dispersive X-ray spectroscopy (EDS) mapping. Uniform distributions of three elements in individual NPs were observed for both L10-Pt(Co,Zn) and L10-Pt(Ni,Zn) NPs, indicating no phase segregation after reductive annealing with or without subsequent acid treatment (Fig. 3C, F and S7–S11). These results strongly confirmed that the disordered A1 structure of the as-synthesized NPs with various Zn/M ratios was converted to ordered L10 structure by reductive annealing.
3.3 Zn-promoted formation of highly ordered L10-Pt(M,Zn) NPs
S is a critical parameter that determines both the activity and durability of catalysts. S of the NPs was evaluated by calculating the integrated intensity ratio I110/I111 obtained from the Rietveld refinement of XRD data (see Fig. S12 and S13 and Methods in the SI).29,30 L10-Pt5Co5/C and L10-Pt5Ni5/C obtained by reductive annealing for 6 h at 600 and 550 °C, respectively, showed S of 65% and 47%, respectively. L10-Pt5Co4Zn1/C and L10-Pt5Ni4Zn1/C obtained under the same conditions possessed higher S of 78% and 81%, respectively, which was induced by the small amount of Zn. When the annealing temperature of A1-Pt5Co5/C was increased from 600 to 650 °C, S of the resulting L10-Pt5Co5/C reached 79%, although obvious sintering took place in areas with high NP density (Fig. S14). Different from L10-Pt5Co5/C, S of L10-Pt5Ni5/C obtained by annealing A1-Pt5Ni5/C at 600 °C was 36%, probably because of the low TPT of PtNi of ∼630 °C (Fig. S15). These results demonstrate that the initiation of ordering at lower relative temperature in the presence of Zn is effective for obtaining highly ordered L10-Pt(M,Zn)/C. The S values of L10-Pt(Co,Zn)/C and L10-Pt(Ni,Zn)/C obtained at various annealing temperatures are summarized in Table S5. These values clearly show that S was increased by the presence of Zn even for small NPs. To study the effect of NP size on S without Zn incorporation, L10-Pt5Co5/C and L10-Pt5Ni5/C with a size of around 5 nm were also synthesized. These samples exhibited lower S than their larger counterparts (Fig. S16, S17 and Table S5). These results further emphasize the importance of Zn incorporation for achieving high S in small NPs.To consider the origin of the increase of S achieved by the addition of Zn, the formation energies (Eform) of A1-and L10-Pt(M,Zn) with various compositions were estimated by theoretical calculations. Calculated Eform of binary PtM at 0 K are more negative in the order PtCo < PtNi < PtFe < PtZn for both A1 and L10 structures (Fig. S18 and S19). The L10 structure is the thermodynamically stable phase with larger Eform than the A1 structure for all PtM in the low temperature region. The difference between Eform of the A1 and L10 structures (ΔEform = Eform[A1] − Eform[L10]) of each PtM is shown in Fig. S19B. ΔEform is an indicator of the difficulty of phase transformation from L10 to A1 at high temperature. A larger positive value of ΔEform indicates that the phase transformation can occur at higher temperature. ΔEform increased in the order PtNi < PtCo < PtFe < PtZn, corresponding to the ascending order of TPT obtained from bulk Pt–M binary phase diagrams (TPT: PtNi ∼630 °C < PtCo ∼820 °C < PtFe ∼1300 °C, PtZn over 900 °C [data over 900 °C are not available]). ΔEform of Pt1M1−xZnx with various compositions (0 < x < 1) are summarized in Fig. S19. The tendency of ΔEform to increase with Zn content in all systems strongly suggests that the incorporation of Zn contributes to the increase of TPT by making the L10 structure more stable than the A1 structure with the same composition, resulting in the promotion of L10 ordering even for small NPs.
The high degree of ordering induced by the introduction of Zn was further confirmed by magnetic measurements, as shown in Fig. S20. L10-Pt5Co5, Pt5Co4Zn1, and Pt5Co2.5Zn2.5 obtained by reductive annealing at 600 °C for 6 h exhibited superparamagnetic behavior at 27 °C and almost no coercivity (Hc), which could be explained by the low blocking temperature caused by their small NP size.31 Magnetic hysteresis loops were then measured at −263 °C. L10-Pt5Co5 showed hard magnetic properties with low Hc (2.6 kOe) and high saturation magnetization. In contrast, both L10-Pt5Co4Zn1 and L10-Pt5Co2.5Zn2.5 exhibited harder magnetic properties with higher Hc of 10.0 and 12.0 kOe, respectively, and lower saturation magnetization.32 This change in magnetic properties induced by Zn substitution could be explained by the increase in Hc caused by the increase in magnetic anisotropy resulting from the increase in the tetragonality of the L10 structure (see Table S5) and the decrease in magnetization caused by the decreased content of 3d lone-pair electrons. The approximately single-phase loops of L10-Pt5Co4Zn1 and L10-Pt5Co2.5Zn2.5 also indicate the uniform distribution of Zn atoms at an atomic level in the NPs. These results confirmed that Zn incorporation promoted atom diffusion at lower temperature, resulting in the formation of L10 structures with high S.33
3.4 Formation of compressed Pt-rich shells on L10-Pt(M,Zn) cores
To use the series of L10-Pt(M,Zn)/C as ORR catalysts, the samples were first subjected to acid treatment to generate Pt-rich shells through the etching of M from the NP surface. Elemental compositions before and after acid treatment were analyzed by SEM-EDX, from which it was confirmed that around 50% of M remained in the ordered L10 NPs (Table S4). Contrary to the ordered L10 NPs, dramatic decreases of M content were observed in disordered A1-PtM NPs (PtNi: 48% to 18%, PtCo: 50% to 32%) after acid treatment, indicating the superior resistance of the ordered alloy structure towards M etching.Detailed structural analysis of L10-Pt(M,Zn)@Pt NPs was conducted by HAADF-STEM and EDS mapping (Fig. 4, S7 and S8). Fig. 4A shows an HAADF-STEM image of a typical L10-Pt5Co2.5Zn2.5 NP enclosed by major {111} and {001} facets and minor {110} facets. The NP consists of a thin Pt shell and intermetallic L10 core of alternating Pt and Co/Zn columns, represented by dots with brighter and darker contrast, respectively. Note that it is difficult to distinguish Zn from Co and Ni in the HAADF-STEM image because of their similar Z contrast. Fig. 4B presents the fast Fourier transform (FFT) image of Fig. 4A, which shows superlattice spots such as 001 and 1−10 characteristic of L10 structure. The interplanar spacing of {001} planes (d{001}) measured from the masked inverse FFT image was 0.372 nm (Fig. 4C and D), which agreed well with the lattice constant c of L10-Pt5Co2.5Zn2.5 NPs determined from XRD refinement. The L10 structure core was further verified by the elemental mapping images (Fig. 4E–I), which showed alternating layers of Pt and Co/Zn. The acid treatment of L10-Pt5Co2.5Zn2.5 at 60 °C for 10 h provided a Pt-rich shell with a thickness of two to three atomic layers, as clearly seen in EDS line-scanning profiles (Fig. 4J and K), HAADF-STEM Z-contrast profiles (Fig. 4L and M), and electron energy loss spectroscopy mapping images (Fig. S21). XRD patterns before and after the acid treatment of representative L10-Pt5Co2.5Zn2.5/C (Fig. S22) further confirmed the formation of core–shell structure. That is, no shift of the superlattice peaks was observed after acid treatment, indicating the L10 structure was robust. The small negative shift of the non-superlattice peaks was caused by the etching of M to form the shell structure.
To experimentally confirm the compressive strain in the Pt-rich shell, we measured the actual interatomic distances in the Pt-rich shell from the HAADF-STEM Z-contrast image in Fig. 4A (also see Fig. S23 and S24). The interatomic distance between neighboring atoms in the {001} planes projected from the 〈110〉 direction corresponds to d{110}. The average measured d{110} values in the surface atomic layers were 0.266, 0.261, and 0.266 nm for the first, second, and third layer, respectively, which are considerably shorter than d{110} of both pure Pt (0.277 nm), despite the Pt-rich composition, and the highly ordered alloy core (0.271 nm). A similar trend was observed for the {111} plane. These results strongly support that the Pt-rich shells are subjected to compressive strain induced by the smaller lattice of the L10-PtM core relative to that of the shell. However, because it is complicated to determine the average compressive surface strain of a sample macroscopically, the surface strain of Pt shells (see the Methods section in the SI for definition) was estimated by calculating the lattice mismatch between L10-Pt(M,Zn) and pure Pt from the average lattice constant determined macroscopically by XRD measurements. The calculation results plotted in Fig. S3 showed that higher Zn content leads to lower surface strain. This is logical because the atomic radius of Zn (134 pm) is larger than those of Co (125 pm) and Ni (125 pm). However, the incorporation of low content of Zn can still lead to high S and large compressive surface strain.
Overall, acid treatment provided NPs with L10-Pt(M,Zn)@Pt core–shell structure by the effective etching of M/Zn components from the L10-Pt(M,Zn) NP surface. The Pt shells showed compressive strain caused by the lattice mismatch between the Pt shells and L10 cores, which is promising for achieving simultaneous improvements of ORR durability and activity.
3.5 ORR electrocatalytic performance of highly ordered L10-Pt(M,Zn)@Pt/C
The electrocatalytic performance of highly ordered L10-Pt(M,Zn)@Pt/C in the acidic ORR was evaluated using a typical thin-film rotating disc electrode technique. Cyclic voltammetry (CV) measurements were conducted in N2-saturated 0.1 M HClO4 aqueous solution at room temperature until stable CV curves were obtained (Fig. 5A and D). ECSA of the samples was calculated from the charge of hydrogen underpotential deposition and normalized by the Pt loading mass (see the SI for details). The ECSA values of L10-Pt(M,Zn)@Pt/C (40–60 m2 gPt−1) and commercial standard Pt/C (67 m2 gPt−1) tended to increase with decreasing NP size (Table S6). The surface areas of L10-Pt(M,Zn)@Pt/C are much higher than those of recently reported PtM IMCs catalysts because of the smaller initial particle size with narrower distribution and their morphology retention without aggregation after low-temperature annealing.6,7,9,14After obtaining stable CV curves, linear sweep voltammetry (LSV) was used to study the ORR catalytic activity of the highly ordered catalysts. As examples, the LSV polarization curves measured in O2-saturated 0.1 M HClO4 aqueous solution at 1600 rpm are shown in Fig. 5B and E. In both L10-Pt(Co,Zn)@Pt and L10-Pt(Ni,Zn)@Pt systems, the half-wave potential increased with the amount of Co or Ni. Specific activity (is) and mass activity (im) at 0.9 V vs. RHE were calculated from kinetically controlled current (ik) and ECSA (Fig. 5C and F), where ik was obtained from Koutecky–Levich plots of current extracted from LSVs measured at various rotation rates. The highest catalytic activity among the series of ternary L10-Pt(M,Zn)@Pt/C was obtained for L10-Pt5Co4Zn1@Pt/C, which showed is of 1.71 mA cmPt−2 and im of 0.69 A mgPt−1. These values are comparable with those of binary L10-Pt5Co5@Pt/C and are approximately 5.6 and 3.3 times higher than those of commercial Pt/C, respectively.
To quantitatively discuss the effect of compressive strain in Pt-rich shells on ORR catalytic activity, the surface strain of each highly ordered sample with S > 0.7, which was estimated from the lattice mismatch of the ORR-active {111} facets shown in Fig. S3, was plotted against is (Fig. 5G). This plot showed the monotonic increase of is with compressive surface strain. Therefore, the high ORR activity of L10-Pt(M,Zn)@Pt/C likely results from the high compressive strain in the Pt-rich shell with a thickness of two to three atomic layers derived from the smaller atomic radius of Co/Ni compared to that of Zn. The compressive strain induced in {111} planes is believed to modify the electronic structure, causing a downward shift of the d-band centre. This shift reduces the binding energy of oxygen intermediates and kinetically enhances ORR activity.34–37 In contrast, the poorly ordered L10-PtNi (∼38%) exhibits a significantly lower ECSA (25 m2 gPt−1) and specific activity (0.491 mA cmPt−2) compared with the highly ordered L10-Pt(M,Zn) counterparts (Table S6). The inferior ORR performance of L10-PtNi can be primarily attributed to its low degree of ordering, which restricts the beneficial strain and structural effects associated with ordered intermetallic phases. These results suggest that dealloying by M leaching and coarsening by agglomeration and Ostwald ripening of NPs during electrochemical cleaning processes prior to activity evaluation decrease activity and ECSA;38 that is, the high S of L10-Pt(M,Zn)@Pt/C contributes the improvement of both catalytic activity and durability.
To further confirm the exceptional durability of the highly ordered L10-Pt(M,Zn)@Pt/C, accelerated durability tests (ADTs) were performed on Pt/C, A1-PtNi@Pt/C, and the representative L10-Pt5Ni4Zn1@Pt/C in a half-cell configuration for comparison. The ECSA retention as a function of cycle number revealed a continuous decline for commercial Pt/C, retaining only ∼69% after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 5H). In contrast, both L10-Pt5Ni4Zn1@Pt/C and disordered A1-PtNi@Pt/C exhibited an initial increase in ECSA during the early stages of ADT. This phenomenon is generally attributed to electrochemical surface activation, including selective dissolution of less noble metals and surface reconstruction, which exposes previously inaccessible Pt active sites.39–41 With continued cycling, the disordered A1-PtNi@Pt/C catalyst experienced rapid ECSA decay following the initial activation, indicative of accelerated Ni leaching and structural degradation. Conversely, the highly ordered L10-Pt5Ni4Zn1@Pt/C maintained a sustained increase in ECSA throughout the ADT, suggesting that atomic ordering effectively suppresses excessive metal dissolution while enabling controlled surface activation. These findings are corroborated by TEM images before and after ADT (Fig. S26), which revealed significant particle growth for Pt/C and A1-PtNi@Pt/C, whereas the particle size of L10-Pt5Ni4Zn1@Pt/C remained essentially unchanged. These observations underscore the superior structural stability of the ordered intermetallic catalyst under prolonged cycling conditions.
000 cycles (Fig. 5H). In contrast, both L10-Pt5Ni4Zn1@Pt/C and disordered A1-PtNi@Pt/C exhibited an initial increase in ECSA during the early stages of ADT. This phenomenon is generally attributed to electrochemical surface activation, including selective dissolution of less noble metals and surface reconstruction, which exposes previously inaccessible Pt active sites.39–41 With continued cycling, the disordered A1-PtNi@Pt/C catalyst experienced rapid ECSA decay following the initial activation, indicative of accelerated Ni leaching and structural degradation. Conversely, the highly ordered L10-Pt5Ni4Zn1@Pt/C maintained a sustained increase in ECSA throughout the ADT, suggesting that atomic ordering effectively suppresses excessive metal dissolution while enabling controlled surface activation. These findings are corroborated by TEM images before and after ADT (Fig. S26), which revealed significant particle growth for Pt/C and A1-PtNi@Pt/C, whereas the particle size of L10-Pt5Ni4Zn1@Pt/C remained essentially unchanged. These observations underscore the superior structural stability of the ordered intermetallic catalyst under prolonged cycling conditions.
4. Conclusions
A feasible strategy to synthesize highly ordered IMC NPs with well-defined structures and compositions was developed. The ordering degree of L10-PtM NPs was increased and aggregation was prevented by Zn incorporation at low temperature. The ordering degree was greatly improved while maintaining a strain effect by decreasing the amount of Zn, which gave high ORR specific activity under harsh electrochemical conditions. In particular, L10-Pt5Co4Zn1 showed a mass activity of 0.69 A mgPt−1 and specific activity of 1.71 mA cmPt−2, demonstrating its potential for fuel-cell applications. By precisely controlling NP composition, a comprehensive map of the correlation between the strain effect and ORR activity was constructed, which showed that specific activity increases monotonically with the compressive strain of surface Pt. These results provide us with insight into the tuning of ORR catalytic activity by finely controlling the structures of IMC NPs.Author contributions
W. T., R. S., T. T. conceived the study. W. T., R. S. designed the synthesis scheme. W. T. performed the synthesis. W. T., R. S., K. M. were responsible for the characterization. W. T., R. S., T. U., Y. U. measured the electochemical catalytic properties. W. T., R. S. collected and analyzed the HAADF-STEM images and EDX elemental maps. Y. T. performed theoretical calculations. W. T., R. S., T. T. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: SI figures (Fig. S1–S26) and tables (Tables S1–S7) (PDF). See DOI: https://doi.org/10.1039/d5ta08256k.Acknowledgements
The PEMFC project commissioned by the New Energy and Industrial Technology Development Organization (NEDO) (Grant No. 20001199-0 [T. T.]). Japan Science and Technology Agency, Core Research for Evolutionary Science and Technology (JST-CREST) (Grant No. JPMJCR21B4 [T. T.]). The Japan Society for the Promotion of Science (JSPS) KAKENHI for Scientific Research (S) (Grant No. JP24H00053 [T. T.]) and Scientific Research (B) (Grant No. JP23K26627 [R. S.]). High-resolution STEM analyses were performed under the NEDO FC-Platform. We thank Natasha Lundin, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.References
- R. Borup, J. Meyers, B. Pivovar, Y. S. Kim, R. Mukundan, N. Garland, D. Myers, M. Wilson, F. Garzon, D. Wood, P. Zelenay, K. More, K. Stroh, T. Zawodzinski, J. Boncella, J. E. McGrath, M. Inaba, K. Miyatake, M. Hori, K. Ota, Z. Ogumi, S. Miyata, A. Nishikata, Z. Siroma, Y. Uchimoto, K. Yasuda, K.-i. Kimijima and N. Iwashita, Chem. Rev., 2007, 107, 3904–3951 CrossRef CAS PubMed.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef PubMed.
- C. Chen, Y. Kang, Z. Huo, Z. Zhu, W. Huang, H. L. Xin, J. D. Snyder, D. Li, J. A. Herron, M. Mavrikakis, M. Chi, K. L. More, Y. Li, N. M. Markovic, G. A. Somorjai, P. Yang and V. R. Stamenkovic, Science, 2014, 343, 1339–1343 CrossRef CAS PubMed.
- X. Tian, X. Zhao, Y.-Q. Su, L. Wang, H. Wang, D. Dang, B. Chi, H. Liu, E. J. M. Hensen, X. W. Lou and B. Y. Xia, Science, 2019, 366, 850–856 CrossRef CAS.
- D. Wang, H. L. Xin, R. Hovden, H. Wang, Y. Yu, D. A. Muller, F. J. DiSalvo and H. D. Abruña, Nat. Mater., 2013, 12, 81–87 CrossRef CAS.
- J. Li, S. Sharma, X. Liu, Y.-T. Pan, J. S. Spendelow, M. Chi, Y. Jia, P. Zhang, D. A. Cullen, Z. Xi, H. Lin, Z. Yin, B. Shen, M. Muzzio, C. Yu, Y. S. Kim, A. A. Peterson, K. L. More, H. Zhu and S. Sun, Joule, 2019, 3, 124–135 CrossRef CAS.
- J. Li, Z. Xi, Y. T. Pan, J. S. Spendelow, P. N. Duchesne, D. Su, Q. Li, C. Yu, Z. Yin, B. Shen, Y. S. Kim, P. Zhang and S. Sun, J. Am. Chem. Soc., 2018, 140, 2926–2932 CrossRef CAS.
- J. Liang, Z. Zhao, N. Li, X. Wang, S. Li, X. Liu, T. Wang, G. Lu, D. Wang, B.-J. Hwang, Y. Huang, D. Su and Q. Li, Adv. Energy Mater., 2020, 10, 2000179 CrossRef CAS.
- W. J. Zeng, C. Wang, Q. Q. Yan, P. Yin, L. Tong and H. W. Liang, Nat. Commun., 2022, 13, 7654 CrossRef CAS.
- T.-W. Song, C. Xu, Z.-T. Sheng, H.-K. Yan, L. Tong, J. Liu, W.-J. Zeng, L.-J. Zuo, P. Yin, M. Zuo, S.-Q. Chu, P. Chen and H.-W. Liang, Nat. Commun., 2022, 13, 6521 CrossRef CAS PubMed.
- C.-L. Yang, L.-N. Wang, P. Yin, J. Liu, M.-X. Chen, Q.-Q. Yan, Z.-S. Wang, S.-L. Xu, S.-Q. Chu, C. Cui, H. Ju, J. Zhu, Y. Lin, J. Shui and H.-W. Liang, Science, 2021, 374, 459–464 CrossRef CAS.
- J. Liang, N. Li, Z. Zhao, L. Ma, X. Wang, S. Li, X. Liu, T. Wang, Y. Du, G. Lu, J. Han, Y. Huang, D. Su and Q. Li, Angew. Chem., Int. Ed., 2019, 58, 15471–15477 CrossRef CAS.
- J. Li, S. Sharma, K. Wei, Z. Chen, D. Morris, H. Lin, C. Zeng, M. Chi, Z. Yin, M. Muzzio, M. Shen, P. Zhang, A. A. Peterson and S. Sun, J. Am. Chem. Soc., 2020, 142, 19209–19216 CrossRef CAS PubMed.
- T. Wang, J. Liang, Z. Zhao, S. Li, G. Lu, Z. Xia, C. Wang, J. Luo, J. Han, C. Ma, Y. Huang and Q. Li, Adv. Energy Mater., 2019, 9, 1803771 CrossRef.
- Y. Yan, J. S. Du, K. D. Gilroy, D. Yang, Y. Xia and H. Zhang, Adv. Mater., 2017, 29, 1605997 CrossRef.
- J. Liang, Y. Wan, H. Lv, X. Liu, F. Lv, S. Li, J. Xu, Z. Deng, J. Liu, S. Zhang, Y. Sun, M. Luo, G. Lu, J. Han, G. Wang, Y. Huang, S. Guo and Q. Li, Nat. Mater., 2024, 23, 1259–1267 CrossRef CAS PubMed.
- R.-Y. Shao, X.-C. Xu, Z.-H. Zhou, W.-J. Zeng, T.-W. Song, P. Yin, A. Li, C.-S. Ma, L. Tong, Y. Kong and H.-W. Liang, Nat. Commun., 2023, 14, 5896 CrossRef CAS.
- Z. Chen, J. Liu, B. Yang, M. Lin, C. Molochas, P. Tsiakaras and P. Shen, J. Colloid Interface Sci., 2023, 652, 388–404 Search PubMed.
- Y. Yu, W. Yang, X. Sun, W. Zhu, X. Z. Li, D. J. Sellmyer and S. Sun, Nano Lett., 2014, 14, 2778–2782 CrossRef CAS PubMed.
- A. Cebollada, D. Weller, J. Sticht, G. R. Harp, R. F. C. Farrow, R. F. Marks, R. Savoy and J. C. Scott, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 3419–3422 CrossRef CAS PubMed.
- T. Degen, M. Sadki, E. Bron, U. König and G. Nénert, Powder Diffr., 2014, 29, S13–S18 CrossRef CAS.
- T. Ozaki, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 155108 CrossRef.
- A. van de Walle, P. Tiwary, M. de Jong, D. L. Olmsted, M. Asta, A. Dick, D. Shin, Y. Wang, L. Q. Chen and Z. K. Liu, Calphad, 2013, 42, 13–18 CrossRef CAS.
- S. Takamoto, C. Shinagawa, D. Motoki, K. Nakago, W. Li, I. Kurata, T. Watanabe, Y. Yayama, H. Iriguchi, Y. Asano, T. Onodera, T. Ishii, T. Kudo, H. Ono, R. Sawada, R. Ishitani, M. Ong, T. Yamaguchi, T. Kataoka, A. Hayashi, N. Charoenphakdee and T. Ibuka, Nat. Commun., 2022, 13, 2991 CrossRef CAS.
- C. Huang, H. Liu, Y. Tang, Q. Lu, S. Chu, X. Liu, B. Shan and R. Chen, Appl. Catal., B, 2023, 320, 121986 CrossRef CAS.
- A. Miura, H. Wang, B. M. Leonard, H. D. Abruña and F. J. DiSalvo, Chem. Mater., 2009, 21, 2661–2667 Search PubMed.
- S. Mourdikoudis and L. M. Liz-Marzán, Chem. Mater., 2013, 25, 1465–1476 Search PubMed.
- G. Feng, F. Ning, Y. Pan, T. Chen, J. song, Y. Wang, R. Zou, D. Su and D. Xia, J. Am. Chem. Soc., 2023, 145, 11140–11150 Search PubMed.
- Z. Cui, H. Chen, M. Zhao and F. J. DiSalvo, Nano Lett., 2016, 16, 2560–2566 Search PubMed.
- K. Takanashi, S. Mitani, M. Sano, H. Fujimori, H. Nakajima and A. Osawa, Appl. Phys. Lett., 1995, 67, 1016–1018 Search PubMed.
- S. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989–1992 Search PubMed.
- G. Kim, T. Hiratsuka, H. Naganuma, M. Oogane and Y. Ando, J. Phys.: Conf. Ser., 2010, 200, 052011 Search PubMed.
- R. A. Ristau, K. Barmak, L. H. Lewis, K. R. Coffey and J. K. Howard, J. Appl. Phys., 1999, 86, 4527–4533 Search PubMed.
- A. Kulkarni, S. Siahrostami, A. Patel and J. K. Nørskov, Chem. Rev., 2018, 118, 2302–2312 Search PubMed.
- Z. Wang, Y. Mai, Y. Yang, L. Shen and C. Yan, ACS Appl. Mater. Interfaces, 2021, 13, 38138–38146 CrossRef CAS.
- Z. Wang, Y. Yang, X. Wang, Z. Lu, C. Guo, Y. Shi, H. Tan, L. Shen, S. Cao and C. Yan, J. Mater. Chem. A, 2022, 10, 12141–12149 Search PubMed.
- Y. Zhou, Z. Zhou, R. Shen, R. Ma, Q. Liu, G. Cao and J. Wang, Energy Storage Mater., 2018, 13, 189–198 CrossRef.
- X. Xie, A. L. M. Sandhya, L. Piliai, M. Vorokhta, I. Motolínová and I. Khalakhan, Appl. Catal., B, 2023, 325, 122328 CrossRef CAS.
- C. Lim, A. R. Fairhurst, B. J. Ransom, D. Haering and V. R. Stamenkovic, ACS Catal., 2023, 13, 14874–14893 CrossRef CAS.
- M. Gatalo, A. M. Bonastre, L. J. Moriau, H. Burdett, F. Ruiz-Zepeda, E. Hughes, A. Hodgkinson, M. Šala, L. Pavko, M. Bele, N. Hodnik, J. Sharman and M. Gaberšček, ACS Appl. Energy Mater., 2022, 5, 8862–8877 CrossRef CAS PubMed.
- X. Tong, J. Zhang, G. Zhang, Q. Wei, R. Chenitz, J. P. Claverie and S. Sun, Chem. Mater., 2017, 29, 9579–9587 CrossRef CAS.
Footnote |
| † Present address: Department of Metallurgy, Materials Science and Materials Processing, Tohoku University, Sendai, Miyagi 980-8579, Japan. |
| This journal is © The Royal Society of Chemistry 2026 |





