Pd composition and dispersion control selectivity in photocatalytic methane oxidation
Jiawei
Wu†
a,
Hu
Ding†
a,
Yan
Wang
a,
Ulfi
Muliane
b,
Shuji
Anabuki
c,
Naoya
Murakami
 d,
Xinli
Zhu
d,
Xinli
Zhu
 a,
Akira
Yamakata
a,
Akira
Yamakata
 cef,
Teruhisa
Ohno
cef,
Teruhisa
Ohno
 g and
Shi Nee
Lou
g and
Shi Nee
Lou
 *a
*a
aCollaborative Innovation Center of Chemical Science and Engineering, Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Haihe Laboratory of Sustainable Chemical Transformations, Tianjin University, Tianjin, 300072, China. E-mail: snlou@tju.edu.cn
bDepartment of Energy Engineering, Korea Institute of Energy Technology (KENTECH), Naju, 58330, Korea
cGraduate School of Natural Science and Technology, Okayama University, 3-1-1 Tsushima-naka, Kita-ku, Okayama 700-8530, Japan
dGraduate School of Life Science and Systems Engineering, Kyushu Institute of Technology, Fukuoka, 804-8550, Japan
eResearch Institute for Interdisciplinary Science, Okayama University, 3-1-1 Tsushima-naka, Kita-ku, Okayama 700-8530, Japan
fInstitute for Aqua Regeneration, Shinshu University, 4-17-1 Wakasato, Nagano, 380-8553, Japan
gGraduate School of Engineering, Kyushu Institute of Technology, Fukuoka, 804-8550, Japan
First published on 29th September 2025
Abstract
Selective methane oxidation to methanol under mild conditions presented a significant challenge due to the high bond dissociation energy of methane and the difficulty in achieving high selectivity. This study investigated how Pd composition and dispersion influenced photocatalytic oxidation of methane over brookite TiO2 (BTO) nanorods, using molecular O2 and water as oxidants. In situ time-resolved transient absorption (TA) spectroscopy in the visible to mid-infrared (IR) range, along with X-ray photoelectron spectroscopy (XPS) analysis before and after methane oxidation, revealed that BTO nanorods with high Pd loading and lower Pd dispersion exhibited significant electron trapping in the Pd/PdO nanoparticles under light irradiation. This electron trapping promoted the self-reduction of PdO to metallic Pd (Pd0), which in turn drove the selective production of CH3OH with 98% selectivity. In contrast, a moderate Pd loading and dispersion on the BTO nanorods enhanced electron transfer to O2, reducing electron trapping, and resulted in a higher concentrations of mixed Pd0/PdO nanoparticles, favoring the formation of primary oxygenates – CH3OOH and CH3OH. DFT calculations and experimental findings showed that Pd0 facilitates the direct three-electron reduction of O2 to *OH or ˙OH, which subsequently coupled with *CH3 or ˙CH3 to form CH3OH. Meanwhile PdO promotes the one-electron reduction of O2 to *OOH or ˙OOH, leading to CH3OOH formation. This work provides valuable insights into the design of efficient photocatalysts for selective methane oxidation and underscores the critical role of Pd composition and dispersion in modulating charge dynamics and steering product selectivity in methane.
Introduction
Methane (CH4), a potent greenhouse gas and key natural gas component, holds promise as a feedstock for methanol (CH3OH), a clean transportable fuel and valuable chemical building block. However, its strong C–H bond (∼439 kJ mol−1) complicates selective oxidation.1–4 Currently, industrial CH3OH synthesis relies on high-temperature steam reforming (>700 °C) to produce syngas.1–4 Direct methane oxidation is a more energy-efficient alternative5,6 but faces challenges, such as high reaction temperatures,1,2 or the use of costly or environmentally harmful oxidants (e.g., H2O2 or N2O).1–4Photocatalytic methane oxidation under mild conditions using molecular O2 and water as oxidants presents a promising solution. Titanium dioxide (TiO2), particularly in its mixed anatase/rutile7–14 and pure anatase forms,15–22 is widely studied for methane oxidation due to its high stability, efficient light-to-energy conversion, and cost-effectiveness. To enhance charge separation and surface reactions, metal cocatalysts (e.g., Au,7,12,19,21 Ag,7,15 Pd,7,12,14,18 Ni11) and oxygen vacancy10,16,17 modifications are often employed. However, over-oxidation of products to formaldehyde (HCHO) and carbon dioxide (CO2) diminishes CH3OH selectivity.8,11–14,16,17,19 Although the anatase–rutile interface promotes charge separation and photocatalytic performance, excessive hydroxyl radical (˙OH) generation during water photo-oxidation contributes to over-oxidation, limiting CH3OH yield.7
Surface modifications with hole acceptors7,10,14,20 like CoOX,7 CuOX,14 and N–Fe2Ti3O9,20 and W-doping9 (to adjust the valence band potential), help control ˙OH formation and improve oxygenate selectivity and overall efficiency. Despite these improvements, over-oxidation to HCHO and CO2 persists.7,9,14 Although alternative catalysts such as ZnO,23,24 BiVO4,25,26 WO3,27,28 and In2O3 (ref. 29 and 30) show potential, they face long-term stability issues, highlighting the need for further development of TiO2-based photocatalysts with better selectivity and reduced over-oxidation.
Brookite TiO2, a less-studied polymorph, shows significant potential as a photocatalyst.31–39 Previous studies have demonstrated that brookite TiO2 nanorods with well-developed {210} and {212} facets can be synthesized through hydrothermal reactions using TALH (dihydroxybis(ammonium lactato) titanium(IV)) and urea as precursors,34,35,37,38 with sodium lactate enhancing crystallinity.32,39 These {210} and {212} facets serve as reduction and oxidation sites, respectively, improving electron–hole separation and photocatalytic performance.33–35,38 Additionally, brookite TiO2 possesses a moderate electron trap depth, which could facilitate the regulation of ˙OH radical production during oxygen reduction, thereby helping to address the issue of over-oxidation.36 Photo-deposition of metal cocatalysts is a promising approach for selectively loading them onto reduction-active sites, thereby enhancing photocatalytic performance. Compared to non-site-selective chemical reduction methods, photo-deposition allows for more precise placement of cocatalyst particles, maximizing their effectiveness.40,41 While Au, Ag, and Pd are active cocatalysts for generating reactive oxygen species in CH4 oxidation, Au and Ag tend to exhibit weak interactions with the TiO2 surface, leading to particle aggregation at high metal loadings and reduced active sites.37,42–44 In contrast, Pd forms a strong metal-support interaction with TiO2, facilitating better dispersion and smaller, more effective Pd nanoparticles for photocatalytic reaction.44
Herein, we uncover how the composition and dispersion of Pd critically govern the photocatalytic oxidation of methane to primary oxygenates – methanol (CH3OH) and methyl hydroperoxide (CH3OOH) – over brookite-phase TiO2 nanorods. By engineering Pd-modified BTO (Pd/BTO) nanorods with controlled Pd0/PdO ratios and dispersion profiles, we systematically evaluated their activity using molecular O2 and water as oxidants. In situ time-resolved transient absorption (TA) spectroscopy, spanning the visible to mid-infrared (IR) range, alongside X-ray photoelectron spectroscopy (XPS) analysis conducted before and after methane oxidation, revealed that catalysts with higher Pd loadings and lower dispersion exhibited pronounced electron trapping under light irradiation. This trapping effect facilitates the self-reduction of PdO to metallic Pd0, thereby enabling highly selective CH3OH production with 98% selectivity. In contrast, lower Pd loadings and higher dispersion favor electron transfer to O2 and reduce electron trapping, leading to a mixed Pd0/PdO state that promotes the formation of both CH3OOH and CH3OH. Density functional theory (DFT) calculations, supported by experimental evidence, show that Pd0 mediates the three-electron reduction of O2 to *OH or ˙OH species, which couple with methyl intermediates (*CH3 or ˙CH3) to form CH3OH. Meanwhile, PdO catalyzes the one-electron reduction of O2 to *OOH or ˙OOH, steering the reaction toward CH3OOH. These results highlight the fundamental role of Pd composition and dispersion in directing charge carrier behavior and controlling product selectivity during methane oxidation.
Results and discussion
Morphological and physicochemical characterization
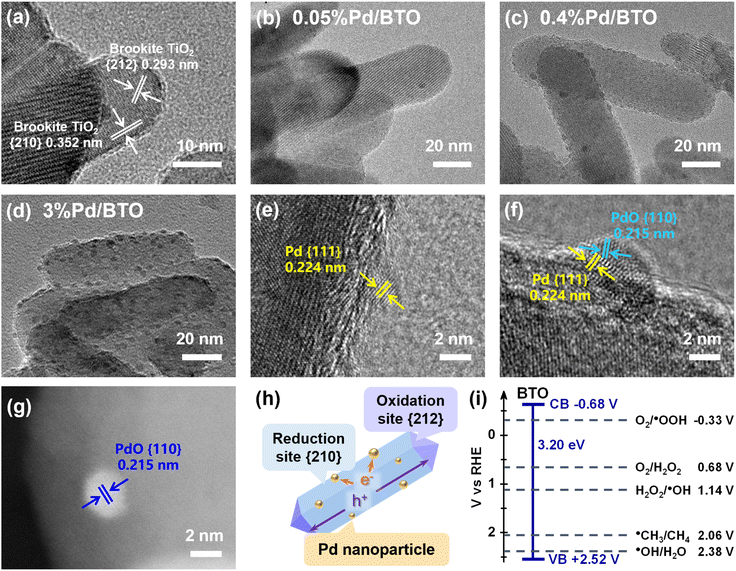
The BTO nanorods were synthesized using hydrothermal reactions with aqueous solutions containing TALH, sodium lactate and urea. Pd/BTO samples with different amount of Pd nanoparticles (0.05, 0.2, 0.4, 1 and 3 wt%) were photo-deposited on the BTO nanorod surface by a conventional photo-reduction technique. Inductively coupled plasma optical emission spectrometry (ICP-OES) showed that in all the Pd/BTO samples, the Pd precursor was loaded onto the BTO nanorod surface, with the nominal Pd loading corresponding to the actual Pd content in the samples (Table S1). These Pd/TiO2 samples are hereafter named as x%Pd/BTO, where x% is the weight percentage of Pd photo-deposited on the BTO nanorods. X-ray diffraction (XRD) patterns (Fig. S1) showed the as-prepared BTO and Pd/BTO nanorods exhibited only the diffraction peaks of brookite phase TiO2 (JCPDS no. 29-1360). No characteristic diffraction peaks associated with anatase or rutile phase TiO2 was observed indicating the purity of the BTO nanorods. The Raman spectrum of BTO also indicated the formation of a pure-phase brookite TiO2 (Fig. S2).38 The scanning electron microscopy (SEM) image of the BTO sample (Fig. S3) showed the BTO sample exhibited a nanorod morphology with an average length and width of 112 ± 17 nm and 31.5 ± 3.8 nm, respectively.Fig. 1a–f and S4 show the high-resolution transmission electron microscopy (HR-TEM) images of BTO and Pd/BTO with varying Pd loadings. HR-TEM imaging revealed that the BTO nanorods exhibited exposed {210} crystalline body facets, which were surrounded by {212} facets (Fig. 1a). Additionally, the HR-TEM images of Pd/BTO samples demonstrated that the Pd nanoparticles were selectively photo-deposited on the {210} facet of BTO. As the Pd concentration was increased from 0.05% to 3%, more Pd nanoparticles appeared on the {210} facet of BTO (Fig. 1b–d), indicating that the {210} facet of BTO acted as a reduction site. In contrast, previous research by Ohno et al. demonstrated that the {212} facets of BTO served as oxidation sites through the photo-deposition of PbO2.33 Lattice spacing imaging of the Pd nanoparticles on the BTO nanorods revealed that the deposition of three distinct types of Pd nanoparticles on the {210} facet of BTO. The first type of Pd nanoparticles consisted of pure metallic Pd0 nanoparticles, which were highly crystalline and exhibited a single lattice spacing of 0.224 nm, corresponding to the {111} planes of metallic Pd0 (Fig. 1e).45 The second type of Pd nanoparticles observed were metallic Pd0/PdO nanoparticles, which displayed two distinct sets of lattice spacings: one corresponding to metallic Pd0 with a {111} lattice spacing, and the other to PdO with a lattice spacing of 0.215 nm, aligning with the {110} planes of PdO (Fig. 1f).46 The third type of Pd particles identified were PdO {110} nanoparticles, as confirmed by the high-angle annular dark-field scanning transmission electron microscope (HAADF-STEM) image (Fig. 1g). Fig. 1h illustrates a schematic representation of the oxidation and reduction sites corresponding to the {212} and {210} facets of a BTO nanorod. In the typical mechanism of photo-deposition, photogenerated electrons are produced on the surface of a semiconductor (e.g., BTO) upon photoexcitation. Metal precursors such as Pd2+, are initially reduced on this surface, followed by further reduction of the metal-deposited layer. As a result, the core of the cocatalyst nanoparticles consists of highly reduced metal species (e.g., Pd0), while the exposed surface forms an oxide layer (e.g., PdO) due to electron transfer from the metal surface to the aqueous environment (water) during the photo-deposition process.47
Fig. S5 shows the Pd particle size and distribution for Pd/BTO with different Pd loadings. It is observed, on average, the size of the Pd nanoparticles increased marginally from 2.0 ± 0.8 nm to 3.0 ± 0.8 nm with increasing Pd loading. Specifically, the average diameters of the Pd nanoparticles for 0.05%Pd/BTO, 0.2%Pd/BTO, 0.4%Pd/BTO, 1%Pd/BTO and 3%Pd/BTO are 2.0 ± 0.8, 2.4 ± 0.6, 2.6 ± 0.7, 3.1 ± 0.7 and 3.0 ± 0.8 nm, respectively. HRTEM imaging revealed no evidence of particle agglomeration, even at higher Pd loadings. The Pd nanoparticles remained well-dispersed on the BTO nanorods with narrow size distributions, based on measurements of over 100 individual particles per sample. No large clusters or fused particles were observed, indicating that agglomeration did not occur during the photo-deposition process. Additional representative TEM images are provided in the SI to support this observation (Fig. S6–S8). Thus, as the Pd loading increased, the average particle size increased slightly, leading to a decrease in Pd dispersion due to a lower proportion of surface-exposed atoms relative to the total Pd content.
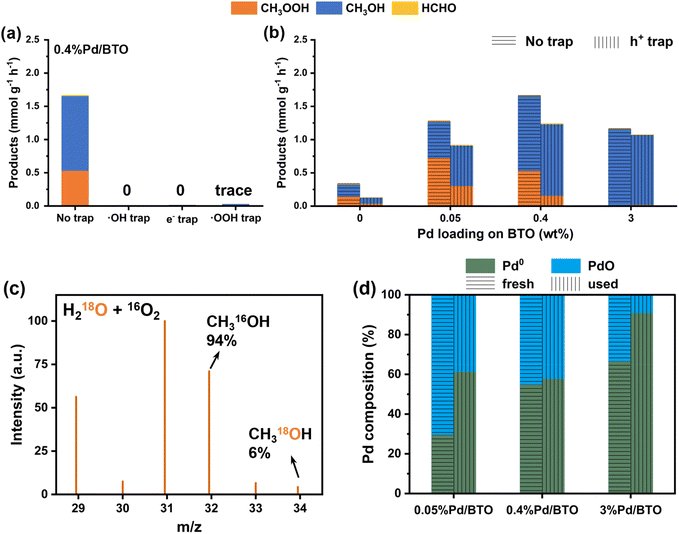
XPS was used to investigate the surface composition of BTO and Pd/BTO samples, as shown in Fig. S9. The fitted Ti 2p spectra revealed a Ti 2p3/2 at 458.5 eV, corresponding to Ti4+ (Fig. S9a).14,15 The fitted O 1s spectra primary exhibited the lattice oxygen (Olat) peak at 529.9 eV, with a minor concentration of O vacancies at 531.7 eV, suggesting that the BTO nanorods possessed high crystallinity (Fig. S9b).48 The fitted Pd 3d spectra displayed the two peaks for the Pd 3d5/2 band: one at 335.0 eV, attributed to metallic Pd0, and another at 336.5 eV, corresponding to PdO (Fig. S9c).49,50 The Pd 3d spectra did not show any evidence of PdCl2, the precursor used in the photo-deposition of Pd, which would exhibit a peak around 337.9 eV if present.51 The fractional compositions of Pd0 and PdO in the Pd/BTO samples were estimated based on the area ratios of Pd0 or PdO peaks to the total area of both the metallic Pd0 and PdO peaks. As shown in Fig. S9d, the concentration of metallic Pd0 in the 0.05%Pd/BTO sample was 29.6%, which increased to 54.9% and 66.7% for the 0.4%Pd/BTO and 3%Pd/BTO samples, respectively. The increase in concentration of metallic Pd0 suggests that the Pd nanoparticles on the BTO nanorod surface undergo greater reduction as the Pd dispersion increases.
Fig. S10 shows the UV-vis diffused reflectance spectra (UV-vis DRS) of BTO and Pd/BTO. The minimum absorbance at λ = ∼380 nm was attributed to the band gap charge transition from the O 2p state (valence band) to Ti 3d state (conduction band) of BTO. The Tauc plot of BTO showed an optical band gap (Eg) of 3.2 eV (Fig. S11a). Mott–Schottky analysis of the BTO sample at different measurement frequencies showed positive slopes with increasing potential, confirming an n-type characteristic (Fig. S11b). The flatband potential was estimated to be approximately −0.68 V. Assuming that the flatband potential corresponds to the conduction band potential for n-type semiconductors, the valence band potential of BTO with Eg of 3.20 eV was determined to be +2.52 V, which also coincided with the measured valence band potential obtained from XPS (Fig. S11c). The proposed band gap structure of BTO, along with the water oxidation reaction (WOR), oxygen reduction reaction (ORR), and methane oxidation reaction (MOR) redox potentials, is shown in Fig. 1i. The BTO nanorods displayed appropriate conduction and valence band potentials to effectively drive these reactions.
MOR performance
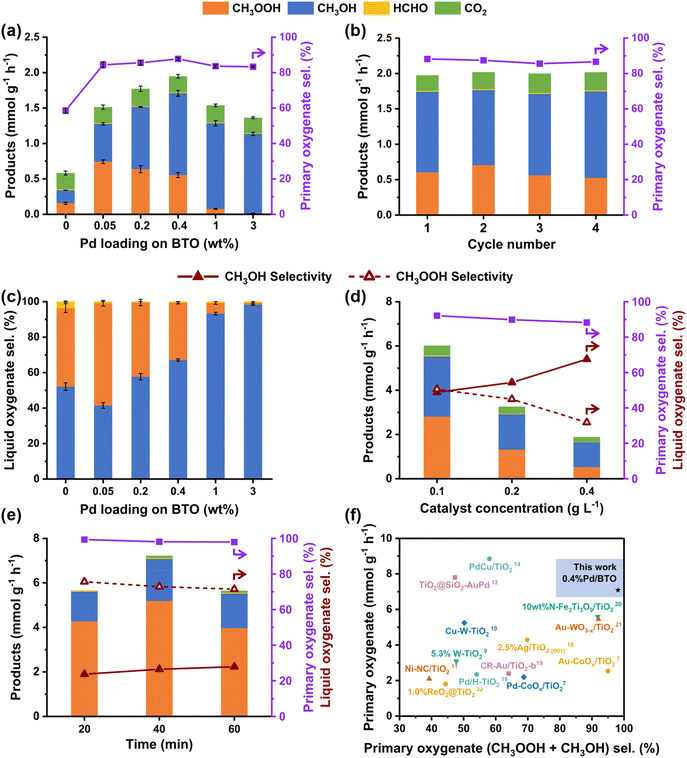
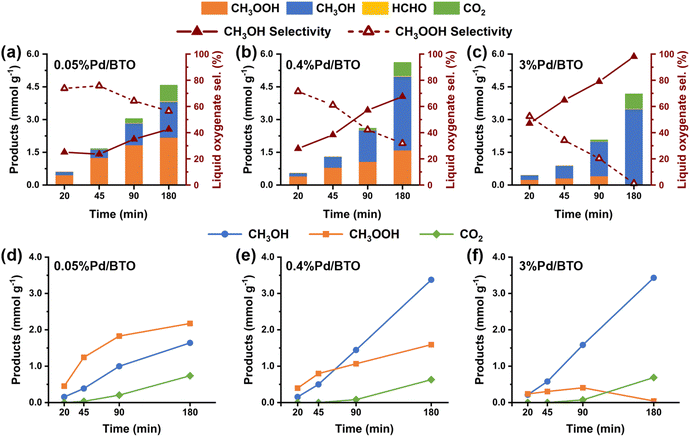
The as-prepared BTO and Pd/BTO were tested for photocatalytic oxidation of CH4 with O2. The photocatalytic MOR were conducted at room temperature under 19 bar CH4 and 1 bar O2 in a batch reactor with 50 mL of water and a catalyst concentration of 0.4 g L−1. The reactions were performed for 3 hours with a 300 W Xe lamp (wavelength λ = 320–780 nm) as the light source at a light intensity of 200 mW cm−2. In this study, CH3OH and CH3OOH are considered the primary desired oxygenates of MOR. Fig. 2a shows the MOR product yield and the selectivity for primary oxygenates on BTO and Pd/BTO. The MOR oxygenates detected were primarily CH3OOH and CH3OH with a trace amount of HCHO. Gaseous CO2 was also detected. Bare BTO exhibited the lowest MOR product yield. However, in the presence of Pd cocatalysts, the MOR product yield increased significantly, following a volcanic trend with higher Pd loading. 0.4%Pd/BTO exhibited the highest MOR productivity among the tested samples, achieving a rate of 1.95 mmol g−1 h−1. It also exhibited a high primary oxygenate selectivity of 87.8%, indicating excellent selectivity towards desired products. Furthermore, 0.4%Pd/BTO showed remarkable stability over repeated use, with negligible decreases in both productivity and selectivity after four consecutive reaction cycles (Fig. 2b), underscoring its potential practical and sustainable catalytic applications.Notably, the MOR experiments revealed a shift in the liquid oxygenates from CH3OOH to CH3OH with increased Pd loading. The CH3OH selectivity was 98% for 3%Pd/BTO, compared to 52%, 41%, 58%, 67% and 93% for bare BTO, 0.05%Pd/BTO, 0.2%Pd/BTO, 0.4%Pd/BTO and 1%Pd/BTO, respectively (Fig. 2c). Furthermore, control experiments in the absence of light, under similar CH4/O2 atmosphere and reaction conditions did not yield any products, confirming that the formation of MOR products is driven by a photocatalytic process (Fig. S12a and b). Control experiments in Ar/O2 atmosphere (19 bar Ar and 1 bar O2) did not yield any liquid oxygenates (Fig. S12c).
The catalyst concentration and reaction time of 0.4%Pd/BTO catalyst were further optimized to enhance MOR performance. The MOR product productivity of the catalyst increased threefold, from 1.95 mmol g−1 h−1 to 6.01 mmol g−1 h−1, when the catalyst concentration was reduced from 0.4 g L−1 to 0.1 g L−1, as shown in Fig. 2d. Additionally, the selectivity towards primary oxygenates improved from 87.8% to 92.1%, indicating a reduced extent of over oxidation to CO2. Moreover, the decrease in catalyst concentration led to a shift in the distribution of liquid oxygenates from CH3OH to CH3OOH. At a catalyst concentration of 0.4 g L−1, the selectivities for CH3OH and CH3OOH were 67.1% and 32.2%, respectively. When the catalyst concentration was reduced to 0.1 g L−1, the selectivities shifted to 48.8% for CH3OH and 50.5% for CH3OOH.
Subsequently, the reaction time was optimized. As shown in Fig. 2e, at an optimal reaction time of 40 min and catalyst concentration of 0.1 g L−1, the 0.4%Pd/BTO achieved an MOR productivity of 7.22 mmol g−1 h−1, along with a high primary oxygenate selectivity of 98.1%. Under these reaction conditions, the selectivities for CH3OOH and CH3OH were 72.8% and 26.5%, respectively. Furthermore, the apparent quantum efficiency (AQY) for primary oxygenates was 4.6% under photo-irradiation with a 365 nm LED at a light intensity of 12 mW cm−2 (Table S2). To better assess the photocatalytic performance of 0.4%Pd/BTO, the findings were compared with those of mixed anatase/rutile and pure anatase TiO2 catalysts reported in the literature. With a primary oxygenate selectivity of 98.1% and productivity of 7.22 mmol g−1 h−1, 0.4%Pd/BTO demonstrated superior selectivity and productivity for CH3OOH and CH3OH, outperforming most TiO2 catalysts (Fig. 2f and Table S3).
Elucidating the photocatalytic MOR mechanism on Pd/BTO nanorods
Earlier, TEM analysis revealed that increasing Pd loading on BTO nanorods decreased the dispersion of Pd particles. XPS analysis showed that Pd/BTO samples with higher Pd loading contained a higher fractional composition of metallic Pd0 compared to PdO. These observations, along with the results from the time-dependent MOR experiments, suggest that MOR activity and product selectivity are influenced by Pd composition and dispersion on the BTO nanorods.
˙CH3 formation:
| CH4 + h+ → ˙CH3 + H+ | (1) |
| CH4 + ˙OH → ˙CH3 + H+ | (2) |
Water oxidation reaction (WOR):
| H2O + h+ → ˙OH + H+ | (3) |
O2 reduction reaction (ORR):
| O2 + e− + H+ → *OOH (or ˙OOH) | (4) |
| *OOH + e− + H+ → *O + H2O | (5) |
| *O+ e− + H+ → *OH (or ˙OH) | (6) |
| *OOH + e− + H+ → H2O2 | (7) |
| O2 + 2 (e− + H+) → H2O2 | (8) |
| H2O2 + e− + H+→ *OH (or ˙OH) + H2O | (9) |
CH3OOH & CH3OH formation:
| ˙CH3 + ˙OOH → CH3OOH | (10) |
| ˙CH3 + ˙OH → CH3OH | (11) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and ∼12
000 cm−1 and ∼12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1.36,56 The peak at ∼24
000 cm−1.36,56 The peak at ∼24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 is attributed to photogenerated holes, while the peak at ∼12
000 cm−1 is attributed to photogenerated holes, while the peak at ∼12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 corresponds to trapped electrons.36,56 The effects of Pd dispersion on trapped electrons were further investigated by monitoring the decay of TA signal at 12
000 cm−1 corresponds to trapped electrons.36,56 The effects of Pd dispersion on trapped electrons were further investigated by monitoring the decay of TA signal at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1.
000 cm−1.
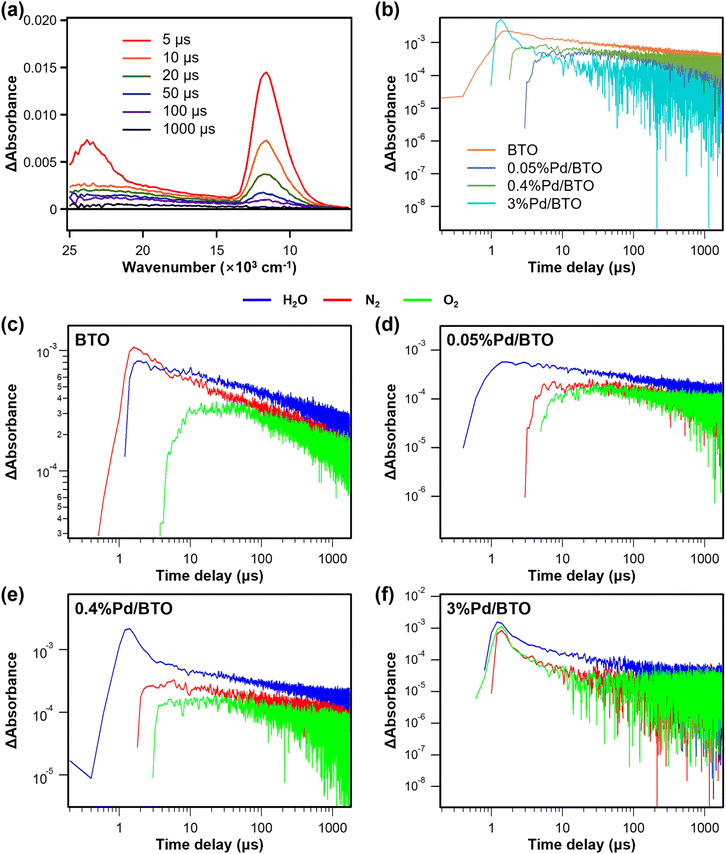
Fig. 5b shows the TA spectra of BTO and Pd/BTO measured at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 in vacuum. In the presence of Pd nanoparticles, the intensities of the TA signals from trapped electrons are lower than those of BTO, indicating that the population of trapped electrons in Pd-loaded BTO is reduced due to electron capture by the Pd cocatalysts. Moreover, the loading of Pd cocatalysts also led to accelerated electron decay. The delay in detecting trapped electrons in Pd-loaded BTO suggests rapid electron transfer from BTO to Pd (within a few μs). Among the Pd-loaded samples, 3%Pd/BTO exhibited the fastest electron trapping (∼1 μs), with a sharp increase in trapped electrons within 0.5 μs, followed by the fastest decay rate.
000 cm−1 in vacuum. In the presence of Pd nanoparticles, the intensities of the TA signals from trapped electrons are lower than those of BTO, indicating that the population of trapped electrons in Pd-loaded BTO is reduced due to electron capture by the Pd cocatalysts. Moreover, the loading of Pd cocatalysts also led to accelerated electron decay. The delay in detecting trapped electrons in Pd-loaded BTO suggests rapid electron transfer from BTO to Pd (within a few μs). Among the Pd-loaded samples, 3%Pd/BTO exhibited the fastest electron trapping (∼1 μs), with a sharp increase in trapped electrons within 0.5 μs, followed by the fastest decay rate.
TA spectra of BTO and Pd/BTO were also investigated in the presence and absence of reactant molecules (O2 or H2O) (Fig. 5c–f), with the TA measurement in N2, used as a reference. For BTO, the intensity of TA decreased upon exposure to O2, and electron decay occurred with a fast rate, indicating that adsorbed O2 on BTO surface rapidly consumed electrons and accelerated electron decay. For 0.4%Pd/BTO, the TA spectrum also showed reduced intensity and accelerated electron decay, indicating that electrons were readily consumed by O2 on its Pd cocatalysts. However, the TA spectra of 0.05%Pd/BTO and 3%Pd/BTO showed less significant reductions in TA upon exposure to O2, suggesting that O2 adsorption is less efficient, and electron trapping occurs in the Pd cocatalysts of these samples.
On the other hand, TA measurements with photocatalysts in the presence of H2O vapor reflect hole oxidation kinetics, as the consumption of photogenerated holes by H2O vapor prevents electron recombination and extends electron lifetime.56 For bare BTO, a relatively low enhancement in TA intensity was observed, indicating that charge separation efficiency in BTO is limited by poor hole oxidation kinetics. In contrast, for the Pd-loaded BTO samples, a significant increase in TA intensity was observed upon exposure to H2O vapor, suggesting that efficient electron capture by Pd cocatalysts simultaneously boosts hole oxidation kinetics.
In addition, we investigated the population of surviving electrons at a specific delay time – 10 μs after photoexcitation – as an indicator of the number of electrons that successfully evade recombination and remain available for redox reactions. To assess the reactivity of electrons and holes, we compared the relative electron populations under different atmospheric conditions: N2 (inert), O2 (electron scavenger), and H2O vapor (hole scavenger). The results are summarized in Tables S6 and S7. Under an O2 atmosphere, 0.05%Pd/BTO and 3%Pd/BTO exhibited higher relative electron population than BTO and 0.4%Pd/BTO, which is attributed to increased electron trapping. In the presence of H2O vapor, BTO catalysts with Pd cocatalysts exhibited significantly higher relative electron populations compared to bare BTO, indicating enhanced hole scavenging and improved charge separation dynamics due to the presence of Pd cocatalysts. Notably, 0.05%Pd/BTO and 3%Pd/BTO exhibited the highest relative electron population because electron trapping increase the lifetime of photogenerated electrons. Overall, the results from in situ time-resolved TA spectroscopy are in good agreement with the XPS findings, indicating more efficient photogenerated electron transfer between 0.4%Pd/BTO and O2, while significant electron trapping occurred in 0.05%Pd/BTO and 3%Pd/BTO.
We also calculated the Gibbs free energy barriers for *OOH and *OH formation on Pd0/BTO and PdO, as shown in Fig. 6c and d. The results show that the hydrogenation of *O2 to form *OOH is more favorable on the PdO catalyst (Ga = 0.70 eV) compared to the Pd0/BTO catalyst (Ga = 0.90 eV). Conversely, the hydrogenation of *O to form *OH is more favorable on the Pd0/BTO catalyst (Ga = 0.70 eV) than on PdO (Ga = 1.07 eV). These findings suggest that metallic Pd0 is more effective than oxidized PdO in facilitating the three-electron reduction of O2 to *OH while one-electron reduction of O2 to *OOH is more easily achieved on PdO.
In the experiments, we observed CH4 can be activated by ˙OH to form ˙CH3 radicals. Therefore, we further explored via DFT calculations, the reaction pathways for ˙CH3/*CH3 and ˙OH/*OH coupling to form CH3OH, as well as the possible pathway for ˙CH3/*CH3 and ˙OOH/*OOH coupling to form CH3OOH. As shown in Fig. 6e and f, when the Pd0 or PdO surface contains *OOH or *OH intermediates, the free ˙CH3 radical readily adsorbs onto the Pd site forming *CH3 on the catalyst surface in an exothermic reaction, with *CH3 being more easily adsorbed on Pd0/BTO than on PdO. The simultaneous adsorption of *CH3 and *OH on Pd sites induces a coupling reaction forming CH3OH, which is endothermic on Pd0/BTO but even more so on PdO (Fig. 6f). This suggests that CH3OH formation is more favorable on Pd0/BTO. On the other hand, the coupling reaction between *CH3 and *OOH on PdO is less endothermic than on Pd0/BTO indicating that CH3OOH formation is more favorable on PdO (Fig. 6e). The difference in the free energy required for CH3OOH and CH3OH formation on Pd0/BTO and PdO supports the experimental observations. In particular, 3%Pd/BTO, which exhibited a high surface concentration of metallic Pd0 due to in situ Pd reduction during MOR, predominantly produced CH3OH. In contrast, 0.05%Pd/BTO and 0.4%Pd/BTO, which contained a mixture of Pd0 and PdO on their surface, produced both CH3OH and CH3OOH.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
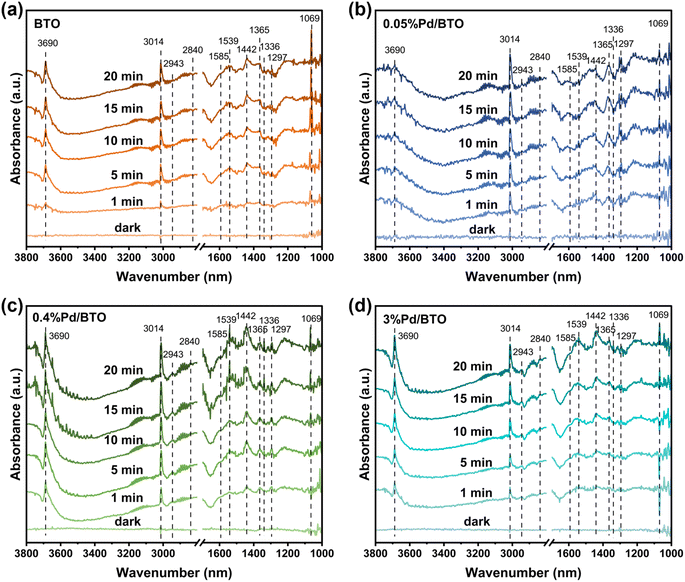
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Fig. 8a–d presents the in situ DRIFTS spectra of the BTO and Pd/BTO samples obtained under these conditions. The peaks at 3014 cm−1 and 1297 cm−1 are attributed to the C–H deformation vibration of CH4, while the peak at 1442 cm−1, assigned to adsorbed *CH3 indicates the dissociation of CH4.46 The intensities of these peaks increased with prolonged light irradiation time, suggesting the progressive activation of CH4. Similarly, characteristic peaks corresponding to CH3O* species were observed at 2943 cm−1, 2840 cm−1, and 1069 cm−1,57 confirming the formation of CH3OH. Moreover, the peak at 3690 cm−1 associated with adsorbed *OH, the key reaction intermediate, were more intense on 0.4%Pd/BTO and 3%Pd/BTO compared to 0.05%Pd/BTO, indicating a higher coverage of *OH on 0.4%Pd/BTO and 3%Pd/BTO, which aids the formation of CH3OH.58
1). Fig. 8a–d presents the in situ DRIFTS spectra of the BTO and Pd/BTO samples obtained under these conditions. The peaks at 3014 cm−1 and 1297 cm−1 are attributed to the C–H deformation vibration of CH4, while the peak at 1442 cm−1, assigned to adsorbed *CH3 indicates the dissociation of CH4.46 The intensities of these peaks increased with prolonged light irradiation time, suggesting the progressive activation of CH4. Similarly, characteristic peaks corresponding to CH3O* species were observed at 2943 cm−1, 2840 cm−1, and 1069 cm−1,57 confirming the formation of CH3OH. Moreover, the peak at 3690 cm−1 associated with adsorbed *OH, the key reaction intermediate, were more intense on 0.4%Pd/BTO and 3%Pd/BTO compared to 0.05%Pd/BTO, indicating a higher coverage of *OH on 0.4%Pd/BTO and 3%Pd/BTO, which aids the formation of CH3OH.58
On the other hand, peaks at 1365 cm−1 and 1336 cm−1, attributed to the vibrations of adsorbed CH3OOH species were observed across all samples.59 Notably, 0.05%Pd/BTO exhibited the highest intensity for these peaks, reflecting more favorable formation of CH3OOH on its surfaces, consistent with its higher CH3OOH selectivity in MOR. Additionally, peaks at 1585 cm−1 and 1539 cm−1 can be assigned to *HCOO species, indicative of overoxidation byproducts.60 The results are consistent with the proposed mechanism that Pd0-rich BTO catalyst facilitates a direct three-electron reduction of O2, leading to the formation of *OH species, which can then react with *CH3 to produce CH3OH. In contrast, PdO-rich catalyst, favors a one-electron O2 reduction pathway, generating *OOH species that couple with *CH3 to form CH3OOH. This mechanistic divergence accounts for the different product distributions observed among the catalysts.
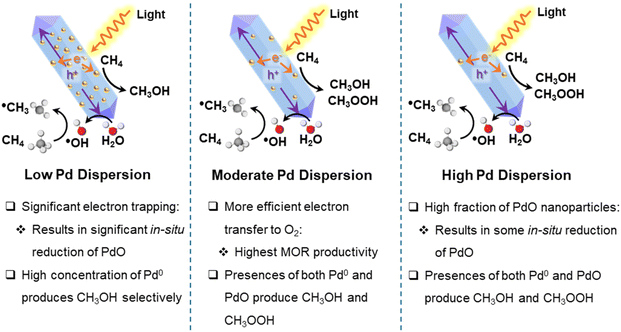 | ||
| Scheme 1 Pd dispersion and composition on BTO nanorods regulate the activity and selectivity of photocatalytic MOR products. | ||
For 0.4%Pd/BTO, with an optimal Pd particle loading and dispersion, electron transfer to O2 is more efficient, yielding the highest MOR product output with minimal in situ reduction of PdO, as supported by the XPS and TA spectroscopy. However, due to the co-existence of both Pd0 and PdO active sites in 0.05%Pd/BTO and 0.4%Pd/BTO, these catalysts produce both CH3OOH and CH3OH. DFT calculations and the experimental observations indicate that Pd0 facilitates the direct three-electron reduction of O2 to form *OH or ˙OH, which then reacts with *CH3 or ˙CH3 to produce CH3OH. In contrast, PdO favors one-electron reduction of oxygen and the formation of CH3OOH by coupling of *CH3 or ˙CH3 with *OOH or ˙OOH.
Conclusion
In conclusion, the study highlights the crucial role of Pd composition and dispersion in enhancing the photocatalytic oxidation of methane to primary oxygenates – CH3OH and CH3OOH over BTO nanorods. Pd/BTO nanorods with higher Pd loading and lower Pd dispersion, enhanced electron trapping in the Pd0/PdO nanoparticles and promoted the self-reduction of PdO to metallic Pd0 upon light irradiation. The higher surface concentration of Pd0 resulted in selective CH3OH production. Conversely, BTO with optimum Pd loading and dispersion promoted electron transfer to O2; however, the presence of both Pd0 and PdO, led to the formation of both CH3OH and CH3OOH. DFT calculations together with experimental findings show that Pd0 enables the direct three-electron reduction of O2 to form *OH or ˙OH, which then reacts with *CH3 or ˙CH3 to produce CH3OH. In contrast, PdO favors one-electron reduction of oxygen and the formation of CH3OOH by coupling of *CH3 or ˙CH3 with *OOH or ˙OOH. The distinct reaction pathways provide valuable insights into optimizing Pd-based photocatalysts for selective methane oxidation, highlighting the importance of Pd composition and dispersion in influencing charge carrier dynamics and determining the selectivity of products in methane oxidation.Author contributions
Jiawei Wu: investigation, formal analysis, methodology, validation, visualization. Hu Ding: methodology, formal analysis, validation, visualization. Yan Wang: formal analysis, validation. Ulfi Muliane: investigation, methodology, validation. Shuji Anabuki: investigation, methodology, validation. Naoya Murakami: validation. Xinli Zhu: resources, methodology, validation. Akira Yamakata: resources, methodology, validation. Teruhisa Ohno: validation. Shi Nee Lou: conceptualization, methodology, validation, funding acquisition, data curation, project administration, resources, supervision, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its SI. Supplementary information is available. See DOI: https://doi.org/10.1039/d5ta04478b.Acknowledgements
This work was supported by National Nature Science Foundation of China (grant no. 22250410262), Tianjin Natural Science and Technology Project (grant no. 22JCYBJC01410) and National Key Research and Development Program of China (grant no. 2022YFB4101702).References
- M. Ravi, M. Ranocchiari and J. A. van Bokhoven, Angew. Chem., Int. Ed., 2017, 56, 16464–16483 CrossRef CAS
.
- P. Schwach, X. Pan and X. Bao, Chem. Rev., 2017, 117, 8497–8520 CrossRef CAS PubMed
.
- A. I. Olivos-Suarez, À. Szécsényi, E. J. M. Hensen, J. Ruiz-Martinez, E. A. Pidko and J. Gascon, ACS Catal., 2016, 6, 2965–2981 CrossRef CAS
.
- P. Tang, Q. Zhu, Z. Wu and D. Ma, Energy Environ. Sci., 2014, 7, 2580–2591 RSC
.
- N. Agarwal, S. J. Freakley, R. U. McVicker, S. M. Althahban, N. Dimitratos, Q. He, D. J. Morgan, R. L. Jenkins, D. J. Willock, S. H. Taylor, C. J. Kiely and G. J. Hutchings, Science, 2017, 358, 223–227 CrossRef CAS PubMed
.
- H. Ceri, M. F. Michael, H. A. R. Mohd, T. Adam, H. Qian, D. Nikolaos, K. Joo and H. Henk, Angew. Chem., Int. Ed., 2012, 51, 5129–5133 CrossRef PubMed
.
- H. Song, X. Meng, S. Wang, W. Zhou, S. Song, T. Kako and J. Ye, ACS Catal., 2020, 10, 14318–14326 CrossRef CAS
.
- Y. Jiang, W. Zhao, S. Li, S. Wang, Y. Fan, F. Wang, X. Qiu, Y. Zhu, Y. Zhang, C. Long and Z. Tang, J. Am. Chem. Soc., 2022, 144, 15977–15987 CrossRef CAS PubMed
.
- M. Huang, S. Zhang, B. Wu, X. Yu, Y. Gan, T. Lin, F. Yu, Y. Sun and L. Zhong, ChemSusChem, 2022, 15, e202200548 CrossRef CAS
.
- M. Huang, S. Zhang, B. Wu, Y. Wei, X. Yu, Y. Gan, T. Lin, F. Yu, F. Sun, Z. Jiang and L. Zhong, ACS Catal., 2022, 12, 9515–9525 CrossRef CAS
.
- H. Song, H. Huang, X. Meng, Q. Wang, H. Hu, S. Wang, H. Zhang, W. Jewasuwan, N. Fukata, N. Feng and J. Ye, Angew. Chem., Int. Ed., 2023, 62, e202215057 CrossRef CAS
.
- C. Xie, E. Sun, G. Wan, J. Zheng, R. Gupta and A. Majumdar, Nano Lett., 2023, 23, 2039–2045 CrossRef CAS
.
- R. Zhang, J. Shi, L. Fu, Y.-G. Liu, Y. Jia, Z. Han, K. Yuan and H.-Y. Jiang, ACS Nano, 2024, 18, 12994–13005 CrossRef CAS
.
- Y. Li, K. Sun, S. Ning, P. Qiao, S. Wang, Z.-j. Wang, L. Zhu, X. Zhang, K. Peng, X.-s. Wang, D. Wang, L. Liu, H. Song and J. Ye, J. Catal., 2024, 440, 115840 CrossRef CAS
.
- N. Feng, H. Lin, H. Song, L. Yang, D. Tang, F. Deng and J. Ye, Nat. Commun., 2021, 12, 4652 CrossRef CAS
.
- P.-P. Luo, X.-K. Zhou, Y. Li and T.-B. Lu, ACS Appl. Mater. Interfaces, 2022, 14, 21069–21078 CrossRef CAS
.
- X.-K. Zhou, Y. Li, P.-P. Luo and T.-B. Lu, ACS Appl. Mater. Interfaces, 2023, 15, 36280–36288 CrossRef CAS
.
- X. Zhang, Y. Wang, K. Chang, S. Yang, H. Liu, Q. Chen, Z. Xie and Q. Kuang, Appl. Catal., B, 2023, 320, 121961 CrossRef CAS
.
- K. Chang, X. Zhang, Y. Hua, Z. Wang, Z. Song, Y. Wang, Q. Chen, W. Yan, Q. Kuang and Z. Xie, ACS Appl. Nano Mater., 2024, 7, 21453–21462 CrossRef CAS
.
- F. Si, M. Lv, X. Cai, Y. Li, M. Zhao, T. Hou and Y. Li, Appl. Catal., B, 2024, 358, 124417 CrossRef CAS
.
- F. Si, G. Wang, M. Lv, J. Bai, Y. Li, T. Hou and Y. Li, Appl. Catal., B, 2025, 365, 124934 CrossRef CAS
.
- L. Yan, S. Wang, C. Qian and S. Zhou, J. Mater. Chem. A, 2025, 13, 4662–4672 RSC
.
- H. Song, X. Meng, S. Wang, W. Zhou, X. Wang, T. Kako and J. Ye, J. Am. Chem. Soc., 2019, 141, 20507–20515 CrossRef CAS PubMed
.
- W. Zhou, X. Qiu, Y. Jiang, Y. Fan, S. Wei, D. Han, L. Niu and Z. Tang, J. Mater. Chem. A, 2020, 8, 13277–13284 RSC
.
- W. Zhu, M. Shen, G. Fan, A. Yang, J. R. Meyer, Y. Ou, B. Yin, J. Fortner, M. Foston, Z. Li, Z. Zou and B. Sadtler, ACS Appl. Nano Mater., 2018, 1, 6683–6691 CrossRef CAS
.
- Y. Fan, W. Zhou, X. Qiu, H. Li, Y. Jiang, Z. Sun, D. Han, L. Niu and Z. Tang, Nat. Sustain., 2021, 4, 509–515 CrossRef
.
- K. Wang, L. Luo, C. Wang and J. Tang, Chin. J. Catal., 2023, 46, 103–112 CrossRef CAS
.
- Y. Fan, Y. Jiang, H. Lin, J. Li, Y. Xie, A. Chen, S. Li, D. Han, L. Niu and Z. Tang, Nat. Commun., 2024, 15, 4679 CrossRef CAS PubMed
.
- L. Luo, L. Fu, H. Liu, Y. Xu, J. Xing, C.-R. Chang, D.-Y. Yang and J. Tang, Nat. Commun., 2022, 13, 2930 CrossRef CAS
.
- Y. Jiang, S. Li, S. Wang, Y. Zhang, C. Long, J. Xie, X. Fan, W. Zhao, P. Xu, Y. Fan, C. Cui and Z. Tang, J. Am. Chem. Soc., 2023, 145, 2698–2707 CrossRef CAS PubMed
.
- K.-i. Katsumata, Y. Ohno, K. Tomita, T. Taniguchi, N. Matsushita and K. Okada, ACS Appl. Mater. Interfaces, 2012, 4, 4846–4852 CrossRef CAS PubMed
.
- H. Lin, L. Li, M. Zhao, X. Huang, X. Chen, G. Li and R. Yu, J. Am. Chem. Soc., 2012, 134, 8328–8331 CrossRef CAS PubMed
.
- T. Ohno, T. Higo, N. Murakami, H. Saito, Q. Zhang, Y. Yang and T. Tsubota, Appl. Catal., B, 2014, 152–153, 309–316 CrossRef CAS
.
- T. Ohno, T. Higo, H. Saito, S. Yuajn, Z. Jin, Y. Yang and T. Tsubota, J. Mol. Catal. A: Chem., 2015, 396, 261–267 CrossRef CAS
.
- A. Naldoni, T. Montini, F. Malara, M. M. Mróz, A. Beltram, T. Virgili, C. L. Boldrini, M. Marelli, I. Romero-Ocaña, J. J. Delgado, V. Dal Santo and P. Fornasiero, ACS Catal., 2017, 7, 1270–1278 CrossRef CAS
.
- J. J. M. Vequizo, H. Matsunaga, T. Ishiku, S. Kamimura, T. Ohno and A. Yamakata, ACS Catal., 2017, 7, 2644–2651 CrossRef CAS
.
- Y. Ma, K. Kobayashi, Y. Cao and T. Ohno, Appl. Catal., B, 2019, 245, 681–690 CrossRef CAS
.
- Y. Cao, S. N. Lou, S. Wang, H. Yang, Q. Zhang, C. Wang, N. Murakami and T. Ohno, Appl. Catal., A, 2022, 634, 118539 CrossRef CAS
.
- M. Kamran, T. A. Kandiel, S. Abdel-Azeim, M. A. Morsy and D. W. Bahnemann, J. Phys. Chem. C, 2023, 127, 7707–7717 CrossRef CAS
.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed
.
- K. Fuku, R. Takioka, K. Iwamura, M. Todoroki, K. Sayama and N. Ikenaga, Appl. Catal., B, 2020, 272, 119003 CrossRef CAS
.
- P. D. Cozzoli, R. Comparelli, E. Fanizza, M. L. Curri, A. Agostiano and D. Laub, J. Am. Chem. Soc., 2004, 126, 3868–3879 CrossRef CAS
.
- J. Ohyama, A. Yamamoto, K. Teramura, T. Shishido and T. Tanaka, ACS Catal., 2011, 1, 187–192 CrossRef CAS
.
- Z. H. N. Al-Azri, M. AlOufi, A. Chan, G. I. N. Waterhouse and H. Idriss, ACS Catal., 2019, 9, 3946–3958 CrossRef CAS
.
- J. Yang, H. Zhou, J. Luo and M. Wang, ACS Catal., 2025, 15, 1663–1671 CrossRef CAS
.
- W. Zhang, D. Xi, Y. Chen, A. Chen, Y. Jiang, H. Liu, Z. Zhou, H. Zhang, Z. Liu, R. Long and Y. Xiong, Nat. Commun., 2023, 14, 3047 CrossRef CAS PubMed
.
- O. Tomita, T. Otsubo, M. Higashi, B. Ohtani and R. Abe, ACS Catal., 2016, 6, 1134–1144 CrossRef CAS
.
- X. Wu, Q. Zhang, W. Li, B. Qiao, D. Ma and S. L. Wang, ACS Catal., 2021, 11, 14038–14046 CrossRef CAS
.
- T. Liu, Z. Pan, J. J. M. Vequizo, K. Kato, B. Wu, A. Yamakata, K. Katayama, B. Chen, C. Chu and K. Domen, Nat. Commun., 2022, 13, 1034 CrossRef CAS PubMed
.
- H. Zhang, P. Sun, X. Fei, X. Wu, Z. Huang, W. Zhong, Q. Gong, Y. Zheng, Q. Zhang, S. Xie, G. Fu and Y. Wang, Nat. Commun., 2024, 15, 4453 CrossRef CAS PubMed
.
- Y. Yu, T. He, L. Guo, Y. Yang, L. Guo, Y. Tang and Y. Cao, Sci. Rep., 2015, 5, 9561 CrossRef CAS PubMed
.
- Y. Nosaka and A. Y. Nosaka, Chem. Rev., 2017, 117, 11302–11336 CrossRef CAS PubMed
.
- A. Kulkarni, S. Siahrostami, A. Patel and J. K. Nørskov, Chem. Rev., 2018, 118, 2302–2312 CrossRef CAS PubMed
.
- S. N. Lou, J. Lim, T. H. Jeon and W. Choi, ACS ES&T Eng., 2022, 2, 1116–1129 Search PubMed
.
- Q. Zhou, X. Tan, X. Wang, Q. Zhang, C. Qi, H. Yang, Z. He, T. Xing, M. Wang, M. Wu and W. Wu, ACS Catal., 2024, 14, 955–964 CrossRef CAS
.
- A. Yamakata, M. Kawaguchi, N. Nishimura, T. Minegishi, J. Kubota and K. Domen, J. Phys. Chem. C, 2014, 118, 23897–23906 CrossRef CAS
.
- J. Wang, G. Li, Z. Li, C. Tang, Z. Feng, H. An, H. Liu, T. Liu and C. Li, Sci. Adv., 2017, 3, e1701290 CrossRef PubMed
.
- H. Gong, L. Zhang, C. Deng, M. Liu, X. Liu, Y. Huang, K. Zhou, P. He, J. Li, Y. Yang, L. Wang, Q. Yang, Z. Bao, Q. Ren, T. Tan, S. Yao and Z. Zhang, J. Am. Chem. Soc., 2025, 147, 9134–9146 CrossRef CAS PubMed
.
- Y. Xu, C. Wang, X. Li, L. Xiong, T. Zhang, L. Zhang, Q. Zhang, L. Gu, Y. Lan and J. Tang, Nat. Sustain., 2024, 7, 1171–1181 CrossRef
.
- Z. Xiao, J. Shen, J. Jiang, J. Zhang, S. Liang, S. Han, J. Long, W. Dai, Y. Li, X. Wang, H. Xi, X. Fu and Z. Zhang, Adv. Funct. Mater., 2025, 35, 2422726 CrossRef CAS
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |