Effectively controlling Ni photodeposition on polyhedron SrTiO3via double-doped Al and Co
Qibo
Jia
*a,
Yang
Wang
a,
Yan
Li
a,
Xiaodong
Zhang
a,
Li
Zhong
a,
Yan
Liu
a,
E.
Zhou
a,
Yuzhi
Ren
a,
Ting
Li
a and
Dongping
Duan
 *ab
*ab
aCAS Key Laboratory of Green Process and Engineering, National Engineering Research Center of Green Recycling for Strategic Metal Resources, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: qibojia@126.com
bUniversity of Chinese Academy of Sciences, Beijing 100049, China. E-mail: douglass@ipe.ac.cn
First published on 19th November 2025
Abstract
SrTiO3 micron-nano-sized particles (MNPs) have been widely applied in the photocatalysis field. Currently, many studies focus on the morphological control and heterojunction construction of SrTiO3; however, unsuitable active sites limit their performance improvements. In this study, we developed polyhedron SrTiO3 MNPs double doped with Al and Co, which showed outstanding in situ photo-reduction performance of Ni2+ (from Ni2+ to Ni0), achieving about 39% improvement in 10 minutes compared with the traditional sample doped only with Al, benefiting from the unique strengthening function of the crystal facet effect. The photo-reduction reaction of Ni2+ exhibited a significant selectivity on the (112) crystal facet mainly due to the changes in the surface structure, including the atomic nanoclusters (ACs) and the satellite single atom (SAs) of Co. The femtosecond transient absorption spectrum (fs-TA) revealed the difference in the excited states of photo-induced electrons for the same delay time, which was the essence of the synergistic effect between ACs and SAs. Benefitting from the above characteristics, the double-doped SrTiO3 has high efficiency in the selective photodeposition of Ni. In addition, we established an experimental platform to assess the photo-quantum absorption efficiency of an aqueous dispersion system to verify that Ni may be an effective active site in the photocatalytic process for SrTiO3. Therefore, this work demonstrates that these special SrTiO3 MNPs have a huge potential application in the field of Ni recycling in wastewater or cocatalyst construction.
Introduction
Strontium titanate (SrTiO3), as a metallic oxide with a typical perovskite structure, has been proven to be an efficient photocatalytic material for solar-driven water splitting, organic matter degradation and so on.1–3 Due to the controllable regulation of defective structures and exposed crystal facets,4 SrTiO3 micron-nano-sized particles (MNPs) show potential in highly photocatalytic oxidation and reduction activities, though there are still significant challenges to overcome. The photoredox ability of SrTiO3 to different metal ions still needs detailed research, which plays a decisive role in the photocatalytic performance of SrTiO3.For example, in the water splitting process, as a basic fundamental thermodynamic requirement, the external input energy must be greater than the positive value of the Gibbs free energy of water splitting (ΔG = 237 kJ mol−1).1 Simultaneously, semiconductor materials should not only meet the potential requirements of hydrogen and oxygen evolution (1.23 eV) but also have enhanced electron–hole pair separation efficiency, each of which is of significant importance.5 Constructing active sites and heterojunction structures are two effective means, which can enhance the photonic utilization ratio and the range of photo-response.6–9 From the perspective of reducing the work function of carriers, the active or defect sites on a semiconductor's surface can realize the oriented aggregation of electrons and holes, called the hydrogen evolution reaction (HER) site and oxygen evolution reaction (OER) site, respectively.10–12 Rh and Co are proven to be effective HER and OER sites, respectively, for solar-driven water splitting.2,13 Furthermore, our previous work revealed that SrTiO3 MNPs have strong photooxidation ability for Co2+, originating from the binding energy change of the exciton (Ee) at the crystal defect.14,15 The SrTiO3-Rh-Co photocatalyst has been widely investigated for the water splitting field in recent researches,2,16 but the H2 production efficiency achieved in the recent studies are still considerably lower than the expected values.17,18 Expanding the types of cocatalysts can be used to further enhance the photo redox ability of SrTiO3, and assist in raising the photocatalytic performance of active sites, such as Ni-, Mn-, and Zn-based cocatalysts. Simultaneously, in the preparation process of cocatalysts, a photo-deposition reaction, as a kind of well-adopted treating method, is often used to construct effective cocatalysts.19–21 Therefore, strong photooxidation and reduction abilities to the metal or metal oxide ions of semiconductor materials play crucial roles and guarantee achieving an active site with high efficiency. However, the selectivity of the deposition ions is uncontrollable, and the types of cocatalysts are also restricted. Herein, developing more varied cocatalysts or improving the efficiency of the photo-deposition of different metal ions is a long-term and critical task in the photocatalysis field.
Based on the widely studied crystal facet effect of polyhedral SrTiO3, in situ photooxidation and photoreduction reactions can be easily realized on different crystal facets. However, crystal facet engineering is not enough, and the reaction efficiency of different metal ions is quite different in the actual process of photodeposition. How to enhance the reaction efficiency of specific metal ions by controlling the surface structure of SrTiO3 is a challenge. Specifically, auxiliary active sites can induce directional migration of the carriers and desirably inhibit electron–hole recombination, such as single atoms (SAs) or atomic clusters (ACs) of different kinds.22–25 Compared with the crystal facet effect, less attention has been paid to the specific mechanism of the effect of the surface structure on the photoredox behavior, especially the relationship between the crystal facet effect and surface structure. Simultaneously, the effect of ion doping on the photoredox ability or facet control in the SrTiO3 system is negligible.
In this work, we redesigned the dopant elements and regulated the transfer properties of photoinduced carriers through Al and Co double-doping, after which the Co SAs and nano ACs were created side by side on the (112) facets using a melt-blending process. The new surface structure had stronger selectivity for different types of metal ions, and we found out that this structure enhanced the photoelectron anisotropy and photo-reduction efficiency of the (112) facet only for Ni2+. We put forward the theory by considering the synergy effect on electron transfer to explain the new reaction mechanism of Ni2+ photo-reduction to Ni0, which was also proved by density functional theory (DFT) calculations. Consequently, from the DFT calculations, the work function differences of electrons along different facets of SrTiO3 were significantly decreased by the unique structure, indicating that the charge carriers exhibited stronger anisotropic mobility. Based on the enhanced polyhedral crystal facet effect, the photo-reduction efficiency of Ni2+ on the SrTiO3 MNPs was improved. Simultaneously, SrTiO3 with a new active site has potential applications in water splitting under solar light irradiation.
Experimental
Synthesis of Al and Co-doped SrTiO3 MNPs
The Al- and Co-doped SrTiO3 MNPs were synthesized using molten-salt mediation technology at a temperature of 1423 K in this study.2 First, SrCl2 (AR, Macklin Inc., China), Al2O3 and Co(NO3)2 (or only Al2O3) (AR, Macklin Inc., China) and SrTiO3 (AR, Macklin Inc., China) were mixed by grinding in an agate mortar in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02
0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02
0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. Then, the mixture was subsequently heated in an alumina crucible at 1423 K for 10 h in air and then allowed to cool to room temperature. The product was first separated from residual Al2O3 by the addition of a small amount of diluted hydrochloric acid, and then separated from SrCl2 and related compounds as well as by washing with distilled water several times, at which point the supernatant solution was neutral. Here, the SrTiO3 MNP samples doped with Al and Al/Co were named STOAl and STOAlCo, respectively.
1 molar ratio. Then, the mixture was subsequently heated in an alumina crucible at 1423 K for 10 h in air and then allowed to cool to room temperature. The product was first separated from residual Al2O3 by the addition of a small amount of diluted hydrochloric acid, and then separated from SrCl2 and related compounds as well as by washing with distilled water several times, at which point the supernatant solution was neutral. Here, the SrTiO3 MNP samples doped with Al and Al/Co were named STOAl and STOAlCo, respectively.
Photoredox reaction of metal ions
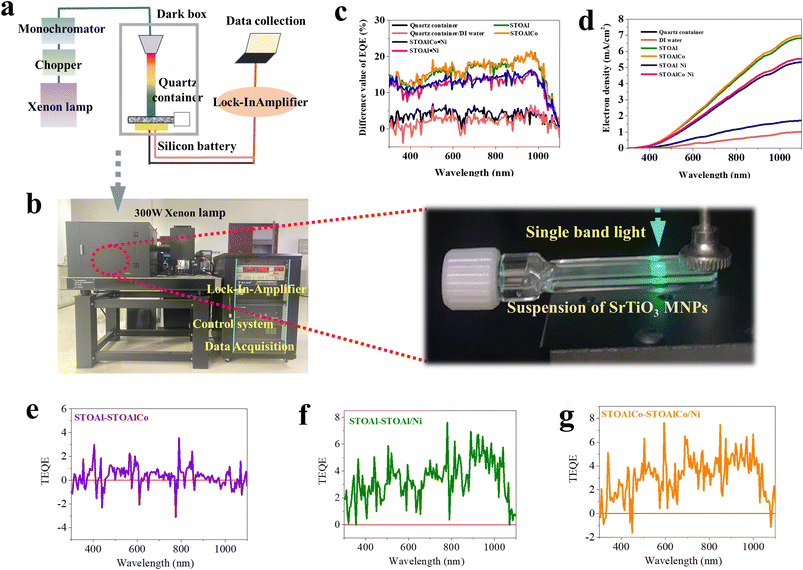
In this study, the photo-deposition reaction experiment was performed under illumination conditions with all-wave band light. First, aqueous solutions of RhCl3·6H2O, K2CrO4, Mn(NO3)2, Co(NO3)2 and Ni(NO3)2·6H2O (AR, Macklin Inc., China) were all prepared with a concentration of 2 mg ml−1. Then, 0.1 g of SrTiO3 is dispersed in 100 ml of distilled water and placed in a 200 ml glass bottle with brief sonication (20 min). To investigate the photoredox ability of SrTiO3 to different metal ions, the 2 ml aqueous solution prepared above was added to the dispersion of SrTiO3 with magnetic stirring, and the resulting mixture was irradiated with a Xe lamp (300 W, full arc; MC-SCI Inc., China) for 10 min, after which the mixture was heated in a hot water bath until dry. In particular, STOAl and STOAlCo with Ni photodeposition were named STOAl-Ni and STOAlCo-Ni, respectively.The light intensity irradiation on the photocatalyst dispersion was measured using a THORLABS GmbH S425C thermopile detector with a sensor area of 4.9 cm2 (SI Fig. S14). The light intensity and actual light-receiving area were 100 mw cm−2, 1700 mw cm−2 and 4.9 cm2, respectively. In this study, to reduce the impact of maximum temperature, we used magnetic stirring and a water bath for the temperature stability of the aqueous system shown in SI Fig. 15, and the reaction temperature only increased 5° after the reaction proceeded completely (the entire reaction process was controlled below 30 °C).
Before and after the photoredox reaction, the concentrations of different ions in the aqueous solution were analysed using an Agilent ICAOES730 inductively coupled plasma atomic emission spectrometry (ICP-AES).
Morphology and spectroscopic measurements
All the spectra were measured at room temperature unless otherwise noted. The morphology and energy-dispersive spectroscopy (EDS) mappings of the samples were studied using a JEOL JSM-7100 scanning electron microscope (SEM) operating at 5 kV. Atomic-resolution high-angle annular dark-field scanning transmission electron microscope (HAADF-STEM) images were taken using an FEI Titan Cubed Themis G2 300 S/TEM with a probe corrector operating at 200 keV. In situ X-ray diffraction (XRD) measurements were performed using a Rigaku D/max-2500 diffractometer with Cu Kα radiation (40 kV, 100 mA) at a scan rate of 10° min−1 in the 2θ range of 10–90° under different temperatures. Spin-trapped electron paramagnetic resonance (EPR) experiments were performed using a Bruker A300-10/12 electron spin resonance spectrometer. Raman measurements were obtained using a Horiba HR-800 spectrometer. X-ray photoelectron spectroscopy (XPS) patterns, including the full and high-resolution XPS spectra, were collected using a VGScientific ESCALab220IXL spectrometer with an Al/Kα (hν = 1486.6 eV) anode mono X-ray source. Diffuse reflectance spectroscopy (DRS) measurements were made using an Agilent Cary5000 spectrophotometer equipped with a solid sample holder, and the band gaps of the two samples were calculated using the following Kubelka–Munk formula: (αhν)1/n = A(hν − Eg), (n = 2 for a direct band gap of SrTiO3 reported in our previous study14), where α, h, ν, Eg and A are the absorption coefficient, Planck constant, light frequency, band gap, and a constant, respectively. Ultraviolet photoelectron spectroscopy (UPS) measurements were performed using an unfiltered He(I) (21.22 eV) gas discharge lamp with a total instrumental energy resolution of 100 meV.Transient absorption spectroscopy on the femtosecond scale
A femtosecond-transient absorption (fs-TA) spectrometer was used for transient absorption measurements, as described in a previous report.26 This system consisted mainly of a coherent amplified femtosecond laser system with a pulse width of 25 fs and a Helios pump-probe system, which was a product of Ultrafast Systems for fs-TA data recording. In this study, a TOPAS-800 fs optical parametric amplifier was used to provide pump pulses, the wavelength of which was 325 nm (∼40 nJ per pulse at the sample). The stable white-light continuum (WLC) probe pulses were 400–800 nm in this work, which were generated by focusing the fundamental 800 nm beam into a sapphire plate. All samples were well dispersed onto the surface of quartz plates with an area of 4 cm2 by applying the spin coating method with full drying. The collected data were finally processed using Surface Xplorer software.Photo-quantum absorption efficiency under consecutive wavelengths (AD-PQAE system)
A novel experimental platform for testing the photo-quantum absorption efficiency of an aqueous dispersion system (AD-PQAE) of SrTiO3 MNPs in water dispersion was designed and applied in this study. The AD-PQAE system was based on the basic structure of the absolute spectral response system (BSRS) (incident-photon-to-charge conversion efficiency (IPCE) measurement system) structure. The major components of this system are described in detail in the section on results and discussion. The parameters obtained from this system were different values of the external quantum efficiency (EQE) and the short circuit current density (Jsc) between a standard silicon battery and the aqueous suspension of SrTiO3 MNPs, in which the test wavelength range was 290–800 nm and the scanning speed was 10 nm min−1.Theoretical simulation
The plane-wave density of functional theory (DFT) calculations were carried out using the CASTEP code.3 As another important parameter for studying electron transfer within duplicate semiconductor heterostructures, work function (Φ) was estimated from the energy difference between the vacuum and Fermi levels according to the electrostatic potential of a crystal model. In the calculation of electrostatic potentials, geometry optimizations were performed with the BFGS minimization algorithm for 2 × 2 × 1 supercells of Al- and Co-doped SrTiO3 crystal. Based on the supercells, the (110) and (112) facets were cleaved to simulate the corresponding exposed facet, and the vacuum slab of the crystal was set at 30 Å. The OTFG ultrasoft and Koelling-Harmon modes were selected for the pseudopotential and relativistic treatment optimization, respectively, and the band energy tolerance was 1.0 × 10−5 eV in the calculation.Results and discussion
Morphology and surface structure
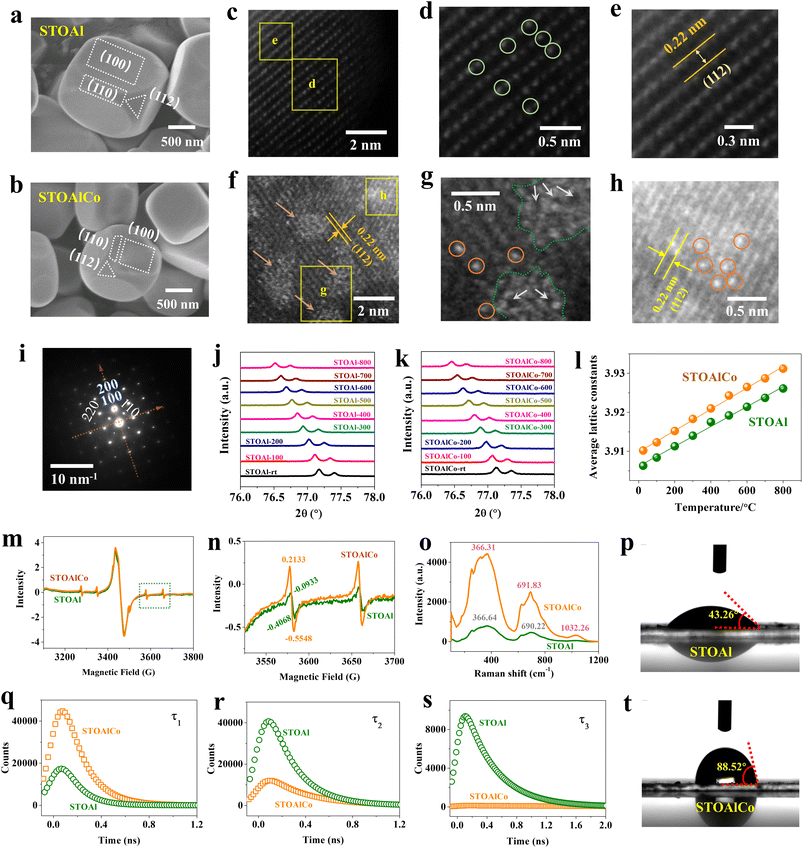
In this article, using molten-salt mediation technology, STOAl and STOAlCo particles with (100)-truncated rhombic dodecahedra exposing (100), (110) and (112) facets are obtained, as shown in Fig. 1(a) and (b).3 The field-emission scanning electron microscopy (FESEM) images present STOAl and STOAlCo with the morphology of a typical edge-truncated cube with a diameter of approximately 200 nm. The atomic scale distribution of SrTiO3 crystals with clusters and SAs is investigated by high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), as shown in Fig. 1(c–e), for STOAl and Fig. 1(f–h) for STOAlCo. For STOAl, the existence of Al in the lattice is observed on the carbon support, as shown in the magnified image of Fig. 1(d), which highlights the Al atoms in the crystal lattice of SrTiO3 and proves that Al has filled the SrTiO3 crystal perfectly and repaired the lattice defect. In addition, Fig. 1(e) illustrates that the lattice distance is 0.22 nm, a typical crystal lattice arrangement of SrTiO3 along the (111) facet,27 which is the (112) facet in this study. In contrast, also on the (112) facet, Fig. 1(f) depicts the typical coexistence characteristics of both the clusters and SAs in STOAlCo. From Fig. 1(g), the magnified image of the specific position marked in Fig. 1(f) shows that several Co atoms (orange circles) closely surround the cluster (dark yellow arrow and green dotted circles) with a distance of <2 nm, clearly indicating the successful construction of Co clusters and satellite SAs. What is more important, it is observed that the clusters are loosely coupled clusters with single vacancies and related point defects, as indicated by the white arrows, which can be considered direct evidence of O or Co vacancies in the cluster in this study. More similar details can also be observed in SI Fig. S1. Notably, it is not well established whether all the loosely coupled clusters are distributed in the perfect lattice (bulk) or surface. From the above analysis results, it can be estimated that the dispersibility of the Co atom is quite different from that of Al, and benefiting from the clusters, STOAlCo has more vacancies than STOAl. Moreover, in quantitative analysis, the quantity of Co SA is much more than that of the cluster because more Co SAs are dispersed in the crystal and do not form an effective cluster, as shown in Fig. 1(h), suggesting that Co should behave as a combined action of regular single-atom and cluster. Significantly, using the present molten salt method, the Co SAs and clusters are obtained simultaneously. Moreover, the reproducibility experiment of these nanostructures is performed, and the HAADF-STEM results also confirm that the Co SAs and clusters can be obtained steadily with good repeatability. However, for the STOAlCo, energy-dispersive spectroscopy (EDS) elemental mappings further confirm the element types and reveal the uniform distribution of the hybrid doped elements on (100), (110) and (112) crystal facets of SrTiO3 particles (SI Fig. S2) (The specific contents of different elements can be observed in SI Table S1). However, the Co SAs and clusters appear primarily in the (112) facets from the HAADF-STEM results. Simultaneously, the crystal structure of STOAlCo is analyzed by selected area electron diffraction (SAED), as shown in Fig. 1(k), and X-ray diffraction (XRD), all confirming the strict SrTiO3 crystal structure, as shown in SI Fig. S3,2,14 which indicates that the doped Al and Co do not destroy the perovskite structure of SrTiO3.In order to investigate the crystal structure better, changes in the crystal structure of the two samples are examined using the in situ XRD method, and the results summarize the lattice distance changes of STOAl and STOAlCo under different temperatures in Fig. 1(l) based on the data of Fig. 1(j) and (k), respectively. In general, the lattice distance difference between STOAl and STOAlCo is about 0.004 Å, and there is a slight difference in the extent of lattice expansion as the temperature increases. Obviously, from the fitted straight line, it is observed that STOAlCo has greater lattice expansion, mainly attributed to the atomic vacancies in Co ACs. This is confirmed by spin-trapped electron paramagnetic resonance (EPR) and Raman measurements. As observed from the EPR results (Fig. 1(m)), STOAl and STOAlCo present similar O vacancy (VO) signals (g = 2.003), which should be attributed to the replacement of Al atoms with O vacancies. The same weak signal corresponding to g = 1.989 (assigned to Ti3+) indicates that there are also a few Ti3+ ions in the two samples. However, the appropriate magnified image from Fig. 1(n) more clearly shows that there are great differences in the metal atomic vacancies corresponding to g = 1.989, considered as the Co3+ and Al3+ vacancy, and strongly relevant signals occurring in STOAlCo should be referred to as Co ACs. Additionally, the relative intensities of these characteristic peaks are compared quantitatively, with STOAlCo showing 0.7681, which is 0.4546 higher than that of STOAl. Simultaneously, as shown in Fig. 1(o), the Raman measurements reveal the intensity of characteristic peaks of metal atomic vacancies enhanced significantly in STOAlCo, as well as the corresponding peaks shift apparently, which are all consistent with the analysis results of EPR.
However, limited by the analysis methods above, the research results cannot definitely show the specific location of Co ACs and SAs, especially the nanoclusters with atomic vacancies. Therefore, to study the characterization of surface and bulk defects of SrTiO3 MNPs precisely, the positron annihilation life spectrum (PALS) technique is used in this article. Although PALS is a proven technology used to study defects and the chemical nature of various solid oxides and semiconductors, including SrTiO3,28,29 to date, very few studies have used it to characterize the nature of defects of Co ACs. In PALS, lifetime and intensity are employed to identify each positron state delocalized in the lattice and trapped in various kinds of vacancies. Thus, the positron trapped at a defect lives longer than the delocalized positron because the electron density in open volume defects is less than the delocalized lattice.30 According to the two-state capture model, three-lifetime components are generally used for spectrum decomposition.31 In this article, the first (τ1), second (τ2) and third (τ2) component lifetime values represent the information of positron annihilation at the bulk lattice, crystal interface and vacancy cluster, and surface lattice of SrTiO3 MNPs, respectively, as observed in the summarized fitting results of Fig. 1(q–s) and Table 1. The results show the intensity comparison of fitting PALS of STOAl and STOAlCo, from which STOAlCo has a higher intensity of positron annihilation in the bulk crystal (τ1−STOAlCo > τ1−STOAl). However, in the interface and surface, STOAl has lower intensity signals (τ2−STOAlCo < τ2−STOAl, τ3−STOAlCo < τ3−STOAl), proving that STOAlCo has the AC structure with a specific number of cluster defects; hence, a high concentration of structural defects like dislocations and vacancies is expected. The calculated positron state lifetime values for STOAl and STOAlCo are illustrated in Table 1, showing that most of the defective structures are located in the perfect lattice interior (intensity ratio above 98%). However, comparing the two samples, the differential values of positron lifetimes are 0.9 ± 0.1, 2.1 ± 0.1 and 7.3 ± 0.1 ps obtained from the fitting spectra of STOAlCo and STOAl crystals, respectively, concluding that STOAlCo possesses defects with a still higher concentration level in the crystal interface and surface, especially on the surface, than that in the crystal bulk. These results elucidate that ACs containing a large number of atomic vacancies are mainly concentrated on the crystal surface of STOAlCo. However, the increase in ACs will inevitably lead to the microzone characteristic change of the surface. Accordingly, a contact angle meter is employed to characterize the effect of ACs on the surface hydrophobicity of SrTiO3 MNPs, as depicted in Fig. 1(p and t), showing that STOAlCo has a more hydrophobic surface with a contact angle of 91.4° than STOAl (136.74°). Therefore, the result reveals that most ACs introduce atomic vacancies located on the particle surface and rough surface caused by Co ACs, reducing the hydrophilicity of SrTiO3 MNPs, which is consistent with the experimental conclusions of PALS.
| Samples | τ 1 (ns) | Intensity % | τ 2 (ns) | Intensity % | τ 3 (ns) | Intensity % |
|---|---|---|---|---|---|---|
| STOAl | 0.166 | 63.3 | 0.302 | 35.5 | 1.833 | 1.2 |
| STOAlCo | 0.175 | 71.3 | 0.323 | 27.7 | 1.906 | 1.0 |
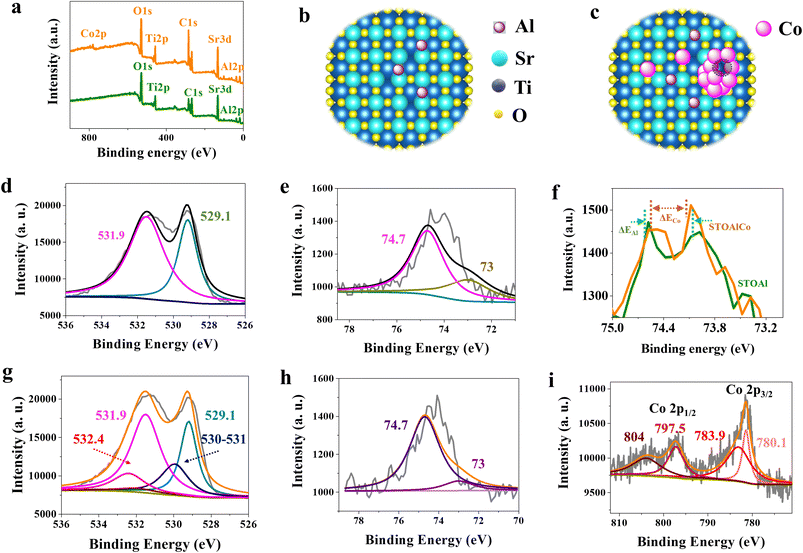
In this study, XPS spectra are used to measure the content range of the main elements with different valence states of the SrTiO3 MNPs, including Sr, Ti, O, Al and Co, the monitoring depth of which in metal oxides is about 10 nm, also aiming to evaluate the chemical environment difference of the elements caused the Al and Co. Fig. 2(a) presents the survey full XPS spectrum, showing the presence of the apparent Sr 3d, Ti 2p, O 1s, Al 2p and Co 2p peaks. The high-resolution XPS spectra of Sr and Ti reveal that these two elements of STOAl and STOAlCo have neither a change in valence states nor a peak shift in binding energy, as shown in SI Fig. S4, also indicating that for the two samples. There is no difference in O vacancy content around Sr and Ti. For the high-resolution XPS spectra of O 1s, the major two peaks can be fitted by five peaks, which are mainly attributed to the O2− ions in the Sr-Ti-O lattice at around 529.1 eV; the O2− of Al2O3 at 530–531 eV; the O− species in the Ti-O lattice at around 531.5 eV, which also corresponds to Ti vacancy; the O defect at 531.9 eV; and O species in Al-O lattice (alumina) at around 532.4 eV (Fig. 2(d and g)).11,32,33 However, for STOAl, the characteristic peaks at 530, 531.9 and 532.4 eV disappear, providing direct evidence that Al has filled the crystal of SrTiO3 of STOAl perfectly, and STOAl has the same amount of Ti vacancies as STOAlCo; however, STOAlCo has more O defects. Based on the analysis above, the results illustrate two points. First, the doped Co has little effect on the O and metal vacancies in the bulk crystal. Thus, for STOAlCo, the O vacancies detected by XPS and the new metal atomic vacancies, which mainly refer to Co, detected by other measurements (EPR and Raman spectra), are located in Co ACs certainly but not in the bulk crystal. Second, the stable valence state of Sr and Ti reveals that, except for Al, Co does not enter the bulk crystal lattice but is located on the surface in the form of SAs or ACs, which is well consistent with the results of PALS.
Furthermore, the corresponding high-resolution spectra of Al 2p are illustrated in Fig. 2(e and h), and they can be divided into two peaks, which are mainly attributed to a main peak at 74.7 eV and another peak at 73 eV, corresponding to Al-Sr/Al-Ti and Al-Al, respectively.33 The contrast between the two results demonstrates two points: on the one hand, most Al is filled in the SrTiO3 crystal, other than in the crystal surface in the form of clusters, especially for STOAlCo. Specifically, to analyse the electron binding energy change of Al atoms, the interval between the two main peaks of the highest point of Al in the two samples is compared here, showing that the interval between them in STOAlCo is less than that in STOAl, as shown in Fig. 2(f). It can be inferred that Al or Co has a binding effect and that some Co SAs or ACs may be adsorbed on Al atoms for STOAlCo. On the other hand, it is almost certain that there is no Al3+ of Al2O3 and Al-Co lattice (at 75.6 eV based on previous studies33) in the two samples. Thus, the ACs on the STOAlCo surface contain only Co. However, if it only contains isolated Co atoms, it needs to be further confirmed by XPS analysis of Co 2p. As shown in Fig. 2(i), the Co 2p3/2 XPS mainly includes a major peak at 781 eV, which can be fitted by two peaks at 780.1 and 783.9 eV contributed from the Co3+ in the SrTiO3 and Co composite, respectively. Another apparent peak in Co 2p1/2 is mainly attributed to the main Co2+ peak at 797.5 eV and a satellite peak at 804 eV. Although the peaks shift to the position of higher binding energy, Co with different valence states exists in the cluster, confirming that the Co cluster contains not only Co metal but also a few CoO or CoOOH clusters. It also indicates that vacancies are easily formed in the clusters due to the different valence states of Co and oxygen releasing under high temperature. Thus, a unified crystal structural model of SrTiO3 doped with Al and Co is shown intuitively in Fig. 2(b) and (c). The two models reveal that the Al atoms do not form the atomic clusters and are regularly embedded in the lattices of both STOAl and STOAlCo. In contrast, doped Co atoms simultaneously form SAs and clusters on the surface of SrTiO3, which possesses more atomic vacancies.
Based on the above data from various measurements, a unified structural model can be established to explain the relationship between the structure and photocatalytic performance of the SrTiO3. What is important about these structural characteristics is mainly reflected in two aspects: first, Co clusters and satellite SAs, and second, the CoO cluster with vacancies. Although the positions of vacancies are often difficult to distinguish, HRTEM, PALS and XPS are used to analyse this issue effectively in this article, providing a reference to similar problems. The result of structural changes also paves the way for studying the carrier dynamic features and photoredox performance of metal ions of SrTiO3 in subsequent studies.
Carrier dynamic features
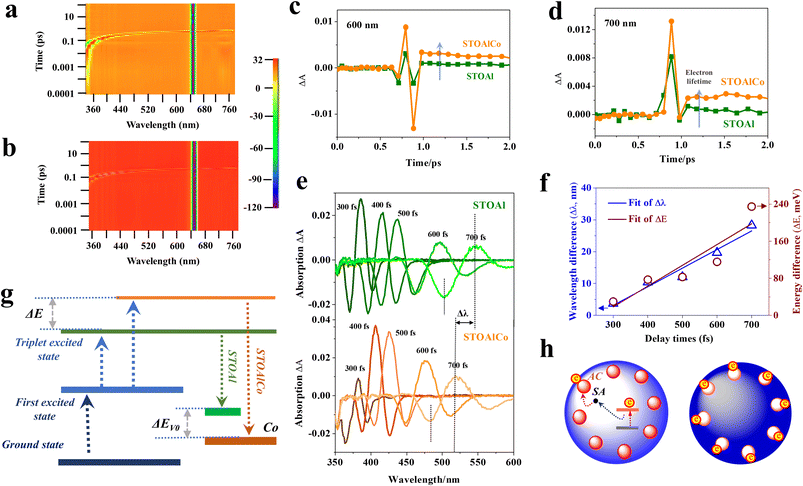
In this article, the optical absorption range of SrTiO3 MNPs is investigated using a UV-vis diffuse reflectance spectrometer (DRS), as shown in SI Fig. S5(a), suggesting that all exhibit strong absorption peaks in the ultraviolet region, although the absorption capability to ultraviolet light of STOAlCo is a little weaker than that of STOAl. Based on the results of the DRS, the band gaps of the two samples are calculated by applying the Kubelka–Munk formula,34 showing that the maximum values (Eg) of the band gaps are 3.01 and 2.97 eV for STOAl and STOAlCo, respectively, as presented in SI Fig. S5(b). This comparison demonstrates that the double-doped Al and Co lower the band gaps and strengthen the band bending effect of SrTiO3, but this change is small. Simultaneously, ultraviolet photoelectron spectroscopy (UPS) was used to determine the ionization potential (equivalent to the valence band energy (Ev)) of SrTiO3 MNPs, which are calculated to be 6.47 and 6.8 eV for STOAl and STOAlCo, respectively, by subtracting the width of the He(I) UPS spectra from the excitation energy (21.22 eV), as shown in SI Fig. S5(c and d).35 Thus, the conduction band energy (Ec) values of STOAl and STOAlCo are estimated at 3.46 and 3.83 eV from Ev − Eg, respectively. Based on the above data, the entire energy band structure of SrTiO3, including Ev and Ec, can be summarized, as shown in SI Fig. S5(e), indicating that STOAlCo may have a stronger reduction ability compared with STOAl, which is greatly beneficial for the photoreduction of metal ions and H2 evolution reactions.Our previous studies have shown that the transition mechanism between the exciton and free carrier states is of strategic importance for the separation efficiency of electrons and holes based on ultrafast photo-induced carrier dynamics.14,15 Therefore, we use femtosecond multiphoton transient absorption (fs-TA) spectroscopy to clarify the existence forms and transition process of photo-induced carriers in SrTiO3 MNPs caused by changes in surface structure, including Co ACs and SAs. Three-dimensional (3D) pictures of the reflective TA spectroscopic measurements of STOAl and STOAlCo are given, as shown in Fig. 3(a and b), within the range of a probe photon of 350–800 nm and a delay time of 0–100 ps when excited by pump light of 325 nm, indicating that STOAlCo has a higher density and intensity of photo-induced carriers compared with STOAl obviously. Significantly, there exists a noticeable feature of intense excited-state absorption (ESA) of the free carriers at the delay time range of 0–1 ps, showing that the free carrier is still dominant in the form of photo-induced carrier here. Simultaneously, Fig. 3(c) and (d) illustrate the dynamic profiles at 600 and 700 nm of the two samples, respectively, from which it can be found that the carriers in STOAlCo actually have a longer delay lifetime at two wavelengths of the probe light. Longer delay lifetimes of carriers have more beneficial implications for decreasing the recombination probability of electrons and holes; however, effective diffusion from the bulk or surface to the active site is more important.
To confirm the details of the dynamics change characteristics of carriers, the ESA spectra at different response times are summarized in Fig. 3(e), and the results include not only the different intensities of signal strength varying with delay time and wavelength of probe light (the maximum occurs at 300 fs at 385 nm for STOAl and 400 fs at 405 nm for STOAlCo) but also strongly indicate that STOAl has a remarkably faster decaying rate of carrier's density with an increase in the wavelength of the probe light. Therefore, at the same delay time, the relative excited state of electrons in STOAl and STOAlCo is different, and the concrete values can be measured by the wavelength difference of the probe light (Δλ = λSTOAl − λSTOAlCo). As the delay time increases, the results show a very significant linearity increase in Δλ from 3.55 nm (300 fs) to 56.4 nm (700 fs), portending the linear variation of the excited states of electrons. Thus, the calculation formula of the electromagnetic radiation energy (E = hc/λ) can be used to calculate the energy difference of electrons (ΔE) at different excited states. The specific values and variation tendencies of Δλ and ΔE are summarized, as shown in Fig. 3(f), and the ΔE can reach 234.69 meV (at 700 fs) from 29.9 meV (at 300 fs), through which method the binding energy of excitons in polyhedron SrTiO3 was also estimated in our previous studies.14 The above analysis has richly validated that the electrons are excited at a higher energy level in STOAlCo, which probably originates from the unique synergistic effect of ACs and satellite SAs, because the separation ability for electron–holes of the synergistic effect of Co may be better than that of Al. From the interaction viewpoint between electron and hole, the excited electrons of free carriers may be analogous to a triplet excited state with a metastable state other than excitons because remarkably broad photoinduced absorption (PIA) signals are not detected accurately in the fs-TA measurement results, which is a typical feature of the self-trapped exciton (STE) in the semiconductor.
However, the fs-TA measurement results present deep level bleaching at around 648 nm of probe light (ΔA < 0), as depicted in Fig. 3(a and b),36 the signals of which almost double the excitation wavelength (325 nm) and seem probably the overtone band of excitation light. Therefore, they are not usable for discussing carrier dynamics in this article.
Additionally, a whole excitation and recombination process of electrons has been proposed as a model involving all energy level conversion of carriers, as shown in Fig. 3(g), in which the ΔE and Ev0 are defined and marked clearly. Considering this, Fig. 3(j) and (k) illustrate the carrier transfer process in the STOAlCo MNPs, mainly including the excited electrons intuitively trapped by Co ACs and SAs. Compared with STOAl, ACs and SAs at the surface of STOAlCo play the critical role of a synergistic trap site for electrons, which is mainly reflected in two aspects. First, in the excitation stage, electrons are excited to a higher excited state by the activity of the SAs. Second, in the carrier trapping stage, STOAlCo with lots of defect structures, including the atom vacancies, provides more active centers for electron traps than STOAl with only Al atoms,14 further supplying more electrons involved in the photo-redox reaction. Consequently, the determining factor influencing photocatalytic efficiency is the synergistic effect of ACs and SAs, which is seldom mentioned in other studies.
Therefore, based on the above analysis, the synergistic effect of Co ACs and SAs on electron transfer is first put forward here. The theory has revealed the basic model of the electron transfer route in photocatalytic materials, which have both nano-ACs and SAs, mainly including two important viewpoints. First, AC with a defective structure supplied carrier trapping sites other than the SA site. Second, the electron transfer process has two separate stages: the electron is first captured by the SA site and then captured by the AC next (Fig. 3(h)). Specially, at different delay times, the captured electron has different electronic excited states due to the properties of the defect modes of AC. In summary, combined with the above research, for the (112) facets of STOAlCo, electronic transmission efficiency has increased more than for the other facets.
In addition to the Co and Al atoms covered in this article, the synergistic effect theory can explain all similar situations of the photocatalytic process for materials with the same structural features. To the best of our knowledge, this is the first report on the synergistic effects of ACs and SAs on photo-induced carrier characteristics in semiconductor photocatalysts. Here, an approximate function obtained from the fit curve of ΔE (Fig. 3(f)) is used to describe the mechanism of the synergistic effect:
| y(ΔE, eV) = −116.06 + 0.44x(delay times, s), |
Photoredox performance of metal ions
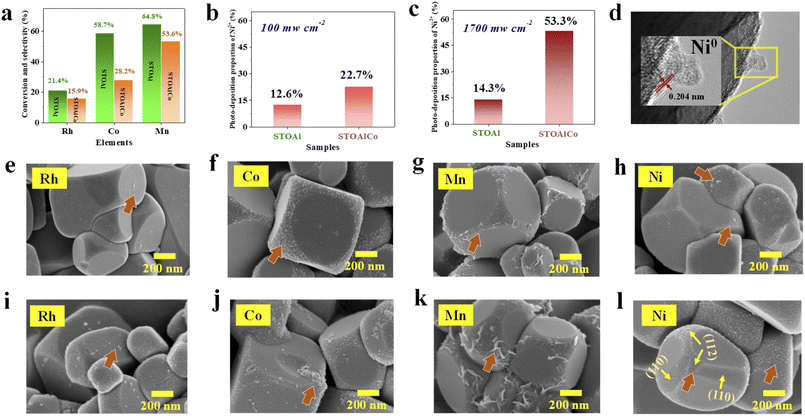
First, to verify our above analysis of the synergistic effect caused by Co ACs and SAs and filter the appropriate cocatalysts, i.e., active site, SrTiO3 and several typical metal ions, including Rh, Co, Mn and Ni, are applied in the photo-deposition reaction, which is performed under a 300 W Xe lamp irradiation. Unexpectedly, the SrTiO3 MNPs exhibit remarkable selectivity for different types of metal ions with the same valence state. For Rh3+, Co2+ and Mn2+, the results show that the STOAl has stronger photoredox capacity, and the photocatalytic efficiency is enhanced by about 5.5%, 30.5%, and 11.2% compared with the STOAlCo, as shown in Fig. 4(a), respectively. However, for Ni2+, STOAlCo has a higher photo-deposition efficiency than STOAl under AM1.5G (Fig. 4(b)). Moreover, STOAlCo exhibits an excellent photo-deposition efficiency to Ni2+ under a light intensity of about 1700 mw cm−2 irradiation, which is 39% higher than STOAl (Fig. 4(c)), indicating that STOAlCo has high selectivity for Ni2+. Moreover, the stability of the photoredox reaction of Ni2+ is tested under the same experimental conditions, and the experiments indicate that this reaction has good stability, as shown in SI Fig. S6. However, further research is needed to prove that the STOAlCo-Ni system can be used in light-driven photocatalytic reactions more effectively than the STOAl-Rh-Co catalyst system.Simultaneously, FESEM is used to analyse the distribution of the photo-deposited metal oxide on different crystal facets, as shown in Fig. 4(e–l). First, the results indicate that both STOAl and STOAlCo exhibit a crystal facet effect for the photo redox reaction of all metal ions, which has been studied extensively. Obviously, for STOAl and STOAlCo, Rh is adsorbed onto the (100) or (111) facets, and the Co with Mn is absorbed on the surface of (110) facets, which are consistent with the laws and characteristics of the location of the cocatalyst on the surface of SrTiO3, as summarized in the previous studies.2,3,27 However, Fig. 4(h) clearly shows that a few Ni elements are distributed on the surface of STOAl, mainly including the (100) and (112) facets. A large amount of Ni element is deposited and arranged tightly on the (112) facets rather than on the (100) and (110) facets of STOAlCo, as shown in Fig. 4(l). According to relevant research, photo-induced electrons have always been enriched at (112) facets; therefore, the Ni2+ is photo-reduced to Ni metal (Ni0) here, indicating that the photoreduction effect of the (112) facet is strengthened. As an evidence, high resolution transmission electron microscopy (HRTEM) is used to prove the successful loading of Ni0 onto the SrTiO3 surface of the metallic oxide, as shown in Fig. 4(d), which is judged based on the morphology and lattice spacing (0.204 nm corresponds to the (111) plane of metal Ni) according to the literature.37–39 To a certain extent, this phenomenon indicates that STOAlCo has maintained and strengthened the crystal facet effect in the process of photoreduction of Ni2+, and Ni metal is distributed only in the (112) facets. On the contrary, the anisotropic migration effect of STOAl is weakened in this process. Therefore, the crystal facets of traditional STOAl and STOAlCo show opposite selective photodeposition activity to different metal ions. This also explains that Rh, Co or Mn is more suitable for preparing the cocatalysts on STOAl. Through the analysis above, the results illustrate that the changes in the surface structure of SrTiO3 (appearance of ACs and SAs of Co) can regulate the crystal facet effect and then alter the photoredox efficiency of different metal ions. This provides a new solution method for removing several metal ions from industrial wastewater or for developing a new potential metal active site for the photocatalyst.
Photo-quantum absorption efficiency of Ni-loaded SrTiO3
Inspired by the testing principle of photoelectric conversion efficiency under homogeneous light in solar photovoltaic cells and based on the absolute spectral response system (BSRS), we have established a novel experimental platform for testing the photo-quantum absorption efficiency of SrTiO3 MNPs in aqueous dispersion (AD-PQAE), realizing rapid measurement under consecutive wavelengths. The main components in the AD-PQAE system include a Xenon lamp with full-wavelengths (300 W), a chopper, a monochromator and a dark box, in which there is a quartz container containing a sample dispersion of SrTiO3 MNPs and a standard silicon battery connecting to an external lock-in-amplifier equipped with a computer for data acquisition and processing, as shown in Fig. 5(a). The digital photograph of the practical test equipment is shown in Fig. 5(b) and SI S7. In order to get more accurate and reliable experimental data, a special quartz container with flat shape is used in this test, which practicality digital photograph is shown in Fig. 5(b) and SI S8. is used to obtain a stable suspension of SrTiO3 MNPs result of surface tension of water. Generally, the BSRS test results include the external quantum efficiency (EQE) spectra, also called incident-photon-to-charge conversion efficiency (IPCE) spectra, and the short circuit current density (Jsc) values versus wavelength integrated from the spectra of EQE.39 Thus, for the EQE and Jsc of the standard silicon battery as the control sample, the difference value of EQE (EQEd) and Jsc (Jsc−d) between the standard silicon battery and the aqueous suspension of MNPs can be used to characterize the AD-PQAE of SrTiO3 MNPs in this system. Consequently, for the photocatalyst systems of MNPs, the greater the EQEd and Jsc−d, the higher the AD-PQAE. It is worth noting that the quartz container and DI water do not affect the AD-PQAE measurement. SI Fig. S9 shows the EQEd and Jsc−d of DI water with different depths according to the data of EQE and Jsc of the DI water (SI Fig. S10), indicating that the water molecules have a strong selective absorbing effect for infrared light (900–1100 nm) in this system but not an effect for ultraviolet and visible light. Based on the results of EQE and Jsc, as shown in SI Fig. S11, EQEd and Jsc−d of STOAl, STOAlCo and the corresponding SrTiO3 MNPs with photo-deposition of Ni (STOAl Ni and STOAlCo Ni) are calculated, as presented in Fig. 5(c) and (d), respectively. First, as depicted in Fig. 5(c), the result demonstrates that the EQEd values of STOAl and STOAlCo are similar. However, the same samples with the active site of Ni have an enhanced photo-response of about 5% across 400–600 and 700–1000 nm compared to those without Ni0. The results indicate that Co ACs and SAs have not contributed very largely to the photo-quantum absorption efficiency across the whole wave-band. In contrast, the active site of Ni0 improves it greatly in this system, whether for STOAl or STOAlCo, as shown in Fig. 5(e–g), which has not been reported in previous studies. Obviously, the curves of the experimental data reflect the same rule and trend: the longer the wavelength of light, the smaller the AD-PQAE response. More perversely, besides around 650 nm, the photo-response is still at a relatively low level from the 350–400 nm region, which is seemingly contradictory to the high quantum utilization rate of SrTiO3 at around 360 nm from a previous report.2 An important factor that should not be neglected is the effect of Rayleigh scattering or Mie scattering in certain practical fields of aqueous dispersion systems, which has clarified that the scattering intensity of light becomes stronger as the wavelength decreases. Most particle diameters of both STOAl and STOAlCo are less than 350 nm in the water dispersion system, which fully meets the conditions for the occurrence of Rayleigh scattering. Similarly, when the incident occurs at around 650 nm, few particles with a diameter of around 500–600 nm cause a Mie scattering effect, resulting in low values of AD-PQAE. Notably, the scattering process is more effective in STOAl-Ni and STOAlCo-Ni than in STOAl and STOAlCo, which probably originates from the rougher surface of SrTiO3 MNPs caused by active site Ni0. Moreover, all the Jsc−d values summarized from the curves of Jsc well match the values obtained from the EQEd measurement and show the same varying tendency, as shown in Fig. 5(d), confirming the accuracy of the device measurement. To sum up, we establish a simple and effective method for estimating AD-PQAE at a single and consecutive wavelength scale, and the AD-PQAE measurement of SrTiO3 MNPs is not much affected by different elemental doping but is obviously affected by the active site of Ni and light scattering phenomena. However, the EQEd of STOAlCo-Ni is obviously higher than that of STOAl-Ni, as shown in Fig. 5(c), based on which it can be inferred that the photo-deposited Ni0 has strengthened the photocatalytic performance of SrTiO3. The above result indicates that Ni0 may be an effective active site in metal ion removal or water splitting reactions for SrTiO3.Theoretical simulation of electron migration
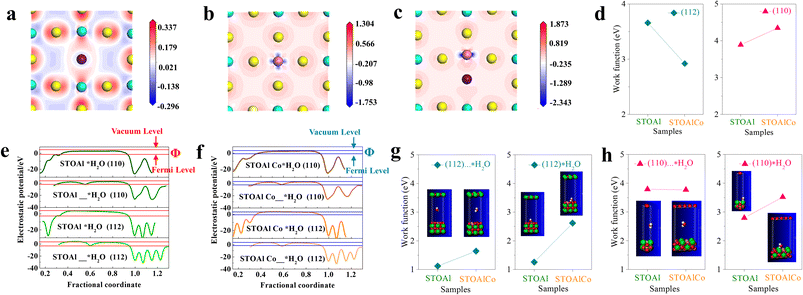
To understand the role of doped Al and Co in promoting the activity and transfer route of photo-induced electrons of SrTiO3 MNPs, DFT calculations are performed. The photocatalytic properties of STOAl or STOAlCo with polyhedral morphology still follow the theory of anisotropic high charge carrier mobility, as confirmed by our studies and other studies. For studying the activity of doping sites, the effects of the crystal facet orientation and Co ACs and SAs should be considered in the simulated calculation process. Therefore, we have created the three models of SrTiO3 crystal doped with Al, Co and Al/Co, and to analyse the electronic distribution on the SrTiO3 crystal surface, the charge density differences of the three models are calculated, as shown in Fig. 6(a–c). The results show that there is a low-density electron distribution around the Al atom, indicating that Al shows the characteristic of an electron donor in the Al-doped SrTiO3 lattice. However, the Co-doped samples give a reliable conclusion that Co has a significantly lower electron density than Al due to its stronger electron-donating property, especially in the STOAlCo in this article. In most cases, electron-donating effects are more conducive to surface electron escape from the crystal facet; in other words, this effect can distinctively promote the reduction reaction of photo-induced electrons, which also fully proves the point that the reduction ability difference of Co- and Al-doped SrTiO3.However, in the photoreduction process of Ni2+, the effect of water molecules on charge migration at the surface of SrTiO3 MNPs cannot be negligible in this system. Accordingly, in order to predict the difficulty degree of electron migration along different facets of STOAl and STOAlCo in aqueous dispersion systems, values of work function (Φ) along (110) and (112) facets are calculated based on the DFT calculation method (computational models are shown in SI Fig. S12 and S13). First, as shown in Fig. 6(d), the calculated result along the (112) facet shows that the Φ of STOAlCo (2.92 eV) is much lower than that of STOAl (3.65 eV), which is mainly attributed to Co doping, signaling an easily reduced reaction in the (112) facets. However, in the direction of (110), it can be observed that the Φ of STOAlCo has a relatively large value (4.34 eV) compared with STOAl (3.88 eV), indicating that Co adds further opportunities for oxidation reaction at the (110) facet. Specially, although for STOAl and STOAlCo, the Φ of the (112) facet is lower than that of (110), and the result is quite consistent with those of previous studies.2 We have defined the difference of work function of (112) and (110) as ΔΦ(112)–(110), and there is a significant difference in ΔΦ(112)–(110) for STOAl (0.231 eV) and STOAlCo (1.426 eV) caused by Co, predicting that STOAlCo has a larger electrical potential difference between (112) and (110) than STOAl, and a higher separation efficiency potential of photo-induced electrons and holes. Moreover, before and after adsorbing water molecules on (112) and (110), the ΔΦwater molecule values of STOAl and STOAlCo are summarized in Fig. 6(g) and (h) based on the corresponding variation curves of the electrostatic potential (Fig. 6(e) and (f)). Overall, under the condition of water molecules, ΔΦ values of (112) facets are generally lower than those of (110), following the same rule discussed above, which shows that the water molecules exhibit no effects on the basic properties of SrTiO3 crystal facets. For the (112) facet, the Φ values of STOAl and STOAlCo increase by about 0.139 and 0.976 eV, respectively. In contrast, all the Φ values of (110) facets decrease, and the variational range of ΔΦ of STOAlCo (0.251 eV) is smaller than that of STOAl (0.99 eV). The results clearly demonstrate that the Φ values of (112) and (110) of STOAlCo become more balanced after a water molecule is absorbed.
Therefore, the calculation results of work functions can better reflect the advantages of surface structure in the photoredox process. The above theoretical simulation results show that the Co SA site decreases the electron transfer barriers, and in this situation, carriers prefer to follow the rules of the crystal facet effect. Thus, it is reasonable that Ni is more easily adsorbed and photo-reduced on the (112) or (111) facet of STOAlCo than that of STOAl. However, in the photoredox process of Rh, Co and Mn, the above analysis is unable to explain the difference between the STOAlCo and STOAl in this article.
Conclusions
In summary, based on the findings, SrTiO3 with ACs and SAs of Co on the crystal surface is successfully prepared by molten salt synthesis, which has not been reported in other studies about SrTiO3 MNPs. The surface structure changes in SrTiO3 MNPs doped with bimetallic (Al and Co) are studied systematically in this article. The synergistic effects of Co ACs and SAs on electron transfer are also proposed in this article. More importantly, the SrTiO3 MNPs exhibit remarkable selectivity for different types of metal ions with the same valence state, and according to the experimental results, we find that Ni can be photo-reduced to Ni0 efficiently by the (112) facet of double-doped SrTiO3 for the first time. Furthermore, a new AD-PQAE system employing an aqueous dispersion of SrTiO3 MNPs under consecutive wavelengths has been proved to be effective, indicating that the Ni-loaded STOAlCo has the potential to be an available photocatalyst to be used in the field of industrial wastewater treatment, water splitting and so on. However, the definite mechanism of photoredox efficiency of different metal ions is still not explained clearly, and the relationship of this mechanism and photo-quantum absorption efficiency also deserves further investigation in depth. Finally, this study enriches the pool of photocatalytic SrTiO3-based materials and contributes new insights into the novel active site characteristics of SrTiO3, which can be widely applied to the development of photocatalytic materials for the water splitting process.Author contributions
Conceptualization, Q. B. J.; methodology, Q. B. J.; investigation, Q. B. J.; writing – original draft, Q. B. J; funding acquisition, Q. B. J.; resources, Q. B. J., Y. W., Y. Li, X. D. Z., L. Z.; Y. Liu; E. Z.; Y. Z. R and T. L.; computing support, Q. B. J.; supervision, D. P. D.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article are included in the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ta08073h.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 22379148).Notes and references
- Y. F. Bao, C. Li, K. Domen and F. X. Zhang, Strategies and Methods of Modulating Nitrogen-Incorporated Oxide Photocatalysts for Promoted Water Splitting, Acc. Mater. Res., 2022, 3, 449–460 CrossRef CAS.
- T. Takata, J. Z. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi and K. Domen, Photocatalytic water splitting with a quantum efficiency of almost unity, Nature, 2020, 581, 411 CrossRef CAS.
- C. Y. Wang, Q. B. Jia, X. D. Zhang, X. Chen, Y. Wang, G. Q. Yu and D. P. Duan, Effect of Different Molten Salts on Structure and Water Splitting Performance of Al-Doped Fillet Polyhedral SrTiO3, Small, 2025, 21, 2407963 CrossRef CAS.
- L. Q. Dong, H. Shi, K. Cheng, Q. Wang, W. J. Weng and W. Q. Han, Shape-controlled growth of SrTiO3 polyhedral submicro/nanocrystals, Nano Res., 2014, 9, 1311 CrossRef.
- T. Higashi, H. Nishiyama, V. Nandal, Y. Pihosh, Y. Kawase, R. Shoji, M. Nakabayashi, Y. Sasaki, N. Shibata, H. Matsuzaki, K. Seki, K. Takanabe and K. Domen, Design of semitransparent tantalum nitride photoanode for efficient and durable solar water splitting, Energy Environ. Sci., 2022, 15, 4761 RSC.
- X. Cao, A. J. Huang, C. Liang, H. C. Chen, T. Han, R. Lin, Q. Peng, Z. W. Zhuang, R. A. Shen, H. M. Chen, Y. Yu, C. Chen and Y. D. Li, Engineering Lattice Disorder on a Photocatalyst: Photochromic BiOBr Nanosheets Enhance Activation of Aromatic C-H Bonds via Water Oxidation, J. Am. Chem. Soc., 2022, 144, 3386–3397 CrossRef CAS PubMed.
- B. Wu, S. Gong, Y. C. Lin, T. Li, A. Y. Chen, M. Y. Zhao, Q. J. Zhang and L. Chen, A Unique NiOOH@FeOOH Heteroarchitecture for Enhanced Oxygen Evolution in Saline Water, Adv. Mater., 2022, 34, 2108619 CrossRef CAS.
- B. Y. Zhai, H. G. Li, G. Y. Gao, Y. Wang, P. Niu, S. L. Wang and L. Li, A Crystalline Carbon Nitride Based Near-Infrared Active Photocatalyst, Adv. Funct. Mater., 2022, 32, 220737 Search PubMed.
- T. T. Xu, X. Liu, S. L. Wang and L. Li, Ferroelectric Oxide Nanocomposites with Trimodal Pore Structure for High Photocatalytic Performance, Nano-Micro Lett., 2019, 11, 37 CrossRef CAS.
- A. Kudo and Y. Miseki, Heterogeneous photocatalyst materials for water splitting, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- T. Takata and K. Domen, Defect engineering of photocatalyst by doping of aliovalent metal cations for efficient water splitting, J. Phys. Chem. C, 2009, 113, 19386–19388 CrossRef CAS.
- B. Y. Zhai, J. Zeng, Y. Wang, P. Niu, S. L. Wang and L. Li, Achieving near-infrared photocatalytic overall water splitting with singular crystalline C3N4 from semi-molten-salt treatment, Appl. Catal. B Environ., 2024, 359, 124496 CrossRef CAS.
- T. Suguro, F. Kishimoto, N. Kariya, T. Fukui, M. Nakabayashi, N. Shibata, T. Takata, K. Domen and K. Takanabe, A hygroscopic nano-membrane coating achieves efficient vapor-fed photocatalytic water splitting, Nat. Commun., 2022, 13, 5698 CrossRef CAS PubMed.
- Q. B. Jia, C. Y. Wang, J. Liu, X. J. Cai, L. Zhong, S. M. Chen, T. Li, G. Q. Yu, L. Z. Wu and D. P. Duan, Synergistic effect of Sr-O divacancy and exposing facets in SrTiO3 micro/nano particle: accelerating exciton formation and splitting, highly efficient Co2+ photooxidation, Small, 2022, 18, 2202659 CrossRef CAS PubMed.
- Q. B. Jia, C. Y. Wang, J. Liu, X. J. Cai, L. Zhong, G. Q. Yu and D. P. Duan, Strong synergistic effect of the (110) and (100) facets of the SrTiO3 perovskite micro/nanocrystal: decreasing the binding energy of exciton and superb photooxidation capability for Co2+, Nanoscale, 2022, 14, 12875 RSC.
- P. Zhou, I. A. Navid, Y. J. Ma, Y. X. Xiao, P. Wang, Z. W. Ye, B. W. Zhou, K. Sun and Z. T. Mi, Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting, Nature, 2023, 613, 66–70 CrossRef CAS PubMed.
- Z. L. Wang, T. Hisatomi, R. G. Li, K. Sayama, G. Liu, K. Domen, C. Li and L. Z. Wang, Efficiency Accreditation and Testing Protocols for Particulate Photocatalysts toward Solar Fuel Production, Joule, 2021, 5, 344–359 CrossRef CAS.
- H. Nishiyama, T. Yamada, M. N, Y. Maehara, M. Yamaguchi, Y. Kuromiya, Y. Nagatsuma, H. Tokudome, S. Akiyama, T. Watanabe, R. Narushima, S. Okunaka, N. Shibata, T. Takata, T. Hisatomi and K. Domen, Photocatalytic solar hydrogen production from water on a 100-m2 scale, Nature, 2021, 598, 304–307 CrossRef CAS.
- M. G. Alemseghed, T. P. A. Ruberu and J. Vel, Controlled Fabrication of Colloidal Semiconductor–Metal Hybrid Heterostructures: Site Selective Metal Photo Deposition, Chem. Mater., 2011, 23(15), 3571–3579 CrossRef CAS.
- J. Cure, H. Assi, K. Cocq, L. Marìn, K. Fajerwerg, P. Fau, E. Bêche, Y. J. Chabal, A. Estève and C. Rossi, Controlled Growth and Grafting of High-Density Au Nanoparticles on Zinc Oxide Thin Films by Photo-Deposition, Langmuir, 2018, 34(5), 1932–1940 CrossRef CAS.
- J. Lian, D. Li, Y. Qi, N. Yang, R. Zhang, T. Xie, N. Guan, L. Li and F. Zhang, Metal-seed assistant photodeposition of platinum over Ta3N5 photocatalyst for promoted solar hydrogen production under visible light, J. Energy Chem., 2021, 55, 444–448 CrossRef CAS.
- C. W. Hsu, K. Awaya, M. Tsushida, T. Miyano and M. Koinuma, Shintaro Ida, Water Splitting Using a Photocatalyst with Single-Atom Reaction Sites, J. Phys. Chem. C, 2020, 124(38), 20846–20853 CrossRef CAS.
- H. Su, W. Che, F. M. Tang, W. R. Cheng, X. Zhao, H. Zhang and Q. H. Liu, Valence Band Engineering via PtII Single-Atom Confinement Realizing Photocatalytic Water Splitting, J. Phys. Chem. C, 2018, 122(37), 21108–21114 CrossRef CAS.
- Q. Guo, Q. Zhao, R. C. Crespo-Otero, D. D. Tommaso, J. W. Tang, S. D. Dimitrov, M. M. Titirici, X. H. Li and A. B. J. Sobrido, Single-Atom Iridium on Hematite Photoanodes for Solar Water Splitting: Catalyst or Spectator?, J. Am. Chem. Soc., 2023, 145(3), 1686–1695 CrossRef CAS PubMed.
- L. F. Zhang, Q. Q. Luo, S. L. Hu, Z. P. Hu, W. H. Zhang and J. L. Yang, Enhanced Electron–Hole Separation in Phosphorus-Coordinated Co Atom on g-C3N4 toward Photocatalytic Overall Water Splitting, J. Phys. Chem. Lett., 2022, 13(51), 11961–11967 CrossRef CAS.
- X. Ma, L. Wang, Q. Zhang and H. L. Jiang, Switching on the Photocatalysis of Metal–Organic Frameworks by Engineering Structural Defects, Angew. Chem., Int. Ed., 2019, 58, 12175–12179 CrossRef CAS PubMed.
- B. Li, F. X. Tong, M. Lv, Z. Y. Wang, Y. Y. Liu, P. Wang, H. F. Cheng, Y. Dai, Z. K. Zheng and B. B. Huang, In Situ Monitoring Charge Transfer on Topotactic Epitaxial Heterointerface for Tetracycline Degradation at the Single-Particle Level, ACS Catal., 2022, 12(15), 9114–9124 CrossRef CAS.
- M. L. Guan, C. Xiao, J. Zhang, S. J. Fan, R. An, Q. M. Cheng, J. F. Xie, M. Zhou, B. J. Ye and Y. Xie, Vacancy Associates Promoting Solar-Driven Photocatalytic Activity of Ultrathin Bismuth Oxychloride Nanosheets, J. Am. Chem. Soc., 2013, 135, 10411–10417 CrossRef CAS PubMed.
- A. Uedono, K. Shimayama, M. Kiyohara, Z. Q. Chen and K. Yamabe, Study of oxygen vacancies in by positron annihilation, J. Appl. Phys., 2002, 92, 2697–2702 CrossRef CAS.
- A. Musiienko, J. Čížek, H. Elhadidy, P. Praus, K. Higgins, B. Dryzhakov, A. Kanak, F. Sureau, J. Pipek, E. Belas, M. Betušiak, M. Brynza, E. Lukosi, B. Hu and M. Ahmadi, Origin of Defects and Positron Annihilation in Hybrid and All-Inorganic Perovskites, Chem. Mater., 2022, 34, 297–306 CrossRef CAS.
- D. J. Keeble, J. Wiktor, S. K. Pathak, L. J. Phillips, M. Dickmann, K. Durose, H. J. Snaith and W. Egger, Identification of lead vacancy defects in lead halide perovskites, Nat. Commun., 2021, 12, 5566 CrossRef CAS PubMed.
- M. Schneider, V. A. Gasparov, W. Richter, M. Deckwerth and C. Riissel, XPS studies on oxynitride glasses in the system Si-A1-O-N, J. Non-Cryst. Solids., 1997, 215, 201–207 CrossRef CAS.
- P. Motamedi and K. Cadien, XPS analysis of AlN thin films deposited by plasma enhanced atomiclayer deposition, Appl. Surf. Sci., 2014, 315, 104–109 CrossRef CAS.
- J. Tauc, R. Grigorovici and A. Vancu, Optical Properties and Electronic Structure of Amorphous Germanium, Phys. Stat. Sol., 1966, 15, 627 CrossRef CAS.
- J. Liu, Y. Liu, N. Y. Liu, Y. Z. Han, X. Zhang, H. Huang, Y. Lifshitz, S. T. Lee, J. Zhong and Z. H. Kang, Metal-free c for stable visible water splitting via a two-electron pathway, Science, 2015, 347, 970–974 CrossRef CAS.
- R. Berera, R. V. Grondelle and J. T. M. Kennis, Ultrafast transient absorption spectroscopy: principles and application to photosynthetic systems, Photosynth. Res., 2009, 101, 105–118 CrossRef CAS.
- X. C. Zheng, Y. D. Wang, S. T. Ren, Q. X. Gai, W. J. Liu and Q. L. Dong, Construction of Interfacial P−Ni Bonding for Enhanced Hydrogen Evolution Performance of P-Doped C3N4/Ni Photocatalyst, ACS Appl. Energy Mater., 2022, 5, 5756–5765 CrossRef CAS.
- C. Liu, J. Liu and R. Godin, ALD-Deposited NiO Approaches the Performance of Platinum as a Hydrogen Evolution Cocatalyst on Carbon Nitride, ACS Catal., 2023, 13, 573–586 CrossRef CAS.
- Y. Qi, J. W. Zhang, Y. Kong, Y. Zhao, S. S. Chen, D. Li, W. Liu, Y. F. Chen, T. F. Xie, J. Y. Cui, C. Li, K. Domen and F. X. Zhang, Unraveling of cocatalysts photodeposited selectively on facets of BiVO4 to boost solar water splitting, Nat. Commun., 2022, 13, 484 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |