Activated carbon microtube electrodes with cement and fly ash for enhanced supercapacitor performance
S.
Nagarani
 a,
Jih-Hsing
Chang
a,
Jih-Hsing
Chang
 *a,
Mohanraj
Kumar
a,
Krishnan Vancheeswaran
Prasad
ab and
Raman
Arunpandian
ab
*a,
Mohanraj
Kumar
a,
Krishnan Vancheeswaran
Prasad
ab and
Raman
Arunpandian
ab
aDepartment of Environmental Engineering and Management, Chaoyang University of Technology, Taichung City, 413310, Taiwan. E-mail: changjh@cyut.edu.tw
bDepartment of Applied Chemistry, Chaoyang University of Technology, Taichung 413310, Taiwan
First published on 17th November 2025
Abstract
We developed chemically activated carbon microtubes (ACMTs) from waste surgical face masks and combined them with fly ash (FA) and cement (CE) to create symmetric electrodes for high-performance supercapacitors. The facemasks were carbonized at 700 °C and then activated with KOH at 900 °C to produce ACMTs with hierarchical porosity and tubular structure, as confirmed by SEM and HR-TEM analyses. Raman spectroscopy showed a higher defect density in ACMTs (ID/IG = 1.30) compared to pristine carbon microtubes (CMTs; ID/IG = 1.19), while BET analysis indicated an improved surface area of 1310 m2 g−1. The unique combination of FA and CE is vital for electrode performance, FA acts as a conductive additive, aiding charge transport through its metal oxide content, while CE functions as a stable binder, enhancing structural integrity and ion diffusion pathways. The optimized ACMT2/FA1/CE1 electrode delivers a high specific capacitance of 669 F g−1 at 1.0 A g−1 in 3 M KOH. The corresponding solid-state symmetric device (NF/ACMT2/FA1/CE1) retains 94.2% of its capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and achieves an energy density of 87 Wh kg−1 at 750.5 W kg−1. Expanding the potential window with an alkaline electrolyte further boosts the energy density, demonstrating that ACMT-based, waste-derived electrodes provide a sustainable and low-cost pathway towards high-performance supercapacitors.
000 cycles and achieves an energy density of 87 Wh kg−1 at 750.5 W kg−1. Expanding the potential window with an alkaline electrolyte further boosts the energy density, demonstrating that ACMT-based, waste-derived electrodes provide a sustainable and low-cost pathway towards high-performance supercapacitors.
1. Introduction
The global pursuit of environmentally friendly energy storage solutions has become increasingly urgent as the demand for renewable and portable power sources grows. Modern society's reliance on energy storage systems for portable electronics, electric vehicles, and grid stability requires devices that are efficient, durable, sustainable, and economically viable.1–3 Traditional technologies such as batteries and electrolytic capacitors, though effective, face inherent limitations. Batteries provide high energy density but poor power capability and cycling stability, while capacitors deliver excellent power output but limited energy storage.4,5 These constraints have driven intensive research into supercapacitors (SCs), which bridge the gap between conventional capacitors and batteries.6 Supercapacitors combine high power density, fast charge–discharge ability, long cycle life, and environmental friendliness.7,8 Structurally, they consist of three main components: an electrolyte, a separator, and electrodes. The electrodes are the primary sites of charge storage, the electrolyte enables ionic transport, and the separator prevents short-circuiting while maintaining ion flow. The electrodes largely determine performance factors such as specific capacitance, energy density, and stability. Based on their charge storage mechanism, SCs are generally classified into electric double-layer capacitors (EDLCs), which store energy through electrostatic ion adsorption, and pseudocapacitors, which rely on rapid surface redox reactions.9 Despite their advantages, SCs still suffer from relatively low energy density, primarily due to electrode material limitations. Hence, developing advanced, sustainable, and high-surface-area electrode materials remains critical for improving SC performance and practical applicability.10–12Among the various materials explored for high-performance and sustainable SCs electrodes, carbon-based materials, conductive polymers, and transition metal oxides have emerged as the most promising candidates.13 Specifically, carbon-based materials such as graphene, graphene oxide (GO), carbon nanotubes (CNTs), carbon nano-onions, bio mass derived activated carbon (AC), carbon quantum dots, and carbon aerogels are renowned for their affordability, excellent conductivity, high specific surface area, thermal stability, ease of handling, and structural tunability, which make them highly suitable for SCs applications.14,15 Among these, activated carbon (AC) has garnered significant attention due to its abundant raw material sources, low cost, high surface area, tuneable porosity, non-toxic nature, and satisfactory electrical conductivity, all contributing to improved capacitance performance in SCs.16 Over the past two decades, it has become feasible to produce activated carbons (ACs) from various waste-derived precursors, including coal,17 cassava starch,18 PET face shields,19 corncobs,20 wood,21 biomass residues,22 and other solid wastes.23 The production process is straightforward and cost-effective, while the inherent porous structure enhances their electrochemical properties and boosts the sustainable value of these waste materials. Additionally, a range of organic and inorganic functional groups contributes to the thermal stability of ACs, making them particularly appealing for energy storage applications.
Recently, there has been a growing focus on producing AC from used surgical face masks (FMs), which presents a promising strategy for upcycling plastic waste. FM-derived carbons have been studied for a range of applications beyond energy storage, including transistors, triboelectric self-powering devices, smart wearable sensors, CO2 capture, and stretchable SCs.24–28 After the COVID-19 pandemic began in late 2019, the global consumption of disposable surgical masks, mainly made of polypropylene (PP), rose sharply. Since PP is a non-biodegradable plastic, its widespread use has significantly worsened the global plastic waste crisis.29 In 2020, National Geographic estimated that over three billion masks were discarded daily, a trend that continued for years and posed serious environmental and ecological risks.30 Addressing this problem requires innovative recycling and upcycling strategies. One promising approach is to convert discarded FMs into activated carbon, which can serve as a sustainable electrode material for SCs.31 Activated carbon is typically synthesised via pyrolysis and hydrothermal carbonization, followed by chemical activation using agents such as KOH, H3PO4, or ZnCl2.32,33 Transforming FMs into activated carbon microtubes (ACMTs) reduces plastic pollution. It creates a high-surface-area, porous material that can be combined with other functional components to enhance capacitance, expand the potential window, and improve electrical conductivity.34 Meanwhile, industrial by-products as supplementary components in electrode design have gained significant interest. Fly ash (FA), a residue from coal combustion in power plants, mainly consists of inorganic oxides such as Al2O3, SiO2, CaO, and MgO, along with trace amounts of carbonaceous and metallic species.35 Although many of these oxides are not inherently conductive, fly ash (FA) can impart structural stability, porosity, and surface reactivity. When combined with conductive carbon phases, FA can significantly enhance the electrochemical performance of supercapacitor electrodes. Several studies have demonstrated this potential: Pransisco et al.36 incorporated FA into a three-dimensional graphene framework on Ni foam, improving capacitive behavior; Zheng et al.37 employed FA microspheres as templates to develop spongy carbon composites; Wang et al.38 synthesized FA-derived cobalt–iron silicate electrodes with a high specific capacitance of 493.3 F g−1; and Chanut et al.39 reported that carbon-doped cement composites effectively enhanced electrochemical performance. These findings collectively demonstrate that waste-derived oxides, such as FA, not only augment mechanical robustness but also enhance electrochemical activity when effectively integrated with carbon-based structures. Recent research has demonstrated that cement-based composites are capable of displaying detectable electrochemical activity, which makes them attractive options for energy storage applications.39,40 During hydration, calcium hydroxide and calcium silicate hydrate (C–S–H) phases form in cement, creating nanoscale porosity and promoting ionic transport. By forming conductive channels, these hydration-induced microstructures can accommodate electrolytes and facilitate charge storage via both faradaic and double-layer processes.41–43 Expanding upon this idea, the current study uses cement (CE) as an inexpensive and environmentally friendly binder material in place of traditional polymeric binders like polyvinylidene fluoride (PVDF) and Nafion, which, despite being effective adhesives, have limited electrochemical activity, high costs, and require the use of hazardous organic solvents. In this work, we propose a cost-effective and sustainable strategy for fabricating high-performance supercapacitor electrodes by integrating activated carbon microtubes (ACMTs) derived from waste surgical face masks with fly ash (FA) as a functional additive and cement (CE) as a novel binder. Among several compositions, the optimized ACMT2/FA1/CE1 electrode exhibited superior electrochemical performance with hybrid charge-storage behavior, combining electric double-layer capacitance and pseudocapacitive contributions from FA and CE. This synergistic interaction enhances conductivity, ion diffusion, and structural stability, offering a simple and scalable route toward eco-friendly, high-performance, and low-cost supercapacitor technologies.
2. Experimental section
2.1. Materials
Surgical face masks (FMs) were collected from a hospital in Taichung, Taiwan. The masks, originally manufactured by Motex Modern Tech Company (Taiwan), were selected owing to their uniform polypropylene fiber structure. Fly ash (FA), rich in metal oxides, was obtained from a local thermal power plant in Taichung, Taiwan, and employed as a conductive additive. Ordinary Portland cement (OPC; Type I) was purchased from Taiwan Cement Corporation (113 Chung Shan N. Rd, Taipei, Taiwan) and used as the binder material during electrode fabrication. Table S1 (SI) shows the full elemental makeup of OPC. Analytical-grade sulfuric acid (H2SO4), potassium hydroxide (KOH), hydrochloric acid (HCl), polyvinylidene fluoride (PVDF), and ethanol were procured from JT Baker Chemicals and used without further purification. Deionized (DI) water (18.2 MΩ cm) was used in all solution preparations.2.2. Preprocessing of surgical masks and fly ash
To remove residual moisture and volatile contaminants, the collected waste surgical face masks were first immersed in 70% ethanol for 1 hour and then dried in a hot-air oven at 80 °C for 24 hours. To ensure material uniformity, the dried masks were manually separated into outer, middle, and inner layers, and the metal nose strips and elastic ear loops were removed. The polypropylene (PP) layers were subsequently cut into small strips (∼0.5 cm2) to facilitate uniform carbonization. Fly ash (FA) from the nearby thermal power plant was oven-dried for seven hours at 90 °C to eliminate moisture that had been absorbed. After drying, the FA was utilized directly in the electrode fabrication process, requiring no additional purification. Before being used, the substance was stored in airtight containers to prevent moisture absorption.2.3. Preparation of activated carbon microtube from face mask
After preprocessing, the waste surgical face masks (FMs) were used as the primary carbon source. The preprocessed masks were treated with concentrated H2SO4 under solvothermal conditions at 160 °C for 8 h in a 100 mL Teflon-lined stainless-steel autoclave to promote polymer stabilization and partial cross-linking of the fibrous matrix. The resulting material was thoroughly washed with deionized (DI) water, dried at 80 °C, and subsequently carbonized at 700 °C for 2 h under a continuous N2 flow (100 mL min−1) to obtain carbon microtubes (CMT). For chemical activation, 1 g of as-prepared carbon microtubes (CMT) was impregnated with 2 g of potassium hydroxide (KOH), corresponding to a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (CMT
2 (CMT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH). The KOH was dissolved in 100 mL of deionized water, resulting in a concentration of 0.02 g mL−1. The suspension was stirred at room temperature for 24 hours to ensure uniform impregnation. Afterward, the mixture was dried at 90 °C for 12 hours to remove moisture. The dried CMT–KOH mixture was thermally activated in a tubular furnace under continuous nitrogen (N2) flow (∼100 mL min−1). The temperature was ramped from room temperature to 900 °C at 20 °C min−1 and maintained at this temperature for 2 hours. These activation parameters were chosen based on prior studies demonstrating that KOH activation above 800 °C promotes extensive micropore and mesopore formation while preserving the carbon framework. After cooling under nitrogen, the activated product was washed repeatedly with 1 M hydrochloric acid (HCl) to remove residual KOH and inorganic impurities, followed by rinsing with deionized water until the filtrate reached neutral pH (∼7.0). The final product was dried at 100 °C for 24 hours and stored for further use. This material was designated as activated carbon microtubes (ACMT). A schematic representation of the complete preparation and electrode fabrication procedure is given in Scheme 1.
KOH). The KOH was dissolved in 100 mL of deionized water, resulting in a concentration of 0.02 g mL−1. The suspension was stirred at room temperature for 24 hours to ensure uniform impregnation. Afterward, the mixture was dried at 90 °C for 12 hours to remove moisture. The dried CMT–KOH mixture was thermally activated in a tubular furnace under continuous nitrogen (N2) flow (∼100 mL min−1). The temperature was ramped from room temperature to 900 °C at 20 °C min−1 and maintained at this temperature for 2 hours. These activation parameters were chosen based on prior studies demonstrating that KOH activation above 800 °C promotes extensive micropore and mesopore formation while preserving the carbon framework. After cooling under nitrogen, the activated product was washed repeatedly with 1 M hydrochloric acid (HCl) to remove residual KOH and inorganic impurities, followed by rinsing with deionized water until the filtrate reached neutral pH (∼7.0). The final product was dried at 100 °C for 24 hours and stored for further use. This material was designated as activated carbon microtubes (ACMT). A schematic representation of the complete preparation and electrode fabrication procedure is given in Scheme 1.
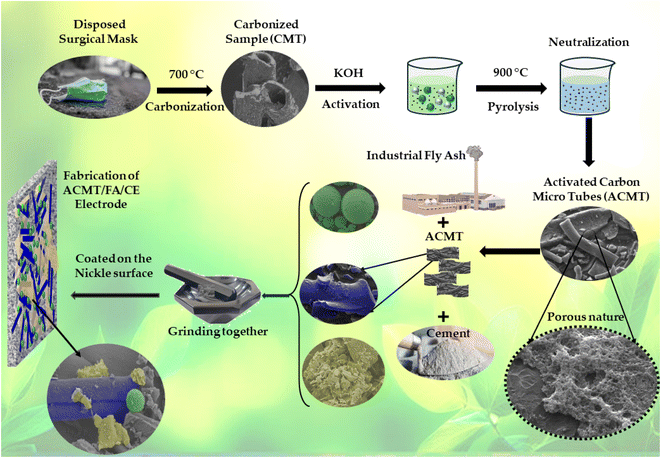 | ||
| Scheme 1 A schematic representation of the synthesis of ACMT and fabrication of ACMT/FA/CE electrode for symmetric supercapacitor applications. | ||
2.4. Materials characterization
A comprehensive set of advanced characterization techniques was employed to analyze the structural, chemical, and morphological properties of the synthesized materials. X-ray diffraction (XRD) was conducted using a Rigaku Rotaflex diffractometer (Japan) equipped with Cu Kα radiation (λ = 1.5418 Å, 40 kV, 40 mA) to determine the crystalline structure, with data collected at a scan rate of 5° min−1 over a 2θ range of 10–80°. Raman microspectroscopy (Nano Finder 3D, Tokyo Instruments) provided insight into the vibrational modes and degree of structural order within the carbon framework. Thermogravimetric analysis (TGA, Mettler-Toledo Pac Rim AG) was performed to evaluate the thermal stability of the samples. Surface functional groups were identified via Fourier transform infrared spectroscopy (FTIR, Nicolet iS5, Thermo Fisher), while N2 adsorption–desorption isotherms were employed to calculate the specific surface area and porosity using the Brunauer–Emmett–Teller (BET) method. The elemental composition and oxidation states were further investigated by X-ray photoelectron spectroscopy (XPS, PHI 5000 Versa Probe, ULVAC-PHI). Finally, morphological characteristics were examined using field-emission scanning electron microscopy (FE-SEM, JEOL JSM-7800F) coupled with energy-dispersive X-ray spectroscopy (EDX), along with high-resolution transmission electron microscopy (HR-TEM, JEOL JEM-1400). The Electrochemical measurements were performed using a CHI6279C electrochemical workstation.2.5. Electrochemical characterization
Nickel foam substrates (1 cm × 1 cm) were first rinsed with deionized (DI) water and cleaned by ultrasonication. Electrode slurries were then prepared by mixing fly ash (FA), carbon microtubes (CMT), activated carbon microtubes (ACMT), and cement (CE) in different weight ratios. The specific formulations included 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ACMT1/FA1/CE1), 2
1 (ACMT1/FA1/CE1), 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ACMT2/FA1/CE1), 3
1 (ACMT2/FA1/CE1), 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ACMT3/FA1/CE1), 1
1 (ACMT3/FA1/CE1), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ACMT1/FA2/CE1), 1
1 (ACMT1/FA2/CE1), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ACMT1/FA3/CE1), (ACMT2/FA1/PVDF1), and a binary mixture of ACMT and CE (ACMT/CE). In addition, a control electrode containing non-activated CMT (CMT2/FA1/CE1) was prepared to compare the performance before and after chemical activation. In each case, CE acted as the binder, and its proportion was varied according to the specified ratios rather than being kept constant. The subscripts denote the relative weight proportions of each component. The active material loading on the Ni foam was maintained at 2 mg cm−2. The electrochemical performance was then evaluated in a 3 M KOH electrolyte using a three-electrode configuration, comprising a platinum wire as the counter electrode and an Hg/HgO electrode as the reference. Cyclic voltammetry (CV) was carried out at different scan rates within a potential window of −1.0 to 0.3 V. Galvanostatic charge–discharge (GCD) tests were carried out at varies current densities, and the specific capacitance (Cs) was calculated from the discharge curves using eqn (1).44 Electrochemical impedance spectroscopy (EIS) measurements were conducted with an amplitude of 5 mV over the frequency range of 0.1 Hz to 105 Hz.
1 (ACMT1/FA3/CE1), (ACMT2/FA1/PVDF1), and a binary mixture of ACMT and CE (ACMT/CE). In addition, a control electrode containing non-activated CMT (CMT2/FA1/CE1) was prepared to compare the performance before and after chemical activation. In each case, CE acted as the binder, and its proportion was varied according to the specified ratios rather than being kept constant. The subscripts denote the relative weight proportions of each component. The active material loading on the Ni foam was maintained at 2 mg cm−2. The electrochemical performance was then evaluated in a 3 M KOH electrolyte using a three-electrode configuration, comprising a platinum wire as the counter electrode and an Hg/HgO electrode as the reference. Cyclic voltammetry (CV) was carried out at different scan rates within a potential window of −1.0 to 0.3 V. Galvanostatic charge–discharge (GCD) tests were carried out at varies current densities, and the specific capacitance (Cs) was calculated from the discharge curves using eqn (1).44 Electrochemical impedance spectroscopy (EIS) measurements were conducted with an amplitude of 5 mV over the frequency range of 0.1 Hz to 105 Hz.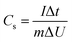 | (1) |
The coulombic efficiency (η) was calculated using eqn (2):
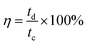 | (2) |
2.6. Solid-state symmetric supercapacitors
The solid-state symmetric supercapacitor (SSSC) was fabricated using two identical NF/ACMT2/FA1/CE1 electrodes prepared with the same binder composition. For the electrolyte, a PVA/KOH gel was used (see SI, Section S1 for details of the preparation process). A butter sheet, pre-soaked in the PVA/KOH gel electrolyte for 30 minutes, was employed as the separator. The loading mass of the active material on nickel foam electrodes in the two-electrode solid-state configuration was approximately 4 mg cm−2. The electrochemical performance of the assembled device was evaluated using CV, GCD, and EIS measurements within a voltage window of 0 to 2.0 V. Long-term cycling stability was also tested at a current density of 1 A g−1 for up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles under ambient conditions.
000 cycles under ambient conditions.
3. Results and discussion
3.1. Structural and functional groups analysis
The preparation of the ACMT/FA/CE electrode materials was carried out as follows. Waste surgical face masks were first carbonized and subsequently activated using KOH to obtain activated carbon microtubes (ACMT). The activated carbon was then mixed with varying proportions of fly ash (FA), while cement (CE) was employed as the binder. All components were thoroughly blended to form a uniform slurry, which was evenly coated onto cleaned nickel foam substrates. The resulting electrodes were then dried and used for electrochemical evaluation, schematically illustrated in Scheme 1. The structural and crystalline properties of the synthesized CMT and ACMT materials were analyzed using XRD. As shown in Fig. 1a, the XRD pattern of CMT displays diffraction peaks at 2θ values of 21.4°, 23.6°, 29.7°, 31.0°, and 43.4°, corresponding to the (002), (222), (026), and (100) planes. The prominent (002) and (100) peaks confirm that CMT primarily exhibits an amorphous carbon structure, while the weaker (222) and (026) reflections suggest the presence of sulfur species, consistent with JCPDS no. 08-0247.34 In contrast, the ACMT sample shows two distinct diffraction peaks at (002) (2θ = 23.8°) and (100) (2θ = 43.7°), characteristic of amorphous carbon.45 Chemical activation of CMT made it easier to get rid of surface impurities and allowed graphitic and amorphous domains to exist at the same time. The sharper and more intense peaks observed for ACMT indicate enhanced interlayer ordering and micropore development following activation. Raman spectroscopy was employed to analyze the graphitic and disordered features of the synthesized carbon materials. As shown in Fig. 1b, both CMT and ACMT exhibit two characteristic peaks at 1344.6 cm−1 (D band) and 1593.5 cm−1 (G band). The D band originates from the double-resonance Raman process associated with structural defects and disorder in the carbon framework, while the G band corresponds to the in-plane vibrational mode of sp2-hybridized carbon atoms in graphitic domains. The intensity ratio of the D to G bands (ID/IG) offers an estimate of the degree of disorder within the material.46,47 The calculated ID/IG values are 1.19 for CMT and 1.30 for ACMT, indicating that both materials possess relatively high defect densities and lower graphitic ordering. The higher ID/IG ratio for ACMT further confirms that KOH activation introduces additional surface defects and edge sites, which can enhance electrochemical activity. | ||
| Fig. 1 (a) XRD pattern of CMT and ACMT, (b) Raman spectra, (c) FTIR spectra of CMT and ACMT, and (d) TGA analysis. | ||
FT-IR spectra were employed to identify the surface functional groups in the synthesized materials. As shown in Fig. 1c, the FT-IR spectrum of CMT exhibits several characteristic absorption bands, primarily originating from the polymeric components of the surgical face masks. The bands at 1096 cm−1, 1391.3 cm−1, and 1578 cm−1 correspond to C–O stretching vibrations, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C conjugated bonds, and C
C conjugated bonds, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl groups, respectively.48,49 Additional peaks observed below 1000 cm−1 fall within the fingerprint region, indicating complex molecular vibrations. In contrast, the FT-IR spectrum of ACMT reveals that many of these dominant functional groups either disappeared or were markedly reduced after high-temperature KOH activation. After activation, a new absorption band at 1184.6 cm−1 appeared. This band was linked to C–O stretching. These observations confirm that the activation process effectively removed oxygen-containing surface groups and altered the chemical structure of the carbon framework. Thermogravimetric analysis (TGA) was conducted to assess the thermal stability of CMT and ACMT. As shown in Fig. 1d, the TGA curves of both materials were recorded over a temperature range of 25–900 °C. The initial weight loss observed below ∼100 °C for ACMT corresponds to the evaporation of adsorbed moisture and residual solvents, which is excluded from the analysis of the carbon framework. The CMT sample loses weight slowly, and at high temperatures, it keeps about 60% of its original mass. In contrast, ACMT demonstrates superior thermal stability, with the major weight loss occurring at higher temperatures, indicating the formation of a more robust carbon structure after chemical activation.50 These results confirm that ACMT maintains strong thermal stability under elevated temperatures, while the removal of moisture-related components during activation further improves its structural integrity.
O carbonyl groups, respectively.48,49 Additional peaks observed below 1000 cm−1 fall within the fingerprint region, indicating complex molecular vibrations. In contrast, the FT-IR spectrum of ACMT reveals that many of these dominant functional groups either disappeared or were markedly reduced after high-temperature KOH activation. After activation, a new absorption band at 1184.6 cm−1 appeared. This band was linked to C–O stretching. These observations confirm that the activation process effectively removed oxygen-containing surface groups and altered the chemical structure of the carbon framework. Thermogravimetric analysis (TGA) was conducted to assess the thermal stability of CMT and ACMT. As shown in Fig. 1d, the TGA curves of both materials were recorded over a temperature range of 25–900 °C. The initial weight loss observed below ∼100 °C for ACMT corresponds to the evaporation of adsorbed moisture and residual solvents, which is excluded from the analysis of the carbon framework. The CMT sample loses weight slowly, and at high temperatures, it keeps about 60% of its original mass. In contrast, ACMT demonstrates superior thermal stability, with the major weight loss occurring at higher temperatures, indicating the formation of a more robust carbon structure after chemical activation.50 These results confirm that ACMT maintains strong thermal stability under elevated temperatures, while the removal of moisture-related components during activation further improves its structural integrity.
3.2. Specific surface area and chemical composition analysis
Fig. 2a and b displays the nitrogen adsorption–desorption isotherms of CMT and ACMT. The isotherm of CMT is a type IV curve, and the chemically activated carbon (ACMT) exhibits features of both type I and type IV isotherms.51 ACMT shows a clear hysteresis loop in the relative pressure range of P/P0 = 0.45–1.0, which means that mesoporous structures are present. This observation confirms the development of both mesopores and heteropores after KOH activated CMT at elevated temperatures. The formation of additional mesopores is due to the widening of pre-existing micropores during high-temperature activation, as further supported by the pore size distribution in Fig. 2b. The BET surface areas of CMT and ACMT are measured at 86.5 m2 g−1 and 1315 m2 g−1, respectively. For comparison, previously reported activated carbon derived from surgical face masks showed a surface area of 143 m2 g−1,52 indicating that the prepared ACMT has a substantially higher surface area. This significant increase demonstrates the effectiveness of KOH activation in creating a hierarchical porous structure and enhancing the overall surface morphology of the material. XPS was employed to analyze the surface compounds of ACMT. The XPS survey spectrum of ACMT is shown in Fig. S1, displaying two main peaks corresponding to carbon (C 1s) and oxygen (O 1s) at binding energies of approximately 284.7 eV and 532.4 eV, respectively. The high-resolution C 1s spectrum (Fig. 2c) reveals three distinct components at 284.2 eV, 285.4 eV, and 288.6 eV, attributed to C–C, C–OH, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively. These features suggest partial graphitization and the presence of sp2-hybridized carbon domains.53,54 The O 1s spectrum (Fig. 2d) shows peaks at 531.5 eV, 532.3 eV, and 533.1 eV, which are for the C
O bonds, respectively. These features suggest partial graphitization and the presence of sp2-hybridized carbon domains.53,54 The O 1s spectrum (Fig. 2d) shows peaks at 531.5 eV, 532.3 eV, and 533.1 eV, which are for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH, and C–O–C functional groups, respectively.55,56 These data confirm that oxygen-containing functionalities are present on the surface of ACMT, which can enhance its wettability and electrochemical performance.
O, C–OH, and C–O–C functional groups, respectively.55,56 These data confirm that oxygen-containing functionalities are present on the surface of ACMT, which can enhance its wettability and electrochemical performance.
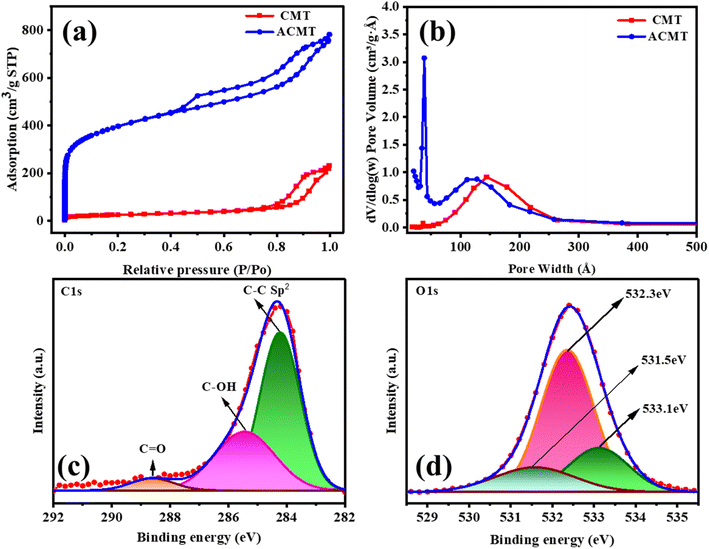 | ||
| Fig. 2 (a) N2 sorption isotherm of CMT and ACMT, (b) BHJ Pore size distributions, and (c and d) C 1s and O 1s spectra of ACMT. | ||
3.3. Morphology and microstructure analysis
SEM was used to examine the surface morphology of the synthesized CMT and ACMT materials. Fig. 3a shows the SEM image of a raw surgical face mask, revealing a macroscopic tube-like structure with dimensions around 10 µm. Fig. 3a–d displays SEM images of CMT at various magnifications, illustrating the formation of microtube structures. After carbonization, the original morphology of the raw material changed, with shorter tube lengths and agglomerated particles on the CMT surface. Following KOH activation, as shown in Fig. 3e–i, the agglomerates were mostly removed, although many microtube structures were partially destroyed. The SEM images of ACMT show both preserved microtube structures and the development of hierarchical pores on the tube surfaces. Additionally, the ACMT microtubes are smaller compared to CMT. Fig. 3h highlights the hierarchical pore network on the ACMT surface, aligning with the BET analysis results. EDX spectroscopy was performed to determine the elemental composition of ACMT. Fig. 3i–j presents the elemental spectra and mapping for oxygen and carbon. The ACMT material contains 8.85 wt% oxygen and 91.15 wt% carbon, with elemental mapping confirming a uniform distribution of these heteroatoms across the sample. Fig. 4 displays SEM images of unprocessed fly ash (FA), cement (CE), and the ACMT2/FA1/CE1 electrode. The SEM images of raw FA (Fig. 4a–c) display smooth, dense surfaces characteristic of fine fly ash particles formed during the rapid cooling of coal combustion ash at high temperatures in the boiler.35,36 Raw FA primarily contains crystalline Al2O3, SiO2, and CaO, with minor contributions from other metal oxides. Incorporating ACMT into the FA significantly enhances its conversion into a functional energy storage material, improving both morphology and crystal structure. Fig. 4d shows cement particles exhibiting a sheet-like morphology, serving as the binder in electrode preparation. SEM images of the ACMT2/FA1/CE1 nickel foam electrode (Fig. 4e–h) reveal a porous network combining microtubes, spherical, and sheet-like structures, indicating a uniform dispersion of ACMT, FA, and cement on the nickel foam surface. EDX analysis of FA (Fig. 4i) confirms the presence of oxygen (O), aluminum (Al), silicon (Si), calcium (Ca), magnesium (Mg), iron (Fe), and potassium (K), consistent with the XRD results shown in Fig. S2. The EDX spectrum of the ACMT2/FA1/CE1 composite (Fig. 4j) indicates that the composite contains significant amounts of FA-derived metal oxides along with ACMT carbon. The analysis further reveals the presence of carbon, oxygen, silica, aluminum, iron, and nickel, which are beneficial for supercapacitor electrode applications.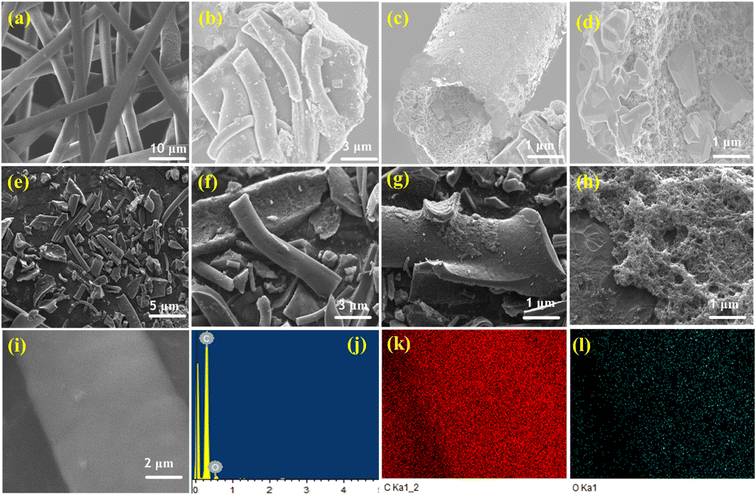 | ||
| Fig. 3 HR-SEM image of (a) surgical face mask, (b–d) CMT, (e–i) ACMT, (j) EDAX spectra of ACMT, and (k and l) Elemental mapping of C 1s, and O 1s. | ||
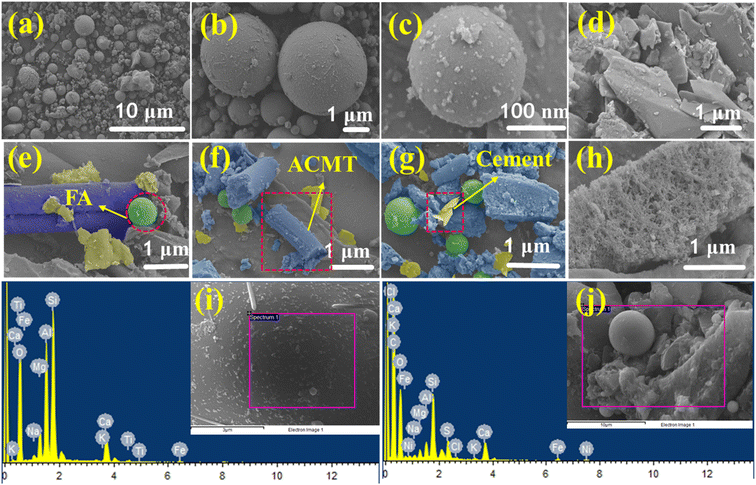 | ||
| Fig. 4 HR-SEM image of (a–c) fly ash, (d) cement, (e–h) ACMT2/FA1/CE1, (i) EDAX spectrum of fly ash, and (j) EDAX of ACMT2/FA1/CE1. | ||
HR-TEM was used to further examine the microstructural features of CMT, ACMT, and ACMT2/FA1/CE1 as shown in Fig. 5. The TEM image of CMT (Fig. 5a) shows a clear microtube structure with several agglomerated particles attached to the surface, indicating incomplete carbonization. In contrast, the HR-TEM images of ACMT (Fig. 5b and c) display a porous and rough surface, confirming that KOH activation effectively removes agglomerated particles and creates additional porosity. The pore sizes in ACMT range from 2 to 8 nm, which aligns with the mesoporous nature observed from BET analysis. The selected area electron diffraction (SAED) pattern of ACMT (Fig. 5d) shows diffuse diffraction rings, confirming the material's amorphous structure, consistent with the XRD results in Fig. 1a. Moreover, HR-TEM images of the optimized ACMT2/FA1/CE1 electrode (Fig. 5e–i) demonstrate a well-structured morphology, comprising porous carbon microtubes, spherical fly ash particles, and sheet-like cement components evenly distributed on the nickel foam surface. The close contact between these components ensures efficient electron transport and mechanical stability, which benefits supercapacitor electrode performance.
 | ||
| Fig. 5 HR-TEM image of (a) CMT, (b and c) ACMT, (d) SADE pattern of ACMT, and (e–i) optimal sample of ACMT2/FA1/CE1 at different scales. | ||
3.4. Electrochemical performance
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA ratio is 1
FA ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ACMT1/FA1/CE1), the CV curves resemble a battery-type profile (see Fig. S5b), indicating significant PCs contribution from FA's metal oxides in addition to EDLC from ACMT.57 By incorporating cement (CE) as a binder, the electrode's pseudocapacitive behavior is enhanced, supporting both EDLC and redox contributions across −1.0 to +0.3 V.39 In contrast, for ACMT2/FA1/CE1 and ACMT3/FA1/CE1the EDLC contribution becomes dominant and the redox-peak signal decreases. This phenomenon is attributed to the greater ACMT content, which increases the accessible surface area, and the lower FA content, which reduces the number of faradaic (redox-active) sites. As a result, EDLC behaviour increases and redox peaks become reduced (see Fig. 6b for CV and 6c for GCD). This observation indicates that excessively high ACMT content boosts EDLC but reduces PCs. Moreover, beyond the optimal composition, ACMT microtubes tend to agglomerate, which hinders charge transfer during redox reactions and decreases effective capacitance.58 Conversely, in ACMT1/FA2/CE1 and ACMT1/FA3/CE1, a higher FA proportion improves PCs, but the reduced ACMT content limits EDLC, leading to different electrochemical responses (see Fig. S5d and e). GCD measurements were conducted to further confirm these results (Fig. 6b). The GCD curves for all electrodes align with the CV results, confirming the hybrid charge storage mechanism. The curved, non-ideal triangular shape reflects the coexistence of EDLC and PCs. ACMT2/FA1/CE1 exhibits a much longer discharge time than the other samples. This demonstrates how ACMT's large surface area, microporous structure, and favorable electrical conductivity facilitate charge transfer. Fig. 6c displays the calculated specific capacitances at 1 A g−1 (eqn (1)). It is evident that ACMT2/FA1/CE1 is the optimal electrode composition. The results from both CV and GCD indicate that when either ACMT or FA exceeds the balanced 2
1 (ACMT1/FA1/CE1), the CV curves resemble a battery-type profile (see Fig. S5b), indicating significant PCs contribution from FA's metal oxides in addition to EDLC from ACMT.57 By incorporating cement (CE) as a binder, the electrode's pseudocapacitive behavior is enhanced, supporting both EDLC and redox contributions across −1.0 to +0.3 V.39 In contrast, for ACMT2/FA1/CE1 and ACMT3/FA1/CE1the EDLC contribution becomes dominant and the redox-peak signal decreases. This phenomenon is attributed to the greater ACMT content, which increases the accessible surface area, and the lower FA content, which reduces the number of faradaic (redox-active) sites. As a result, EDLC behaviour increases and redox peaks become reduced (see Fig. 6b for CV and 6c for GCD). This observation indicates that excessively high ACMT content boosts EDLC but reduces PCs. Moreover, beyond the optimal composition, ACMT microtubes tend to agglomerate, which hinders charge transfer during redox reactions and decreases effective capacitance.58 Conversely, in ACMT1/FA2/CE1 and ACMT1/FA3/CE1, a higher FA proportion improves PCs, but the reduced ACMT content limits EDLC, leading to different electrochemical responses (see Fig. S5d and e). GCD measurements were conducted to further confirm these results (Fig. 6b). The GCD curves for all electrodes align with the CV results, confirming the hybrid charge storage mechanism. The curved, non-ideal triangular shape reflects the coexistence of EDLC and PCs. ACMT2/FA1/CE1 exhibits a much longer discharge time than the other samples. This demonstrates how ACMT's large surface area, microporous structure, and favorable electrical conductivity facilitate charge transfer. Fig. 6c displays the calculated specific capacitances at 1 A g−1 (eqn (1)). It is evident that ACMT2/FA1/CE1 is the optimal electrode composition. The results from both CV and GCD indicate that when either ACMT or FA exceeds the balanced 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the capacitance decreases due to surface blockage or limitations on ion transport. According to the GCD curves, the coulombic efficiency of the 2
1 ratio, the capacitance decreases due to surface blockage or limitations on ion transport. According to the GCD curves, the coulombic efficiency of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was found to be higher compared to other weight percentages, as shown in Fig. 6d. These findings highlight that carefully optimizing the ratio of ACMT, FA, and CE is crucial for maximizing hybrid supercapacitor performance.
1 ratio was found to be higher compared to other weight percentages, as shown in Fig. 6d. These findings highlight that carefully optimizing the ratio of ACMT, FA, and CE is crucial for maximizing hybrid supercapacitor performance.
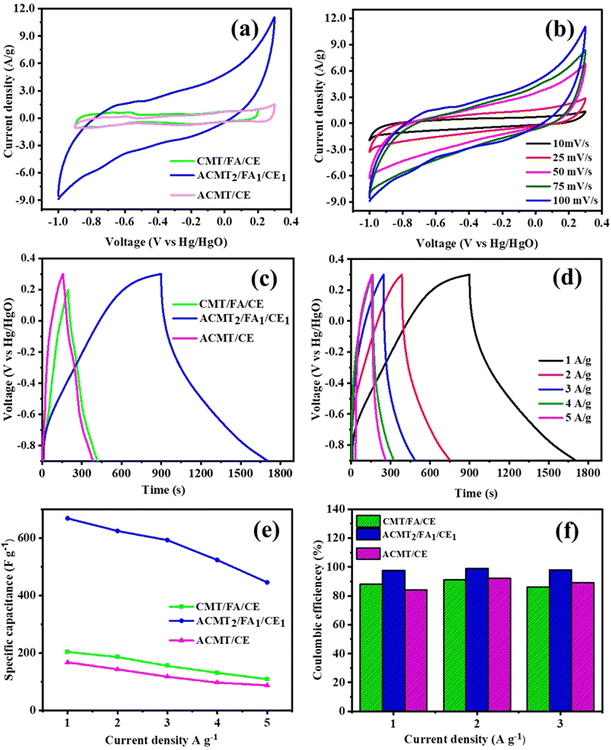
Fig. 7a presents the CV graphs of CMT/FA/CE, ACMT2/FA1/CE1, and ACMT/CE at a scan rate of 100 mV s−1. The quasi-rectangular shape with redox peaks suggests both reversible and non-reversible redox processes during the charge storage mechanism. Notably, ACMT2/FA1/CE1 exhibits the largest CV area compared to the other electrode materials at the same scan rate. The CV profile of ACMT2/FA1/CE1 features two pairs of Faraday peaks in the −0.6 to −0.5 V range, indicating the partial pseudocapacitance contribution from FA and CE.31,59,60 During the charging and discharging processes, the volume of the FA nanoparticles increases, creating optimal interfacial spacing within the mixed materials and facilitating more 2D pathways for rapid ion transport. The uniform distribution of FA nanoparticles densifies the ACMT surface, narrows the ion transport channels, and exposes new active sites. In contrast, the highly conductive ACMT and CE structures provide a flexible buffer to accommodate the volume expansion of FA nanoparticles during the redox reaction, ensuring stability.36,61 As a result, the FA/CE mixture significantly enhances ion mobility, increases the CV integral area, and boosts the current density—all crucial factors for efficient redox reactions. Moreover, the FA/CE mixture supports the microporous structure of ACMT, promoting better electrochemical performance.15–17,38 The addition of FA, with its excellent electrical conductivity, improves the performance of ACMT2/FA1/CE1 over CMT/FA/CE. Without FA, the ACMT/CE electrode exhibits a smaller CV integral area and lower current density compared to ACMT2/FA1/CE1. Overall, these results demonstrate that adding FA/CE to ACMT significantly enhances the electrochemical efficiency of the final optimal electrode material. Fig. S11(a and b) shows that the PVDF-based electrode (using polyvinylidene fluoride as the binder) has a significantly smaller CV integral area and lower current density compared to our cement-based electrode (calcium aluminate cement binder) for the composition ACMT2/FA1/CE1. These results demonstrate that the cement-binder electrode (ACMT2/FA1/CE1) outperforms the standard PVDF-binder version in terms of electrochemical activity, highlighting the advantages of using cement as the binder in this system.
Fig. 7b shows the CV curves of ACMT2/FA1/CE1 at various scan rates. The curves maintain their quasi-rectangular shape and redox peaks even at 100 mV s−1, indicating the rapid EDLC performance of the ACMT2/FA1/CE1 electrode. When the scan rate is lower, electrolyte ions have more time to move into the active sites of the electrode, which increases the specific capacitance. Conversely, at higher sweep rates, capacitance decreases due to the limited time for ion diffusion to reach the active regions on the electrode surface. Despite changes in scan rate, the voltammograms keep their shape, owing to fast faradic redox kinetics and charge transfer at the electrode/electrolyte interface. As the scan rate increases, peak current density rises, and peak positions shift either more negatively or positively, as noted in ref. 62 and 63. Similarly, in Fig. S5(a–f), the CV graphs of CMT/FA/CE and ACMT/CE from 10 to 100 mV s−1 display quasi-rectangular shapes with stable Faraday peaks, confirming the efficient and stable ion transport even at high scan rates.39 To further evaluate the electrochemical performance of CMT/FA/CE, ACMT2/FA1/CE1, and ACMT/CE, GCD tests were performed at a current density of 1 A g−1 (Fig. 7c). Consistent with the CV results, all electrodes demonstrate hybrid capacitive behavior. The ACMT2/FA1/CE1 electrode offers the best electrochemical response, significantly surpassing both CMT/FA/CE and ACMT/CE. This enhancement can be attributed to the synergistic effect between FA/CE and ACMT, which improves charge storage and transport within the electrode. The GCD curves for ACMT2/FA1/CE1, CMT/FA/CE, and ACMT/CE at various current densities are shown in Fig. 7d and S6(a–f). From these curves, the specific capacitance and coulombic efficiency values were derived, as shown in Fig. 7e and f. Notably, the ACMT2/FA1/CE1 electrode achieves a specific capacitance of 669 F g−1 along with high coulombic efficiency, confirming its superior energy storage capability. Nevertheless, at elevated current densities, the structural constraints of the composite limit complete charge–discharge utilization, leading to a slight performance reduction.63,64 Importantly, the specific capacitance of ACMT2/FA1/CE1 (669 F g−1) surpasses that of many previously reported face mask and FA-derived carbons tested in three-electrode systems (Table 1), underscoring its competitive advantage.
| Synthesized materials | Electrode system | Specific capacitance (F g−1) | Energy density (Wh kg−1) | Cycling stability (after cycle) | Ref. |
|---|---|---|---|---|---|
| SWAC 800 (sanitary pad) | 3 (6 M KOH) | 306 at 1 A g−1 | 7.1 at 250 W Kg−1 | 95% 5000 | 15 |
| 2 (1 M Na2SO4) | 92 at 1 A g−1 | 93% 5000 | |||
| ACFM-850/TS composite | 3 (1.0 M Na2SO4) | 421 at 1 A g−1 | 9.2 µWh cm−2 at 0.13 mW cm−2 | — | 28 |
| 2 (CMC/NaClO4) | 33.8 mF cm−2 at 0.2 mA cm−2 | 64% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| Face mask | 3 (6 M KOH) | 366.22 at 4 A g−1 | 35.54 at 1.5 W Kg−1 | 100% 8000 | 31 |
| 2 (PVA/KOH) | 113.73 at 1.3 A g−1 | 83% 8000 | |||
| N/P/O self-co-doped hierarchically porous carbons | 3 (6 M KOH) | 522.1 at 1 A g−1 | 39.3 at 500 W kg−1 | — | 37 |
| 2 (1 M Na2SO4) | 283.3 at 0.5 A g−1 | 100% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| CNFs/FA-CoFeSiSx | 3 (6 M KOH) | 493.33 F g−1 at 0.5 A g−1 | 25.43 at 359.07 W kg−1 | 79.50% 1800 | 38 |
| 2 (PVA/KOH) | 50.87 F g−1 at 1 mA cm−2 | 75% 2000 | |||
| Face mask 700 | 3 (1 M KOH) | 108 at 1 A g−1 | 15.7 at 360 W kg−1 | — | 65 |
| 2 (PVA/KOH) | 92.67 at 0.2 A g−1 | 92.67%1000 | |||
| Biomass fly ash | 3 (6 M KOH) | 207 at 1 A g−1 | 8.04 at 50 W kg−1 | 93.57% 5000 | 66 |
| Sin-CNTs | 3 (1 M H2SO4) | 133.2 at 1 A g−1 | 4.84 at 799 W kg−1 | 98.11% 5000 | 67 |
| 2 (1 M H2SO4) | 54.5 at 0.5 A g−1 | 100% 5000 | |||
| Barbary figs Husk | 3 (6 M KOH) | 497 at 0.5 A g−1 | 35.8 at 478.6 W kg−1 | 96.5% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
68 |
| 2 (PVA/KOH) | 96 at 0.5 A g−1 | 97.5% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| Fold-carbon spheres | 3 (6 M KOH) | 405 at 1 A g−1 | 24.7 at 697 W kg−1 | — | 69 |
| 2 (1 M Na2SO4) | 114 at 1 A g−1 | 91% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| Linum usitatissimum L. stem | 3 (6 M KOH) | 434 at 1 A g−1 | 12.4 at 125 W kg−1 | — | 70 |
| 2 (1 M Na2SO4) | 185 at 1 A g−1 | 100% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| Turnip | 3 (6 M KOH) | 570 at 0.5 A g−1 | 47.5 at 372 W kg−1 | 86% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
71 |
| 2 (1 M Na2SO4) | 193 at 0.5 A g−1 | 93.5% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| Activated carbon | 3 (1 M Na2SO4/0.08 M KBr) | 957.8 at 0.5 A g−1 | 133 at 859.6 W kg−1 | 86% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
72 |
| 2 (1 M Na2SO4/0.08 M KBr) | 127 at 20 mA/4 cm2 | 57.15 at 5262 W kg−1 | 82.8% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| Heteroatom-doped lignin-derived carbon material | 3 (6 M KOH) | 264 at 0.5 A g−1 | 15.97 at 450 W kg−1 | 91.34% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
73 |
| 2 (1 M Na2SO4) | 142.1 at 0.5 A g−1 | 75% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| Olive husk | 3 (6 M KOH) | 549 at 1 A g−1 | 38.8 at 650 W kg−1 | 95% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
74 |
| 2 (6 M KOH) | 259 at 1 A g−1 | 93% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| ACMT 2 /FE 1 /CE 1 | 3 M KOH | 669 at 1 A g− 1 | 134 at 600 W kg− 1 |
96.7% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
This work |
| 2 (PVA/KOH) | 156.5 at 1 A g− 1 | 87 at 750.5 W kg− 1 |
94.2% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
Electrochemical impedance spectroscopy (EIS) was used to examine various resistance components, including electrolyte–electrode resistance, internal cell resistance, and charge transfer resistance (Rct).74Fig. 8a displays the Nyquist plots of the samples, with the real part of the impedance on the x-axis and the imaginary part on the y-axis. For clarity, an enlarged view is provided in the inset. The measurements were conducted over a frequency range of 0.1 Hz to 105 kHz. From the EIS profiles, four main characteristics can be identified: (i) resistance originating from the electrolyte, (ii) losses due to wiring, current collectors, and electrode conductivity, (iii) ion transport within the electrode matrix, and (iv) Rct. The intercepts on the x-axis indicate the series resistance (Rs) of the electrodes, which are 1.16 Ω for CMT/FA/CE, 0.63 Ω for ACMT2/FA1/CE1, and 1.19 Ω for ACMT/CE. The ACMT2/FA1/CE1 electrode has the lowest Rs, indicating better electrical conductivity. Additionally, compared with CMT/FA/CE and ACMT/CE, the Nyquist curve of ACMT2/FA1/CE1 is more vertical at low frequencies, showing a reduced Warburg impedance (Wd). Since Wd relates to ion diffusion length, its narrower width suggests more efficient ion transport. The ACMT2/FA1/CE1 electrode benefits from shorter diffusion pathways, which improve electrolyte ion mobility. Overall, the EIS analysis demonstrates that ACMT2/FA1/CE1 offers superior electrochemical conductivity and faster charge transfer than the other electrode materials.
Fig. 8b displays the Bode phase–frequency plots of the three electrode samples. The ACMT2/FA1/CE1 electrode shows a phase angle of approximately 75.5°, which is closer to the ideal capacitive response of −90° than those of CMT/FA/CE and ACMT/CE. This indicates superior capacitive behavior. Using τ0 = 1/f0, the relaxation time constant was calculated as 0.019 s at a phase angle of 45°, indicating a rapid ion transport rate.44,75 To better understand the charge storage mechanism, Dunn's model was applied to the CV curves, separating the total capacitance into capacitive and diffusion-controlled contributions. This analysis offers additional details about the balance between surface-controlled processes and diffusion-limited ion transport within the prepared electrodes.76,77
| i(V) = k1v + k2v1/2 | (3) |
Eqn (3) separates the current response into two components: the capacitive current (k1v), which results from ion adsorption, and the diffusive current (k2v1/2), reflecting diffusion-controlled processes. At lower scan rates, the device exhibits a dominant diffusive contribution, as ions have enough time to participate in faradaic redox reactions. However, at higher scan rates, diffusion becomes limited because ions cannot fully access the active sites within the limited time frame. Fig. 8c summarizes the quantitative contributions of capacitive and diffusive processes. The bar chart indicates that both mechanisms play significant roles in charge storage, confirming the hybrid nature of the ACMT2/FA1/CE1 electrode. To further assess this dual charge storage behavior, Power's law was applied.78,79 The governing relationship is expressed as follows:
| i = avb | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the ESR slightly increased to 10.48 Ω, demonstrating that the device maintained high conductivity. This slight rise indicates that electrolyte ions continue to efficiently penetrate the porous ACMT material and reach the current collector during cycling. The EIS results confirm fast and effective ion transport within the NF/ACMT2/FA1/CE1 electrode. The Ragone plots in Fig. 9f show that the NF/ACMT2/FA1/CE1 device achieves a maximum energy density of 87 Wh kg−1 at 1 A g−1, which is higher than previously reported values for similar devices.15,28,37,66,82,83 After 10
000 cycles, the ESR slightly increased to 10.48 Ω, demonstrating that the device maintained high conductivity. This slight rise indicates that electrolyte ions continue to efficiently penetrate the porous ACMT material and reach the current collector during cycling. The EIS results confirm fast and effective ion transport within the NF/ACMT2/FA1/CE1 electrode. The Ragone plots in Fig. 9f show that the NF/ACMT2/FA1/CE1 device achieves a maximum energy density of 87 Wh kg−1 at 1 A g−1, which is higher than previously reported values for similar devices.15,28,37,66,82,83 After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles at 1 A g−1, the device retains over 94.2% of its initial specific capacitance (Fig. 9e). These findings demonstrate that the NF/ACMT2/FA1/CE1 device combines high performance, cost-effectiveness, and long-term stability, making it a promising candidate for supercapacitor applications.
000 charge–discharge cycles at 1 A g−1, the device retains over 94.2% of its initial specific capacitance (Fig. 9e). These findings demonstrate that the NF/ACMT2/FA1/CE1 device combines high performance, cost-effectiveness, and long-term stability, making it a promising candidate for supercapacitor applications.
3.5. Limitations and future directions
This study illustrates the efficiency of inexpensive and environmentally friendly materials, cement, coal fly ash (FA), and activated carbon microtubes (ACMT) made from used surgical masks as useful constituents for high-performance supercapacitor electrodes. Using waste materials from homes and businesses has both financial and environmental benefits. For example, fly ash can be preprocesses and then used right away to make electrodes. Through quantitatively improved specific capacitance, energy density, and cycling stability, the optimized ACMT2/FA1/CE1 electrode confirms its competitive performance when compared to previously reported carbon- and fly ash-based work. However, some constraints persist. Even though the existing fabrication and activation techniques work well in the lab, more improvement is needed to guarantee rigidity, scalability, and uniformity in large-scale applications. Furthermore, the extended durability of the cement-based binder across different humidity and temperature conditions necessitates additional investigation. Future efforts will concentrate on fine-tuning activation parameters to manage porosity, investigating alternative electrolytes to expand the operating voltage range, and incorporating the electrode design into flexible or asymmetric device structures to improve practical use. The recent developments have an opportunity to greatly enhance the performance and longevity of energy storage systems, establishing the stage for sustainable and scalable supercapacitor technologies.4. Conclusion
The present investigation outlines a sustainable and cost-effective approach for fabricating high-performance supercapacitor electrodes. It involves transforming waste surgical face masks into activated carbon microtubes (ACMT) through a chemical activation process, followed by integrating these materials with coal fly ash (FA) and cement (CE). The synthesized ACMT exhibited a highly microtubular porous structure, characterized by an extensive surface area of 1315 m2 g−1 and numerous defect sites that promote fast ion diffusion and enhance its charge storage capacity. When combined with FA and CE, the optimized ACMT2/FA1/CE1 composite formed an interconnected porous microtube network, providing efficient electron transport pathways and structural stability. FA contributed redox-active metal oxides (Si, Al, Mg, Fe, and Ca), enhancing conductivity and pseudocapacitance, while CE served as an electrochemically active binder that improved adhesion and mechanical integrity. The optimized ACMT2/FA1/CE1 electrode demonstrated an impressive specific capacitance of 669 F g−1 at 1 A g−1 within a three-electrode setup. In contrast, the associated two-electrode solid-state symmetric device (NF//ACMT2/FA1/CE1//NF/ACMT2/FA1/CE1) achieved an energy density of 87 Wh kg−1 and a power density of 750.5 W kg−1, maintaining a capacitance retention of 94.2% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. It highlights the advantages of cement as a sustainable alternative to traditional binders and emphasizes that the collective incorporation of ACMT, FA, and CE provides a viable and environmentally friendly pathway for developing advanced carbon-based supercapacitors suitable for real-world energy storage solutions.
000 cycles. It highlights the advantages of cement as a sustainable alternative to traditional binders and emphasizes that the collective incorporation of ACMT, FA, and CE provides a viable and environmentally friendly pathway for developing advanced carbon-based supercapacitors suitable for real-world energy storage solutions.
Author contributions
S. Nagarani: conceptualization, experimental work, methodology, writing – original draft. Jih-Hsing Chang: formal analysis, investigation, supervision, revised the articles, project administration, funding acquisition, Mohanraj Kumar: revised the articles, resources, conceptualization, project administration, funding acquisition, VP Krishnan: formal analysis. Raman Arunpandian: revised the articles.Conflicts of interest
The authors declare no conflicts of interest.Data availability
Data will be made available on request.Supplementary information is available. See DOI: DOI: https://doi.org/10.1039/d5ta06902e.
Acknowledgements
The authors from the Chaoyang University of Technology thank the National Science and Technology Council of Taiwan (grant no. MOST 111-2221-E-324-004-MY3) for supporting this work.References
- M. Amir, R. G. Deshmukh, H. M. Khalid, Z. Said, A. Raza, S. M. Muyeen, A. S. Nizami, R. M. Elavarasan, R. Saidur and K. Sopian, Energy storage technologies: An integrated survey of developments, global economical/environmental effects, optimal scheduling model, and sustainable adaption policies, J. Energy Storage, 2023, 72, 108694, DOI:10.1016/j.est.2023.108694.
- S. Tian, S. Wu, S. Cui, Y. Tian, K. J. Balkus, L. Zhou and G. Xiong, High-performance solid-state supercapacitors integrated with thermal management systems based on phase change materials: All in one, Chem. Eng. J., 2022, 446, 136787, DOI:10.1016/j.cej.2022.136787.
- Q. C. Zhu, D. Y. Zhao, M. Y. Cheng, J. Q. Zhou, K. A. Owusu, L. Q. Mai and Y. Yu, A new view of supercapacitors: integrated supercapacitors, Adv. Energy Mater., 2019, 9, 1901081, DOI:10.1002/aenm.201901081.
- M. N. Sakib, S. Ahmed, S. M. S. M. Rahat and S. B. Shuchi, A review of recent advances in manganese-based supercapacitors, J. Energy Storage, 2021, 44, 103322, DOI:10.1016/j.est.2021.103322.
- P. Phogat, S. Dey and M. Wan, Powering the sustainable future: a review of emerging battery technologies and their environmental impact, RSC Sustainability, 2025, 3, 3266–3306, 10.1039/D5SU00127G.
- S. B. Srinivasan, S. Devendiran, K. V. Savunthari, P. Arumugam and S. Mukerjee, Insights into multifarious heteroatom-doped/enriched carbon-based materials and their composites: Synthesis and Supercapacitor applications A crucial review, Prog. Mater. Sci., 2025, 153, 101470, DOI:10.1016/j.pmatsci.2025.101470.
- Y. Y. Dai, C. L. Liu, Y. Bai, Q. Q. Kong and H. Pang, Framework materials for supercapacitors, Nanotechnol. Rev., 2022, 11, 1005–1046, DOI:10.1515/ntrev-2022-0042.
- C. Li, Q. Yue, Y. Gao, Z. Li, J. Zhang, M. Zhang, S. He, Z. Wu, Y. Yang, J. Gan, C. Li, X. Xue, F. Qi, L. She, C. Zheng, J. Miao, D. Zhang, Z. Xia and H. Pan, Toward Rational Design of Carbon-Based Electrodes for High Performance Supercapacitors, ACS Appl. Mater. Interfaces, 2025, 17, 24675–24700, DOI:10.1021/acsami.4c21036.
- R. Dhilip Kumar, A. Jagadeesh Kumar, S. Balachandran, F. V. Kusmartsev, A. B. G. Trabelsi, F. H. Alkallasf, S. Nagarani, V. Sethuraman and B. Lee, High-performance chrysanthemum flower-like structure of Ni doped ZnO nanoflowers for pseudo-supercapacitors, J. Energy Storage, 2023, 72, 108441, DOI:10.1016/j.est.2023.108441.
- M. Maher, S. Hassan, K. Shoueir, B. Yousif and M. E. A. Abo-Elsoud, Activated carbon electrode with promising specific capacitance based on potassium bromide redox additive electrolyte for supercapacitor application, J. Mater. Res. Technol., 2021, 11, 1232–1244, DOI:10.1016/j.jmrt.2021.01.080.
- Y. Yan, W. R. Wu, Y. Yang, T. Xu and X. F. Li, Stepwise construction of MoS2@CoAlLDH/NF 3D core-shell nanoarrays with high hole mobility for high-performance asymmetric supercapacitors, ACS Appl. Mater. Interfaces, 2024, 16, 32434–32444, DOI:10.1021/acsami.4c05421.
- Y. Y. Chen, H. W. Hou, B. Liu, M. Y. Li, L. Chen, C. Z. Chen, S. F. Wang, Y. Y. Li and D. Y. Min, Wood-derived scaffolds decorating with nickel cobalt phosphate nanosheets and carbon nanotubes used as monolithic electrodes for assembling high-performance asymmetric supercapacitor, Chem. Eng. J., 2023, 454, 140453, DOI:10.1016/j.cej.2022.140453.
- S. A. Hashmi, S. Suematsu and K. Naoi, All solid-state redox supercapacitors based on supramolecular 1,5-diaminoanthraquinone oligomeric electrode and polymeric electrolytes, J. Power Sources, 2004, 137, 145–151, DOI:10.1016/j.jpowsour.2004.05.007.
- A. Gaber, A. Gaber, S. Bilge, Y. O. Donar, B. Ozoylumlu, Z. Kara and A. Sına, A first comprehensive review of ionothermal carbon-based supercapacitors, J. Power Sources, 2025, 653, 237760, DOI:10.1016/j.jpowsour.2025.237760.
- R. J. Wesley, S. Sowmya, A. Durairaj, R. Justinabraham, V. Vijaikanth, A. Obadiah and S. Vasanthkumar, Synthesis and electrochemical evaluation of porous carbon derived from sanitary pad waste for high performance in symmetric supercapacitor application, J. Energy Storage, 2023, 72, 108662, DOI:10.1016/j.est.2023.108662.
- Z. Zhai, L. Zhang, T. Du, B. Ren, Y. Xu, S. Wang, J. Miao and Z. Liu, A review of carbon materials for supercapacitors, Mater. Des., 2022, 221, 111017, DOI:10.1016/j.matdes.2022.111017.
- Y. Pei, Z. Ren, X. Wu, Y. Lv, N. Liang, H. Gao, P. Dong, X. Luo and J. Guo, Iodine intercalation-assisted alkali activation constructs coal-based porous carbon for high-performance supercapacitors, J. Colloid Interface Sci., 2024, 669, 518–528, DOI:10.1016/j.jcis.2024.04.207.
- T. Jorn-am, P. Supchocksoonthorn, W. Pholauyphon, J. Manyam, C. Chanthad and P. Paoprasert, Quasi-Solid, Bio-Renewable Supercapacitors Based on Cassava Peel and Cassava Starch and the Use of Carbon Dots as Performance Enhancers, Energy Fuels, 2022, 36, 7865–7877, DOI:10.1021/acs.energyfuels.2c01263.
- R. K. K. Reddy, C. Andrew, S. Lidija, F. Karen, B. Leonard and I. Aruna, Scalable slot-die coated flexible supercapacitors from upcycled PET face shields, RSC Adv., 2024, 14, 12781–12795, 10.1039/D2RA06809E.
- T. Ru-Ling and T. Szu-Kung, Pore structure and adsorption performance of the KOH-activated carbons prepared from corncob, J. Colloid Interface Sci., 2005, 287, 428–437, DOI:10.1016/j.jcis.2005.02.033.
- M. Li, L. Wang, W. Xiong, L. Zhao, Y. Tian, M. Cheng, Y. Wang, Z. Li, X. Wang, Q. Sheng and Y. Luo, Self-supported porous wood carbon electrode with a MoC/carbon nanocage composite for application in a high-performance supercapacitor, J. Colloid Interface Sci., 2024, 672, 392–400, DOI:10.1016/j.jcis.2024.06.014.
- P. Manasa, S. Sambasivam and F. Ran, Recent progress on biomass waste derived activated carbon electrode materials for supercapacitors applications—A review, J. Energy Storage, 2022, 54, 105290, DOI:10.1016/j.est.2022.105290.
- G. Tatrari, M. Karakoti, C. Tewari, S. Pandey, B. S. Bohra, A. Dandapat and N. G. Sahoo, Solid waste-derived carbon nanomaterials for supercapacitor applications: a recent overview, Mater. Adv., 2021, 2, 1454–1484, 10.1039/D0MA00871K.
- J. Yeh, S. P. Prakoso, L. L. Santoso, S. Chen, B. Chiang, J. Cheng, R. Zhang and Y. Chiu, A sustainable biomass-based electret for face mask and non-volatile transistor memory, Org. Electron., 2024, 124, 106944, DOI:10.1016/j.orgel.2023.106944.
- B. Ghatak, S. Banerjee, Sk. B. Ali, R. Bandyopadhyay, N. Das, D. Mandal and B. Tudu, Design of a self-powered triboelectric face mask, Nano Energy, 2021, 79, 105387, DOI:10.1016/j.nanoen.2020.105387.
- Y. Song, X. Wang, L. Wang, L. Qu and X. Zhang, Functionalized Face Masks as Smart Wearable Sensors for Multiple Sensing, ACS Sens., 2024, 9, 4520–4535, DOI:10.1021/acssensors.4c01705.
- J. Serafin, J. Srénscek-Nazzal, A. Kamínska, O. Paszkiewicz and B. Michalkiewicz, Management of surgical mask waste to activated carbons for CO2 capture, J. CO2 Util., 2022, 359, 132043, DOI:10.1016/j.jcou.2022.101970.
- R. R. Kiran Kumar, S. Lidija and I. Aruna, Screen-Printed Stretchable Supercapacitors Based on Tin Sulfide-Decorated Face-Mask-Derived Activated Carbon Electrodes with High Areal Energy Density, ACS Appl. Energy Mater., 2024, 7, 3558–3576, DOI:10.1021/acsaem.3c02902.
- D. Hantoko, X. Li, A. Pariatamby, K. Yoshikawa, M. Horttanainen and M. YanHantoko, Challenges and practices on waste management and disposal during COVID-19 pandemic, J. Environ. Manage., 2021, 286, 112140, DOI:10.1016/j.jenvman.2021.112140.
- K. Selvaranjan, S. Navaratnam, P. Rajeev and N. Ravintherakumaran, Environmental challenges induced by extensive use of face masks during COVID-19: A review and potential solutions, Environ. Chall., 2021, 3, 100039, DOI:10.1016/j.envc.2021.100039.
- A. Ahmed, S. Verma, P. Mahajan, A. K. Sundramoorthy and S. Arya, Upcycling of surgical facemasks into carbon based thin film electrode for supercapacitor technology, Sci. Rep., 2023, 13, 12146, DOI:10.1038/s41598-023-37499-x.
- Y. Li, C. Kong, Z. Du, J. Zhang, X. Qin, J. Zhang, C. Li, Y. Jin and S. Wang, Oxygen-rich hierarchical porous carbon nanosheets derived from the KOH/KNO3 co-activation treatment of soybean straw for high-performance supercapacitors, Energy Adv., 2024, 3, 904–915, 10.1039/d4ya00076e.
- L. Feng, B. Yan, J. Zheng, J. Chen, R. Wei, S. Jiang, W. Yang, Q. Zhang and S. He, Soybean protein-derived N, O co-doped porous carbon sheets for supercapacitor applications, New J. Chem., 2022, 46, 10844–10853, 10.1039/D2NJ01355J.
- R. Mendoza, J. Oliva, K. P. Padmasree, A. I. Oliva, A. I. Mtz-Enriquez and A. Zakhidov, Highly efficient textile supercapacitors made with face masks waste and thermoelectric Ca3Co4O9-δ oxide, J. Energy Storage, 2022, 46, 103818, DOI:10.1016/j.est.2021.103818.
- X. Li, C. Bai, Y. Qiao, X. Wang, K. Yang and P. Colombo, Preparation, properties and applications of fly ash-based porous geopolymers: A review, J. Clean. Prod., 2022, 359, 132043, DOI:10.1016/j.jclepro.2022.132043.
- P. Pransisco, M. S. Balbir Singh, M. Shuaib, M. F. Shukur and E. Joseph, 3D graphene/fly ash waste material for hybrid supercapacitor electrode: specific capacitance Analysis, Materialwiss. Werkstofftech., 2020, 51, 713–718, DOI:10.1002/mawe.201900249.
- G. Zheng, Z. Huang and Z. Liu, Cooperative utilization of beet pulp and industrial waste fly ash to produce N/P/O self-co-doped hierarchically porous carbons for high-performance supercapacitors, J. Power Sources, 2021, 482, 228935, DOI:10.1016/j.jpowsour.2020.228935.
- H. Wang, M. Wang, J. Zhang, N. Wang, J. Wang and J. Yang, Preparation of fly ash-based cobalt-iron silicate as supercapacitor electrode material, Chem. Eng. J., 2022, 434, 134661, DOI:10.1016/j.cej.2022.134661.
- N. Chanut, D. Stefaniuk, J. C. Weaver and F. Ulm, Carbon–cement supercapacitors as a scalable bulk energy storage solution, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2304318120, DOI:10.1073/pnas.2304318120.
- T. Liu, Yu. Feng, Z. Song and Y. Li, Revitalizing carbon supercapacitor electrodes with hierarchical porous structures, J. Mater. Chem. A, 2017, 5, 07, 10.1039/C7TA05646J.
- M. J. A. Qomi, M. Bauchy, F. J. Ulm and R. J.-M. Pellenq, Anomalous composition-dependent dynamics of nanoconfined water in the interlayer of disordered calcium-silicates, J. Chem. Phys., 2014, 140, 054515, DOI:10.1063/1.4864118.
- A. Allen, J. Thomas and H. Jennings, Composition and density of nanoscale calcium-silicate-hydrate in cement, Nat.Mater., 2007, 6, 311–316, DOI:10.1038/nmat1871.
- T. C. Powers and T. L. Brownyard, Studies of the physical properties of hardened Portland cement paste, Am. Concr. Inst. J. Proc., 1946, 43, 249–336, DOI:10.14359/15304.
- X. Yang, M. Zhang, C. Wang, M. Bi, J. Xie, W. Bai, Y. Zhang, S. Pan, M. Liu, X. Pan, Z. Lu, Z. Han and Y. Fu, S, N co-doped rGO/fluorine-free Ti3C2Tx aerogels for high performance all-solid-state supercapacitors, J. Energy Storage, 2023, 71, 108140, DOI:10.1016/j.est.2023.108140.
- K. K. R. Reddygunta, A. Callander, L. Šiller, K. Faulds, L. Berlouis and A. Ivaturi, A. Sono-exfoliated graphene-like activated carbon from hazelnut shells for flexible supercapacitors, Int. J. Energy Res., 2022, 46, 16512–16537, DOI:10.1002/er.8314.
- D. Liu, J. Gao, Q. Cao, S. Wu and Y. Qin, Improvement of Activated Carbon from Jixi Bituminous Coal by Air Preoxidation, Energy Fuels, 2017, 31, 1406–1415, DOI:10.1021/acs.energyfuels.6b02875.
- Y. Zhai, X. Liu, Y. Zhu, C. Peng, T. Wang, L. Zhu, C. Li and G. Zeng, Hydrothermal carbonization of sewage sludge: The effect of feed-water pH on fate and risk of heavy metals in hydrochars, Bioresour. Technol., 2016, 218, 183–188, DOI:10.1016/j.biortech.2016.06.085.
- A. Omidvar, M. R. Rashidianvaziri, B. Jaleh, N. Partovi Shabestari and M. Noroozi, Metal-enhanced fluorescence of graphene oxide by palladium nanoparticles in the blue-green part of the spectrum, Chin. Phys. B., 2016, 25, 118102, DOI:10.1088/1674-1056/25/11/118102.
- T. Yang, L. H. Liu, J. W. Liu, M. L. Chen and J. H. Wang, Cyanobacterium metallothionein decorated graphene oxide nanosheets for highly selective adsorption of ultra-trace cadmium, J. Mater. Chem., 2012, 22, 21909–21916, 10.1039/C2JM34712A.
- M. M. Tobías, M. Åhlén, O. Cheung, D. G. Bucknall, M. R. S. McCoustra and H. H. P. Yiu, Plasma degradation of contaminated PPE: an energy-efficient method to treat contaminated plastic waste, npj Mater. Degrad., 2023, 7(33), 1–9, DOI:10.1038/s41529-023-00350-9.
- A. B. F. M. Sevilla, Hierarchical Microporous/Mesoporous Carbon Nanosheets for High-Performance Supercapacitors, CS Appl. Mater. Interfaces, 2015, 77, 4344–4353, DOI:10.1021/am508794f.
- U. Manojkumar and P. Senthilkumar, Activated carbon from single use surgical mask as an efficient adsorbent for 4-nitrophenol and 2,4-dicholorophenol from aqueous solution: adsorption kinetics, isotherms and thermodynamic study, Int. J. Environ. Anal. Chem, Int., 2023, 1–21, DOI:10.1080/03067319.2023.2300749.
- R. Haerle, E. Riedo, A. Pasquarello and A. Baldereschi, sp2/sp3 hybridization ratio in amorphous carbon from C 1s core-level shifts: X-ray photoelectron spectroscopy and first-principles calculation, Phys. Rev. B:Condens. Matter Mater. Phys., 2001, 65, 045101, DOI:10.1103/PhysRevB.65.045101.
- Z. Liang, H. Xia, H. Liu, L. Zhang, Y. Zhao, J. Zhou, H. Li and W. Xie, In Situ Pore Self-Formed Strategy for the Preparation of Sulfur-Doped Hierarchical Porous Carbon Spheres for Supercapacitors, ECS J. Solid State Sci.Technol., 2020, 9, 021005, DOI:10.1149/2162-8777/ab6ab0.
- Z. Liu, Y. Qi, M. Gui, C. Feng, X. Wang and Y. Lei, Sulfonated carbon derived from the residue obtained after recovery of essential oil from the leaves of Cinnamomum longe paniculatum using Brønsted acid ionic liquid, and its use in the preparation of ellagic acid and gallic acid, RSC Adv., 2019, 9, 5142–5150, 10.1039/C8RA08685K.
- J. Zhou, H. Shen, Z. Li, S. Zhang, Y. Zhao, X. Bi, Y. Wang, H. Cui and S. Zhuo, Porous carbon materials with dual N, S-doping and uniform ultra-microporosity for high performance supercapacitors, Electrochim. Acta., 2016, 209, 557–564, DOI:10.1016/j.electacta.2016.05.127.
- M. Z. Iqbal, M. M. Faisal, S. R. Ali, S. Farid and A. M. Afzal, Co-MOF/polyaniline-based electrode material for high performance supercapattery devices, Electrochim. Acta, 2020, 346, 136039, DOI:10.1016/j.electacta.2020.136039.
- H. Ansarinejad, M. Shabani-Nooshabadi and S. M. Ghoreishi, Facile synthesis of crumpled-paper like CoWO4-CoMn2O4/N-doped graphene hybrid nanocomposites for high performance all-solid-state asymmetric supercapacitors, J. Energy Storage, 2022, 45, 103513, DOI:10.1016/j.est.2021.103513.
- Z. Chen, Y. Chen, Q. Wang, T. Yang, Q. Luo, K. Gu and W. Yang, Molecularly-regulating oxygen-containing functional groups of ramie activated carbon for high-performance supercapacitors, J. Colloid Interface Sci., 2024, 665, 772–779, DOI:10.1016/j.jcis.2024.03.177.
- X. Dong, Y. Yu, X. Jing, H. Jiang, T. Hu, C. Meng, C. Huang and Y. Zhang, Sandwich-like honeycomb Co2SiO4/rGO/honeycomb Co2SiO4 structures with enhanced electrochemical properties for high-performance hybrid supercapacitor, J. Power Sources, 2021, 492, 229643, DOI:10.1016/j.jpowsour.2021.229643.
- Y. L. Ma, H. W. Sheng, W. Dou, Q. Su, J. Y. Zhou, E. Q. Xie and W. Lan, Fe2O3 nanoparticles anchored on the Ti3C2Tx mXene paper for flexible supercapacitors with ultrahigh volumetric capacitance, ACS Appl. Mater. Interfaces, 2020, 12, 41410–41418 CrossRef CAS PubMed.
- X. Chen, H. Li, J. Xu, F. Jaber, F. Musharavati, E. Zalezhad, S. Bae, K. S Hui, K. N Hui and J. Liu, Synthesis and characterization of a NiCo2O4@NiCo2O4 hierarchical mesoporous nanoflake electrode for supercapacitor applications, Nanomaterials, 2020, 10(7), 1292, DOI:10.3390/nano10071292.
- H. Ansarinejad, M. Shabani-Nooshabadi and S. M. Ghoreishi, Introducing of WO3@NiCo2O4/rGO ternary nanocomposites as active material for high-performance supercapacitor applications, J. Energy Storage, 2023, 74, 109256, DOI:10.1016/j.est.2023.109256.
- C. Wang, H. Wang, B. Dang, Z. Wang, X. Shen, C. Li and Q. Sun, Ultrahigh yield of nitrogen doped porous carbon from biomass waste for supercapacitor, Renew. Energy., 2020, 156, 370–376, DOI:10.1016/j.renene.2020.04.092.
- V. P. Krishnan, K. Mohanraj, C. Ching-Lung, H. Mon-Shu, M. Khursheed, Z. Yousef, M. A. Musa and C. Jih-Hsing, A novel carbon microtubes derived from the used surgical face mask for the Ni-F/FMs symmetric supercapacitor device, J. Saudi Chem. Soc., 2024, 28, 101865, DOI:10.1016/j.jscs.2024.101865.
- S. Chuan-lin, R. Ke, L. Shen-wei, Z. Ying-quan, T. Zhao-cai, W. Mei-mei, Z. Ji-gang and H. Kui-hua1, Preparation of activated carbon from unburned carbon in biomass fly ash and its supercapacitor performance, J. Fuel Chem. Technol., 2021, 49(12), 1936–1942, DOI:10.1016/S1872-5813(21)60129-9.
- O. Khiter, A. H. Hammad, A. O. A Zahrani, A. Melaibari, M. O. Ansari and N. Salah, High performance supercapacitors based on oil fly ash-derived carbon nanotubes, Electrochim. Acta, 2025, 540, 147190, DOI:10.1016/j.electacta.2025.
- R. Nasser, G.-F. Zhang, H. Liang, N.-N. Zhou and J.-M. Song, Lamellar hierarchically porous carbon derived from discarded Barbary figs husk: Preparation, characterization, and its excellent capacitive, J. Electroanal. Chem., 2021, 888, 114930, DOI:10.1016/j.jelechem.2020.114930.
- J. Du, M. Li, J. Song, X. Gao, S. Hou and A. Chen, In-situ activator-induced evolution of morphology on carbon materials for supercapacitors, J. Colloid Interface Sci., 2023, 630, 61–69, DOI:10.1016/j.jcis.
- W. Tian, P. Ren, X. Houa, Z. Guo, R. Xue, Z. Chen and Y. Jin, Biomass derived N/O self-doped porous carbon for advanced supercapacitor electrodes, Ind. Crop. Prod., 2023, 202, 117032, DOI:10.1016/j.indcrop.2023.117032.
- T. Jiang, R. Nasser, X. Wang, A. B. G. Trabelsi, F. H. Alkallas, H. Elhouichet and J.-M. Song, 2D porous carbon nanosheets derived from turnip: Preparation, characterization and its electrochemical performance, J. Energy Storage, 2023, 59, 106552, DOI:10.1016/j.est.2022.106552.
- M. Maher, S. Hassan, K. Shoueir, B. Yousif and M. E. A. Abo-Elsoud, Activated carbon electrode with promising specific capacitance based on potassium bromide redox additive electrolyte for supercapacitor application, J. Mater. Res. Technol., 2021, 11, 1232–1244, DOI:10.1016/j.jmrt.2021.01.080.
- W. Li, C. Li, Y. Xu, G. Wang, T. Xu, W. Zhang and C. Si, Heteroatom-doped and graphitization-enhanced lignin-derived hierarchically porous carbon via facile assembly of lignin-Fe coordination for high-voltage symmetric supercapacitors, J. Colloid Interface Sci., 2024, 659, 374–384, DOI:10.1016/j.jcis.2023.12.162.
- R. Nasser, J. Tiantian and J.-M. Song, Hierarchical porous activated carbon derived from olives: Preparation, (N, S) co-doping, and its application in supercapacitors, J. Energy Storage, 2022, 51, 104348, DOI:10.1016/j.est.2022.104348.
- P. Ren, D. Wu, T. Wang, P. Zeng and D. Jia, K2CO3-KCl acts as a molten salt flame retardant to prepare N and O doped honeycomb-like carbon in air for supercapacitors, J. Power Sources, 2022, 532, 231072, DOI:10.1016/j.jpowsour.2022.231072.
- M. Z. Iqbal, U. Aziz, N. Amjad, S. Aftab and S. M. Wabaidur, Porous activated carbon and highly redox active transition metal sulfide by employing multi-synthesis approaches for battery-supercapacitor applications, Diam. Relat. Mater., 2023, 136, 110019, DOI:10.1016/j.diamond.2023.110019.
- R. Li and J. Liu, Mechanistic investigation of the charge storage process of pseudocapacitive Fe3O4 nanorod film, Electrochim. Acta, 2014, 120, 52–56, DOI:10.1016/j.electacta.2013.12.040.
- H. Yin, C. Song, Y. Wang, S. Li, M. Zeng, Z. Zhang, Z. Zhu and K. Yu, Influence of morphologies and pseudocapacitive contributions for charge storage in V2O5 micro/nano-structures, Electrochim. Acta, 2013, 111, 762–770, DOI:10.1016/j.electacta.2013.08.005.
- M. Sathiya, A. Prakash, K. Ramesha, J. M. Tarascon and A. K. Shukla, V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage, J. Am. Chem. Soc., 2011, 133, 16291–16299, DOI:10.1021/ja207285b.
- J. Zhou, J. Yu, L. Shi, Z. Wang, H. Liu, B. Yang, C. Li, C. Zhu and J. Xu, A conductive and highly deformable all-pseudocapacitive composite paper as supercapacitor electrode with improved areal and volumetric capacitance, Small, 2018, 14, 1803786, DOI:10.1002/smll.201803786.
- F. Mo, H. X. Zhang, Y. X. Wang, C. X. Chen and X. L. Wu, Heteroatom-doped hierarchical porous carbon for high performance flexible all-solid-state symmetric supercapacitors, J. Energy Storage, 2022, 49, 104122, DOI:10.1016/j.est.2022.104122.
- J. Lie, H. Shuwanto, H. Abdullah, S. Ismadji, I. D. A. A. Warmadewanthi and F. E. Soetaredjo, Fly ash electrodes fabricated by an acid-assisted subcritical water extraction method for supercapacitor applications, New J. Chem., 2023, 47, 3802–3809, 10.1039/D2NJ05087K.
- J.-H. Chang, R. Arunpandian, S. Nagarani, P. Sivasubramanian, M. Kumar, R. A. Lenin and S. Balachandran, Activated carbon derived from rice husk for highly enhanced symmetric supercapacitor application, Mater. Lett., 2025, 384, 138117, DOI:10.1016/j.matlet.2025.
| This journal is © The Royal Society of Chemistry 2026 |