 Open Access Article
Open Access ArticleGreen extraction of cellulose fibers from pineapple crown waste for the development of pH-sensitive bioplastic films based on starch and purple cabbage anthocyanins
Nguyen Bui Anh
Duy
 ab,
Nguyen Thanh
Huy
ab,
Nguyen Thanh
Huy
 ab,
Bui Phuong
Dong
c,
Pham Nguyen Hong
Nhu
c,
Phan Quoc
Phu
ab,
Bui Phuong
Dong
c,
Pham Nguyen Hong
Nhu
c,
Phan Quoc
Phu
 *ab and
Nguyen Chi
Thanh
*ab and
Nguyen Chi
Thanh
 *c
*c
aDepartment of Polymer Materials, Faculty of Materials Technology, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, Dien Hong Ward, Ho Chi Minh City, Vietnam. E-mail: nbaduy.sdh241@hcmut.edu.vn; nthuy.sdh241@hcmut.edu.vn; pqphu@hcmut.edu.vn
bVietnam National University Ho Chi Minh City (VNUHCM), Thu Duc Ward, Ho Chi Minh City, Vietnam
cFaculty of Applied Sciences, Ho Chi Minh City University of Technology and Education, 01 Vo Van Ngan, Thu Duc Ward, Ho Chi Minh City, Vietnam. E-mail: 20130020@student.hcmute.edu.vn; 21130086@student.hcmute.edu.vn; thanhnc@hcmute.edu.vn
First published on 9th December 2025
Abstract
Environmental concerns over plastic waste and food safety have driven the development of smart and biodegradable active packaging materials. This study reports an intelligent bioplastic film capable of real-time monitoring of food freshness. Cellulose fibers (CFs) were extracted from pineapple crown waste through alkali and hydrogen peroxide treatment, followed by citric acid hydrolysis to enhance crystallinity. The extraction yield of cellulose fibers was 48.25 ± 0.37%, with a crystallinity index of 78.54%, confirming the effective removal of amorphous components. The obtained cellulose fibers were incorporated as reinforcing agents into cassava starch films containing a fixed amount of purple cabbage anthocyanin extract (2 mL, 255.49 mg L−1). Mechanical analysis revealed that the optimal cellulose concentration was 16 wt%. The resulting intelligent bioplastic film exhibited an apparent color change from red to green or yellow, consistent with the behavior of an anthocyanin solution. During shrimp storage, the film functioned as a freshness indicator, changing color from purple to blue upon exposure to volatile amines such as trimethylamine (TMA), dimethylamine (DMA), and ammonia (NH3). These findings demonstrate the potential of this intelligent biodegradable packaging for real-time food quality monitoring and environmental sustainability.
Sustainability spotlightThis study addresses the pressing need for sustainable utilization of agricultural residues by transforming pineapple crown waste into high purity cellulose through an environmentally friendly citric acid process. The approach eliminates the use of strong mineral acids, thereby reducing chemical waste, corrosion risk, and water consumption. The recovered cellulose was applied to fabricate starch-based bioplastic films incorporated with purple cabbage anthocyanins, which function as color changing indicators under different pH conditions. This work represents a practical step toward green innovation in the valorization of biomass resources and the development of intelligent and biodegradable packaging materials. The study aligns with the United Nations Sustainable Development Goals 9 (Industry, Innovation and Infrastructure), 12 (Responsible Consumption and Production), and 13 (Climate Action). |
Introduction
During storage, food products are highly susceptible to spoilage due to microbial growth and biochemical reactions such as lipid oxidation and protein degradation.1 These processes generate volatile compounds, including ammonia (NH3), trimethylamine (TMA), and organic acids, which cause fluctuations in the pH of the packaging environment.2 Although packaging helps protect food from external influences, most current materials are synthetic plastics derived from petroleum.3 These materials are not biodegradable and have the risk of chemical contamination in food, raising serious concerns for both human health and environmental sustainability.4–6 To address these challenges, biodegradable intelligent packaging has emerged as a promising and sustainable solution.7–9 Intelligent packaging not only offers physical protection but also integrates sensing capabilities to monitor and respond to biochemical changes occurring in food during storage.10 Among various indicators, the pH was considered a critical parameter due to its direct correlation with food freshness. Therefore, pH-sensitive materials can be effectively employed as visual indicators in active packaging.11Anthocyanins are natural pigments found in abundance in purple plants such as purple cabbage, butterfly pea flowers, and purple sweet potato, and they can change their structure in response to pH, resulting in distinct, visible color changes.12–15 In acidic environments, anthocyanins appear red as the pH decreases, and the color changes to purple, blue, green, or yellow, depending on the degree of alkalinity.16–18 However, to develop pH-responsive packaging films that are also mechanically stable and biodegradable, careful selection and combination of matrix materials is required.
Cassava starch is a natural polysaccharide widely available in Vietnam, known for its good film-forming ability and low cost.19–21 Nevertheless, films made from pure starch typically exhibit poor mechanical strength and high moisture sensitivity.22 Reinforcement with cellulose is therefore necessary to improve the film's durability and stability.23 Among various cellulose-rich sources, pineapple crown waste – a common agricultural by-product in Vietnam – is considered a promising candidate for cellulose extraction.24,25
One significant challenge in utilizing cellulose is its extraction, which traditionally relies on strong mineral acids such as sulfuric or hydrochloric acid.26–28 Although effective, these methods pose risks of environmental contamination and equipment corrosion and require complex neutralization steps.29 According to recent studies, citric acid has demonstrated the ability to effectively remove lignin and hemicellulose while maintaining a high crystallinity index in the cellulose structure.30,31 Moreover, this method generates minimal hazardous by-products, reduces the need for chemical neutralization, and consumes less water. With a moderate acidity of around pH 3.5, citric acid facilitates sufficient hydrolysis while preserving the structural integrity of the cellulose fibers. However, research on the application of citric acid for cellulose hydrolysis from agricultural residues in Vietnam remains scarce, particularly concerning pineapple crown waste. Previous studies have mainly applied this green hydrolysis approach to other biomass sources such as eucalyptus kraft pulp,32 commercial microcrystalline cellulose,33 bean husks,34 and date palm residues.35 Therefore, this study underscores the novelty, practical significance, and potential of this environmentally friendly extraction strategy for the efficient isolation of cellulose from pineapple crown waste.
Based on the above discussion, this study aims to develop an intelligent biodegradable packaging film capable of visually monitoring food freshness through pH-induced color changes. The material design integrates cellulose fibers extracted from pineapple crown waste, a largely unexplored agricultural by-product in Vietnam, using citric acid-assisted hydrolysis as a reinforcing agent, and anthocyanin extracted from purple cabbage as a natural pH-sensitive indicator within a cassava starch matrix. This approach addresses the limited research on eco-friendly cellulose extraction and the application of natural color indicators in starch-based films while valorizing agricultural waste. The outcomes of this study are expected to advance sustainable, intelligent food packaging systems, providing a multifunctional, environmentally friendly solution that aligns with circular economy principles.
Materials and methods
Materials
Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v) ratio to remove most of the hemicellulose and some of the lignin. After treatment, the samples were washed several times with distilled water until the pH was neutral, and then bleached with a 5% hydrogen peroxide (H2O2) solution at 80 °C for 2 hours. Finally, the obtained cellulose fibers were hydrolyzed with 60% citric acid at 90 °C for 6 hours to remove the amorphous regions. After hydrolysis, the samples were washed with distilled water until neutral and freeze-dried at −40 °C for 48 h. The schematic diagram in Fig. 1a shows the cellulose fiber extraction process.
20 (w/v) ratio to remove most of the hemicellulose and some of the lignin. After treatment, the samples were washed several times with distilled water until the pH was neutral, and then bleached with a 5% hydrogen peroxide (H2O2) solution at 80 °C for 2 hours. Finally, the obtained cellulose fibers were hydrolyzed with 60% citric acid at 90 °C for 6 hours to remove the amorphous regions. After hydrolysis, the samples were washed with distilled water until neutral and freeze-dried at −40 °C for 48 h. The schematic diagram in Fig. 1a shows the cellulose fiber extraction process.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) ratio. The extraction was conducted in a tightly sealed glass container at 5 ± 2 °C for 24 hours to minimize anthocyanin degradation. After soaking, the mixture was filtered to remove solid residues, and the anthocyanin-containing extract was collected. The obtained extract was then concentrated using a rotary vacuum evaporator (Buchi R210, Switzerland; ID: RE-01HL) at 40 °C for about 2 hours to remove ethanol and part of the water, leaving 10% of the initial volume. The concentrated purple cabbage extract (PCE) was stored in dark glass bottles at 5 ± 2 °C until further use. A schematic diagram of the anthocyanin extraction process is illustrated in Fig. 1b.
10 (w/v) ratio. The extraction was conducted in a tightly sealed glass container at 5 ± 2 °C for 24 hours to minimize anthocyanin degradation. After soaking, the mixture was filtered to remove solid residues, and the anthocyanin-containing extract was collected. The obtained extract was then concentrated using a rotary vacuum evaporator (Buchi R210, Switzerland; ID: RE-01HL) at 40 °C for about 2 hours to remove ethanol and part of the water, leaving 10% of the initial volume. The concentrated purple cabbage extract (PCE) was stored in dark glass bottles at 5 ± 2 °C until further use. A schematic diagram of the anthocyanin extraction process is illustrated in Fig. 1b.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v), followed by the addition of CFs at different concentrations of 0, 8, 16, and 24 wt% (based on the weight of starch), and labeled as CS 0.0, CS 0.2, CS 0.4, and CS 0.6, respectively. The mixture was ultrasonicated for 15 min at room temperature, then stirred at 400 rpm and heated to 80 °C for 15 min to complete gelatinization. After cooling to 40 °C, 2 mL of anthocyanin extract and 30% glycerol (w/w based on starch) were added. The resulting mixture was stirred for an additional 10 min to ensure homogeneity. To ensure uniform film thickness across all samples, 25 mL of the homogeneous film-forming solution was poured into Petri dishes with a 10 cm diameter, and 20 mL into those with an 8 cm diameter, under identical conditions. The films were then dried at 45 °C for 12 h. A schematic illustration of the film preparation process is shown in Fig. 1c.
20 (w/v), followed by the addition of CFs at different concentrations of 0, 8, 16, and 24 wt% (based on the weight of starch), and labeled as CS 0.0, CS 0.2, CS 0.4, and CS 0.6, respectively. The mixture was ultrasonicated for 15 min at room temperature, then stirred at 400 rpm and heated to 80 °C for 15 min to complete gelatinization. After cooling to 40 °C, 2 mL of anthocyanin extract and 30% glycerol (w/w based on starch) were added. The resulting mixture was stirred for an additional 10 min to ensure homogeneity. To ensure uniform film thickness across all samples, 25 mL of the homogeneous film-forming solution was poured into Petri dishes with a 10 cm diameter, and 20 mL into those with an 8 cm diameter, under identical conditions. The films were then dried at 45 °C for 12 h. A schematic illustration of the film preparation process is shown in Fig. 1c.
Characterization analysis
 | (1) |
 | (2) |
 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (v/v). The sample was then fixed in a dark environment for about 30 minutes at room temperature, and its absorbance was measured at 520 and 700 nm using a UV-vis spectrophotometer. The relative absorbance of the purple cabbage extract was calculated using eqn (4).46
9 (v/v). The sample was then fixed in a dark environment for about 30 minutes at room temperature, and its absorbance was measured at 520 and 700 nm using a UV-vis spectrophotometer. The relative absorbance of the purple cabbage extract was calculated using eqn (4).46| A = (Aλ520 − Aλ700)pH 1.0 − (Aλ520 − Aλ700)pH 4.5 | (4) |
The total monomeric anthocyanin (TMA, mg L−1) content in the extract was calculated using eqn (5).46
 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 L mol−1 cm−1), and L is the cuvette width (1 cm).
900 L mol−1 cm−1), and L is the cuvette width (1 cm).
 | (6) |
 | (7) |
 | (8) |
Young's modulus (E, MPa) is related to its tensile strength and elongation, as shown in eqn (9).39
 | (9) |
L*: lightness, values from 0 (black) to 100 (white),
a*: red–green axis, in which positive values represent red and negative values represent green,
b*: yellow–blue axis, in which positive values represent yellow and negative values represent blue.
To evaluate the degree of color change after pH treatment, the overall color difference (ΔE) was calculated using eqn (10):
 | (10) |
 ,
,  ,
,  is the initial color value of the film (before pH treatment), and L*, a*, and b* are the color values after treatment. Each sample was measured at three random locations to increase the precision and eliminate local errors. This measurement enables accurate quantification of biofilm color changes across different pH environments.
is the initial color value of the film (before pH treatment), and L*, a*, and b* are the color values after treatment. Each sample was measured at three random locations to increase the precision and eliminate local errors. This measurement enables accurate quantification of biofilm color changes across different pH environments.
Results and discussion
Extraction yield of CFs
The extraction yield of cellulose fibers from PCF was 48.25 ± 0.37%, calculated according to eqn (1). This value was higher than those reported for other biomass sources such as corn cob (37.15%),51 wheat straw (36.1%),52 aloe peel (32%),53 and rice husk (30%),54 all of which were processed through acid hydrolysis with inorganic acids. These results confirm that the sequential treatment with NaOH and H2O2, followed by citric acid hydrolysis, provides an effective route for cellulose recovery from pineapple crown waste.Although the yield obtained in this study was lower than that reported by Fitriani et al. for cellulose fibers extracted from the same raw material, pineapple crown fibers (76.23%),55 this difference cannot be explained solely by variations in the original biomass source. It also reflects the distinct extraction and purification strategies adopted. In the above study, a one-step combined treatment using sodium hydroxide (NaOH) and hydrogen peroxide (H2O2) was applied to the pineapple crown fibers for 1 hour before hydrolysis with 3 M sulfuric acid (H2SO4) at 45 °C. This procedure is known to accelerate the removal of amorphous domains and enhance cellulose recovery, though it may be less effective in eliminating hemicellulose and lignin residues. In contrast, the present study employed separate alkaline and bleaching treatments, each conducted for two hours at 80 °C, to ensure more thorough delignification and dehemicellulization, both of which account for a large proportion of pineapple crown fiber biomass.28,56 Before hydrolysis with 60% w/w citric acid at 90 °C for six hours, this process enhances the removal of impurities while preserving the structural integrity of cellulose; however, the repeated washing steps required to remove residual chemicals can lead to partial cellulose loss, resulting in a lower overall extraction yield.
Compared with other studies employing citric acid as a green hydrolyzing agent, the extraction yield obtained in this work falls within the expected range for organic acid hydrolysis. Specifically, Aisy et al. reported the extraction of cellulose nanofibrils (CNFs) from date by-products that had undergone NaOH and H2O2 pretreatments, followed by hydrolysis with 5.5 M citric acid (approximately 80% w/w) at 100 °C for 6 h under ultrasonic assistance, achieving a yield of 51.73%.35 Similarly, Hui Ji et al. utilized pre-bleached bagasse as the feedstock and conducted hydrolysis with 80% w/w citric acid at 100 °C for 4 h, resulting in a CNF yield of 63.4%.38 The slightly lower yield observed in the present study can be attributed to the absence of ultrasonic enhancement and the adoption of milder hydrolysis conditions (60% w/w citric acid, 90 °C, 6 h), which were deliberately selected to minimize cellulose degradation while maintaining a practical balance between the recovery yield and economic feasibility. Overall, these findings substantiate that citric acid hydrolysis represents a sustainable and effective alternative to mineral acids for cellulose extraction from pineapple crown waste.
Moisture content of CFs
The moisture content of cellulose fibers extracted from pineapple crowns was determined using eqn (2).57 The analysis yielded an average moisture content of 11.47 ± 0.58%.Scanning electron microscopy
The morphological changes on the surface of PCF and CFs were analyzed by scanning electron microscopy, as illustrated in Fig. 2. As shown in Fig. 2a and b, PCF had a rough surface, and the cellulose chains tended to aggregate into fiber bundles. This behavior was attributed to the presence of compounds such as lignin, hemicellulose, pectin, and other non-cellulose substances, which acted as binders.58 In contrast, the CFs observed in Fig. 2c and d have a well-defined fibrous structure with a smooth and uniform surface. These findings indicated that chemical treatment, especially hydrolysis with citric acid, effectively removed amorphous impurities.59 The CFs had an average diameter in the range of 4.21–5.18 µm, as shown in Fig. 3. Compared with the study of Kassim et al., the cellulose fibers were extracted from the same plant source through hydrolysis with H2SO4, and the average fiber diameter was 3.66 µm, which is relatively close to the value reported in this study.60 This indicates the potential of using citric acid as an adequate replacement for H2SO4 to reduce its environmental impacts. | ||
| Fig. 2 SEM images of (a and b) fibers from pineapple crown waste at ×300 and ×1000 magnification, respectively, and (c and d) obtained cellulose fibers at ×300 and ×1000 magnification, respectively. | ||
Fourier transform infrared spectroscopy
The FTIR spectra of PCF and CFs are shown in Fig. 4. The absorption bands in the range of 3700–3000 cm−1, with a maximum peak at approximately 3345 cm−1, correspond to the stretching vibration of the hydroxyl group (O–H) present in the chemical structure of the cellulose molecule in both samples. The peaks observed at 2919 cm−1 and 1639 cm−1 are attributed to the stretching vibration of the C–H and O–H groups, respectively. Furthermore, the peak at 1740 cm−1 in the spectrum of PCF is assigned to the stretching vibration of the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the chemical structure of hemicellulose, or from the ester bond of the carboxylic group in the chemical structure of lignin.60 In addition, the absorption peaks at 1050 cm−1 and 892 cm−1 were assigned to the stretching vibration of the (C–O–C) bond in the pyranose ring and the glycosidic bond between glucose units in the cellulose chain. Interestingly, compared to the FTIR spectrum of PCF, the FTIR spectrum of CFs showed a decrease in absorption intensity at 1740 cm−1, meaning that a part of the hemicellulose was removed. Furthermore, the disappearance of the peak at 1245 cm−1, which is typically associated with syringyl-type lignin, was also not observed in the FTIR spectrum of the CFs, indicating that the chemical treatments significantly removed the amorphous components while preserving the chemical structure of the obtained cellulose fibers.55
O) in the chemical structure of hemicellulose, or from the ester bond of the carboxylic group in the chemical structure of lignin.60 In addition, the absorption peaks at 1050 cm−1 and 892 cm−1 were assigned to the stretching vibration of the (C–O–C) bond in the pyranose ring and the glycosidic bond between glucose units in the cellulose chain. Interestingly, compared to the FTIR spectrum of PCF, the FTIR spectrum of CFs showed a decrease in absorption intensity at 1740 cm−1, meaning that a part of the hemicellulose was removed. Furthermore, the disappearance of the peak at 1245 cm−1, which is typically associated with syringyl-type lignin, was also not observed in the FTIR spectrum of the CFs, indicating that the chemical treatments significantly removed the amorphous components while preserving the chemical structure of the obtained cellulose fibers.55
X-ray diffraction
The X-ray diffraction patterns of the PCF and CFs are shown in Fig. 5. The results showed that both PCF and CFs exhibited characteristic diffraction peaks at 2θ values of approximately 15.2°, 16.1°, 22.6° and 34.6°, corresponding to the (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0), (110), (200) and (040) crystal planes, respectively, representing the structure of type I cellulose.61 In addition, the crystallinity index of CFs calculated from eqn (3) was 78.54%, which was significantly higher than that of PCF (36.41%). This indicated that the chemical treatments, especially citric acid hydrolysis, effectively removed amorphous components such as hemicellulose, lignin, and other non-cellulose substances, thereby increasing the crystallinity of the CFs.62 These results are consistent with those reported by Diana Choquecahua Mamani et al.63
0), (110), (200) and (040) crystal planes, respectively, representing the structure of type I cellulose.61 In addition, the crystallinity index of CFs calculated from eqn (3) was 78.54%, which was significantly higher than that of PCF (36.41%). This indicated that the chemical treatments, especially citric acid hydrolysis, effectively removed amorphous components such as hemicellulose, lignin, and other non-cellulose substances, thereby increasing the crystallinity of the CFs.62 These results are consistent with those reported by Diana Choquecahua Mamani et al.63
Crystallinity was considered a critical parameter that determined many mechanical and physicochemical properties of cellulose-based materials. Fig. 6 visually illustrates the crystallinity of the CFs in this study in comparison with previously reported values for cellulose hydrolyzed from various biomass sources using inorganic acids.54,55,60,64–70 This result indicated that, although citric acid was weaker in acidity compared to H2SO4 and HCl, it was still able to disrupt amorphous and paracrystalline regions within the cellulose structure when suitable hydrolysis conditions were applied. The variation in crystallinity among samples was influenced not only by the type of acid used but also by the microstructure of the raw biomass, fiber size, the initial ratio of crystalline to amorphous regions, and specific treatment parameters.71 Achieving a high degree of crystallinity using an organic acid demonstrated the effectiveness and sustainability of the proposed method compared to conventional inorganic acid-based processes.
UV-vis analysis of PCE
The UV-vis absorption spectra of PCE at pH values ranging from 2 to 12 are shown in Fig. 7, showing distinct changes in both the position and intensity of the absorption peaks. These changes reflected the characteristic structural transformations of anthocyanins under varying pH conditions. In the acidic medium (pH 2 to 4), a strong absorption peak at approximately 524 nm was observed, corresponding to the flavylium cation form. This form exhibited a deep red color and was considered the most stable under low pH conditions.72 As the pH was increased to near-neutral levels (pH 5–6), the absorbance at approximately 524 nm decreased significantly. This reduction was attributed to the structural conversion of anthocyanins into colorless forms, such as carbinol bases and chalcones, which showed weak or negligible absorption in the visible region.73 When the pH was further increased to alkaline conditions (pH 8 to 12), the absorption maximum shifted to a longer wavelength (600 to 620 nm). This shift was interpreted as resulting from the formation of quinonoid anion species or degradation products exhibiting blue or green coloration.74 However, the absorbance at high pH values was markedly decreased, indicating that anthocyanins degraded or became less stable under alkaline conditions.75 These results confirmed the pH sensitivity of anthocyanins and suggested their potential as natural indicators in innovative packaging.Anthocyanin content
The UV-vis spectra of PCE at pH 1 and 4.5 are presented in Fig. S1. The total anthocyanin concentration extracted from purple cabbage using ethanol solvent was calculated using eqn (4) and (5) as 255.49 mg L−1. Compared to other studies related to the anthocyanin concentration obtained from purple cabbage, this value is lower than that of Abderrahim Bouftou et al. (790.69 mg L−1),76 while it is higher than the value reported by Mohammad Shayan et al. (154 mg L−1).40 The difference in anthocyanin content can be attributed to several factors, including species, growth period, growing conditions, season, soil, and geographical origin, as well as different cultivation and extraction methods.76pH-sensitivity of the colorimetric indicator
The pH sensitivity of the color indicator was evaluated by adding a fixed amount of PCE to buffer solutions with different pH values and to PCE-containing film samples. Fig. 8 shows that the pH-induced color changes in the observed film samples followed a similar trend to PCE. This result suggests that the fabricated bioplastic film has potential application as a pH sensor indicator in the field of innovative packaging. The color changes observed with increasing pH include purple or dark red in acidic environments, fading to red as the pH approaches neutral, and turning green or yellow in alkaline environments. These color change characteristics are consistent with the results reported by Alizadeh-Sani et al.77 | ||
| Fig. 8 (a) Tensile strength, (b) elongation at break and (c) Young's modulus of bioplastic film samples. | ||
Solubility in water and moisture content of bioplastic films
Water solubility and moisture content are two critical indicators for evaluating the stability of bioplastic films in humid environments, as they directly affect the mechanical properties and performance of the indicator film during use. The results presented in Table 1 show that the CS 0.0 film has the highest water solubility (22.74 ± 1.47%) and moisture content (18.35 ± 1.26%), reflecting its strong hydrophilicity and weakening of the polymer network due to the hydroxyl-rich structure of starch and glycerol.78 This makes it susceptible to degradation in aqueous environments and is mechanically unstable. However, when CFs were added in increasing proportions (CS 0.2, CS 0.4, and CS 0.6), both solubility and moisture content were significantly reduced. This demonstrates that cellulose fibers play an essential role in reinforcing the polymer network through hydrogen bonding with the hydroxyl groups of starch and the phenolic functional groups of anthocyanins, thereby reducing the water contact space and limiting water absorption and retention capacity. This trend is consistent with previous studies.79–81 It suggests the potential application of pH indicator films with good water resistance for packaging high-moisture foods such as fresh meat, seafood, pre-cut fruits, and dairy products.| Film samples | Solubility in water (%) | Moisture contents (%) |
|---|---|---|
| a Different superscripts in the column indicate significant differences between bioplastic films at p <0.05. | ||
| CS 0.0 | 22.74 ± 1.47a | 18.35 ± 1.26a |
| CS 0.2 | 17.24 ± 0.96b | 12.92 ± 1.12b |
| CS 0.4 | 16.83 ± 1.12b | 12.28 ± 0.84b |
| CS 0.6 | 16.35 ± 1.28b | 12.52 ± 1.04b |
Mechanical properties of bioplastic films
Fig. 9 and S2 show the mechanical properties of starch-based bioplastic films reinforced with the CFs and anthocyanins from purple cabbage. The results showed that both the tensile strength and Young's modulus of the films increased significantly with the addition of CFs, reaching the highest value at a concentration of 16 wt% (CS 0.4). At this concentration, the tensile strength reached 6.11 ± 0.12 MPa. At the same time, the Young's modulus increased to 318.96 ± 23.24 MPa, approximately 2.7 times higher than that of the unreinforced sample (CS 0.0), reflecting the improvement in both the tensile strength and stiffness of the material. This mechanical improvement is explained by the relatively good dispersion of cellulose fibers in the starch matrix, which promotes the formation of hydrogen bonds between the phases and helps transfer stress effectively.82 However, when the CF concentration increased to 24 wt% (CS 0.6), the tensile strength tended to decrease slightly. The reason may be that the addition of high CF content hindered the interaction between starch molecules, leading to agglomeration and affecting the uniformity of the film.83In contrast, the elongation at break gradually decreased with increasing CF content, indicating that the material became more brittle. Specifically, the CS 0.0 sample had an elongation at break of about 16.63 ± 1.28%, whereas the CS 0.6 sample showed a sharp decrease to about 6.48 ± 0.45%. This implies that at high concentrations, CFs reduced the flexibility of polymer chains, thereby reducing the film's elastic deformation capacity under tensile force. Overall, the CS 0.4 sample exhibited the best mechanical properties, suggesting potential applications in biodegradable packaging.
Color property analysis
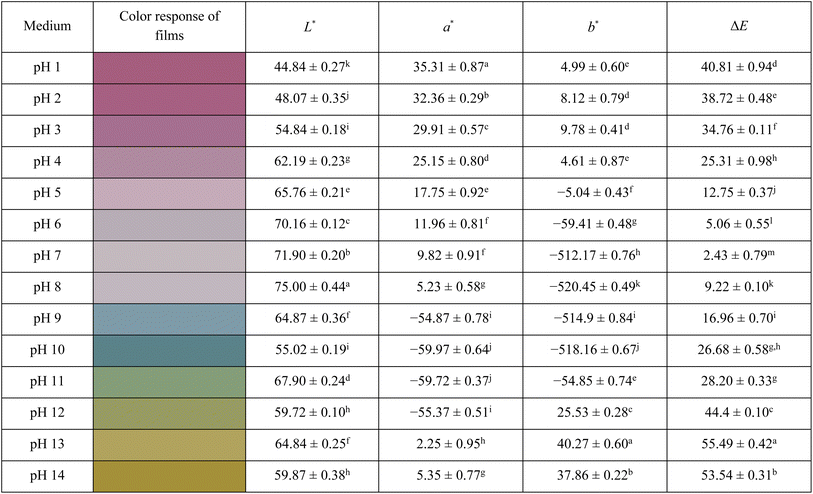
The variation in L*, a*, and b* values clearly reflected the color transition of the CS 0.4 bioplastic film under different pH conditions (Table 2), which was associated with transformations among key anthocyanin molecular structures, including the flavylium cation, quinoidal base, carbinol base, and chalcone. In strongly acidic media (pH 1–3), anthocyanins predominantly existed in the flavylium cation form, characterized by an intense purplish-red color, as indicated by very high a* values (35.31–29.91) and slightly positive b* values (4.99–9.78). Simultaneously, the low L* values (44.84–54.84) suggested dark tones and high light absorption. As the pH increased to the neutral range (pH 4–7), anthocyanins transformed from the flavylium form to unstable intermediates, such as the carbinol base (colorless) and the quinoidal base (purple-blue).84 This was reflected by a sharp decrease in a*, a shift of b* to negative values, and an increase in L*, reaching a peak at pH 7 (71.90), which indicated a lighter color. From pH 8 to 10, anthocyanins mainly existed in the anionic quinoidal base form, resulting in blue to greenish hues, as evidenced by strongly negative a* values (−4.87 to −9.97) and profoundly negative b* values (−20.45 to −18.16).85 Under strong alkaline conditions (pH 11–14), the anthocyanin structure was further transformed into the open-ring chalcone form, a more stable structure in alkaline environments, exhibiting intense yellow coloration. This was confirmed by significantly high positive b* values (25.53–40.27) and slightly positive a* values (2.25–5.35). These color changes are shown in Fig. S3.| a Different superscripts in the column indicate significant differences between films in different pH environments at p <0.05. |
|---|
|
The total color difference (ΔE) provided quantitative insight into the extent of color variation in the film under each pH conditions relative to its initial state.85 Very high ΔE values at pH 1–3 (40.81–34.76) indicated drastic color changes when anthocyanins were mainly in the flavylium form. From pH 4 to 7, ΔE values decreased gradually and reached a minimum at pH 7 (2.43), indicating a color appearance closest to that of the untreated film. However, in alkaline environments (pH 8–14), ΔE significantly increased again, reaching the highest levels at pH 13 and 14 (55.49 and 53.54), due to the presence of yellow-colored chalcone or colorless degradation products. The observed color transitions across various pH levels demonstrated the high sensitivity and reliable color-sensing capability of the developed bioplastic film.
Application of films as a pH indicator for food
The color change of the bioplastic film reflects the quality deterioration of shrimp during storage, as shown in Fig. 10. During the decomposition of seafood, especially shrimp, volatile nitrogen compounds such as trimethylamine (TMA), dimethylamine (DMA), and ammonia (NH3) are produced by the activity of microorganisms and proteolytic enzymes.76 As the storage time increases, the concentration of these compounds in the surrounding space increases and tends to diffuse into the indicator film. The absorption of these alkaline gases increases the density of hydroxyl ions on the film surface, thereby altering the chemical structure of anthocyanin molecules. Specifically, the conversion to the quinoidal base anion form leads to a color change of the bioplastic film from purple to blue,85 and the processes are illustrated in Fig. 11. These interesting findings demonstrate the potential application of pH-sensitive films in monitoring the freshness of shrimp as well as other fresh foods during storage. | ||
| Fig. 10 Color response of bioplastic films containing the anthocyanin extract for real-time monitoring of shrimp freshness at room temperature. | ||
Limitations
Although the study demonstrated the effectiveness of citric acid as a green hydrolyzing agent to replace mineral acids in cellulose fiber extraction and highlighted the potential application of pH-sensitive bioplastic films, several limitations remain to be addressed. Specifically, the hydrolysis process involved a high citric acid concentration (60% w/w) and a prolonged reaction time (6 hours), which may result in significant energy costs if scaled up for industrial production. In addition, although anthocyanins exhibited a distinct color response to pH changes, the long-term color stability of the film under practical storage conditions, such as exposure to light, oxygen, or high humidity, was not evaluated. These factors may influence the reliability and consistency of the colorimetric signal. Furthermore, the color change observed during shrimp storage was assessed only qualitatively. Critical quantitative parameters, such as the limit of detection, response time, and the correlation between color intensity and the concentration of volatile compounds, including trimethylamine and ammonia, were not determined. Migration of compounds from the films into food, including overall and specific migration, was also not investigated and should be evaluated in future studies to ensure food safety. Finally, the long-term stability of the film has not been verified, including its storage lifespan and its ability to retain pH-responsive functionality over time, which are essential considerations for potential commercialization.Conclusion
In this study, a green method was successfully employed to extract cellulose fibers from pineapple crown waste using alkali treatment, hydrogen peroxide bleaching, and citric acid hydrolysis. The obtained cellulose demonstrated a high extraction yield (48.25 ± 0.37%) and a crystallinity index of 78.54%, indicating the effective removal of amorphous components. These fibers were subsequently incorporated into cassava starch films as reinforcement. When 16 wt% cellulose fibers (CFs) were incorporated into the film formulation, the film exhibited significantly enhanced mechanical properties, with a Young's modulus of 318.96 ± 23.24 MPa and a tensile strength of 6.11 ± 0.12 MPa. Furthermore, anthocyanins extracted from purple cabbage were incorporated into the bioplastic film and exhibited excellent color-changing behavior. The bioplastic film labeled CS 0.4 demonstrated enhanced mechanical properties and effective pH-sensing capabilities, suggesting its potential as an intelligent packaging material. Overall, the use of agricultural by-products and natural pigments provided an eco-friendly, promising solution for real-time food freshness monitoring and contributed to advancing sustainable food packaging technologies.Author contributions
Nguyen Bui Anh Duy: conceptualization; performance of experiments; analysis and interpretation of data; writing – original draft; project administration; investigation. Nguyen Thanh Huy: performance of experiments; analysis and interpretation of data. Bui Phuong Dong: collection of raw materials; analysis and interpretation of data. Pham Nguyen Hong Nhu: assistance in conceptualization; performance of experiments. Phan Quoc Phu: validation; review and editing. Nguyen Chi Thanh: supervision; validation; review and editing.Conflicts of interest
There are no conflicts to declare.Data availability
Data supporting this article have been included as part of the supplementary information (SI). Supplementary information: additional characterization results (UV-Vis, tensile test, and color changes). See DOI: https://doi.org/10.1039/d5su00648a.Acknowledgements
We acknowledge the Ho Chi Minh City University of Technology (HCMUT), VNU-HCM, for supporting this study.References
- S. Chen, M. Wu, P. Lu, L. Gao, S. Yan and S. Wang, Int. J. Biol. Macromol., 2020, 149, 271–280 CrossRef CAS PubMed
.
- P. A. V. Freitas, R. R. A. Silva, T. V. de Oliveira, R. R. A. Soares, N. S. Junior, A. R. F. Moraes, A. C. d. S. Pires and N. F. F. Soares, Lwt, 2020, 132, 109780 CrossRef CAS
.
- J. Rajesh Banu and V. Godvin Sharmila, Sustain. Energy Fuels, 2023, 7, 3165–3184 RSC
.
- X. Zhang, X. Chen, J. Dai, H. Cui and L. Lin, Food Packag. Shelf Life, 2023, 40, 101215 CrossRef CAS
.
- M. Anwar, M. E. Konnova and S. Dastgir, RSC Sustain., 2025, 3, 3724–3840 RSC
.
- N. N. T. Tien, H. T. Nguyen, N. L. Le, T. T. Khoi and A. Richel, Food Packag. Shelf Life, 2023, 37, 101084 CrossRef CAS
.
- J. R. Westlake, M. W. Tran, Y. Jiang, X. Zhang, A. D. Burrows and M. Xie, Sustainable Food Technol., 2023, 1, 50–72 RSC
.
- Y. Amaregouda and K. Kamanna, Sustainable Food Technol., 2023, 1, 738–749 RSC
.
- M. Meenu, A. K. Pujari, S. Kirar, A. Thakur, M. Garg and J. Bhaumik, Sustainable Food Technol., 2025, 3, 414–424 RSC
.
- Y. Palanisamy, V. Kadirvel and N. D. Ganesan, Sustainable Food Technol., 2025, 3, 161–180 RSC
.
- R. R. Koshy, A. Reghunadhan, S. K. Mary, K. Thomas, A. Kr, S. Thomas and L. A. Pothen, New J. Chem., 2022, 46, 9036–9047 RSC
.
- M. Raji, L. El Foujji, M. E. M. Mekhzoum, M. El Achaby, H. Essabir, R. Bouhfid and A. e. K. Qaiss, J. Bionic Eng., 2022, 19, 837–851 CrossRef
.
- S. U. Haq, M. Aghajamali and H. Hassanzadeh, RSC Adv., 2021, 11, 24387–24397 RSC
.
- S. K. Mary, R. R. Koshy, J. Daniel, J. T. Koshy, L. A. Pothen and S. Thomas, RSC Adv., 2020, 10, 39822–39830 RSC
.
- L. Prietto, T. C. Mirapalhete, V. Z. Pinto, J. F. Hoffmann, N. L. Vanier, L.-T. Lim, A. R. G. Dias and E. da Rosa Zavareze, Lwt, 2017, 80, 492–500 CrossRef CAS
.
- Q. Gao, R. Ma, L. Shi, S. Wang, Y. Liang and Z. Zhang, Food Funct., 2023, 14, 2034–2044 RSC
.
- S. B. H. Hashim, H. E. Tahir, L. Liu, J. Zhang, X. Zhai, A. A. Mahdi, F. N. Awad, M. M. Hassan, Z. Xiaobo and S. Jiyong, Food Chem., 2022, 373, 131514 CrossRef CAS PubMed
.
- F. Xie, Z. Qin, Y. Luo, Z. He, Q. Chen and J. Cai, Food Chem., 2025, 478, 143560 CrossRef CAS PubMed
.
- A. Ancy, M. Lazar, A. S. Chandran and M. Ushamani, Sustainable Chem. Pharm., 2024, 37, 101377 CrossRef CAS
.
- B. K. Dejene and T. M. Geletaw, Polym.-Plast. Technol. Mater., 2024, 63, 540–569 CAS
.
- A. Surendren, A. K. Mohanty, Q. Liu and M. Misra, Green Chem., 2022, 24, 8606–8636 RSC
.
- N. Pooja and S. Shashank, RSC Adv., 2024, 14, 23943–23951 RSC
.
- Y. Altynov, K. Bexeitova, M. Nazhipkyzy, S. Azat, A. Konarov, D. Rakhman, N. Sahiner and K. Kudaibergenov, Nanoscale, 2025, 17, 12580–12619 RSC
.
- A. A. Reichert, M. R. Sá, T. Castilhos de Freitas, R. Barbosa, T. S. Alves, E. H. Backes, J. H. Alano and A. D. Oliveira, J. Nat. Fibers, 2022, 19, 8541–8554 CrossRef CAS
.
- M. Buvaneswaran, A. Gopan and S. Vr, Biomass Convers. Biorefin., 2025, 1–14 Search PubMed
.
- M. Cheng, Z. Qin, J. Hu, Q. Liu, T. Wei, W. Li, Y. Ling and B. Liu, Carbohydr. Polym., 2020, 231, 115701 CrossRef CAS PubMed
.
- S. M. Mohomane, S. V. Motloung, L. F. Koao and T. E. Motaung, Cellul. Chem. Technol., 2022, 56, 691–703 CrossRef
.
- L. U. S. Faria, B. J. S. Pacheco, G. C. Oliveira and J. L. Silva, J. Mater. Res. Technol., 2020, 9, 12346–12353 CrossRef CAS
.
- B. Ahmed, J. Gwon, M. Thapaliya, A. Adhikari, S. Ren and Q. Wu, Cellulose, 2023, 30, 2895–2911 CrossRef CAS
.
- C. Liu, H. Du, G. Yu, Y. Zhang, Q. Kong, B. Li and X. Mu, Pap. Biomater., 2017, 2, 19–26 CrossRef
.
- N. Kaur, P. Chandel, A. J. Capezza, A. Pandey, R. T. Olsson and N. Banik, RSC Sustain., 2025, 3, 2970–2983 RSC
.
- T. J. Bondancia, J. de Aguiar, G. Batista, A. J. G. Cruz, J. M. Marconcini, L. H. C. Mattoso and C. S. Farinas, Ind. Eng. Chem. Res., 2020, 59, 11505–11516 CrossRef CAS
.
- X. Kang, B. Wang, Y. Ding, L. Xu and Y. Zhang, Ind. Crops Prod., 2024, 219, 119100 CrossRef CAS
.
- M. S. Wahyudi, H. Bahruji, D. Prasetyoko, A. W. Pratama, D. W. Indriani, L. Suryanegara, R. H. F. Faradilla, M. Mahardika, R. K. Wardani and B. Piluharto, Case Stud. Chem. Environ. Eng., 2025, 11, 101179 CrossRef CAS
.
- L. A. R. Aisy, T. Kemala, L. Suryanegara and H. Purwaningsih, Sci. Technol. Indones., 2024, 9, 818–827 CrossRef
.
- N. C. Thanh, N. B. A. Duy, H. T. H. Xuyen, N. T. Huy, B. P. Dong and T. Q. Giang, Polym. Sci., 2024, 66, 376–386 Search PubMed
.
- P. H. F. Pereira, H. L. Ornaghi Jr, V. Arantes and M. O. H. Cioffi, Carbohydr. Res., 2021, 499, 108227 CrossRef CAS PubMed
.
- H. Ji, Z. Xiang, H. Qi, T. Han, A. Pranovich and T. Song, Green Chem., 2019, 21, 1956–1964 RSC
.
- F. W. Hailu, S. W. Fanta, A. A. Tsige and M. A. Delele, Discov. Sustain., 2025, 6, 1–12 CrossRef
.
- M. Shayan, J. Gwon, M. S. Koo, D. Lee, A. Adhikari and Q. Wu, Cellulose, 2022, 29, 9731–9751 CrossRef CAS
.
- M. L. Hassan, L. Berglund, W. S. Abou Elseoud, E. A. Hassan and K. Oksman, Cellulose, 2021, 28, 10905–10920 CrossRef CAS
.
- J. Ahmed, M. A. Balaji, S. S. Saravanakumar and P. Senthamaraikannan, J. Nat. Fibers, 2021, 18, 1460–1471 CrossRef
.
- J. Chandrasekhar, M. C. Madhusudhan and K. Raghavarao, Food Bioprod. Process., 2012, 90, 615–623 CrossRef CAS
.
- K. Devarayan and B.-S. Kim, Sens. Actuators, B, 2015, 209, 281–286 CrossRef CAS
.
- M. M. Giusti and R. E. Wrolstad, Curr. Protoc. Food Anal. Chem., 2001,(1), F1.2.1–F1.2.13 Search PubMed
.
- K. Pusty, K. K. Dash, A. Tiwari and V. M. Balasubramaniam, Food Sci. Biotechnol., 2023, 32, 2025–2042 CrossRef CAS PubMed
.
- D. Thakur, Y. Kumar, V. S. Sharanagat, T. Srivastava and D. C. Saxena, Sustainable Chem. Pharm., 2023, 36, 101236 CrossRef CAS
.
- L. Gao, P. Liu, L. Liu, S. Li, Y. Zhao, J. Xie and H. Xu, Process Biochem., 2022, 121, 463–480 CrossRef CAS
.
- S. Pang, Y. Wang, H. Jia, R. Hao, M. Jan, S. Li, Y. Pu, X. Dong and J. Pan, Int. J. Biol. Macromol., 2023, 230, 123156 CrossRef CAS PubMed
.
- K. Akhila, A. Sultana, D. Ramakanth and K. K. Gaikwad, Food Biosci., 2023, 52, 102397 CrossRef CAS
.
- C. Rovera, D. Carullo, T. Bellesia, D. Büyüktaş, M. Ghaani, E. Caneva and S. Farris, Front. Sustain. Food Syst., 2023, 6, 1087867 CrossRef
.
- S. Sihag, J. Pal and M. Yadav, J. Water Environ. Nanotechnol., 2022, 7, 317–331 CAS
.
- K. J. Nagarajan, A. N. Balaji and N. R. Ramanujam, Int. J. Polym. Anal. Char., 2020, 25, 51–64 CrossRef CAS
.
- A. N. Vu, L. H. Nguyen, H.-C. V. Tran, K. Yoshimura, T. D. Tran, H. Van Le and N.-U. T. Nguyen, RSC Adv., 2024, 14, 2048–2060 RSC
.
- Fitriani, N. A. S. Aprilia and N. Arahman, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 796, 012007 CrossRef CAS
.
- A. Ganesan and J. Rengarajan, Iran. Polym. J., 2024, 33, 1157–1170 CrossRef CAS
.
- D. Agarwal, W. MacNaughtan, R. Ibbett and T. J. Foster, Carbohydr. Polym., 2019, 211, 91–99 CrossRef CAS PubMed
.
- M. A. Guancha-Chalapud, L. Serna-Cock and D. F. Tirado, Appl. Sci., 2022, 12, 6956 CrossRef CAS
.
- P. H. F. Pereira, H. L. Ornaghi Júnior, L. V. Coutinho, B. Duchemin and M. O. H. Cioffi, Cellulose, 2020, 27, 5745–5756 CrossRef CAS
.
- N. A. Kassim, A. Z. Mohamed, E. S. Zainudin, S. Zakaria, S. K. Z. Azman and H. H. Abdullah, BioResources, 2019, 14, 1198–1209 Search PubMed
.
- P. H. F. Pereira, V. Arantes, B. Pereira, H. L. Ornaghi Jr, D.
M. De Oliveira, S. H. Santagneli and M. O. H. Cioffi, Cellulose, 2022, 29, 8587–8598 CrossRef CAS
.
- F. Fitriani, S. Aprilia, N. Arahman, M. R. Bilad, A. Amin, N. Huda and J. Roslan, Polymers, 2021, 13, 4188 CrossRef CAS PubMed
.
- D. Choquecahua Mamani, K. S. Otero Nole, E. E. Chaparro Montoya, D. A. Mayta Huiza, R. Y. Pastrana Alta and H. Aguilar Vitorino, Recycling, 2020, 5, 24 CrossRef
.
- H. A. Silvério, W. P. F. Neto, N. O. Dantas and D. Pasquini, Ind. Crops Prod., 2013, 44, 427–436 CrossRef
.
- P. Panyamao, S. Charumanee, J. Ruangsuriya and C. Saenjum, ACS Sustain. Chem. Eng., 2023, 11, 13962–13973 CrossRef CAS
.
- K. S. Prado and M. A. S. Spinacé, Int. J. Biol. Macromol., 2019, 122, 410–416 CrossRef CAS PubMed
.
- L. Q. Dien and T. K. Anh, J. Jpn. Inst. Energy, 2021, 100, 135–143 CrossRef CAS
.
- M. R. K. Sofla, R. J. Brown, T. Tsuzuki and T. J. Rainey, Adv. Nat. Sci.:Nanosci. Nanotechnol., 2016, 7, 035004 Search PubMed
.
- R. K. Punnadiyil, M. P. Sreejith and E. Purushothaman, J. Chem. Pharm. Sci, 2016, 974, 2115 Search PubMed
.
- G. P. Bernardes, M. de Prá Andrade and M. Poletto, J. Mater. Res. Technol., 2023, 23, 64–76 CrossRef CAS
.
- R. M. Vieira, P. B. Sanvezzo, M. C. Branciforti and M. Brienzo, Bioenergy Res., 2023, 16, 2192–2203 CrossRef CAS
.
- F. Zeng, H. Zeng, Y. Ye, S. Zheng, Y. Zhuang, J. Liu and P. Fei, Food Funct., 2021, 12, 6821–6829 RSC
.
- M. Cheng, X. Yan, Y. Cui, M. Han, X. Wang, J. Wang and R. Zhang, J. Food Eng., 2022, 321, 110943 CrossRef CAS
.
- H. He, Y. Song, M. Li, H. Zhang, J. Li, H. Huang and Y. Li, Anal. Methods, 2023, 15, 228–239 RSC
.
- A. Boonsiriwit, M. Lee, M. Kim, P. Inthamat, U. Siripatrawan and Y. S. Lee, Food Biosci., 2021, 44, 101392 CrossRef CAS
.
- A. Bouftou, K. Aghmih, D. Belfadil, F. Lakhdar, S. Gmouh and S. Majid, Food Sci. Biotechnol., 2025, 34, 337–348 CrossRef CAS PubMed
.
- M. Alizadeh-Sani, E. Mohammadian, J.-W. Rhim and S. M. Jafari, Trends Food Sci. Technol., 2020, 105, 93–144 CrossRef CAS
.
- J. Yan, R. Cui, Y. Qin, L. Li and M. Yuan, Int. J. Biol. Macromol., 2021, 177, 328–336 CrossRef CAS PubMed
.
- W. Zhang, X. Li and W. Jiang, Int. J. Biol. Macromol., 2020, 154, 1205–1214 CrossRef CAS PubMed
.
- C. M. P. Yoshida, V. B. V. Maciel, M. E. D. Mendonça and T. T. Franco, LWT–Food Sci. Technol., 2014, 55, 83–89 CrossRef CAS
.
- M. Shakouri, M. Salami, L.-T. Lim, M. Ekrami, M. Mohammadian, G. Askari, Z. Emam-Djomeh and D. J. McClements, J. Food Meas. Char., 2023, 17, 472–484 CrossRef
.
- J. L. Li, M. Zhou, G. Cheng, F. Cheng, Y. Lin and P. X. Zhu, Starch/Staerke, 2019, 71, 1800114 CrossRef
.
- M. Lubis, M. B. Harahap, M. H. S. Ginting, M. Sartika and H. Azmi, J. Eng. Sci. Technol, 2018, 13, 381–393 Search PubMed
.
- N. A. Azlim, A. Mohammadi Nafchi, N. Oladzadabbasabadi, F. Ariffin, P. Ghalambor, S. Jafarzadeh and A. A. Al-Hassan, Nutr. Food Sci., 2022, 10, 597–608 CrossRef CAS PubMed
.
- K. Zhang, T.-S. Huang, H. Yan, X. Hu and T. Ren, Int. J. Biol. Macromol., 2020, 145, 768–776 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2026 |








