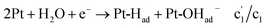Gradient-wettability oxide-Pt/C electrocatalysts for stable seawater hydrogen evolution via superaerophobicity and surface acidity
Jing-Fang
Huang
 *,
Jung-Hung
Huang
and
Che-Jung
Hsu
*,
Jung-Hung
Huang
and
Che-Jung
Hsu
Department of Chemistry, National Chung Hsing University, Taichung 402, Taiwan, Republic of China. E-mail: jfh@dragon.nchu.edu.tw
First published on 22nd September 2025
Abstract
This study reports the successful development of a series of surface gradient wettability electrocatalysts, Pt5/C/oxide-50, by integrating hydrophilic oxides (SiO2, Al2O3, CeO2, and TiO2) with hydrophobic carbon-supported Pt (Pt/C) without the need for sophisticated surface microstructuring. The hybrid composites exhibited exceptional superaerophobicity, which facilitated the rapid detachment of H2 bubbles from the Pt active sites, effectively preventing blockage, as confirmed by the H2 oxidation signals in cyclic voltammograms and water droplet contact-angle measurements. Unlike conventional Pt/C, which suffers from alkaline poisoning during seawater electrolysis, Pt5/C/oxide-50 mitigates this issue by introducing surface acidic sites via oxides. Linear sweep voltammetry was employed to specifically monitor the oxidation signal associated with OH− adsorption on Pt (Pt-OHad−) during the hydrogen evolution reaction (HER), revealing that the incorporation of oxides effectively suppresses the formation of Pt-OHad−. This work demonstrates a facile and scalable approach that combines microwettability modulation with surface acid site engineering to enhance both the durability of the HER and the gas-repelling performance, enabling efficient direct seawater splitting for H2 production.
Introduction
Direct seawater electrolysis provides a sustainable route for H2 production without freshwater consumption.1,2 However, the complex ionic environment of natural seawater (e.g., Mg2+, Ca2+, Cl−, and SO42−) poses significant challenges, including catalyst deactivation, electrode scaling, and local alkalization (Scheme 1).3–5 During the hydrogen evolution reaction (HER), the local pH near the cathode often exceeds 9.5, leading to the in situ precipitation of Mg(OH)2 and Ca(OH)2, which block the active sites and rapidly degrade the performance.3–7 Additionally, poor H2 bubble detachment further limits the catalytic activity by dynamically obstructing mass transfer and surface accessibility.8–14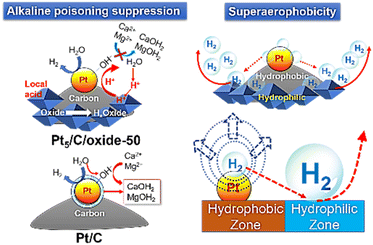 | ||
| Scheme 1 Insoluble precipitate poisoning of Pt/C, alkaline poisoning suppression of Pt5/C/oxide-50, and superaerophobicity of Pt5/C/oxide-50 from the gradient wettability surface. | ||
Although alkali-assisted pretreatment and Lewis-acidic coatings (e.g., Cr2O3 and V2O3) have been explored to reduce precipitation, their long-term stability remains limited.7,15,16 Creating an acid-like microenvironment at the electrode surface has recently emerged as a promising route to improve the HER selectivity and stability in untreated seawater.6,7 Although carbon-supported Pt (Pt/C) is a benchmark HER catalyst,17–20 it is highly susceptible to alkaline poisoning during seawater electrolysis. Replacing carbon supports with oxide materials enriched in surface acidic sites offers a solution, as these oxides can buffer the local pH by reversibly storing and releasing protons.21,22 However, their poor conductivities can compromise their catalytic performances.
To address these issues, we report a series of gradient wettability electrocatalysts (Pt5/C/oxide-50) that integrate hydrophilic oxides (SiO2, Al2O3, CeO2, and TiO2) with hydrophobic Pt/C. Without relying on elaborate surface microstructuring, these hybrid composites exhibited superaerophobic behavior that enabled rapid H2 bubble detachment (Scheme 1), as confirmed by H2 oxidation signals in the cyclic voltammograms (CVs) and water droplet contact-angle (θw) measurements. More importantly, the oxide components serve as surface-acidic reservoirs that buffer the local alkalinity and suppress the formation of poisoning Pt-OHad− species,6 as tracked by linear sweep voltammetry. This dual-function design, which combines interfacial wettability control and local microenvironment engineering, offers a scalable and effective strategy to overcome the major bottlenecks in direct seawater electrolysis and achieve stable and efficient HER performance under realistic saline conditions.
Results and discussion
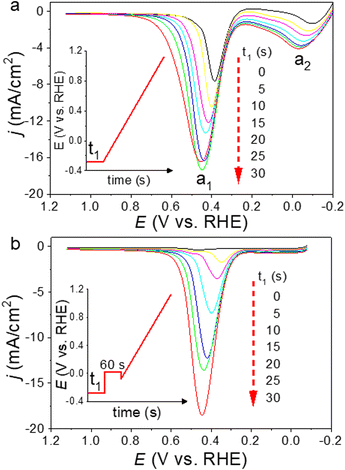
CVs of the Pt/C catalysts (Ptm/C, where m denotes the Pt wt%) were measured in a 0.5 M NaCl aqueous solution (NaClaq, pH 8.3) to simulate seawater without Ca2+ and Mg2+ (Fig. 1). For Pt5/C, two pairs of redox waves (c1/a1 and c2/a2) were observed that were absent in pure carbon. H2 bubbles appeared only at potentials more negative than −0.1 V, indicating that c2 is associated with the HER. Holding the potential at 0.0 V for varying periods (td) in the reverse cyclic voltammetry scan causes the disappearance of H2 bubbles from c2, but no change in a1 (Fig. 1b). This indicates that H2 oxidation only occurs at a2. These redox signals originated from water electrolysis because they were independent of the electrolyte type (Fig. S1). CVs were recorded between 1.1 and 0.0 V, 1.1 and 0.4 V, and 1.1 and 0.43 V, paused at reversed potentials for varying periods, and then restored to 1.1 V (Fig. 1c). The value of a1 remained constant after the oxidation of the surface-saturated adsorbed species.23,24 In water splitting, c1 is the first step (Volmer step), generating adsorbed hydrogen (Pt-Had) and hydroxide (Pt-OHad−) in neutral aqueous solutions. A smaller pair of shoulder waves,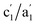 , appeared before c1/a1, corresponding to hydrogen underpotential deposition (Hupd) and oxidation (Hupd-o). Although a1 is derived from the surface-saturated adsorbed species, lowering the reverse potential to a negative value leads to a significant increase in adsorbed species. Additional HCl titration experiments confirmed that a1 originated from an alkaline species (Pt-OHad−) because a1 decreased with decreasing pH (Fig. 1d). Based on the above observations, the overall mechanism of direct water electrolysis in NaClaq can be summarized as follows:
, appeared before c1/a1, corresponding to hydrogen underpotential deposition (Hupd) and oxidation (Hupd-o). Although a1 is derived from the surface-saturated adsorbed species, lowering the reverse potential to a negative value leads to a significant increase in adsorbed species. Additional HCl titration experiments confirmed that a1 originated from an alkaline species (Pt-OHad−) because a1 decreased with decreasing pH (Fig. 1d). Based on the above observations, the overall mechanism of direct water electrolysis in NaClaq can be summarized as follows:| Pt-Had + Pt-OHad− + H2O + e− → Pt + Pt-OHad− + H2 + OH− c2 |
| Pt + Pt-OHad− + H2 + OH− → Pt + Pt-OHad− + 2H+ + 2e− + OH− a2 |
| Pt-OHad− → Pt-Oad− + H+ + e− a1 |
Pt-Had and Pt-OHad− form at 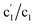 . When the potential reaches ∼−0.1 V, the HER proceeds via the Heyrovsky step, producing H2. A related Tafel slope of ∼105.9 mV dec−1 (Fig. S2) indicates that the Volmer–Heyrovsky step is the HER rate-determining step.17,19,20,24,25 In addition to Pt-OHad−, more OH− is produced during the HER. As a result, a2 (H2 oxidation) increases, and concurrently, a1 associated with the oxidative desorption of H+ from Pt-OHad− greatly intensifies. This mechanistic insight indicates that direct electrolysis in neutral seawater is not only challenged by the local pH increase but also by the formation of Pt-OHad−. A decrease in pH effectively suppresses OH− formation, mitigating this deactivation pathway. However, adjusting the pH of the solution only mitigated the soluble OH− generated during the HER. The Pt-OHad− formed in the initial Volmer step was unaffected by changes in solution pH (Fig. S3). In real seawater environments, cations such as Ca2+ and Mg2+ further interact with Pt-OHad−, leading to the poisoning of insoluble precipitates (Scheme 1). Linear scan voltammograms (LSVs) were used to monitor the generation of Pt-OHad− (the anodic peak a1) (Fig. 2a). To eliminate interference from a2, a preconditioning step at 0.0 V for 60 s was applied to remove the H2 bubbles before the potential scan (p-LSV) (Fig. 2b). LSVs and p-LSVs were recorded after varying durations of the HER at −0.28 V. In the p-LSVs, a2 was effectively suppressed by the preconditioning step, allowing the distinguishable growth of peak a1 with increasing Pt-OHad−. CVs and p-LSVs were further employed to assess H2 bubble accumulation and the effects of local surface pH elevation to evaluate the rationally designed electrocatalysts for mitigating these detrimental influences.
. When the potential reaches ∼−0.1 V, the HER proceeds via the Heyrovsky step, producing H2. A related Tafel slope of ∼105.9 mV dec−1 (Fig. S2) indicates that the Volmer–Heyrovsky step is the HER rate-determining step.17,19,20,24,25 In addition to Pt-OHad−, more OH− is produced during the HER. As a result, a2 (H2 oxidation) increases, and concurrently, a1 associated with the oxidative desorption of H+ from Pt-OHad− greatly intensifies. This mechanistic insight indicates that direct electrolysis in neutral seawater is not only challenged by the local pH increase but also by the formation of Pt-OHad−. A decrease in pH effectively suppresses OH− formation, mitigating this deactivation pathway. However, adjusting the pH of the solution only mitigated the soluble OH− generated during the HER. The Pt-OHad− formed in the initial Volmer step was unaffected by changes in solution pH (Fig. S3). In real seawater environments, cations such as Ca2+ and Mg2+ further interact with Pt-OHad−, leading to the poisoning of insoluble precipitates (Scheme 1). Linear scan voltammograms (LSVs) were used to monitor the generation of Pt-OHad− (the anodic peak a1) (Fig. 2a). To eliminate interference from a2, a preconditioning step at 0.0 V for 60 s was applied to remove the H2 bubbles before the potential scan (p-LSV) (Fig. 2b). LSVs and p-LSVs were recorded after varying durations of the HER at −0.28 V. In the p-LSVs, a2 was effectively suppressed by the preconditioning step, allowing the distinguishable growth of peak a1 with increasing Pt-OHad−. CVs and p-LSVs were further employed to assess H2 bubble accumulation and the effects of local surface pH elevation to evaluate the rationally designed electrocatalysts for mitigating these detrimental influences.
Various oxides (SiO2, Al2O3, CeO2, and TiO2) were mixed with Pt20/C at different weight ratios via grinding to obtain hybrid composites. To compare the HER activities, the composites were normalized to 5 wt% Pt by the addition of C (denoted as Pt5/C/oxide-x, where x = oxide wt%). The TEM images of Pt20/C and Pt5/C/oxide-50 are shown in Fig. S4. The TEM image of Pt20/C revealed a uniform dispersion of Pt nanoparticles (nPts) with sizes ranging from 5 to 8 nm. The images of Pt5/C/oxide-50 showed not only a reduced nPt content but also nPts surrounded by oxides (Fig. 3). The energy-dispersive spectroscopy (EDS) elemental mapping images of Pt5/C/oxide-50 are presented in Fig. 3 and S5–S8. Taking Pt5/C/SiO2-50 as an example, the microstructure of the hybrid composites featured hydrophilic SiO2 domains surrounding the hydrophobic Pt/C regions. The θw value of Pt/C was 139.45° (Fig. 3), indicating strong hydrophobicity, whereas that of SiO2 was 47.14° (Fig. S9), reflecting its hydrophilic nature. The θw of Pt5/C/SiO2-50 was 63.2°, which falls between those of Pt/C and SiO2 (Fig. 3). These results, along with microscopic imaging, confirmed the formation of a gradient wettability surface on Pt5/C/SiO2-50. All Pt5/C/oxide-50 composites exhibited similar microstructures (Fig. 3 and S9). X-ray diffraction and X-ray photoelectron spectroscopy analyses of Pt5/C/oxide-50 revealed that the crystalline structure and binding energies of Pt remained unchanged upon oxide addition (Fig. S10 and S11). These results confirm that the oxides act solely as physically blended components, modifying microwettability while preserving the intrinsic electronic properties of Pt.
The CVs of Pt5/C/oxide-x in NaClaq showed unchanged c1 and c2 potentials but increased c1 current with higher oxide content (Fig. 4). Given that oxides are inert toward the HER and have low conductivity, the c1 peak current enhancement can be attributed to the improved hydrophilicity of the catalysts, which promotes water accessibility and facilitates the Volmer step on Pt. Furthermore, the slope of the HER current at c2 increases with oxide incorporation, and the corresponding Tafel slope decreases from 105 mV dec−1 to ∼92–82 mV dec−1 (Fig. S2). In electrochemical impedance spectroscopy (EIS) at an overpotential of 0.24 V, the charge-transfer resistance (Rct) decreased from 26.7 Ω cm2 (Pt/C) to 16.5 Ω cm2 (Pt5/C/oxide-50), with consistent trends across all oxide types (Fig. S12). This indicates that the oxide enhances the HER kinetics.26 Notably, both a2 and a1 signals decreased significantly with increasing oxide content. Unlike simply lowering the solution pH, which only reduces the soluble OH− generated during the HER, oxide addition in catalysts uniquely suppresses the formation of Pt-OHad−. The decline in a2 does not reflect reduced H2 generation but rather a substantial decrease in H2 bubble retention. This indicates that oxide addition significantly improves the aerophobicity of the catalyst, facilitating H2 bubble detachment and reducing surface blockage. To visualize H2 bubble formation during the HER, the current density was controlled at 10 mA cm−2 to avoid vigorous bubbling, which could hinder the observation. H2 bubbles were imaged every 5 s after the HER began (Fig. 5 and Videos S1 and S2). On Pt5/C, 2–3 small bubbles (diameters of ∼0.1 mm) were initially formed and gradually increased in size. No new small bubbles were observed, indicating limited nucleation. By contrast, on Pt5/C/oxide-50, numerous fine bubbles appeared immediately across the electrode surface. These bubbles were noticeably smaller than those on Pt5/C and detached rapidly once they reached ∼1.2-fold their original size. Subsequently, a continuous stream of bubbles was generated. These observations demonstrate the superaerophobicity of Pt5/C/oxide-50 compared with that of Pt5/C, aligning well with the reduced a2 signals in the CV, indicating decreased bubble retention on the catalyst surface. Hydrophilic surfaces (θw < 90o) typically exhibit aerophobicity (high gas-bubble contact angle and high θg), enabling rapid bubble release (details in the SI). θw and θg exhibit the relationship θg = 180oθw. By contrast, hydrophobic surfaces (θw > 90o) tend to be aerophilic (low θg), causing bubble retention.23,27–32 Controlling the surface wettability is essential for enhancing gas-evolution efficiency. The superaerophobicity of Pt5/C/oxide-50 was related to its unique gradient wettability surface. Bubbles nucleate in hydrophobic/aerophilic regions (Pt/C) but spontaneously migrate toward hydrophilic/aerophobic zones (oxide) owing to an asymmetric wettability-driven force, analogous to the Marangoni effect (Scheme 1).33–37 Bubbles detached rapidly from the hydrophilic/aerophobic zone. p-LSVs of Pt5/C/oxide-x were recorded in NaClaq after 60 s of the HER at −0.2 V (Fig. 4). Anodic peak a1 decreased with increasing oxide content in the composites. When the oxide content reaches 50%, the a1 anodic current is down to the 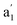 (Hupd-o) background. This indicated that the incorporation of oxides, owing to their higher surface acidity, effectively suppressed the formation of Pt-OHad−. In natural seawater electrolysis, Pt5/C suffers from OH− accumulation and surface precipitation (e.g., Mg(OH)2 and Ca(OH)2), leading to activity loss. By contrast, Pt5/C/oxide-50 forms surface acidic sites that act as H+ reservoirs, facilitating H+ transfer to Pt and promoting the HER.6 The generated H+ lowers the local pH, neutralizes excess OH−, and effectively suppresses precipitation, enhancing HER durability in seawater (Scheme 1).
(Hupd-o) background. This indicated that the incorporation of oxides, owing to their higher surface acidity, effectively suppressed the formation of Pt-OHad−. In natural seawater electrolysis, Pt5/C suffers from OH− accumulation and surface precipitation (e.g., Mg(OH)2 and Ca(OH)2), leading to activity loss. By contrast, Pt5/C/oxide-50 forms surface acidic sites that act as H+ reservoirs, facilitating H+ transfer to Pt and promoting the HER.6 The generated H+ lowers the local pH, neutralizes excess OH−, and effectively suppresses precipitation, enhancing HER durability in seawater (Scheme 1).
Long-term electrocatalytic stability tests with Pt5/C/oxide-50 and Pt5/C at a constant overpotential of 100 mV (chronoamperometry, CA) and 50 mA cm−2 (chronopotentiometry, CP) in natural seawater were performed for 20 h (Fig. 6). Natural seawater collected from the west coast of Central Taiwan was filtered before use. Pt5/C suffered from activity decay and bubble-induced current oscillations, while Pt5/C/oxide-50 maintained stable performance. In the chronoamperogram of Pt5/C (Fig. 6a), the current initially decreased sharply, followed by a series of periodic fluctuations. These current oscillations arose from the dynamic growth and detachment of H2 bubbles at the electrode surface. Each current minimum corresponds to the accumulation of H2 bubbles that partially block the active surface area, whereas each subsequent increase reflects the sudden release of bubbles that temporarily restore the electrochemically active surface. The waiting time between the bubble detachment events is governed by the critical size required for buoyant lift-off, which is related to the hydrophobicity/aerophilicity of Pt5/C. In addition to the bubble dynamics, the progressive decline in the overall cathodic current is attributed to an increase in the local surface alkalinity and the formation of Mg(OH)2 and Ca(OH)2, which block the Pt sites.6 In the chronopotentiogram of Pt5/C (Fig. 6b), the potential dropped sharply from the initial −0.4 V to below −0.8 V within a short duration. This rapid potential drift, accompanied by a marked decline in the H2 bubble-generation efficiency, confirms the severe catalyst deactivation caused by local alkalization. By contrast, Pt5/C/oxide-50 was much more durable, with negligible current or potential fluctuations during CA and CP. Post-HER CV tests were recorded after 20 h electrolysis in natural seawater. The Pt/C electrode exhibited a clear loss of HER activity. In contrast, Pt5/C/oxide-50 retained well-defined CV features with minimal degradation (Fig. S13). This indicates that the incorporation of oxides not only mitigates the alkaline poisoning effect but also enhances the superaerophobicity of the catalyst, promoting more stable H2 bubble release and sustained HER activity.
Conclusions
Herein, we demonstrated a facile and scalable strategy to overcome the key limitations of the seawater HER by combining wettability control and microenvironment engineering. The Pt5/C/oxide-50 catalyst exhibited superior superaerophobicity and local acid-site functionality, enabling rapid H2 bubble release and suppressing alkaline poisoning. This design significantly improves HER durability in natural seawater and is broadly applicable to other gas-evolving electrochemical reactions where surface deactivation, bubble accumulation, or pH shifts limit the performance. These findings pave the way for practical decentralized hydrogen production using untreated seawater.Experimental
Chemicals
Graphite powder (XC-72 Vulcan carbon); a commercial Pt/C catalyst (20 wt% Pt, Pt particle size ∼6 nm, E-TEK); and metal oxide nanopowders, including Al2O3 (13 nm), SiO2 (10–20 nm), CeO2 (23 nm), and TiO2 (P25, 25 nm) were purchased from Uni-Onward Corp., TW. Sodium chloride (NaCl, ≥99.0%, ACS reagent), sulfuric acid (H2SO4, 95–98%), hydrochloric acid (HCl, 37%, ACS reagent), and Nafion perfluorinated resin solution (5 wt% in a mixture of lower aliphatic alcohols and water) were obtained from Sigma-Aldrich. All chemicals were used as received without further purification.Preparation of Pt5/C and Pt5/C/oxide-x composites
To prepare the composites with a normalized Pt content of 5 wt%, Pt20/C, XC-72 Vulcan carbon, and various oxides (SiO2, Al2O3, CeO2, and TiO2) were used in different proportions, as summarized in Table 1. In a typical procedure, 5 mg of Pt20/C and the designated amount of oxide powder were first ground thoroughly in an agate mortar to ensure uniform mixing of Pt/C and the oxide. Subsequently, the appropriate amount of C was added and ground until a homogeneous composite was obtained. The resulting material was dried at 60 °C for 3 h to remove residual moisture. The total mass of each composite was kept constant (20 mg), and the Pt content was normalized to 5 wt% by adjusting the amount of added C accordingly. These composites are denoted as Pt5/C/oxide-x, where x represents the weight percentage of the oxide component in the final catalyst (e.g., Pt5/C/oxide-10 contains 10 wt% SiO2).| Catalysts | Pt20/C | C | Oxides |
|---|---|---|---|
| a “Oxide” refers to the selected hydrophilic metal oxide additive (SiO2, Al2O3, CeO2, or TiO2) used for wettability modulation. | |||
| Pt5/C | 5 | 15 | 0 |
| Pt5/C/oxide-10 | 5 | 13 | 2 |
| Pt5/C/oxide-20 | 5 | 11 | 4 |
| Pt5/C/oxide-30 | 5 | 9 | 6 |
| Pt5/C/oxide-40 | 5 | 7 | 8 |
| Pt5/C/oxide-50 | 5 | 5 | 10 |
Characterization
The surface compositions and chemical states of the catalysts were analyzed using X-ray photoelectron spectroscopy (XPS; ULVAC-PHI PHI5000 VersaProbe Scanning ESCA Microprobe). Powder X-ray diffraction (XRD) patterns were acquired using Cu Kα radiation (λ = 1.5406 Å), with the catalyst samples supported on 10 × 10 mm Ti foil. The Pt content of each catalyst was quantified using inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500ce). Prior to the analysis, the samples were digested in 2 mL of freshly prepared aqua regia and incubated overnight. The solution was diluted with deionized water, boiled to remove excess HCl, and further diluted to a suitable concentration for measurement. The micromorphological and structural features were examined using field-emission transmission electron microscopy (JEOL JEM-1400 and JEM-2100F). High-resolution transmission electron microscopy and high-angle annular dark-field scanning TEM were used to evaluate the nanoscale structures and metal dispersions.Electrochemical experiments
Electrochemical experiments were conducted using a CHI 760E potentiostat (CH Instruments) with a three-electrode configuration. A glassy carbon electrode (GCE) or glassy carbon screen-printed electrode (GC-SPE, 0.196 cm2) served as the working electrode, a graphite rod was used as the counter electrode, and a Hg/HgSO4 reference electrode was employed (depending on the electrolyte). All potentials were converted to the reversible hydrogen electrode (RHE) scale using ERHE = EHg/HgSO4 + 0.68 + 0.059pHelectrolyte. To eliminate interference from anodic chlorine evolution (CER) during cathodic HER measurements, the counter electrode was physically separated in a fritted glass tube filled with the same electrolyte (NaCl solution or natural seawater). This effectively isolates anodic CER products from reaching the working electrode compartment.First, 2 mg of the catalyst was dispersed in a solvent mixture of 0.83 mL deionized water (18.2 MΩ cm), 0.17 mL of isopropanol, and 5 μL of 5 wt% Nafion solution. The ink was sonicated for 20 min to ensure uniform dispersion. The polished GCE (0.07 cm2) was prepared by sequential polishing with 1.0, 0.3, and 0.05 μm alumina slurries, followed by sonication in deionized water and drying. A 4 μL aliquot of catalyst ink was drop-cast onto the GCE surface (catalyst/GCE, Pt loading ≈ 5.7 μg cm−2) and dried under Ar flow at room temperature. Cyclic voltammetry (CV) was performed in 0.5 M H2SO4 to pretest the catalytic activity. The as-prepared catalyst/GCEs as the working electrodes were cleaned electrochemically by potential cycling 10 times between 0.0 V and 1.0 V vs. RHE in an Ar-purged 0.5 M H2SO4 aqueous electrolyte (scan rate, 0.2 V s−1). The electrochemical surface areas (ECSAs) were determined by measuring the areas (charges) under the hydrogen adsorption/desorption peaks (Hupd) in the cyclic voltammograms. A conversion factor of 0.21 mC cm−2 was used to determine the ECSA. The measured ECSA values ranged between approximately 32 and 43 m2 g−1 across all catalyst variations, indicating good reproducibility and confirming that the oxide blending process did not compromise the electrochemical accessibility of Pt. To simulate the seawater HER test, CV measurements were acquired in an Ar-saturated 0.5 M NaCl aqueous solution (NaClaq, pH 8.3) to simulate seawater without Ca2+ and Mg2+. For measurements in natural seawater electrolytes, 10 μL of ink was drop-cast onto a GC-SPE and dried under ambient conditions, yielding a Pt loading of 5.1 μg cm−2. Linear sweep voltammetry was conducted at 5 mV s−1 without iR compensation to evaluate the HER activity and extract Tafel slopes from the overpotential (η) vs. log current density (j) plots. Long-term stability was assessed using chronopotentiometry at 50 mA cm−2 and chronoamperometry at an overpotential of 100 mV for 20 h in natural seawater. Natural seawater was collected from the west coast of central Taiwan, filtered through a 0.22 μm membrane, and used without further purification.
Gas-bubble imaging and water droplet contact-angle measurements
All bubble-imaging experiments for the HER were performed in a custom-fabricated electrochemical cell with a transparent quartz window to allow for unobstructed visualization. The working electrode (GC-SPE) coated with the catalyst was placed vertically at the bottom of the cell. The electrochemical HER was triggered under galvanostatic control using a CHI 760E workstation. Videos and sequential images of gas bubble nucleation, growth, and detachment were recorded during operation in natural seawater. Images were recorded at a rate of one frame every 5 s. The comparative bubble behavior of the Pt5/C and Pt5/C/oxide-50 electrodes was analyzed based on the bubble size, coverage, residence time, and detachment dynamics at identical HER current densities. To visualize H2 bubble formation during the HER, the current density was controlled at 10 mA cm−2 to avoid vigorous bubbling, which could hinder the observation. The water droplet contact angles (θw) were extracted by image analysis using ImageJ software equipped with the First Ten Angstroms/1000 B, allowing quantitative evaluation of the gas–solid–liquid interface.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: gas bubble dynamics, Scheme S1, Fig. S1–S13, and Videos S1 and S2. See DOI: https://doi.org/10.1039/d5ta06647f.Acknowledgements
This work was supported by the National Science and Technology Council of the Republic of China, Taiwan (MOST 111-2113-M-005-015-MY3 and NSTC 114-2113-M-005-019-MY3).35–37Notes and references
- H. P. Xie, Z. Y. Zhao, T. Liu, Y. F. Wu, C. Lan, W. C. A. Jiang, L. Y. Zhu, Y. P. Wang, D. S. Yang and Z. P. Shao, Nature, 2022, 612, 673–678 CrossRef CAS.
- J. Y. Liu, S. Duan, H. Shi, T. Y. Wang, X. X. Yang, Y. H. Huang, G. Wu and Q. Li, Angew. Chem., Int. Ed., 2022, 61, e202210753 CrossRef CAS.
- L. Chen, C. Yu, J. T. Dong, Y. N. Han, H. L. Huang, W. B. Li, Y. F. Zhang, X. Y. Tan and J. S. Qiu, Chem. Soc. Rev., 2024, 53, 7455–7488 RSC.
- H. Y. Jin, J. Xu, H. Liu, H. F. Shen, H. M. Yu, M. Jaroniec, Y. Zheng and S. Z. Qiao, Sci. Adv., 2023, 9, adi7755 CrossRef.
- Y. Liu, Y. Wang, P. Fornasiero, G. Tian, P. Strasser and X. Y. Yang, Angew. Chem., Int. Ed., 2024, 63, e202412087 CrossRef CAS PubMed.
- D. Y. Bao, L. S. Huang, Y. J. Gao, K. Davey, Y. Zheng and S. Z. Qiao, J. Am. Chem. Soc., 2024, 146, 34711–34719 CrossRef CAS PubMed.
- J. X. Guo, Y. Zheng, Z. P. Hu, C. Y. Zheng, J. Mao, K. Du, M. Jaroniec, S. Z. Qiao and T. Ling, Nat. Energy, 2023, 8, 264–272 CAS.
- A. Bashkatov, S. Park, Ç. Demirkir, J. A. Wood, M. T. M. Koper, D. Lohse and D. Krug, J. Am. Chem. Soc., 2024, 146, 10177–10186 CrossRef CAS.
- R. C. Iwata, L. N. Zhang, K. L. Wilke, S. Gong, M. F. He, B. M. Gallant and E. N. Wang, Joule, 2021, 5, 887–900 CrossRef CAS.
- P. A. Kempler, R. H. Coridan and L. Luo, Chem. Rev., 2024, 124, 10964–11007 CrossRef CAS PubMed.
- Z. H. Liu, Y. Du, R. H. Yu, M. B. Zheng, R. Hu, J. S. Wu, Y. Y. Xia, Z. C. Zhuang and D. S. Wang, Angew. Chem., Int. Ed., 2023, 62, e202212653 CrossRef CAS PubMed.
- C. H. Zhang, Z. Xu, N. A. Han, Y. Tian, T. Kallio, C. M. Yu and L. Jiang, Sci. Adv., 2023, 9, add6978 CrossRef PubMed.
- K. G. Shi, Z. H. Ren, Z. Meng and X. F. Feng, ChemCatChem, 2024, 16, e202301308 CrossRef CAS.
- J. Ryu and D. W. Lee, J. Mater. Chem. A, 2024, 12, 10012–10043 RSC.
- Q. H. Sha, S. Y. Wang, L. Yan, Y. S. Feng, Z. Zhang, S. H. Li, X. L. Guo, T. S. Li, H. Li, Z. B. Zhuang, D. J. Zhou, B. Liu and X. M. Sun, Nature, 2025, 639, 360–367 CrossRef CAS.
- H. S. Hu, Z. R. Zhang, L. J. Liu, X. L. Che, J. C. Wang, Y. Zhu, J. P. Attfield and M. H. Yang, Sci. Adv., 2024, 10, adn7012 CrossRef.
- J. F. Huang, W. J. Hsieh and J. L. Chen, ACS Appl. Mater. Interfaces, 2024, 16, 27504–27510 CrossRef CAS.
- J. F. Huang, J. R. Sie and R. H. Zeng, Electrochim. Acta, 2021, 372, 8 CrossRef.
- J. F. Huang and Y. C. Wu, ACS Sustain. Chem. Eng., 2018, 6, 8285–8290 CrossRef CAS.
- J. F. Huang, R. H. Zeng and J. L. Chen, J. Mater. Chem. A, 2021, 9, 21972–21980 RSC.
- S. Dresp, F. Dionigi, S. Loos, J. F. de Araujo, C. Spöri, M. Gliech, H. Dau and P. Strasser, Adv. Energy Mater., 2018, 8, 201800338 Search PubMed.
- W. M. Tong, M. Forster, F. Dionigi, S. Dresp, R. S. Erami, P. Strasser, A. J. Cowan and P. Farràs, Nat. Energy, 2020, 5, 367–377 CrossRef CAS.
- P. Daubinger, J. Kieninger, T. Unmüssig and G. A. Urban, Phys. Chem. Chem. Phys., 2014, 16, 8392–8399 RSC.
- A. J. Bard and L. R. Faulkner, Electrochemical Method: Fundamentals and Applications, John Wiley & Son, New York, 2nd edn, 2001 Search PubMed.
- J. F. Huang, L. J. Chen, B. Z. Yang and J. L. Chen, J. Mater. Chem. A, 2025, 13, 13734–13742 RSC.
- T. Shinagawa, A. T. Garcia-Esparza and K. Takanabe, Sci. Rep., 2015, 5, srep13801 CrossRef.
- M. Bae, Y. Kang, D. W. Lee, D. Jeon and J. Ryu, Adv. Energy Mater., 2022, 12, 202201452 Search PubMed.
- Y. Kang, S. Lee, S. Han, D. Jeon, M. Bae, Y. Choi, D. W. Lee and J. Ryu, Adv. Funct. Mater., 2024, 34, 202308827 Search PubMed.
- Z. Y. Lu, M. Sun, T. H. Xu, Y. J. Li, W. W. Xu, Z. Chang, Y. Ding, X. M. Sun and L. Jiang, Adv. Mater., 2015, 27, 2361–2366 CrossRef CAS PubMed.
- Q. Song, Z. J. Xue, C. Liu, X. Z. Qiao, L. Liu, C. H. Huang, K. Y. Liu, X. Li, Z. L. Lu and T. Wang, J. Am. Chem. Soc., 2020, 142, 1857–1863 CrossRef CAS PubMed.
- D. Jeon, J. Park, C. Shin, H. Kim, J. W. Jang, D. W. Lee and J. Ryu, Sci. Adv., 2020, 6, aaz3944 CrossRef.
- Q. Zhang, P. S. Li, D. J. Zhou, Z. Chang, Y. Kuang and X. M. Sun, Small, 2017, 13, 201701648 Search PubMed.
- J. Lin, W. Wang, W. L. Bai, M. N. Zhu, C. Zheng, Z. L. Liu, X. F. Cai, D. D. Lu, Z. W. Qiao, F. Q. Chen and J. X. Chen, Chem. Eng. J., 2017, 315, 262–273 CrossRef CAS.
- E. Ruckenstein, Colloids Surf., A, 2012, 412, 36–37 CrossRef CAS.
- T. Bánsági, M. M. Wrobel, S. K. Scott and A. F. Taylor, J. Phys. Chem. B, 2013, 117, 13572–13577 CrossRef PubMed.
- N. D. Patil, P. G. Bange, R. Bhardwaj and A. Sharma, Langmuir, 2016, 32, 11958–11972 CrossRef CAS.
- S. Le Roux, M. Roché, I. Cantat and A. Saint-Jalmes, Phys. Rev. E, 2016, 93, 01310713 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |