Electrocatalytic activity of 1D/3D TiO2 tubular layers/Ni-modified MXene microsphere heterojunction electrodes
Dujearic-Stephane
Kouao
 *a,
Agnieszka
Kramek
b,
Justyna
Gumieniak
b,
Karol
Załęski
*a,
Agnieszka
Kramek
b,
Justyna
Gumieniak
b,
Karol
Załęski
 c,
Emerson
Coy
c,
Jakub
Karczewski
d,
Guowei
Li
c,
Emerson
Coy
c,
Jakub
Karczewski
d,
Guowei
Li
 ef and
Katarzyna
Siuzdak
ef and
Katarzyna
Siuzdak
 *a
*a
aCentre for Plasma and Laser Engineering, Institute of Fluid-Flow Machinery, Polish Academy of Sciences, 14 Fiszera St., 80-231 Gdańsk, Poland. E-mail: dkouao@imp.gda.pl; ksiuzdak@imp.gda.pl
bThe Faculty of Mechanics and Technology, Rzeszów University of Technology, Kwiatkowskiego 4 St., 37-450 Stalowa Wola, Poland
cNanoBioMedical Centre, Adam Mickiewicz University, Wszechnicy Piastowskiej 3 St., 61-614 Poznań, Poland
dFaculty of Applied Physics and Mathematics, Institute of Nanotechnology and Materials Engineering, Gdańsk University of Technology, Narutowicza 11/12 St., 80-233 Gdańsk, Poland
eZhejiang Provincial Key Laboratory of Magnetic Materials and Applications, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201, China
fCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, 19 A Yuquan Rd, Shijingshan District, Beijing 100049, China
First published on 15th December 2025
Abstract
Controlling the morphology and size of MXene-based materials through top–down, electrochemical-assisted synthesis methods is crucial for improving catalytic performance and environmental safety issues compared with traditional etching techniques. However, achieving precise morphological control while avoiding structural and catalytic performance degradation remains a key scientific challenge. In this study, we introduce a mild and versatile approach that reshapes MXene flakes into microspheres via cyclic voltammetry. After 300 cycles at a scan rate of 20 mV s−1, spherical particles averaging 2.1 ± 0.1 µm were obtained. Despite the disruption of the strong Ti–C covalent bonds revealed by X-ray photoelectron spectroscopy (C1s spectrum), transmission electron microscopy confirmed a homogeneous distribution of Ti and C across the structure. Raman spectroscopy further verified that the electrochemically treated product preserved the characteristic Ti3C2Tx MXene chemical framework. To demonstrate the practical impact of this morphological tuning, a TiO2 nanotube/Ni–Ti3C2Tx heterostructure was fabricated and evaluated for water splitting catalysis. Remarkably, after only one minute of thermal annealing in air at 450 °C, the electrochemically derived MXenes demonstrated a substantially reduced overpotential of 260 mV for the oxygen evolution reaction (OER), surpassing most reported Ni-MXene-based catalysts in alkaline media.
1. Introduction
Electrochemical-assisted synthesis methods are considered green, straightforward, and efficient approaches used for the exfoliation of two-dimensional (2D) materials, such as graphite,1,2 phosphorene,3 transition metal dichalcogenides,4 and transition metal carbides.5 In the electrochemical approach, the electric field generated by the potential difference between the electrodes, namely anode and cathode, induces an effective attack of ions such as SO4−, F−, and Cl− present in the electrolyte (i.e., the etching solvent) on the bulk material, leading to the formation of open fringes distributed across its surface.2 The etching process is a crucial step that enables the intercalation of solvent molecules and cations (or anions) into the bulk material, thereby facilitating its subsequent exfoliation (or delamination). Making this process facile and requiring fewer toxic compounds is highly desirable. Finally, delamination of the multi-layered material can be achieved by ultrasonic treatment or mechanical shaking.Recently, there has been an emerging focus on the development of an efficient electrochemical-assisted etching process targeting the extraction of aluminum layers in 2D MAX phases towards the production of MXenes.6 The main advantages of the electrochemical synthesis over the traditional one based on HF-based etching method for MXenes production include not only the use of moderately toxic salts such as NH4Cl7 or NH4F,8 but also the ability to control the entire etching process by simply adjusting the electrochemical parameters, including the used method (cyclic voltammetry or chronoamperometry), applied potential or the range of potentials, and the composition of the electrolyte. In general, the electrochemical etching of MAX phases is carried out by direct polarization of the bulk material (i.e., anodic or cathodic polarization), which acts as the electrode material in a two-5 or three-electrode system.9 When the material is in powder form, the working electrode can be fabricated by packing the powder material against a current collector substrate using a porous membrane to avoid the utilization of a binder in the deposited MAX phases.8 It has even been pointed out that the anodic polarization of the MAX phases precursor can increase the Fermi potential of the aluminum element in the MAX phases precursor, which allows its anodic dissolution and the formation of MXenes.10 Moreover, as Zhao et al.11 have shown, the anodic polarisation of the bulk Ti3AlC2 MAX phase immersed in a mixed solution of tetramethylammonium hydroxide and NH4Cl at 0.1 V for 1 h resulted in the formation of nitrogen-doped Ti3C2 MXene quantum dots. This demonstrates the possibility of tuning both the flake sizes and their shape, as well as the surface chemistry properties of the synthesised MXenes, in a shorter time compared to the traditional hydrothermal method, directly via a one-step electrochemical process. However, reports also show that the polarisation of the bulk MAX phase can result in amorphous carbide-derived carbon instead of MXene.12 Although several approaches to electrochemically assisted etching of MXenes have been reported, and only the study by Zhao et al.,11 addresses tuning the size of MXene flakes, and this type of synthesis is still not well explored. Furthermore, to date, there has been no report on the electrochemical activity towards water splitting of these small-sized MXene flakes, a few micrometres in size, fabricated using the electrochemical etching method.
In this work, MXenes with an average size of 2.1 ± 0.1 µm were produced via an electrochemical etching route from a 325 mesh MAX phase precursor. Compared to the widely reported anodic polarisation method, the procedure adopted in this work involves producing Ti3C2Tx MXenes in a three-electrode cell, where the MAX powder is suspended in the electrolyte, with immersed glassy carbon electrodes serving as the counter and working electrodes. Changes in morphology and structure were investigated using electron microscopy and spectroscopic methods. Following the electrochemical synthesis of the microspherical MXene, a heterojunction catalyst was fabricated by depositing the as-prepared Ti3C2Tx MXene, previously modified with Ni, onto a platform composed of highly ordered titania nanotubes and peforming thermal treatment in two different atmosphere, namely, in air and under hydrogen atmosphere. It should be noted that the synergistic behaviour of MXenes and TiO2 nanotubes (TN) heterostructure material in water splitting catalysis has been previously studied by Mishra et al.,13 and showed that the 1D/2D heterostructure of TiO2 nanotubes and Ti3C2Tx MXenes promotes efficient charge carrier separation. As a result, a significant improvement in the electrocatalytic performance of the electrode compared to pristine MXenes has been achieved.13 However, according to their study, the nanotubes were synthesized via a hydrothermal process, which typically yields a final product in the form of a powder composed of randomly distributed hierarchical tubes exhibiting multimodal length distributions.
Unlike the hydrothermal method, herein a highly ordered tubular layer was first formed on a stable substrate through the anodic oxidation of titanium foil. Subsequently, the heterostructure is fabricated by depositing Nickel decorated MXenes onto the anodized substrate. Other works have also demonstrated that TiO2 nanotube arrays can serve as a promising, stable substrate hosting catalyst for electrocatalysis testing. For example, Krstajić et al.14 showed that osmium loaded onto hydrogenated TiO2 nanotube arrays exhibits superior catalytic performance toward the HER reaction. Although the tubular layer was almost totally covered by the osmium film, the resultant Os@TiO2 nanotubes catalyst exhibited a record-low overpotential (η) of 61 mV at a current density of 100 mA cm−2. Similarly, the sandwich-structure C/NiO/TiO2 nanotube arrays prepared by Yang et al.15 exhibited superior HER activity, with an overpotential of 86 mV at 10 mA cm−2 and a Tafel slope of 67.1 mV dec−1. The catalyst was prepared by first embedding NiO nanoparticles within the nanotube structure, followed by coating the top of the tubular layer with a carbon nanofilm via carbonization of a polydopamine membrane. Overall, TiO2 nanotube arrays not only act as an ordered scaffold for catalyst deposition but also facilitate charge transport. It is worth mentioning that the substrate with nanotubes also serves directly as the current collector.
The aim in this work is not to embed the MXene microspheres within the nanotubes, but rather to establish a 1D/3D heterojunction (i.e., TiO2 nanotubes/MXene microspheres) that effectively facilitates charge carrier separation.13 The influence of the annealing atmosphere on the electrocatalytic properties of the resulting Ni–Ti3C2Tx@TN heterostructure towards hydrogen and oxygen production are discussed in detail, taking into account both complex analysis of the structure and electrochemical performance. The experimental section and descriptions of all characterization techniques are provided in the SI file.
2. Results
2.1. Physicochemical characterization of the as-prepared Ti3C2Tx MXene
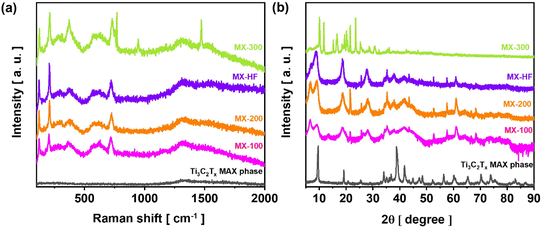
The morphologies of the samples obtained for 100 and 200 CV cycles exhibit some open fringes at the edges, similar to those of the MX-HF sample (shown in the SI file, Fig. S3). This change in the microstructure of the sample after the electrochemical treatment will be further analysed and discussed in the XRD section. However, one can observe non-uniformity in the obtained structures, as both spherical and 2D layered structures were achieved. The morphologies of the as-synthesized MX-300 is presented in Fig. 1a. It can be noticed that the MXene flakes are fragmented into spherical grains with an average size of 2.1 ± 0.1 µm after 300 CV cycles. In general, MXenes obtained via the widely reported anodic polarization method of MAX phases preserve the 2D morphology of their parent MAX phases.16–18 However, studies have shown that the pronounced self-stacking of 2D MXenes hinders their electrochemical performance.19 Related to this, it has been proposed to construct MXene assemblies from 2D to 3D to address this issue.19 In this regard, the method described in this work towards shape tuning of 2D MXenes into a 3D architecture is a simple way for direct electrochemical production of MXene microspheres without the need of any spherical precursor templates. The elemental composition of the MX-300 sample was analysed by EDX elemental mapping (Fig. 1c–e), and a uniform distribution of the C, Ti and Al elements within the MX-300 sample has been identified.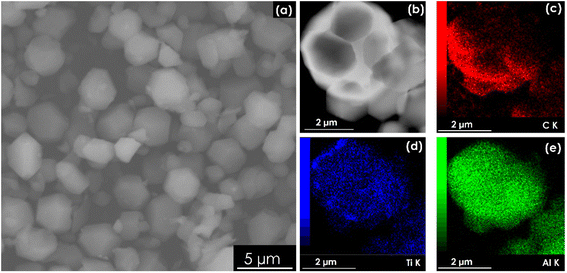 | ||
| Fig. 1 (a) SEM and (b) TEM images of the powder obtained after 300 CV cycles (i.e., MX-300). (c)–(e) distribution mapping recorded for (b) C, (c) Ti and (d) Al of MX-300. | ||
The presence of a residual aluminum layer in MX-300 is not unusual. Indeed, regardless of the synthesis route used, namely the traditional etching method,9 electrochemically assisted etching16 or the hydrothermal method,20 the presence of residual aluminum in MXenes is often reported. The mapping performed for F element is also shown in Fig. S4. It should be noted that the etching solvent used in this work for the electrochemical removal of the aluminum layers is a fluorine-containing electrolyte (HBF4:TMATFB). Therefore, F− can also interact with Ti, resulting in the formation of a Ti–F termination group on the MX-300 surface. Indeed, Yin et al.,9 have also obtained highly fluorinated Ti3C2Tx after the electrochemical process for only after 5 h in [BMIM][PF6]/MeCN electrolyte at 5 V vs. Ag/AgCl. Similarly, other studies have also reported Cl− contamination when using a chlorine-containing electrolyte (NH4Cl:TMAOH) during the electrochemical synthesis of MXens quantum dots.11
The crystal structures of MX-HF and MXenes obtained after different numbers of cycles were identified by Raman measurements and shown in Fig. 2a. Peak positions and corresponding vibrational modes for MXene based materials are presented in Table S1. Compared to the Raman spectrum of Ti3AlC2 MAX phase, the spectra of the prepared samples i.e., MX-HF and MX-100, MX-200 and MX-300, exhibit four distinct vibrational modes, namely the resonance peak at 120 cm−1, the out-of-plane A1g (Ti, C, O) vibrations at 206 cm−1 and 736 cm−1, and the in-plane Eg (Ti, C, O) vibration at 361 cm−1. The appearance of these characteristic Raman bands indicates the successful synthesis of Ti3C2Tx MXenes after the electrochemical treatment. Similar Raman bands were also detected for Ti3C2Tx prepared by electrochemical etching in tetrafluoroboric acid for 24 h at 1 V.10
However, no Raman peak was detected on the spectrum recorded by Zhao et al.,11 for Ti3C2Tx quantum dots electrochemically produced in a mixed solution of tetramethylammonium hydroxide and NH4Cl at 0.1 V for 1 h. This could probably be due to the power of the laser beam used during Raman spectra measurements, which interacts with the sample. Indeed, with relatively high laser power, the structure of MXenes may collapse, resulting in no signal being detected.21 However, as shown in Fig. 2a, besides the Raman peaks characteristic of the MXenes structure mentioned above, three additional peaks at 771 cm−1, 952 cm−1 and 1470 cm−1 were found in the spectrum of MX-300 sample. The Raman spectrum of TMATFB, provided in the SI file (Fig. S5), indicates that these signals result from TMATFB used during the electrochemical etching process. This contamination can result from TMA+/TBF− ions (TMATBF → TMA+ + TBF−) incorporated into the MXenes structure during the synthesis. Indeed, a previous report pointed out that, similarly to HF, the TBF− anions can also act as an effective etchant for aluminum extraction in MAX phases.22 Thus, the chemical reaction between TBF− and Ti3AlC2 may be responsible for the presence of TBF− contaminant in the synthesized material. It is also common to detect some amount of etchant in the structure of the prepared material. For example, in the case of Ti3C2Tx obtained via HF etching, fluorine is often found via physicochemical analyses of the reaction product,23 as was shown on EDX maps. Another evidence for the presence of TMA+ and TBF− adsorbed on the MXenes surface will be discussed later in the section focused on XPS analysis of MX-300.
The difference in the crystal structure of MX-300 and MX-HF samples was further analysed by XRD (Fig. 2b). The intense diffraction peaks in the XRD pattern of the Ti3AlC2 MAX phase indicate a highly crystalline material. In agreement with the JCPDS card no. 52-0875, the characteristics peaks at 9.77°, 19.34°, 34.24°, 36.98°, 39.01°, 41.77°, 48.60°, 52.41°, 56.54° and 60.32° correspond to the (002), (004), (101), (103), (008), (104), (105), (107), (108), (109) and (110) diffraction planes of Ti3AlC2.24,25 However, after the HF-etching (i.e., MX-HF sample) or the electrochemical process (i.e., MX-100, MX-200 and MX-300 sample) the characteristic diffraction signal at 39.01° almost disappeared from the X-ray diffraction patterns. This indicates that most of the aluminum layers were selectively extracted from the MAX phase used as a substrate.24,25 One can notice that, compared to the MX-300 sample, the diffraction peaks corresponding to the (002), (004), and (101) planes for the MX-HF, MX-100, and MX-200 samples have become broader and shifted towards lower angles. These results demonstrate a widening of the lattice spacing in the MX-HF, MX-100, and MX-200 crystal structures compared to the MX-300 sample.24,25 In addition, compared to samples MX-100, MX-200, and MX-HF, the TEM images of MX-300 (Fig. S6) show no visible open fringes, indicating that the electrochemically fragmented sample, i.e., MX-300, is not delaminated. This can presumably explain the sharper (002) diffraction peak of MX-300 compared to those of MX-100, MX-200, and MX-HF, similarly to the Ti3AlC2 MAX phase, intense diffraction peaks are visible in the XRD pattern of the MX sample at diffraction angles of 11° and 25°. These intense signals arise from the crystalline structure of TMA+/TBF− that could be adsorbed (or intercalated) on the MX-300 surface.
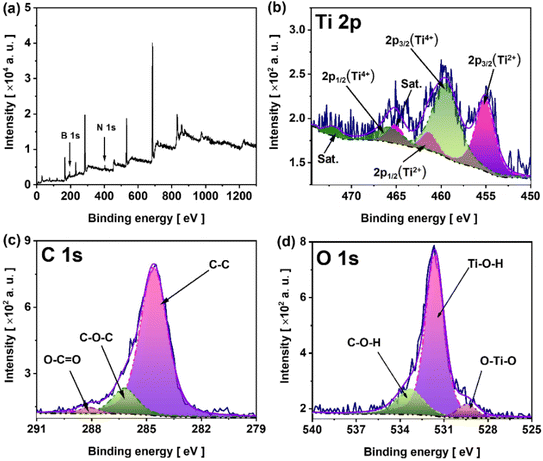
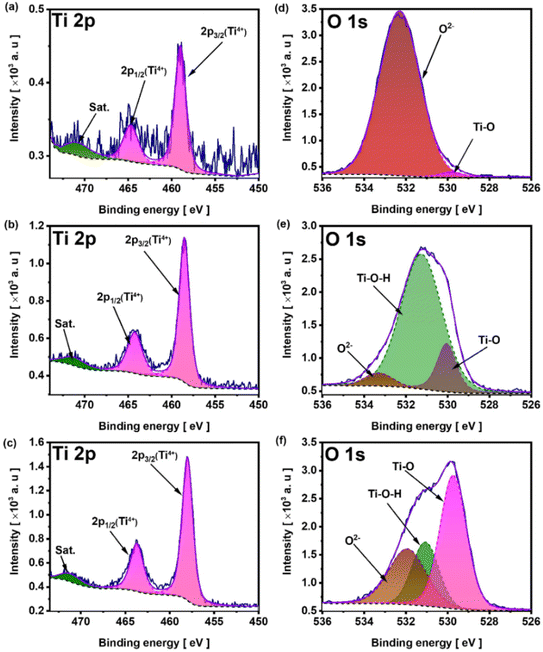
The XPS spectrum of sample MX-300, shown in Fig. 3, was recorded to describe the chemical nature of elements after electrochemically assisted etching. The survey spectrum (Fig. 3a) exhibits characteristic peaks at 187.2 eV and 401 eV, corresponding to the B 1s and N 1s core levels, respectively. The presence of these two elements indicates the adsorption of TMA+ and TBF− on the MX surface.26,27 The Ti 2p spectrum presents two sets of spin–orbit doublets corresponding to 2p3/2 and 2p1/2 components, with a splitting energy of 5.7 eV for each doublet. The 2p3/2 component at 455.3 eV is assigned to titanium bond to one carbon atom and three oxygen atoms (i.e., in the Ti2+ oxidation state),28,29 while the other 2p3/2 component at 459 eV is attributed to titanium in the Ti4+ chemical state (Fig. 3b).28,30 The other two peaks at 454.6 eV and 472.1 eV are attributed the satellite peaks of the 2p3/2 orbitals. The C 1s spectrum (Fig. 3c) has been fitted by three singlets with the maxima at 284.4 eV, 286.2 eV and 288.1 eV corresponding to C–C bonds,31 C–O–C bonds,32 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds,33 respectively. The characteristic peak of MXenes corresponding to C–Ti bonds, typically found between 283 eV and 281 eV, was not detected on the C 1s spectrum. Similar results were also obtained for Ti3C2Tx quantum dots produced by Zhao et al.11via electrochemical etching of Ti3AlC2 in an electrolyte containing the quaternary ammonium cation TMA+. Yun et al.9 also observed the absence of C–Ti bonding in the deconvoluted C 1s XPS spectrum during the electrochemical production of Ti3C2Tx MXenes, following anodic polarization of the Ti3AlC2 MAX phase at 5 V in a [BMIM][PF6]/MeCN electrolyte. The breaking of these covalent bonds can presumably occur during the electrochemical fragmentation of the Ti3C2Tx flakes into small, grain-sized fragments.11
O bonds,33 respectively. The characteristic peak of MXenes corresponding to C–Ti bonds, typically found between 283 eV and 281 eV, was not detected on the C 1s spectrum. Similar results were also obtained for Ti3C2Tx quantum dots produced by Zhao et al.11via electrochemical etching of Ti3AlC2 in an electrolyte containing the quaternary ammonium cation TMA+. Yun et al.9 also observed the absence of C–Ti bonding in the deconvoluted C 1s XPS spectrum during the electrochemical production of Ti3C2Tx MXenes, following anodic polarization of the Ti3AlC2 MAX phase at 5 V in a [BMIM][PF6]/MeCN electrolyte. The breaking of these covalent bonds can presumably occur during the electrochemical fragmentation of the Ti3C2Tx flakes into small, grain-sized fragments.11
The high-resolution O 1s spectrum (Fig. 3d) consists of three peaks with maxima at 530.4 eV, 531.3 eV, and 533.3 eV, which belong to O–Ti, Ti–OH, and adsorbed water, respectively.34 Although the EDX elemental mapping of Al revealed a uniform distribution, the Al 2p XPS spectra of the MAX phase and MX-300 were recorded to identify the corresponding Al bonding in these materials (Fig. S7). The Al 2p spectrum of MX-300 shows only the presence of AlO(OH) and Al(OH)3. The peak associated with Ti–Al bonds in the 70–72 eV binding energy range is below the detection limit. In contrast, for the MAX phase the Ti–Al bond was found. This indicates that the Al detected by EDX arises from the formation of AlO(OH) and Al(OH)3 surface terminations after Ti–Al bond breaking. Although the generated AlO(OH)-terminated surface groups induce Al contamination, the presence of these termination groups can further improve the hydrophilicity of the resulting materials, thereby enhancing their electrochemical performance.35
In summary, the primary outcome is the production of small-sized MXenes using a single-step electrochemical process, which is relatively short compared to existing methods.36 However, before discussing the comparative electrochemical performance of the as-synthesised MX-300 and MX-HF materials, we would like to focus on the mechanism responsible for the etching and formation of small-size MXenes during the cyclic voltammetry process in the TMATFB:HBF4 electrolyte. The applied potential to the arrangement of two glassy carbon substrates, acting together as the working electrode, leads to the hydrolysis of HBF4 according to eqn (1).
| HBF4 + H2O → BF4− + H3O+ | (1) |
The presence of water in eqn (4) results from the composition of the reaction mixture under the initial conditions, i.e., tetrafluoroboric acid was used at a concentration of 48 wt% concentrated solution in H2O. Additional TFB− anions (i.e. BF4−) are also produced during the dissolution of TMATFB in DMSO (i.e., TMATBF → TMA+ + TBF−). Then, the selective Al extraction out of Ti3AlC2 MAX phase occurs according to the fluorine etching the reaction (2):22
| Ti3AlC2 + 3TBF− → Al(TBF)3 + Ti3C2 | (2) |
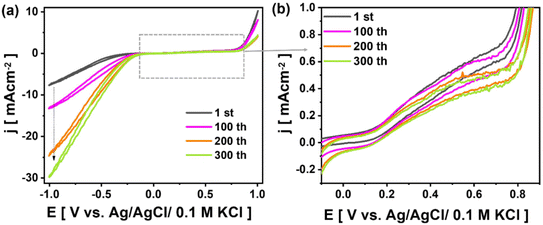
During the cyclic voltammetry measurements, the Ti3AlC2 boundaries are attacked by both TMA+ and TBF− ions present in the electrolyte. These ions then intercalate into the open fringes formed at the Ti3AlC2 edges during etching. As Zhao et al.11 pointed out, fragmentation of the MXene flakes occurs via interactions between the intercalated ions and MXene layers at the open edges under the applied potential. Specifically, the large organic ions (e.g., TMA+ ∼0.322 nm) embedded within the MXene galleries weaken the flake structure, causing fragmentation when the electrochemical etching time is extended from about 5 h 34 min (i.e., 100 CV cycles) to about 16 h 42 min (i.e., 300 CV cycles). Consequently, extending the electrochemical etching time enables the direct synthesis of MXene microspheres via fragmentation of the 2D flakes, without the need for spherical precursor templates such as monodispersed poly(methyl methacrylate) (PMMA) spheres37,38 or polystyrene spheres.39,40 It is worth noting that continuous mechanical agitation of the electrolyte through stirring during the electrochemical process can also support fragmentation process.41,42 Herein, cyclic voltammetry was employed to identify some possible electrochemical reactions associated with the Al-etching process.43 The recorded CV curves throughout the entire etching procedure are presented in Fig. 4a. It can be observed that increasing the number of CV cycles results in an increase in the current density in the cathodic direction. This change in current density, with polarisation toward a more negative direction, corroborates with the decomposition of water during the hydrolysis of HBF4. As shown in Fig. 4b, compared to the curve recorded during the first cycle, the 100th, 200th, and 300th CV cycles exhibit a wide oxidation hump before the rapid increase in current density occurs when the working electrode is polarised above +0.7 V. The occurrence of such a hump with the increase in CV cycles can be associated with the deposition of the extracted Al on the working electrode in the form of Al(TBF)3.12 Indeed, unlike the capacitor-like CV shape recorded by Chan et al.,10 during the electrochemical treatment of Ti3AlC2 in HBF4 electrolyte, the curves presented in Fig. 4 are similar in shape to the CVs recorded by Rodriguez et al.,43 during electrodeposition on a glassy carbon electrode of Al suspended in a deep eutectic solvent-based electrolyte.
2.2. Physicochemical characterization of heterojunction catalyst
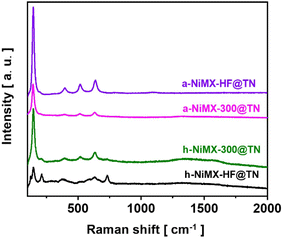
To demonstrate the potential applications of electrochemically synthesized MXene, MX-300 was employed in the fabrication of an electrode capable of catalysing both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) during electrochemical water splitting. For this purpose, h-NiMX-300@TN and a-NiMX-300@TN materials were fabricated according to the procedure described in Section 1.5. The SEM images of the anodic nanotubes and the NiMX-300 deposits on the tubular layer, after annealing in air and hydrogen atmospheres, labelled as a-NiMX-300@TN and h-NiMX-300@TN respectively, are presented in Fig. S8 and S9, while the morphological details of the titania nanotubes are provided in the Experimental section. The change in the crystal structure of the as-synthesized catalyst, before and after thermal annealing of the samples in air and in a hydrogen atmosphere, was analyzed using Raman measurements. Raman spectra of the prepared materials are presented in Fig. S10 and 5. The Raman spectrum of the as-anodized titania (Fig. S10) exhibits two broad bands at 144 cm−1 and 580 cm−1. The observed peaks at 580 cm−1 correspond to Ti–O vibration in amorphous titania, whereas the wide band at 144 cm−1 corresponds to the Eg(1) active mode of anatase TiO2. As shown in Fig. S10 and 5, after annealing in air or in a hydrogen atmosphere the peak at 144 cm−1 becomes very sharp and more intense. In addition, the broad bands at 580 cm−1, observed in the spectra before the thermal annealing, disappeared, and three additional signals at 399, 516 and 639 cm−1 are detected on the spectra of TN, MX-300@TN and NiMX-300@TN samples. Listed peaks are ascribed to B1g, A1g + B1g and Eg(3) modes, of the anatase phase of titanium dioxide, respectively.These findings indicate that, even with NiMX-300 or NiMX-HF present on the tubular layer, the amorphous titania phase converts to anatase due to the treatment at 450 °C. As shown in Fig. S10 and 5, the peak corresponding to MXenes at 720 cm−1 appear in the Raman spectra of all samples annealed under hydrogen atmosphere.
The changes in the surface chemistry of the NiMX-300 sample before and after annealing in various atmospheres were also analysed by XPS. The spectra obtained for the Ti 2p cores are shown in Fig. 6a–c. Compared to NiMX-300@TN, the only noticeable difference in the h-NiMX-300 @TN and a-NiMX-300@TN samples is the increased intensity of the peaks corresponding to 2p3/2 (Ti4+) chemical state. Indeed, the corresponding atomic content of titanium was found to be 0.72%, 3.23%, and 8.22% for NiMX-300@TN, h-NiMX-300@TN, and a-NiMX-300@TN, respectively. This result indicates the formation titanium dioxide through the partial oxidation of the deposited MXene,44 during the annealing process. The presence of the oxygen atoms that established these bonds results from the chemisorption of oxygen and water on the surface of the sample after its chemical modification with nickel in an acetone solvent.45 Indeed, as shown in Fig. 6d, a very intense peak at 532.3 eV, corresponding to the chemisorption of oxygen and water, is observed in the O1s spectrum of the NiMX-300@TN sample before its annealing. Moreover, after the heat treatment under hydrogen atmosphere (Fig. 6e) or in air (Fig. 6f) the peak at 532.3 eV decreased significantly, leading both to an increase in the signal at 530.4 eV, corresponding to the Ti–O bonds, and to the appearance of a strong band at 531.25 eV, associated with the Ti–OH bonds.
 | ||
| Fig. 6 Ti 2p spectra of (a) NiMX-300@TN, (b) h-NiMX-300@TN (c) a-NiMX-300@TN. O 1s spectra of (d) NiMX-300@TN, (e) h-NiMX-300@TN (f) a-NiMX-300@TN. | ||
Overall, the oxygen content has increased from 32.24% in NiMX-300@TN to 37.29% and 58.53% in h-NiMX-300@TN and a-NiMX-300@TN samples, respectively. The change in oxygen surface content is quite small and results from the absence of oxygen in the annealing chamber. Indeed, a vacuum was previously created before filling the chamber with hydrogen gas, whereas the presence of oxygen in air affect its content in Ni-MX modified titania. The C 1s spectrum NiMX-300@TN before (Fig. 7a) and after (Fig. 7b and c) sample annealing exhibit three peaks at 284.5 eV, 285.8 eV and 288.2 eV, corresponding to the presence of graphite-like carbon i.e., C–C bond,46 C–O–Ti and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.47
O bonds, respectively.47
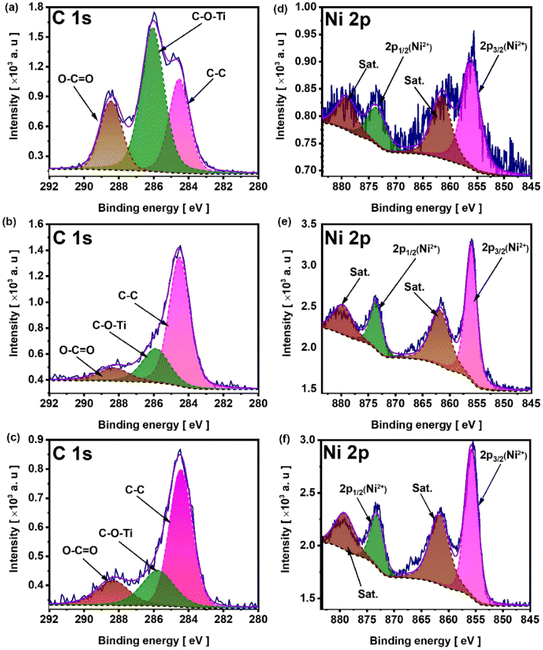 | ||
| Fig. 7 C 1s spectra of (a) NiMX-300@TN, (b) h-NiMX-300@TN (c) a-NiMX-300@TN. Ni 2p spectra of (d) NiMX-300@TN, (e) h-NiMX-300@TN (f) a-NiMX-300@TN. | ||
The reduction in the surface group functionalities anchored to carbon atoms can be observed by the decrease in the intensities of the peaks at 285.8 eV and 288.2 eV after the thermal treatment under hydrogen (Fig. 7b) and in air (Fig. 7c). The Ni 2p spectrum of the NiMX-300@TN (Fig. 7d), h-NiMX-300@TN (Fig. 7e) and a-NiMX-300@TN (Fig. 7f) shows two spin–orbit doublet peaks at 856.1 eV and 873.7 eV, with splitting energies of 17.6 eV. These two peaks correspond to Ni 2p3/2, and are characteristic of Ni(OH)2.47 The two additional bands, located at 861.1 eV and 879.6 eV, are identified as the satellite peaks of the 2p3/2 orbitals.48,49 The increase in peak intensities after the annealing under hydrogen atmosphere (Fig. 7e) and in air (Fig. 7f) indicates the formation of nickel hydroxides, namely Ni(OH)2.
2.3. Electrochemical performance of NiMX-300@TN heterojunction catalyst
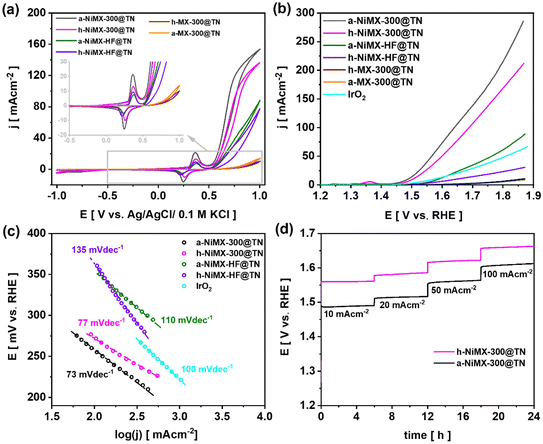
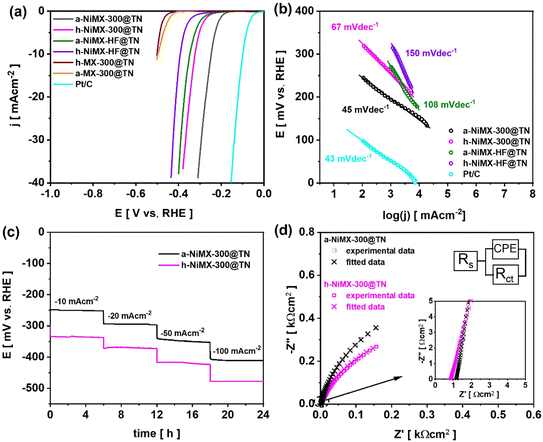
The electrochemical activity of the prepared samples after annealing in air and under hydrogen atmosphere was first characterized by cyclic voltammetry. Voltammograms recorded for MX-300@TN, NiMX-300@TN, and NiMX-HF@TN samples annealed in air or under hydrogen using glassy carbon rod as the counter electrode are presented in Fig. 8a.The reduction and oxidation peaks located at −1.12 and −0.05 V vs. Ag/AgCl/0.1 M KCl on the voltammograms of samples modified with nickel results from the redox reaction of Ni+2/Ni+3 couple. As the consequence, the samples modified with Ni exhibit larger CV loops in the anodic regime with respect to a-MX-300@TN and h-MX-300@TN. In addition, the voltammograms of the annealed NiMX-300@TN and NiMX-HF@TN catalysts exhibit a sharp increase in current density in the polarisation direction toward the positive side, immediately after the oxidation peak. This result reveals that unlike the a-MX-300@TN and h-MX-300@TN samples, all annealed NiMX-300@TN and NiMX-HF@TN electrodes i.e., whether treated in air or under a hydrogen atmosphere are the most promising materials for catalysing the oxygen evolution reaction. The OER catalytic activities of the materials were analysed using LSV (Fig. 8b). The OER did not take place when using MX-300@TN sample as the catalyst due to the fact that the oxygen termination groups present on the surface of the pristine Ti3C2Tx can be easily oxidized in aqueous electrolytes.50,51 This leads to the formation of an oxide layer on the MXenes surface.50,51 The poor conductivity of the formed oxide layers reduces the electrocatalytic performance of the MXene-based material.50 Thus, tuning the surface chemistry or modifying the as-synthesised MXenes with other transition metal catalysts (e.g., Ni, as in this work) is essential to improve their electrochemical activities.50 As shown in Fig. 8b, a-NiMX-300@TN sample exhibits the best OER activity compared to other electrodes. In particular, the overpotential at 10 mA cm−2 for a-NiMX-300@TN is 260 mV, which is much lower than those found for h-NiMX-300@TN (280 mV), a-NiMX-HF@TN (365 mV) and commercial IrO2 (360 mV) catalysts. Previous studies have revealed that Ni–OH bonding is typically the main contributor to the enhanced catalytic activity in water splitting processes.52,53 XPS analysis indicates a significant increase in Ni(OH)2 formation after thermal treatment. Specifically, Ni–OH bonding increased from 1% for NiMX-300@TN to 5.7% for h-NiMX-300@TN and 8.4% for a-NiMX-300@TN. The pronounced increase in Ni–OH bonding for a-NiMX-300@TN can support its improved electrocatalytic performance. To compare the electrochemical activities of a-NiMX-300@TN with those of NiMX-200@TN and NiMX-100@TN thermally treated under the same conditions (i.e., in air), the CV and LSV or a-NiMX-200@TN and a-NiMX-100@TN are also recorded and presented in Fig. S11 and S12. The results revealed that a-NiMX-300@TN exhibit better OER performance compared to NiMX-200@TN and NiMX-100@TN (Fig. S12). As shown in Table S2, the a-NiMX-300@TN sample exhibits a good OER performance and outperforms most of the recently reported value for Ti3C2Tx MXenes modified with Ni-compounds in alkaline electrolytes. It should be noted that many studies focus on Ni-compounds such as NiFe, Ni3S2 and NiFeS rather than on Ni particles as catalysts for modifying MXenes to enhance their performance.54 This strategy enables the acceleration of the electrochemical reaction rate of the catalyst, thereby improving the overall performance of the Ni-MXenes material.54 The kinetics characteristic towards OER of the electrodes were evaluated by Tafel analysis (Fig. 8c). Table S2 also shows that the Tafel slopes of the a-NiMX-300@TN sample is comparable to those recently reported for Ti3C2Tx MXenes modified with Ni compounds. Fig. 8d shows the long-term OER stability performance of the a-NiMX-300@TN and h-NiMX-300@TN samples. For both electrode materials, no potential drop has been observed after 24 hours, demonstrating the excellent stability of the prepared catalysts. The HER performances were investigated as well, through the LSV measurement presented in Fig. 9a.
The a-NiMX-300@TN sample exhibits the best performance among fabricated materials towards HER reaction with an overpotential of 247 mV at 10 mA cm−2. The Tafel slope of the a-NiMX-300@TN catalyst equals only 45 mV dec−1 (Fig. 9b), which is comparable to that of Pt/C electrode (43 mV dec−1). This indicates that the rate-determining step of the HER process on both electrodes proceeds via the Heyrovsky reaction.:55
| H* + H2O + e− → H2 + OH− | (3) |
The comparison of HER activity of a-NiMX-300@TN, a-NiMX-200@TN and a-NiMX-100@TN samples is provided in the SI file Fig. S12. Table S3, shows the comparison of the catalytic performance of the prepared a-NiMX-300@TN sample towards HER with recently reported performances of Ti3C2Tx modified by Ni-compounds. As shown in Fig. 9c, the NiMX-300@TN catalyst exhibits good stability during 24 h test.
For comparison, the CV and LSV curves for the same set of electrodes shown in Fig. 8, recorded using a Pt mesh as the counter electrode instead of a glassy carbon rod, are presented in Fig. S13–S15. This comparison aims to analyse potential changes in the catalytic activity of the as-prepared NiMX-based electrodes when a highly conductive noble metal material is used as the counter electrode, compared to a glassy carbon rod. Moreover, as pointed out by Gregory,56 the use of Pt as the counter electrode can lead to the electrodeposition of Pt species such as Pt2+ and Pt4+ on the working electrode. This electrodeposited Pt layer can abruptly increase the overall electrocatalytic performance of the working electrode. The possible electrodeposition of Pt layer on the working electrode was first analysed by cyclic voltammetry. Five CV cycles were recorded, and for each electrode the fifth cycle is presented in Fig. S13. Typically, as shown in Fig. S13, and also discussed in the work of Nacys et al.,57 the presence of a Pt layer deposited on the working electrode is characterised by a cathodic peak at −0.4 V vs. Ag/AgCl in 1 M NaOH electrolyte. The absence of this peak in the voltammograms of a-NiMX-300@TN, h-NiMX-300@TN, a-NiMX-HF@TN and h-NiMX-HF@TN shown in the inset of Fig. S13, indicates that no electrodeposition of Pt species has occurred on the working electrodes after five CV cycles. Afterwards, LSV curves were recorded to assess the OER and HER activities of the as-prepared samples (Fig. S13). In general, for both cases (i.e., for OER or for HER) as show in Table S4 the overpotentials at a current density of 10 mA cm−2 obtained for a-NiMX-300@TN, h-NiMX-300@TN, a-NiMX-HF@TN and h-NiMX-HF@TN electrodes using a Pt mesh as the counter electrode are lower compared to those obtained with the glassy carbon counter electrode. In general, when electrodeposition occurs, the Pt layer continuously grows on the electrode surface as the electrochemical test progresses. Consequently, the electrochemical activity arising from the presence of the Pt layer on the working electrode becomes more pronounced. In this regard, a stability test lasting approximately 6 hours, using Pt mesh as the counter electrode was also performed to identify any changes in the electrocatalytic performance of the material over time, that can be potentially caused by continuous electrodeposition of a Pt layer on the working electrode due to degradation of the Pt mesh during the electrochemical test. The overpotential was recorded over 6 hours at a current density of 10 mA cm−2, and the results are shown in Fig. S15. As shown in Fig. S15, there is no abrupt changes in the overpotential of the electrodes over time for both HER and OER. The electrochemical activity typical for Pt in not present at the voltammogram shown in Fig. S13, where only the redox reaction of Ni was observed. Moreover, the good stability of the electrodes over time can support the conclusion that no Pt layer is electrodeposited on the working electrode during the electrochemical test. In consequence, the changes in overpotentials observed when using Pt mesh as the counter electrode, compared to those obtained with a glassy carbon rod as the counter electrode, as presented in Table S4, can be attributed to the superior electron transfer kinetics of the Pt material and are not primarily due to electrodeposited Pt species on the working electrode. It should also be noted that NiMX-300@TN exhibits better performance towards HER and OER compared to NiMX-HF@TN, where MXene was obtained via a traditional HF-assisted method. Electrochemical impedance spectroscopy measurements were carried out for a comparative study of the electrical properties of these two electrodes. For those measurements, Pt mesh was used as the counter electrode. The Nyquist EIS spectra were fitted using the equivalent electrical circuit (EEC) shown in Fig. 9d (inset) and S16. The equivalent circuit model contains a series resistance (Rs) attributed typically to the electrolyte resistance, a charge transfer resistance at the electrode/electrolyte interface (Rct), and a constant phase element (CPE). The goodness of the fitting (χ2) is about 10−3, and the values of all parameters are summarized in Table S5. Regardless of the annealing atmosphere, i.e. in air or under hydrogen, as shown in Table S5, the NiMX-300@TN sample exhibits significantly lower series resistance and charge transfer resistance after annealing compared to NiMX-HF@TN. In particular, the a-NiMX-300@TN sample has a higher oxygen surface termination content compared to h-NiMX-300@TN, as detected by the XPS measurements discussed in Section 2.2. This can explain the increase in the Rct value for a-NiMX-300@TN (0.9 kΩ cm2) relative to h-NiMX-300@TN (0.8 kΩcm2). The main reason of the superior catalytic performance of the a-NiMX-300@TN electrode can be justified by difference inthe double-layer capacitance (Cdl). Indeed, the double-layer capacitance is positively and linearly related to the active surface area of the catalyst that is effectively available for electrochemical reactions, such as the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER).13 Bakovic et al.58 also emphasized that the enhanced electrocatalytic activity of a material is primarily due to an increase in its electrochemically active surface area. Taking into account values of the electrical circuit parameters obtained from the supported with the EEC analysis of electrochemical impedance spectra given Fig. 9d and S16, the double-layer capacitance (Cdl) can be calculated with eqn (4) taking into account the geometric surface area of the electrode:59
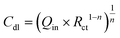 | (4) |
As summarized in Table S5, the superior electrocatalytic activity of a-NiMX-300@TN electrode compared to h-NiMX-300@TN can be primarily attributed to its relatively larger Cdl.
3. Conclusions
In summary, MXene microspheres with an average diameter of 2.1 ± 0.1 µm were synthesised using cyclic voltammetry. The transformation of the 325-mesh two-dimensional Ti3AlC2 MAX phase into three-dimensional microspheres was achieved after 300 CV cycles at a scan rate of 20 mV s−1, demonstrating an effective strategy for morphological tuning. XPS analysis revealed that the electrochemical process enabled particle size tuning and nitrogen incorporation into the MXene structure. Electrocatalysts based on TiO2 nanotube heterostructures and nickel-modified MXenes were fabricated to evaluate the catalytic performance for water splitting. XPS analysis revealed that a-NiMX-300@TN exhibit the highest Ni(OH)2 formation of about 8.4%, compared to its counterparts h-NiMX-300@TN and NiMX-300@TN, which show 5.7% and 1%, respectively. Although the sample annealed under hydrogen shows relatively better electrical properties in terms of lower charge transfer resistance, its calculated double-layer capacitance was found to be smaller than that of the catalyst annealed in air. As a result, the a-NiMX-300@TN electrode demonstrated enhanced catalytic activity. A facile and scalable electrochemical synthesis route for MXenes has been introduced, enabling precise control over structural and compositional features. These findings pave the way for the use of NiMX-300 as a promising catalyst in energy conversion systems, particularly for efficient and durable water-splitting applications.Author contributions
Dujearic-Stephane Kouao: conceptualization, data curation, writing – original draft, writing – review & editing, methodology, visualization, investigation. Agnieszka Kramek: investigation, writing – review & editing, methodology. Justyna Gumieniak: investigation, writing – review & editing, methodology. Karol Załęski: investigation, methodology. Emerson Coy: investigation, visualization, methodology. Jakub Karczewski – investigation, writing – review & editing. Guowei Li – methodology, writing – review & editing, supervision. Katarzyna Siuzdak: conceptualization, data curation, formal analysis, project administration, writing – original draft, writing – review & editing, methodology, visualization, supervision, funding acquisition.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data are available on request from the corresponding authors.Supplementary information (SI): electrochemical etching conditions of Ti3AlC2 MAX phase; modification method of MXene with Ni; fabrication procedure of the hierarchical NiMX-300@TN heterojunction catalyst; electrochemical testing conditions of the as-prepared catalysts; SEM images of Ti3AlC2 and MXenes synthesized by the electrochemical method; TEM image and elemental distribution mapping (O, Al, F) of MXene produced after 300 cyclic voltammetry cycles (MX-300); Raman spectra of tetramethylammonium tetrafluoroborate and MX-300; high-resolution TEM images of MX-300; high-resolution XPS spectra of Al 2p for Ti3AlC2 MAX phase and MX-300; SEM images of Ni-modified MXene deposited on titania nanotubes; Raman spectra of Ni-modified MXene on titania nanotubes, after annealing in air and hydrogen atmospheres; linear sweep voltammetry curves recorded for a-NiMX-100@TN, a-NiMX-200@TN, and a-NiMX-300@TN, tested for OER and HER in 1 M NaOH; OER and HER performance of electrocatalysts based on Ni-modified MXenes; linear sweep voltammetry curves recorded for a-NiMX-300@TN, h-NiMX-300@TN, a-NiMX-HF@TN, h-NiMX-HF@TN, a-MX-300@TN, and h-MX-300@TN catalysts, tested in 1 M NaOH using Pt mesh as the counter electrode; chronopotentiometric curves for h-NiMX-300@TN and a-NiMX-300@TN recorded over 6 hours under galvanostatic conditions, using Pt mesh as the counter electrode. See DOI: https://doi.org/10.1039/d5ta07124k.
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (NSFC 52471214) and CAS-ANSO Fellowship Program.References
- K. Verguts, J. Coroa, C. Huyghebaert, S. De Gendt and S. Brems, Nanoscale, 2018, 10, 5515–5521 RSC.
- S. Yang, A. G. Ricciardulli, S. Liu, R. Dong, M. R. Lohe, A. Becker, M. A. Squillaci, P. Samorì, K. Müllen and X. Feng, Angew. Chem., Int. Ed., 2017, 56, 6669–6675 CrossRef CAS PubMed.
- C. Shu, Ph. D. J. Zhou, Ph. D. Z. Jia, H. Zhang, Z. Liu, W. Tang and X. Sun, Chem.–Eur. J., 2022, 28, e202200857 CrossRef CAS PubMed.
- A. Ambrosi and M. Pumera, Chem.–Eur. J., 2018, 24, 18551–18555 CrossRef CAS PubMed.
- J. Chen, M. Chen, W. Zhou, X. Xu, B. Liu, W. Zhang and C. Wong, ACS Nano, 2022, 16, 2461–2470 CrossRef CAS PubMed.
- K. Nasrin, V. Sudharshan, K. Subramani and M. Sathish, Adv. Funct. Mater., 2022, 32, 2110267 CrossRef CAS.
- S. Yang, P. Zhang, F. Wang, A. G. Ricciardulli, M. R. Lohe, P. W. M. Blom and X. Feng, Angew. Chem., 2018, 130, 15717–15721 CrossRef.
- Z. Huang, J. Qin, Y. Zhu, K. He, H. Chen, H. Y. Hoh, M. Batmunkh, T. M. Benedetti, Q. Zhang, C. Su, S. Zhang and Y. L. Zhong, Carbon Energy, 2023, 5, e295 CrossRef CAS.
- T. Yin, Y. Li, R. Wang, O. A. Al-Hartomy, A. Al-Ghamdi, S. Wageh, X. Luo, X. Tang and H. Zhang, Ceram. Int., 2021, 47, 28642–28649 CrossRef CAS.
- K. C. Chan, X. Guan, T. Zhang, K. Lin, Y. Huang, L. Lei, Y. Georgantas, Y. Gogotsi, M. A. Bissett and I. A. Kinloch, J. Mater. Chem. A, 2024, 12, 25165–25175 RSC.
- L. Zhao, Z. Wang, Y. Li, S. Wang, L. Wang, Z. Qi, Q. Ge, X. Liu and J. Z. Zhang, J. Mater. Sci. Technol., 2021, 78, 30–37 CrossRef CAS.
- M. R. Lukatskaya, J. Halim, B. Dyatkin, M. Naguib, Y. S. Buranova, M. W. Barsoum and Y. Gogotsi, Angew. Chem., 2014, 126, 4977–4980 CrossRef.
- K. Mishra, J. Rawat, H. Sharma and C. Dwivedi, Int. J. Hydrogen Energy, 2024, 95, 710–720 CrossRef CAS.
- M. N. Krstajić Pajić, A. S. Dobrota, A. Mazare, S. Đurđić, I. Hwang, N. V. Skorodumova, D. Manojlović, R. Vasilić, I. A. Pašti, P. Schmuki and U. Lačnjevac, ACS Appl. Mater. Interfaces, 2023, 15, 31459–31469 CrossRef PubMed.
- J. Yang, L. Cheng, L. Wan, J. Yan, R. Chen and H. Ni, Electrochem. Commun., 2018, 97, 68–72 CrossRef CAS.
- M. Shen, W. Jiang, K. Liang, S. Zhao, R. Tang, L. Zhang and J. Wang, Angew. Chem., 2021, 133, 27219–27224 CrossRef.
- W. Sun, S. A. Shah, Y. Chen, Z. Tan, H. Gao, T. Habib, M. Radovic and M. J. Green, J. Mater. Chem. A, 2017, 5, 21663–21668 RSC.
- M. Ostermann, M. Piljević, E. Akbari, P. Patil, V. Zahorodna, I. Baginskiy, O. Gogotsi, C. Gachot, R. M. Rodríguez, M. Valtiner and P. Bilotto, Small, 2025, 21, 2500807 CrossRef CAS PubMed.
- Y. Wu, A. Lin, J. Zhang, D. Zhao, X. Bai, C. Lu, S. Wang, L. Fan, L. Cao, S. Liu and F. Gu, Adv. Eng. Mater., 2022, 24, 2101556 CrossRef CAS.
- M. Kulkarni, A. Lalic, R. Balu, H. Zhang, N. K. Dutta and N. Roy Choudhury, MRS Adv., 2024, 9, 1310–1317 CrossRef CAS.
- Y. Gogotsi, MXenes: from Discovery to Applications of Two-Dimensional Metal Carbides and Nitrides, Jenny Stanford Publishing, Singapore, 2023 Search PubMed.
- J. Hao Ran Huang, S.-W. Tseng and I.-W. Peter Chen, Chem. Eng. J., 2025, 503, 158232 CrossRef CAS.
- M. He, H. Chen, H. Peng, Y. Zhou, Z. Song, Y. Wang, S. Feng and X. Bu, Chem. Eng. J., 2023, 456, 140985 CrossRef CAS.
- Y. Wen, T. E. Rufford, X. Chen, N. Li, M. Lyu, L. Dai and L. Wang, Nano Energy, 2017, 38, 368–376 CrossRef CAS.
- S. Gasso, M. K. Sohal and A. Mahajan, Sens. Actuators, B, 2022, 357, 131427 CrossRef CAS.
- Z. Li, H. Yu, Y. Zhang, D. Wu, Y. Bai, S. Liu and H. Zhao, Chem. Commun., 2023, 59, 4535–4538 RSC.
- C. W. Ong, H. Huang, B. Zheng, R. W. M. Kwok, Y. Y. Hui and W. M. Lau, J. Appl. Phys., 2004, 95, 3527–3534 CrossRef CAS.
- Y. Peng, P. Cai, L. Yang, Y. Liu, L. Zhu, Q. Zhang, J. Liu, Z. Huang and Y. Yang, ACS Omega, 2020, 5, 26486–26496 CrossRef CAS PubMed.
- J. Vida, P. Gemeiner, M. Pavličková, M. Mazalová, P. Souček, D. Plašienka and T. Homola, Nanoscale, 2023, 15, 1289–1298 RSC.
- S. J. Kim, H.-J. Koh, C. E. Ren, O. Kwon, K. Maleski, S.-Y. Cho, B. Anasori, C.-K. Kim, Y.-K. Choi, J. Kim, Y. Gogotsi and H.-T. Jung, ACS Nano, 2018, 12, 986–993 CrossRef CAS PubMed.
- S. Wang, Z. Ma, Q. Lü and H. Yang, ChemElectroChem, 2019, 6, 2748–2754 CrossRef CAS.
- Z. Lv, W. Ma, M. Wang, J. Dang, K. Jian, D. Liu and D. Huang, Adv. Funct. Mater., 2021, 31, 2102576 CrossRef CAS.
- A. Li, X. Wang, J. Chen, C. Dong, D. Wang and Z. Mao, Coatings, 2022, 12, 1005 CrossRef CAS.
- L. Zhang, S. Wang and C. Lu, Anal. Chem., 2015, 87, 7313–7320 CrossRef CAS PubMed.
- A. Qian, H. Wu, G. Wang, N. Sun, H. Cheng, K. Zhang and F. Cheng, ACS Appl. Mater. Interfaces, 2023, 15, 9203–9211 CrossRef CAS PubMed.
- G. Li, K. Jiang, S. Zaman, J. Xuan, Z. Wang and F. Geng, Inorg. Chem., 2019, 58, 9397–9403 CrossRef CAS PubMed.
- W. Huang, Z. Ma, L. Zhong, K. Luo, W. Li, S. Zhong and D. Yan, Small, 2024, 20, 2304690 CrossRef CAS PubMed.
- M. Zhao, X. Xie, C. E. Ren, T. Makaryan, B. Anasori, G. Wang and Y. Gogotsi, Adv. Mater., 2017, 29, 1702410 CrossRef PubMed.
- M. Zhu, Y. Yue, Y. Cheng, Y. Zhang, J. Su, F. Long, X. Jiang, Y. Ma and Y. Gao, Adv. Electron. Mater., 2020, 6, 1901064 CrossRef CAS.
- G. Zhang, L. Wang, R. Sa, C. Xu, Z. Li and L. Wang, Environ. Sci.: Nano, 2022, 9, 204–213 RSC.
- M. Malaki, A. Maleki and R. S. Varma, J. Mater. Chem. A, 2019, 7, 10843–10857 RSC.
- A. Thakur, N. Chandran B.S., K. Davidson, A. Bedford, H. Fang, Y. Im, V. Kanduri, B. C. Wyatt, S. K. Nemani, V. Poliukhova, R. Kumar, Z. Fakhraai and B. Anasori, Small Methods, 2023, 7, 2300030 CrossRef CAS PubMed.
- E. Rodríguez-Clemente, T. L. Manh, C. E. Guinto-Pano, M. Romero-Romo, I. Mejía-Caballero, P. Morales-Gil, E. Palacios-González, M. T. Ramírez-Silva and M. Palomar-Pardavé, J. Electrochem. Soc., 2019, 166, D3035–D3041 CrossRef.
- D. Kuang, L. Wang, X. Guo, Y. She, B. Du, C. Liang, W. Qu, X. Sun, Z. Wu, W. Hu and Y. He, J. Hazard. Mater., 2021, 416, 126171 CrossRef CAS PubMed.
- D. Wang, N. He, L. Xiao, F. Dong, W. Chen, Y. Zhou, C. Chen and S. Wang, Angew. Chem., Int. Ed., 2021, 60, 24605–24611 CrossRef CAS PubMed.
- L.-Å. Näslund and I. Persson, Appl. Surf. Sci., 2022, 593, 153442 CrossRef.
- H. Yan, J. Bai, J. Wang, X. Zhang, B. Wang, Q. Liu and L. Liu, CrystEngComm, 2013, 15, 10007 RSC.
- X. Zhang, S. Yang, W. Lu, D. Lei, Y. Tian, M. Guo, P. Mi, N. Qu and Y. Zhao, J. Colloid Interface Sci., 2021, 592, 95–102 CrossRef CAS PubMed.
- H. Wang, L. Meng, S. Liu, H. Liu, F. Wang and H. Wu, Chem. Eng. J., 2023, 476, 146929 CrossRef CAS.
- Z. Wang, K. Yu, Y. Feng, R. Qi, J. Ren and Z. Zhu, Appl. Surf. Sci., 2019, 496, 143729 CrossRef CAS.
- J. Wang, Y. Guan, Q. Zhang, H. Zhu, X. Li, Y. Li, Z. Dong, G. Yuan and Y. Cong, Appl. Surf. Sci., 2022, 582, 152481 CrossRef CAS.
- B. Jansi Rani, N. Dhivya, G. Ravi, S. S. Zance, R. Yuvakkumar and S. I. Hong, ACS Omega, 2019, 4, 10302–10310 CrossRef CAS PubMed.
- L. Tie, N. Li, C. Yu, Y. Liu, S. Yang, H. Chen, S. Dong, J. Sun, S. Dou and J. Sun, ACS Appl. Energy Mater., 2019, 2, 6931–6938 CrossRef CAS.
- J. Huang, M. Feng, Y. Peng, C. Huang, X. Yue and S. Huang, Small, 2023, 19, 2206098 CrossRef CAS PubMed.
- K. Gothandapani, G. Tamil Selvi, R. Sofia Jennifer, V. Velmurugan, S. Pandiaraj, M. Muthuramamoorthy, S. Pitchaimuthu, V. Raghavan, A. C. Josephine Malathi, A. Alodhayb and A. Nirmala Grace, Int. J. Hydrogen Energy, 2024, 52, 1164–1171 CrossRef CAS.
- G. Jerkiewicz, ACS Catal., 2022, 12, 2661–2670 CrossRef CAS.
- A. Nacys, D. Šimkūnaitė, A. Balčiūnaitė, A. Zabielaitė, D. Upskuvienė, B. Šebeka, V. Jasulaitienė, V. Kovalevskij, E. Norkus and L. Tamašauskaitė-Tamašiūnaitė, Crystals, 2022, 12, 362 CrossRef CAS.
- S. I. Perez Bakovic, P. Acharya, M. Watkins, H. Thornton, S. Hou and L. F. Greenlee, J. Catal., 2021, 394, 104–112 CrossRef CAS.
- V. D. Jović, Zas Mat, 2022, 63, 50–57 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |