 Open Access Article
Open Access ArticleSemi-crystalline and recyclable pressure sensitive adhesives from non-edible rapeseed oil-based hyperbranched polyester vitrimers
Virgile
Ayzac
a,
Boris
Bizet
*b,
Marie
Reulier
b,
Guillaume
Chollet
b,
Cédric
Le Coz
 a,
Etienne
Grau
*a and
Henri
Cramail
a,
Etienne
Grau
*a and
Henri
Cramail
 *a
*a
aUniversity of Bordeaux, CNRS, Bordeaux INP, LCPO, UMR 5629, 16 Avenue Pey-Berland, F, 33600 Pessac, France. E-mail: cramail@enscbp.fr; egrau@enscbp.fr
bITERG, 43 rue Thomas Edison - ZA Pessac-Canéjan, CS 20428 - 33610 Canéjan, France
First published on 28th November 2025
Abstract
Hyperbranched polymers exhibit distinctive properties attributed to their highly branched architecture and the abundant functional groups they carry. In this study, we synthesized innovative hyperbranched polyesters from vegetable oil derivatives without utilizing solvents. The reaction conditions were optimized and these polymers were comprehensively characterized based on size distribution, degree of branching and molecular structure. These hyperbranched polymers manifest as sticky viscous liquids with semi-crystalline behavior, featuring glass transition temperatures (Tg) and melting temperatures (Tm) as low as 18 °C. Subsequently, the latter were utilized as precursors for the design of unique thermosets with potential self-healing and recyclable pressure-sensitive adhesive properties.
Sustainability spotlightThis work advances green chemistry by designing recyclable and self-healing pressure-sensitive adhesives (PSAs) from hyperbranched polyesters derived from erucic acid, a non-edible and renewable vegetable oil. The synthesis is performed solvent-free, valorizing a non-food biobased synthon and yielding vitrimers that are recyclable and reusable while maintaining adhesive performance. |
Introduction
Manufacturing bio-based materials that offer both competitive properties and sustainability has emerged as a highly important industrial matter.1 In the field of pressure sensitive adhesives (PSAs),2 which are reversible adhesives via non-covalent bonding used in a large variety of applications from tapes to electronics, the market is dominated by oil-based and non-sustainable materials with extremely cheap costs. The most common systems are based on either polyacrylates, styrene–butadiene/isoprene block copolymers or polysiloxanes.2 Consequently, recyclable materials3 and sustainable alternatives based on renewable resources have been developed.4–10Vegetable oils, which are triglycerides composed of various saturated and unsaturated fatty acid moieties, are considered potential resources for such applications due to their availability, low toxicity and low cost. Additionally, their fatty acid chains contribute to the flexibility, hydrophobicity and low glass-transition temperatures of PSAs, providing the tackiness such materials require.11
Various methods are available for converting vegetable oils into pressure-sensitive adhesives (PSAs). One prevalent method involves the use of epoxidized vegetable oils, which are produced through the oxidation of double bonds with peroxide acids. Recently, Li et al. designed a resin based on epoxidized soybean oil (ESO) and a highly branched polyamine, which exhibits excellent adhesion and film-forming properties.12 In a previous study, ESO was used by Wang et al. with rosin acid to obtain bio-based PSAs with high adhesivity.13 The authors also designed PSAs using carboxylic acid-terminated polyricinoleate and ESO as well as flame-retardant PSAs with ESO and phosphorus containing dicarboxylic acids.14 Epoxidized fatty acids have also been used by Li et al. to prepare bio-based PSAs by reaction with di-carboxylic acids or anhydrides.15 PSAs based on linseed oil were developed by Pan et al.16 Oleic methyl ester obtained by transesterification of the corresponding triglyceride was epoxidized with peracetic acid/H2O2 and acrylated with acrylic acid. The resulting acrylated monomer was then polymerized through a miniemulsion process to obtain PSAs with properties comparable to petroleum-based systems.17 Following a similar strategy, Badía et al. recently demonstrated that removable biobased waterborne PSAs could be obtained by forming bio-based 2-octyl acrylate-containing latexes by seeded semi-batch emulsion polymerization.18 This monomer contributed to the soft phase of the formulation. The properties of the resulting latexes were adjusted through the incorporation of piperonyl methacrylate and isosorbide dimethacrylate, which constituted a harder phase.19,20 Using this strategy, high bio-content (71%) waterborne PSAs were thus obtained.
Nevertheless, due to their nature, thermoset-based adhesives are non-recyclable. In order to overcome this issue, bio-based vitrimers were developed by different teams for adhesive applications.21–31 Vitrimers are associative covalent adaptable networks that are built from reversible covalent bonds giving them a dynamic behavior and granting them both recyclability and thermoset properties such as mechanical strength and solvent resistance.21 The dynamic covalent network can be based on multiple types of bonds, among which boronic esters,22 which can present a high naturality index,23,24 are of particular interest in this study. Accessing recyclable thermosets is of utmost importance in today's context and many bio-based vitrimers were designed in anticipation of a post-petroleum era.25 Some of these systems present adhesive properties such as the one synthesized from ESO and glycyrrhizic acid based on transesterification reactions26 or an ESO based epoxy adhesive using dynamic borates designed by Qiu et al.27 Similar to ESO, lignin is a highly available bio-resource and has been reported to manufacture vitrimer materials with adhesive properties.28 Vitrimeric PSAs were developed as well29–31 but, to the best of our knowledge, no bio-based system has been reported.
The objective of this study was to enhance the adhesive properties of rapeseed oil-based hyperbranched polyesters (HBPEs) for the development of pressure-sensitive adhesives (PSAs) with customizable mechanical and adhesive characteristics. Hyperbranched polymers (HBPs) are synthesized in a single step and exhibit a highly branched, compact, and globular structure. This unique structure endows them with distinctive properties when compared to their linear counterparts, including lower viscosities in both molten and solution states, improved solubility, and high functionality.32,33 Their synthesis via self-condensation of ABn type monomers was predicted by Flory in the 1950s34 but the first intentional synthesis was reported in 1987 by Kim and Webster with polyphenlenes.35 Our group explored the possibility to produce bio-based hyperbranched polyesters (HBPEs) with ABn type monomers derived from vegetable oils.36
There are examples of HBP based PSAs in the literature37 but none of them are made from renewable resources. Some examples of bio-based HBPs with adhesive properties were, however, described.38,39
In this study, a PSA was synthesized using a HBPE derived from erucic acid. The HBPE was prepared by transesterification polymerization of dihydroxylated methyl erucate at 160 °C under reduced pressure with a basic catalyst. The resulting HBPE exhibits high degrees of polymerization and branching, appearing as either a very viscous liquid or a visco-elastic solid at room temperature and turning into a flowing liquid at higher temperatures. Cold grinding of the HBPE with phenyl-diboronic acid and curing at 120 °C overnight under reduced pressure produced elastic materials with PSA properties. These materials were thermally recyclable at 120 °C through low pressure molding or injection molding.
Experimental section
Materials
TBD (triazabicyclodecene) and phenyl-1,4-diboronic acid were purchased from Sigma Aldrich and used as received. Dihydroxylated methyl erucate was kindly provided by ITERG (and was synthesized according to the procedure reported by Testud et al. with a GC purity of 85%).36Analyses
Nuclear magnetic resonance (NMR) spectroscopy and 1H and 13C NMR experiments were conducted on a Bruker Avance 400 spectrometer (400.20 MHz or 400.33 MHz and 100.63 MHz for 1H and 13C, respectively) at room temperature, in CDCl3 as solvent unless otherwise mentioned. Chemical shifts (δ) are reported in parts per million relative to the known solvent residual peak (δ = 7.26 ppm). DEPT-135 (Distortion Enhanced Polarization Transfer) and two-dimensional analyses such as 1H–1H COSY (Homonuclear correlation spectroscopy), 1H–13C-HSQC (Heteronuclear single quantum coherence) and 1H–13C-HMBC (Heteronuclear multiple bond correlation) were also performed.Size exclusion chromatography (SEC) analyses were performed in THF as the eluent (1 mL min−1) at 40 °C, on a PL-GPC 50 plus Integrated GPC from Polymer Laboratories–Varian with a series of four columns from TOSOH [TSKgel TOSOH: HXL-L (guard column 6.0 mm ID × 4.0 cm L); G4000HXL (7.8 mm ID × 30.0 cm L); G3000HXL (7.8 mm ID × 30.0 cm L); and G2000HXL (7.8 mm ID × 30.0 cm L)]. The elution times of the filtered samples were monitored using RI detectors with a calibration curve based on low dispersity polystyrene standards (PSs). Trichlorobenzene was added as a flow marker.
Differential scanning calorimetry (DSC) measurements were carried out on a TA Instruments DSC Q200. For each sample, two cycles from −100 to 150 °C (unless otherwise mentioned) were performed at 10 °C min−1. Glass transition and melting temperatures were calculated based on the second heating run. Thermogravimetric analyses (TGAs) were performed on a TA Q500 at a heating rate of 10 °C min−1 under a nitrogen atmosphere from room temperature to 700 °C.
Rheological analyses were performed using an Anton Paar MCR 302 rheometer equipped with parallel plates (Ø = 8 mm) and with 3% strain. The frequency dependence of the moduli was measured between 0.01 Hz and 100 Hz at 20 °C. The evolution of the moduli with temperature was measured at 10 rad s−1 and 2 °C min−1 between 20 and 120 °C.
The adhesion properties of the synthesized HBPE were assessed with a simple tack test. The upper plate of the rheometer was put in contact with the sample for 60 seconds in order for residual forces to disappear and then the plate was moved up at 1 mm s−1 and, the strength, imposed by the sample on the plate, was measured. This allowed us to obtain surface adhesion strengths in J m−2 and to compare the different materials. This experiment was performed multiple times in order to obtain reproducible results.
Stress relaxation tests were performed at 10% strain.
Synthesis of the HBPE
20 g of the monomer were heated and magnetically stirred under reduced pressure (ca. 35 mbar) at 90 °C for one hour in order to remove remaining traces of water. 300 mg of TBD were then added and the reaction mixture was heated at 120 °C under a nitrogen flux for one hour in order to eliminate the formed methanol. The reaction was then put under reduced pressure until no more bubbling was observed. The temperature was then increased to 160 °C for forty-nine hours. An increase of viscosity with time was observed but the product remained liquid at 160 °C. Upon cooling, a colorless sticky visco-elastic solid was obtained and used without any further purification.The conversion of the reaction was followed by 1H NMR (see SI Fig. S1–S6).
Synthesis of the HBPE – phenyl diboronic acid vitrimer
2 g of the HBPE and different amounts of phenyldiboronic acid (from 10.7 mg (0.06 eq. to diols) to 44.7 mg (0.25 eq. to diols)) were mixed and finely ground in liquid nitrogen. The resulting powder was cured under reduced pressure at 120 °C overnight and molded at 120 °C for 15 min. A sticky, elastic, non-colored and transparent material was obtained (Fig. 1 shows the image of the material produced by curing a mixture of HBPE with 8 equivalents of phenyldiboronic acid at 120 °C under vacuum overnight.) | ||
| Fig. 1 Image of the material produced by curing a mixture of the HBPE with 8 equivalents of phenyldiboronic acid at 120 °C under vacuum overnight. | ||
Characterization
The degree of branching (DB) was expressed by Fréchet and coll. as a function of dendritic (D), terminal (T) and linear (L) unit proportions40 but Frey and coll. lately simplified the equation for high molar mass HBPEs, which only takes into account the dendritic and linear units and is written as follows:41The Frey equation was used in this study. In order to calculate this DB, the proportions of dendritic and linear units can be obtained by 1H NMR as the signals corresponding to dendritic (5.0 ppm) and linear (4.7 and 3.6 ppm) units are well defined. The degree of polymerization was assessed by NMR and the signal corresponding to the methyl ester is well separated (3.7 ppm). The dispersity of the polymer was assessed in SEC. It is important to note that the molar masses obtained by Size Exclusion Chromatography (SEC) using polystyrene standards were significantly underestimated. This discrepancy arises because these standards do not account for the dense structure of the hyperbranched polyester (HBPE), which consequently appears smaller than its real size in solution.
The thermal properties of both the HBPE and the vitrimers were analyzed. The melting temperature and the glass transition temperature of the different materials were measured by Differential Scanning Calorimetry (DSC) during the second heating and their thermal stability was assessed by Thermal Gravimetric Analysis (TGA). Moreover, the gel content of these materials was measured by immersing the sample in acetone for 48 h, drying overnight at 90 °C and measuring the resulting loss of weight, corresponding to the non-soluble fraction.
Results and discussion
Due to their high availability and chemical versatility, vegetable oils have emerged as starting materials of high interest since they can serve as building blocks, the reactivity and properties of which can be adjusted through chemical modification. In addition, developing solutions that do not compete with the food industry is highly desirable. In this regard, erucic acid-enriched oils originating from rapeseed appear as relevant non-edible products, allowing further valorization by chemical transformation.Successive transesterification, epoxidation, and hydrolysis of these erucic acid-enriched oils were performed, resulting in the production of dihydroxylated methyl erucate (Fig. S1). This compound, produced at the kg-scale at the technical institute, ITERG, was then polymerized in bulk by heating under vacuum with TBD as a basic catalyst to produce hyperbranched polyesters (HBPEs) (Scheme 1).
 | ||
| Scheme 1 HBPE obtained by polymerization of dihydroxylated methyl erucate in bulk at 160 °C under vacuum for two days and catalysed by TBD. | ||
Characterization of the HBPE
The 1H NMR analysis of the HBPE (Fig. 2) allowed the quantification of methyl ester moieties, dendritic units (D), linear units (L), and terminal units (T).36 A DBFrey of 0.46 was calculated from the integrals of the dendritic unit at 5 ppm and the linear unit at 4.6 ppm. The ratio between the terminal CH3 of the fatty acid chain at 0.8 ppm and the focal methyl ester group at 3.7 ppm allowed calculation of the average molar mass of the resulting polymer, with a Mn of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 (a degree of polymerization (DPn) of 114.4). Finally, the ratio between the integrations of the focal methyl ester group at 3.7 ppm and the terminal units gave an average value of 30.6 (1,2-diol) units per HBPE molecule. Complementary NMR and FTIR characterization results of the HBPE are given in Fig. S2–S7.
600 g mol−1 (a degree of polymerization (DPn) of 114.4). Finally, the ratio between the integrations of the focal methyl ester group at 3.7 ppm and the terminal units gave an average value of 30.6 (1,2-diol) units per HBPE molecule. Complementary NMR and FTIR characterization results of the HBPE are given in Fig. S2–S7.
 | ||
| Fig. 2 1H NMR spectrum of the HBPE in CDCl3, indicating methyl ester groups and terminal, linear, and dendritic units with their respective assignments. | ||
The SEC analysis of this dihydroxylated methyl erucate-based HBPE (Fig. 3) in THF indicated an Mn value of 5200 g mol−1 with a large dispersity value around 12, in agreement with the formation of a hyperbranched structure. While this Mn value could appear to be in disagreement with the NMR result, one should remember that the molar mass is given according to the linear PS standard and thus highly underestimated in this specific case. This is mostly due to the limited swelling capacity of a densely constrained hyperbranched polymeric structure, making it appear smaller as one could expect.
The HBPE thus obtained was subsequently cross-linked with phenyldiboronic acid by means of cold grinding, followed by an overnight curing process at 120 °C under vacuum conditions (Scheme 2). FTIR analysis of the cross-linked materials reveals the appearance of bands at 650 cm−1 and 1350 cm−1 characteristic of the boronic ester function (see SI Fig. S7) without substantial variation according to the ratio of phenyldiboronic acid. In order to determine the influence of the cross-linking density on the properties of the material, different quantities of phenyldiboronic acid were used. The appearance of the resulting gel varies significantly according to differences in the phenyldiboronic acid ratio. Four materials ranging from 2 to 8 equivalents of phenyldiboronic acid to HBPE (i.e. 0.06 to 0.25 eq. to diol) were manufactured and characterized via thermal and rheological analyses.
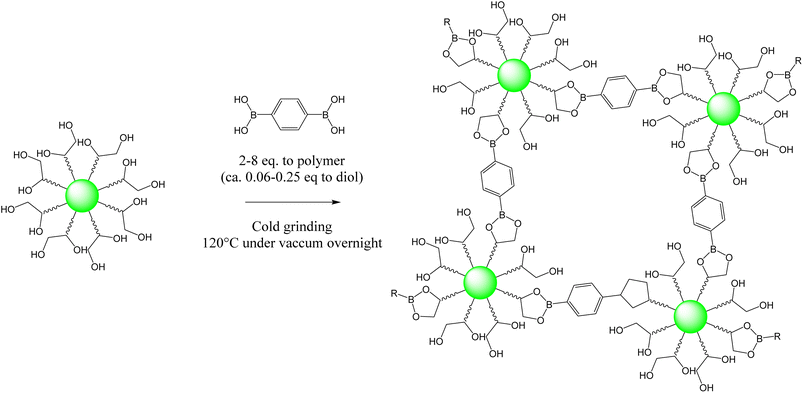 | ||
| Scheme 2 Simplified view of dihydroxylated methyl erucate-based HBPE cross-linking with phenyldiboronic acid at 120 °C under vacuum overnight. | ||
To confirm the effective cross-linking, the gel content was measured by immersing the materials in acetone and recording weight loss. The results indicated gel content values between 79 and 85%, irrespective of the quantity of phenyldiboronic acid used. The materials also demonstrated a swelling ratio in acetone of approximately 200% for 2 eq. of phenyldiboronic acid crosslinker against 100% for 8 eq.
Thermal properties
All materials showed similar thermal stability with Td,5% = 326.1–331.7 °C according to TGA under nitrogen (see SI Fig. S8). DSC analyses of the cross-linked materials (Fig. 4) reveal a decrease in the crystallinity ratio and the melting temperature with an increase in the phenylboronic acid ratio. The melting temperature ranges from 18 °C to 13.6 °C and the melting enthalpy ranges from 24 J g−1 to 17 J g−1. In addition, the glass transition temperature decreases with the boronic acid content, from 5 °C for the HBPE precursor to −12 °C for the network with 8 eq. of boronic acid. The observation of uncommon remaining crystallinity in the networks is attributed to the alignment of pendant alkyl chains. This original feature observed with the erucic acid precursor may open up new functionalities such as thermomechanical actuation or controlled adhesion. Moreover, the increase in phenyldiboronic acid crosslinker content logically reduces the chain mobility, which decreases the ability of the alkyl chains to crystallize.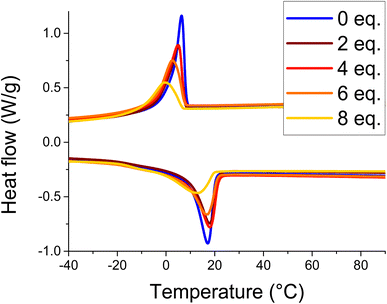 | ||
| Fig. 4 DSC traces of the dihydroxylated methyl erucate-based HBPE (blue) and the cross-linked HBPE at different phenyldiboronic acid ratios. | ||
Mechanical properties
Rheological experiments were performed to measure the mechanical properties of the cross-linked HBPEs. The variations of materials' moduli with frequency were measured to obtain their visco-elastic window, which is a usual characteristic of pressure-sensitive adhesives, useful to predict the adhesive behavior of the materials.42 The visco-elastic window of the synthesized materials is represented in Fig. 5 (see SI Fig. S9). It represents the moduli window between a situation where the material is strongly solicited (102 Hz) and a situation where the material is weakly solicited (10−2 Hz).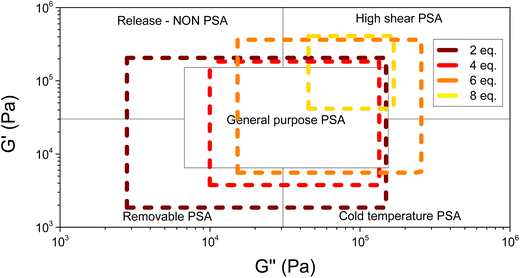 | ||
| Fig. 5 Visco-elastic windows (visualization specific to PSA) of cross-linked dihydroxylated methyl erucate-based HBPE at different phenyldiboronic acid ratios (from 2 eq. to 8 eq.). | ||
As the quantity of phenyldiboronic acid increases (the lighter the color of the visco-elastic window in Fig. 5), the window reduces and the moduli increase. This relationship indicates the potential to develop materials with specific properties ranging from general purpose PSAs (for low diboronic loading) to high shear PSAs (for high diboronic acid loading).
The variation of the moduli with temperature was measured to determine how phenyldiboronic acid affects the mechanical properties of the cross-linked HBPE upon heating (Fig. 6). The uncross-linked material (HBPE, black curve) shows a typical thermoplastic behavior, with a sharp decrease in G′ over 30 °C, corresponding to the melting of its crystalline phase (see Fig. 4).
 | ||
| Fig. 6 Variation, with temperature, of the moduli of the cross-linked HBPE at different phenyldiboronic acid ratios (down: zoomed-in view). | ||
As expected, the loss of mechanical properties upon heating was directly related to the amount of cross-linker used. Highly cross-linked materials had higher and more stable moduli upon heating (Fig. 6). For cross-linked materials, all curves show three temperature domains with different behaviors. The first domain shows a loss in both moduli (G″ faster than G′), with a steeper slope for the formulations including lower concentrations of cross-linker. The second domain exhibits a continued decrease in G′ but an increase in G″ until it equals G′, indicating that the material starts flowing. The last domain shows a faster decrease in both G′ and G″, with a more liquid behavior (G″ is higher than G′). Finally, all materials exhibited vitrimeric behavior, with flowing temperatures increasing with higher cross-linker concentrations.
Adhesion properties
To evaluate the adhesion properties, the samples were placed in a rheometer between two steel plates under low stress at a given temperature and the upper plate was moved upward while measuring the adhesion force over time (see SI Fig. S10–S12). The adhesion strength was determined by analyzing the variation area of the adhesion force as a function of time. The results are summarized in Fig. 7 and 8. An inversely proportional relationship between the amount of phenyldiboronic acid and the adhesion strength was observed. This could be attributed to the reduced number of hydroxyl groups that reacted with the crosslinker leading to fewer hydrogen bonding interactions. | ||
| Fig. 7 Evolution of the surface adhesion of the cross-linked HBPE onto steel as a function of the quantity of phenyldiboronic acid cross-linker at 20 °C and 30 °C, respectively. | ||
 | ||
| Fig. 8 Evolution of the surface adhesion with temperature of the HBPE cross-linked with 8 eq. of phenyl diboronic acid cross-linker. | ||
Stress relaxation
Boronic ester, due to its constant chemical equilibrium, exhibits vitrimer properties and can relax stress within the network. This behavior is temperature-dependent, similar to the kinetics of the reactions occurring in the vitrimers. To assess the ability of these cross-linked materials, stress relaxation tests were performed on the HBPE crosslinked with 8 eq. of phenyl diboronic acid with a rheometer at different temperatures (Fig. 9) to measure the stress relaxation time τ (G(τ) = 1/e). The principle involves applying stress (here 10% strain) to the vitrimer and observing the evolution of this stress over time until complete relaxation. The evolution of the stress relaxation time with temperature followed an Arrhenius law (Fig. 10), thus demonstrating the vitrimeric feature of these materials. The latter exhibited an activation energy of 55.62 ± 1.31 kJ mol−1. | ||
| Fig. 9 Stress relaxation curves of the cross-linked HBPE with 8 eq. of phenyl diboronic acid cross-linker with respect to temperature. | ||
Mechanical recycling
Due to their vitrimer properties, these materials can be recycled. Mechanical recycling was performed on the highly cross-linked material (8 eq. of cross-linker). It can be reshaped at 120 °C using low pressure casting or injection molding (Fig. 11). The mechanical and adhesion properties of the recast material are comparable to those of the original. The moduli remain unchanged at low temperatures and slightly vary at higher temperatures (Fig. 12). Surface adhesion at 20 °C is consistent, maintaining 15 J m−2 (compared to 14.5 J m−2 for the original). | ||
| Fig. 11 Pictures of the cut pristine (left) and recast (at 120 °C) (right) dihydroxylated methyl erucate-based cross-linked HBPEs with 8 eq. of phenyl diboronic acid used as the cross-linker. | ||
 | ||
| Fig. 12 Evolution of the moduli with temperature of the cross-linked HBPE with 8 eq. of phenyl diboronic acid used as the cross-linker, before and after mechanical recycling. | ||
Conclusion
We have developed a bio-sourced, recyclable, and semi-crystalline pressure-sensitive adhesive (PSA) exhibiting vitrimeric behavior. This result leverages hyperbranched polyesters (HBPEs), which offer multifunctionality with approximately 30 diol units per HBPE and flexibility due to their unique molecular structure. Notably, the HBPE utilized in this study is derived from erucic acid, a resource that is not in competition with the food industry, positioning it as a sustainable alternative.Interestingly, the cross-linked networks display a degree of crystallinity that is unusual, a very specific feature that is attributed to the presence of long pendant alkyl chains derived from the fatty acid structure. This crystalline character may contribute to the materials' shape-memory behavior and may offer additional functionality worth exploring.
It has been established that the degree of crosslinking via boronic esterification can modulate both adhesive and mechanical properties, highlighting the interdependence of these characteristics. Owing to their vitrimeric nature, these materials can be reshaped and recycled mechanically, and their performance is maintained after reprocessing.
These PSA materials thus offer tunable properties and multiple recyclability pathways, supporting their potential in sustainable adhesive technologies.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5su00868a.Acknowledgements
This work was supported by the Carnot 3BCAR.References
- Y. Zhu, C. Romain and C. K. Williams, Sustainable Polymers from Renewable Resources, Nature, 2016, 354–362, DOI:10.1038/nature21001.
- Handbook of Pressure-Sensitive Adhesives and Products, ed. I. Benedek and M. M. Feldstein, CRC Press, 2019, DOI:10.1201/9780429151699.
- K. R. Mulcahy, A. F. R. Kilpatrick, G. D. J. Harper, A. Walton and A. P. Abbott, Debondable Adhesives and Their Use in Recycling, Green Chem., 2022, 7, 36–61, 10.1039/d1gc03306a.
- E. Cohen, O. Binshtok, A. Dotan and H. Dodiuk, Prospective Materials for Biodegradable and/or Biobased Pressure-Sensitive Adhesives: A Review, J. Adhes. Sci. Technol., 2013, 27(18–19), 1998–2013, DOI:10.1080/01694243.2012.696901.
- S. V. Pradeep, B. Kandasubramanian and S. Sidharth, A Review on Recent Trends in Bio-Based Pressure Sensitive Adhesives, J. Adhes., 2023, 99(14), 2145–2166, DOI:10.1080/00218464.2023.2176761.
- A. Beharaj, I. Ekladious and M. W. Grinstaff, Poly(Alkyl Glycidate Carbonate)s as Degradable Pressure-Sensitive Adhesives, Angew. Chem., 2019, 131(5), 1421–1425, DOI:10.1002/ange.201811894.
- T. T. D. Chen, L. P. Carrodeguas, G. S. Sulley, G. L. Gregory and C. K. Williams, Bio-Based and Degradable Block Polyester Pressure-Sensitive Adhesives, Angew. Chem., Int. Ed., 2020, 59(52), 23450–23455, DOI:10.1002/anie.202006807.
- R. Paul, M. Singh, V. J. Vidhya, G. Manik and S. K. Sahoo, Bio-Based Pressure Sensitive Adhesives Derived from Cardanol, Vanillin, and Sebacic Acid for Removable Nonstructural Applications, Ind. Eng. Chem. Res., 2023, 62(1), 423–434, DOI:10.1021/acs.iecr.2c03601.
- Y. F. Lei, X. L. Wang, B. W. Liu, L. Chen and Y. Z. Wang, Bio-Based Removable Pressure-Sensitive Adhesives Derived from Carboxyl-Terminated Polyricinoleate and Epoxidized Soybean Oil, Chin. Chem. Lett., 2021, 32(2), 875–879, DOI:10.1016/j.cclet.2020.06.015.
- H. J. Kim, K. Jin, J. Shim, W. Dean, M. A. Hillmyer and C. J. Ellison, Sustainable Triblock Copolymers as Tunable and Degradable Pressure Sensitive Adhesives, ACS Sustainable Chem. Eng., 2020, 8(32), 12036–12044, DOI:10.1021/acssuschemeng.0c03158.
- Y. Ma, Y. Ji, J. Zhang, Y. Sha, P. Jia and Y. Zhou, Research Advances in Vegetable-Oil-Based Pressure-Sensitive Adhesives, Green Mater., 2023, 11(4), 147–161, DOI:10.1680/jgrma.22.00061.
- Z. Qian, S. Liu, G. Du, S. Wang, Y. Shen, X. Zhou, S. Jiang, H. Niu, Z. Duan and T. Li, Versatile Epoxidized Soybean Oil-Based Resin with Excellent Adhesion and Film-Forming Property, ACS Sustainable Chem. Eng., 2023, 11(13), 5315–5324, DOI:10.1021/acssuschemeng.3c00743.
- Y.-F. Lei, X.-L. Wang, B.-W. Liu, X.-M. Ding, L. Chen and Y.-Z. Wang, Fully Bio-Based Pressure-Sensitive Adhesives with High Adhesivity Derived from Epoxidized Soybean Oil and Rosin Acid, ACS Sustainable Chem. Eng., 2020, 8(35), 13261–13270, DOI:10.1021/acssuschemeng.0c03451.
- X. L. Wang, L. Chen, J. N. Wu, T. Fu and Y. Z. Wang, Flame-Retardant Pressure-Sensitive Adhesives Derived from Epoxidized Soybean Oil and Phosphorus-Containing Dicarboxylic Acids, ACS Sustainable Chem. Eng., 2017, 5(4), 3353–3361, DOI:10.1021/acssuschemeng.6b03201.
- A. Li and K. Li, Pressure-Sensitive Adhesives Based on Epoxidized Soybean Oil and Dicarboxylic Acids, ACS Sustainable Chem. Eng., 2014, 2(8), 2090–2096, DOI:10.1021/sc5003853.
- J. Fan, Y. Tang, L. Lu, X. Qiu and L. Pan, Pressure-Sensitive Adhesive Tapes with High Peel Strength Derived from Linseed Oil, Macromol. Chem. Phys., 2024, 225(5), 2300331, DOI:10.1002/macp.202300331.
- S. Bunker, C. Staller, N. Willenbacher and R. Wool, Miniemulsion Polymerization of Acrylated Methyl Oleate for Pressure Sensitive Adhesives, Int. J. Adhes. Adhes., 2003, 23(1), 29–38, DOI:10.1016/S0143-7496(02)00079-9.
- A. Badía, A. Agirre, M. J. Barandiaran and J. R. Leiza, Easy Removable and UV Tunable Biobased Waterborne Pressure Sensitive Adhesives, Int. J. Adhes. Adhes., 2021, 108, 102860, DOI:10.1016/j.ijadhadh.2021.102860.
- A. Badía, J. I. Santos, A. Agirre, M. J. Barandiaran and J. R. Leiza, UV-Tunable Biobased Pressure-Sensitive Adhesives Containing Piperonyl Methacrylate, ACS Sustainable Chem. Eng., 2019, 7(23), 19122–19130, DOI:10.1021/acssuschemeng.9b05067.
- A. Badía, A. Agirre, M. J. Barandiaran and J. R. Leiza, Removable Biobased Waterborne Pressure-Sensitive Adhesives Containing Mixtures of Isosorbide Methacrylate Monomers, Biomacromolecules, 2020, 21(11), 4522–4531, DOI:10.1021/acs.biomac.0c00474.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Silica-Like Malleable Materials from Permanent Organic Networks, Science, 2011, 334(6058), 965–968, DOI:10.1126/science.1212648.
- M. Gosecki and M. Gosecka, Boronic Acid Esters and Anhydrates as Dynamic Cross-Links in Vitrimers, Polymers, 2022, 14(4), 842, DOI:10.3390/polym14040842.
- A. Zych, J. Tellers, L. Bertolacci, L. Ceseracciu, L. Marini, G. Mancini and A. Athanassiou, Biobased, Biodegradable, Self-Healing Boronic Ester Vitrimers from Epoxidized Soybean Oil Acrylate, ACS Appl. Polym. Mater., 2021, 3(2), 1135–1144, DOI:10.1021/acsapm.0c01335.
- Y. Shi, Y. Hong, J. Hong, A. Yu, M. W. Lee, J. Lee and M. Goh, Bio-Based Boronic Ester Vitrimer for Realizing Sustainable and Highly Thermally Conducting Nanocomposites, Composites, Part B, 2022, 244, 110181, DOI:10.1016/j.compositesb.2022.110181.
- T. Vidil and A. Llevot, Fully Biobased Vitrimers: Future Direction toward Sustainable Cross-Linked Polymers, Macromol. Chem. Phys., 2022, 223(13), 2100494, DOI:10.1002/macp.202100494.
- J. Wu, X. Yu, H. Zhang, J. Guo, J. Hu and M.-H. Li, Fully Biobased Vitrimers from Glycyrrhizic Acid and Soybean Oil for Self-Healing, Shape Memory, Weldable, and Recyclable Materials, ACS Sustainable Chem. Eng., 2020, 8(16), 6479–6487, DOI:10.1021/acssuschemeng.0c01047.
- C. Li, Y. Chen, Y. Zeng, Y. Wu, W. Liu and R. Qiu, Strong and Recyclable Soybean Oil-Based Epoxy Adhesives Based on Dynamic Borate, Eur. Polym. J., 2022, 162(6058), 110923, DOI:10.1016/j.eurpolymj.2021.110923.
- S. Zhang, T. Liu, C. Hao, L. Wang, J. Han, H. Liu and J. Zhang, Preparation of a Lignin-Based Vitrimer Material and Its Potential Use for Recoverable Adhesives, Green Chem., 2018, 20(13), 2995–3000, 10.1039/c8gc01299g.
- Y. Xu, S. Dai, H. Zhang, L. Bi, J. Jiang and Y. Chen, Reprocessable, Self-Adhesive, and Recyclable Carbon Fiber-Reinforced Composites Using a Catalyst-Free Self-Healing Bio-Based Vitrimer Matrix, ACS Sustainable Chem. Eng., 2021, 9(48), 16281–16290, DOI:10.1021/acssuschemeng.1c05586.
- K. M. Herbert, N. D. Dolinski, N. R. Boynton, J. G. Murphy, C. A. Lindberg, S. J. Sibener and S. J. Rowan, Controlling the Morphology of Dynamic Thia-Michael Networks to Target Pressure-Sensitive and Hot Melt Adhesives, ACS Appl. Mater. Interfaces, 2021, 13(23), 27471–27480, DOI:10.1021/acsami.1c05813.
- L. Pettazzoni, F. Leonelli, A. G. Marrani, L. M. Migneco, F. Vetica, L. Celio, V. Napoleone, S. Alfano, A. Colecchia, F. Amato, V. Di Lisio and A. Martinelli, Self-Healing and Reprocessable Oleic Acid-Based Elastomer with Dynamic S-S Bonds as Solvent-Free Reusable Adhesive on Copper Surface, Polymers, 2022, 14(22), 4919, DOI:10.3390/polym14224919.
- Hyperbranched Polymers, ed. D. Yan, C. Gao and H. Frey, Wiley, 2011. DOI:10.1002/9780470929001.
- Y. Zheng, S. Li, Z. Weng and C. Gao, Hyperbranched Polymers: Advances from Synthesis to Applications, Chem. Soc. Rev., 2015, 44(12), 4091–4130, 10.1039/C4CS00528G.
- P. J. Flory, Molecular Size Distribution in Three Dimensional Polymers. VI. Branched Polymers Containing A—R—Bf-1 Type Units, J. Am. Chem. Soc., 1952, 74(11), 2718–2723, DOI:10.1021/ja01131a008.
- Y. H. Kim and O. W. Webster, Hyperbranched Polyphenylenes, Macromolecules, 1992, 25(21), 5561–5572, DOI:10.1021/ma00047a001.
- B. Testud, D. Pintori, E. Grau, D. Taton and H. Cramail, Hyperbranched Polyesters by Polycondensation of Fatty Acid-Based AB: N-Type Monomers, Green Chem., 2017, 19(1), 259–269, 10.1039/c6gc02294d.
- C. Cui, B. Liu, T. Wu, Y. Liu, C. Fan, Z. Xu, Y. Yao and W. Liu, A Hyperbranched Polymer Elastomer-Based Pressure Sensitive Adhesive, J. Mater. Chem. A, 2022, 10(3), 1257–1269, 10.1039/d1ta08091a.
- T. Jin, H. Zeng, Y. Huang, L. Liu, W. Yao, H. Lei, S. Shi, G. Du and L. Zhang, Bio-Hyperbranched Polyesters Synthesized from Citric Acid and Bio-Polyols and Applications on Wood Adhesives, Polym. Test., 2023, 120, 107974, DOI:10.1016/j.polymertesting.2023.107974.
- P. B. Smilth, B. A. Howell and C. Zhang, US Pat., US20200262974, 2020 Search PubMed.
- C. J. Hawker, R. Lee and J. M. J. Fréchet, One-Step Synthesis of Hyperbranched Dendritic Polyesters, J. Am. Chem. Soc., 1991, 113(12), 4583–4588, DOI:10.1021/ja00012a030.
- D. Hölter, A. Burgath and H. Frey, Degree of Branching in Hyperbranched Polymers, Acta Polym., 1997, 48(1–2), 30–35, DOI:10.1002/actp.1997.010480105.
- E. P. Chang, Viscoelastic Windows of Pressure-Sensitive Adhesives, J. Adhes., 1991, 34(1–4), 189–200, DOI:10.1080/00218469108026513.
| This journal is © The Royal Society of Chemistry 2026 |



