Radiative cooling materials and strategies for suppressing ice melting and enabling passive cold-chain management
Cheng-Yu
He†
 a,
Xu-Yan
Xu†
a,
Ying-Ying
Wu
a,
Ge-Ting
Sun
ab,
Qi-Sen
Wang
a,
Yong-Zhi
Zhang
a,
Rui-Ting
Gao
c and
Xiang-Hu
Gao
a,
Xu-Yan
Xu†
a,
Ying-Ying
Wu
a,
Ge-Ting
Sun
ab,
Qi-Sen
Wang
a,
Yong-Zhi
Zhang
a,
Rui-Ting
Gao
c and
Xiang-Hu
Gao
 *ab
*ab
aKey Laboratory of Energy Conservation and Energy Storage Materials of Gansu Province, Research Center for Resource Chemistry and Energy Materials, State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China. E-mail: gaoxh@licp.cas.cn
bCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
cCollege of Chemistry and Chemical Engineering, Inner Mongolia Key Laboratory of Low Carbon Catalysis, Inner Mongolia University, Hohhot, China
First published on 14th November 2025
Abstract
Ice loss from glaciers and snowpacks poses a growing threat to freshwater resources and coastal communities through sea-level rise, while also placing unprecedented demands on global cold-chain logistics. Passive daytime radiative cooling (PDRC) has emerged as a zero-energy strategy to counteract solar heating by reflecting over 95% of sunlight (0.3–2.5 µm) and emitting thermal radiation through the atmospheric window (8–13 µm) into outer space. In this review, we first outline the governing radiative and non-radiative heat-transfer principles and define performance targets. We systematically summarize recent advances in material design, highlighting architectures that achieve 5–15 °C sub-ambient cooling under direct sunlight, together with robust self-cleaning, UV durability, mechanical strength, and humidity tolerance for real-world deployment. Two major application frontiers are emphasized: (1) passive cold-chain management, where radiative-cooling films and packaging materials provide energy-free refrigeration for perishable goods; and (2) glacier and ice preservation, where PDRC covers significantly prolong ice and snow longevity under intense sunlight. Finally, we identify major challenges for practical adoption, such as scalable manufacturing, all-weather reliability, and environmental sustainability, and propose future research directions to accelerate the large-scale implementation of PDRC technologies.
1. Introduction
Suppressing ice melting has become a critical imperative in the face of accelerating climate change.1–4 Glaciers worldwide are retreating at record rates, threatening freshwater supplies for nearly two billion people who depend on seasonal meltwater for drinking, agriculture, and hydropower.1 The loss of ice also contributes directly to sea-level rise, undermining coastal defenses and exposing low-lying communities to flooding. Moreover, the reduction of ice cover decreases Earth's surface albedo, diminishing its capacity to reflect solar radiation and thereby intensifying warming through a positive feedback loop that further destabilizes polar and alpine regions.5–8 Notably, the Arctic has warmed nearly four times faster than the global average since 1979.9 As such, arresting or even slowing this melt is therefore essential not only for preserving vital water resources but also for stabilizing global climate systems. In parallel to safeguarding natural glaciers, many industrial and humanitarian applications rely on ice or subzero storage.10–12 Cold-chain logistics preserve roughly 40% of the world's food and vital pharmaceuticals, consuming over 10% of global electricity and accounting for significant greenhouse-gas emissions.13,14 In regions with unreliable power infrastructure, food spoilage and vaccine wastage are severe public-health and economic concerns. Traditional refrigeration methods are energy-intensive, costly, and often impractical in remote or disaster-affected areas. Likewise, construction of shade structures or deployment of reflective blankets to retard ice melt on glaciers or in refrigerated warehouses is laborious, weather-sensitive, and only partially effective.3 What is needed is a passive, zero-energy strategy that can be deployed at scale to curb ice loss in both natural and engineered contexts.Passive daytime radiative cooling (PDRC) has recently emerged as a promising solution to this dual challenge.15–20 PDRC materials are engineered to reflect the majority of incoming solar radiation (0.3–2.5 µm) while emitting thermal radiation through the atmospheric window (8–13 µm) directly to outer space, which sits at a background temperature of ∼3 K. When a surface reflects more solar energy than it absorbs and concurrently emits heat to the sky, it achieves net cooling below ambient air temperature, even at midday under direct sun. Early demonstrations showed building surfaces reaching 5–15 °C below ambient, validating the concept's viability for large-area, zero-energy cooling.21–24 Applying PDRC to glacier protection leverages these same radiative principles but requires exceptionally high performance.3 A glacier surface at noon can receive 800–1000 W m−2 of solar irradiance; to suppress melt, a PDRC cover must reflect over 90% of that incident power and emit >100 W m−2 through the 8–13 µm band. Recent laboratory studies have met these criteria. For example, hierarchically porous polymer composites boasting >95% solar reflectance and >90% mid-IR emissivity achieved ∼9 °C sub-ambient cooling, extending the lifetime of ice samples by a factor of four compared to bare ice.25 Similarly, cellulose-acetate membranes demonstrated 6–8 °C sub-ambient cooling in field tests, effectively slowing snowpack loss over multi-day exposures.26 These results confirm that passive radiative cooling can tip the energy balance of ice surfaces toward net heat rejection, offering a sustainable method to retard glacier melt without any power input.
Beyond glaciers, PDRC shows great promise for cold-chain preservation. Food and vaccine packaging films made from biodegradable polymers, such as nanoporous cellulose acetate (CA) infused with ZnO nanoparticles, have delivered 10–14 °C temperature reductions under sunlight.27 These films combine high solar reflectance, strong thermal emissivity, antimicrobial action, and self-cleaning superhydrophobic surfaces to maintain extended cooling performance in outdoor markets. The prospect of wrapping perishable goods in a single layer of radiative-cooling material offers an elegant, energy-free adjunct or alternative to powered refrigeration, particularly in off-grid and resource-constrained settings. To translate these advances into real-world solutions, several challenges must be addressed, both optical performance and environmental durability.28–30 In this review, we discuss the fundamental radiative mechanisms and performance benchmarks for sub-ambient cooling under sunlight, survey recent materials design innovations, and highlight applications in glacier protection and food preservation, as shown in Fig. 1. We then identify remaining challenges and propose future research directions to realize large-scale, zero-energy solutions for protecting Earth's ice resources and preserving perishable goods.
2. Fundamentals of passive daytime radiative cooling
2.1. Basic principles and spectral requirements
A high-performance PDRC is crucial to suppress ice melting, and this hinges on spectral control, i.e. engineering a surface's optical properties across solar and thermal wavelengths,31–33 as shown in Fig. 2a. In the Earth-atmosphere system, an energy equilibrium is maintained when outgoing thermal radiation balances incoming solar irradiance. During daytime, incoming short-wave solar radiation (0.3–2.5 µm) is partly absorbed by the ground or atmosphere and partly reflected back to space (Fig. 2b). Meanwhile, the Earth and all objects on it emit long-wave infrared radiation (2.5–25 µm); much of this is absorbed by atmospheric gases, but a portion in the 8–13 µm band can transmit through clear sky directly to outer space. This transmission window (8–13 µm), often called the atmospheric window, arises because atmospheric components (notably H2O and CO2) absorb very weakly in that band.34,35 The 8–13 µm window overlaps with the peak thermal emission of a ∼300 K blackbody (Fig. 2c), providing an avenue to dump heat from Earth to the 3 K sink of deep space. The core design principle for PDRC is to ensure that, under sunlight, a surface emits more radiative power through the atmospheric window than it absorbs from solar irradiance, maximizing heat outflow and minimizing heat inflow. | ||
| Fig. 2 Fundamentals of PDRC. (a) Basic principles for PDRC: it reflects short-wave solar radiation in the wavelength range of 0.3–2.5 µm and emits long-wave infrared radiation within the atmospheric window of 8–13 µm. (b) Solar spectrum across UV-visible-near-IR wavelengths. (c) The 8–13 µm atmospheric window, which overlaps with the peak thermal emission of a blackbody at approximately 300 K. (d) Absorption spectra comparison between broadband and selective thermal emitters. (e) Net radiative cooling power as a function of the temperature difference between the emitter surface and the ambient environment, calculated under the assumptions of a heat transfer coefficient of 3 W m−2 K−1, an ambient temperature of 20 °C, and a total precipitable water vapor of 10 mm. Reproduced from ref. 37 with permission from the AIP Publishing, copyright 2019. | ||
Depending on the application scenario and operating temperature, two main types of radiative cooling surfaces are adopted: spectrally selective emitters and broadband emitters (Fig. 2d). Spectrally selective emitters have high emissivity only within the 8–13 µm atmospheric window and low emissivity elsewhere, while broadband emitters feature high emissivity across the entire infrared range.36 The choice between broadband and selective emitters should be determined by the specific temperature regime and environmental context. Zhao et al.37 demonstrated an almost linear dependence of net radiative-cooling power on the temperature differential between an emitter's surface and its surroundings for spectrally selective and broadband emitters (Fig. 2e). Their results indicate that a spectrally selective emitter offers superior passive cooling when the surface is below ambient temperature, whereas a broadband emitter outperforms once the surface temperature approaches or exceeds ambient.
To attain effective net cooling under sunlight and thereby suppress ice melting, a PDRC surface should be a selective emitter satisfying three spectral criteria: (1) near-zero absorptance in the solar spectrum (0.3–2.5 µm). This is equivalent to having very high solar reflectance across UV-visible-near-IR wavelengths so that the surface absorbs as little sunlight as possible. In practice, the solar absorptivity should be close to 0 to limit solar heat gain. (2) High emissivity in the mid-IR atmospheric window. Ideally the emissivity ≈1 in this band, so that the surface can radiate heat at thermal wavelengths to the sky at the maximum possible rate.38 This ensures most thermal radiation is funneled into the sky window and away from the surface. (3) Low emissivity outside the atmospheric window (i.e. in the 2.5–8 µm and >13 µm bands).39 Since thermal radiation outside 8–13 µm is mostly absorbed by atmospheric gases and re-radiated back to Earth, a low emissivity in these regions is necessary to minimize parasitic energy recycling and optimize net cooling.
In special conditions such as very low humidity, high altitude, or polar regions, another atmospheric window (16–28 µm) becomes accessible due to reduced water vapor.40 For such environments, enhancing emissivity in both the primary (8–13 µm) and secondary (16–28 µm) windows can further improve radiative cooling performance. However, under typical lowland and humid conditions, the benefit from this secondary window is usually negligible.
2.2. Energy analysis of the PDRC system
The cooling performance of a PDRC material under sunlight is usually evaluated by the achieved sub-ambient temperature drop and cooling power. However, these metrics can vary with testing conditions such as geographic location, wind speed, and humidity; even tests on the same material often yield different results. In general, when a radiative cooler is exposed to sunlight, it exchanges heat with its environment via multiple pathways: it absorbs solar irradiance and downward atmospheric IR, and it loses heat via thermal radiation to the sky, as well as conductive and convective transfer to the air. Therefore, considering the energy flow per unit area of the PDRC surface exposed to the atmosphere, if the radiative outflow power exceeds the total heat input to the surface, net cooling will occur. According to conservation of energy, the net cooling power can be expressed conceptually as:| Pnet = Prad − (Psolar + Patm + Pnonrad) | (1) |
The thermal radiation power Prad emitted from a PDRC surface is related to the surface temperature (Ts) and the emissivity spectrum. It can be expressed by integrating the spectral radiance over all wavelengths and angles. Under isotropic emission, this yields the Stefan–Boltzmann law:
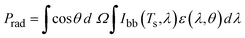 | (2) |
| Prad = εσTs4 | (3) |
Historically, absorption of solar radiation was not a concern for radiative sky-cooling applications focused on nighttime use only. However, daytime radiative cooling, reflection of solar radiation by the surface must be maximized. Solar irradiation has minimal spectral overlap with 300 K thermal emission, meaning it is difficult to develop a spectrally selective surface that has extremely low absorptance (high reflectance) in the solar spectrum while maintaining high emissivity in the infrared. In practice, the PDRC surface should greatly reduce solar heat gain by reflecting most sunlight. Quantitatively, if the average solar reflectance is below ∼90%, the absorbed solar power (>100 W m−2) would negate much of the cooling power of PDRC. Thus, typical PDRC surfaces target solar reflectance above 90%. In other words, the material's solar absorptance must be kept very small to prevent solar heating from overwhelming the radiative cooling effect.
It is worth noting that under humid or cloudy conditions when the atmospheric transmittance in the 8–13 µm window is low and the sky is nearly opaque, a spectrally selective emitter is preferred. By restricting emission to the 8–13 µm window and suppressing it elsewhere, the cooler avoids wasting radiative power at wavelengths that would be absorbed by the moist atmosphere, thus maintaining a low net exchange with the warm sky. Conversely, under dry, clear-sky conditions or when the surface temperature is at or above ambient, a broadband emitter can radiate more heat overall and may be advantageous for maximizing total cooling power. In summary, the optimal emissive spectrum can depend on atmospheric conditions: selective emitters excel when the atmosphere strongly absorbs IR, whereas broadband emitters can perform better when the sky is very transparent.
| Pnonrad = hc(Tamb − Ts) | (4) |
With these principles in mind, researchers have established guidelines for practical PDRC performance. Generally, to achieve a meaningful cooling effect under peak sunlight, the material should reflect >95% of solar radiation and emit >90% of 8–13 µm thermal radiation. State-of-the-art PDRC devices can attain daytime cooling powers on the order of 100–120 W m−2 and stabilize surfaces at 5–15 °C below ambient in dry, sunny weather.20,42,43 In the next sections, we review how material design innovations enable these spectral properties (Section 3) and how PDRC is being applied to real-world cooling challenges (Section 4), especially for preserving ice and perishable goods without energy input.
3. Design of high-performance PDRC materials to suppress ice melting
To passively keep ice and snow from melting under sunlight, radiative cooling materials must be carefully engineered for both high optical performance (maximizing solar reflectance and thermal emittance) and robust environmental durability. This section discusses key design principles and strategies, from enhancing broadband scattering and infrared emission to incorporating hierarchical structures and protective surface functionalities, and explains how these translate to suppressed ice melting in real-world conditions.3.1. Achieving high solar reflectance and infrared emissivity
An ideal radiative cooling material should absorb minimal sunlight while efficiently emitting thermal radiation through the 8–13 µm atmospheric window. By minimizing solar absorption (0.3–2.5 µm) and maximizing mid-IR emissivity, the material can achieve a net heat loss to the cold sky, even under intense solar illumination. Researchers have employed several optical design approaches to boost solar reflectance and to enhance thermal emittance.3.1.1.1. Mie scattering. By introducing particles or structures comparable to or larger than the wavelength of incident light, one can induce strong Mie scattering of solar radiation.31,44–48 Such scatterers with size on the order of 0.1–1 µm or larger for visible/near-IR light cause incoming sunlight to undergo multiple elastic scattering events, effectively producing a high diffuse reflectance. For example, Mandal et al. used a solvent-non-solvent phase inversion technique to create hierarchically porous fluoropolymer coatings (Fig. 3a).32 By exploiting differences in solvent evaporation rates, PVDF-HFP films formed interconnected micro- and nano-pores, yielding exceptional optical properties: solar reflectance of ∼96% and mid-infrared emissivity of ∼97% (Fig. 3b). Under natural sunlight, these coatings achieved a sub-ambient temperature reduction of ∼6 K and a cooling power up to 96 W m−2. Moreover, this phase-separation method extends to diverse polymers (PMMA, PS, ethyl cellulose, cellulose acetate), demonstrating broad applicability for passive radiative cooling materials. Xiang et al. introduced a three-dimensional porous cellulose acetate film with one-sided auto-deposited SiO2 microspheres.49 The hybrid film features ∼5 µm pores and ∼10 vol% SiO2, delivering ultrahigh solar reflectance (96–97% across 0.3–2.5 µm) and enhanced infrared emittance (∼95% in the 8–13 µm band). Outdoor tests reveal sub-ambient cooling of ∼6.2 °C under sunlight and ∼8.6 °C at night, illustrating full-day passive radiative cooling performance. The scattering efficiency of embedded particles generally increases with their refractive-index contrast relative to the matrix (for instance, n ≈ 2.5 for TiO2vs. n ≈ 1.45 for Al2O3 in a polymer of n ≈ 1.5). Thus, TiO2 spheres scatter sunlight more effectively than Al2O3, and embedding scatterers in an even lower-index medium (like air voids) further enhances scattering. However, narrow-bandgap materials like TiO2 can introduce UV absorption losses that reduce overall solar reflectance.50 Consequently, wide-bandgap alternatives such as Al2O3 (7.0 eV), BaSO4 (6.0 eV), and CaCO3 (7.0 eV) are preferred for high-efficiency optical scattering without significant intrinsic absorption. For example, Wei et al. coated textiles with a cellulose acetate matrix embedding Al2O3 nanoparticles via dip-coating, achieving a substantial increase in solar reflectance from 62.6% to 80.1% across 0.3–2.6 µm (Fig. 3c).51 These examples show that incorporating microscale scatterers within porous networks or fiber-based structures is an effective route to maximize solar reflection for PDRC, thereby minimizing solar heating of the underlying surface such as ice.
 | ||
| Fig. 3 Approaches for maximizing solar reflectance. (a) Top-view and cross-section micrographs of PVDF-HFP films, with inset highlighting nanoporous architecture. Reproduced from ref. 32 with permission from the American Association for the Advancement of Science, copyright 2018. (b) Measured reflectance spectra spanning the solar band (0.3–2.5 µm) and the atmospheric window (8–13 µm). Reproduced from ref. 32 with permission from the American Association for the Advancement of Science, copyright 2018. (c) UV-visible-near-IR reflectivity profile of the Al2O3-cellulose cooler. Reproduced from ref. 51 with permission from Elsevier, copyright 2020. (d) Cross-section schematics of the fluff-model scatterer under illumination from various angles, alongside simulated electric-field intensity distributions at λ = 0.49 µm. Reproduced from ref. 55 with permission from the PNAS license, copyright 2020. (e) Conceptual diagram and corresponding SEM of the bioinspired flexible hybrid film. Reproduced from ref. 55 with permission from the PNAS license, copyright 2020. (f) SEM of the photonic radiative cooler, with its wavelength-dependent emissivity/absorptivity measured under unpolarized light. Reproduced from ref. 59 with permission from the Springer Nature, copyright 2014. (g) Structural schematic of the implemented multilayer architecture and its emissivity spectrum. Reproduced from ref. 60 with permission from Elsevier, copyright 2017. | ||
3.1.1.2. Total internal reflection (TIR). When light travels from a medium of higher refractive index to a lower-index medium, it can be completely reflected at the interface if the incident angle exceeds the critical angle.52 By designing geometries that promote multiple TIR events such as hollow microcavities, cones, or pyramid-textured surfaces, sunlight can be repeatedly reflected within a material rather than transmitted, thereby increasing the overall reflectivity.43,53 Many natural systems exploit TIR for cooling; for instance, the triangular cross-sectional hairs of certain desert beetles and the tapered setae of Saharan silver ants internally reflect sunlight, helping these insects stay cool in intense sun.54,55 In man-made radiative coolers, Zhang et al.'s photonic film benefited from TIR within its micro-imprinted cavities (Fig. 3d and e),55 while Guo et al.'s metafabric used a fibrous structure that induced internal reflections.56 In both cases, TIR contributed to high solar reflectance and enhanced the cooling effect. These designs underscore that creating refractive index contrast and light-trapping geometries is key: by embedding air voids or using high-index particles in a polymer matrix, one can force sunlight to undergo internal reflections (“mirror-like” behavior) without relying on metallic mirrors. Such light-trapping via TIR complements Mie scattering, further reducing solar transmittance.
3.1.1.3. Multilayer interference coatings. Another classical approach to reflect sunlight is to use thin-film interference stacks (alternating layers of high- and low-refractive-index materials), which act as one-dimensional photonic crystals.52,57,58 By tuning the thickness and refractive index contrast of each layer, one can design a multilayer film that selectively reflects certain wavelength bands via constructive interference (Bragg reflection). A sufficiently broadband multilayer stack can thus achieve high reflectance across the entire solar spectrum. Raman et al.59 famously used a seven-layer dielectric mirror (HfO2/SiO2 on an Ag substrate) to achieve ∼97% reflectance of solar irradiance while maintaining strong thermal emission, enabling >5°C daytime cooling under direct sun (Fig. 3f). Kecebas et al.60 later optimized similar multilayer designs by using TiO2 and Al2O3 in place of HfO2, which improved mid-IR emissivity around 10 µm due to the phonon absorption features of Al2O3 (Fig. 3g). Beyond inorganic films, polymer-based multilayers have also been explored: Gentle and Smith61 demonstrated an all-polymer interference coating that achieved sub-ambient cooling under the open sky. Metamaterial approaches have further extended this concept. For instance, Huang et al.62 created an “invisible” radiative cooling coating using a stack of seven CaF2/Ge layers on a thin NiCr film; this design achieved high reflectance while being visually inconspicuous.
In summary, multilayer and photonic-crystal coatings represent a powerful method to tailor reflectance spectra and can be engineered to reflect nearly all incoming solar energy while remaining highly emissive in the IR. The trade-off is that such coatings can be angle- and polarization-sensitive and often require precise fabrication, but they have demonstrated the feasibility of near-ideal spectral selectivity for radiative cooling. By employing Mie scatterers, light-trapping structures, or interference stacks (or hybrids thereof), modern PDRC materials can achieve solar reflectance >95%, drastically reducing solar heat uptake on surfaces like ice.
3.1.2.1. Molecular vibrational absorption. Most polymers and certain functional oxides have a rich tapestry of vibrational modes (bond stretches, bends, rotations) that interact with infrared light (Fig. 4a).63–67 When the vibrational resonances of molecular bonds overlap with thermal IR wavelengths, the material can absorb and re-emit IR radiation effectively. Polymers, in particular, contain various chemical bonds (C–H, C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O–H, etc.) that produce broad absorption bands across a wide IR range (Fig. 4b and c).68,69 The superposition of multiple vibrational modes leads to broadband IR emissivity in materials like cellulose, PDMS, or other organics. For example, the C–O and O–H groups in cellulose acetate contribute to strong emissivity in the mid-IR, which has been leveraged in several passive cooling films.66,67,70 By selecting materials with appropriate functional groups, one can ensure the emitter has ε > 90% over a broad thermal spectrum. Multiple vibrational excitations can also couple or overlap, further strengthening the absorption/emission in those bands. Thus, organic polymers and composites rich in IR-active bonds are naturally good emitters, a property harnessed in many recent cooling coatings.
O, O–H, etc.) that produce broad absorption bands across a wide IR range (Fig. 4b and c).68,69 The superposition of multiple vibrational modes leads to broadband IR emissivity in materials like cellulose, PDMS, or other organics. For example, the C–O and O–H groups in cellulose acetate contribute to strong emissivity in the mid-IR, which has been leveraged in several passive cooling films.66,67,70 By selecting materials with appropriate functional groups, one can ensure the emitter has ε > 90% over a broad thermal spectrum. Multiple vibrational excitations can also couple or overlap, further strengthening the absorption/emission in those bands. Thus, organic polymers and composites rich in IR-active bonds are naturally good emitters, a property harnessed in many recent cooling coatings.
 | ||
| Fig. 4 Strategies for enhancing mid-infrared emissivity. (a) Schematic of key vibrational modes in common polymer functional groups. Reproduced from ref. 67 with permission from Elsevier, copyright 2019. (b) Fourier transform infrared spectroscopy absorbance spectra of PMMA, PVDF, and their PMMA/PVDF blend. Reproduced from ref. 67 with permission from Elsevier, copyright 2019. (c) Normal-incidence spectral emissivity (or absorptivity) across the thermal IR window. Reproduced from ref. 67 with permission from Elsevier, copyright 2019. (d) Illustration of phonon–polariton resonance mechanisms in dielectric particles. Reproduced from ref. 74 with permission from Elsevier, copyright 2022. (e) Monte Carlo-predicted reflectance and emissivity curves. Reproduced from ref. 74 with permission from Elsevier, copyright 2022. (f) Process flow diagram for fabricating the composite emitter. Reproduced from ref. 76 with permission from John Wiley and Sons, copyright 2024. (g) Calculated effective refractive index versus porosity (0–100%) for 500 nm pores under different light polarizations. Reproduced from ref. 76 with permission from John Wiley and Sons, copyright 2024. (h) SEM micrographs showing arrays of micro-pyramids with varied feature sizes. Reproduced from ref. 78 with permission from Springer Nature, copyright 2024. | ||
3.1.2.2. Phonon–polariton resonance. Certain dielectric materials support strong IR resonances due to lattice vibrations (optical phonons) coupling with electromagnetic waves, which is a phenomenon known as a phonon–polariton.71,72 Polar dielectric particles such as SiO2, Al2O3, or BaSO4 exhibit a characteristic Reststrahlen band in the mid-IR where they have high reflectivity and conversely strong absorption just outside that band.73 In a composite, these materials can enhance emissivity at specific IR wavelengths corresponding to their resonant phonon modes. For instance, SiO2 and Al2O3 have phonon resonances that supplement the 8–13 µm emission when combined with a polymer matrix.60 BaSO4, widely used in ultra-white paints, also has vibrational modes contributing to IR emission (Fig. 4d).74 Within the Reststrahlen region, a material's permittivity allows surface phonon–polaritons that increase the local density of states for radiation, thus boosting thermal emission at those frequencies (Fig. 4e). By incorporating a mix of dielectric particles with different phonon resonances, one can broaden the overall emissive spectrum. For example, adding Al2O3 into a multilayer stack was found to significantly improve emission around 10 µm due to its lattice phonon mode.60 In addition, Cheng et al. combined high concentrations of BaSO2 and SiO2 particles in a ∼100 µm layer. The coating achieves ∼95% solar reflectance (0.3–2.5 µm) and ∼96% emissivity in the 8–13 µm atmospheric window, yielding a peak sub-ambient temperature drop of 8.1 °C and an average cooling power of 89.6 W m−2 at solar noon. These examples show that leveraging phonon–polariton effects in microparticle fillers or coatings is an effective tactic to raise emissivity at targeted IR bands and push cooling performance closer to the blackbody limit, thereby drawing more heat away from surfaces like ice.
3.1.2.3. Gradual refractive index (GRIN) structures. A GRIN interface is one where the refractive index changes gradually rather than abruptly. In optical design, a graded index can suppress Fresnel reflections and enable light to penetrate and be absorbed more deeply. Applying this concept to radiative cooling, structures such as functionally graded porous layers or tapered surface features can reduce reflection of IR radiation.75 By matching the optical impedance across an interface, a GRIN layer allows IR photons to enter the emitting material rather than reflecting off the surface. Examples include foams or aerogels with a gradient porosity, or pyramid/cone surface textures that present a continuous effective index transition to the environment. Such structures effectively lengthen the optical path for IR inside the emitter and increase the chances of absorption and re-emission. Li et al.46 employed a hierarchically porous polymer design with a gradient porosity from top to bottom to create a selective emitter. More recently, Liu et al.76 reported a gradient-index porous metamaterial where the porosity and refractive index vary gradually through the thickness, producing a wide-band high reflectance (Fig. 4f). Their hierarchically porous polymer film exhibited 97.3% solar reflectance and over 97% mid-IR emissivity by combining multiple optical mechanisms (Mie scattering from pores plus graded-index interference, Fig. 4g). Likewise, pyramid or cone-array surfaces on infrared emitters can act as an anti-reflection layer for thermal wavelengths (Fig. 4h).77,78 Many advanced radiative coolers now utilize multiscale roughness or porosity not only for scattering sunlight (as discussed earlier) but also for enhancing IR emission by reducing internal reflection losses.
By combining the above strategies, researchers have achieved spectrally selective PDRC materials that come very close to the ideal performance outlined in Section 2. For instance, a polymer film with embedded TiO2 or BaSO4 particles hierarchical porosity, and intrinsic vibrational emissivity can exhibit >95% solar reflectance and >90% thermal emissivity. In the next subsections, we turn to equally important aspects of material design: ensuring that these high-performance optical properties persist in real outdoor conditions through self-cleaning surfaces, UV/weather resistance, mechanical durability, and environmental stability.
3.2. Self-cleaning and anti-fouling
For radiative cooling materials deployed in the field such as covering a glacier, contamination by dust, dirt, water, or organic growth can degrade the optical performance. Accumulation of dust or other particles on a cooling surface significantly reduces its solar reflectance and IR emissivity over time, thereby diminishing the cooling effect.79 Likewise, if the surface becomes wet from rain or dew, the presence of liquid water can temporarily eliminate the cooling performance by filling porous structures or absorbing IR radiation. Thus, self-cleaning and anti-fouling surface designs are essential to maintain long-term cooling efficacy. One effective solution is to impart superhydrophobic self-cleaning characteristics to the surface. A superhydrophobic surface is defined by a water contact angle >150° and a droplet sliding angle <10°; water droplets bead up and roll off, carrying away dust and debris (the classic lotus-leaf effect).80 Such surfaces are achieved by combining a rough micro/nanostructured topography with a low-surface-energy coating. Integrating superhydrophobicity into PDRC materials prevents dust adhesion and rain wetting, thus preserving high reflectance and emissivity over long outdoor exposures.81–83The surface roughness needed for superhydrophobicity can be made compatible with the optical requirements of radiative cooling. In fact, the same micro/nanoscale textures that promote light scattering can also serve as the basis for hydrophobic roughness. For example, a hierarchical porous film with pores or fibers on multiple length scales can be post-treated with a fluoropolymer or silane to create a superhydrophobic coating that both scatters sunlight and repels water. This dual-function approach has been demonstrated in several studies. Wang et al.84 developed a bioinspired dual-layer coating with a hierarchical micro-pattern (Fig. 5a): the coating exhibited high solar reflectance and thermal emissivity for radiative cooling, while exhibiting superhydrophobicity for diverse liquid droplets (Fig. 5b); it retained its optical and hydrophobic performance after 80 mechanical abrasion cycles (Fig. 5c), prolonged outdoor exposure (Fig. 5d), extreme pH exposure (Fig. 5e), and 100 thermal-shock cycles (Fig. 5f). Xue et al.85 prepared a hierarchically porous PVDF-HFP membrane via a phase separation process embedding hydrophobic SiO2 nanoparticles, achieving ∼92% solar reflectance and a ∼160° static water contact angle; under one-sun outdoor tests, the film provided a ∼10 °C sub-ambient temperature drop and showed negligible performance loss after soiling and rinsing. Jiang et al.86 report the development of a durable, self-cleaning daytime radiative-cooling paint based on a PVDF/PDMS composite. The coating's hierarchical micro-nanostructure confers superhydrophobicity, exhibiting a static water contact angle of 158.2° and a roll-off angle of just 7.9°, thereby enabling efficient self-cleaning, as dust and other particulates are readily removed by rolling water droplets. Wang et al.87 created a superhydrophobic porous film by embedding hydrophobic SiO2 nanoparticles into a polymer matrix via solvent exchange. The resulting film had a solar reflectance of 96% and superhydrophobic performance (Fig. 5g), achieving an impressive 12 °C sub-ambient cooling under direct sunlight. Importantly, after chemical immersion, the film's self-cleaning property prevented performance degradation (Fig. 5h), underscoring the benefit of anti-fouling for longevity. Similarly, Liu et al.88 reported a low-cost phase-separation method to fabricate a multi-bioinspired self-cleaning porous coating made of PVDF-HFP/PDMS. This coating maintained stable optical properties even after prolonged exposure to harsh conditions, enabling sustained radiative cooling performance. Dust and dirt did not adhere strongly, and any that settled were easily removed by water or wind, so the cooling effect remained intact over time.
 | ||
| Fig. 5 Self-cleaning and anti-fouling performance of PDRC coatings. (a) Schematic of the bioinspired superhydrophobic radiative cooling coating. Reproduced from ref. 84 with permission from American Chemical Society, copyright 2021. (b) Diverse liquid droplets bead up (high contact angle) and roll off the coating with minimal tilt. Reproduced from ref. 84 with permission from American Chemical Society, copyright 2021. (c) Water contact angle measured after successive abrasion cycles; inset shows a droplet on the surface following the 80th abrasion, confirming retained hydrophobicity. Reproduced from ref. 84 with permission from American Chemical Society, copyright 2021. (d) Contact angle stability over prolonged outdoor exposure. Reproduced from ref. 84 with permission from American Chemical Society, copyright 2021. (e) pH-dependent contact angle tests (from acidic to alkaline). Reproduced from ref. 84 with permission from American Chemical Society, copyright 2021. (f) Contact angle and surface morphology after 100 thermal-shock cycles. Reproduced from ref. 84 with permission from American Chemical Society, copyright 2021. (g) Photographs of the EPDM/SiO2 film repelling various common liquids such as dyed water, milk, cola, tea, and orange juice, and the progressive removal of stains as water droplets roll across the surface. Reproduced from ref. 87 with permission from Elsevier, copyright 2021. (h) Temperature evolution after chemical immersion. Reproduced from ref. 87 with permission from Elsevier, copyright 2021. | ||
While superhydrophobic self-cleaning surfaces are highly effective in preventing contamination, maintaining their function under environmental stresses such as rain, abrasion, or repeated cleaning remains a significant challenge for real-world applications. To address this, several robust design strategies have been proposed: (i) constructing hierarchical micro/nanostructures distributes external forces and improves resistance to collapse;89 (ii) embedding self-healing agents within the coating matrix enables dynamic regeneration of surface hydrophobicity following mechanical or chemical damage;90 (iii) utilizing tough, flexible polymer substrates increases resilience to abrasion and deformation;91 (iv) incorporating crosslinked networks or reinforcing nanofillers further enhances structural stability.92
By implementing robust self-cleaning surfaces, radiative cooling materials can sustain their high reflectance/emittance over long periods. This is particularly important for remote ice-covered areas where maintenance is infeasible. A field study showed that a dust-resistant radiative cooling film remained nearly as reflective after weeks outdoors as it was at deployment, whereas traditional radiative cooling surface quickly became dirty and absorbed more sunlight.93,94 In summary, superhydrophobic and anti-fouling coatings prevent performance loss due to environmental soiling, thereby prolonging the cooling protection for ice and snow. Keeping the surface clean ensures that the material continues to reflect sunlight and radiate heat effectively, extending the time that covered ice can remain frozen under real-world conditions.
3.3. UV stability and weathering resistance
Long-term durability under solar ultraviolet radiation is another critical requirement for PDRC materials, especially for applications like glacier protection where the material may be exposed to intense sunlight and harsh weather for extended periods. Polymeric materials, which are widely used in radiative cooling films due to their light weight and processability, are particularly susceptible to UV-induced degradation. Prolonged UV exposure can break chemical bonds in polymers, leading to chain scission, cross-linking, and the formation of chromophores that cause yellowing.95 This photodegradation not only embrittles the material but also increases its absorption in the visible spectrum due to yellowing or other photo-products, thereby reducing solar reflectance and cooling performance. Traditional approaches to improve polymer UV stability include adding UV absorbers or stabilizers; however, conventional organic UV stabilizers themselves strongly absorb UV, which conflicts with the requirement of low solar absorptance in PDRC systems. Therefore, enhancing the UV durability of radiative cooling materials requires special strategies that do not compromise their optical properties.One approach is to integrate inorganic UV blockers into the polymer matrix. Oxide particles like Al2O3 and BaSO4 have wide band gaps and are essentially transparent (non-absorbing) to UV wavelengths above ∼300 nm; instead of absorbing UV, they tend to reflect or scatter it. Additionally, these materials have high refractive indices that also help scatter sunlight contributing to reflectance. For these reasons, Al2O3 and BaSO4 are often incorporated as pigments in PDRC coatings to serve dual roles: improving UV resistance by preventing UV from penetrating into the polymer, and enhancing solar reflectance by Mie scattering of visible light.96 Al2O3 in particular has been shown to effectively inhibit UV-induced polymer degradation when used as a filler. For example, Zhang et al. reported a hierarchically designed hybrid porous radiative film (HPRF) comprised of cost-effective Al2O3 particles and poly(dimethylsiloxane) (PDMS) via a simple phase separation method.97 After exposure to UV for 70 h in the lab (and 150 h outdoors), the film retained stable reflectance and emissivity. However, experiments have revealed that simply mixing these inorganic particles into polymers doesn't fully eliminate UV absorption by the polymer itself. The composite's reflectance spectrum may still show the polymer's intrinsic UV absorption edge, limiting the overall UV-blocking capability.98 This suggests that a synergistic approach is needed, combining different types of UV protection.
Researchers have developed composite designs that use both UV-reflective and UV-absorbing components in a way that shields the polymer without adding broadband absorption. One clever design by Zhou et al.99 was a hollow/porous structured film inspired by the natural leaf-insect exoskeleton. They integrated hollow TiO2 particles which reflect and scatter UV within a porous fluorinated polyurethane matrix which provides hydrophobicity and a controlled amount of UV absorption at the surface. The hollow particles reflect most incoming UV before it can penetrate, while any transmitted UV is absorbed in a thin superficial layer of the polymer, preventing it from reaching deeper into the bulk. This hybrid film showed excellent weatherability and self-cleaning, significantly improving durability under long-term outdoor UV exposure. Another approach by Li et al.100 involved spray-coating a thin layer of TiO2 nanoparticles on the exterior of a polymer radiative cooling film as a UV-reflective shield (Fig. 6a). The TiO2 overlayer effectively blocked UV radiation from reaching the polymer substrate (Fig. 6b and c). While this method provided comprehensive UV protection, the added inorganic shell can interfere with some advantages of the polymer film such as flexibility or infrared transparency; thus, there is a trade-off when adding external coatings.
 | ||
| Fig. 6 UV Stability and weathering resistance performance of PDRC coatings. (a) Cross-sectional schematic of the UV-stable cooling film, highlighting the protective TiO2-Al2O3 layer atop the PES substrate. Reproduced from ref. 100 with permission from John Wiley and Sons, copyright 2023. (b) Solar-spectrum reflectance of the pristine PES film and the PES-TiO2-Al2O3 composite before and after accelerated UV exposure. Reproduced from ref. 100 with permission from John Wiley and Sons, copyright 2023. (c) Photographic comparison of the PES-TiO2-Al2O3 coating's surface appearance pre- and post-UV irradiation. Reproduced from ref. 100 with permission from John Wiley and Sons, copyright 2023. (d) Optical images and SEM micrographs of the TPU membrane and the BST@TPU composite before and after 216 h of continuous UV aging. Reproduced from ref. 101 with permission from John Wiley and Sons, copyright 2024. (e) DFT results comparing adsorption energies and reaction free energies for TPU degradation pathways, indicating reduced photochemical reactivity in the presence of BST. Reproduced from ref. 101 with permission from John Wiley and Sons, copyright 2024. (f) Mechanistic illustration of TPU photo-aging alongside the protective role of BST nanorods. Reproduced from ref. 101 with permission from John Wiley and Sons, copyright 2024. (g) Schematic of the K2Ti6O13 enhancing UV resistance mechanism. Reproduced from ref. 102 with permission from John Wiley and Sons, copyright 2022. (h) Outdoor aging study of PT@PEO versus pure PEO films over 30 days. Reproduced from ref. 102 with permission from John Wiley and Sons, copyright 2022. | ||
A particularly successful strategy to improve UV endurance without sacrificing cooling performance was demonstrated by Li et al.101 (Fig. 6d). They used coaxial electrospinning to fabricate a composite membrane of barium strontium titanate nanorods (BST) and thermoplastic polyurethane (TPU). The BST nanorods act as UV scatterers owing to their high refractive index and also scatter visible light, compensating for any reflectance loss due to residual UV absorption by the TPU. The resulting BST@TPU fibrous membrane achieved a very high solar reflectance of 97.2% while significantly improving UV stability. In accelerated UV-aging tests (continuous 216 hours of UV exposure), its reflectance dropped only to ∼92%, whereas a comparable film without the BST nanorods degraded much more rapidly. In effect, the inorganic nanorods acted as built-in UV shields and light scatterers, disrupting the polymer's photodegradation pathways and effectively suppressing yellowing (Fig. 6e and f). This technique is especially attractive because it maintains the simplicity and flexibility of a polymer-based film, and electrospinning is scalable. Moreover, Yao et al.102 presented a spider-silk-inspired nanocomposite by incorporating potassium titanate (K2Ti6O13) nanofibers into a porous poly(ethylene oxide) matrix via scalable roll-to-roll electrospinning. The K2Ti6O13 nanofibers improve photostability under prolonged UV exposure by efficiently absorbing ultraviolet photons and promoting the recombination of photogenerated charge carriers into nonradiative pathways, thereby inhibiting harmful photochemical reactions (Fig. 6g). The composite maintains ∼93% solar reflectance (Fig. 6h), delivering a continuous sub-ambient cooling power of 92 W m−2 under sunlight and stable operation over 720 h of UV aging and 30 days of outdoor exposure.
In summary, UV durability is addressed by integrating UV-resistant components (oxide particles, reflective pigments, or protective coatings) and by drawing bioinspiration for durable architectures. The goal is to prevent polymer photodegradation without compromising the cooling film's low solar absorptance. Using these strategies, researchers have created radiative cooling materials that endure weeks or months of intense UV exposure with minimal performance loss. Such longevity is essential for real-world applications like glacier preservation, where materials must survive summer-long sun to meaningfully slow ice melting.
3.4. Mechanical durability and abrasion resistance
Radiative cooling films and coatings for ice protection will face mechanical stresses: wind and snow abrasion, thermal expansion/contraction, and handling during deployment. Mechanical integrity is therefore a key design criterion. The materials should be sufficiently robust to withstand scratching and bending without losing their optical properties. Flexible polymer-based coolers are advantageous here, but polymers can tear or wear down, especially in cold, brittle conditions. Recent work has focused on improving the toughness, tear resistance, and abrasion resistance of PDRC materials.The incorporation of rigid oxide nanoparticles into polymer hosts has proven effective in enhancing toughness and abrasion resistance without compromising optical performance. For example, Wang et al.103 employed a phase-separation and biaxial-stretching protocol to disperse nano-Al2O3 uniformly within a polyurethane matrix (Fig. 7a), yielding films with 93% solar reflectance (0.38–0.5 µm) and 95% emissivity (8–13 µm) that retained >98% of their optical performance after multiple bending, tensile, and Taber abrasion cycles. Likewise, Farooq et al.104 incorporated a bimodal ZnO particle distribution into PVDF-HFP to replicate bamboo's gradient porosity, resulting in a composite film with nearly doubled tensile strength (∼9 MPa) and over 200% elongation (Fig. 7b). Song et al.105 formulated a silicone–acrylate paint loaded with 60 vol% ZrO2 (particle size ∼2.6 µm), attaining 95.7% reflectivity and 97.8% emissivity. The coating exhibited a Taber wear index of 5310 m N mm−3, representing an order of magnitude improvement compared to commercial benchmark coatings, and it maintained over 99% of its optical performance after thousands of abrasion cycles (Fig. 7c and d).
 | ||
| Fig. 7 Mechanical durability and abrasion resistance of PDRC materials. (a) Nano-Al2O3 reinforced APU films showing enhanced solar reflectance and mechanical strength. Reproduced from ref. 103 with permission from American Chemical Society, copyright 2024. (b) Roll-to-roll fabricated 1500 cm2 gradient-porosity PDRC coating demonstrating high flexibility (bending and torsion); inset compares its tensile strength with that of conventional non-gradient porous films. Reproduced from ref. 104 with permission from Elsevier, copyright 2024. (c) Abrasion-resistance test setup: reciprocating wear by a steel ball under constant load. Bar charts contrast wear volume (left) and retained solar reflectance (right) for coatings filled with 2.6 µm (blue) versus 3.4 µm (red) ZrO2 particles. Reproduced from ref. 105 with permission from Elsevier, copyright 2024. (d) Sandpaper abrasion protocol: sample mounted under a 1 kg weight and reciprocated over 400-grit sandpaper for a total sliding distance of 100 × 20 cm. Reproduced from ref. 105 with permission from Elsevier, copyright 2024. (e) Radial compressive stress-strain curves of 3D-printed MSC and CNF lattice coolers. Reproduced from ref. 106 with permission from Elsevier, copyright 2024. (f) Radial compressive stress-strain curves of 3D-printed MSC and CNF lattice coolers. Reproduced from ref. 106 with permission from Elsevier, copyright 2024. (g) Cyclic compressive loading profiles of silica–alumina nanofibrous aerogels (SAFAs), demonstrating structural resilience over repeated cycles. Reproduced from ref. 110 with permission from Elsevier, copyright 2023. | ||
Beyond planar films, three-dimensional scaffolds and fibrous networks provide mechanical reinforcement via structural hierarchy. Direct-ink-written lattices of cellulose nanofiber/silica exhibit 94.2% solar reflectance, 96.1% emissivity, and a specific strength of 9.7 kN m kg−1, enduring compressive loads and temperatures up to 100 °C while cooling electronic devices by ∼6 °C under one-sun illumination (Fig. 7e).106 Coaxial electrospun PU-core/PVDF-sheath nanofibers, loaded with SiO2/TiO2, deliver 4.3 MPa tensile strength and 127% elongation with >90% retention of radiative properties after extensive folding.107 Inspired by spider silk, Du et al.108 have templated natural nanofibrils within PEO/SiO2 membranes, boosting toughness and tensile strength by nearly seven-fold, achieving an elongation of 34.8% (Fig. 7f), and maintaining high optical performance (95.6% reflectance and 89.9% emissivity).
Ultralight aerogel composites have also been made mechanically robust through polymer and ceramic network reinforcement. Chitosan/PVA aerogels hybridized with cost-effective wollastonite silica sols achieve compressive strengths of 1.57 MPa and stable porous architectures that withstand thermal cycling and handling, while imparting passive cooling.109 Silica–alumina nanofiber aerogels further combine >95% reflectance, 93% emissivity, >5 °C sub-ambient cooling under sunlight, exceptional compression-fatigue resistance (Fig. 7g), and inherent fire retardancy, making them suitable for remote polar installations.110 Thermoplastic polyurethane (TPU) has emerged as a versatile platform for PDRC applications due to its intrinsic low-temperature flexibility, elasticity, and wear resistance. Hydrophobic silica aerogel particles dispersed within TPU produce self-supporting, waterproof, and breathable films that can be cut, folded, rolled out over uneven ice surfaces without cracking or optical degradation.111 By employing either a hard overcoat or a bulk homogeneous composite design where optical scatterers are uniformly distributed through the material, these films preserve radiative performance even after surface wear exposes underlying layers of equivalent reflectivity.
In conclusion, mechanically robust PDRC materials have been achieved by using durable polymers (like TPU) and designing composite structures that tolerate bending and abrasion. These advances bring PDRC technology closer to practical deployment; one can imagine rolling out large flexible cooling sheets on rooftops or ice fields without fear of tearing or performance loss. Future work may explore other tough polymers or fiber-reinforced composites, and standardized tests for wear (e.g. sand abrasion, cyclic bending) will be important to validate long-term durability under field conditions. Overcoming mechanical challenges ensures that radiative cooling films remain effective on glaciers, snowpacks, or refrigerated containers throughout their service life.
3.5. Low-temperature and high-humidity stability
Finally, materials design must account for the environmental conditions specific to ice and snow applications. One issue is that many polymer-based coatings are developed and tested at ambient laboratory conditions, but glacier covers will operate at sub-freezing temperatures. The material should retain its flexibility and performance at low temperatures (some polymers become brittle in the cold).112–114 Additionally, any thermal contraction of the material in cold environments should not cause cracking or delamination.115 Incorporating reinforcing fibers or using crosslinked elastomers can mitigate thermal stress. In practice, cooling films have been applied on ice at 0 °C or below and shown to function as expected.A second crucial issue is ambient humidity and moisture. Paradoxically, while radiative cooling works best under dry, clear-sky conditions, the process of manufacturing or deploying porous polymer coatings can be sensitive to humidity. It was recently discovered that some ultra-white coatings such as phase-separation induced porous paints suffer from “humidity fragility” during curing: if the paint dries in humid air, capillary effects collapse the porous network, drastically reducing reflectance. Li et al. reported that their BaSO4 paint (which achieved >4.5 °C cooling with 117 W m−2 net cooling power) would lose its cooling ability if dried above 30% relative humidity.96 In fact, at >45% RH the porous coating could even turn into a net absorber (becoming a solar heating material) due to pore collapse and increased absorption.116 This represents a serious challenge for real-world application, since field deployment or large-scale painting can rarely be done in perfectly dry conditions.
To tackle this, Hong et al. introduced a humidity-tolerant porous polymer coating.116 They added a small amount of a polymer reinforcement agent (a hydrophobic binder and fumed silica nanoparticles) to the standard phase-separating paint formulation. The fumed silica particles helped retain the porous architecture during drying by providing structural support, even in moist air (Fig. 8a). As a result, the modified coating maintained high solar reflectance and a positive cooling power up to 60% RH, whereas the original formulation failed above 30% RH (Fig. 8b–d). When typical afternoon relative-humidity data across the United States are overlaid, this modification enlarges the coating's viable geographic coverage by roughly 950% (Fig. 8e). In short, the mechanically stabilized porous architecture effectively prevents humidity-driven collapse, enabling consistent radiative cooling performance across a much wider range of environmental conditions.
 | ||
| Fig. 8 Mechanical durability and abrasion resistance of PDRC materials. (a) SEM cross-sectional views of porous polymer coatings (PPC): PPC dried at 33%, 46% and 64% RH and FSPPC dried at 34%, 46% and 59% RH. Yellow ellipsoids indicate modeled pore geometry with horizontal and vertical diameters. Reproduced from ref. 116 with permission from Springer Nature, copyright 2024. (b–d) Daytime subambient cooling performance of PPC and FSPPC samples prepared at (b) low (∼35% RH), (c) moderate (∼46% RH), and (d) high (∼60% RH) humidity. Reproduced from ref. 116 with permission from Springer Nature, copyright 2024. (e) Annual afternoon relative humidity map of the United States, with colored overlays denoting the climatic regions where PPC and FSPPC maintain stable cooling; source data in supplementary data. Reproduced from ref. 116 with permission from Springer Nature, copyright 2024. (f) Photographs of AFTPU-10 elastomer film demonstrating water shedding of dyed droplets. Reproduced from ref. 111 with permission from John Wiley and Sons, copyright 2022. (g) SEM micrographs of neat TPU and AFTPU composites containing 10, 15, and 25 wt% fumed silica, showing hierarchical porosity (scale bar: 100 µm). Reproduced from ref. 111 with permission from John Wiley and Sons, copyright 2022. | ||
When in service on a glacier or roof, the cooling material will also face moisture from rain, dew, or melting ice. In these conditions, waterproofing and breathability become important. A successful ice cover should repel liquid water (so that its pores don't fill with water or ice, which would ruin the optical function) but ideally allow water vapor to diffuse out (to avoid trapping moisture beneath the cover). The aerogel-infused TPU film by Shan et al. is an example of achieving this balance. It is waterproof to liquid water but breathable to vapor (Fig. 8f and g).111 Field tests on snowpack covered with porous radiative coolers have shown that a breathable cover lets sublimation occur and prevents meltwater pooling, which helped preserve more snow mass compared to an impermeable tarp.26
In summary, addressing low-temperature flexibility and humidity tolerance is the final piece in designing radiative cooling materials for ice and snow preservation. By formulating polymers that remain pliable in the cold and engineering pore structures that are robust against moisture, researchers have created cooling covers capable of real-world deployment. These developments give confidence that radiative cooling materials can function reliably in the dynamic, wet environments associated with ice and snow.
In conclusion of Section 3, the practical performance of PDRC materials relies not only on advanced optical engineering such as broadband scattering, total internal reflection, and multilayer or gradient-refractive-index architectures, but also on the integration of robust durability features, including superhydrophobicity, UV stability, and mechanical resilience. The combination of these strategies ensures that PDRC materials maintain high solar reflectance and mid-infrared emissivity under harsh outdoor conditions. Such synergy is essential for glacier preservation, snowpack management, and cold-chain applications, and offers a promising, energy-free approach to mitigating the impacts of climate change.
4. Practical applications
Passive daytime radiative cooling has rapidly progressed from conceptual designs to functioning devices that complement or even replace traditional cooling systems. Thanks to advances in scalable fabrication and multifunctional material design, PDRC technologies are now being explored for a wide range of real-world applications. In this section, we discuss two major application areas that have gained attention: food preservation via passive cooling (Section 4.1) and glacier/ice protection to slow ice melting (Section 4.2). These examples illustrate how PDRC can be harnessed to achieve low-temperature preservation with zero energy input.4.1. Food preservation and cold-chain management
Maintaining the freshness of food through refrigeration is energy-intensive and environmentally costly. Cold-chain logistics consume enormous amounts of electricity and often rely on diesel generators or coal-fired grid power, contributing substantially to greenhouse gas emissions.10,13,117 The primary value of PDRC materials in cold-chain management lies in their ability to passively reduce the temperature of perishable goods in outdoor or off-grid scenarios where powered refrigeration is unavailable or unreliable.26 In developing regions, rural markets, and disaster-relief operations, fresh produce, dairy, meat, and vaccines are frequently stored, displayed, or transported in the open.118,119 Here, PDRC-based wraps, films, and box liners can provide significant temperature reduction under direct sunlight, improving product quality and shelf life. Furthermore, portable PDRC-lined coolers can be used for last-mile delivery of vaccines and biological samples, ensuring safe temperature control without electricity.30 In these situation, packaging or storage containers made with radiative cooling materials could passively keep food chilled without power, especially in arid, sunny climates.120,121One promising avenue is radiative cooling packaging films that wrap perishable goods. Cellulose acetate (CA), a widely available and biodegradable cellulose derivative, has attracted attention as a sustainable packaging material.51,122,123 CA is derived from cellulose, is hydrophobic, easily cast into films, and is considered one of the most commercially important bio-based polymers.124 Given increasing concerns over plastic waste and petrochemical resource use, CA provides an eco-friendly alternative for functional food packaging.125–127
Recently, Zhang et al.27 developed a novel layered porous CA/ZnO film that integrates passive radiative cooling with antibacterial and self-cleaning functions, designed for long-term food preservation (Fig. 9a). Using a simple water-assisted phase separation method, they fabricated a film with impressive optical and functional properties. The film exhibits an impressive ∼97% solar reflectance and ∼94% mid-IR emissivity, yielding a temperature drop of 13.8 °C under direct sunlight. In other words, at midday sun the wrapped item stays nearly 14 °C cooler than ambient purely by radiative cooling. The CA/ZnO film also demonstrated excellent weather resistance, mechanical strength, and thermal stability. When applied to real produce (fruit and vegetable models), the radiative cooling packaging significantly extended their freshness compared to conventional packaging, outperforming other commercial food packaging materials (Fig. 9b and c). The antimicrobial ZnO component helped prevent spoilage, and the self-cleaning surface kept the film effective over time. Such CA-based radiative cooling films are promising as long-lasting, renewable passive cooling packaging to prolong the shelf-life of food without electricity. The cooling mechanism by which the CA/ZnO film achieves cooling is rooted in its nanostructure. The film's aligned cellulose nanofibers strongly scatter sunlight, and the ZnO nanoparticles along with CA's molecular bonds provide high IR emissivity, giving it strong cooling power.
 | ||
| Fig. 9 Applications of PDRC films for food preservation and cold-chain management. (a) Schematic of the custom test rig used to evaluate the cooling performance of the CA/ZnO composite film on fresh produce. Reproduced from ref. 27 with permission from American Chemical Society, copyright 2023. (b) Photographs of strawberries stored for 9 days at ambient outdoor conditions, wrapped in various packaging materials. Reproduced from ref. 27 with permission from American Chemical Society, copyright 2023. (c) Weight-loss profiles of strawberries as a function of storage time under different packaging films. Reproduced from ref. 27 with permission from American Chemical Society, copyright 2023. (d) Temperature trajectories (left axis) of ice samples exposed to one-sun illumination, comparing different wrap materials. Reproduced from ref. 26 with permission from the Creative Commons Attribution-NonCommercial license, copyright 2022. (e) Photographs of ice-cream samples after ∼80 minutes of sunlight exposure (≈540 W m−2); only the hierarchically porous CA film maintains ice integrity (scale bar: 2 cm). Reproduced from ref. 26 with permission from the Creative Commons Attribution-NonCommercial license, copyright 2022. (f) Photograph of a flexible, custom-fabricated portable bag lined with the CA cooling film, designed for outdoor preservation of ice and iced foods (pink slider indicates seal). Reproduced from ref. 26 with permission from the Creative Commons Attribution-NonCommercial license, copyright 2022. (g) Photographs of vials containing trypsin solution subjected to: unprotected control, standard packaging, and CA/ZnO-wrapped packaging. Reproduced from ref. 120 with permission from Elsevier, copyright 2024. (h) Temperature profiles recorded in Guangzhou (July 2022) for trypsin vials with different treatments during midday sun exposure. Reproduced from ref. 120 with permission from Elsevier, copyright 2024. (i) Residual enzymatic activity (degradation index) of trypsin solutions after sun exposure, demonstrating enhanced thermal protection by the CA/ZnO film. Reproduced from ref. 120 with permission from Elsevier, copyright 2024. | ||
Additionally, radiative cooling wraps could be especially useful for keeping ice-cream cold during transport, or for preserving food in outdoor markets where refrigeration is unavailable.26Fig. 9d plots the surface temperature of iced food under one sun illumination (∼540 W m−2) when wrapped in different materials. Iced food enclosed in the hierarchically designed CA film remains below 0 °C for approximately 5.5 h, whereas identical samples wrapped in white paper, Al foil, and PET-Al-PE film only stay below freezing for about 3.7 h, 3.8 h, and 2.6 h, respectively. Fig. 9e shows photographs of ice cream samples after ∼80 minutes of sunlight exposure: those packaged in the CA film retain ≈98% of their original shape, while ice creams in conventional wrappers (white paper, Al foil, PET-Al-PE) are less than 50% intact, visually confirming the superior passive preservation afforded by the CA radiative cooling film. Moreover, the CA film's mechanical flexibility allows it to be shaped into bespoke containers or liners for outdoor ice or iced-food storage (Fig. 9f). The above results hint that PDRC might be integrated into portable coolers or food storage units for off-grid use.
Xu et al.120 devised a dual-function hydrogel packaging system composed of a PAAm/PVA network loaded with PTFE and ZrO2 nanoparticles, which synergistically combines evaporative cooling with daytime radiative cooling to protect temperature-sensitive payloads during ambient transport. To illustrate the gradual enhancement in solar shielding, Fig. 9g presents vials under five conditions, including unprotected control, amber shading, aluminum-foil wrap, PAAm/PVA hydrogel, and the nanoparticle reinforced PAAm/PVA composite hydrogel, revealing markedly improved opacity with each successive treatment. Quantitative evaluation in Fig. 9h shows that, during outdoor exposure from 11:00 to 13:00 in Guangzhou, the average vial temperatures follow the sequence amber (53.6 °C) > blank (46.8 °C) > aluminum foil (40.0 °C) > PAAm/PVA hydrogel (30.2 °C), reaching a minimum with the NPs@PAAm/PVA composite, thereby confirming the nanocomposite's superior heat suppression. Consistent with these thermal data, Fig. 9i compares post-exposure degradation indices of trypsin solution: the NPs@PAAm/PVA group exhibits the lowest index, while all other treatments suffer significantly greater enzyme breakdown, demonstrating the nanocomposite hydrogel's enhanced preservation of enzyme activity under combined thermal and photonic stress.
Overall, passive radiative cooling for food preservation is an emerging area with great potential. Future research may explore other materials and address challenges such as performance in humid climates or during nighttime.128–133 Moreover, for direct-contact food packaging, PDRC materials must comply with relevant food safety regulations such as those set by the U.S. food and drug administration (FDA), European food safety authority (EFSA), and other national agencies to ensure non-toxicity, chemical inertness, and the absence of harmful leachates or additives.134,135 The use of biocompatible and biodegradable materials, such as CA,26 not only meets these requirements but also addresses environmental concerns associated with traditional plastics, which is safe for food contact, biodegradable, and capable of providing efficient passive cooling for cold-chain and food preservation applications. This combination of performance, safety, and sustainability makes them promising candidates for future regulatory-compliant food packaging solutions.
4.2. Glacier and ice protection
PDRC materials are suited for application on exposed glacier surfaces, vulnerable snowpacks, and man-made ice storage sites in high-altitude or polar regions experiencing accelerated melting.3 In practice, flexible PDRC films or sheets are unrolled and placed directly onto the ice or snow surface. Edges are commonly secured using stakes, weighted bags, or by embedding the film ends beneath snow/ice to resist wind uplift.136 For large areas, multiple sheets can be joined or overlapped for comprehensive coverage. Recent field deployments on glaciers in the Arctic have demonstrated that such method is feasible and can extend ice longevity by reducing melt rates during the warm season.137 Materials should be installed before peak melt and can be removed at season's end, allowing for adaptive, low-impact interventions. It is essential that installation minimizes disturbance to local ecosystems and considers terrain, accessibility, and prevailing weather.However, preserving ice under sunlight is extremely challenging and imposes stricter requirements than ordinary ambient cooling. Ice is usually at 0 °C or below, meaning the temperature difference to warm ambient air can be above 20 °C on a sunny day. Thus, very high cooling power is needed to keep ice from warming. It has been estimated that increasing the radiative cooling power from ∼70 W m−2 typical for many current coolers to ∼110 W m−2 could prevent ice from melting under sun without any active refrigeration.26 Additionally, materials used for ice protection must be environmentally benign and deployable over large areas with minimal ecological impact.
Recent studies have demonstrated that PDRC materials can indeed substantially delay ice melting under sunlight. Yue et al.25 reported a cost-effective polymer composite film combining a porous polymer matrix with inorganic dielectric particles via a phase inversion technique. The resulting hierarchically porous composite showed remarkable cooling performance: under direct sunlight, it achieved a 9.1 °C sub-ambient temperature and extended the melting time of ice by a factor of four compared to bare ice (Fig. 10a–c). In other words, ice covered with this radiative cooling film lasted four times longer before completely melting. This result provides a strong proof-of-concept that scalable and sustainable ice-protecting films can be created. The composite's micro/nanostructure was optimized for efficient solar scattering (using hierarchical pores and particles) and strong IR emission (leveraging the polymer's vibrational bands and the particles' phonon resonances). The tests showed that covering an ice-filled cavity with hierarchically porous radiative film (HPRF) kept it on average 7 °C colder than covering the cavity with a conventional white cover (Fig. 10b).25 This temperature difference is critical in slowing melt rates (Fig. 10c). The HPRF's performance inspires the possibility of scaling up passive ice protection—imagine covering parts of a glacier or a snow storage pile with reflective cooling blankets to reduce melting in summer.
 | ||
| Fig. 10 Applications of PDRC films for glacier and ice protection. (a) Schematic and photograph of the custom setup used to evaluate radiative cooling on ice blocks under direct solar illumination. Reproduced from ref. 25 with permission from Royal Society of Chemistry, copyright 2023. (b) Temperature profiles of bare ice, ice covered with standard white paperboard, and ice covered with the hierarchically porous CA film during one sun exposure. Reproduced from ref. 25 with permission from Royal Society of Chemistry, copyright 2023. (c) Melting rates of ice under the three conditions, illustrating slowed melt beneath the CA film. Reproduced from ref. 25 with permission from Royal Society of Chemistry, copyright 2023. (d) Photographs showing mass loss of ice samples with (left) and without (right) the CA film after a fixed irradiation period (scale bar = 2 cm). Reproduced from ref. 26 with permission from the Creative Commons Attribution-NonCommercial license, copyright 2022. (e) Time-resolved mass decay curves (left axis) and remaining mass fraction (right axis) for ice blocks covered by the CA film versus uncovered controls. Reproduced from ref. 26 with permission from the Creative Commons Attribution-NonCommercial license, copyright 2022. (f) Snowpack preservation: images of natural snow patches at deployment and after 20 days of outdoor sun exposure, demonstrating significantly reduced melt under the CA film. Reproduced from ref. 26 with permission from the Creative Commons Attribution-NonCommercial license, copyright 2022. (g) High-latitude protection simulations. Reproduced from ref. 26 with permission from the Creative Commons Attribution-NonCommercial license, copyright 2022. (h) Comparative plots of ice volume, mass, and internal cavity temperature over time under HPRF and paperboard covers, highlighting the superior cooling and melt-delay performance of the PDRC coating. Reproduced from ref. 97 with permission from American Chemical Society, copyright 2023. | ||
To explore the factors influencing ice melt, Li et al.26 conducted a comprehensive study on how surface cooling and environmental conditions like high latitude where solar intensity is lower affect melting. They developed a layered cellulose acetate (CA) radiative cooling film (mentioned earlier as well) specifically for ice protection. Tests under sunlight showed that ice blocks covered with the CA film barely melted at all, while adjacent uncovered ice rapidly shrank and disappeared (Fig. 10d and e). In outdoor field experiments on snow, after 20 days of exposure, the snow patches protected by the CA cooling film retained about 50% more mass than the unprotected snow nearby (Fig. 10f). Such dramatic results underscore the power of radiative cooling in preserving ice: by keeping the surface colder and reflecting sunlight, the film slowed both sensible heating and direct solar absorption by the ice. The CA-based film was made of abundant and environmentally friendly materials, which is important to avoid introducing pollutants to sensitive glacier areas. This work also noted that the typical granular morphology of fresh snow in high latitudes which is naturally very reflective already offers some protection, but adding the radiative cooling cover can enhance it further (Fig. 10g).
Another recent effort by Zhang et al.97 integrated robust durability into a radiative cooling film for ice, ensuring it could endure outdoor exposure for extended periods. They demonstrated that their durable film significantly delayed ice melting over time, consistent with the above studies (Fig. 10h). The convergence of results from different research groups, quadrupling ice survival time, maintaining sub-freezing surface conditions under sun, and halving melt loss over weeks, strongly indicates that PDRC is a viable strategy to protect ice and snow in various contexts.
Traditional PDRC films are generally unsuitable for artistic ice sculptures due to their opacity; however, development of transparent PDRC coatings may open new possibilities for aesthetic preservation.138 Most feasibly, PDRC materials can be effectively used to cover glacier surfaces, snowpacks, and artificial ice or snow reserves where direct human or vehicular access is not required, but maintaining low temperature and minimizing melt is critical. By focusing on these target applications, PDRC technology can deliver its greatest practical impact for ice and snow management under climate change.
5. Conclusions and perspectives
This review has highlighted recent breakthroughs in PDRC materials, with particular emphasis on their application to ice preservation and cold-chain management. The advanced PDRC designs, ranging from hierarchical porous polymers and dielectric composites to multilayered and bio-inspired architectures, now routinely combine high solar reflectance (>95%) with strong thermal emissivity in the 8–13 µm window (>90%). Such innovations enable substantial sub-ambient cooling even under intense sunlight. Importantly, the latest generation of materials is engineered not just for optical performance, but also for environmental durability: incorporating superhydrophobic self-cleaning surfaces, UV-resistant additives, and mechanically resilient matrices to withstand real-world challenges like dust, rain, and temperature extremes. These advances are already translating into impactful applications. In food preservation and cold-chain logistics, PDRC films are being used to passively extend shelf life and maintain low temperatures without grid power. The potential to slow glacier and snowpack melt is demonstrating, providing a novel tool for safeguarding water resources and ecosystems amid climate warming. While PDRC is not a cure-all, its scalable, energy-free cooling offers significant promise for targeted protection of vulnerable cold assets and for reducing the energy burden of refrigeration in a warming world.Looking ahead, several future directions are apparent for this field:
(1) Developing cost-effective manufacturing methods (spray coating, roll-to-roll processing) for PDRC materials is crucial. Paint-like formulations (e.g., nanoparticle-based paints) have shown promise for easy application on buildings. Scaling production to cover large surface areas (ice field) will require collaboration between materials scientists and engineers to optimize production and deployment techniques.
(2) Much of the reported success has been in dry, clear-sky environments. Future studies should explore PDRC performance in humid or cloudy conditions and during nighttime. Combining radiative cooling with other passive cooling methods (like evaporative cooling, daytime shading, or nocturnal insulation/thermal storage) could yield all-weather cooling systems. Recent works integrating evaporative and radiative cooling in textiles hint at such hybrid approaches.
(3) Vast-area blanket coverage of glaciers or plateaus is unrealistic due to the enormous material demand and the challenges posed by wind, slopes, and rough surfaces. More practical is a strategy of targeted, modular, and seasonal interventions at melt hotspots, where localized deployment can yield measurable benefits without excessive footprint. To improve stability, modules should integrate reinforced seams, slope-adapted anchoring systems (stakes, ballast, or cross-strips), and breathable yet waterproof laminates that allow vapor transport while resisting liquid water intrusion. Such modular designs align with temporary, reversible, and low-impact interventions suited for field conditions.
(4) Large-scale outdoor deployment raises questions about potential ecosystem disruption and material shedding. Continuous surface coverage could alter local light balance and airflow, while polymer degradation may lead to microplastic release into glacier meltwater or surrounding soils. To mitigate these risks, future PDRC designs should emphasize durable, abrasion-resistant composites, laminated or filled-through-thickness structures, and bio-derived or biodegradable polymers (such as cellulose-based films). Operational strategies, including end-of-season retrieval and reuse, mandatory inspection for wear, and water-quality monitoring in sensitive areas, will also be essential to ensure sustainable use.
Conflicts of interest
There are no conflicts to declare.Data availability
All data supporting the findings of this study are available within the article. No new datasets were generated or analyzed during the current study.Acknowledgements
This work was supported by the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (GZC20250037), the China Postdoctoral Science Foundation (2025M770042), the National Natural Science Foundation of China (52573373), the CAS “Light of West China”, the Basic Research Innovation Group Project of Gansu Province (No: 24JRRA785), and the Major Science and Technology Projects of Gansu Province (25ZDGF001).References
- M. Huss, Nat. Water, 2024, 2, 606–607 CrossRef.
- A. Witze, Nature, 2019, 573, 320–321 CrossRef CAS.
- N. Cao, H. Chi, B. Zhu, H. Pang, C. Ke, X. Zhang, J. Chen and J. Zhu, Nat. Water, 2025, 3, 251–255 CrossRef.
- A. Chen, C. Zhao, H. Zhang, Y. Yang, J. Li, Y. Yu, Q. Zhang and J. Li, Natl. Sci. Rev., 2025, 12, nwaf116 CrossRef PubMed.
- R. E. Bell and H. Seroussi, Science, 2020, 367, 1321–1325 CrossRef CAS.
- K. Pistone, I. Eisenman and V. Ramanathan, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 3322–3326 CrossRef CAS.
- S. Manabe and R. T. Wetherald, J. Atmos. Sci., 1975, 32, 3–15 CrossRef CAS.
- X. Lu, Y. Hu, Z. Liu, S. Rodier, M. Vaughan, P. Lucker, C. Trepte and J. Pelon, Remote Sens. Environ., 2017, 194, 248–263 CrossRef.
- M. Rantanen, A. Y. Karpechko, A. Lipponen, K. Nordling, O. Hyvärinen, K. Ruosteenoja, T. Vihma and A. Laaksonen, Commun. Earth Environ., 2022, 3, 168 CrossRef.
- N. Aste, C. Del Pero and F. Leonforte, Sustain. Energy Technol. Assess., 2017, 22, 150–160 Search PubMed.
- D.-K. Liu, C.-C. Xu, C.-X. Guo and X.-X. Zhang, J. Food Eng., 2020, 275, 109881 CrossRef.
- G. Li, Y. Hwang, R. Radermacher and H.-H. Chun, Energy, 2013, 51, 1–17 CrossRef CAS.
- Y. Dong and S. A. Miller, J. Cleaner Prod., 2021, 303, 126982 CrossRef CAS.
- H. Gao, IOP Conf. Ser.: Earth Environ. Sci., 2019, 252, 032007 CrossRef.
- P. A. Raman, E. Rephaeli, S. Fan, M. A. Anoma and L. Zhu, Nature, 2014, 515, 540–544 CrossRef PubMed.
- N. N. Shi, C. C. Tsai, F. Camino, G. D. Bernard, N. Yu and R. Wehner, Science, 2015, 349, 298–301 CrossRef CAS PubMed.
- Y. Zhai, Y. Ma, S. N. David, D. Zhao, R. Lou, G. Tan, R. Yang and X. Yin, Science, 2017, 355, 1062 CrossRef CAS PubMed.
- B. Zhu, W. Li, Q. Zhang, D. Li, X. Liu, Y. Wang, N. Xu, Z. Wu, J. Li, X. Li, P. B. Catrysse, W. Xu, S. Fan and J. Zhu, Nat. Nanotechnol., 2021, 16, 1342–1348 CrossRef CAS PubMed.
- X. Zhao, T. Li, H. Xie, L. He, L. Wang, Y. Qu, S. C. Li, S. Liu, A. H. Brozena, Z. Yu, J. Srebric and L. Hu, Science, 2023, 382, 684–691 CrossRef CAS PubMed.
- Y. Wang, H. Ji, B. Liu, P. Tang, Y. Chen, J. Huang, Y. Ou and J. Tao, J. Mater. Chem. A, 2024, 12, 9962–9978 RSC.
- M.-C. Huang, M. Yang, X.-J. Guo, C.-H. Xue, H.-D. Wang, C.-Q. Ma, Z. Bai, X. Zhou, Z. Wang, B.-Y. Liu, Y.-G. Wu, C.-W. Qiu, C. Hou and G. Tao, Prog. Mater. Sci., 2023, 137, 101144 CrossRef.
- Q. Zou, K. Zhang, X. Tai, Z. Zhang, F. Xiao and S. Jiao, Build. Sci., 2024, 266, 112053 Search PubMed.
- R. Liu, S. Wang, Z. Zhou, K. Zhang, G. Wang, C. Chen and Y. Long, Adv. Mater., 2025, 37, 2401577 CrossRef CAS.
- Z. Li, Q. Chen, Y. Song, B. Zhu and J. Zhu, Adv. Mater. Technol., 2020, 5, 1901007 CrossRef CAS.
- Q. Yue, L. Zhang, C.-Y. He, B.-H. Liu, W.-M. Wang, Z.-W. Lu, G. Liu and X.-H. Gao, J. Mater. Chem. A, 2023, 11, 3126–3135 RSC.
- J. Li, Y. Liang, W. Li, N. Xu, B. Zhu, Z. Wu, X. Wang, S. Fan, M. Wang and J. Zhu, Sci. Adv., 2022, 8, eabj9756 CrossRef CAS.
- K. Zhang, C. Mo, X. Tang and X. Lei, ACS Sustain. Chem. Eng., 2023, 11, 10 Search PubMed.
- Y. Fu, L. Chen, Y. Guo, Y. Shi, Y. Liu, Y. Zeng, Y. Lin and D. Luo, Adv. Sci., 2024, 11, 2404900 CrossRef CAS PubMed.
- J. Song, Q. Shen, H. Shao and X. Deng, Adv. Sci., 2024, 11, 2305664 CrossRef.
- A. Aili, T. Jiang, J. Chen, Y. Wen, R. Yang, X. Yin and G. Tan, Next Energy, 2024, 3, 100121 CrossRef.
- P. C. Hsu, A. Y. Song, P. B. Catrysse, C. Liu, Y. Peng, J. Xie, S. Fan and Y. Cui, Science, 2016, 353, 1019–1023 CrossRef CAS.
- J. Mandal, Y. Fu, A. C. Overvig, M. Jia, K. Sun, N. N. Shi, H. Zhou, X. Xiao, N. Yu and Y. Yang, Science, 2018, 362, 315–319 CrossRef CAS.
- T. Li, Y. Zhai, S. He, W. Gan, Z. Wei, M. Heidarinejad, D. Dalgo, R. Mi, X. Zhao, J. Song, J. Dai, C. Chen, A. Aili, A. Vellore, A. Martini, R. Yang, J. Srebric, X. Yin and L. Hu, Science, 2019, 364, 760–763 CrossRef CAS PubMed.
- D. J. Fixsen, Astrophys. J., 2009, 707, 916 CrossRef.
- P. Berdahl and R. Fromberg, Sol. Energy, 1982, 29, 299–314 CrossRef.
- Z. Chen, L. Zhu, A. Raman and S. Fan, Nat. Commun., 2016, 7, 13729 CrossRef CAS PubMed.
- D. Zhao, A. Aili, Y. Zhai, S. Xu, G. Tan, X. Yin and R. Yang, Appl. Phys. Rev., 2019, 6, 021306 Search PubMed.
- W. Cai, J. Wang, W. Wang, S. Li, M. Z. Rahman, B. Tawiah, Y. Ming, X. Zhou, W. Xing, Y. Hu, J. Zhu and B. Fei, Small, 2024, 20, 2402349 CrossRef CAS.
- C.-Y. He, P. Zhao, H. Zhang, K. Chen, B.-H. Liu, Z.-W. Lu, Y. Li, P.-Q. La, G. Liu and X.-H. Gao, Adv. Sci., 2023, 10, 2204817 CrossRef CAS.
- E. Rephaeli, A. Raman and S. Fan, Nano Lett., 2013, 13, 1457–1461 CrossRef CAS.
- C. Wang, G.-T. Sun, C.-Y. He, B.-H. Liu, Z.-W. Lu and X.-H. Gao, Adv. Mater., 2025, e08636, DOI:10.1002/adma.202508636.
- S. So, J. Yun, B. Ko, D. Lee, M. Kim, J. Noh, C. Park, J. Park and J. Rho, Adv. Sci., 2024, 11, 2305067 CrossRef PubMed.
- T. Wang, Y. Liu, Y. Dong, X. Yin, D. Lei and J.-G. Dai, Adv. Mater., 2025, 37, 2414300 CrossRef CAS PubMed.
- J. Liu, H. Tang, C. Jiang, S. Wu, L. Ye, D. Zhao and Z. Zhou, Adv. Funct. Mater., 2022, 32, 2206962 CrossRef CAS.
- Y. Peng, J. Chen, A. Y. Song, P. B. Catrysse, P.-C. Hsu, L. Cai, B. Liu, Y. Zhu, G. Zhou, D. S. Wu, H. R. Lee, S. Fan and Y. Cui, Nat. Sustain., 2018, 1, 105–112 CrossRef.
- D. Li, X. Liu, W. Li, Z. Lin, B. Zhu, Z. Li, J. Li, B. Li, S. Fan, J. Xie and J. Zhu, Nat. Nanotechnol., 2021, 16, 153–158 CrossRef CAS PubMed.
- S. Zeng, S. Pian, M. Su, Z. Wang, M. Wu, X. Liu, M. Chen, Y. Xiang, J. Wu, M. Zhang, Q. Cen, Y. Tang, X. Zhou, Z. Huang, R. Wang, A. Tunuhe, X. Sun, Z. Xia, M. Tian, M. Chen, X. Ma, L. Yang, J. Zhou, H. Zhou, Q. Yang, X. Li, Y. Ma and G. Tao, Science, 2021, 373, 692–696 CrossRef CAS PubMed.
- X.-J. Kang, H. Zhang, C.-Y. He, B.-H. Liu, Y.-Z. Zhang and X.-H. Gao, Mater. Today Energy, 2024, 43, 101579 CrossRef CAS.
- B. Xiang, R. Zhang, Y. Luo, S. Zhang, L. Xu, H. Min, S. Tang and X. Meng, Nano Energy, 2021, 81, 105600 CrossRef CAS.
- D. J. Y. Lai, E. M. Chua, A. K. Koyande, W. T. Hong and I. Khoiroh, Appl. Energy, 2025, 377, 124510 CrossRef CAS.
- W. Wei, Y. Zhu, Q. Li, Z. Cheng, Y. Yao, Q. Zhao, P. Zhang, X. Liu, Z. Chen, F. Xu and Y. Gao, Sol. Energy Mater. Sol. Cells, 2020, 211, 110525 CrossRef CAS.
- W. Xie, C. Xiao, Y. Sun, Y. Fan, B. Zhao, D. Zhang, T. Fan and H. Zhou, Adv. Funct. Mater., 2023, 33, 2305734 CrossRef CAS.
- S. Fan and W. Li, Nat. Photonics, 2022, 16, 182–190 CrossRef CAS.
- D. Xie, Z. Yang, X. Liu, S. Cui, H. Zhou and T. Fan, Soft Matter, 2019, 15, 4294–4300 RSC.
- H. Zhang, K. C. Ly, X. Liu, Z. Chen, M. Yan, Z. Wu, X. Wang, Y. Zheng, H. Zhou and T. Fan, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 14657–14666 CrossRef PubMed.
- H. Guo, B. Ma, J. Yu, X. Wang and Y. Si, Adv. Fiber Mater., 2025, 7, 106–116 CrossRef.
- Q. Zhang, S. Wang, X. Wang, Y. Jiang, J. Li, W. Xu, B. Zhu and J. Zhu, Small Methods, 2022, 6, 2101379 CrossRef PubMed.
- T. Xiao, H. Ji, Z. Zeng, P. Long and S. Jin, J. Mater. Chem. C, 2025, 13, 13827–13835 RSC.
- A. P. Raman, M. A. Anoma, L. Zhu, E. Rephaeli and S. Fan, Nature, 2014, 515, 540–544 CrossRef CAS PubMed.
- M. A. Kecebas, M. P. Menguc, A. Kosar and K. Sendur, J. Quant. Spectrosc. Radiat. Transfer, 2017, 198, 179–186 CrossRef CAS.
- A. R. Gentle and G. B. Smith, Adv. Sci., 2015, 2, 1500119 CrossRef.
- Y. Huang, M. Pu, Z. Zhao, X. Li, X. Ma and X. Luo, Opt. Commun., 2018, 407, 204–207 CrossRef CAS.
- K. Feng, Y. Wu, X. Pei and F. Zhou, Mater. Today Energy, 2024, 43, 101575 CrossRef.
- H. Y. Woo, Y. Choi, H. Chung, D. W. Lee and T. Paik, Nano Convergence, 2023, 10, 17 CrossRef CAS PubMed.
- Y. Wang, H. Ji, Y. Chen, B. Liu, J. Huang, M. Lu, Y. Ou, Y. Zhao, J. Tao, Y. Huang and J. Wang, Ceram. Int., 2023, 49, 40297–40304 CrossRef CAS.
- J. Jaramillo-Fernandez, H. Yang, L. Schertel, G. L. Whitworth, P. D. Garcia, S. Vignolini and C. M. Sotomayor-Torres, Adv. Sci., 2022, 9, 2104758 CrossRef CAS.
- A. Aili, Z. Y. Wei, Y. Z. Chen, D. L. Zhao, R. G. Yang and X. B. Yin, Mater. Today Phys., 2019, 10, 100127 CrossRef.
- K. Zhang and B. Wu, Mater. Today Phys., 2025, 51, 101643 CrossRef CAS.
- C. Fan, Y. Zhang, Z. Long, A. Mensah, Q. Wang, P. Lv and Q. Wei, Adv. Funct. Mater., 2023, 33, 2300794 CrossRef CAS.
- H. Du, Y. Zhang, Y. Li, Q. Xu and P. Wang, Renewable Sustainable Energy Rev., 2025, 218, 115792 CrossRef CAS.
- D. G. Baranov, Y. Xiao, I. A. Nechepurenko, A. Krasnok, A. Alù and M. A. Kats, Nat. Mater., 2019, 18, 920–930 CrossRef CAS PubMed.
- W.-M. Wang, B.-H. Liu, C.-Y. He, P. Zhao, S.-J. Zhao, Z.-Q. Wang, Z.-W. Lu, H.-X. Guo, G.-Y. Ren, G. Liu and X.-H. Gao, Adv. Funct. Mater., 2023, 33, 2303197 CrossRef CAS.
- C. G. Ribbing and E. Wäckelgård, Thin Solid Films, 1991, 206, 312–317 CrossRef CAS.
- Z. Tong, J. Peoples, X. Li, X. Yang, H. Bao and X. Ruan, Mater. Today Phys., 2022, 24, 100658 CrossRef CAS.
- H. Zhong, T. Meng, W. Ding, Y. Xiao and P. Zhang, ACS Omega, 2025, 10, 1012–1018 CrossRef CAS PubMed.
- J. Liu, Y. Wei, Y. Zhong, L. Zhang, B. Wang, X. Feng, H. Xu and Z. Mao, Adv. Funct. Mater., 2024, 34, 2406393 CrossRef CAS.
- J. He, Q. Zhang, Y. Zhou, Y. Chen, H. Ge and S. Tang, ACS Nano, 2024, 18, 11120–11129 CrossRef CAS PubMed.
- G. Huang, A. R. Yengannagari, K. Matsumori, P. Patel, A. Datla, K. Trindade, E. Amarsanaa, T. Zhao, U. Köhler, D. Busko and B. S. Richards, Nat. Commun., 2024, 15, 3798 CrossRef CAS PubMed.
- F. Fan, J. Wang, H. Pan, Z. Li and D. Zhao, Renewable Sustainable Energy Rev., 2024, 205, 114878 CrossRef.
- M. Ma and R. M. Hill, Curr. Opin. Colloid Interface Sci., 2006, 11, 193–202 CrossRef CAS.
- H. Zhong, S. Gao and Z. Wang, Langmuir, 2024, 40, 2792–2799 CrossRef CAS PubMed.
- X. Liu, M. Zhang, Y. Hou, Y. Pan, C. Liu and C. Shen, Adv. Funct. Mater., 2022, 32, 2207414 CrossRef CAS.
- C. Jin, W. Zhang, J. Ni, L. Li, Y. Hao, G. Pei and B. Zhao, Sol. Energy Mater. Sol. Cells, 2025, 286, 113577 CrossRef CAS.
- S. Wang, Y. Wang, Y. Zou, G. Chen, J. Ouyang, D. Jia and Y. Zhou, ACS Appl. Mater. Interfaces, 2021, 13, 21888–21897 CrossRef CAS.
- C.-H. Xue, R.-X. Wei, X.-J. Guo, B.-Y. Liu, M.-M. Du, M.-C. Huang, H.-G. Li and S.-T. Jia, Compos. Sci. Technol., 2022, 220, 109279 CrossRef CAS.
- T. Jiang, W. Fan and F. Wang, Colloids Surf., A, 2023, 666, 131296 CrossRef CAS.
- H.-D. Wang, C.-H. Xue, X.-J. Guo, B.-Y. Liu, Z.-Y. Ji, M.-C. Huang and S.-T. Jia, Appl. Mater. Today, 2021, 24, 101100 CrossRef.
- B.-Y. Liu, C.-H. Xue, H.-M. Zhong, X.-J. Guo, H.-D. Wang, H.-G. Li, M.-M. Du, M.-C. Huang, R.-X. Wei, L.-G. Song, B. Chang and Z. Wang, J. Mater. Chem. A, 2021, 9, 24276–24282 RSC.
- K. Park, H. Kim, K. Kim, C. Lee, A. Asadi, H. J. Kim and K. Lee, Mater. Des., 2025, 253, 113980 CrossRef CAS.
- H. Zhang and Z. Guo, Nano Today, 2023, 51, 101933 CrossRef CAS.
- M. Mohseni, H. S. Far, M. Hasanzadeh and K. Golovin, Prog. Org. Coat., 2021, 157, 106319 CrossRef CAS.
- H. Shen, Z. Tan, Y. Li, L. Yang and D. Ge, Colloids Surf., A, 2024, 686, 133350 CrossRef CAS.
- J. Zhang, C. Zhu, J. Lv, W. Zhang and J. Feng, ACS Appl. Mater. Interfaces, 2018, 10, 40219–40227 CrossRef CAS PubMed.
- M.-C. Huang, C.-H. Xue, J. Huang, B.-Y. Liu, X.-J. Guo, Z.-X. Bai, R.-X. Wei, H.-D. Wang, M.-M. Du, S.-T. Jia, Z. Chen and Y. Lai, Chem. Eng. J., 2022, 442, 136239 CrossRef CAS.
- Z. Zhang, R. Tian, P. Zhang, C. Lu and X. Duan, ACS Cent. Sci., 2020, 6, 771–778 CrossRef CAS PubMed.
- X. Li, J. Peoples, P. Yao and X. Ruan, ACS Appl. Mater. Interfaces, 2021, 13, 21733–21739 CrossRef CAS.
- L. Zhang, H. Zhan, Y. Xia, R. Zhang, J. Xue, J. Yong, L. Zhao, Y. Liu and S. Feng, ACS Appl. Mater. Interfaces, 2023, 15, 31994–32001 CrossRef CAS.
- C. Fu, M. Zhu, D. Zhao, L. Yu, Y. Ding and D. Liu, Sol. Energy, 2022, 245, 322–331 CrossRef CAS.
- J. Zhou, C. Ding, X. Zhang, D. Li, D. Yang, B. You and L. Wu, ACS Appl. Mater. Interfaces, 2023, 15, 57514–57524 CAS.
- M. Li, C. Lin, K. Li, W. Ma, B. Dopphoopha, Y. Li and B. Huang, Small, 2023, 19, 2301159 CrossRef CAS PubMed.
- X. Li, L. Pattelli, Z. Ding, M. Chen, T. Zhao, Y. Li, H. Xu, L. Pan and J. Zhao, Adv. Funct. Mater., 2024, 34, 2315315 CrossRef CAS.
- P. Yao, Z. Chen, T. Liu, X. Liao, Z. Yang, J. Li, Y. Jiang, N. Xu, W. Li, B. Zhu and J. Zhu, Adv. Mater., 2022, 34, 2208236 CrossRef CAS PubMed.
- Q. Wang, J. Luo, Z. Lv, T. Wu, L. Zhang, Y. Zhong, H. Xu and Z. Mao, ACS Appl. Mater. Interfaces, 2024, 16, 46761–46770 CrossRef CAS.
- A. S. Farooq, X. Song, D. Wei, L. Liu and P. Zhang, Mater. Today Energy, 2024, 45, 101666 CrossRef CAS.
- X. Song, H. Gong, H. Li, M. Zhang, L. Jiang, C. Wang, P. Jiang, H. Wang, K. Cao and G. Liu, Sol. Energy Mater. Sol. Cells, 2024, 275, 113003 CrossRef CAS.
- X. Shi, C. Liu, B. Lin, G. Zhou, C. Liu, C. Mei and M.-C. Li, Addit. Manuf., 2024, 92, 104392 CAS.
- W. Liu, W. Guo, Y. Wang, L. Song, P. Du, N. Li and J. Xiong, Appl. Therm. Eng., 2024, 255, 123955 CrossRef CAS.
- Y. Du, W. Wang, J. Mei and L. Zhang, Chem. Eng. J., 2024, 485, 149976 CrossRef CAS.
- C. Deng, H. Zhang, B. Zhao, Y. Chen, Y. Li, T. Zhang and F. Qiu, Sol. Energy, 2024, 279, 112840 CrossRef CAS.
- T. Li, H. Sun, M. Yang, C. Zhang, S. Lv, B. Li, L. Chen and D. Sun, Chem. Eng. J., 2023, 452, 139518 CrossRef CAS.
- X. Shan, L. Liu, Y. Wu, D. Yuan, J. Wang, C. Zhang and J. Wang, Adv. Sci., 2022, 9, 2201190 CrossRef CAS PubMed.
- D. Garcia-Gonzalez, M. Rodriguez-Millan, A. Rusinek and A. Arias, Compos. Struct., 2015, 134, 440–449 CrossRef.
- H. Dal, O. Gültekin, S. Başdemir and A. K. Açan, Comput. Methods Appl. Mech. Eng., 2022, 401, 115639 CrossRef.
- G. O. Shonaike, Eur. Polym. J., 1988, 24, 1107–1110 CrossRef CAS.
- M. Naebe, M. M. Abolhasani, H. Khayyam, A. Amini and B. Fox, Poly. Rev., 2016, 56, 31–69 CrossRef CAS.
- D. Hong, Y. J. Lee, O. S. Jeon, I.-S. Lee, S. H. Lee, J. Y. Won, Y. P. Jeon, Y. La, S. Kim, G.-S. Park, Y. J. Yoo and S. Y. Park, Nat. Commun., 2024, 15, 4457 CrossRef CAS PubMed.
- M. Heydari, J. Cleaner Prod., 2024, 442, 141037 CrossRef.
- J.-W. Han, M. Zuo, W.-Y. Zhu, J.-H. Zuo, E.-L. Lü and X.-T. Yang, Trends Food Sci. Technol., 2021, 109, 536–551 CrossRef CAS.
- S. Munné-Bosch and C. Vincent, Crit. Rev. Plant Sci., 2019, 38, 140–157 CrossRef.
- L. Xu, D.-W. Sun, Y. Tian and Z. Zhu, Food Chem., 2024, 437, 137804 CrossRef CAS PubMed.
- Y. Yin, M. Zhang and Z. Wang, Adv. Mater. Technol., 2025, 2402112 CrossRef.
- J. Jaramillo-Fernandez, H. Yang, L. Schertel, G. L. Whitworth, P. D. Garcia, S. Vignolini and C. M. Sotomayor-Torres, Adv. Sci., 2022, 9, 2104758 CrossRef CAS.
- N.-S. Hon, J. Polym. Sci., Polym. Chem. Ed., 1977, 15, 725–744 CrossRef CAS.
- D. M. Gouvêa, R. C. S. Mendonça, M. L. Soto and R. S. Cruz, LWT--Food Sci. Technol., 2015, 63, 85–91 CrossRef.
- A. Rajeswari, E. J. S. Christy, E. Swathi and A. Pius, Environ. Chem. Ecotoxicol., 2020, 2, 107–114 CrossRef.
- S. Gemili, A. Yemenicioğlu and S. A. Altınkaya, J. Food Eng., 2009, 90, 453–462 CrossRef.
- D. A. Marrez, A. E. Abdelhamid and O. M. Darwesh, Food Packag. Shelf Life, 2019, 20, 100302 CrossRef.
- G. Kim, Mater. Today Sustain., 2024, 27, 100928 Search PubMed.
- A. Hazarika, B. K. Deka, H. Park, Y. J. Hwang, A. P. Jaiswal, Y.-B. Park and H. W. Park, Chem. Eng. J., 2023, 471, 144536 CrossRef CAS.
- J. He, Q. Zhang, Y. Wu, Y. Ju, Y. Wang and S. Tang, Chem. Eng. J., 2023, 466, 143127 CrossRef CAS.
- Y. Liu, X. Bu, R. Liu, M. Feng, Z. Zhang, M. He, J. Huang and Y. Zhou, Chem. Eng. J., 2024, 481, 148780 CrossRef CAS.
- S. Ouyang, Q. Jiang, Y. Wan, X. Qu, Z. Yu, H. He and J. Wang, Int. J. Biol. Macromol., 2024, 275, 133533 CrossRef CAS PubMed.
- Z. Li, J.-H. Zhang, J. Li, S. Wang, L. Zhang, C.-Y. He, P. Lin, S. Melhi, T. Yang, Y. Yamauchi and X. Xu, Small, 2024, 20, 2309397 CrossRef CAS.
- J. Muncke, J. P. Myers, M. Scheringer and M. Porta, J. Epidemiol. Community Health, 2014, 68, 592 CrossRef PubMed.
- K.-H. Choi, J. W. Yoon, M. Kim, H. J. Lee, J. Jeong, M. Ryu, C. Jo and C.-K. Lee, Compr. Rev. Food Sci. Food Saf., 2021, 20, 429–457 CrossRef PubMed.
- F. Wang, Y. Xie, L. Wang, S. Liu and X. Jin, Sci. Total Environ., 2025, 963, 178450 CrossRef CAS PubMed.
- A. Malinka, E. Zege, G. Heygster and L. Istomina, Cryosphere, 2016, 10, 2541–2557 CrossRef.
- B. Ko, J. Noh, D. Chae, C. Lee, H. Lim, H. Lee and J. Rho, Adv. Funct. Mater., 2024, 34, 2410613 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |



