Dual-amide strategy for quaternary aqueous electrolytes
Insu
Jeong†‡
 a,
Younsang
Cho†
b,
Youngbi
Kim†
c,
Hyungmin
Park
d,
Jeong Woo
Han
a,
Younsang
Cho†
b,
Youngbi
Kim†
c,
Hyungmin
Park
d,
Jeong Woo
Han
 *e,
Soojin
Park
*e,
Soojin
Park
 *a and
Jaegeon
Ryu
*a and
Jaegeon
Ryu
 *bf
*bf
aDepartment of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang 37673, Republic of Korea. E-mail: soojin.park@postech.ac.kr
bDepartment of Chemical and Biomolecular Engineering, Sogang University, Seoul 04107, Republic of Korea. E-mail: jryu@sogang.co.kr
cDepartment of Chemical Engineering, Pohang University of Science and Technology (POSTECH), Pohang 37673, Republic of Korea
dKorea Conformity Laboratories (KCL), Jeonnam CCU (Carbon Capture and Utilization) Center, Yeosu 59631, Republic of Korea
eDepartment of Materials Science and Engineering, Research Institute of Advanced Materials, Seoul National University, Seoul 08826, Republic of Korea. E-mail: jwhan98@snu.ac.kr
fInstitute of Energy and Environment, Sogang University, Seoul 04107, Republic of Korea
First published on 2nd December 2025
Abstract
Aqueous Li-ion batteries (LIBs) offer promising advantages in terms of safety, sustainability, and cost-effectiveness, however, their practical application is fundamentally limited by the narrow electrochemical stability window (∼1.23 V) of water. To overcome this issue, solvation structure design is essential—not only for suppressing water reactivity but also for the formation of stable solid-electrolyte interphase (SEI) layers. Building on this concept, we introduce a dual-amide strategy through a quaternary aqueous electrolyte composed of LiTFSI, water, and two structurally complementary amide-based diluents—acetamide and ε-caprolactam. Their contrasting molecular characteristics enable modular tuning of Li+ solvation, strengthening Li+-anion association that serves as a foundation for anion-derived SEI formation, without compromising Li+ conductivity. The dual amide quaternary electrolyte retains 82% capacity after 1200 cycles at 1C—the longest cycle life reported for aqueous LIBs.
Introduction
Interfacial stability remains a unifying bottleneck across emerging battery chemistries, from lithium-metal and all-solid-state systems to more distinct alternatives such as sodium-ion, zinc-ion, multivalent-ion, dual-ion, and lithium-air batteries. Diverse battery systems share a common bottleneck: unstable electrode–electrolyte interfaces that limit cycle life, rate capability, and safety.1–6 These interfacial instabilities arise from issues such as uncontrolled side reactions, dendrite growth, mechanical delamination, and insufficient solid-electrolyte interphase (SEI) formation.1,7 Given the central role of the electrolyte in mediating ion transport and interfacial reactions, it is increasingly regarded as a critical design parameter for enabling interface stability.5,8 Accordingly, recent research has shifted toward electrolyte designs that not only facilitate ion conduction but also actively regulate chemical reactivity and phase evolution at the electrode–electrolyte boundary.Aqueous Li-ion batteries (LIBs) present compelling advantages in terms of safety, sustainability, and manufacturing cost owing to the use of water as a non-flammable, environmentally benign solvent.9 However, the inherently narrow electrochemical stability window (ESW) of water (∼1.23 V) poses a fundamental constraint, particularly under reductive potentials at the anode interface.10 This limitation leads to parasitic hydrogen evolution and continuous electrolyte decomposition, which severely undermine interfacial stability and cycling durability. Unlike organic electrolyte systems, where a SEI forms spontaneously to passivate the anode surface, aqueous systems require deliberate suppression of water activity—both chemically and structurally—to mitigate reductive side reactions and enable the formation of stable electrode–electrolyte interfaces.11,12
Rational design of Li+ solvation structures has emerged as a powerful strategy to modulate interfacial reactivity in aqueous LIBs. The local solvation environment—classified into solvent-separated ion pairs (SSIPs), contact ion pairs (CIPs), and aggregates (AGGs)—dictates the spatial arrangement and interaction strength between Li+, anions, and water molecules.13 Particularly, strong Li+-anion coordination in CIPs and AGGs disrupts the extended hydrogen-bond network of bulk water and effectively confines water molecules. Such molecular confinement enables the selective decomposition of anion and co-solvent species at the electrode surface, leading to the formation of a bilayer SEI: an inner LiF-rich layer that electronically passivates the interface and an outer organic layer that imparts mechanical flexibility and surface conformity.14 The bilayer architecture improves adhesion, accommodates electrode volume changes, and enhances long-term electrochemical performance.15 The feasibility of this approach was first demonstrated in “water-in-salt” electrolytes, wherein high concentrations of anions such as bis(trifluoromethanesulfonyl)imide (TFSI−) were employed to shift the solvation equilibrium toward anion-rich coordination environments.11 This strategy successfully suppressed free water activity and promoted SEI formation, validating solvation structure design as a powerful method for interfacial stabilization in aqueous systems. Building on this foundation, eutectic electrolyte systems have emerged as an extension of the above concept, incorporating hydrogen-bonding diluents such as urea, acetamide, or ε-caprolactam to further tailor the local solvation environment. These systems enhance Li+-anion coordination and suppress water reactivity more effectively than simply increasing the salt concentration.16–20
However, numerous eutectic formulations rely on a single diluent to perform several distinct roles. These include Li+ coordination, modulation of hydrogen bonding as well as controlling concentration. Relying on a single molecule for these multiple roles often leads to design conflicts and limits structural tunability. Organic LIB electrolyte design gave a valuable inspiration, as it consists of binary solvents to balance complementary properties. For example, linear carbonates such as dimethyl carbonate reduce viscosity and enhance ionic mobility, while cyclic carbonates like ethylene carbonate support SEI formation due to their high dielectric strength.8 While aqueous systems differ fundamentally, this modular solvent design principle provides a valuable framework for constructing tunable solvation environments that enable interfacial stabilization and water suppression.21
Herein, we present a quaternary aqueous electrolyte system comprising LiTFSI, water, and two structurally distinct amide-based hydrogen-bonding diluents: acetamide and ε-caprolactam. These diluents were selected for their complementary molecular characteristics. Acetamide, a small linear molecule with a high dielectric constant (ε = 66) and a primary amide group, exhibits strong hydrogen-bond donation and efficient Li+ coordination. On the other hand, ε-caprolactam, with its cyclic structure, secondary amide group, and low dielectric constant (ε = 1.8), introduces steric modulation and distinct solvation behavior. By combining these structurally and electronically divergent diluents, we construct a solvation environment that allows modular tuning of Li+-anion–solvent interactions. As illustrated in Fig. 1, this dual-amide strategy yields a compact solvation sheath enriched with TFSI− closely associated with Li+. Such structural organization facilitates direct anion transport to the electrode surface and enhances the likelihood of selective anion decomposition. The resulting interfacial reaction leads to the formation of a uniform, LiF-rich SEI layer that effectively suppresses hydrogen evolution while ensuring mechanical robustness through complementary organic byproducts. Compared to a ternary electrolyte with a single diluent, the quaternary formulation significantly improves interfacial passivation and electrochemical performance. The optimized quaternary electrolyte achieves a discharge capacity of 154.8 mAh g−1 at 1C and retains 82% capacity after 1200 cycles—representing the longest cycle life reported to date for aqueous LIBs under these conditions.
Results and discussion
Characterization of dual amide-based quaternary eutectic electrolyte
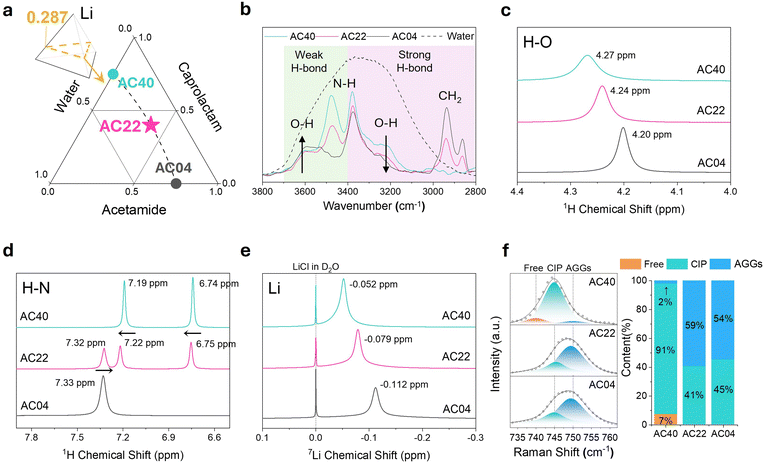
Both acetamide and ε-caprolactam are water-miscible amide compounds known to form eutectic mixtures with LiTFSI, owing to their bifunctional hydrogen bonding capabilities.22–24 A quaternary eutectic electrolyte was formulated by combining both amides with LiTFSI and water, to investigate their synergistic effects on solvation structure and water reactivity. The number of amide molecules was fixed at four, and the Li+ to H2O molar ratio was maintained at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Li mole fraction = 0.287), as depicted in the tetrahedral diagram (Fig. 2a). These compositional constraints enabled systematic variation of the amide ratio through a ternary composition projection. To investigate the cooperative solvation behavior of linear and cyclic amides, a series of electrolytes denoted as ACXY (X = molar ratio of acetamide (A), Y = caprolactam (C)) were selected: AC22 (2
1 (Li mole fraction = 0.287), as depicted in the tetrahedral diagram (Fig. 2a). These compositional constraints enabled systematic variation of the amide ratio through a ternary composition projection. To investigate the cooperative solvation behavior of linear and cyclic amides, a series of electrolytes denoted as ACXY (X = molar ratio of acetamide (A), Y = caprolactam (C)) were selected: AC22 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a representative quaternary composition, AC40 (2
1) as a representative quaternary composition, AC40 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing only acetamide, and AC04 (2
1) containing only acetamide, and AC04 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing only caprolactam. This comparative framework enables a systematic evaluation of the structural and electrochemical consequences of dual-amide integration within the solvation matrix.
1) containing only caprolactam. This comparative framework enables a systematic evaluation of the structural and electrochemical consequences of dual-amide integration within the solvation matrix.
Solvation structure modulations induced by dual-amide integration were investigated using FT-IR (Fig. 2b). In the N–H stretching region (∼3400 cm−1), the acetamide-only system (AC40) exhibits two bands corresponding to symmetric and asymmetric –NH2 stretches, while the caprolactam-only system (AC04) shows a single peak from the cyclic –NH group. The appearance of methylene (–CH2) stretching bands (2800–3000 cm−1) further confirms caprolactam's growing contribution in AC22 and AC04. As expected, the dual-amide system AC22 presents intermediate spectral features across these regions, indicating the coexistence of both functional groups and the formation of a hybridized hydrogen-bonding network. The O–H stretching region (3200–3800 cm−1) provides insight into the hydrogen-bonding environment of water. With increasing caprolactam content, the intensity of the strong hydrogen-bonded O–H band (∼3200 cm−1) decreases, while the weakly bonded band (∼3600 cm−1) becomes more prominent, reflecting a progressive breakdown of the extended water–water hydrogen-bond network. AC22 again shows a blended profile, consistent with its dual-amide composition. Systematic variation of the acetamide-to-caprolactam ratio under different Li+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and amide content conditions revealed that a 2
H2O and amide content conditions revealed that a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Li+
1 Li+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio and a total of four amide molecules (X + Y = 4) offer optimal bulk properties for designing a stable quaternary eutectic electrolyte (Fig. S1 and S2).
H2O ratio and a total of four amide molecules (X + Y = 4) offer optimal bulk properties for designing a stable quaternary eutectic electrolyte (Fig. S1 and S2).
The 1H NMR spectra of the water O–H proton (Fig. 2c) show a gradual upfield shift with increasing caprolactam content (AC40 → AC22 → AC04), indicating enhanced shielding. This trend aligns with FT-IR results and reflects a progressive weakening of the water hydrogen-bond network. The amide N–H signals (Fig. 2d) further illustrate solvation differences. AC40 displays two distinct –NH2 peaks from acetamide, arising from asymmetric hydrogen bonding with water, TFSI−, or other amide molecules. Upon addition of caprolactam in AC22, both –NH2 peaks shift downfield, while the caprolactam –NH peak shifts slightly upfield compared to AC04. These opposite trends indicate strengthened hydrogen bonding for acetamide and weakened interaction for caprolactam, highlighting a hybridized solvation structure unique to AC22. The 7Li spectra (Fig. 2e) reveal increasing electron density around Li+ from AC40 to AC04, suggesting tighter coordination and reduced local polarity with higher caprolactam content. Ion pairing states were evaluated by Raman analysis of the TFSI− S–N–S bending mode (730–760 cm−1) (Fig. 2f). AC40 is dominated by CIP, whereas AC22 and AC04 exhibit mixed populations of CIP and AGGs. AC22 contains a marginally greater proportion of AGGs than AC04. Yet, its lower Li+ electron density, as observed in 7Li NMR, suggests a different aggregate structure—likely mediated by acetamide's influence on solvation geometry and coordination balance. This likely stems from acetamide's role in modulating the solvation environment and stabilizing aggregate motifs without significantly increasing local charge concentration.
A detailed investigation into the spatial configuration of the primary solvation shell around Li+ ions was conducted using 7Li–1H heteronuclear Overhauser effect spectroscopy (HOESY) (Fig. 3a). Compared to AC40, where dominant cross-peaks were observed between 7Li and the C–H protons of acetamide, the AC22 and AC04 systems exhibited strong 7Li–1H (H–O) cross-peaks, indicating closer spatial proximity and stronger interactions between Li+ and water molecules. Further insight into ion association and the spatial organization of Li+ in varying solvation environments was obtained through molecular dynamics (MD) simulations. The resulting Li–Li radial distribution functions (RDFs) and coordination numbers (CNs) (Fig. S3) revealed that AC04 exhibits the shortest Li–Li distance, indicative of the most compact solvation sheath, followed by AC22 and AC40. This trend aligns with the observations from 7Li NMR and Raman spectroscopy, which also indicate enhanced ion association in AC04. Li-solvent and Li–H2O RDF analyses (Fig. S4) revealed minimal differences in coordination number across compositions, suggesting that neither the amide solvents nor water exhibits significant variation in their average coordination to Li+. These results suggest that the spectroscopic differences arise primarily from local interactions within the solvation shell. These structural insights are summarized schematically in Fig. 3b. AC40 is characterized by a relatively expanded solvation sheath dominated by contact ion pairs (CIPs), reflecting weaker interactions between Li+, the anion, and solvent molecules. In contrast, AC22 and AC04 exhibit more aggregates (AGGs), characterized by enhanced cation–anion coordination. Among them, AC04 presents the most compact and densely coordinated configuration, attributed to the low dielectric constant and sterically constrained ring structure of caprolactam, which facilitates TFSI−-bridged Li+ clustering and promotes short-range ion association.
Based on the solvation structure insights obtained from MD simulations, electronic structure calculations were conducted to further elucidate the interfacial stability of each electrolyte system. Representative solvation clusters surrounding Li+ were selected from the MD trajectories and analyzed using density functional theory (DFT) calculations (Fig. 4a). Among the various configurations within 4 Å of the Li+ center, the most statistically dominant clusters were chosen. These consistently involved water molecules for charge neutrality, along with coordinating TFSI− anions and amides. Bader charge analysis revealed that the oxygen atom of water in AC04 carried a more electron rich (−0.10e) compared to those in AC22 and AC40 (both −0.06e), implying stronger confinement of water molecules within the first solvation shell. Here, the reported Bader values denote relative electron accumulation, where more negative values correspond to higher electron density. This observation supports earlier spectroscopic and structural results that indicated a more compact solvation environment in AC04. The influence of these solvation structures extends to the electronic energy levels of each system, as shown in the projected density of states (pDOS) plots (Fig. 4b). The lowest unoccupied molecular orbital (LUMO) levels of AC22 and AC04 are noticeably upshifted relative to AC40, indicating a greater resistance to reduction. In all AC electrolytes, the frontier orbitals near the Fermi level are dominated by anions and amides, suggesting that these species serve as preferential reduction targets during initial interfacial reactions. This orbital alignment facilitates the selective formation of a stable SEI under aqueous conditions.
Electrochemical performance of AC electrolytes and interface characterization
The electrochemical stability of the AC electrolytes was evaluated using linear sweep voltammetry (LSV) (Fig. S5).19 The results revealed a progressive increase in electrochemical stability from AC40 to AC22 and AC04. However, for practical cell operation, electrolyte stability alone is insufficient, as the physical properties of the electrolyte, such as viscosity and ionic conductivity, must also be considered. As the proportion of caprolactam increases, the viscosity of the electrolyte rises sharply while ionic conductivity decreases (Fig. 4c and Table S1). Excessive viscosity slows charge transfer and exaggerates the observed ESW by delaying electrochemical reactions. This increased resistance not only hinders hydrogen evolution but also interferes with Li+ redox reactions, leading to enhanced polarization. Despite this trade-off, AC22 demonstrates a well-regulated balance between electrochemical stability and ion transport. This balance enables kinetically accessible redox reactions at both LiMn2O4 (LMO) and Li4Ti5O12 (LTO) electrodes with minimal polarization, maintaining an effective ESW of 4.0 V (1.2–5.2 V) (Fig. 4d).Electrochemical performances were evaluated on AC electrolytes in LMO‖LTO full cell (Fig. 5a). Among the tested cells, the AC22 cell exhibited the most outstanding cycling stability, delivering an initial discharge capacity of 154.8 mAh g−1 and retaining 82% of this capacity after 1200 cycles at 1C, demonstrating excellent long-term durability. In contrast, the AC40 cell, despite achieving a relatively high initial capacity, suffered from rapid capacity degradation over a limited number of cycles, likely due to unstable interfacial reactions. Notably, the AC04 cell demonstrated the lowest initial discharge capacity mainly due to its inferior ionic conductivity. This is supported by the CV profiles of LMO and LTO electrodes in the AC04 system, which display broader and weaker redox peaks, indicative of sluggish kinetics (Fig. S6). These results underscore that, beyond thermodynamic stability, ion transport efficiency plays a more critical role in determining practical cycling performance. The voltage profiles of AC22 cell during 1C cycling revealed minimal polarization and consistent redox reversibility with corresponding energy density of 102 Wh kg−1 (Fig. S7). This stability was supported by impedance analysis, which revealed stable interfacial resistance that remained well-regulated during prolonged cycling (Fig. S8). The practical applicability of the electrolyte was further examined using a pouch cell, which was cycled at 1C and delivered an initial specific capacity of 153.4 mAh g−1, retaining 65% of its capacity after 500 cycles (Fig. S9). Furthermore, long-term cycling at a lower current density of 0.5C also showed stable capacity retention and high coulombic efficiency over 400 cycles, confirming that the electrolyte remains effective across a range of operating conditions (Fig. S10). Comparison with other aqueous electrolyte systems reported in the literature highlights the advantages of AC22 (Fig. 5b).14,16,19,25–30 This optimized electrolyte not only achieves superior cycling stability at 1C but also maintains a competitive specific capacity relative to previously reported values. These performance outcomes corroborate the thermodynamic stability previously suggested by spectroscopic and electrochemical analyses, evidencing its practical impact on the long-term operational performance of the cell.
 | ||
| Fig. 5 Electrochemical performance and post-mortem analysis of AC electrolytes. (a) Cycling performance of LMO‖LTO full cells with AC40, AC22, and AC04 at 1C. (b) Benchmark comparison of specific capacity and cycle life at 1C across aqueous LIB systems.14,16,19,25–30 (c) TEM images of the SEI layer on cycled LTO anodes in AC22 and AC04. (d) F 1s, O 1s and C 1s XPS depth profiles of the SEI layer on cycled LTO anodes in AC22 and AC04. | ||
To gain insight into the origin of this stable cycle life, detailed post-mortem analyses were carried out using atomic-resolution transmission electron microscopy (AR-TEM) (Fig. 5c). Given the inherently low ionic conductivity of AC04, it was excluded from further structural comparison. Interfacial analyses revealed that AC22 forms a compact, inorganic-rich SEI that provides strong electronic insulation through a LiF-dominated inner layer while maintaining mechanical compliance through a thinner, more deformable outer region. In contrast, AC40 develops a significantly thicker, compositionally mixed SEI in which extensive organic accumulation increases interfacial resistance and reduces structural flexibility. These architectural differences rationalize the superior interfacial stability observed in AC22. In the case of AC22 cell, a uniform SEI layer was formed in thickness of approximately 10 nm. Distinct crystalline domains corresponding to LiF, Li2O, and Li2CO3 were clearly identified based on their characteristic d-spacings: 0.20 nm for LiF (200), 0.14 nm for LiF (220), 0.27 nm for Li2O (111), and 0.295 nm for Li2CO3 (−202).31 In contrast, AC40 cell exhibited a substantially thicker SEI layer of approximately 18 nm, which is attributed to excessive electrolyte decomposition. This thick interphase is detrimental to cell performance, as it impedes Li+ transport and increases interfacial resistance, likely accelerating polarization and capacity degradation over extended cycling. X-ray photoelectron spectroscopy (XPS) depth profiling of the cycled LTO revealed distinct SEI architectures for AC22 and AC40 cells (Fig. 5d). AC22 showed a well-defined bilayer SEI structure. F 1s spectra showed an increasing LiF signal (686 eV) with depth, while O 1s spectra exhibited a marked reduction in surface-bound organic species such as C–N–O (532.8 eV) and C–O (533.7 eV), indicating a configuration consisting of an inorganic-rich inner layer and an organic-rich outer layer. This layered architecture provides both electronic insulation and mechanical flexibility, effectively mitigating continuous side reactions and promoting interfacial stability. In contrast, the AC40 electrode exhibited a thick SEI layer, with both LiF and organic components distributed throughout the interphase. This extensive accumulation may impede efficient ion transport and contribute to increased interfacial resistance, thereby adversely affecting long-term cycling performance time-of-flight secondary ion mass spectroscopy (ToF-SIMS) analysis revealed consistent trends, with LiF2− detected at deeper depths and organic fragments such as CNO− and C2HO− enriched at the outermost surface (Fig. S11).
Despite its outstanding cycle life at 1C, AC22 exhibited poor rate performance, with significant capacity loss at higher current densities such as 3C (Fig. S15). This limitation is attributed to the high viscosity and low ionic mobility associated with the inclusion of bulky ε-caprolactam. To address this limitation, ε-caprolactam was replaced with a smaller lactam, pyrrolidone, yielding AP22, which showed a marked reduction in viscosity and enhanced ionic conductivity (Fig. S16). As shown in Fig. S17, AP22 delivered markedly higher discharge capacities than AC22 under fast cycling conditions, confirming its advantage in high-rate transport behavior. However, while this substitution improved bulk properties, the AP22 cell suffered from rapid capacity fading and overall degradation in electrochemical performance, with particularly poor stability observed at the LTO anode interface (Fig. S18–S21). This instability was particularly evident at the LTO anode, accompanied by rapid capacity fading and reduced coulombic efficiency. Spectroscopic analyses revealed weakened participation of pyrrolidone in Li+ coordination, as indicated by minimal changes in vibrational and NMR features (Fig. S22–S25). These observations support a hypothesis that pyrrolidone undergoes self-association via stable hydrogen-bonded dimers, thereby limiting its availability for effective Li+ coordination. Previous studies have shown that pyrrolidone can form such dimers even in the presence of water, and based on this, it is reasonable to infer that this self-association behavior contributes to the interfacial instability observed in AP22.32,33 Supporting this interpretation, radial distribution function analyses and DFT calculations revealed negligible changes in Li+ coordination number upon pyrrolidone addition, suggesting its limited coordination activity (Fig. S26 and S27). This outcome indicates a strong correlation between pyrrolidone dimerization and the diminished electrochemical performance of AP22. These findings underscore the need for a more sophisticated electrolyte design strategy that systematically accounts for intermolecular interactions in aqueous eutectic systems.
Conclusions
This study demonstrates the effectiveness of solvation structure engineering through dual-amide integration as a strategy for interfacial stabilization in aqueous LIBs. By employing acetamide and ε-caprolactam with distinct dielectric properties, steric profiles, and coordination tendencies, the AC22 quaternary eutectic electrolyte forms a compact and well-regulated solvation environment. This facilitates selective anion decomposition and supports bilayer SEI formation, effectively suppressing hydrogen evolution while maintaining ionic conductivity and interfacial stability during prolonged cycling. AC22 achieved a stable discharge capacity of 154.8 mAh g−1 with 82% retention over 1200 cycles at 1C, setting a new performance benchmark under ambient conditions. Post-mortem analyses revealed spatially coherent and chemically graded SEI and CEI layers, shaped by the tailored solvation structure. In contrast, the pyrrolidone-substituted electrolyte system (AP22) showed reduced stability and faster capacity fading. These issues stemmed from persistent pyrrolidone dimerization, which hindered effective cation coordination and exposed a design limitation of self-associating lactams in multifunctional electrolytes. These findings highlight the importance of carefully selecting amide components, as their intrinsic intermolecular interactions can significantly influence solvation behavior and interfacial stability in aqueous electrolytes. The dual-amide approach presented here provides an effective framework for molecular-level tuning, offering valuable insights for future high-performance aqueous battery systems.Author contributions
Insu Jeong: conceptualization, methodology, data curation, investigation, visualization, writing – original draft. Younsang Cho: methodology, data curation, investigation, visualization, writing – original draft. Youngbi Kim: formal analysis, software. Hyungmin Park: investigation (X-ray photoelectron analysis). Jeong Woo Han: supervision, Writing – review & editing. Soojin Park: supervision, writing – review & editing. Jaegeon Ryu: supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
All data supporting the findings of this study are available within the article and its supporting information (SI). Supplementary information: additional experimental details, characterization data, electrochemical measurements, and computational analyses. See DOI: https://doi.org/10.1039/d5ta08103c.Acknowledgements
The authors acknowledge the financial support from the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021R1A2C3004019 and RS-2023-NR077019) and Korea Toray Science Foundation.References
- X.-B. Cheng, R. Zhang, C.-Z. Zhao and Q. Zhang, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS PubMed.
- H.-D. Lim, J.-H. Park, H.-J. Shin, J. Jeong, J. T. Kim, K.-W. Nam, H.-G. Jung and K. Y. Chung, Energy Storage Mater., 2020, 25, 224–250 CrossRef.
- Y. Gao, Z. Pan, J. Sun, Z. Liu and J. Wang, Nano-Micro Lett., 2022, 14, 94 CrossRef CAS PubMed.
- M. Balaish, A. Kraytsberg and Y. Ein-Eli, Phys. Chem. Chem. Phys., 2014, 16, 2801–2822 RSC.
- X. Yu and A. Manthiram, Energy Environ. Sci., 2018, 11, 527–543 RSC.
- J. Park and Y. Son, Korean J. Chem. Eng., 2025, 42, 1491–1505 CrossRef CAS.
- H. Zhao, X. Bo, H. Xu, L. Wang, W. A. Daoud and X. He, Energy Storage Mater., 2024, 72, 103696 CrossRef.
- K. Xu, Chem. Rev., 2004, 104, 4303–4418 CrossRef CAS PubMed.
- H. Kim, J. Hong, K.-Y. Park, H. Kim, S.-W. Kim and K. Kang, Chem. Rev., 2014, 114, 11788–11827 CrossRef CAS.
- Z. Liu, Y. Huang, Y. Huang, Q. Yang, X. Li, Z. Huang and C. Zhi, Chem. Soc. Rev., 2020, 49, 180–232 RSC.
- L. Suo, O. Borodin, T. Gao, M. Olguin, J. Ho, X. Fan, C. Luo, C. Wang and K. Xu, Science, 2015, 350, 938–943 CrossRef CAS PubMed.
- L. Suo, D. Oh, Y. Lin, Z. Zhuo, O. Borodin, T. Gao, F. Wang, A. Kushima, Z. Wang, H.-C. Kim, Y. Qi, W. Yang, F. Pan, J. Li, K. Xu and C. Wang, J. Am. Chem. Soc., 2017, 139, 18670–18680 CrossRef CAS PubMed.
- N. Dubouis, P. Lemaire, B. Mirvaux, E. Salager, M. Deschamps and A. Grimaud, Energy Environ. Sci., 2018, 11, 3491–3499 RSC.
- J. Chen, J. Vatamanu, L. Xing, O. Borodin, H. Chen, X. Guan, X. Liu, K. Xu and W. Li, Adv. Energy Mater., 2020, 10, 1902654 CrossRef CAS.
- D. Aurbach, J. Power Sources, 2000, 89, 206–218 CrossRef CAS.
- R. Lin, C. Ke, J. Chen, S. Liu and J. Wang, Joule, 2022, 6, 399–417 CrossRef CAS.
- J. Xu, X. Ji, J. Zhang, C. Yang, P. Wang, S. Liu, K. Ludwig, F. Chen, P. Kofinas and C. Wang, Nat. Energy, 2022, 7, 186–193 CrossRef CAS.
- L. Zhou, S. Tian, X. Du, T. Liu, H. Zhang, J. Zhang, S. Hu, Z. Chen, J. Zhang and G. Cui, ACS Energy Lett., 2023, 8, 40–47 CrossRef CAS.
- I. Jeong, S. Kim, Y. Kim, C. Kim, J. Kang, J. H. Ha, Y. Cho, S. J. Kang, J. Ryu, J. W. Han and S. Park, Adv. Mater., 2025, 37, 2412652 CrossRef CAS PubMed.
- J. Han, A. Mariani, S. Passerini and A. Varzi, Energy Environ. Sci., 2023, 16, 1480–1501 RSC.
- C. Yuan, J. Xiao, C. Liu and X. Zhan, J. Mater. Chem. A, 2024, 12, 19060–19068 RSC.
- C.-C. Yang, H.-Y. Hsu and C.-R. Hsu, Z. Naturforsch. A, 2007, 62, 639–646 CrossRef CAS.
- M. S. Ding and K. Xu, J. Phys. Chem. C, 2018, 122, 16624–16629 CrossRef CAS.
- Z. Liu, F. Feng, W. Feng, G. Wang, B. Qi, M. Gong, F. Zhang and H. Pang, Energy Environ. Sci., 2025, 18, 3568–3613 RSC.
- J. Xie, Z. Liang and Y.-C. Lu, Nat. Mater., 2020, 19, 1006–1011 CrossRef CAS PubMed.
- D. Liu, L. Yuan, X. Li, J. Chen, R. Xiong, J. Meng, S. Zhu and Y. Huang, ACS Appl. Mater. Interfaces, 2022, 14, 17585–17593 CrossRef CAS.
- Z. Ma, J. Chen, J. Vatamanu, O. Borodin, D. Bedrov, X. Zhou, W. Zhang, W. Li, K. Xu and L. Xing, Energy Storage Mater., 2022, 45, 903–910 CrossRef.
- D. Liu, X. Li, R. Xiong, R. Ning, C. Xu, J. Meng, Y. Huang and L. Yuan, ACS Appl. Mater. Interfaces, 2023, 15, 32376–32384 CrossRef CAS PubMed.
- Y. Wang, T. Ou, Y. Dong, L. Chen, Y. Huang, D. Sun, W. Qiang, X. Pei, Y. Li and Y. Tan, Adv. Mater., 2024, 36, 2311009 CrossRef CAS PubMed.
- C. Zhang, B. Chen, Q. Chen, C. Tian, M. Zhou, X. Zhao, Z. Li, L. Fan, X. Kong and H. Pan, ACS Energy Lett., 2024, 9, 4691–4698 CrossRef CAS.
- B. Luo, J. Wu, M. Zhang, Z. Zhang, X. Zhang, Z. Fang, Z. Xu and M. Wu, Chem. Sci., 2023, 14, 13067–13079 RSC.
- H. Yekeler, A. Guven aattin and R. Ozkan, J. Comput.-Aided Mol. Des., 1999, 13, 589–596 CrossRef CAS PubMed.
- A. E. Shchavlev, A. N. Pankratov, V. B. Borodulin and O. A. Chaplygina, J. Phys. Chem. A, 2005, 109, 10982–10996 CrossRef CAS PubMed.
Footnotes |
| † Dr I. Jeong, Y. Cho and Y. Kim contributed equally to this work. |
| ‡ Present address – Battery R&D, LG Energy Solution, Daejeon 34112, Republic of Korea. |
| This journal is © The Royal Society of Chemistry 2026 |