The application of low-temperature processed metal oxide electron transport layers in flexible perovskite solar cells
Jianghao
Tian
ab,
Kun
Wang
ab,
Zhipeng
Zhou
ab,
Lexiang
Zhang
ab,
Pu
Fan
*b,
Huajing
Zheng
*ac,
Ding
Zheng
*a and
Junsheng
Yu
 *ab
*ab
aState Key Laboratory of Electronic Thin Films and Integrated Devices, School of Optoelectronic Science and Engineering, University of Electronic Science and Technology of China (UESTC), Chengdu, 610054, P. R. China. E-mail: jsyu@uestc.edu.cn; dingzheng@uestc.edu.cn
bUniversity of Electronic Science and Technology of China Yangtze Delta Region Institute, Huzhou, 313001, P. R. China. E-mail: fanpu@csj.uestc.edu.cn
cUniversity of Electronic Science and Technology of China Guangdong Institute of Electronic Information Engineering, Dongguan, 523808, P. R. China. E-mail: zhj12@163.com
First published on 29th October 2025
Abstract
Flexible perovskite devices have garnered significant attention due to their promising applications in wearable electronics and portable energy systems. Metal oxide electron transport layers (ETLs), such as SnO2 and ZnO, processed at low temperatures, are particularly well-suited for fabrication processes that require compatibility with flexible substrates. This review presents recent advancements in the low-temperature preparation of SnO2 and ZnO, followed by a summary of their latest applications, categorized into additive and interface engineering. Additionally, other metal oxide ETMs treated at low temperatures are briefly discussed. Finally, the review concludes with an analysis of the challenges and limitations associated with perovskite solar cells (PSCs) that incorporate low-temperature processed metal oxide ETLs.
1. Introduction
The photovoltaic effect has built a bridge between light and electricity. Solar cells, a product of the photovoltaic effect, are slowly developing a new energy way in the interweaving of light and electricity. In 2009, Miyasaka's team introduced the first Perovskite Solar Cell (PSC),1 achieving a photoelectric conversion efficiency (PCE) of 3.8%. This breakthrough injected new vitality into the photovoltaic industry. Extensive research has been conducted on PSCs, which has increased the conversion efficiency of PSCs to 26.95% (ref. 2) in just 16 years (2009–2025). It should be noted that the highest single junction efficiency of the organic solar cell (OSC), which was discovered in 1986,3 is 20.2%,4 and the highest efficiency of the dye-sensitized solar cell(DSSC), which was discovered in 1991,5 is 14.3%.6 The rapid development of PSCs is evident.Fig. 1 shows the typical structure and working principle of the n–i–p PSCs. The ETL is known as a key functional layer in PSC devices. The main task of the ETL is to transport electrons and prevent the transport of holes.7 To achieve high performance, the ETL's energy band should be matched with the perovskite layer's; specifically, the highest valence band of the ETL should be lower than that of the perovskite layer for blocking transport of holes into the ETL.8 Moreover, the transmittance and electron mobility should be high, so that the light will be absorbed by the active layer and more electrons will be collected by the electrode.
Nowadays, many types of ETLs have been used in different PSCs. C60, as one of the most famous fullerene materials, has been used in ETLs for a long time because of its high electron mobility and energy level alignment, and researchers can greatly improve the performance of devices by adding other functional groups to form new fullerene materials.9–11 Phenyl-C61 butyric acid methyl ester (PCBM) is also a widely used ETL in many studies.12–16 Because PCBM has low carrier mobility and conductivity,12 researchers have improved PSC efficiency by doping various materials into the PCBM layer, such as oleamide,13 SnS2,14 CoSe16etc. ICBA is another fullerene derivative that can act as an ETL. Its LUMO (Lowest Unoccupied Molecular Orbital) is higher than that of PCBM, so the Voc (open circuit voltage) can be improved.17,18 Fullerene materials and their derivatives are one of the main materials for ETLs. Other materials have also been reported in the last decade. Graphene has high electron mobility, and its work function is suitable for FTO.19,20 Small molecule materials,21 carbon nanotubes,22 amino functionalized carborane derivative CB-NH2,23 3D molecules based on perylene diimide (PDI)(TPE-PDI4),24etc. can also be used as ETLs.
With the fast development of flexible electronic equipment and solar cells, people tend to combine the two to produce portable and wearable flexible solar cell equipment. Flexible solar cells can be achieved by continuous roll-to-roll technology, which has faster manufacturing speed and lower cost, and their applications are more extensive, from civilian to military, and flexible photovoltaic devices can complete the corresponding work well.25 However, flexible PSCs require a flexible substrate, which is not resistant to high temperature. Take polyethylene terephthalate (PET) and polyethylene naphthalate (PEN) as examples, and their glass transition temperatures are about 80 °C and 120 °C, respectively.26 Additionally, the roll-to-roll technology also has strict requirements for low temperature.27 This means that the traditional high-temperature preparation process is not suitable for flexible equipment, so it is very important to develop and improve the preparation process at low temperature, even at room temperature. At present, many low-temperature processed ETLs have been applied to flexible perovskite solar cells. In 2016, a new electron transport material (ETM), amorphous Bi2S3 (a-Bi2S3), was prepared by Li et al.28 at room temperature through a simple thermal evaporation process. Bi2S3 has inherent high electron concentration and carrier mobility, which is much higher than those of PCBM, showing high electron collection efficiency and low carrier recombination loss. Cadmium sulfide (CdS) has excellent electron extraction and transmission characteristics, which help to reduce the hysteresis of solar cells and improve efficiency.29 WS2 can also be deposited by solution-treated low-temperature technology or the radio frequency (RF) sputtering method,30 and the optimized solar cell structure (FTO/WS2/CsSnI3/rGO/Pt) showed photovoltaic performance with a power conversion efficiency of 31% from the solar cell capacitance simulator (SCAPS-1D).31 Yoon et al. used C60 (fullerene) treated in a vacuum at room temperature as the ETL, achieving a photoelectric conversion efficiency of 19.1%.32 Other materials like TiS2,33 In2S3,34 ZrSnO4,35 and mesoporous graphene36 have also been reported as low-temperature ETLs in recent studies.
Metal oxide (MO) materials like TiO2/TiOx, ZnO, SnO2/SnOx, WO3, NiOx, Al2O3, etc. have many advantages and the potential to be the ETLs of efficient PSCs. MO usually has a high electron affinity, which can effectively transport electrons and inhibit the transmission of holes, so as to reduce carrier recombination and improve the efficiency of the cells. MO also has good broadband transparency,37 and its chemical and thermal stability is high, so that the stability of PSCs will be improved. As we can see from the energy level of MO in Fig. 2, the conduction band minimum (CBM) level of the MO material is generally lower than that of the perovskite, facilitating electron extraction from the perovskite layer into the electron transport layer. Additionally, its valence band maximum (VBM) level is lower than that of the active layer, thereby blocking and suppressing holes. This fulfills the requirements for an excellent ETL solution as previously mentioned. As a very popular ETM in the progress of PSCs, TiO2 has attracted a lot of attention. Some research progress of TiO2 is shown in Table 1. As we can see, researchers used different ways for improving the performances of TiO2-based PSCs by optimizing fabrication technology, doping, adding a modification layer, and so on. It is worth noting that metal oxides with a mesoporous structure tend to have better performance as ETLs, for the reason that the mesoporous structure can provide a channel for perovskite molecules to fully come into contact with the electron transport layer, which is equivalent to increasing the contact area between the two functional layers and greatly increasing the electron transport efficiency. Moreover, it can provide good mechanical properties.38 However, as shown in Table 1, a traditional TiO2 ETL is usually prepared by the spin coating method, which usually requires two high-temperature sintering processes;54 so the TiO2 ETL prepared by high-temperature technology(mainly above 400 °C) is not suitable for flexible PSCs. Moreover, the high temperature process will also increase the preparation difficulty of PSCs and is not conducive to the stability of devices. The inherent excellent properties of inorganic metal oxides cannot make people ignore this ETL material. Therefore, it is of great significance to study low-temperature metal oxides used as ETLs in PSCs, which can not only promote the progress of solar cell performance but also be conducive to the development of wearable photovoltaic devices.
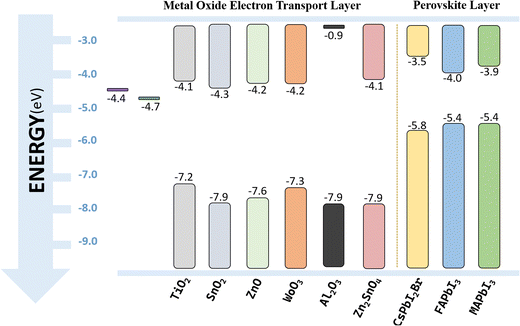 | ||
| Fig. 2 Conduction band (CB) and valence band (VB) of metal oxide materials, and comparison with that of perovskite materials. | ||
| Year | Fabrication | Optimal methods and materials | TiO2 structure | Champion PCE | Reference |
|---|---|---|---|---|---|
| 2014 | Thermal oxidation sputtering | — | — | 15.07% | 39 |
| 2015 | Spin-coating | Modify with rGO (reduced graphene oxide) | Mesoporous | 18% | 41 |
| 2016 | Sputtering | — | Anatase | 12.5% | 40 |
| 2016 | Anodic oxidation | — | Nanorod | 15.2% | 40 |
| 2016 | Spin-coating | Interface modification with ionic liquid | — | 19.62% | 42 |
| 2016 | Spin-coating | La2O3 as the interface modification layer | Mesoporous | 15.81% | 43 |
| 2017 | Atomic layer deposition method (ALD) and spin coating technology | Bilayer ETL structure | Anatase | 16.5% | 44 |
| 2018 | Hydrolysis-pyrolysis method | Nb-doping ETL | Nanoparticle | 18.88% | 45 |
| 2018 | Spin-coating | Interface modification with graphene quantum dots (GQDs) | Mesoporous | 20.45% | 46 |
| 2019 | Spin-coating | — | Nanoparticle | 18.72% | 47 |
| 2020 | Spin-coating with acid-treated | Acid-treated amorphous TiO2 as the buffer layer | Anatase | 17.8% | 48 |
| 2020 | Radio frequency (RF) magnetron sputtering | Li+-doping ETL | Rutile | 24.23% | 49 |
| 2020 | Electrochemical anodic oxidation | TiCl4-doping ETL | Leaves and needles like (LNT) | 9% | 50 |
| 2021 | Spin-coating | Li2CO3-doping ETL | Mesoporous | 25.28% | 51 |
| 2021 | Liquid phase deposition (LPD) | 2D TiS2 as the interface modification layer | Nanograss | 18.73% | 52 |
| 2024 | Chemical bath deposition (CBD) | Embed Ti0.936O2 nanocrystals into TiO2 | — | 25.5% | 53 |
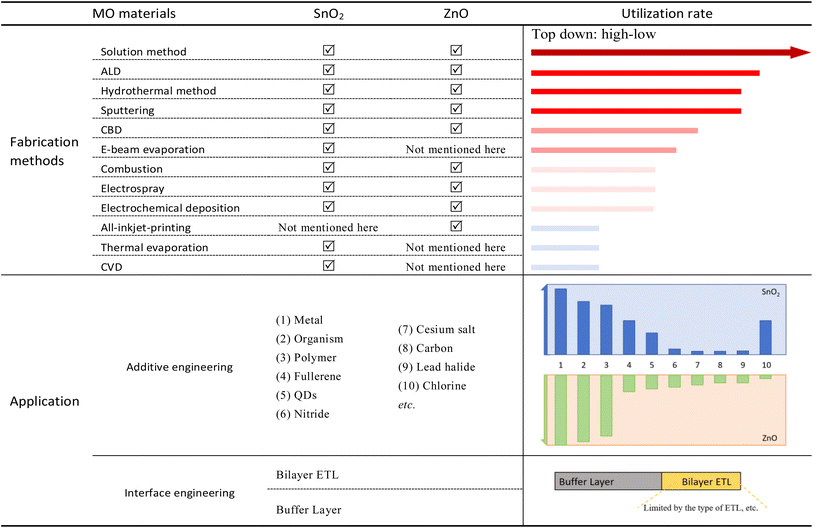
In this review, we will focus on the frequently used low-temperature MO ETLs—SnO2 and ZnO, and their inherent properties, traditional preparation methods, current research progress, and application in PSCs will be described in detail. We found that the functional groups or functional atoms provided by different materials play key roles in the doping or interface modification of the ETL. This review will pay more attention to the microscopic mechanism of different materials and make some inferences and comparisons. We also summarized and compared the preparation methods and modification methods of SnO2 and ZnO, and drew a table. In the table, we counted the commonly used modified materials and main preparation methods, compared the use of different materials on different ETLs, and the research on different low-temperature preparation methods. We also conducted an in-depth analysis of the fabrication processes for different MO ETLs, with particular emphasis on their compatibility with roll-to-roll processes and large-area fabrication techniques. Later, we will present the recent application of TiO2 in a low-temperature process. In addition, we will introduce several excellent low temperature metal oxides like Al2O3, ZrO2, In2O3, etc. It is worth mentioning that at the end of this review, we compared the traditional preparation methods that need high temperature with the current reported low temperature methods, and formed an image to make an intuitive comparison, so as to clarify the advantages of these low temperature preparation methods. Finally, prospects of MO ETLs prepared at low temperatures will be discussed.
2. Tin oxide
2.1 Basic properties and advantages
Tin oxide (SnO2) is a common MO ETL in PSCs. Compared with TiO2, SnO2 has many advantages. Firstly, from Fig. 2, SnO2 has a deeper conduction band and valence band,55 so the ETL will have better electron extraction capability, and the recombination of electrons and holes will also be reduced. Secondly, the bandgap is wide (3.6–4.0 eV), so that more light will be able to pass through the ETL to reach the perovskite layer and the efficiency will be improved. Thirdly, the high bulk electron mobility (>240 cm2 (V−1 s−1))56 will help improve electron transmission capability. Fourthly, the temperature required for preparation is usually low (≤200 °C), sometimes even room temperature. Additionally, it has the characteristics of stable chemical properties, diverse preparation methods, and strong environmental stability. All these properties make SnO2 a promising ETM for PSCs.2.2 Fabrication methods of SnO2
The methods of depositing SnO2 are various. Generally speaking, the preparation temperature is not too high, which is suitable for preparation on a flexible substrate. The main technologies are summarized here.The sol–gel method is one of the primary solution preparation methods.57–59 For obtaining SnO2 films with high quality, the gels need to be formed from metal alkoxide precursors through hydrolysis and condensation reactions, and drying and heat treatment are also essential. In this way, Wang et al. prepared a SnO2 nanocrystalline ETL with a small particle size and a large specific surface area.57 This is a SnO2/Sn composite ETL made by controlling the annealing temperature and can help to achieve 8.7% PCE. However, in the traditional wet synthesis, a high temperature is required to improve the crystallinity, so another team proposed a new method to prepare the SnO2 ETL by the sol–gel method at low temperature (below 80 °C).59 As shown in Fig. 3a, this method omits the 150 °C high-temperature step and realizes the oxidation of Sn+ by refluxing SnCl4·2H2O alcohol solution. The reflux process makes O2 and H2O in the atmosphere participate in the oxidation of Sn2+, reducing the crystallization barrier of SnO2. Because of colloidal aging at room temperature, the unreacted precursor solvent or organic solvent is volatilized, and the dispersion and uniformity of the whole solution are also improved. As shown in Fig. 3b, the light absorption ability is enhanced, and finally, a steady-state efficiency of 18.48% is achieved.
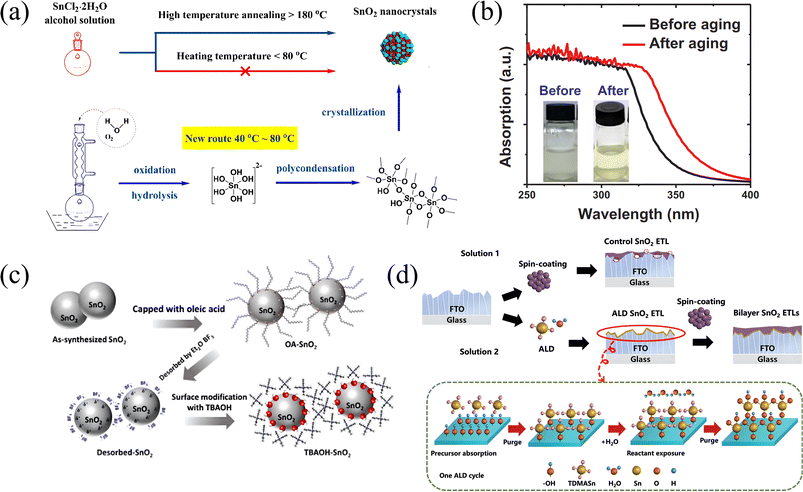 | ||
| Fig. 3 (a) Schematic diagram of the new route to SnO2 nanocrystals (NCs) at low temperature, (b) the SnO2 sol before and after aging at room temperature for 24 h;59 Copyright 2017, Elsevier. (c) Schematic illustration of the ligand exchange procedure for TBAOH-capped SnO2 nanoparticles;63 Copyright 2020, American Chemical Society. (d) Sketch processes for the preparation of a control SnO2 ETL with the sol–gel solution; schematic illustration of one atomic layer deposition cycle for a SnO2 ETL; a bilayer SnO2 ETL preparation process combining ALD and spin-coating techniques, respectively.66 Copyright 2023, John Wiley and Son. | ||
Thermal decomposition is also a useful solution method. Ke et al. spin-coated SnCl2·2H2O precursor solution at room temperature and then conducted annealing at 180 °C to prepare SnO2 ETLs directly.60 This involves dehydration of the solution and hydrolysis of the metal salt, resulting in the formation of a tin oxide nanocrystalline film. Due to the special structure of the nanocrystal, this SnO2 ETL film is compact, uniform, and has strong antireflection ability. Yang's group also used the SnCl2·2H2O dissolved in ethanol as a precursor solution, but they aged it by vigorously stirring for 1 hour at room temperature in an ambient atmosphere, like the research mentioned before; in this way, it may be easier for the solution to crystallize to form an ETL film, and finally achieve the champion PCE of 20.2%.61 This is the typical operation technique of this method, and the precursor solution can also be SnCl4 mixed with ethanol.
Zhu et al. used another solution method—hydrothermal method—to make SnO2 nanocrystals, which can produce highly crystallized SnO2 nanocrystals and meanwhile help maintain effective electron transport in thicker films.62 We deem that the sealed environment and constant temperature provided by the hydrothermal method make the solution more stable and uniform, the film growth will be more orderly, and the film morphology and photoelectric properties will be better. To improve the dispersibility of SnO2 nanoparticles in ethanol, Lee and co-workers proposed a ligand exchange method.63 Generally, this is a solvothermal method for preparing SnO2 nanoparticles. In detail, TBAOH replaces the insulating OA molecules on the surface of SnO2 nanoparticles (Fig. 3c), and thus, a more uniform and compact film can be obtained; this benefits from the large volume and appropriate polarity of the TBAOH material, which can prevent the aggregation of nanoparticles, and the hydroxyl (–OH) in it can also adjust the energy level of SnO2 to obtain a better energy level structure. Tu's team used the hydrothermal method and they achieved a PCE of 7.81% with the CH3NH3PbI3 active layer.83 In the same year, Zhu et al. also used this method for making a SnO2 ETL, and they achieved 18.8% PCE with the use of this ETL in inverted PSCs.84 We conducted a series of comparative analyses and found that the latter uses a p–i–n type device structure. In order to prevent the SnO2 solution from damaging the perovskite (MAPbI3), a layer of C60 was added between the two functional layers. This avoids direct contact between SnO2 and the perovskite, and also provides an additional hole blocking layer to suppress carrier recombination and optimize the device energy level structure. This seems to be an important reason for its improved efficiency.
The above methods all involve hydrolysis reactions, and in essence, they all chemically generate SnO2 through reaction and heat treatment. Currently, tin oxide dispersion solutions can be purchased directly on the market and can be directly applied in the spin-coating process by direct dilution. After annealing at 150 °C to evaporate the diluted liquid, the SnO2 ETL film is obtained by physical volatilization. Based on the above analysis, we will temporarily categorize the preparation methods into chemical and physical preparation for comparison. Chemical preparation methods produce denser and more uniform films, but they require consideration of the sufficiency of the hydrolysis reaction and the formation of by-products in a water–oxygen environment, both of which significantly impact film quality. The process is more complicated, too. A simple and direct physical preparation process can obtain an effective ETL, but its surface morphology is generally unsatisfactory, and particle aggregation is inevitable. Therefore, we believe that the development of simulated self-assembled materials (SAMs) based on SnO2 by introducing some anchoring functional groups can be explored in depth as a research topic to prepare ETLs with a simple spin-coating process and excellent morphology.
Xiao et al. found that the performance of the device based on the ALD SnO2 ETL is significantly higher than that of the ETL-free one, and after modifying the ETL with a fullerene SAM, the PCE of the PSC can be improved to 17.25%.64 This is due to the special properties of the SAM. By combining the anchoring group with the SnO2 ETL, it is very likely that an effective dipole moment is formed, so as to improve the energy level of the ETL and make it fully match that of the perovskite layer. In addition, with the addition of a SAM fullerene material, its end functional group can capture additional oxygen vacancies or excess metal ions, which can greatly reduce the defect state between perovskite and the ETL, thus reducing the probability of carrier recombination, and alleviating the hysteresis effect of the whole device. However, traditional ALD also has some problems. For example, insufficient temperature (<150 °C) will lead to the appearance of amorphous SnO2, which will affect the electrical performance of the ETL. Additionally, an incomplete precursor reaction will lead to an increase in defect states in the ETL, affecting carrier transport. Plasma-assisted atomic layer deposition (PAALD) for the SnO2 ETL is an innovation because, with the help of plasma, higher reaction barriers can be overcome, thereby reducing defects. Moreover, this method can further optimize the band structure between the ETL and perovskite layers, and reduce the preparation temperature. As shown in Kuang and co-workers’ research, though the efficiency of 50 °C SnO2 is slightly lower than that of 200 °C (former 16.2 ± 0.7%; later 16.1 ± 1%), other key properties are similar, which means that this method can be used in flexible PSCs well.65 In 2023, Zhang et al. used two different methods for preparing the SnO2 ETL—ALD and sol–gel—and combined them to form a double-layer ETL device (as Fig. 3d shows).66 The unique conformal effect of ALD-SnO2 can effectively regulate the roughness of FTO substrates, improve ETL quality, and induce the growth of the PbI2 crystal phase, thereby developing the crystallinity of perovskite layers. Finally, 23.86% PCE and good device stability are achieved. Additionally, it is worth noting that the post-annealing of ALD is essential to the performance of the interface between the ETL and perovskite layer. As the post-annealing temperature increases, the crystal structure of the film gradually becomes orderly, and the content of Sn4+ is also increasing, resulting in the valence band shift of the SnO2 thin film gradually decreasing, indicating a decrease in its bandgap, and the reduction of defect states will also make the energy levels near the valence band more concentrated.67
Yoo and co-workers prepared a SnO2 ETL by the CBD method, and a certification efficiency of 25.2% was obtained by optimizing this device.71 This process mentioned in the report can be seen as two stages: Sn+ is dissolved in a strong acidic environment (pH = 1–1.5) and hydrolyzed to form the Sn(OH)+ intermediate. The Sn(OH)+ intermediate is further oxidized in solution and reacts with OH− provided by urea decomposition to form Sn(OH)4, which is then dehydrated to form SnO2. This is stage A. Next, the pH of the solution will be increased, and different Sn intermediates will be formed in the solution at the same time. This is stage B. After condensation and dehydration reactions, a mixture of Sn6O4(OH)4 and SnO compounds will be formed from the intermediates. The SnO2 film formed in stage A-ii (pH = 1.5) completely covers FTO and has better performance. This also shows a characteristic of CBD, that is, it is necessary to strictly control the reaction time and environment to obtain relatively high-quality film materials.
Jeong et al. also used CBD to deposit the SnO2 ETL, and by using Ga(acac)3 in the P3HT HTL, the device exhibited a PCE of up to 24.6%.72 It is worth mentioning that the traditional CBD method typically only produces relatively dense SnO2 films, making it difficult to form mesoporous structures with strong mechanical stability. Wang's group proposed a method to optimize the preparation of SnO2 in CBD.73 One key part of the study is adding thioglycolic acid (TGA) into the CBD solution, which has a sulfhydryl group (–SH) that can form a strengthening bond with SnO2 nanoparticles, and a weakly acidic carboxyl group (–COOH). The former can prevent excessive aggregation or deposition of nanoparticles, and the latter can adjust the nucleation rate of SnO2. After that, through pre-aging treatment, TGA molecules were consumed spontaneously and the aggregation of SnO2 nanoparticles was promoted for forming a mesoporous structure. The approach used in this study is quite interesting. Basically, it involves modifying SnO2 through doping. However, unlike typical doping (simply blending two different substances), this study incorporates the dopant directly as a reactant in the SnO2 formation reaction, thereby altering the inherent properties of the ETL. The use of specific substances blended with the ETL precursor or solution to induce a reaction could provide new insights into doping processes and could even lead to the development of new ETMs based on mainstream ETLs.
For preparation by sputtering, attention should be paid to the damage of high-energy particles to other functional layers (similar to breaking through the film surfaces). This can be alleviated by sputtering in steps (first at low speed and then at high speed) or by adding a sacrificial layer.
Through literature reading and experimental comparison, we believe that among many preparation methods, the solution method (especially the spin coating method) has broader application prospects, low cost, and a simple and easy-to-understand preparation process, and is also suitable for the preparation of flexible devices and large-sized devices. Other preparation methods require complex reaction processes and harsh reaction conditions, while some require expensive preparation equipment, and the requirement of high-energy particles or plasma will also increase the cost and difficulty of preparation. For the solution method, in addition to directly purchasing SnO2 dispersions, the spontaneous formation of SnO2 through appropriate reactions, such as chemical interactions between different substances in solution and annealing processes, also has corresponding research value. Given the growth environment of SnO2, these studies require attention to the key role of the reaction range and the detection of ETL components to minimize the formation of by-products. Alleviating the surface roughness of the film prepared by the solution method is also an important research direction.
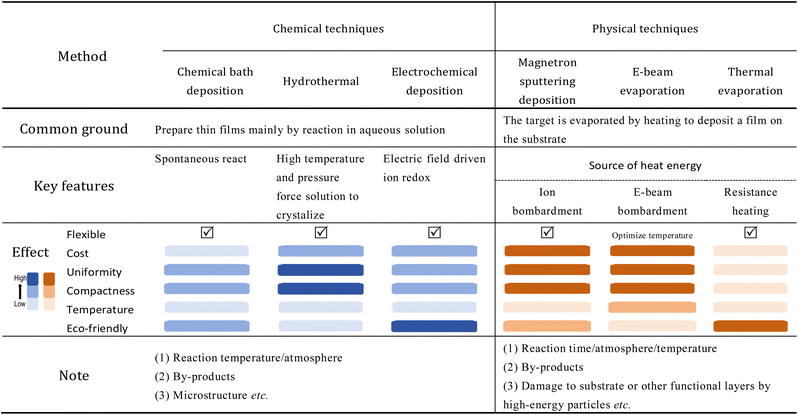
In the process of sorting out the film preparation methods, we found that the working environment or working principle of many methods is roughly similar. For example, CBD, the electrochemical deposition method, and the hydrothermal method all put the substrate in solution for film preparation; magnetron sputtering, E-beam evaporation, and thermal evaporation all seem to involve target heating or target bombardment. In order to clarify the similarities and differences of these methods, we designed Table 2 to summarize and distinguish them. Sometimes researchers combine different methods in their studies to prepare two different ETLs, which can also achieve excellent performances. The best approach is to select the method that is most suitable for the preparation of the entire device.
2.3 Modifications of the SnO2 ETL
Metal oxide, as mentioned above, has many excellent electrical and optical properties that other materials do not possess, such as high conductivity, high chemical stability, wide band gap, etc. However, when they work with other materials like perovskite or ITO/FTO, the interactions between the microscopic particles will have a great impact on the overall performance. For example, the metal ions in the MO ETL may be extended to the perovskite layer to destroy the perovskite crystal structure, and the ions in perovskite, such as I−, may be oxidized to form an intermediate to destroy the interface morphology. Furthermore, a single MO material cannot meet people's needs for higher performance ETLs sometimes. So, it's necessary to make some changes to the MO ETL layer. Additive engineering and interface modification are used to accomplish such a task.2.3.1.1 Metals and metal cations. Metals and metal cations are ideal additives. They can change the band structure of the original ETL to optimize the electrical limit. Some can also improve the interface performance and reduce defects.
Park and co-workers used metal Li to dope SnO2 (Li:SnO2) at a low temperature (185 °C) and achieved a flexible and wearable device.91 Li+ replaces Sn4+ and acts on the lattice of SnO2, thus reducing its energy level position, and the defect states in the film are reduced accordingly, thus promoting the growth of grains and bringing fewer charge defects, so the conductivity is also improved. What needs to be noted is that different doping concentrations can be used to prepare thin films with different grain sizes as shown in Fig. 4a.92 Zhuang et al. realized that ion diffusion induced double-layer doping by directly adding LiOH to SnO2 colloidal dispersion solution.93 As shown in Fig. 4b, a large number of Li+ ions diffuse into the SnO2/perovskite interface and the perovskite layer, forming a gradient concentration distribution, promoting the carrier to move from the high concentration region to the low concentration region, thus improving the effective transport and extraction of carriers at the interface. The doped device showed higher PCE, reaching 21.31%. Zn doping can also help to improve the energy level alignment by raising the conduction band energy level, so as to reduce energy loss and improve the charge extraction efficiency. Liao's team from Huazhong University of Science and Technology used Zn to dope the SnO2 ETL for the first time at low temperature and achieved the highest PCE of 17.78%.95 Co doping can reduce the charge traps.99 Nd3+ doping can effectively improve electron mobility and reduce surface defects, thus reducing the interfacial recombination between perovskite and the ETL.81 The bismuthene-doped SnO2 ETL has a smoother surface, and the energy band moves up after doping, which reduces the interface resistance between the composite layer and the perovskite layer, effectively accelerates electron extraction, and does not reduce the hole blocking ability.100 It is worth mentioning that all the research above found that the doping of these metal ions will make the grain size of the SnO2 ETL larger, and then lead to the perovskite film structure with larger grains.
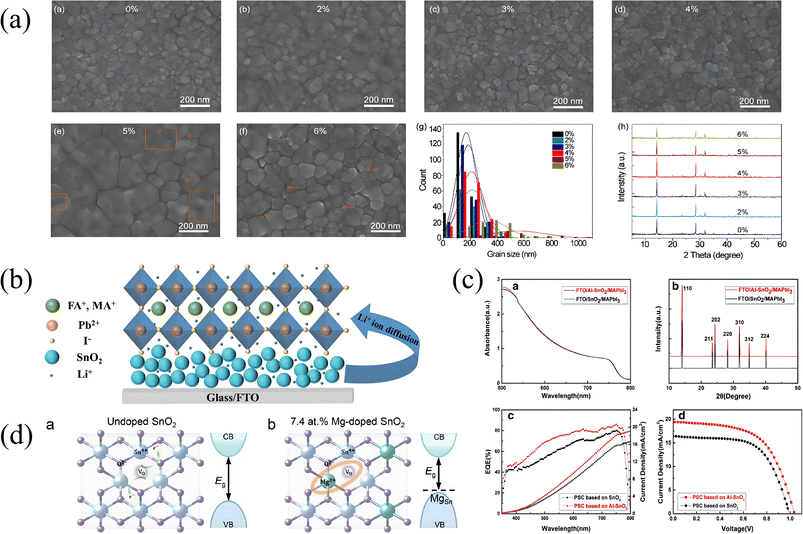 | ||
| Fig. 4 (a) The top-view SEM images of the perovskite film of (a) pristine SnO2, (b) 2% Li: SnO2, (c) 3% Li: SnO2, (d) 4% Li: SnO2, (e) 5% Li: SnO2, and (f) 6% Li: SnO2, (g) grain size distributions obtained from SEM images, abd (h) XRD patterns of perovskite films;92 Copyright 2020, Elsevier. (b) Schematic illustration of Li+ ion diffusion;93 Copyright 2022, Springer Nature. (c) (a) UV-vis absorbance spectra and (b) XRD patterns of perovskite films grown on SnO2 and Al–SnO2 substrates, (c) EQE curves and integrated current density of perovskite solar cells based on SnO2/Al–SnO2 and (d) J–V curves of the best-performing PSC using SnO2/Al–SnO2 ETLs;94 Copyright 2017, Hao Chen et al. (d) 7.4-MTO thin films and the corresponding schematic diagrams of the energy band structures.96 Copyright 2022, American Chemical society. | ||
Grain size has a great impact on device stability, interface defects, and electron transmission efficiency. As for the problem that Li, Zn, Co and Nd doping will increase the grain size of the SnO2 ETL, we conclude that they have similar ion radii and properties, so they can well replace Sn4+ or occupy its sites to form a matching lattice, thus reducing the generation of defect states, inhibiting the effect of oxygen vacancies, and increasing the chance of grain growth. For bismuthene, it mainly depends on its self-adaptive characteristics to form a lattice-matched heterojunction with SnO2 and reduce interface mismatch and defect density, so as to realize the growth of large-size grains. Because of the high quality, uniform, and compact ETL, it provides a better growth environment for perovskite films with large grain size. Of course, excessive doping may lead to additional defect states or lattice mismatch, which can explain why the grain size increases and then decreases with the increase in doping concentration.
Quy and Bark dissolved Ni(OCOCH3)2·4H2O directly in the commercial SnO2 colloidal solution to realize the doping of Ni.97 The roughness of Ni:SnO2 is more, and this may because the difference between the radius of Ni+ and Sn4+ causes lattice distortion, or the doping of Ni ions may affect the phase transition of SnO2. However, the perovskite layer grown on the doped ETL is more uniform, which also greatly improves the performance of the device. This may be because the doping of Ni reduces the defect states. In addition, it is mentioned that the ETL may have a suitable wetting surface, which is conducive to the formation of a smooth perovskite surface. Another useful metal material is Al. As shown in Fig. 4c, Al doping will not affect the formation and light absorption of perovskite; in contrast, it can improve the photoelectric performance of SnO2, optimize its energy level structure, and improve the performance of the whole device.94 Eu3+ doping helps to reduce the charge accumulation at the interface by adjusting the band structure of SnO2, and reducing the trap density in perovskite films by passivating Pb vacancies and I vacancies, and improving the carrier transport efficiency.98 Magnesium (Mg) doping can passivate oxygen vacancy defects(Vo defect), for the reason that when Mg2+ replaces Sn4+, they consume the additional electrons originally provided by oxygen vacancies, thereby reducing the concentration of free electrons in the material, and as shown in Fig. 4d, the energy level structure can be optimized in this way.96
2.3.1.2 Chlorine atoms and chlorides. Chlorine atoms and chlorides are another common dopant. Since the SnO2 precursor solutions used in a large number of studies contain Cl− ions, we believe that the presence of Cl− is beneficial to ETLs. In the above discussion, metal ions are almost always provided by metal salt solutions, the most commonly used of which are chlorides, nitrates, and acetates. The latter two can volatilize during annealing, but the Cl− provided by chlorides does not volatilize in large quantities and may combine with the main components of the ETL. However, the research mentioned above did not elaborate on this, focusing instead on metal particles. Here, we will focus on the Cl− in chlorides for a summary. Compared with metal doping, they are more involved in changing the interface and chemical properties of ETLs. Cl can successfully combine with the SnO2 surface to form enhanced bonds, which can reduce the existence of defect states, regulate energy levels, inhibit carrier recombination and improve electron transport performance, and this is main reason for the improvement of the device. Liang and co-workers used a chlorine-doped SnO2 ETL to achieve high Voc (1.195 V) on planar PSCs and the PCE reached 20% in this research.101 Ren et al. developed a direct-contact reaction process (DCRP) and used 1′2-dichlorobenzene to prepare SnO2–Cl. Compared with the original SnO2 ETL, the efficiency of the SnO2–Cl ETL is improved (17.01% to 17.81%), and the hysteresis phenomenon is significantly reduced.102
TiCl4 is a common material for chlorine incorporation. The electronegativity of Ti4+ being lower than that of Sn4+ indicates that Ti4+ has a relatively weaker ability to attract electrons. Consequently, its multivalency is lower than that of Sn4+. Due to its multivalency, Sn4+ is highly susceptible to oxygen vacancies, readily forming defect states such as Sn2+. The incorporation of Ti4+, however, enables the formation of more stable Ti–O bonds, effectively reducing oxygen vacancies. Furthermore, the addition of Ti4+ can alter the energy level structure of the ETL. By introducing a small amount of TiCl4 into the SnO2 precursor solution, Cai et al. effectively reduced the active layer surface roughness (from 29.3 to 23.3 nm, with the surface roughness of the ETL decreased from 16.7 to 13.9 nm).103 This is primarily due to the reduction in defects within the ETL, which enhances its uniformity and hydrophilicity, thereby yielding superior perovskite films. Liu et al. also used TiCl4 for dissolution in the SnO2 NCs solution.104 Different from the research above, they stirred the solution at 70 °C, so SnO2:TiO2 solution was prepared. From Fig. 5a, compared with other thin films, the SnO2:TiO2 mixed film has better surface conditions, and the device based on it achieves a PCE of up to 23.19%. Chen and co-workers regulated the performance of the ETL by introducing small molecule copper(II) chloride (CuCl2)(a low concentration and low molecular weight chloride material).105 The introduction of CuCl2 improves the dispersion of the SnO2 colloid, reduces the aggregation of SnO2 nanoparticles, and improves the uniformity and quality of the ETL. A large number of Cl ions were released at the interface between the ETL and perovskite layer, which played a great role in passivating the interface defects. As shown in the diagram in Fig. 5b, SnO2–CuCl2 has better conductivity, and the PCE and stability of the device based on it can be improved a lot. Zhu et al. from Nanjing University106 and Lin's team from the University of Tokyo107 used KCl and NaCl, respectively, to dope the SnO2 ETL, and both achieved good passivation effects and energy level optimization, finally achieving breakthroughs in device performance.
 | ||
| Fig. 5 (a) AFM height images of (a) TiO2, (b) SnO2, and (c) SnO2:TiO2 NCs deposited on FTO substrates;104 Copyright 2023, Elsevier. (b) Conductivity of SnO2 and SnO2–CuCl2 on FTO, measured in the dark, steady-state PCE at the maximum power point of different PSCs (the bias voltages are 0.90 and 0.95 V, respectively), and PCE histograms of 30 PSCs;105 Copyright 2024, American Chemical Society. (c) The schematic of the distribution of SnO2 nanocrystals in the dispersions, arrangement of SnO2 nanocrystals in the thin films, and the crystal growth of the perovskite layer on the SnO2 thin films with and without HP.113 Copyright 2020, John Wiley and Sons. | ||
Many common chloride dopants are accompanied by the doping of metal ions, such as Ti4+, K+, Cu2+, Na+, etc. We compared and summarized the working mechanism of metal ions and Cl− in the research studies and found that compared with most metal ions directly acting on Sn4+ to change the ETL energy level, Cl− may participate in the defect passivation of the ETL and perovskite interface in addition to forming a chemical bond with SnO2. Notably, when Cl− binds with Pb2+, it can mitigate the impact of lead vacancies on the entire device. The use of metal chlorides for dual-ion doping is highly effective and provides a viable approach for doping optimization in the future.
Ammonium chloride has also been used in doping for inducing modification of the tin oxide colloid.108 This modification not only improves the electron mobility of the ETL but also improves the energy band alignment with perovskite. The introduction of NH4+ and Cl− helps to optimize the ETL/perovskite interface and reduce the interface defects by inhibiting the formation of deep level defects. It is worth noting that NH4+ interacts with the surface of SnO2 to alter the zeta potential of SnO2 colloids, leading to colloidal aggregation and the formation of a denser ETL. Lin et al. proposed a new method of ETL modification by introducing perovskite precursors (methylammonium chloride MACl or formamide chloride FACl) into the SnO2 layer.109 These two types of doping materials are common core components in the preparation of perovskite solutions. Their cations can interact with the surface of SnO2, providing nucleation sites for the growth of perovskite crystals. Cl− can effectively passivate the defect states of the ETL, making the growth of perovskite crystals more uniform and compact, and with sufficiently large grain size.
2.3.1.3 Polymers. Polymers are also widely used in the modification of ETLs. Common polymers include conjugated polymers, organic polymers, etc. The electrochemical properties of conjugated polymers can be adjusted by adjusting their microstructure. Organic polymers also have a feature that other substances do not have, that is, they are eco-friendly. These polymer materials have special functional groups that can be utilized to achieve binding and modification with SnO2.
Wei et al. studied novel SnO2 in a polymer matrix (SPM) as an electron transport layer (ETL).111 Hydrophilic polyethylene glycol (PEG) molecules have an ether bond, which can form hydrogen bonds with the surface of SnO2, allowing it to adsorb onto the surface of SnO2. The long chains in their molecules increase the distance between particles, thereby limiting the aggregation of nanoparticles. Adding PEG to SnO2 colloidal solution can improve the quality of the film, and enhance the hydrophilicity, so that perovskite precursor solution can completely cover the SnO2 film without plasma treatment. The non-ionic polymer polyacrylamide (PAM) contains a large number of amide groups, and its chemical activity can lead to the formation of hydrogen bonds with SnO2, making the entire ETL have good hydrophilicity and surface morphology, which enables researchers to obtain devices with a high conversion rate (21.61%) and stability.112
According to the fabrication process shown in Fig. 5c, the high negative charge density of biopolymers (heparin potassium, HP) helps to stabilize SnO2 nanocrystals in the dispersion and then enhances interface contact.113 The –COO− group contained in HP can interact with Sn atoms, allowing the polymer to adsorb on the surface of SnO2. In this way, the negatively charged polymer will cause electrostatic repulsion between particles, preventing SnO2 from agglomerating. This can explain why HP can improve the stability of the SnO2 colloid precursor. In addition, the abundant –SO3− in HP will bind with Pb2+, which appears to enhance the contact between perovskite and the ETL, thereby reducing the defect states at the interface. The SnO2 composite, which is regulated by crystalline polymer carbonitride (cPCN), provides better energy level alignment with the perovskite layer. This is because the rich π-conjugated electron system in cPCN can adjust the band structure of SnO2 and improve the electron extraction and migration efficiency of the entire ETL.114
Kwon and co-workers also conducted research on the polymer-capped SnO2.115 The study has shown that polymers with carboxylic acid (–COOH), phosphoric acid (–PO3H2), and sulfonic acid functional groups can chemically interact with oxides and have strong binding affinity. All of them can form chemical bonds with the SnO2 surface, improve film uniformity, and reduce interface defects. Strong binding affinity is an effective means of inhibiting nanoparticle aggregation, and it can also control the growth of nanoparticles and tightly connect conductive substrates and ETLs. However, it is worth noting that the bonding strength of these three functional groups is different, and their working mechanism on ETLs is also different. Carboxylic acid groups have the strongest bonding ability, and are anchored on the surface of SnO2 by coordination or hydrogen bonds, effectively inhibiting the agglomeration of nanoparticles. The weak phosphate group mainly forms coordination bonds with SnO2 metal sites, reducing local thickness differences. The weakest sulfonic acid group mainly acts on the ETL by adsorption, so its device performance improvement is limited. The essence of doping and incorporating modification is actually the interaction of different functional groups, ions, molecules, etc., in the microstructure. Therefore, the choice of blending materials is actually the choice of functional groups.
2.3.1.4 Others. Other materials can also be used in the modification of the SnO2 ETL, such as quantum dots. This material, known as “artificial atoms”, is widely used as an additive in the mixed ETL because of its excellent electrochemical properties. Mxene quantum dots (MQDs) have different functional groups on the surface, and they can form chemical bonds with the hydroxyl groups (–OH) on the surface of SnO2, reducing the surface energy of SnO2, which can rapidly induce the nucleation of perovskite in the solution of the perovskite precursor to form an intermediate perovskite phase.116 CdS quantum dots inherently have high electron mobility, and through the coordination effect between S2− and SnO2, the conductivity of ETLs is improved.117 Black phosphorus quantum dots (BPQDs) incorporated in the SnO2 layer can not only improve the inherent defects of the SnO2 layer, but also inhibit the oxidation of BPQDs, thus improving the quality and stability of ETLs.118 Fullerene materials are the research focus for perovskite solar cells. Zhong et al. modified SnO2 by using fullerene derivatives (CPTA).119 The carboxyl groups in CPTA molecules can react chemically with the insufficiently coordinated Sn atoms on the surface of SnO2, forming chemical bonds. This interface modification enhances the adhesion between the MAPbI3 thin film and the SnO2 layer. Other inorganic materials like polyoxometalate,110 graphdiyne(GDY),120 CsI,121 potassium trifluoroacetate(KTFA),122etc., are also used in additive engineering in the previous work.
In addition, the usage of organic compounds in ETLs is also common. Liu's team from UESTC used a very novel way to use additives.123 They pre-buried volatile organic additives (HCOONH4) in the SnO2 ETL, and achieved in situ and overall modification of the ETL, the perovskite layer, and their interface through bottom-up penetration during thermal annealing. The SnO2 ETL treated with HCOONH4 showed enhanced electron extraction ability, reduced surface oxygen vacancies, and inhibited surface charge recombination. These phenomena are attributed to the bonding relationship between the amino and carboxylate functional groups and SnO2. The flexible PSCs based on this ETL achieved a PCE of up to 22.37% and the durability is good. Cobalt amine is a kind of biological macromolecular material, which is compounded with SnO2 and plays a role in interface modification through coordination bond and electrostatic interaction. The SnO2@B12 ETL also has good performance.124 The PO43− in phosphate can form a chemical bond combined with Sn to reduce the suspended bonds and the density of trapped states on the surface of SnO2; in addition to improving the electron transport capacity, it can also enhance the adhesion between the perovskite layer and ETL and reduce unnecessary degradation.142
2.3.2.1 Bilayer ETLs. Making bilayer ETLs is one of the popular directions of developing new ETLs for PSCs. The two types of ETL are often used for different materials or the same material with different preparation methods, so they have their own advantages. Combining them can improve the electrical properties, adjust the energy level, achieve better morphology of the surface, and improve the performance of the device. Broadly speaking, it is a useful way of interface engineering.
Xue et al. used a low-temperature electron beam evaporation technology to make a SnO2/TiO2 bilayer ETL.126 By combining the advantages of the SnO2 layer and the TiO2 layer, the energy band structure can be readjusted, and the surface morphology can be improved. Dropping SnO2 onto a TiO2 thin film can effectively fill the gaps on the surface of the TiO2 thin film prepared by the hydrothermal method, which is beneficial for the growth of high-quality perovskite thin films.127 This is also another major advantage of a double-layer ETL. Dong and co-workers also mentioned that the interface synergy between SnO2 and TiO2 effectively blocks the path of charge recombination and energy loss, and significantly reduces the internal series resistance of the whole PSC.128 Huo et al. constructed a SnO2/TiO2 gradient heterojunction (GHJ) by the two step CBD method.129 Due to the slightly lower conduction band of SnO2 compared to that of TiO2, an energy band gradient is formed between the two ETLs, which is almost a unidirectional electron transport structure, effectively alleviating the problems of electron reflux and carrier recombination. In addition, SnO2 and TiO2 form an interpenetrating GHJ ETL, which is highly beneficial for enhancing conductivity.
SnO2 ETLs prepared by different methods can solve the energy level mismatch between the SnO2 layer and the perovskite layer.67 SnO2 thin films prepared by spin coating on FTO may exhibit issues such as particle aggregation and mismatch with perovskite energy levels. Additionally, the unevenness of FTO can also result in poor film quality. As mentioned above, ALD can precisely control the surface morphology of thin films, resulting in excellent thin film morphology, as shown in Fig. 6a. Moreover, the study suggests that the introduction of ALD-SnO2 increases the Fermi level of the ETL. The built-in electric field created by the double-layer SnO2 structure also helps to overcome the electron accumulation at the ETL/perovskite interface, so as to achieve higher Voc and fill factor (FF). The efficiency of PSCs using this ETL increased from 22.09% to 23.86%. Besides, forming a dual ETL with doped SnO2 is also effective. Guo et al. treated SnO2 with InCl3 to achieve simultaneous doping of the ETL and passivation of interface defects.136 This treatment can reduce the oxygen vacancies on the SnO2 surface, form Pb–Cl bonds, and enhance the chemical interaction between the ETL and perovskite.
 | ||
| Fig. 6 (a) AFM images of the surfaces of the blank, ALD SnO2, control SnO2, and double SnO2 ETL capped FTO, respectively;66 Copyright 2023, John Wiley and Sons. (b) Synthetic process of SnO2 and ‘‘enveloped’’ SnO2 NCs;140 Copyright 2021, Royal Society of Chemistry. (c) Schematic diagram of reducing interface recombination by bandgap alignments. The energy levels of the dense a-WOx are experimentally determined by XPS and UV absorption;138 Copyright 2019, Elsevier. (d) Schematic illustration of NH4F treatment on the SnO2 surface.141 Copyright 2020, American Chemical Society. | ||
Al2O3 can also form a double electron transport layer with SnO2. It is worth mentioning that the UV-irradiated Al2O3 layer effectively enhances the wettability of the electron transport layer and provides a larger interface area, which is conducive to the uniform growth of the perovskite layer and improves the charge extraction efficiency.131 Sulfide can be used as an ETL too. An In2S3 and SnO2 bilayer ETL was studied in the previous work.132 The introduction of the In2S3 layer improves the band bending of the perovskite/ETL interface and increases electron transport. CdS has higher electron mobility than SnO2 and TiO2. When the CdS ETL is deposited on the SnO2 ETL, it can be used as an intermediate step to promote the electron transport from the perovskite layer to SnO2.133
Additionally, the bilayer ETL combined with C60 and ALD-SnO2 can improve the electron transport properties by increasing the density of C60 or SnO2. The conduction band offset (CBO) can indicate the mismatch of different functional layers, and an appropriate CBO value can reduce the electron transport barrier at the C60/SnO2 interface, so that the conductivity could be improved. This can also be controlled by adjusting the density of C60 or SnO2.134
2.3.2.2 Buffer layer. Adding a buffer layer is another method of regulating interface defects. Compared to the bilayer ETL structure, it involves a wider range of materials and may be accompanied by reactions or bonding that determine interface performance at the interface. Many buffer layer materials themselves have not been applied as a separate ETL in PSCs, so we distinguish them from bilayer ETLs. However, both of their key working principles are directly modifying the interface outside the original ETL, avoiding the introduction of additional substances through doping.
2.3.2.2.1 Common buffer layers. Yang et al. used novel histamine diiodate (HADI) for the modification of the SnO2/perovskite interface.135 HADI can bind with Sn4+ ions and hydroxyl groups on the surface of SnO2, which can be imagined as HADI molecules throwing a ‘boat anchor’ onto the SnO2 film, firmly binding the HADI layer and SnO2 layer. Due to the generation of hydrogen bonds, the conduction band of SnO2 may shift upward, reducing the potential barrier for electron extraction. The HADI edge in contact with perovskite can react with lead and iodine vacancies to form stable chemical bonds, reducing interface defects in perovskite. In addition, due to its suitable electrical properties, HADI acts as a ‘bridge’ for electron transport, connecting SnO2 and perovskite layers. This is the typical and main working principle of the buffer layer.
Zuo and co-workers used two different materials—CDSC and DPAH for interface modification.139 The molecules of these two materials react with the –OH group on the surface of SnO2 through an esterification reaction, or coordinate with Sn4+ in SnO2, so as to fill the oxygen vacancies in SnO2, and combine with the cation and anion defects in perovskite films through ion bond interaction, so as to realize the passivation of defects. Fig. 6b shows a novel way to modify the SnO2 layer. Yuan et al. formed an amorphous NbOx layer to reduce the surface defects and promote the favourable growth of perovskite.140 NbOx forms chemical bonds with oxygen atoms on the surface of SnO2, passivating suspended components and defect states on its surface. Nb atoms react with Pb2+and I− to passivate defects on the surface of the perovskite layer, which also leads to a dual interface passivation effect simultaneously. Zhuang and co-workers used two different ways for modifying SnO2—doping and a buffer layer.143 Here, let's focus on the latter. The addition of RbF between SnO2 and perovskite did not change the electron mobility, but the presence of Rb+ can inhibit the ion diffusion of the perovskite layer.
C60 can also be used as a buffer layer in PSCs. Zhu et al. spin-coated C60 with a thickness of approximately 20–30 nm between the perovskite layer and SnO2, and the energy level can be readjusted.146 With the excellent properties of SnO2 nanocrystals and the help of C60, the electronic transmission efficiency of the PSC was greatly improved, and finally, a PCE of 18.8% was obtained. The introduction of a glycine layer can be used to adjust the interfacial stress caused by the lattice mismatch between SnO2, and perovskite and the interface interaction between SnO2 and perovskite is enhanced by hydrogen bonds and/or electrostatic interactions between amino acids and perovskite frameworks.147
The position of the buffer layer is also not fixed; it can be between the perovskite layer and the ETL, or between the ETL and the electrode. In p–i–n PSCs, the materials in the latter application often have high resistance to water and oxygen, preventing water and oxygen in air from damaging the perovskite. In addition, some materials can also prevent metal electrode materials from leaking into the internal functional layer. In n–i–p devices, the buffer layer between the electrode and ETL may provide a smoother ETL growth platform, regulate energy levels, transfer electrons, etc. Two-dimensional carbide (MXene) can be used for modifying the FTO/SnO2 ETL. After the introduction of the MXene layer, a better energy level arrangement of FTO/MXene/SnO2/perovskite is realized.137 In this structure, the working principle of the optimization of the energy level can also be attributed to the functional groups that combine with SnO2. The device achieves 20.65% efficiency, with extremely low saturation current density and negligible hysteresis. Wang et al. used a low temperature treated amorphous tungsten oxide (a-WOx) and SnO2 to form a mixed ETL.138 The a-WOx buffer layer was prepared using vacuum evaporation with a thickness of 5–15 nm. Comparative experiments were conducted at different film thicknesses, revealing that device performance reached its optimum at 10 nm. It is worth noting that a-WOx does not seem to undergo very complex chemical bonding with SnO2, and its function is simple: it acts as a hole isolation barrier, as shown in Fig. 6c. Coincidentally, it also has suitable energy levels and excellent electron mobility, so its existence greatly enhances the overall performance of the device.
It should be noted that sometimes SnO2 can be used as an intermediate layer by itself. Dang's team improved the ZTO/perovskite interface by introducing a thin SnO2 interlayer. It was found that the Zn1Sn1Ox (with the thickness around 40 nm)/SnO2 ETL device exhibited the highest power conversion efficiency (PCE) of 19.01% and better stability.148 The excellent electrical properties of SnO2 are the key reason for the progress of the device.
2.3.2.2.2 Self-growth buffer layer. Almost all the methods for obtaining the buffer layer described above are to prepare the buffer layer solution first, and then directly apply it on the functional layer by spin coating or other methods. This method of preparing a functional layer separately usually leads to a more complicated preparation process and also carries the risk of film growth failure. In fact, the buffer layer can also be obtained by reactive growth between functional layers through technical means. This method of self-growing the buffer layer avoids the above-mentioned problems. Min et al. provided a new idea for the obtention of a buffer layer through direct growth.144 First, a SnO2 thin film (Cl-bSO) was prepared by using ETL solution containing Cl−, and then Cl-cPP (Cl− containing FAPbI3 perovskite precursor) solution was prepared by mixing MACl and other solutions as the Cl− source into FAPbI3 precursor solution. Using Cl− containing FAPbI3 perovskite precursor solution to grow on the perovskite layer on Cl-bSO. Because Cl-bSO contains a large number of Sn–Cl bonds, when Cl− in Cl-cPP comes into contact with these chemical bonds at the interface, it will react and form an FASnClx intermediate layer under the action of FA+. Due to this special synthesis method, the ETL/buffer layer/perovskite layer is almost integrated, which greatly reduces the generation of interface defect states and makes the transmission of electrons more efficient. They finally achieved the certified PCE of 25.5%. Zhou et al. immersed a SnO2 ETL into the TiCl4 solution, and TiCl4 reacts with the –OH on the SnO2 surface to form a Ti–O–Sn bond. The Cl− will also participate in the whole reaction and finally form a TiOxCl4−2x buffer layer after the solution hydrolysis at 75 °C.145 Excess ions or oxygen vacancies will participate in the whole reaction and form the buffer layer. Therefore, the defect states are also reduced, and the ETL level is optimized. The TiOxCl4−2x layer improves the permeability of the SnO2 layer surface to the perovskite precursor, and helps to form a denser, nonporous perovskite film on the surface.
Sargent's team used NH4F to modify the surface of the ETL.141 As shown in Fig. 6d, the NH4+ in NH4F is weakly acidic and can react with the terminal hydroxyl group (OHT) on the surface of SnO2. F− will replace the OHT group and enter the defect site on the surface of SnO2 during the reaction. The samples treated with NH4F had higher quenching efficiency, indicating that NH4F treatment improved the surface of SnO2 and was conducive to the extraction of electrons from perovskite films. A PCE of 23.2% was finally achieved by this solar cell. Therefore, it can be used in interface modification engineering too.
Compared to methods such as spin coating that directly prepare buffer layers on SnO2, self-grown buffer layers are typically extremely thin (approximately several nanometers) and grow directly on the ETL surface. We believe that thicker layers must balance properties such as energy level alignment, surface wettability, and electron transport capability. In contrast, thinner layers, due to the presence of the quantum tunneling effect,270 appear to focus more on enhancing the surface properties of the ETL, such as improving hydrophilicity, providing support or connection for active layer growth, altering the conductivity of the ETL, and reducing defect states. Self-growth of the buffer layer is an interesting research direction, but we believe it also raises several issues that require further exploration. First, the reaction range needs to be strictly controlled to avoid contamination of the ETL. Second, the surface morphology needs to be studied. Extensive surface defects are detrimental to PSCs, and it is necessary to examine the surface of the reactively grown buffer layer and the interface between the buffer layer and the ETL. Third, the impact of by-products on the overall device needs to be minimized.
Actually, interface engineering involves a wide range of technologies, and the boundaries between different approaches are subtle; here, we summarize the technologies of applying an obvious modified layer (ETL/middle layer/buffer layer, etc.) or a modified material for interface engineering to distinguish from doping technology. Fig. 7 shows the working principle of the main materials mentioned above for intuitive comparison.
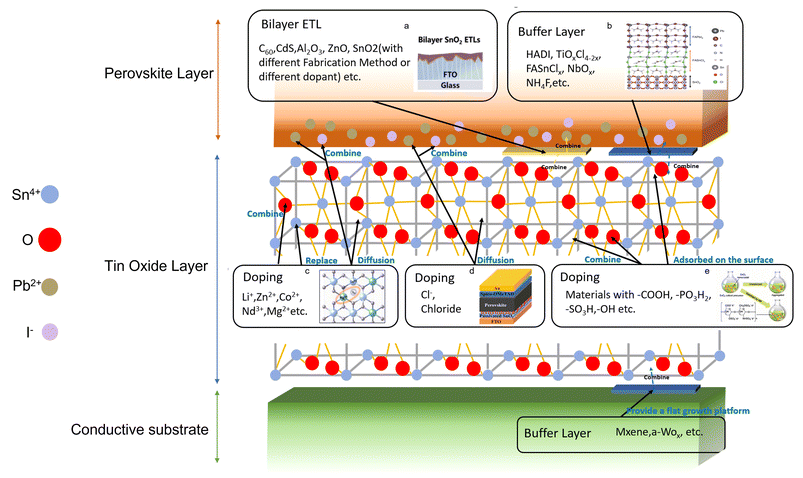 | ||
| Fig. 7 The diagram of basic modification for the SnO2 ETL. (a) Figure reproduced with permission.130 Copyright 2023, John Wiley and Son. (b) Figure reproduced with permission.144 Copyright 2021, The Author, under exclusive licence to Springer Nature Limited. (c) Figure reproduced with permission.96 Copyright 2022, American Chemical society. (d) Figure reproduced with permission.101 Copyright 2019, American Chemical Society. (e) Figure reproduced with permission.113 Copyright 2020, John Wiley and Sons. | ||
3. Zinc oxide
3.1 Basic properties and advantages
As another common metal oxide that can be prepared by a low-temperature processing method, zinc oxide (ZnO) exhibits great performance in the applications of PSCs. The inherent nature of ZnO provides great help for high performance of devices: having (1) good light transmittance in the visible light range; (2) high electron mobility; (3) diverse nanostructure; (4) ease of modification.149–151 Compared with SnO2, ZnO often has higher electron mobility (205–300 cm2 V−1 s−1 (ref. 149)), and lower processed temperature.151 Actually, TiO2 was once the first generation of the MO ETM, and SnO2 follows closely behind. Nowadays, for these excellent properties, ZnO has gained more attention, especially in flexible perovskite solar cells.3.2 Fabrication methods of ZnO
Like the SnO2 ETL, the ZnO ETL has many preparation methods, each of which has its own characteristics, advantages, and application scenarios. In addition, different preparation methods can also lead to different structures of ZnO, resulting in different electrochemical properties. Here, a summary and organization of different methods will be provided.The typical precursor solution for the fabrication of ZnO is zinc acetate dihydrate (ZnAc2),153–155 as shown in Fig. 8a. However, this could induce severe surface defects,156,157 and the organic compounds will remain for quite a while.158,159 Chavan et al. employed a diethylzinc(Et2Zn) DMSO solution as the zinc source.160 Et2Zn was injected into dimethyl sulfoxide (DMSO) solution to form ZnO QDs, which were then spin-coated on FTO. In this way, the quality and purity of the film have been improved, as the surface performance. Runjhun et al. also used Et2Zn as a zinc source, and with a wet organometallic process, successfully fabricated a ZnO QD film.161 By dissolving Et2Zn and betaine (N,N,N-trimethylglycine) in tetrahydrofuran, Zn2+ reacts with amino and carboxyl groups in betaine to form stable intermediates. Finally, these intermediates are stirred in air and oxidized into ZnO quantum dots using H2O and O2 from the atmosphere. This method can be used to precisely control the structure and quality of nanomaterials by selecting different organic ligands and reaction conditions. Zheng and co-workers used another material—Zn(NO3)2·6H2O as the precursor and achieved a ZnO film with low surface roughness and high electron transport efficiency.162 Zhou et al. employed the stable aqueous solution of an ammine–hydroxo zinc complex, [Zn(NH3)x](OH)2 for fabricating the ZnO ETL.163 In this way, the work function of ZnO thin films can be reduced and the Voc can be improved. Kelly's team spin-coated ZnO nanoparticle solution on the glass substrate three times to obtain a continuous, smooth ZnO film and this can also be seen as a solution-processed method.164
 | ||
| Fig. 8 (a) The standard sol–gel process;160 Copyright 2022, John Wiley and Sons. (b) Schematic of a rotating cylinder reactor showing one cycle of ZnO S-ALD using diethylzinc and ozone as reactants;169 Copyright 2015, American Vacuum Society. (c) Device structure of PSCs with a ZnO seed layer;173 Copyright 2016, Tsinghua University Press and Springer-Verlag Berlin Heidelberg. (d) Graphical depiction of the inside of the sputtering chamber;175 Copyright 2019, American Chemical society. (e) The process of the combustion method for preparing the ZnO-ETL;162 Copyright 2019, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) Representation of the synthesis route and the flowchart utilized for producing silver-doped zinc oxide nanoparticles (Ag@ZnO NPs).202 Copyright 2024, Elsevier. | ||
There are various precursors of ZnO, and we will make a brief summary here. First, the traditional acetate dihydrate often decomposes into ZnO by high-temperature annealing. The process is relatively simple, but the morphology of the ZnO film is rough, which can be easily foreseen. Next, the working process and advantages of Et2Zn have been mentioned before, and it is often used for forming ZnO QDs. Zn(NO3)2·6H2O has the same ZnO preparation process as acetate dihydrate, and with NO3−, which is more volatile during annealing, it can form a high-quality polycrystalline structure, but due to incomplete decomposition and other problems, the quality of the film will also be reduced. The last one is [Zn(NH3)x](OH)2, which is also decomposed by annealing to obtain ZnO. However, due to the presence of NH3−, with a faster volatilization rate, the structure of the whole solution may be destroyed before the formation of large-scale ZnO, so its crystallinity should be less than that of Zn(NO3)2·6H2O.
However, magnetron sputtering films will have some traps. To solve these problems, Abhishek and co-workers proposed a new sputtering technology—high-working-pressure sputtering (HWP).175 In the process of HWP, by controlling the working pressure in the sputtering chamber (Fig. 8d), the energy of high-energy particles (such as Ar) is reduced, thus reducing the number of recombination centers and defects. A PCE of 17.3% was achieved by using ZnO PSCs sputtered by HWP. Banerjee et al. used a low-temperature vacuum-based sputter method and achieved a ZnO film with good crystallinity and high transparency.176 This method also revealed the application potential of ZnO in flexible equipment.
As we can see in this part, different nanostructures of ZnO can be formed by the hydrothermal method. Here, we make a brief summary of the various nanostructures achieved by this method in Table 3.
| Structure | Diagram | Cause of formation | Characteristics | Ref. |
|---|---|---|---|---|
| Nanodisk | (a) | At 90 °C, the precursor hydrolyzes rapidly and has no constant growth direction | Disk structure may increase grain boundaries | 178 |
| Nanowell | (b) | At 80 °C, the precursor slowly hydrolyzes to form a porous sponge structure | Perovskite can leak and increase the interface contact with perovskite | 178 |
| Nanorods | (c) | After embedding the ZnO seed layer, one-dimensional growth was promoted by adjusting the pH value | Provide a direct electronic transmission path | 180 |
| Nanocandles | (d) | The doping of Au atoms changes the lattice stress, resulting in delamination or tip growth of nanorods | Due to the introduction of Au, the conductivity has been significantly improved | 184 |
| Nanotubes | (e) | After being grown into nanorods by the hydrothermal method, the more easily soluble polar [001] plane is corroded by ammonia solution | Tube like structure reduces carrier recombination and adapts to flexible substrates | 182 and 183 |
| Nanoparticles | (f) | By adding the corresponding precipitant and reacting with Zn(NO3)2 | There are various preparation methods and a wide range of applications, but due to the possible production of related by-products by precipitants, there may be more impurities or defect states in the film | 185 and 186 |
| Nanowires | (g) | Growing on an AZO substrate, due to the lattice matching properties of AZO, it directly promotes the directional growth of ZnO | High electron mobility and strong light capture capability | 179 |
|
||||
In 2014, Zhang et al. grew nano-ZnO thin films by electrochemical deposition.187 The film grown by this method has high quality and can be accurately controlled at low temperature. Then in 2015, Zhang and colleagues used electrochemical deposition technology to prepare ZnO on flexible substrates.188 They also analysed the effect of different deposition times on the performance. This research deepens people's understanding of electrochemical technology and also proves the feasibility of this technology in flexible solar cells. Christian and co-worker used 0.08 M zinc nitrate (Zn(NO3)2) solution as the deposition solution, and zinc wire as the electrode to deposit ZnO on ITO at 60 °C.189 Like Zn(NO3)2 solution, Zn(NO3)2·5H2O is also the typical deposition material.190 In 2018, Garcia's team chose to use ZnCl2 and KCl mixed solution as the electrolyte and also achieved an ideal result.191
Electrochemical deposition also has some drawbacks: high energy consumption, complex post-processing, accurate control of parameters such as time and temperature, and last but not least, environmental issues.
As mentioned before, different methods have different advantages and drawbacks, and people should use a suitable method according to the specific application. Furthermore, the development of new preparation methods can also be used as a hot direction to promote the development of photovoltaic devices.
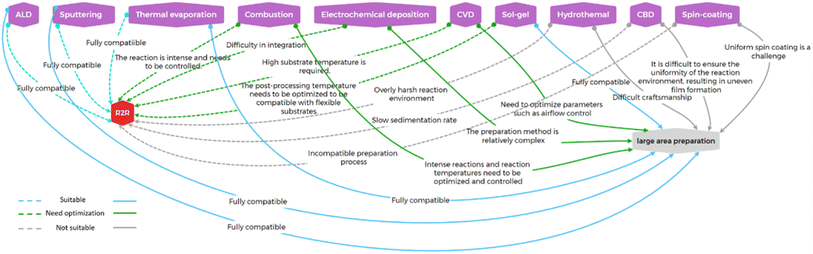
Thus far, the primary low-temperature methods for fabricating MO ETLs have been summarized and explained. Research into low-temperature fabrication techniques aims to integrate them with roll-to-roll processes or large-area device fabrication methods to achieve commercialization. In Table 4, we analyze and discuss the compatibility of the mentioned processes with large-area fabrication techniques.
3.3 Modifications of the ZnO ETL
The ZnO ETL has been widely used in PSCs in the last few decades, and their performance has achieved huge progress. However, ZnO also has some defects: ZnO and perovskite materials (especially lead-containing materials) will have serious interface recombination,196 which is due to the alkalinity of ZnO,197 which will affect the stability of devices. Moreover, when the temperature is too high, ZnO can promote the deprotonation of formamide cations and promote the decomposition of the perovskite layer.198 ZnO has an open hexagonal close-packed lattice structure, and Zn atoms occupy only half of the tetrahedral positions, resulting in a large number of octahedral vacancies. These defects may act as charge recombination centers and leakage current paths, leading to charge transfer barriers and water penetration.199 Modification of the ZnO ETL is the main means to solve these problems.3.3.1.1 Metals and metal ions. For the doping of ZnO, most researchers choose to use metal materials and metal ions as dopants. Metal doping helps to improve the interface morphology, reduce the defect density, modify the energy level structure, and achieve performance improvement and breakthroughs.
Cardozo's team used Ag to dope ETLs.202 They synthesized silver-doped zinc oxide (Ag@ZnO) by using a safe and sustainable by design (SSbD) method. The specific method is mixing Zn(NO3)2·6H2O with AgNO3, then adding VERDEQANT, and finally heating and decomposing into the Ag@ZnO film as shown in Fig. 8f. This method avoids the use of ethylene glycol; instead, the vegetal biocompatible polymers are used as gels, which are renewable and eco-friendly.
Cu doping leads to a decrease in the Fermi energy level of ZnO and the formation of a shallow intermediate energy level, which is better aligned with the conduction band, thus improving the charge extraction efficiency.200 Another transition metal—nickel was also used by Guchhait's team.201 Ni-doped ZnO NRs have a low electronic band gap and good conductivity. Both of these metal materials mainly enter the ZnO lattice by replacing Zn2+, which changes the electron cloud distribution in the ZnO lattice and thus affects the electron transport characteristics and energy levels of the entire ETL.
Gantumur et al. focused on the study of tungsten-doped zinc oxide.203 Tungsten has attracted much attention due to its low reactivity and ion radius (0.064 nm) similar to Zn2+ (0.074 nm).204,205 By using aloe vera leaf extract as a powerful reductant, it can effectively reduce metal ions and prevent the aggregation of ZnO:W nanoparticles. The working principle of W is also similar to Cu and Ni mentioned above, replacing Zn2+ to enter the lattice and improve the conductivity of the ETL. W-doped ZnO nanoparticles (NPs) were synthesized by a green synthesis method, and the whole process can be seen in Fig. 9a. The ZnO:W prepared by this method also has better crystallinity and larger grain size as shown in Fig. 9b(a) and (b), which is helpful to form a uniform perovskite layer, provide better energy level matching, and reduce the recombination of charge carriers.
 | ||
| Fig. 9 (a) Schematic illustration of the W-doped ZnO ETL fabrication, and the inset shows the phytochemicals' effect on the formation of W-doped ZnO NPs; (b) schematic illustration of the degradation of the PSCs with (a) pristine ZnO and (b) ZnO:W ETLs;203 Copyright 2024, American Chemical society. (c) Top-view SEM images of the CsPbI2Br perovskite film based on (a) and (c) ZnO and (b) and (d) ZnO:Ca ETLs. AFM images of the CsPbI2Br films based on (e) ZnO and (f) ZnO:Ca ETLs;213 Copyright 2023, AIP Publishing. | ||
Gallium ion doping also has a good effect.206 Because the ion radii of Ga and Zn are similar, Zn2+ in GZO can be replaced by Ga3+ without interfering with the ZnO structure. The conduction band of GZO is higher than that of CsGeI3, which ensures that electrons can be effectively transferred from the calcium titanite layer to the GZO layer. The doping of Ca2+ is also the same. Because of the doping of Ca2+, which enhances the hydrophilicity of ZnO:Ca, the CsPbI2Br film on the Ca2+-doped ZnO ETL has a more uniform surface and larger grain size, as shown in Fig. 9c, and the efficiency of ZnO:Ca based solar cells reaches 16.39%.213
AZO is the new ETL which uses Al to dope ZnO; it has high electron mobility and good energy level matching with perovskite materials.207 Yang and co-workers used this material.208 We will focus on the PL spectrum test shown in Fig. 10a. Al3+ replacing Zn2+ and entering the ZnO lattice can effectively improve the crystal quality of ZnO and reduce oxygen vacancies. The peak value of PL indicates the level of carrier recombination. From the fact that a doping concentration of 7% has the lowest PL peak, it can be seen that the carrier recombination probability is the lowest at this time. However, as the doping concentration increases, carrier recombination becomes more severe, which may be due to excessive Al doping introducing new defect states or damaging the original ZnO lattice structure. Pramothkumar and co-workers used a novel method; they doped Al and Sn into ZnO.209 Using Al–Sn co-doped ZnO as the ETL can successfully adjust the optical band gap position and enhance the conductivity of the ETL. Research has shown that Al and Sn exhibit a synergistic effect. Replacing Zn2+ with Al3+ will introduce additional charges and improve conductivity. The substitution of Sn2+ will change the local electron cloud distribution, thereby altering the band gap. In addition, the quantum confinement effect introduced by doping makes the energy state of electrons more discrete, which improves the optical absorption and the generation of photogenerated carriers.
 | ||
| Fig. 10 (a) PL spectra of perovskite CsPbBr3 films based on AZO films with different Al doping ratios;208 Copyright 2023, American Chemical society. (b) (a) 1D g-C3N4 additive added ZnO layer charge transport mechanism. (b) Schematic diagram of the fabricated device structure with compounds (c) and (d) the 1D g-C3N4 additive without and with the FTO/perovskite interface electron transportation process;216 Copyright 2023, Elsevier. (c) SEM images of (a) pristine ZnO and (b) PbI2:ZnO films; contact angle tests of the (c) pristine ZnO and (d) PbI2:ZnO films.217 Copyright 2022, John Wiley and Sons. (d) (a) Steady-state PL spectra and (b) XRD patterns of ZnO and Mg0.1Zn0.9O on ITO substrates;223 Copyright 2021, Elsevier. (e) Schematic diagrams of band alignment and charge recombination in the cells with the (a) ZnO single layer and (b) TiO2/ZnO bilayer as the compact layers, respectively;225 Copyright 2015, Royal Society of Chemistry. | ||
Khan's team used different Cd/Zn molar ratios for doping.210 Cd doping can reduce the grain size, help to reduce the grain boundary, and reduce the resistance of electron transmission. Cd doping can also reduce the non-radiative recombination in the material, thus reducing the recombination loss of electron–hole pairs. Moreover, appropriate Cd concentration helps to improve the internal reflection of light in the ETL and perovskite layer, reduce the escape of light, increase the light absorption, and thus improve the Jsc.
Other metal dopants are briefly summarized below, and their main doping principles are similar to those of the above materials. If there are significant differences, we will mention them in the following text. Li doping can change the energy band structure of ZnO, improve the minimum conduction band (CBM), fill some oxygen vacancies, and reduce electron traps, so as to improve the mobility of electrons and the conductivity of devices.211 Mg-doped ZnO nanofibers have a uniform nanometer diameter and porous structure, which is conducive to providing more electron transmission channels.212 Mn doping reduces the alkalinity of ZnO and effectively controls the deprotonation of perovskite at the Mn: ZnO/perovskite interface. Mn4+ doping produces two free electrons in ZnO, which leads to the improvement of the conductivity of Mn:ZnO thin films.214
3.3.1.2 Others. Other materials can also be added into the ZnO ETL for improving the performance.
In the ZnO lattice, carbon doping can replace the position of oxygen atoms or enter the interstitial positions of the lattice, introducing new electronic states, which is helpful to improve the transmission and collection of electrons. Gouda et al. used carbon-doped zinc oxide (ZnO/C) derived from metal–organic framework (MOF) as the electron transport layer in high-performance perovskite solar cells.215 By calcining MOFs, partial carbon loss was achieved, and ZnO/C composite materials with a high specific surface area and uniform pore structure were prepared, which helps to improve the interfacial adhesion between the ETM and perovskite.
Rajaram and co-worker added 1D graphitic carbon nitride into the ZnO ETL.216 1D g-C3N4 and ZnO nanoparticles were mixed by high-energy ball milling to prepare composite materials for ETLs. The existence of additives on the ZnO layer reduces the surface defects. The 1D g-C3N4 additive acts as an electron transport bridge in the ZnO layer, which improves the transmission efficiency of electrons from the perovskite layer to the ETL, as shown in Fig. 10b.
Wu et al. introduced PbI2 to increase oxygen vacancies in ZnO.217 Due to the large ionic radius of Pb2+, it is difficult for it to enter the lattice of Zn2+, which can lead to more defects, such as oxygen vacancies, during the formation of the thin film. The increase in oxygen vacancies in ZnO not only promotes the growth of all inorganic perovskite layers, and improves their morphology and orientation order, but also forms a favorable energy level arrangement with the perovskite layer. As shown in Fig. 10c, PbI2:ZnO thin films exhibit a smoother surface and higher hydrophilicity than the original ZnO thin films, which is conducive to the formation of a more uniform perovskite layer.
Zhang et al. explored cesium salts with functional anions such as acetate (AC−), fluoride (F−), and trifluoroacetate (TFA−) to regulate the deposition of ZnO films.218 These functional anions can coordinate with Zn2+ and Pb2+ ions to achieve defect passivation of ZnO and top perovskite films. Cs+ ions can also reduce hydroxyl defects and form interface dipoles on the ZnO surface through Zn–O–Cs bonds, thus establishing more stable and efficient interface electron transport.
Polymers are widely used too, and we have specifically explained that the interaction between polymers and metal oxides is mainly attributed to the reaction and bonding of different functional groups with the surface of MO. This rule also applies here. Polyethylene glycol (PEG) has a strong interaction with the ZnO surface through its internal functional groups, such as hydroxyl and ether bonds, to achieve the effect of adsorption and filling oxygen vacancies, which can effectively passivate the surface defects of ZnO, increase the conductivity, improve the stability of ZnO solution, and reduce the aggregation of ZnO nanoparticles.219 Poly(vinyl alcohol) (PVA) contains a large number of hydroxyl groups and has good hydrophilicity, which makes the preparation of the solution easier.220 In a polar solution, it can promote the dissolution of ZnO NPs and help to form high-quality and uniform films.
3.3.2.1 Bilayer ETL. The double electron transport layer can greatly improve the electrical performance of devices and the materials for combination are various.
Mahmood's team synthesized a bilayer ZnO ETL by the low-temperature hydrothermal method.177 The structure consists of horizontally arranged nanosheets (as the bottom layer) and vertically arranged nanorods (as the top layer). The former provides a basic support for the growth of thin films with a uniform surface and excellent electron transport performance, while the latter provides a ‘fast road’ for electron transport. In addition, due to the special structure of nanorods, it can also increase the contact area with the perovskite layer. In 2019, they made improvements to the device.192 They used ZnO nanosheets and ZnO nanoparticles as a bilayer ETL with polyethyleneimine (PEI) coated on them. PEI can reduce the working function, accelerate electron transfer, and improve the contact between the perovskite and the ETL. The double-layer ZnO ETL coated with PEI further improves the power conversion efficiency of the device to 16.39%, exhibiting no hysteresis phenomenon.
Mahmud and co-workers studied single, double, and triple ZnO ETLs by preparing layers with different thicknesses (25, 45, and 60 nm, respectively).221 The double-layer ZnO ETL provides high transparency and low reflectivity, ensuring that more light energy is absorbed by the perovskite layer, balancing the needs of electron transport and light absorption. Compared with the double-layer device, the triple-layer ETL device greatly increases the complexity of the device preparation process due to the increase in the number of interfaces, and may also introduce more interface defects. Therefore, the effect is not as good as the double-layer device. This also reflects that blindly increasing the electron transport layer is not an effective means to improve the performance of the device. The greatly increased preparation difficulty, reduced energy level compatibility, negatively affected light-transmittance of the n–i–p device, as well as the increased interface defects or interactions, can all influence the overall device performance. The core of a double-layer ETL to improve device performance is to realize the complementarity or synergy between different ETL performances, which is definitely not a simple superposition of conductive performance. The key is to find suitable and matching ETL materials or preparation methods.
Wu et al. proposed an ETL design of ZnO/Al-doped ZnO (AZO) bilayer films by low-temperature solution treatment based on stepped band alignment.222 The conduction band minimum (CBM) of AZO is slightly higher than that of ZnO, which is helpful for the extraction of electrons from the CH3NH3PbI3 active layer.
Li et al. synthesized a ZnO/Mg-doped ZnO (MgxZn1−xO) bilayer ETL by optimizing the annealing temperature of the ZnO ETL and the Mg content in Mg-doped ZnO thin films.223 As shown in Fig. 10d, the XRD image shows that the doping of Mg does not significantly change the lattice structure of ZnO, which makes the Mg:ZnO film better match the pure ZnO film. In addition, it is not difficult to find from the PL image that the carrier recombination of the double ETL is reduced, and the perovskite grown on it also has larger crystal cells.
TiO2 is a typical MO ETL material. As shown in Fig. 10e, the TiO2/ZnO bilayer may form a type II band structure, which is conducive to the transmission of electrons at the TiO2/ZnO interface and inhibits the charge recombination at the interface of the ZnO layer and ZnO/perovskite absorption layer.225
Samavati's team used Ag-doped ZnO and ZnO to prepare a bilayer ETL.224 Ag-doped ZnO has excellent surface properties, and the surface morphology of the ETL was optimized by adding ethanol to the dispersion of Ag-doped ZnO NPs, as shown in Fig. 11a.
 | ||
| Fig. 11 (a) AFM images of (A) glass/ZnO/H2O–Ag-doped ZnO 1 wt%, and (B) glass/ZnO/H2O–ethanol mixture-Ag-doped ZnO 1 wt% bilayer ETLs;224 Copyright 2024, Springer Nature. (b) (a) Schematic diagram of the preparation of the iodine ligand-modified ZnO/PbS-TBAI ETLs, (b) schematic illustration of the replacement of oleic acid ligands by iodide ion ligands on PbS QD films by TBAI treatment;236 Copyright 2022, Elsevier. (c) Schematic illustrating the extraction and transportion of electrons in flower-like TiO2 ETL based devices;241 Copyright 2016, Royal Society of Chemistry. (d) (a) Schematic device architecture and charge transport pathway in conducting substrates through TiO2 NaPAs and (b) cross-sectional SEM image of TiO2 NaPA based PSCs.249 Copyright 2021, Springer Nature. | ||
SnO2 has many advantages, as shown in the above content, and it can also be used as a part of a double ETL. Wu and co-workers achieved a Voc of up to 1.15 V and PCE of 19.1% by introducing PSCs prepared by using a ZnO/SnO2 bilayer ETL.222 The recombination process in PSCs was analyzed by steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) measurements. It was found that the ZnO/SnO2 bilayer ETL reduced the recombination loss during electron transport. Dkhili et al. also found that this structure, with H2O and KOH treatment on the SnO2 layer, exhibits better wettability on glass substrates than on PET substrates, which helps to form a more uniform and higher quality perovskite layer.226 Good wettability can promote the uniform coating of perovskite precursor solution on the surface of the ETL, thereby facilitating the formation of uniformly dense perovskite thin films.
PCBM is the traditional ETL for PSCs. Qiu et al. used PCBM to combine the ZnO ETL and achieved a device with PCE exceeding 14%.227 As an organic material, PCBM can improve energy level alignment and reduce surface recombination, while the ZnO layer acts as a physical barrier to prevent direct contact between the metal electrode and perovskite layer. Zhang et al. also found that PCBM can be used as an interface modifier, which can not only improve the thermal stability of formamide lead iodide, but also significantly reduce the current voltage hysteresis phenomenon.228
We found that a bilayer ETL can be widely used in p–i–n PSCs. Spin-coating aqueous solutions onto perovskites is undesirable, as it can severely damage the perovskite active layer (AL). Therefore, the electron transport layer (ETL) materials used in conventional p–i–n PSCs are non-aqueous solutions such as PC61BM or C60. In a bilayer ETL structure, these non-aqueous ETMs form a protective film for the perovskite, reducing the impact of aqueous metal oxide solutions on the active layer. In related experiments, we believe that the density of the ETL, acting as a protective film, is crucial. Defects such as pinholes can damage the density of the AL. Furthermore, energy level matching requires attention. Electrodes (such as Ag and Au) can experience corrosion and diffusion after device fabrication, which can severely compromise device stability.271 This necessitates improving the metal oxide ETL's ability to suppress metal ion diffusion. Furthermore, doping is crucial for improving the ETLs' resistance to water and oxygen. Adding a buffer layer such as BCP introduces additional interfaces, significantly increasing fabrication complexity. Therefore, we prioritize doping in these research studies.
3.3.2.2 Buffer layer. As mentioned in the above content, the ZnO ETL and perovskite layer will react and disrupt stability.
Let's take WO3 as a simple example to start this part. WO3 has a more conductive band position than perovskite and has better hydrophilicity. Adding WO3 as a buffer layer between the perovskite layer and ETL can form a good energy level arrangement between the three, making electron transfer more effective. Next, it can make the preparation of perovskite films more uniform and the film quality better.235 This is the main task of a buffer layer. In order to make the buffer layer play a more effective role, many researchers will also focus on the interaction between the buffer layer and the perovskite layer or ETL layer, and make the buffer layer well-connected with other functional layers through chemical bonding, or even change the electrochemical properties.
Cheng et al. from City University of Hong Kong used PEI to prepare a buffer layer to solve this problem.229 PEI has a high molecular weight, which enables it to form a stable and uniform coverage on ZnO without aggregation or diffusion during annealing, and its strong intermolecular force can also prevent the invasion of water and oxygen molecules and protect the perovskite layer. Additionally, the electron affinity of PEI may be more matched with ZnO NPs and perovskite layers, facilitating electron extraction and transport, and reducing electron loss at the interface. Qiu et al. focused on the ZnO ETL modified by the PCBM layer, and they found that the PCBM layer, as an organic molecule, can form a tight bond with the ZnO layer and perovskite layer, which helps accelerate the extraction and transfer of electrons, thereby improving the efficiency of the solar cell.230
Here we need to make some explanations. The combination of PCBM and ZnO has been mentioned in many studies, and they are in the form of a double-layer structure. We distinguish and summarize the ‘buffer layer’ and ‘bilayer ETL’ according to the description of the research. However, for the main working mechanism of PCBM and ZnO, we give the following opinions: when PCBM is combined with ZnO as a double ETL, the research studies will focus more on the synergy between PCBM and ZnO, such as the formation of a gradient energy level; when PCBM is used as a buffer layer, we think that the research studies tend to see the two as a whole, emphasizing the auxiliary role of PCBM, such as isolating the perovskite layer and ZnO to alleviate the degradation of materials, etc., and the main body of the ETL is still ZnO. Finally, we still believe that the added ETL is a special buffer layer. So I hope you don't have to worry too much about the distinction here, just focus on the performance brought by the material itself.
Runjhun and co-workers coated the NH4X solution on a ZnO QD layer.231 The ZnO QD based devices treated with NH4F passivation exhibited a high PCE of 21.9%, which is currently one of the highest performances reported in the literature among ZnO-based PSCs. After NH4F treatment, the Fermi level of ZnO thin films shifts downward, which helps improve the alignment of energy levels between ZnO and perovskite layers and promotes the transfer of electrons from perovskite layers to ZnO layers. Furthermore, NH4F can also passivate the surface of ZnO films and reduce its defects. Murugadoss et al. from Korea University studied different concentrations of NH4F in the application of a modification layer.232 They found that high concentrations of NH4F inhibited the crystallization of ZnO. At appropriate concentrations, NH4F treatment introduces fluorine atoms, which replace the hydroxyl (–OH) and oxygen vacancies on the surface of ZnO, helping to reduce leakage current in the device.
Tulus et al. reduced the defect density on the surface of ZnO nanorods by thermally depositing Au nanoparticles (approximately 4 nm) on the surface of ZnO nanorods.233 This sedimentation method avoids the contamination of the ZnO surface by chemical residues. However, although Au nanoparticles improve the initial performance of the solar cell, they do not enhance the stability of perovskite solar cells under continuous illumination. In 2022, they chose to use 25 nm C60 as the modification layer.234 Research has shown that the C60 layer leads to the formation of PbI2-rich and Br-rich regions in the perovskite absorption layer, which reduces composite losses and improves operational stability. The solar cells with a C60/ZnO ETL exhibit less pronounced or slower electrochemical dynamics, indicating that C60 helps to suppress irreversible electrochemical processes at the ETL interface.
PbS QDs have been selected as surface modification materials due to their wide spectral absorption range, tunable bandgap, multi-exciton generation effect, and low-cost preparation technology.236 Both PbS and perovskite have six coordinated Pb atoms, which can be well matched with perovskite, and the surface morphology of the perovskite layer grown on PbS is better. As shown in Fig. 11b, by further depositing TBAI on the ZnO/PbS layer, the defect state on the surface of the functional layer is greatly suppressed, and its conductivity is improved due to the coordination reaction between iodine ions in TBAI and Pb on PbS QDs.
Liu and co-workers used AZO as the ETL and chose polydopamine (PDA) to conduct interface modification.237 The dopamine group with strong adsorption in PDA can closely connect the perovskite layer and ETL, which helps to reduce defect states and carrier recombination. In addition, the hydroxyl and amino groups in PDA interact with the perovskite precursor solution to induce the vertical growth of perovskite grains and improve the quality of perovskite crystallization. By using AZO:PDA as the ETL, a champion power conversion efficiency of 21.36% was achieved with a minimal hysteresis effect.
Here, we found that, unlike SnO2, the buffer layers of ZnO ETLs are mostly added between the perovskite and the ETL. Few studies have placed it between the electrode and the ETL. This focus is primarily on addressing the problem of ZnO's inherent alkalinity damaging the perovskite and providing a more uniform and flat platform for perovskite growth. Of course, adding a modified layer between the ETL and the electrode to achieve a more suitable energy level alignment is also a viable experimental approach.
In Table 5, we summarized the main contents of the two metal oxide ETLs and also made some comparisons. We take this as the end of the main part of our review for your reference.
4. Other low-temperature processed MO ETLs
Besides SnO2 and ZnO, two typical low-temperature processed MO ETMs, there are also many useful MO ETMs suitable for low-temperature fabrication of flexible PSCs. This part will provide a brief summary.4.1 Titanium dioxide
There are three crystal phases of TiO2: anatase, rutile, and brookite. TiO2 prepared at low temperature generally belongs to anatase and rutile. Many researchers have prepared TiO2 ETLs using different low-temperature techniques, and as mentioned earlier, the TiO2 ETL has also been combined with SnO2 and ZnO to form a bilayer ETL structure. Here, we will introduce some low-temperature processed TiO2 ETLs.In 2014, Yella et al. prepared a nanocrystalline rutile phase TiO2 ETL using the CBD method with TiCl4 solution at 70 °C.238 This method not only achieves low-temperature preparation, but also forms a tight connection between nanocrystalline rutile TiO2 and perovskite layers with a large interface area, which helps to more effectively extract photo-generated electrons, reduce electron–hole pair recombination, and improve charge collection efficiency. TiCl4 is hydrolyzed to produce TiO2, and the hydrolysis reaction itself does not need too high temperatures to occur.
One year later, Kymakis's team prepared amorphous TiO2 by dissolving titanium isopropoxide in isopropanol, adding hydrochloric acid as a stabilizer, and then spin coating on a glass/ITO substrate, followed by annealing at 150 °C for 45 minutes.239 This TiO2 layer exhibits a highly uniform surface, without cracks, and has very low roughness. In the same year, Kim et al. prepared amorphous TiOx layers using plasma-enhanced atomic layer deposition (PEALD) technology, and the entire deposition process was carried out at 80 °C.240 This ETL has been used in flexible PSCs and has achieved great stability.
In 2016, Chen et al. prepared flower-like TiO2 (Fig. 11c) using the CBD method at 80 °C, which is also an anatase TiO2 nanorod material.241 A year later, Wang's team prepared TiO2 thin films at 80 °C and used titanium oxide bis(2,4-pentanedione acid) (TOPD) as a binder for TiO2 NPs to reduce morphological defects in the TiO2:TOPD film.242 After that, Lan's team controlled the growth of TiO2 NRs using oleic acid (OA) ligand assistance, and then replaced the oleic acid ligand with BF4− through ligand exchange treatment to form BF4−-capped TiO2 NRs.243
In 2019, You et al. redispersed TiO2 nanosol by using different solvents (H2O, ethanol, DMSO, and N,N-dimethylformamide (DMF)).244 TiO2 nanosol was formed by hydrolysis of TTIP at 80 °C and the condensation reaction of HNO3 at 60 °C. DMF is the optimal solvent for forming a uniform TiO2 ETL due to its low surface tension and high zeta potential. This solar cell achieved a PCE of 18.2%. The flexible solar cell also demonstrated good mechanical bending stability, maintaining approximately 95% of the initial PCE after 1000 repeated bending cycles.
In 2020, Jo's team adopted a simple oxygen plasma treatment method to improve the low-temperature treatment of mesoporous TiO2 layers.245 Oxygen plasma treatment effectively removes organic additives from the TiO2 layer and reduces oxygen vacancies. Oxygen plasma treatment improves the wettability of the TiO2 layer, promotes the penetration of perovskite precursor solution in the TiO2 layer, and thus enhances the performance of PSCs.
Sanehira et al. synthesized a Nb-doped TiO2 ETL using a one-step low-temperature steam annealing (SA) method.246 Place the substrate coated with precursor solution (TiCl4) in a polytetrafluoroethylene (PTFE) bottle, add 5 ml of water, and then heat at 125 °C for 4 hours. This low-temperature process is beneficial for precise control of the conduction band (CB) level of the ETL.
Ren et al. investigated the effect of UV ozone treatment on the efficiency and stability of the low-temperature TiO2 ETL in planar perovskite solar cells.247 The low-temperature TiO2 interface treated with UV ozone improves the work function and built-in potential of TiO2, and can suppress the photocatalytic activity of TiO2, reducing the decomposition of perovskite films.
Homola's team used a cold (70 °C) plasma treatment method to prepare TiO2 for the first time.248 The polysiloxane adhesive was removed rapidly (1 min) by low-temperature plasma treatment and converted into amorphous silicon dioxide. The photoelectric conversion efficiency of perovskite solar cells using the plasma-treated porous TiO2/SiO2 photoelectric anode is about 12%.
In 2021, Pan et al. used low temperature and a feasible glancing angle deposition (GLAD) method to directly grow TiO2 nanopillar arrays (TiO2 NaPAs) on conductive substrates, as shown in Fig. 11d.249 This material can be used in flexible substrates too.
In 2022, Hong et al. introduced acetylacetone (Acac) into the solution and prepared Acac-TiO2 NPs by the sol–gel method at low-temperature.250 In the same year, Liu and co-workers prepared a TiO2 ETL with s low temperature hydrothermal method and then used acetic acid (AA) and oleic acid (OA) to modify the ETL surface.251 The former surface shows better morphological characteristics, and finally achieved 20.15% PCE in the device modified by using AA.
Fig. 12 shows the difference between some low-temperature fabrication methods and traditional methods. Generally, from the progress summarized above, in order to achieve the low-temperature preparation of the TiO2 ETL, many materials or means need to be involved, which greatly increases the research and development cost and process difficulty. In addition, the properties of TiO2, such as electron mobility, are not as good as those of zinc oxide and tin oxide, which limits the development of this ETL. It is worth noting that the toughness of TiO2 itself is poor, and its application in flexible wearable devices will be greatly limited.
4.2 Others
In 2013, Carnie et al. prepared Al2O3 thin films at low temperature.252 An Al2O3-perovskite layer was co-deposited by spin coating. This method combines two manufacturing steps into one and eliminates the high-temperature sintering step. After co-deposition, low-temperature heating treatment is performed at a temperature not exceeding 110 °C. This step helps with the crystallization of perovskite materials and the formation of electron transport layers. Furthermore, ALD was applied by Zhang et al. for preparing the Al2O3 underlayer.253 They used trimethyl aluminum (TMA) as the aluminum precursor and H2O as the counter reactant for preparation on a substrate at 200 °C. The insulating property of Al2O3 can eliminate the chemical capacitance caused by the high-density sub-band gap state, resulting in higher Voc, and the device with an aluminum support is much more stable,254 so Al2O3 is usually used as the scaffold layer,255,256 encapsulant,257,258 or buffer layer259 for modifying the ETLs or PSCs.Nb2O5 has excellent electro-optical properties: high electron mobility helps to improve the transmission efficiency of electrons and reduce electron recombination; the edge position of the conduction band is well matched with the energy level structure of the perovskite material, which is conducive to the effective extraction and transmission of electrons, while hindering the reverse transmission of holes, and helping to improve the open circuit voltage (Voc) and fill factor (FF); it has less light absorption in the whole visible and near-infrared spectral range, which means that more light energy can be absorbed by the perovskite absorption layer, thus increasing the photogenerated current.260–262 Ye et al. studied the preparation of zinc-doped niobium oxide (Nb2O5) by the low-temperature solution combustion method.263 Nb2O5 thin films were prepared by the low-temperature (200 °C) solution combustion method. This method does not need high-temperature sintering. Nb2O5 and Zn-doped Nb2O5 based devices show better stability than traditional mesoporous TiO2-based devices sintered at high temperature, and can maintain 80% of their initial PCE in air for up to 20 days. Huang and co-workers prepared Nb2O5 thin films by RF sputtering at room temperature.197 The Nb2O5 ETL, perovskite layer, and ZnO ETL form a good step energy level.
SnOx, as another tin oxide material, can also be prepared at low temperatures. Shang et al. proposed a mild dehydration reaction method for the synthesis of amorphous SnOx at a low temperature below 100 °C.264 The synthesis process includes three steps: first, SnCl4·5H2O reacts with ammonia to form Sn(OH)4, then the obtained white precipitate is dissolved in methylamine (MA) solution to form Sn(CH3NH2)x(OH)4, and finally, the amorphous SnOx film is formed by spin coating on an ITO substrate and annealing at 80 °C. The perovskite solar cells using amorphous SnOx as the ETL showed a maximum photoelectric conversion efficiency (PCE) of 20.4%, which was the highest efficiency achieved by the amorphous metal oxide ETL produced at less than 100 °C at that time. Wang's team prepared the SnOx:Cl ETL by chemical deposition at low temperature (about 100 °C).265 They emphasized the collaborative optimization of KX (potassium halide) and SnOx:Cl, and potassium ions not only replace A-site cations, but also occupy a large number of gap sites at grain boundaries, effectively inhibiting iodide migration and organic volatilization, which is a new way to achieve low temperature and high performance PSCs.
As an alternative to ZnO/SnO2, In2O3 has high transparency, high conductivity, and low photocatalytic activity.266 High temperature annealing is required during the formation of traditional In2O3 thin films, which may damage or degrade the underlying perovskite materials. Yang et al. prepared In2O3 and Sn:In2O3 nanoparticles by a low-temperature synthesis method, which can form an effective top ETL without any post-treatment.267 This double-layer ETL improves the interface contact with the perovskite layer, reduces the interface defects and recombination centers, and thus reduces the non-radiation recombination loss.
CeO2 is a new ETL material prepared without annealing treatment, which has excellent thermal and chemical stability, a wide band gap, a high dielectric constant, and excellent ionic conductivity. CeO2 nanocrystals are prepared by the solvothermal method, which can be re-dispersed in cyclohexane to form a stable, uniform and transparent solution, so the ETL can be prepared by the spin coating method.268
Additionally, Guo et al. used SnO2-modified mesoporous ZrO2 as the electron transport layer, which was also prepared by a low-temperature solution method.269
5. Conclusions and prospects
5.1 Conclusions
Flexible perovskite solar cells are one of the most popular parts of the photovoltaic industry, and its ETM plays an important role in the good performance of the device. As mentioned above, the applications of SnO2 and ZnO in flexible PSCs have great potential. In this review, the basic properties of the two materials are first summarized, including their structure characteristics, energy band, electron mobility, and optical properties. Next, we introduce many different ways for fabricating ETL films, such as ALD, sol–gel, CBD, magnetron sputtering deposition, electrochemical deposition, hydrothermal deposition, and so on. We provide brief introductions of the working principles in each method, and various productions achieved by different teams are also sorted. We find that the materials for preparation are varied, and many researchers have prepared ETLs at a very low temperature (even room temperature), which is very suitable for flexible PSCs. Then, we provide a detailed summary of the application of these ETLs, including doping and interface engineering. We mainly classify and summarize from the perspective of materials, and introduce the research results of previous researchers. At last, we also introduce other low-temperature processed MO materials like TiO2, Al2O3, Nb2O5, CeO2, In2O3, etc. They also have many prominent advantages.5.2 Prospectives
Although the technology of low-temperature processed metal oxide ETLs has shown rapid development, there are still many problems to be solved.The development of flexible perovskite solar cells is still in full swing. With the joint efforts of researchers, the performance of devices will certainly be improved a lot.
Author contributions
Jianghao Tian: conceptualization, methodology, formal analysis, investigation, writing – original draft. Kun Wang: methodology, investigation. Zhipeng Zhou: methodology, investigation. Lexiang Zhang: methodology, investigation. Pu Fan: conceptualization, methodology, writing – review & editing. Huajing Zheng: writing – review & editing. Ding Zheng: conceptualization, writing – review & editing. Junsheng Yu: resources, supervision, funding acquisition, writing – review & editing, project administration. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 62275041) and the Project of Science and Technology of Sichuan Province (Grant No. 2023ZYZYTS0016 and 2023ZYD0162). The authors were supported by the Foundation of Yangtze Delta Region Institute (Huzhou) University of Electronic Science and Technology of China (Grant No. U03220143 and U04220079); the Major Technology Project of Sichuan Province (Grant No. 2024ZDZX0030). This work was also sponsored by the Sichuan Province Key Laboratory of Display Science and Technology.References
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS.
- D. I. Kutsarov, E. Rezaee, J. Lambert, W. T. Stroud, A. Panagiotopoulos and S. R. P. Silva, Adv. Mater. Technol., 2025, 2401834 CrossRef CAS.
- E. K. Solak and E. Irmak, RSC Adv., 2023, 13, 12244–12269 RSC.
- Y. Jiang, S. Sun, R. Xu, F. Liu, X. Miao, G. Ran, K. Liu, Y. Yi, W. Zhang and X. Zhu, Nat. Energy, 2024, 9, 8 CrossRef.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J. Fujisawa and M. Hanaya, Chem. Commun., 2015, 51, 15894–15897 RSC.
- K. Mahmood, S. Sarwar and M. Mehran, RSC Adv., 2017, 7, 17044–17062 RSC.
- J. L. Liu, S. Li, A. Y. Mei and H. W. Han, Small Sci., 2023, 3, 2300020 CrossRef CAS PubMed.
- J. Xu, X. Shi, J. Chen, J. Luan and J. Yao, J. Solid State Chem., 2019, 276, 302–308 CrossRef CAS.
- Z. Xing, S. Li, P. Xu, H. Tian, L. Deng, Y. Yao, B. Chen, F. Xie, M. An, D. Yun, S. Xie and L. Zheng, Chem. Res. Chin. Univ., 2022, 38(1), 75–81 CrossRef CAS.
- F. Liu, B. Chen, A. Fan, Z. Chen, Y. Pan, Z. Tang, L. Deng, Z. Xing and S. Li, Adv. Funct. Mater., 2025, 35, 2419582 CrossRef CAS.
- W. Huang, P. Huang and S. Yang, Chem. Commun., 2016, 52, 13572–13575 RSC.
- F. Xia, Q. Wu, P. Zhou, Y. Li, X. Chen, Q. Liu, J. Zhu, S. Dai, Y. Lu and S. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 13659–13665 CrossRef CAS PubMed.
- P. Patil, D. Manna, U. Nakate, Y. Hahn, S. Kwon and S. Na, Chem. Eng. J., 2020, 397, 125504 CrossRef CAS.
- Y. Liu, B. Long, R. Chen, S. Huang, W. Ou-Yang and X. Chen, ACS Appl. Energy Mater., 2021, 4, 3812–3821 CrossRef CAS.
- S. Chen, S. Yang, H. Sun, L. Zhang, J. Peng, Z. Liang, Z. Wang and Z. Wang, J. Power Sources, 2017, 353, 123–130 CrossRef CAS.
- X. Jiang, F. Wang, Q. Wei, H. Li, Y. Shang, W. Zhou, C. Wang, P. Cheng, Q. Chen, L. Chen and Z. Ning, Nat. Commun., 2020, 11, 1245 CrossRef CAS PubMed.
- M. Lee, D. Kim, Y. Lee, H. Koo, K. Lee and I. Chung, ACS Appl. Energy Mater., 2020, 3, 5581–5588 CrossRef CAS.
- L. Chen, Z. Tseng and C. Wang, Mater. Sci. Semicond. Process., 2016, 56, 179–182 CrossRef CAS.
- K. Yokoyama, H. Lin, Q. Shui, X. Wang, N. Saito and Y. Matsuo, Appl. Phys. Express, 2023, 16, 081001 CrossRef CAS.
- W. Lee, C. Chen, C. Li, S. Hsiao, W. Tsai, M. Huang, C. Cheng, C. Wu and H. Lin, Nano Energy, 2017, 38, 66–71 CrossRef CAS.
- R. Ihly, A. Dowgiallo, M. Yang, P. Schulz, N. Stanton, O. Reid, A. Ferguson, K. Zhu, J. Berry and J. Blackburn, Energy Environ. Sci., 2016, 9, 1439–1449 RSC.
- F. Ye, S. Zhang, J. Warby, J. Wu, E. Gutierrez-Partida, F. Lang, S. Shah, E. Saglamkaya, B. Sun, F. Zu, S. Shoaee, H. Wang, B. Stiller, D. Neher, W. Zhu, M. Stolterfoht and Y. Wu, Nat. Commun., 2022, 13, 7454 CrossRef CAS PubMed.
- K. Jiang, F. Wu, H. Yu, Y. Yao, G. Zhang, L. Zhu and H. Yan, J. Mater. Chem. A, 2018, 6, 16868–16873 RSC.
- D. Yang, R. Yang, S. Priya and S. Liu, Angew. Chem., Int. Ed., 2019, 58, 4466–4483 CrossRef CAS PubMed.
- J. Liu, Z. Zhao, J. Qian, Z. Liang, C. Wu, K. Wang, S. Liu and D. Yang, Adv. Mater., 2024, 36, 2401236 CrossRef CAS PubMed.
- X. Li, H. Yu, Z. Liu, J. Huang, X. Ma, Y. Liu, Q. Sun, L. Dai, S. Ahmad, Y. Shen and M. Wang, Nano-Micro Lett., 2023, 15, 206 CrossRef.
- D. Li, L. Hu, Y. Xie, G. Niu, T. Liu, Y. Zhou, L. Gao, B. Yang and J. Tang, ACS Photonics, 2016, 3, 2122–2128 CrossRef CAS.
- J. Jia, J. Wu, J. Dong, L. Fan, M. Huang, J. Lin and Z. Lan, Chem. Commun., 2018, 54, 3170–3173 RSC.
- M. K. S. Bin Rafiq, N. Amin, H. F. Alharbi, M. Luqman, A. Ayob, Y. S. Alharthi, N. H. Alharthi, B. Bais and M. Akhtaruzzaman, Sci. Rep., 2020, 10, 1–11 CrossRef PubMed.
- L. Rani Karna, R. Upadhyay and A. Ghosh, Sci. Rep., 2023, 13, 15212 CrossRef.
- H. Yoon, S. M. Kang, J.-K. Lee and M. Choi, Energy Environ. Sci., 2016, 9, 2262–2266 RSC.
- G. Yin, H. Zhao, J. Feng, J. Sun, J. Yan, Z. Liu, S. Lin and S. Liu, J. Mater. Chem. A, 2018, 6, 9132–9138 RSC.
- B. K. Mohammed, M. K. A. Mohammed and D. S. Ahmed, Solid-State Electron., 2023, 204, 108640 CrossRef CAS.
- Y. W. Noh, I. S. Jin, S. H. Park and J. W. Jung, J. Mater. Sci. Technol., 2020, 42, 38–45 CrossRef CAS.
- S. W. Tong, J. Balapanuru, D. Fu and K. P. Loh, ACS Appl. Mater. Interfaces, 2016, 8, 29496–29503 CrossRef CAS PubMed.
- L. Lin, T. Jones, T. Yang, N. W. Duffy, J. H. Li, L. Zhao, B. Chi, X. B. Wang and G. J. Wilson, Adv. Funct. Mater., 2021, 31, 2008300 CrossRef CAS.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- W. Ke, G. Fang, J. Wang, P. Qin, H. Tao, H. Lei, Q. Liu, X. Dai and X. Zhao, ACS Appl. Mater. Interfaces, 2014, 6, 15959–15965 CrossRef CAS.
- J. Choi, S. Song, M. T. Horantner, H. J. Snaith and T. Park, ACS Nano, 2016, 10, 6029–6036 CrossRef CAS PubMed.
- G. S. Han, Y. H. Song, Y. U. Jin, J. W. Lee, N. G. Park, B. K. Kang, J. K. Lee, I. S. Cho, D. H. Yoon and H. S. Jung, ACS Appl. Mater. Interfaces, 2015, 7, 23521–23526 CrossRef CAS.
- D. Yang, X. Zhou, R. Yang, Z. Yang, W. Yu, X. Wang, C. Li, S. Liu and R. P. H. Chang, Energy Environ. Sci., 2016, 9, 3071–3078 RSC.
- S. F. Shaikh, H.-C. Kwon, W. Yang, H. Hwang, H. Lee, E. Lee, S. Ma and J. Moon, J. Mater. Chem. A, 2016, 4, 15478–15485 RSC.
- H. Lu, W. Tian, B. Gu, Y. Zhu and L. Li, Small, 2017, 13, 1701535 CrossRef PubMed.
- G. Xiao, C. Shi, K. Lv, C. Ying and Y. Wang, ACS Appl. Energy Mater., 2018, 1, 2576–2581 CrossRef CAS.
- D. Shen, W. Zhang, F. Xie, Y. Li, A. Abate and M. Wei, J. Power Sources, 2018, 402, 320–326 CrossRef CAS.
- D. G. Lee, M. Kim, B. J. Kim, D. H. Kim, S. M. Lee, M. Choi, S. Lee and H. S. Jung, Appl. Surf. Sci., 2019, 477, 131–136 CrossRef CAS.
- B. Wang, M. Zhang, X. Cui, Z. Wang, M. Rager, Y. Yang, Z. Zou, Z. L. Wang and Z. Lin, Angew. Chem., Int. Ed., 2020, 59, 1611–1618 CrossRef CAS PubMed.
- R. Teimouri, Z. Heydari, M. P. Ghaziani, M. Madani, H. Abdy, M. Kolahdouz and E. Asl-Soleimani, Superlattices Microstruct., 2020, 145, 106627 CrossRef CAS.
- H. Latif, Z. Azher, S. A. Shabbir, S. Rasheed, E. Pervaiz, A. Sattar and A. Imtiaz, Opt. Mater., 2020, 109, 110281 CrossRef CAS.
- M. Kim, I. Choi, S. J. Choi, J. W. Song, S. Mo, J. An, Y. Jo, S. Ahn, S. K. Ahn, G. Kim and D. S. Kim, Joule, 2021, 5, 659–672 CrossRef CAS.
- N. Alias, A. A. Umar, N. A. A. Malek, K. Liu, X. Li, N. A. Abdullah, M. M. Rosli, M. Y. A. Rahman, Z. Shi, X. Zhang, H. Zhang, F. Liu, J. Wang and Y. Zhan, ACS Appl. Mater. Interfaces, 2021, 13, 3051–3061 CrossRef CAS.
- W. Zhao, P. Guo, J. Wu, D. Lin, N. Jia, Z. Fang, C. Liu, Q. Ye, J. Zou, Y. Zhou, H. Wang and H. Wang, Nano-Micro Lett., 2024, 16, 191 CrossRef CAS.
- W. Ke, G. Fang, J. Wang, P. Qin, H. Tao, H. Lei, Q. Liu, X. Dai and X. Zhao, ACS Appl. Mater. Interfaces, 2014, 6, 15959–15965 CrossRef CAS PubMed.
- P. Wu, S. Wang, X. Li and F. Zhang, J. Mater. Chem. A, 2021, 9, 19554–19588 RSC.
- Q. Jiang, X. Zhang and J. You, Small, 2018, 14, 40 Search PubMed.
- H. Wang, M. A. Sayeed and T. Wang, Aust. J. Chem., 2015, 68, 1783–1788 CrossRef CAS.
- H. B. Lee, N. Kumar, M. M. Ovhal, Y. J. Kim, Y. M. Song and J.-W. Kang, Adv. Funct. Mater., 2020, 30, 1559 Search PubMed.
- Q. Dong, Y. Shi, C. Zhang, Y. Wu and L. Wang, Nano Energy, 2017, 40, 336–344 CrossRef CAS.
- W. Ke, G. Fang, Q. Liu, L. Xiong, P. Qin, H. Tao, J. Wang, H. Lei, B. Li, J. Wan, G. Yang and Y. Yan, J. Am. Chem. Soc., 2015, 137, 6730–6733 CrossRef CAS PubMed.
- L. Zuo, H. Guo, D. W. deQuilettes, S. Jariwala, N. De Marco, S. Q. Dong, R. DeBlock, D. S. Ginger, B. Dunn, M. K. Wang and Y. Yang, Sci. Adv., 2017, 3, e1700106 CrossRef PubMed.
- Z. Zhu, Y. Bai, X. Liu, C.-C. Chueh, S. Yang and A. K.-Y. Jen, Adv. Mater., 2016, 28, 6478–6484 CrossRef CAS PubMed.
- P. Lee, T. Wu, K. Tian, C. Li, C. Hou, J. Shyue, C. Lu, Y. Huang and W. Su, ACS Appl. Mater. Interfaces, 2020, 12, 45936–45949 CrossRef CAS.
- C. Xiao, C. Jiang, W. Ke, C. Wang, B. Gorman, Y. Yan and M. Al-Jassim, Insul. Eng. Electr. Electron, 2016, 1197–1200 Search PubMed.
- Y. Kuang, V. Zardetto, R. van Gils, S. Karwal, D. Koushik, M. A. Verheijen, L. E. Black, C. Weijtens, S. Veenstra, R. Andriessen, W. M. M. Kessels and M. Creatore, ACS Appl. Mater. Interfaces, 2018, 10, 30367–30378 CrossRef CAS.
- X. Zhang, Y. Zhou, M. Chen, D. Wang, L. Chao, Y. Lv, H. Zhang, Y. Xia, M. Li, Z. Hu and Y. Chen, Small, 2023, 19, 39 Search PubMed.
- Y. Lee, S. Lee, G. Seo, S. Paek, K. T. Cho, A. J. Huckaba, M. Calizzi, D. Choi, J. Park, D. Lee, H. J. Lee, A. M. Asiri and M. K. Nazeeruddin, Adv. Sci., 2018, 5, 1800130 CrossRef PubMed.
- J. Bing, S. Huang and A. W. Ho-Baillie, Energy Technol., 2020, 8, 1901114 CrossRef CAS.
- Y. Gu, C. Ye, X. Yin, J. Han, H. Shen, J. Li, X. Hao and H. Lin, Chem. Eng. J., 2018, 351, 791–798 CrossRef CAS.
- Y. Zhou, X. Li and H. Lin, Small, 2020, 16, 1902579 CrossRef CAS.
- J. J. Yoo, G. Seo, M. R. Chua, T. G. Park, Y. Lu, F. Rotermund, Y.-K. Kim, C. S. Moon, N. J. Jeon, J.-P. Correa-Baena, V. Bulović, S. S. Shin, M. G. Bawendi and J. Seo, Nature, 2021, 590, 587–593 CrossRef CAS PubMed.
- M. J. Jeong, K. M. Yeom, S. J. Kim, E. H. Jung and J. H. Noh, Energy Environ. Sci., 2021, 14, 2419–2428 RSC.
- Q. Zhao, D. Liu, Z. Li, B. Zhang, X. Sun, Z. Shao, C. Chen, X. Wang, L. Hao, X. Wang, C. Gao, Y. Li, P. Chen, X. Zhang, S. Pang, G. Cui and L. Wang, Chem. Eng. J., 2022, 443, 136308 CrossRef CAS.
- L. Qiu, Z. Liu, L. K. Ono, Y. Jiang, D.-Y. Son, Z. Hawash, S. He and Y. B. Qi, Adv. Funct. Mater., 2019, 29, 1806779 CrossRef CAS.
- G. Bai, Z. Wu, J. Li, T. Bu, W. Li, A. Li, F. Huang, Q. Zhang, Y.-B. Cheng and J. Zhong, Sol. Energy, 2019, 183, 306–314 CrossRef CAS.
- H. Tao, H. Wang, Y. Bai, H. Long, H. Zhao, Q. Fu and Z. Ma, J. Mater. Sci.: Mater. Electron., 2019, 30, 12036–12043 CrossRef CAS.
- H. Tao, Z. Ma, G. Yang, H. Wang, H. Long, H. Zhao, P. Qin and G. Fang, Appl. Surf. Sci., 2018, 434, 1336–1343 CrossRef CAS.
- J. Li, L. Li, W. Chen, Q. Yi and G. Zou, Nanotechnology, 2021, 32, 025606 CrossRef CAS PubMed.
- Y. Ko, Y. R. Kim, H. Jang, C. Lee, M. G. Kang and Y. Jun, Nanoscale Res. Lett., 2017, 12, 498 CrossRef PubMed.
- D. Zheng, R. Qin, M. Wu and J. Yu, in Optoelectronic Materials and Devices for Sensing and Imaging, ed. Y. Jiang, X. Ma, X. Li, M. Pu, X. Feng and B. Kippelen, 2019, vol. 10843, pp. 1084315 Search PubMed.
- J. Jia, J. Dong, J. Wu, H. Wei and B. Cao, J. Alloys Compd., 2020, 844, 156032 CrossRef CAS.
- K. Mahmood, A. Khalid, F. Nawaz and M. T. Mehran, J. Colloid Interface Sci., 2018, 532, 387–394 CrossRef CAS PubMed.
- P. Zhou, J. Wu, Y. Tu, M. Zhen, J. Huo, Y. Wei and Z. Lan, Sol. Energy, 2016, 137, 579–584 CrossRef CAS.
- Z. Zhu, Y. Bai, X. Liu, C.-C. Chueh, S. Yang and A. K.-Y. Jen, Adv. Mater., 2016, 28, 6478–6484 CrossRef CAS PubMed.
- M. F. M. Noh, N. A. Arzaee, J. Safaei, N. A. Mohamed, H. P. Kim, A. R. Mohd Yusoff, J. Jang and M. A. Mat Teridi, J. Alloys Compd., 2019, 773, 997–1008 CrossRef.
- M. Haghighi, N. Ghazyani, S. Mahmoodpour, R. Keshtmand, A. Ghaffari, H. M. Luo, R. Mohammadpour, N. Taghavinia and M. Abdi-Jalebi, Sol. RRL, 2023, 7, 2201080 CrossRef CAS.
- W. Bin, W. Huang, W. Lin and H. Lee, Crystals, 2021, 11, 1380 CrossRef CAS.
- Y. Guo, X. Yin, J. Liu, W. Chen, S. Wen, M. Que, H. Xie, Y. Yang, W. Que and B. Gao, Org. Electron., 2019, 65, 207–214 CrossRef CAS.
- M. Fieveza, P. J. S. Rana, T. M. Koh, M. Manceau, J. H. Lew, N. F. Jamaludin, B. Ghosh, A. Bruno, S. Cros, S. Berson, S. G. Mhaisalkar and W. L. Leong, Sol. Energy Mater. Sol. Cells, 2021, 230, 111189 CrossRef.
- B. Roose, S. Pathak and U. Steiner, Chem. Soc. Rev., 2015, 44, 8326–8349 RSC.
- M. Park, J. Kim, H. J. Son, C. Lee, S. S. Jang and M. J. Ko, Nano Energy, 2016, 26, 208–215 CrossRef CAS.
- Y. Huang, S. Li, C. Wu, S. Wang, C. Wang and R. Ma, Chem. Phys. Lett., 2020, 745, 137220 CrossRef CAS.
- Q. Zhuang, H. Wang, C. Zhang, C. Gong, H. Li, J. Chen and Z. Zang, Nano Res., 2022, 15, 5114–5122 CrossRef CAS.
- H. Chen, D. Liu, Y. Wang, C. Wang, T. Zhang, P. Zhang, H. Sarvari, Z. Chen and S. Li, Nanoscale Res. Lett., 2017, 12, 238 CrossRef PubMed.
- H. Ye, Z. Liu, X. Liu, B. Sun, X. Tan, Y. Tu, T. Shi, Z. Tang and G. Liao, Appl. Surf. Sci., 2019, 478, 417–425 CrossRef CAS.
- S. Lan, W. T. Zheng, S. Yoon, H. U. Hwang, J. W. Kim, D. W. Kang, J. W. Lee and H. K. Kim, ACS Appl. Energy Mater., 2022, 5, 14901–14912 CrossRef CAS.
- H. V. Quy and C. W. Bark, ACS Omega, 2022, 7, 22256–22262 CrossRef CAS PubMed.
- Y. Chen, X. Zuo, Y. He, F. Qian, S. Zuo, Y. Zhang, L. Liang, Z. Chen, K. Zhao and Z. Liu, Adv. Sci., 2021, 8, 2001466 CrossRef CAS PubMed.
- A. Kumar, A. K. Al-Mousoi, M. J. Saadh, M. K. A. Mohammed, G. V. S. S. Sarma, N. Ahmad and R. Tiwari, Electron. Mater. Lett., 2023, 19, 471–482 CrossRef CAS.
- R. Xue, X. Zhou, S. Peng, P. Xu, S. Wang, C. Xu, W. Zeng, Y. Xiong and D. Liang, ACS Sustainable Chem. Eng., 2020, 8, 10714–10725 CAS.
- J. Liang, Z. Chen, G. Yang, H. Wang, F. Ye, C. Tao and G. Fang, ACS Appl. Mater. Interfaces, 2019, 11, 23152–23159 CrossRef CAS.
- X. Ren, Y. Liu, D. G. Lee, W. B. Kim, G. S. Han, H. S. Jung and S. Liu, InfoMat, 2020, 2, 401–408 CrossRef CAS.
- W. Cai, T. Yang, C. Liu, Y. Wang, S. Wang, Y. Du, N. Wu, W. Huang, S. Wang, Z. Wang, X. Chen, J. Feng, G. Zhao, Z. Ding, X. Pan, P. Zou, J. Yao, S. Liu and K. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202309398 CrossRef CAS PubMed.
- Q. Liu, F. Fei, Y. Xu, L. Gu, X. Ding, K. Wang, K. Du, S. Wang, X. Dong, L. Li, B. Li, N. Yuan and J. Ding, Org. Electron., 2023, 120, 1068 Search PubMed.
- Q. Chen, J. Wu, X. Liu, Y. Du, C. Deng, X. Chen, L. Sun, L. Tan, W. Sun and Z. Lan, ACS Appl. Mater. Interfaces, 2024, 16, 8949–8959 CrossRef CAS.
- P. Zhu, S. Gu, X. Luo, Y. Gao, S. Li, J. Zhu and H. Tan, Adv. Energy Mater., 2019, 1903083 Search PubMed.
- C. Lin, T. Murakami, M. Chikamatsu, T. Bessho, M. Furue and H. Segawa, ACS Omega, 2021, 6, 17880–17889 CrossRef CAS PubMed.
- Z. Liu, K. Deng, J. Hu and L. Li, Angew. Chem., Int. Ed., 2019, 58, 11497–11504 CrossRef CAS PubMed.
- Z. Lin, W. Zhang, Q. Cai, X. Xu, H. Dong, C. Mu and J. Zhang, Adv. Sci., 2021, 8, 2102845 CrossRef CAS PubMed.
- R. Tao, Y. Zhang, Z. Jin, Z. Sun and L. Xu, Electrochim. Acta, 2018, 264, 1017–1025 Search PubMed.
- J. Wei, F. Guo, X. Wang, K. Xu, M. Lei, Y. Liang, Y. Zhao and D. Xu, Adv. Mater., 2018, 30, 1805153 CrossRef.
- L. Chen, X. Li, N. Zhang, L. Yu, Z. Liu, H. Liu and G. Song, Sol. Energy Mater. Sol. Cells, 2024, 272, 112907 CrossRef CAS.
- S. You, H. Zeng, Z. Ku, X. Wang, Z. Wang, Y. Rong, Y. Zhao, X. Zheng, L. Luo, L. Li, S. Zhang, M. Li, X. Gao and X. Li, Adv. Mater., 2020, 32, 2003990 CrossRef CAS.
- Z. Li, Y. Gao, Z. Zhang, Q. Xiong, L. Deng, X. Li, Q. Zhou, Y. Fang, P. Gao and G. Peng, Nano-Micro Lett., 2021, 13, 101 CrossRef CAS.
- N. Kwon and J. Seo, Korean J. Chem. Eng., 2024, 41, 2405–2414 CrossRef CAS.
- Y. Yang, Y. Liu, M. Grätzel and A. Hagfeldt, Energy Environ. Sci., 2021, 14, 3447–3454 RSC.
- Z. Lv, L. He, H. Jiang, X. Ma, F. Wang, L. Fan, M. Wei, J. Yang, L. Yang and N. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 16326–16335 CrossRef CAS.
- B. Gu, Y. Du, B. Chen, R. Zhao, H. Lu, Q. Xu and C. Guo, ACS Appl. Mater. Interfaces, 2022, 14, 11264–11272 CrossRef CAS.
- M. Zhong, Y. Liang, J. Zhang, Z. Wei, Q. Li and D. Xu, J. Mater. Chem. A, 2019, 7, 6659–6664 RSC.
- S. Zhang, H. Si, W. Fan, M. Shi, M. Li, C. Xu, Z. Zhang, Q. Liao, A. Sattar, Z. Kang and Y. Zhang, Angew. Chem., Int. Ed., 2020, 59, 11573–11582 CrossRef CAS PubMed.
- H. Xu, Y. Miao, N. Wei, H. Chen, Z. Qin, X. Liu, X. Wang, Y. Qi, T. Zhang and Y. Zhao, Adv. Energy Mater., 2021, 12, 2103151 CrossRef.
- J. Xi, J. Yuan, J. Du, X. Yan and J. Tian, Small, 2022, 18, 2203519 CrossRef CAS PubMed.
- Z. Zheng, F. Li, J. Gong, Y. Ma, J. Gu, X. Liu, S. Chen and M. Liu, Adv. Mater., 2022, 34, 2109879 CrossRef CAS PubMed.
- X. Pang, J. Huang, C. Lin, Y. Zhang, N. Cheng, W. Zi, Z.-Z. Sun, Z. Yu and Z. Zhao, Chem.–Eur. J., 2023, 29(14), e202202744 CrossRef CAS PubMed.
- J. Xia, M. Sohail and M. K. Nazeeruddin, Adv. Mater., 2023, 35(31), 2211324 CrossRef CAS.
- T. Xue, T. Li, D. Chen, X. Wang, K. Guo, Q. Wang and F. Zhang, Micromachines, 2023, 14, 1549 CrossRef PubMed.
- F. Xie, D. Mei, L. Qiu, Z. Su, L. Chen, Y. Liu and P. Du, J. Electron. Mater., 2023, 52, 4626–4633 CrossRef CAS.
- Q. Dong, Y. Xue, S. Wang, L. Wang, F. Chen, S. Zhang, R. Chi, L. Zhao and Y. Shi, Sci. China Mater., 2017, 60, 963–976 CrossRef CAS.
- Y. Hou, X. Chen, S. Yang, C. Li, H. Zhao and H. G. Yang, Adv. Funct. Mater., 2017, 27, 1700878 CrossRef.
- X. Zhang, Y. Zhou, M. Chen, D. Wang, L. Chao, Y. Lv, H. Zhang, Y. Xia, M. Li, Z. Hu and Y. Chen, Small, 2023, 19, 39 Search PubMed.
- J. Dagar, S. Castro-Hermosa, G. Lucarelli, A. Zampetti, F. Cacialli and T. M. Brown, IEEE J. Photovoltaics, 2019, 9, 1309–1315 Search PubMed.
- M. Hashemib, M. Minbashi, S. M. B. Ghorashib, A. Ghobadic, M. H. Ehsanid, M. Heidariramsheh and A. Hajjiah, Sol. Energy, 2021, 215, 356–366 CrossRef.
- F. Mohamadkhania, S. Javadpoura and N. Taghaviniab, Sol. Energy, 2019, 191, 647–653 CrossRef.
- H. M. Pham, S. D. H. Naqvi, H. Tran, H. V. Tran, J. Delda, S. Hong, I. Jeong, J. Gwak and S. Ahn, Nanomaterials, 2023, 13, 3091 CrossRef CAS.
- Y. Lu, J. Feng, Z. Liu, Y. Duan, S. Zhan, S. Yang, K. He, Y. Li, Y. Zhou, N. Yuan, J. Ding and S. Liu, Adv. Mater., 2022, 34, 2201681 CrossRef.
- X. Guo, J. Du, Z. Lin, J. Su, L. Feng, J. Zhang, Y. Hao and J. Chang, Chem. Eng. J., 2021, 407, 127997 CrossRef CAS.
- Y. Wang, P. Xiang, A. Ren, H. Lai, Z. Zhang, Z. Xuan, Z. Wan, J. Zhang, X. Hao, L. Wu, M. Sugiyama, U. Schwingenschlögl, C. Liu, Z. Tang, J. Wu, Z. Wang and D. Zhao, ACS Appl. Mater. Interfaces, 2020, 12, 53973–53983 CrossRef CAS.
- F. Wang, Y. Zhang, M. Yang, L. Xue, L. Yang, L. Fan, Y. Su, J. Yang and X. Zhang, Nano Energy, 2019, 63, 103825 CrossRef CAS.
- X. Zuo, B. Kim, B. B. Liu, D. M. He, L. Bai, W. Q. Wang, C. Y. Xu, Q. L. Song, C. Y. Jia, Z. G. Zang, D. H. Lee, X. Li and J. Z. Chen, Chem. Eng. J., 2022, 431, 133209 CrossRef CAS.
- R. Yuan, B. Cai, Y. Lv, X. Gao, J. Gu, Z. Fan, X. Liu, C. Yang, M. Liu and W. Zhang, Energy Environ. Sci., 2021, 14, 5074–5083 RSC.
- E. Hyuk Jung, B. Chen, K. Bertens, M. Vafaie, S. Teale, A. Proppe, H. Yi, Z. Tong, C. Zheng and E. H. Sargent, ACS Energy Lett., 2020, 5, 2796–2801 CrossRef.
- E. Jiang, Y. Ai, J. Yan, N. Li, L. Lin, Z. Wang, C. Shou, B. Yan, Y. Zeng, J. Sheng and J. Ye, ACS Appl. Mater. Interfaces, 2019, 11, 36727–36734 CrossRef CAS PubMed.
- J. Zhuang, P. Mao, Y. Luan, N. Chen, X. Cao, G. Niu, F. Jia, F. Wang, S. Cao and J. Wang, Adv. Funct. Mater., 2021, 31, 2010385 CrossRef CAS.
- H. Min, D. Lee, J. Kim, G. Kim, K. S. Lee, J. Kim, M. J. Paik, Y. K. Kim, K. S. Kim and M. G. Kim, Nature, 2021, 598, 444 CrossRef CAS PubMed.
- Q. Zhou, J. Duan, Y. Wang, X. Yang and Q. Tang, J. Energy Chem., 2020, 50, 1–8 CrossRef.
- Z. Zhu, Y. Bai, X. Liu, C.-C. Chueh, S. Yang and A. K.-Y. Jen, Adv. Mater., 2016, 28, 6478–6484 CrossRef CAS PubMed.
- J. Du, L. Feng, X. Guo, X. Huang, Z. Lin, J. Su, Z. Hu, J. Zhang, J. Chang and Y. Hao, J. Power Sources, 2020, 455, 227974 CrossRef CAS.
- M. Thambidurai, F. Shini, P. C. Harikesh, N. Mathews and C. Dang, J. Power Sources, 2020, 448, 227362 CrossRef CAS.
- Q. Zhang, C. S. Dandeneau, X. Zhou and G. Cao, Adv. Mater., 2009, 21, 4087 CrossRef CAS.
- H. Liu, Z. Huang, S. Wei, L. Zheng, L. Xiao and Q. Gong, Nanoscale, 2016, 8, 6209 RSC.
- P. Zhang, J. Wu, T. Zhang, Y. Wang, D. Liu, H. Chen, L. Ji, C. Liu, W. Ahmad, Z. D. Chen and S. Li, Adv. Mater., 2017, 30, 1703737 CrossRef PubMed.
- J. Kim, G. Kim, T. Kim, S. Kwon, H. Back, J. Lee, S. H. Lee, H. Kang and K. Lee, J. Mater. Chem. A, 2014, 2, 17291–17296 RSC.
- X. Xu, H. Zhang, J. Shi, J. Dong, Y. Luo, D. Li and Q. Meng, J. Mater. Chem. A, 2015, 3, 19288–19293 RSC.
- Y. Ko, Y. Kim, S. Y. Kong, S. C. Kunnana and Y. Jun, Sol. Energy Mater. Sol. Cells, 2018, 183, 157–163 CrossRef CAS.
- H. Liang, Y.-C. Hu, Y. Tao, B. Wu, Y. Wu and J. Cao, ACS Appl. Mater. Interfaces, 2019, 11, 43116–43121 CrossRef CAS PubMed.
- D. Lee, M. Wolska-Pietkiewicz, S. Badoni, A. Grala, J. Lewiński and G. De Paëpe, Angew. Chem., Int. Ed., 2019, 58, 17163 CrossRef CAS PubMed.
- Z. Liang, Q. Zhang, L. Jiang and G. Cao, Energy Environ. Sci., 2015, 8, 3442 RSC.
- Y. Guo, X. Li, L. L. Kang, X. He, Z. Q. Ren, J. D. Wu and J. Y. Qi, RSC Adv., 2016, 6, 62522 RSC.
- D. Segets, R. Marczak, S. Schäfer, C. Paula, J.-F. Gnichwitz, A. Hirsch and W. Peukert, ACS Nano, 2011, 5, 4658 CrossRef CAS PubMed.
- R. D. Chavan, M. Wolska-Pietkiewicz, D. Prochowicz, M. Jędrzejewska, M. M. Tavakoli, P. Yadav, C. K. Hong and J. Lewiński, Adv. Funct. Mater., 2022, 32, 2205909 CrossRef CAS.
- R. Runjhun, E. A. Alharbi, Z. Drużyński, A. Krishna, M. Wolska-Pietkiewicz, V. Skorajanc, T. P. Baumeler, G. Kakavelakis, F. Eickemeyer, M. Mensi, S. M. Zakeeruddin, M. Grätzel and J. Leśniowski, Energy Environ. Mater., 2024, 7, e12720 CrossRef CAS.
- D. Zheng, G. Wang, W. Huang, B. Wang, W. Ke, J. L. Logsdon, Z. Wang, W. Zhu, M. R. Wasielewski, M. G. Kanatzidis, T. J. Marks and A. Facchetti, Adv. Funct. Mater., 2019, 29, 1900265 CrossRef.
- J. Zhou, X. Meng, X. Zhang, X. Tao, Z. Zhang, J. Hu, C. Wang, Y. Li and S. Yang, Mater. Chem. Front., 2017, 1, 802–806 RSC.
- J. L. Yang, B. D. Siempelkamp, E. Mosconi, F. De Angelis and T. L. Kelly, Chem. Mater., 2015, 27, 4229–4236 CrossRef CAS.
- X. Dong, H. Hu, B. Lin, J. Ding and N. Yuan, Chem. Commun., 2014, 50, 14405–14408 RSC.
- M. Najafi, V. Zardetto, D. Zhang, D. Koushik, M. S. Dörenkämper, M. Creatore, R. Andriessen, P. Poodt and S. Veenstra, Sol. RRL, 2018, 2, 1800147 CrossRef.
- X. Xu, H. Wang, J. Wang, M. Muhammad, Z. Wang, P. Chen, W. Zhao, B. Kang, J. Zhang, C. Li and Y. Duan, ACS Appl. Energy Mater., 2020, 3, 4208–4216 CrossRef CAS.
- R. Pietruszka, B. S. Witkowski, S. Zimowski, T. Stapinski and M. Godlewski, Surf. Coat. Technol., 2017, 319, 164–169 CrossRef CAS.
- K. Sharma, D. Routkevitch, N. Varaksa and S. M. George, J. Vac. Sci. Technol., A, 2016, 34, 01A146 CrossRef.
- P. Rong, S. Ren and Q. Yu, Crit. Rev. Anal. Chem., 2019, 49, 336–349 CrossRef CAS PubMed.
- A. Thote, I. Jeon, H.-S. Lin, S. Manzhos, T. Nakagawa, D. Suh, J. Hwang, M. Kashiwagi, J. Shiomi, S. Maruyama, H. Daiguji and Y. Matsuo, ACS Appl. Electron. Mater., 2019, 1, 389–396 CrossRef CAS.
- L. S. Liang, Z. F. Huang, L. H. Cai, W. Z. Chen, B. Z. Wang, K. W. Chen, H. Bai, Q. Y. Tian and B. Fan, ACS Appl. Mater. Interfaces, 2014, 6, 20585–20589 CrossRef CAS PubMed.
- S. Li, P. Zhang, Y. Wang, D. Liu, J. Wu, H. Sarvari, Z. D. Chen and H. Sarvari, Nano Res., 2017, 10, 1092–1103 CrossRef CAS.
- X. Yao, J. Liang, T. Li, L. Fan, B. Shi, C. Wei, Y. Ding, Y. Li, Y. Zhao and X. Zhang, Sci. China Mater., 2018, 61, 65–72 CrossRef CAS.
- A. Thote, I. Jeon, H.-S. Lin, S. Manzhos, T. Nakagawa, D. Suh, J. Hwang, M. Kashiwagi, J. Shiomi, S. Maruyama, H. Daiguji and Y. Matsuo, ACS Appl. Electron. Mater., 2019, 1, 389–396 CrossRef CAS.
- A. N. Banerjee, S. W. Joo and B. K. Min, J. Nanosci. Nanotechnol., 2014, 14, 7970–7975 CrossRef CAS PubMed.
- K. Mahmood, B. S. Swain and A. Amassian, Nanoscale, 2014, 6, 14674–14678 RSC.
- K. Mahmood, A. Khalid, M. Hameed, F. Rehman, M. T. Mehran and R. Song, Appl. Phys. A, 2018, 124, 1–6 CrossRef CAS.
- K. Sekar, R. Nakar, J. Bouclé, R. Doineau, K. Nadaud, B. Schmaltz and G. Poulin-Vittrant, Nanomaterials, 2022, 12, 2093 CrossRef CAS PubMed.
- S. Li, P. Zhang, H. Chen, Y. Wang, D. Liu, J. Wu, H. Sarvari, Z. D. Chen and H. Sarvari, J. Power Sources, 2017, 342, 990–997 CrossRef CAS.
- M. M. Tavakoli, R. Tavakoli, Z. Nourbakhsh, A. Waleed, U. S. Virk and Z. Fan, Adv. Mater. Interfaces, 2016, 3, 1500790 CrossRef.
- Q. Yu, R. Lin, L. Jiang, J. Wan and C. Chen, Sci. China Mater., 2018, 61, 1007–1011 CrossRef CAS.
- C. Chung, B. T. Tran, K. Lin, Y. Ho, H. Yu, N. Quan and E. Chang, Nanoscale Res. Lett., 2014, 9, 338 CrossRef PubMed.
- Q. Yu, L. Y. Jiang and T. T. Ai, Mater. Lett., 2015, 152, 142–144 CrossRef CAS.
- G. Zhang, Y. Liu, H. Morikawa and Y. Chen, Cellulose, 2013, 20, 1877–1884 CrossRef CAS.
- S. Wirunchit and W. Koetniyom, Phys. Status Solidi A, 2023, 220, 2200364 CrossRef CAS.
- J. Zhang, P. Barboux and T. Pauporté, Adv. Energy Mater., 2014, 4, 1400932 CrossRef.
- J. Zhang, E. J. Juárez-Pérez, I. Mora-Seró, B. Viana and T. Pauporté, J. Mater. Chem. A, 2015, 3, 4909–4915 RSC.
- C. M. Pelicano and H. Yanagi, J. Energy Chem., 2018, 27, 455–462 CrossRef.
- O. N. Salman, M. O. Dawood, A. K. Ali, D. S. Ahmed and K. I. Hassoon, Dig. J. Nanomater. Bios., 2017, 12, 719–726 Search PubMed.
- V. J. Garcia, C. M. Pelicano and H. Yanagi, Thin Solid Films, 2018, 662, 70–75 CrossRef CAS.
- K. Mahmood, A. Khalid, M. S. Zafar, F. Rehman, M. Hameed and M. T. Mehran, J. Colloid Interface Sci., 2019, 538, 426–432 CrossRef CAS PubMed.
- M. H. Kumar, N. Yantara, S. Dharani, M. Graetzel, S. Mhaisalkar, P. P. Boix and N. Mathews, Chem. Commun., 2013, 49, 11089–11091 RSC.
- V. T. Tran, Y. Wei, H. Yang, Z. Zhan and H. Du, Nanotechnology, 2017, 28, 095204 CrossRef PubMed.
- S. El Ouakili, H. Zahdi, S. Laalioui, A. Rajira, Z. Aqachmar, A. Abounadi, A. Elhichou, A. Almaggoussi and N. Rochdi, Opt. Mater., 2024, 156, 115931 CrossRef CAS.
- Y. Yuan, L. Yang, X. Peng, Q. Chen, X. Li, C. Zuo, Z. Zhou and Z. Bai, J. Mater. Sci., 2024, 59, 11454–11467 CrossRef CAS.
- W. Huang, R. Zhang, X. Xia, P. Steichen, N. Liu, J. Chu and L. Li, Nanomaterials, 2021, 11, 329 CrossRef CAS PubMed.
- J. Cao, B. Wu, R. Chen, Y. Wu, Y. Hui, B.-W. Mao and N.-F. Zheng, Adv. Mater., 2018, 30, 1705596 CrossRef PubMed.
- X. Zhang, D. Zhang, T. Guo, J. Zou, J. Jin, C. Zheng, Y. Zhou, Z. Zhu, Z. Hu, Q. Cao, S. Wu, J. Zhang and Q. Tai, J. Mater. Chem. A, 2023, 11, 9616–9625 RSC.
- K. H. Lee, R. Farheen, Z. Arshad, M. Ali, H. Hassan, M. Alshareef, A. Dahshan and U. Khalid, RSC Adv., 2024, 14, 15391–15407 RSC.
- T. Das, R. Nag, N. K. Rana, M. Nayak, R. Paramanik, A. Bera, S. K. Saha and A. Guchhait, Energy Fuels, 2023, 37, 10642–10651 CrossRef CAS.
- O. Cardozo, A. Stingl and S. Farooq, Mater. Sci. Semicond. Process., 2024, 180, 108537 CrossRef CAS.
- M. Gantumur, M. I. Hossain, M. Shahiduzzaman, A. Tamang, J. H. Rafij, M. Shahinuzzaman, H. T. C. Tu, M. Nakano, M. Karakawa, K. Ohdaira, H. Almohamadi, M. A. Ibrahim, K. Sopian, M. Akhtaruzzaman, J. M. Nunzi and T. Taima, ACS Appl. Mater. Interfaces, 2024, 16, 36255–36271 CrossRef CAS.
- J. H. Kang, A. Song, Y. J. Park, J. H. Seo, B. Walker and K.-B. Chung, Polymers, 2020, 12, 737 CrossRef CAS PubMed.
- P.-C. Pan, H.-S. Koo, D.-X. Chen and C.-M. Chen, Crystals, 2022, 12, 1032 CrossRef CAS.
- S. Ahmed, M. Akhtaruzzaman, W. Zulhafizhazuan, Y. Yusoff, I. Alnaser, M. R. Karim, M. Shahiduzzaman and K. Sobayel, Jpn. J. Appl. Phys., 2023, 62, 092001 CrossRef CAS.
- J. Kruszyńska, J. Ostapko, V. Ozkaya, B. Surucu, O. Szawcow, K. Nikiforow, M. Hołdyński, M. M. Tavakoli, P. Yadav and M. Kot, et al. , Adv. Mater. Interfaces, 2022, 9, 2200575 CrossRef.
- L. Yang, T. Xu, Z. Bai and S. Qin, J. Phys. Chem. C, 2023, 127, 7492–7500 CrossRef CAS.
- A. Pramothkumar, N. Senthilkumar, S. Pitchaiya, N. Eswaramoorthy, V. Madurai Ramakrishnan and I. V. Potheher, J. Mater. Sci.: Mater. Electron., 2023, 34, 627 CrossRef CAS.
- M. Al-Rasheidi, F. Khan, A. Al-Ahmed, S. Rehman and F. Al-Sulaiman, Opt. Mater., 2022, 126, 112144 CrossRef CAS.
- R. Bashir, M. K. Bilal and A. Bashir, Sol. Energy, 2023, 256, 67–75 CrossRef CAS.
- Z. Arshad, S. Wageh, T. Maiyalagan, M. Ali, U. Arshad, N. ul-ain, M. B. Qadir, F. Mateen, A. G. Al-Sehemi and K. Sehemi, Ceram. Int., 2022, 48, 24363–24371 CrossRef CAS.
- Y. Li, N. Liu, Z. Xu, Z. Xu, Y. Pan, J. Zhang, L. Huang, Z. Hu, Y. Zhu and X. Liu, Appl. Phys. Lett., 2023, 123, 163901 CrossRef CAS.
- M. V. Rajendran, S. Ganesan, V. S. Menon, R. K. Raman, A. Alagumalai, S. Ashok Kumar and A. Krishnamoorthy, ACS Appl. Energy Mater., 2022, 5, 6671–6686 CrossRef.
- H. M. Abd El-Lateef, M. M. Khalaf, F. E.-T. Heakal, M. F. Abou Taleb and M. Gouda, Sol. Energy, 2023, 253, 453–461 CrossRef CAS.
- N. Eswaramoorthy and K. Rajaram, Diam. Relat. Mater., 2023, 136, 109917 CrossRef CAS.
- X. Wu, J. Zhang, M. Qin, K. Liu, Z. Lv, Z. Qin, X. Guo, Y. Li, J. Xu, G. Li, H. Yan and X. Lu, EcoMat, 2022, 4, e12192 CrossRef CAS.
- X. Zhang, D. Zhang, T. Guo, J. Zou, J. Jin, C. Zheng, Y. Zhou, Z. Zhu, Z. Hu, Q. Cao, S. Wu, J. Zhang and Q. Tai, J. Mater. Chem. A, 2023, 11, 9616–9625 RSC.
- B. Liu, B. Guo and R. Balamurugan, Nanomaterials, 2020, 10, 1753 CrossRef CAS PubMed.
- P. Fan, J. Tian, K. Wang, D. Zheng, J. Yu and T. P. Russell, J. Mater. Chem. C, 2024, 12, 19219 RSC.
- M. A. Mahmud, N. K. Elumalai, M. B. Upama, D. Wang, K. H. Chan, M. Wright, C. Xu, F. Haque and A. Uddin, Sol. Energy Mater. Sol. Cells, 2017, 159, 251–264 CrossRef CAS.
- S. Wu, M. Lin, S. Chang, W. Tu, C. Chu and Y. Chang, J. Phys. Chem. C, 2018, 122, 236–244 CrossRef CAS.
- J. Li, J. Yang, J. Ma, J. Liang, Y. Liu, X. Hu, C. Chen, W. Yang, J. Min, Q. Bao, G. Fang and C. Tao, Chem. Eng. J., 2021, 417, 129247 CrossRef CAS.
- G. Bagha, K. Samavati, H. Naffakh-Moosavy and L. F. Matin, Sci. Rep., 2024, 14, 4617 CrossRef CAS PubMed.
- X. Xu, H. Zhang, J. Shi, J. Dong, Y. Luo, D. Li and Q. Meng, J. Mater. Chem. A, 2015, 3, 19288–19293 RSC.
- M. Dkhili, G. Lucarelli, F. De Rossi, B. Taheri, K. Hammedi, H. Ezzaouia, F. Brunetti and T. M. Brown, ACS Appl. Energy Mater., 2022, 5, 4096–4107 CrossRef CAS PubMed.
- W. Qiu, M. Buffière, G. Brammertz, U. Paetzold, L. Froyen, P. Heremans and D. Cheyns, Org. Electron., 2015, 26, 30–35 CrossRef CAS.
- J. Zhang, C. H. Tan, T. Du, M. Morbidoni, C.-T. Lin, S. Xu, J. R. Durrant and M. A. McLachlan, Sci. Bull., 2018, 63, 343–348 CrossRef CAS PubMed.
- Y. Cheng, Q. Yang, J. Xiao, Q. Xue, H. Li, Z. Guan, H. Yip and S. Tsang, ACS Appl. Mater. Interfaces, 2015, 7, 19986–19993 CrossRef PubMed.
- Z. Qiu, S. Yuan, H. Gong, H. Zhang, X. Qiu, T. Luo and B. Cao, J. Am. Ceram. Soc., 2017, 100, 176–184 CrossRef CAS.
- R. Runjhun, E. A. Alharbi, Z. Drużyński, A. Krishna, M. Wolska-Pietkiewicz, V. Škorjanc, T. P. Baumeler, G. Kakavelakis, F. Eickemeyer, M. Mensi, S. M. Zakeeruddin, M. Grätzel and J. Lewiński, Energy Environ. Mater., 2024, 7, e12720 CrossRef CAS.
- V. Murugadoss, D. Y. Kang, W. J. Lee, I. G. Jang and T. G. Kim, Adv. Compos. Hybrid Mater., 2022, 5, 1385–1395 CrossRef CAS.
- T. Tulus, S. Olthof, M. Marszalek, A. Peukert, L. A. Muscarella, B. Ehrler, O. Vukovic, Y. Galagan, S. C. Boehme and E. von Hauff, ACS Appl. Energy Mater., 2019, 2, 3736–3748 CrossRef.
- T. Tulus, L. A. Muscarella, Y. Galagan, S. C. Boehme and E. von Hauff, Electrochim. Acta, 2022, 433, 141215 CrossRef.
- S. Tsarev and P. A. Troshin, Synth. Met., 2020, 269, 116547 CrossRef CAS.
- Z. Pang, S. Yang, Y. Sun, L. He, F. Wang, L. Fan, S. Chi, X. Sun, L. Yang and J. Yang, Chem. Eng. J., 2022, 439, 135701 CrossRef CAS.
- G. L. Liu, Y. Zhong, H. D. Mao, J. Yang, R. Y. Dai, X. T. Hu, Z. Xing, W. P. Sheng, L. C. Tan and Y. W. Chen, Chem. Eng. J., 2022, 431, 134235 CrossRef CAS.
- A. Yella, L. Heiniger, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nano Lett., 2014, 14, 2591–2596 CrossRef CAS PubMed.
- N. Vaenas, D. Konios, T. Stergiopoulos and E. Kymakis, RSC Adv., 2015, 5, 107771–107776 RSC.
- B. J. Kim, D. H. Kim, Y. Y. Lee, H. W. Shin, G. S. Han, J. S. Hong, K. Mahmood, T. K. Ahn, Y. C. Joo, K. S. Hong, N. G. Park, S. Lee and H. S. Jung, Energy Environ. Sci., 2015, 8, 916–921 RSC.
- X. Chen, L. J. Tang, S. Yang, Y. Hou and H. G. Yang, J. Mater. Chem. A, 2016, 4, 6521–6526 RSC.
- F. Cai, L. Yang, Y. Yan, J. Zhang, F. Qin, D. Liu, Y. B. Cheng, Y. Zhou and T. Wang, J. Mater. Chem. A, 2017, 5, 9402–9411 RSC.
- Z. Lan, X. Xu, X. Zhang, J. Tang, L. Zhang, X. He and J. Wu, J. Mater. Chem. C, 2018, 6, 3341–3351 RSC.
- M. S. You, J. H. Heo, J. K. Park, S. H. Moon, B. J. Park and S. H. Im, Sol. Energy Mater. Sol. Cells, 2019, 194, 1–6 CrossRef CAS.
- J. Nam, J. H. Kim, C. S. Kim, J.-D. Kwon and S. Jo, ACS Appl. Mater. Interfaces, 2020, 12, 12648–12655 CrossRef CAS PubMed.
- Y. Sanehira, N. Shibayama, Y. Numata, M. Ikegami and T. Miyasaka, ACS Appl. Mater. Interfaces, 2020, 12, 15175–15182 CrossRef CAS PubMed.
- Z. Ren, N. Wang, P. Wei, M. Cui, X. Li and C. Qin, Chem. Eng. J., 2020, 393, 124731 CrossRef CAS.
- T. Homola, J. Pospisil, M. Shekargoftar, T. Svoboda, M. Hvojnik, P. Gemeiner, M. Weiter and P. Dzik, ACS Appl. Energy Mater., 2020, 3, 12009–12018 CrossRef CAS.
- B. Pan, J. Gu, X. Xu, L. Xiao, J. Zhao and G. Zou, Nano Res., 2021, 14, 3431–3438 CrossRef CAS.
- S. Y. Hong, H. J. Lee, J. K. Park, J. H. Heo and S. H. Im, Int. J. Energy Res., 2022, 46, 22819–22831 CrossRef CAS.
- B. Liu, G. Sun, Q. Sun, Y. Lv, M. Huang and B. Qi, Mater. Sci. Semicond. Process., 2022, 138, 106303 CrossRef CAS.
- M. J. Carnie, C. Charbonneau, M. L. Davies, J. Troughton, T. M. Watson, K. Wojciechowski, H. Snaith and D. A. Worsley, Chem. Commun., 2013, 49, 7893–7895 RSC.
- J. Zhang, A. Hultqvist, T. Zhang, L. Jiang, C. Ruan, L. Yang, Y. Cheng, M. Edoff and E. M. J. Johansson, ChemSusChem, 2017, 10, 3810–3817 CrossRef CAS PubMed.
- H. Liu, Z. Huang, S. Wei, L. Zheng, L. Xiao and Q. Gong, Nanoscale, 2016, 8, 6209–6221 RSC.
- Y. Qiang, Y. Xie, Y. Qi, P. Wei, H. Shi, C. Geng and H. Liu, Sol. Energy, 2020, 201, 523–529 CrossRef CAS.
- C. Chen, C. Li, F. Li, F. Wu, F. Tan, Y. Zhai and W. Zhang, Nanoscale Res. Lett., 2014, 9, 457 CrossRef PubMed.
- E. Y. Choi, J. Kim, S. Lim, E. Han, A. W. Y. Ho-Baillie, N. Park and N. Park, Sol. Energy Mater. Sol. Cells, 2018, 188, 37–45 CrossRef CAS.
- R. Singh, S. Ghosh, A. S. Subbiah, N. Mahuli and S. K. Sarkar, Sol. Energy Mater. Sol. Cells, 2020, 205, 110289 CrossRef CAS.
- P. P. Rajbhandari and T. P. Dhakal, J. Vac. Sci. Technol., A, 2020, 38, 032406 CrossRef CAS.
- J. Feng, Z. Yang, D. Yang, X. Ren, X. Zhu, Z. Jin, W. Zi, Q. Wei and S. Liu, Nano Energy, 2017, 36, 1–8 CrossRef CAS.
- T. Yokoyama, Y. Nishitani, Y. Miyamoto, S. Kusumoto, R. Uchida, T. Matsui, K. Kawano, T. Sekiguchi and Y. Kaneko, ACS Appl. Mater. Interfaces, 2020, 12, 27131–27139 CrossRef CAS.
- D. Shen, W. Zhang, Y. Li, A. Abate and M. Wei, ACS Appl. Nano Mater., 2018, 1, 4101–4109 CrossRef CAS.
- X. Ye, H. Ling, R. Zhang, Z. Wen, S. Hu, T. Akasaka, J. Xia and X. Lu, J. Power Sources, 2020, 448, 227419 CrossRef CAS.
- Y. Shang, T. Zhang, D. Yu, Z. Peng, W. Zhou, D. Yin and Z. Ning, ACS Appl. Mater. Interfaces, 2021, 13, 47603–47609 CrossRef CAS PubMed.
- J. Duan, Y. Yang, X. Xie, K. Zhang, H. Wan, J. Zhang, L. Tao and W. Wang, J. Mater. Res., 2022, 37, 2825–2836 CrossRef CAS.
- Q. Dong, F. Z. Liu, M. K. Wong, A. B. Djurisic, Z. W. Ren, Q. Shen, A. Ng, C. Surya and W. K. Chan, Oxide-Based Materials and Devices VII, 2016, vol. 9749, pp. 97491S Search PubMed.
- B. Yang, R. Ma, Z. Wang, D. Ouyang, Z. Huang, J. Lu, X. Duan, L. Yue and W. C. H. Choy, ACS Appl. Mater. Interfaces, 2021, 13, 27179–27187 CrossRef CAS PubMed.
- D. Jia, X. Feng, J. Jia, B. Shi, Y. Wu and B. Cao, J. Solid State Chem., 2023, 317, 123661 CrossRef.
- D. Guo, J. Ma, S. Lin, X. Guo, H. Huang, D. Kong, F. Xu, Y. Gao, W. Zhang, Y. Hu and C. Zhou, Appl. Phys. Lett., 2022, 120, 263502 CrossRef CAS.
- L. J. Chen, G. Wang, L. B. Niu, Y. Q. Yao, Y. X. Guan, Y. T. Cui and Q. L. Song, RSC Adv., 2018, 8, 15961–15966 RSC.
- C. Gong, H. Li, H. Wang, C. Zhang, Q. Zhuang, A. Wang, Z. Xu, W. Cai, R. Li, X. Li and Z. Zang, Nat. Commun., 2024, 15, 4922 CrossRef CAS PubMed.
- W. Shockley and H. J. Queisser, J. Appl. Phys., 1961, 32, 510–519 CrossRef CAS.
- B. Y. Shao, X. Song, H. W. Zhu, Y. A. Bioud, W. T. Wu, M. Abulikemu, H. Saiari, S. Aqeel, I. Gereige, O. F. Mohammed and O. M. Bakr, Chem. Soc. Rev., 2025, 54(21), 9939–9977 RSC.
- X. Qiu, Y. Jiang, H. Zhang, Z. Qiu, S. Yuan, P. Wang and B. Cao, Phys. Status Solidi RRL, 2016, 10, 587–591 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |