Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microplastic removal from wastewater through biopolymer and nanocellulose-based green technologies
Sayam
Sayam
 a,
Tarikul
Islam
a,
Tarikul
Islam
 *bc,
Tasnim Hanan
Tusti
d and
Joyjit
Ghosh
c
*bc,
Tasnim Hanan
Tusti
d and
Joyjit
Ghosh
c
aDepartment of Fabric Engineering, Barishal Textile Engineering College, Barishal 8200, Bangladesh
bDepartment of Textile Engineering, Jashore University of Science and Technology, Jashore 7408, Bangladesh
cDepartment of Textiles, Merchandising, and Interiors, University of Georgia, Athens, Georgia 30602, USA. E-mail: tarikul@uga.edu
dDepartment of Civil Engineering, Khulna University of Engineering & Technology, Khulna 9203, Bangladesh
First published on 28th October 2025
Abstract
Microplastics (MPs) in wastewater are a growing environmental issue that needs effective solutions. This review examines the use of nanocellulose and biopolymers as sustainable options for removing these pollutants from water. Nanocellulose (NC) is efficient due to its large surface area and biodegradable nature, achieving up to 98% removal of microplastics through various processes, including adsorption and filtration. Similarly, biopolymers like polysaccharides, lignin, and pectin can remove up to 99% of particles by clumping and settling them out. However, some microplastics are not easily removed by these materials on their own. Combining different materials, such as cellulose and chitosan, can enhance removal efficiency to about 75%. Integrating these solutions into existing wastewater treatment plants could help reduce microplastics and save costs; however, it is essential to ensure compatibility with current systems and establish appropriate regulations. The review also highlights the need for future research to support the widespread use of these methods in water treatment.
Sustainability spotlightThis review addresses the sustainability challenges associated with microplastic contamination in wastewater and highlights green technologies for their removal. It focuses on nanocellulose and biopolymer-based materials derived from renewable resources, emphasizing their biodegradability, low toxicity, and potential to replace synthetic treatment agents. The paper evaluates the environmental benefits of adsorption, coagulation, and filtration mechanisms compared to conventional chemical methods, outlining strategies to reduce secondary pollution and energy demand. By exploring scalable integration into existing wastewater treatment systems, this review offers a pathway toward sustainable water management practices aligned with circular economy principles. |
1. Introduction
Plastic materials first appeared in the late 19th century, with commercial use beginning in 1870.1 Due to their light weight, durability, and corrosion resistance, plastics have rapidly gained popularity across various industries. As a result, plastic production increased nearly 180 times between 1950 and 2018, reaching over 400.3 million tons globally by 2022.2 These synthetic macromolecular polymers, such as polyethylene terephthalate, polyethylene, polypropylene, polyvinyl chloride, polyamides, polystyrene, and polyurethanes, have become indispensable in daily life,3–5 especially in sectors like textiles, construction, motor vehicles, consumer goods, medical, and food packaging.6,7 A detailed overview of plastic evolution and its key historical milestones is presented in Fig. 1.8 | ||
| Fig. 1 Historical timeline depicting the development and use of plastics (created with MS PowerPoint). | ||
Among these sectors, the textile industry is a leading consumer of synthetic polymers. Since 1995, synthetic fibers have surpassed cotton as the most widely used textile material, accounting for approximately 65% of global fiber production by 2020.9 China and India dominate this sector, accounting for 66% and 8% of global output, respectively, followed by Taiwan and the United States, each at approximately 4%.10,11 However, the widespread use of synthetic fibers has led to increasing environmental concerns, particularly regarding MP pollution. Synthetic fibers shed MPs during washing and wear, contributing significantly to the presence of MPs in water bodies. These particles often end up in wastewater treatment systems, where they persist due to their resistance to degradation.
The rising accumulation of plastic waste, primarily from single-use products such as shopping bags, bottles, and lids, has exacerbated global pollution issues.7,12 While some plastics, such as bottles, are recyclable, most single-use items still accumulate in landfills or are incinerated, adding to environmental degradation.13,14 Plastic pollution has now been reported in a variety of ecosystems, including coastlines,15 oceans,16 deep-sea areas,17 and even remote islands.18 Plastics degrade into MPs, which are categorized into primary and secondary types.19–21 Primary MPs are intentionally produced in small sizes (2 to 5 mm), often found in products such as microbeads in cosmetics,21–23 whereas secondary MPs result from the fragmentation of larger plastics through physical abrasion or UV radiation.22–24 These degradation processes can produce particles as small as 1.6 µm, which have been detected in marine environments.25,26 Interestingly, the concentration of MPs in surface ocean waters is lower than expected due to their tendency to aggregate with marine particles and settle into sediments, often facilitated by biofilm formation.27
Wastewater treatment plants (WWTPs) are significant entry points for MPs into the environment. According to Sun et al.,5 MP concentrations in WWTP effluents can reach up to 447 particles per liter, with polystyrene (PS) being among the most detected polymers. Even with tertiary treatment processes (TTPs), effluents may still contain up to 51 particles per liter, and only 24% of WWTPs globally currently implement TTPs. Consequently, daily median MP discharges can reach approximately 2 million particles.
Traditional methods of removing MPs, such as filtration, coagulation, and sedimentation, face limitations in terms of efficiency and sustainability, especially for nanoscale particles.28,29 Filtration becomes energy-intensive when extremely fine pores are required and often suffers from clogging.29,30 Similarly, Zhang et al.31 demonstrated that coagulation and sedimentation techniques are usually insufficient for the complete removal of both micro- and nanoplastics. Moreover, many conventional filtration materials are non-renewable, non-biodegradable, and relatively expensive, raising concerns over their long-term environmental impact.28,32 As a result, biopolymers and NC have emerged as promising, eco-friendly alternatives due to their renewability, biodegradability, high surface area, and ease of functionalization.33–35 These materials, sourced from plant cellulose and crustacean chitosan, can be tailored for effective MP adsorption while minimizing their ecological footprint.33,34,36–40 Furthermore, the biodegradability of NC is critical for its application in removing MPs, as it ensures that the material itself will not persist in the environment or generate secondary pollution after use. This attribute strengthens its role as a genuinely sustainable, high-performing option for long-term water purification technologies.
Given the urgent need for clean water and a sustainable environment, there is increasing research interest in developing renewable membrane materials for pollutant removal. However, despite numerous studies on MP removal, comprehensive evaluations explicitly linking NC and biopolymer-based adsorbents to practical wastewater treatment performance remain limited. Existing reviews often focus narrowly on material synthesis or laboratory-scale performance without addressing integration challenges, cost-effectiveness, or regulatory considerations. Moreover, recent trends—including the push for sustainable textile processing, tightening environmental regulations, and advances in bio-based nanomaterials—underscore the urgent need for a critical synthesis of current knowledge. This review consolidates and critically evaluates recent advances in NC- and biopolymer-based systems for MP removal from wastewater, focusing on adsorption, filtration, and flocculation mechanisms. It links material performance to practical considerations for integrating wastewater treatment and achieving cost-effectiveness. It situates these findings within emerging regulatory and policy contexts to provide insights that guide future research and sustainable implementation strategies.
1.1 Source of the MPs
According to Babaei et al.,41 MPs in water come from numerous sources, which can be classified into primary and secondary categories. Understanading these sources is essential for formulating effective mitigation strategies. To visualize these origins, the primary and secondary MP pathways are depicted in Fig. 2.42 This study also illustrated that various sources voluntarily produce primary MPs, which typically measure less than 5 mm in size. Familiar primary sources include microbeads found in personal care products such as toothpaste, exfoliating scrubs, and several cosmetics. During use, these microbeads are rinsed down the drain and eventually end up in the water. Plastic pellets, commonly referred to as nurdles, serve as essential raw materials in plastics manufacturing. However, nurdles unintentionally leave the environment during handling processes or transportation.43–45 Another major contributor is the use of synthetic fibers and textiles, as their production, usage, and disposal result in considerable microfiber emissions into the environment.46 Microfibers are notably released from garments during washing due to wear and tear, as shown in a study by Parbhakaran et al.,47 which tested polyester and nylon fabrics using brush-washing methods. In contrast, secondary MPs are formed when larger plastic materials degrade into smaller pieces due to environmental factors, including weathering, UV radiation, and physical abrasion.41,47 The degradation of plastic waste, such as bottles, bags, and packaging materials, occurs through exposure to wind, sunlight, and wave action.48,49 | ||
| Fig. 2 Sources and pathways of MP formation. Primary MPs come directly from products like personal care items, textiles, tires, paints, laundry, and plastic manufacturing. Secondary MPs result from fragmentation of larger plastics such as bottles, bags, fishing nets, and shipping waste. Published under the CC-BY License.42 Copyright 2023, The authors. Published by Springer-Verlag GmbH Germany. | ||
In the field of agriculture, plastic mulches degrade over time, releasing MPs into the soil, and animals may ingest these particles when they graze on crop residues mixed with plastic mulch remnants.48,50 WWTPs can eliminate a considerable amount of MPs, but they remain a significant source of discharge. MPs originating from sources like domestic laundry and industrial wastewater can both enter and exit these facilities, ultimately reaching receiving waters.46,51,52 Urban runoff, via stormwater, transports MPs from polluted soil into aquatic environments,53 while atmospheric deposition also contaminates urban water systems.54 Moreover, abrasion of road markings and tire wear generate plastic particles, making them contributors to MP pollution.46,55
Table 1 summarizes the composition, physical form, and size ranges of MPs from various domestic, industrial, and environmental sources, providing context to their diversity and scale. Particular examples and geographical factors further emphasize the matter: in the Han River, China, the presence of MPs varied from 2315 ± 603 to 8406 ± 2055 n m−3, indicating a rising trend throughout the river.56 Research conducted in textile industrial regions has revealed elevated levels of MPs, predominantly consisting of polyester as the primary polymer type.57 Tsang et al.58 illustrated that in Hong Kong, the levels of particles found in marine waters ranged from 51 to 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 909 particles per m3, while in sediments, they varied from 49 to 46
909 particles per m3, while in sediments, they varied from 49 to 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143 particles per kg. A recent investigation conducted in Indiana rivers has highlighted notable MP contamination, emphasizing the extent of this problem in the Midwest's flowing waters.59
143 particles per kg. A recent investigation conducted in Indiana rivers has highlighted notable MP contamination, emphasizing the extent of this problem in the Midwest's flowing waters.59
| Source | Composition | Physical form | Size range | Ref. |
|---|---|---|---|---|
| Facial cleaners | Polyethylene | Spherical | Higher than 0.5 mm | 60 |
| Beverage products | Polyamide, acrylonitrile–butadiene–styrene, poly(ester-amide), poly(ethylene terephthalate) | Fibres, fragments | 0.1–3 mm | 61 |
| Textile factory | Polyester | Fibres | 0.1–1 mm | 62 |
| Plastic mulch | Polyester, polypropylene | Fibres, fragments, foam, films | Higher than 500 µm | 63 |
| Mariculture activities | Polyester, polypropylene, polyethylene, polyamide (nylon), polystyrene, polyetherurethane, polybutylene terephthalate | Fragments, flakes, fibres, foam | Less than 0.25 mm | 64 |
| Anthropogenic activity | Polystyrene, polyethylene, polypropylene | Fibres, styrofoam, fragments, films, pellets | Less than 0.5 mm | 65 |
| Urban sewage | Polyethylene, polystyrene, polypropylene | Fragments, lines, foam, films | 1–4.75 mm | 66 |
| Construction, fishery activities, and human domestic sewage | Polyvinylchloride, polyethylene, polyamide | Fibres, pellets, films, fragments | Less than 0.5 mm | 67 |
| Industrial area | Polyethylene, polypropylene, nylon | Fibres, fragments | 0.1–5 mm | 68 |
| Tertiary industry | Polyethylene, polypropylene, polyacrylonitrile, polyethylene terephthalate | Fragments, fibers, films | 500 µm to 5 mm | 69 |
| Artificial ecosystems | Polyethylene, rayon, polypropylene | Fibres, flakes, films, granules | Less than 1 mm | 70 |
| Plastic industries | Polypropylene, polyester, nylon, polystyrene | Fibres, lines, spherules, fragments/granules, films | Less than 0.5 mm | 71 |
| Shower gels | Polyethylene | Irregular shapes | 422 ± 185 µm | 72 |
| Car tires | Polypropylene, acrylic, nylon, rubber | Fragments, fibres | Higher than 500 µm | 73 |
| Facial scrubs | Polyethylene, polyvinyl chloride | Spherical, irregular, granular | 85 to 186 µm | 74 |
| Cosmetic products | Polyethylene | Irregular, granular, spherical | 54–115 µm | 75 |
| Industrial sources | Polyethylene, nylon, polypropylene | Films, fragments, lines, granules, sheets, lines | 0.5–1.0 mm | 76 |
| Fishing and shipping activities | Ionomer surlyn, acrylic (acryl fibre), polyetherimide, polyphenylene sulphide, ethylene vinyl alcohol, acrylonitrile, nylon, polyisoprene, polyvinyl chloride, ethylene–vinyl acetate, polyurethane | Fibres, pellets, fragments | 1489 ± 1017 µm | 77 |
| Personal care products, facial cleansers, sewage sludge | Polystyrene, polyester, amino thermoset plastic, polyallyl diglycol carbonate | Fragments, pellets, foam, films, lines | 0.355–0.999 mm | 78 |
| Urbanization | Polyethylene, polypropylene | Pellets, fragments, films, lines, foam | 0.3–4.75 mm | 79 |
| Sludge and WWTPs | Polyamide, polyethylene, polypropylene | Fragments, fibres, films, granules | 0.003–0.05 mm | 80 |
| Local inputs, ocean transport | Polypropylene, polyester, polyester, polyethylene | Fibres, flakes, films, granules | 2.0–2.5 mm | 81 |
| Domestic, agriculture effluent, industry, upstream inflow, and airborne settlement | Polyethylene terephthalate, polyethylene, polypropylene, polystyrene, polycarbonate, polyvinyl chloride, cellulose propionate, polyamide, ethylene–vinyl acetate copolymer | Pellets, fragments | 0.05–5 mm | 82 |
| Commercial fish species | Polyethylene terephthalate, polyethylene, polypropylene, polyamide, phthalocyanine | Fibres, fragments | Higher than 215 µm | 83 |
1.2 Impact of MPs on the ecosystem
The ecological impacts of MP pollution are varied and influence organisms at various trophic levels. A wide range of aquatic organisms, extending from plankton to fish, can ingest MPs, resulting in both chemical and physical toxicity.84Fig. 3 shows the transfer of MPs through different trophic levels.85 In addition to the absorption of harmful substances that build up on the surface of MPs, ingestion of MPs may end in physical injury, such as blockages in the digestive tract.86 It can also disrupt food chains by accumulating within organisms, which leads to higher levels that can affect how ecosystems work and are structured.87,88 A study by Moto et al.89 illustrated that MPs have the potential to change habitats, disturb ecological equilibrium, and pollute water and soil. Sing et al.90 indicate that MPs have negative impacts on plant development, soil pollution, and the contamination of subsurface aquifers. The ability of MPs to absorb and interact with organic pollutants can change their toxic effects and complicate treatment efforts.91 The appearance of MPs may facilitate the movement and colonization of particular microbes, resulting in disruptions within the affected ecosystems.921.3 MPs in drinking water
MPs have been found in both tap and bottled water, emphasizing widespread contamination.93,94 The source of MPs in drinking water can vary, including unfiltered water sources, packaging materials, treatment processes, and distribution systems, as depicted in Fig. 4.94,95 A study by Semmouri et al.96 found the presence of MPs in surface water, ground water, and treated sewage water that serve as sources of drinking water. Another study conducted by Zhou et al.97 indicated that the amount of MPs released from plastic cups was around 556.80 ± 31.39 particles/L. In village areas, MPs can contaminate drinking water systems from purification plants and reservoirs. According to Meshram and Mhatre,98 these particles can pass through the intestinal membrane and aggregate in tissues. They have the potential to promote inflammatory responses and oxidative stress within the body.99,100 Sharma et al.100 discovered that MPs can interfere with typical composition and functioning of the intestinal microbiota, which plays a crucial role in immune and digestion response. | ||
| Fig. 4 Schematic representation of human MP exposure through the use of disposable plastic drinking cups. Published under the CC-BY License.95 Copyright 2024, The authors. Published by MDPI. | ||
To further illustrate these impacts, Table 2 summarizes recent findings on MP sizes, affected organisms, and their observed toxicological effects. Also, they can be found in sources of drinking water, presenting a significant risk to aquatic ecosystems.84 Aquatic organisms have the ability to consume MPs, which can result in stunted growth, reduced feeding efficiency, and reproductive toxicity.101,102 MPs may also function as carriers for harmful chemicals, leading to biomagnification and bioaccumulation within the food chain 101. This trophic transfer and its cascading ecological consequences are illustrated in Fig. 5, which outlines the movement of MPs through various food web levels.103
| Origin | MP exposure/size | Effects | Ref. |
|---|---|---|---|
| Emys orbicularis | 500–1000 mg kg−1 | Induced pathological changes in liver and kidney tissues | 104 |
| Ascidian ciona intestinalis | 1 µm | Impaired food uptake efficiency and reduced growth rate | 105 |
| Nematode | 1 µm | Triggered oxidative stress and induced intestinal injury | 106 |
| Crepidula onyx | 2 µm | Growth reduction | 107 |
| Sardinellagibbose | 1 µm | Decreased body weight and altered feeding behavior | 108 |
| Dania rerio | 70 µm | Disrupted normal gut structure | 109 |
 | ||
| Fig. 5 Transfer routes of MPs from water, soil, and biota to the human body. Published under the CC-BY License.110 Copyright 2024, The authors. Published by Springer Nature Switzerland AG. | ||
In addition, nanoplastics, due to their small size (<1 µm), show unique toxicological characteristics and pose risks distinct from those of MPs. They can translocate across biological barriers and accumulate in vital organs, potentially leading to inflammatory responses, oxidative stress, and genotoxicity.111 Moreover, they can interact with cells at the molecular level, affecting gene expression, signaling pathways, and cellular functions.112 A research conducted by Christopher et al.113 found that nanoplastics have been detected in human placentas, indicating maternal exposure and negative effects on early-life development. Exposure routes include inhalation, dermal contact, and ingestion.114 They can also accumulate biota, leading to histological damage, neurotoxicity, and metabolic disruption in fish. Additionally, they can impact soil function and food-chain transfer.115
2. MP detection
The identification of MPs in wastewater involves a series of steps that include sampling, pre-treatment, and separation, followed by identification and quantification. Many methods, such as Fourier-transform infrared spectroscopy (FTIR), pyrolysis-gas chromatography/mass spectroscopy (Py-GC/MS),† and Raman spectroscopy, are used to find and measure MPs in the environment.116–118 A comprehensive overview of these MP detection techniques, including their analytical capabilities, advantages and limitations, as well as the types of polymers identified in biotic and aquatic environments, is presented in Table 3.| Detection methods | Advantages | Limitations | Source and sample type | Size ranges | Identified polymers | Ref. |
|---|---|---|---|---|---|---|
| a Abbreviations: ABS: acrylonitrile butadiene styrene; ALK: alkyd resin; CPH: cellphone; EPM: ethylene–propylene rubber; EP: epoxy resin; EVOH: ethylene vinyl alcohol copolymer; LLDP/Oct: linear low-density polyethylene–octene copolymer; NY: nylon; PA: polyamide; PAN: polyacrylonitrile; PAS: polyacrylate-stryrene; PCL: polycaprolactone; PE: polyethylene; PEA: polyethylacrylate; PET: polyethylene terephthalate; PEVA: polyethylene-vinyl acetate; PMMA: polymethyl methacrylate; PP: polypropylene; PR: phenoxy resin; PS: polystyrene; PES: polyester; PTFE: polytetrafluoroethylene; PU: polyurethane; PVC: polyvinyl chloride; PVA: polyvinyl alcohol; PVAC: polyvinyl acetate; PVS: polyvinyl sulfate; RY: rayon; SAN: styrene–acrylonitrile; SBR: styrene–butadiene rubber; SR: synthetic rubber. | ||||||
| Optical microscopy (MO) and Fourier transform infrared spectroscopy (FTIR) | • Optical microscopy assists in preliminary sorting and size classification, while FTIR provides chemical identification119,120 | • Still labor-intensive and time-consuming due to manual sorting and FTIR analysis steps120 | Indian white shrimps | 0.157–2.785 mm | PA, PES, PE, PP | 121 |
| • Combining methods reduces error rates compared to optical microscopy alone119 | • FTIR spatial resolution limits detection of smaller particles, and visual sorting may miss small or transparent MPs119 | Deep-sea fish | <1 mm | CPH, PA, PET | 122 | |
| • Requires expertise and is prone to subjective bias during visual pre-sorting120 | Bivalve (oyster, mussel, manila clam and scallop) | 0.1–0.2 mm | PE, PP, PS, PES, PEVA, PET, PU | 123 | ||
| Different fish species | 0.2–5 mm | PE, PP | 124 | |||
| Thamnaconus septentrional | 0.04–5 mm | CPH, PET, PES | 125 | |||
| Mussels (Mytilus edulis) | 0.033–4.7 mm | CPH, PET, PES | 126 | |||
| Benthic organisms | 0.05–5 mm | PP, PE, PS, PET, NY | 127 | |||
| Deep benthic invertebrates | 0.023–6.25 mm | ALK, PES | 128 | |||
| Different fish species | 0.656 mm | PS, PE, PP | 129 | |||
| Benthic and pelagic fish | 0.217–4.81 mm | PP, PE, ALK, RY, PES, NY | 130 | |||
| Pelagic and demersal fish | 0.13–14.3 mm | PA, cellulose, RY | 131 | |||
| Whales | 0.3–7 mm | RY, PES, acrylic, PP, PE | 132 | |||
| Atlantic herring, sprat, common dab, and whiting | 0.300–0.400 mm | PMMA | 133 | |||
| Plastic bottled water | 6.5–>100 µm | PET, PP | 80 | |||
| Glass bottled water | >100 µm | PA, PE, PP | 80 | |||
| Cardboard bottled water | >100 µm | Cellulose, PE, PP | 80 | |||
| Fourier transform infrared spectroscopy (FTIR) | • Widely used and well-established technique with extensive reference databases134 | • Cannot identify MPs smaller than about 20 µm (ref. 119) | Ground drinking water | 50–150 µm | PE, PA, PES, PVC | 135 |
| • Difficult to analyze opaque and black MPs134 | Tap water | 100–5000 µm | — | 136 | ||
| Plastic bottled water | 6.5–>100 µm | PP | 138 | |||
| Glass bottled water | 6.5–>100 µm | — | 138 | |||
| Optical microscopy (MO) and Raman microspectroscopy (RMS) | • MO provides rapid visual identification and preliminary sorting based on size, shape, and color; RMS offers detailed chemical characterization and polymer identification | • Both methods can be affected by MP heterogeneity and environmental aging | European anchovies | 0.124–0.438 mm | PE | 139 |
| Bivalves (Mytilus edulis and Crassostrea gigas) | >0.005 mm | — | 140 | |||
| Mussels (Mytilus edulis) and lugworms (Arenicola marina) | 0.015–1 mm | — | 141 | |||
| Glass bottled water | <5 µm | PE, SBR | 142 | |||
| Plastic bottled water | <5 µm | PE | 142 | |||
| Optical microscopy (MO) | • Convenient and economical method | • Accuracy is relatively low; error rates of 20–70% for transparent MPs | Dogfish, hake, red mullet | 0.38–3.1 mm | — | 143 |
| • Simple operation and low cost | • Time consuming and laborious | Semi-pelagic fish | 0.5 mm | — | 144 | |
| • Can identify MPs with particle sizes of hundreds of microns and above119 | • Cannot provide chemical composition information119 | |||||
| Pyrolysis-gas chromatography-mass spectrometry (pyrolysis-GC-MS) | • Capable of analyzing non-volatile macromolecules by breaking them into volatile fragments for identification | • Sample is decomposed during analysis | Ground drinking water | — | PE | 145 |
| • Provides detailed chemical characterization of polymers and additives in MPs | • Requires specialized instrumentation and expertise | |||||
| • Useful for complex samples where other methods may fail146 | • Does not provide physical information such as particle size, shape, or morphology directly146 | |||||
| Fourier transform infrared spectroscopy (FTIR) & micro-Raman imaging microscopy | • FTIR for larger particles and Raman for smaller particles and detailed chemical imaging.134 | • High cost and complexity due to combining two sophisticated instruments134 | Treated water from water treatment plants | 1–10 µm | PET, PP, PE | 147 |
| • Enhances detection accuracy and chemical identification of MPs across a wide size range119,134 | ||||||
| • Automated or semi-automated systems can reduce analysis time and human bias148 | ||||||
The initial phase involves sampling, an essential procedure focused on gathering samples from WWTPs. The accuracy of this phase depends on the type of water, including freshwater, seawater, or wastewater, since timing is crucial to account for temporal and spatial fluctuations in MP levels.149 Sampling techniques can be customized according to the type of water-be it freshwater, seawater, or wastewater-because of the variations in particulate load and composition.150
After sampling, separation and pretreatment steps are carried out to separate the sample from the wastewater matrices. Filtration stands out as the most prevalent method for separation, generally utilizing filters with stainless steel basket filters that employ smaller and smaller mesh sizes (such as 10 µm, 50 µm, and 100 µm), which are effective at capturing different particle sizes.151 The process becomes better through density separation, which requires the differences in density between MPs and other particulate matter. Researchers used high-density salt solutions to enable the plastics to float, while the heavier materials settle.152 Digestion steps eliminate organic matter that could obstruct spectroscopic identification. Agents for chemical digestion, such as HCl, H2O2, and various enzymes, are applied to break down organic components while preserving the structural integrity of plastic particles.149,152 The identification of MPs is primarily carried out using spectroscopic and thermal analytical techniques. FTIR spectroscopy determines the chemical composition of MPs through the analysis of infrared absorption patterns. Using micro-FTIR along with imaging allows for a close-up and detailed study of individual particles.116,150,153 Raman spectroscopy, a widely used technique, provides information at the molecular level by analyzing rotational and vibrational modes. More advanced methods, such as Surface Enhanced Raman Spectroscopy (SERS), drastically improve detection sensitivity.153–155 Pyrolysis-GC/MS, functioning as a thermoanalytical method, thermally breaks down MPs into smaller molecular fragments for subsequent identification and separation, which is particularly useful for complex or mixed samples where direct spectroscopic identification is difficult.109,155.
Methods of quantification involve both chemical and visual tactics. Optical microscopy is often employed for quantifying microplastic particles, whereas scanning electron microscopy offers a thorough morphological understanding.149,152,153 Research into the topic of spectroscopic quantification continues, utilizing FTIR and Raman spectral data to determine the mass of MPs, although this area is still under development.156 Pyrolysis-GC/MS provides a more detailed characterization of specific polymers by determining the prevalence of their distinct degradation products.157 Innovative detection methods are consistently improving the precision and effectiveness of MP detection. Micro-flow imaging (MFI)‡ assists in the real-time determination of MPs in situ.158 Hyperspectral imaging uses near-infrared technology and FTIR to provide very detailed images, and when combined with chemometric modeling, it helps quickly and accurately identify different types of plastics.152,159 Xue et al.154 demonstrated that new methods, such as the extreme learning machine paired with differential Raman spectroscopy, combine advanced computer learning with light detection, leading to enhanced sensitivity for biological samples. Furthermore, nanoparticle tracking analysis has been used to measure the number of nanoplastics present and their size, in response to growing concerns about plastic particles that are smaller than one micron.119 A study by Reineccius et al.120 illustrated the MP detection workflow shown in Fig. 6, highlighting key steps such as sampling using multi-corers or plankton nets, freeze-drying and digestion with hydrogen peroxide and acetic acid, separation through syringe cascades and detergent desorption, followed by detailed analysis via Raman spectroscopy.
 | ||
| Fig. 6 Workflow for MP detection: (1) sampling via multi-corers or plankton nets; (2) freeze-drying and digestion with H2O2 and acetic acid; (3) separation using syringe cascade and detergent desorption; (4) analysis by Raman spectroscopy. Published under the CC-BY License.120 Copyright 2021, The authors. Published by Elsevier B.V. | ||
3. NC
NC, the most abundant biopolymer on the planet, is an excellent substrate for the massive production of affordable, ecologically sound water treatment media.121 Based on the origin of raw materials, the synthesis method, and the morphological structure, there are multiple kinds of NC materials. A variety of names have been given to these NC materials, such as nanocrystalline cellulose (NCC), cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), cellulose microfibers (CMFs), cellulose nanofibers, nanofibrillated cellulose (NFC), microfibrillated cellulose, and various combinations of the aforementioned types.122 Each type possesses an identical chemical composition, but due to disparities in sources and extraction techniques, they differ in structure, particle size, crystallinity, and other characteristics.123Table 4 provides a comparative overview of these NC types, highlighting their synthesis methods, surface modifications, mechanical properties, recyclability, and MP removal efficiencies.| Type | Source | Size (nm) | Surface area (m2 kg−1) | Synthesis method | Surface chemistry/modification | Mechanical properties | Removal efficiency (%) | Recyclability/stability | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| NCC | Wood pulp, cotton, BC | Width: 2–5; length: 100–300 | 150–250 | Acid hydrolysis (typically H2SO4), enzymatic pretreatment | Sulfate groups from acid hydrolysis, PEI coating, carboxylation | Rigid rod-like crystals | >98% removal of MPs within 20 min of adsorption | — | Used in membranes, composites for rapid adsorption | 144 and 146 |
| CNCs | Wood pulp, cotton, BC | Width: 3–10; length: 100–300 | Up to 150 | Acid hydrolysis (typically H2SO4), mechanical pretreatment | Sulfated groups, PEI, or polymer coatings | Rigid crystalline rods | >98% removal of MPs; rapid adsorption kinetics | — | Incorporated in filtration membranes, aerogels | 144 and 146 |
| CNFs | Wood, cotton, BC, Agave americana | Diameter: 10–30 | High | Mechanical fibrillation (homogenization), enzymatic/chemical pretreatment (carboxymethylation) | Sulfonation (–SO3H), carboxylation (–COOH), PEI coating | Flexible, forms entangled 3D porous aerogels | 88.8% removal of PS-NH2 MPs; adsorption capacity 586.95 mg g−1 | >78% efficiency after 10 cycles | Used in aerogels, filters | 29, 144 and 145 |
| NFC | Wood pulp, agricultural residues | Diameter: 10–30 | High | Mechanical fibrillation, chemical pretreatment (TEMPO oxidation) | TEMPO-oxidized carboxyl groups | Flexible, entangled fibrils | Comparable to CNFs; good adsorption | Good recyclability | Used in filtration membranes, aerogels | 144 and 145 |
| Micro fibrillated cellulose (MFC) | Bleached wood pulp | Width: 5–30 | Moderate to high | Enzymatic or chemical pretreatment + high-pressure homogenization | — | Moderate mechanical strength | Up to 59% removal efficiency | Low recyclability | Used in preliminary MP removal filtration | 144 and 148 |
| CMF | Various cellulose sources | Diameter: 200–300 | Moderate | Microwave-assisted acid hydrolysis + g_C3N4 synergy (10 min) | — | — | 99% removal with an attapulgite (APT) composite | Moderate | Composite filters for MP removal | 148 |
| Bacterial NC | BC (Acetobacter xylinum) | Diameter: 20–100 | High | Bacterial biosynthesis | Typically unmodified, high purity | Uniform size | — | — | — | 144 and 148 |
| Cellulose benzoate (modified CNCs) | Cellulose esterified in an ionic liquid (AmimCl) | — | — | Esterification in an ionic liquid + CNT/MCNT incorporation | Modified with carbon nanotubes (CNTs), magnetic carbon nanotubes (MCNTs) | — | >97% removal of MPS with CNT/MCNT modification | — | Enhanced π–π interactions and zeta potential for adsorption | 147 |
| CNF-coated delignified wood (CNF-CDW) | Balsa wood | CNF diameter up to 10 to 20; wood pores micron scale | — | Delignification (NaClO2, Na2SO3/NaOH, DES) + CNF coating + CaCl2 crosslinking | CNF-film coating on wood; cross-linked | Flux 1146 L m−2 h−1 | 95.97% removal of MPs | Sustainable, reusable filter | Combined mechanical filtration and adsorption | 28 |
NCC—also referred to as CNCs, nanocrystals of cellulose, or cellulose nanowhiskers—is an exceptionally robust form of NC usually retrieved from cellulose fibrils by acid hydrolysis.123 The extended, malleable, and interwoven NC that can be mechanically separated from cellulose fibrils is called NFC, often referred to as cellulose nanofibrils (CNFs) or nanofibrillar cellulose.124,125 Another sort of NC is bacterial NC, which is primarily manufactured by G. xylinus and can be recovered from the ingestion of tiny molecular carbohydrates by bacteria.126 Hydrolysis with extremely potent acids yields the nanocrystalline particles by breaking local crystalline bonds between nanofibrils and rupturing amorphous domains.127 Cellulose fibers undergo mechanical breakdown into CNFs most frequently by refining, high-pressure homogenization, and grinding.125 Bacterial cellulose (BC) can be produced by Acetobacter aceti, and it can be optimized by the fermentation process.128Fig. 7 shows different types of NC materials.129–137
 | ||
| Fig. 7 Microscopy image of (a) CMFs. Reproduced with permission from ref. 129. Copyright 2021, Springer Nature; (b) cellulose fibers (CFs). Published under the CC-BY License.130 Copyright 2017, The authors. Hindawi; (c) cellulose nanoparticles (CNPs). Reproduced with permission from ref. 131. Copyright 2007, Elsevier Ltd.; (d) CNCs. Published under the CC-BY License.132 Copyright 2018, The authors. Published by MDPI; (e) cellulose nanofibers. Reproduced with permission from ref. 133. Copyright 2009, Royal Society of Chemistry; (f) BC and (g) cellulose nanofibers assembled into a material stronger than spider silk. Published under the CC-BY License.134 Copyright 2018, The authors. Published by American Chemical Society; (h) electrospun cellulose nanofibers. Reproduced with permission from ref. 135. Copyright 2012, Elsevier Ltd.; (i) cellulose aerogels. Published under the CC-BY License.136 Copyright 2018, The authors. Published by MDPI. | ||
However, the synthesis of various NC materials faces several challenges primarily related to processing conditions and material properties. Traditional methods for NC preparation, such as mechanical, biological, and chemical treatments, have limitations including long treatment times, high energy consumption, and environmental concerns.138 For instance, the production of CNCs often involves acid hydrolysis, which requires careful control to avoid degrading the cellulose while removing amorphous regions.139,140 CNFs, produced via mechanical methods, can suffer from high energy consumption during fibrillation.141 BNC, although pure, requires specific culture conditions and can be costly to produce at scale.142 Modified CNCs, like cellulose benzoate, and composites like CNF-CDW require additional chemical modification steps that can introduce complexities in maintaining structural integrity and desired properties.143
3.1 NC characteristics relevant to MP removal
3.2 Mechanisms of MP removal using NC
The application of NC in MP remediation operates through several key mechanisms. Fig. 8 provides a schematic overview of four mechanisms such as adsorption, filtration, aggregation, and surface modification, each of which is elaborated below. | ||
| Fig. 8 Schematic representation of four NC-based mechanisms for MP removal (created with MS PowerPoint). | ||
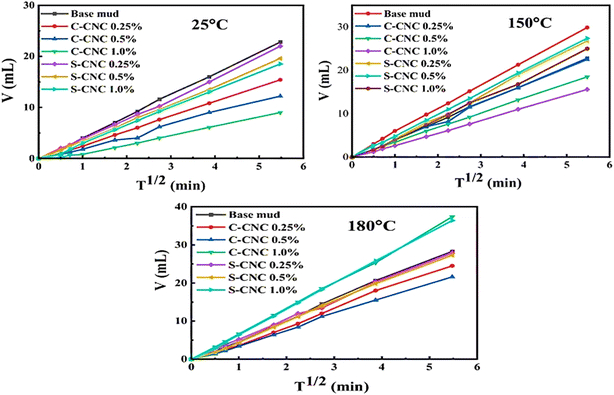 | ||
| Fig. 9 Filtration volume (V) versus square root of time (T1/2) for base mud and CNC-modified muds (C-CNC and S-CNC) at 25 °C, 150 °C, and 180 °C, demonstrating the influence of CNC concentration and temperature on fluid loss behavior. Published under the CC-BY License.170 Copyright 2024, The authors. Published by Springer Nature B.V. | ||
 | ||
| Fig. 10 Micrographs of different types of cellulose-based nanofibers. Published under the CC-BY License.177 Copyright 2022, The authors. Springer Nature B.V. | ||
Several techniques are available for surface modification, each offering unique advantages depending on the application. For instance, TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) is a stable nitroxyl radical widely used in surface modification.180 It is frequently employed to modify CNFs by introducing carboxy groups.181,182 These carboxy groups can then be further functionalized; for instance, primary-amine-terminated polyalkylene glycol (PAG) can react with carboxy groups in TEMPO-CNFs to form amide bonds, improving thermal and dimensional stability.181 Another surface modification technique is amine functionalization, which involves introducing amine groups (–NH2) onto the surface of a material.183 A common method for amine functionalization is silanization using 3-aminopropyltriethoxysilane (APTES).184,185 The ethoxy groups in APTES react with hydroxyl groups on the surface, forming stable siloxane (Si–O–Si) covalent bonds.184 Other surface modification techniques such as cationic surface functionalization using cetrimonium bromide can improve interfacial interactions between CNCs and polymeric matrices in nanocomposites.186
4. Biopolymers for MP removal
Biopolymers are gaining increasing attention as sustainable materials for several applications, including MP removal from wastewater. These polymers offer eco-friendly alternatives to traditional synthetic polymers due to their renewability and biodegradability. Various types of biopolymers have shown promise in MP removal, as shown in Fig. 11 and detailed in Table 5.| Biopolymer | Source | Functional groups | MP removal efficiency | Mechanism | Ref. |
|---|---|---|---|---|---|
| Chitosan | Derived from chitin in crustaceans (shrimp, crab), fungi, insects | –NH2, –OH (primary hydroxyl groups at the C-6 position and secondary hydroxyl groups at the C-3 position) | >90% (depends on pH, dosage, type of MP) | Electrostatic attraction (between cationic amino groups and anionic MPs), H-bonding, van der Waals; enhanced by grafting/cross-linking | 187–189 |
| Alginate | Brown seaweed (phaeophyceae), bacteria | –COOH, –OH | 62–99.79% | Flocculation, electrostatic interaction, hetero-aggregation, physical encapsulation in a Ca-alginate matrix | 190–193 |
| Starch | Corn, potato, wheat, cassava | –OH, N+(CH3)3 (cationic modified) | Up to 90% with additives | Charge neutralization, bridging, flocculation, physical entrapment; performance influenced by particle density & age | 194–197 |
| Polysaccharides | Plants, algae, animals (e.g., laminarin, starch, alginate, chitosan) | –COOH, –OH, –NH2 | 93–99% (e.g., laminarin gels) | H-bonding, sweeping/entrapment, bridging, van der Waals, electrostatic interactions | 195, 197 and 198 |
| Lignin | Wood, pine bark, Miscanthus, paper industry byproduct | –OH (phenolic/aliphatic), –COOH, OCH3 | Raw: up to 26%; modified: up to 99% | π–π stacking, hydrophobic interaction, H-bonding, electrostatic, flocculation (hydrogels, Fe-biochar), magnetic separation | 199–202 |
| Pectin | Citrus fruits, apples | –COOH, –OH | 93–99% (gel systems with coagulants) | H-bonding, sweeping/entrapment, bridging, van der Waals, coagulation & flocculation | 198 and 203 |
| Xanthan gum | Fermentation by Xanthomonas campestris | –OH, –COOH, acetyl groups | >90% (coagulant blends) | H-bonding, electrostatic interactions, bridging, gel floc entrapment; can be enhanced via chemical modification | 204, 205 and 197 |
| Carrageenan | Red algae (e.g., Kappaphycus, Gigartina) | –OSO3− (sulfate), –OH, –OCH3 | >90% (gels and hydrogels) | Physical entrapment in the gel matrix, electrostatic attraction (sulfated groups), H-bonding | 206 and 207 |
| Cyclodextrins | Enzymatic conversion of starch (corn, potato) | –OH (primary and secondary), hydrophobic cavity | >80–90% (for organics) | Host–guest inclusion, H-bonding, van der Waals; enhanced when in composites for aggregation/flocculation | 208 and 209 |
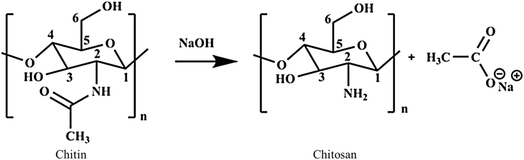
4.1 Chitin and chitosan
Chitosan, a naturally derived biopolymer, is a modified form of chitin.210 It is the most abundant natural polysaccharide found in the exoskeletons of crustaceans (crab, shrimp, and lobster), insects, and fungal cell walls.211,212 The deacetylation of chitin yields chitosan.211,213,214 The structure of chitosan features amino and hydroxyl groups, as depicted in Fig. 12, which play an important role in its ability to interact with pollutants.214,215 The amino groups on the structure of chitosan become positively charged under acidic conditions, facilitating electrostatic interactions with negatively charged MPs.213 This leads to flocculation and coagulation, where small MPs aggregate into larger flocs that can be easily separated from the water.216 The porous structure and reactive functional groups of chitosan enable it to adsorb MPs, effectively trapping them within its matrix.217 Its effectiveness in MP removal stems from its ability to act as an adsorbent and bioflocculant.34,216 As a bioflocculant, chitosan promotes the aggregation of MPs, leading to their sedimentation or easier removal through filtration.216,218 Additionally, shaping chitosan into beds increases its surface area and makes it easier to handle and regenerate.212 Nano-sized chitosan exhibits a high surface area and improved adsorption capacity, and higher MP removal efficiency.2194.2 Alginate
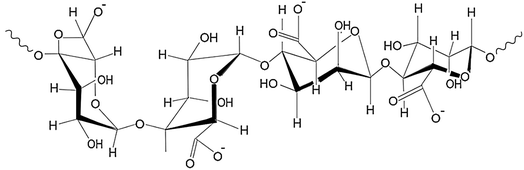
Alginate, a naturally occurring biopolymer extracted from brown algae,220 is a developing sustainable and efficient solution for MP removal from wastewater.220,221 Its biocompatible, cost-effective, and nontoxic features make it suitable for various applications.222 It is a linear polysaccharide composed of two uronic acid monomers, guluronic acid and mannuronic acid.223 The ability of alginate to form gels in the presence of divalent cations, such as calcium ions, is a key property utilized in MP removal.224 Zhang et al.221 showed that calcium alginate hydrogel can be used as a flocculant to remove MPs from water, with a removal efficiency of up to 99.5% for certain MPs. Although the effectiveness of this method can vary depending on the composition and size of the MPs. For more effective removal, Wang et al.222 demonstrated that alginate can be combined with other materials to form composites with enhanced adsorption capabilities. Additionally, alginate can be used to entrap other materials, such as nano-zerovalent iron, to degrade organic pollutants through advanced oxidation processes.225 The functional groups present in alginate can also interact with the surface of MPs, enhancing their removal.152 It can be processed into various useable forms, such as beads or membranes, making it versatile for water treatment technologies.226Fig. 13 illustrates the molecular structure of alginate.2274.3 Starch
Starch is an emerging biopolymer for MP removal from wastewater due to its availability, biodegradability, and low cost.228,229 Modified and unmodified forms of starch are being explored for their ability to capture and remove MPs through various mechanisms, offering an environmentally friendly alternative to traditional methods.194 Gao et al.194 illustrated that cationic-modified starch (CS) is efficient as a bio-coagulant for removing MPs of different sizes, types, and aging conditions under different water conditions. Additionally, starch can destabilize MP suspensions, causing them to aggregate and form larger flocs that can be easily removed.194,230 Another research conducted by Amin et al.231 combined clay and cationic starch to enhance biodegradability and cost-effectiveness. Furthermore, ultralight porous sponges made from crosslinking corn starch and gelatin have been developed for capturing micro- and nano-scale plastics, with the added benefit of being enzymatically decomposable to glucose.232Fig. 14 demonstrated that the primary chemical framework of starch-based polymer.2334.4 Polylactic acid
Polylactic acid (PLA) is an attractive alternative to conventional petroleum-based polymers because of its biocompatibility, biodegradability, and renewability.234–236 It can be processed using techniques like extrusion, injection molding, and electrospinning.237,238 This versatility allows for the creation of various membrane structures and forms suitable for different filtration and adsorption methods in MP removal from wastewater, as shown in Fig. 15.239 For instance, 3D printing methods can be used to create PLA membranes with controlled porosity and dimensional stability.234,240 Khalil et al.241 developed asymmetric ultrafiltration membranes based on PLA for the removal of MPs from wastewater. These membranes, characterized by techniques such as FTIR, XRD, SEM, and porosity analysis, have shown high removal efficiencies for organic matter. Furthermore, advanced PLA-based membranes embedded with functionalized nanomaterials, such as positively charged multi-walled carbon nanotubes/graphene oxide (f-MWCNTs/GO) nanohybrids, have been developed to enhance water flux and nutrient removal. For example, the addition of only 1.5% f-MWCNTs/GO nanohybrid into the PLA matrix increased water flux by 74% compared to unmodified membranes and achieved removal rates up to 90.1% for ammonium nitrogen and 71.3% for phosphate ions from raw wastewater.2424.5 Polysaccharides
Polysaccharides can act as bioflocculants, aggregating MPs into larger, settleable flocs. For instance, an extracellular polymeric substance produced by a freshwater Cyanothece sp. strain demonstrated bioflocculant capacity when exposed to micro- and nano-plastics.243 Jagadeesh and Sundaram244 found that modified polysaccharides can absorb MPs onto their surface, facilitating their removal. Furthermore, organosilanes combined with polysaccharides can be used to agglomerate and fix MPs, enabling their removal from water.245 Biochar, which can be derived from polysaccharide-rich biomass, has shown potential for MP removal through mechanisms such as “Stuck,” “Trapped,” and “Entangled” interactions. However, hybrid biochar-sand filters have shown promise as low-cost systems.246 The chemical structure of polysaccharide chains is shown in Fig. 16. Furthermore, acrylamide (AM) cross-linked psyllium polysaccharide (PLP-AM) has been synthesized and used as a flocculant for removing PS, PVC, and PET MPs from water. Under optimal conditions, PLP-AM achieved removal percentages of 92.55% for PS, 93.85% for PET, and 94.31% for PVC.247 Polysaccharides can be extracted from spirulina platensis using hot water, followed by chitosan flocculation treatment to remove impurities.248,2494.6 Lignin
The complex structure and abundant functional groups of lignin make it a viable option for adsorbing pollutants, including MPs, from wastewater.250,251 Studies have explored the direct application of lignin as an absorbent for MPs. For example, organosolv lignin derived from Miscanthus sp., pine bark, and solid anaerobic digestates has been evaluated as an adsorbent for various types of MPs.199 Additionally, lignin-based materials, especially when processed into activated carbons or porous structures, offer a high surface area and porosity.252,253 MPs can become entrapped within these pores through physical adsorption.253 Cationic lignin polymers can neutralize the negative surface charges commonly found on MPs in wastewater.254 This charge neutralization reduces repulsive forces between MP particles, allowing them to aggregate.255 Furthermore, lignin polymers can act as bridges between MP particles, linking them together to form larger flocs.254 These larger flocs are then easier to remove via sedimentation or filtration.256,257 Kaur et al.258 demonstrated that lignin can degrade MPs under light irradiation. Lignin can be chemically modified with [2-(methacryloyloxy)ethyl]trimethylammonium chloride (METAC) or acrylic acid to improve its charge density, water solubility, and molecular weight, improving its flocculation performance.254,255,259 For example, introducing cationic groups through METAC can enhance the ability of lignin to flocculate negatively charged MPs.255 The underlying polymeric structure of lignin is demonstrated in Fig. 17.5. Coagulation and flocculation mechanisms
Coagulation and flocculation are crucial processes for removing MPs from wastewater.260 These processes involve destabilizing suspended particles, including MPs, and aggregating them into larger flocs that can be easily removed through sedimentation or filtration.261,262 Coagulation involves neutralizing the surface charge of the suspended particles, allowing them to aggregate.262,263 Common coagulants such as aluminum-based salts (such as alum) and iron-based salts (such as ferric chloride) destabilize the MPs by reducing the repulsive forces between them, promoting initial aggregation.264,265 Flocculation, on the other hand, involves the addition of polymers that bridge the destabilized particles, forming larger, more settleable flocs.262,266 These polymers, often polyacrylamides, improve the aggregation of MPs, leading to improved removal efficiency.267 A research by Awan et al.268 illustrated that high dosages of coagulants can lead to the formation of a precipitate that enmeshes MPs, facilitating their removal. This is often referred to as sweep flocculation. Fig. 18 presents the coagulation–flocculation strategy for MP elimination.269 Flocculation thus complements both adsorption and filtration methods, indicating that multi-stage or hybrid systems may provide the most scalable and sustainable solutions. | ||
| Fig. 18 Schematic overview of coagulation–flocculation in MP treatment. Reproduced with permission from ref. 269. Copyright 2024, Institution of Chemical Engineers. Published by Elsevier Ltd. | ||
However, pH affects the surface charge of MPs and the speciation of the coagulants used in flocculation.270 A research conducted by Ummalyma et al.271 have shown that the surface charge of algal cells changes with pH, which affects flocculation efficiency. For instance, maximum flocculation efficiency (94%) was achieved by changing the medium pH from 8.5 to 12 through addition of NaOH. Similarly, in Yellow River water treatment, the pH influences the coagulation behavior and floc properties when using ferric-based coagulants. The pH also affects the structure of humic acid flocs, where flocs formed at pH 5 with low coagulant dosage exhibited a compact structure and high strength.272 Ionic strength also influences the electrostatic interactions between MPs and flocculants by affecting the electrical double layer surrounding the particles.273 Higher ionic strength can compress the double layer, reducing the repulsive forces between particles and promoting aggregation.274 However, excessive ionic strength can lead to destabilization of the suspension and hinder flocculation.275 Studies have shown that increasing ionic strength accelerates aggregation.274 In a similar way, the ionic strength affects floc formation and growth in polymer-clay flocculation.273,275 The effect of total hardness and ionic strength on coagulation performance has been investigated using titanium tetrachloride, showing that these parameters influence the removal of organic matter.276
The combined effect of pH and ionic strength determines the overall efficiency of flocculation. For instance, in aluminum sulfate-induced microalgae flocculation, changes in pH and ionic strength influence algal flocculation by altering the zeta potential of cells. This relationship is described by the classical DLVO theory, where cells with lower total DLVO interaction energy exhibit higher flocculation.270Table 6 provides a summary of coagulant and flocculant performance for MP removal, including dosage, target MP types, efficiency, and key limitations.
| Coagulant/flocculant | Dosage (mg L−1) | MP type(s) | Removal percentage | Drawbacks | Ref. |
|---|---|---|---|---|---|
| Alum (aluminum sulfate) | 50–100 | HDPE, general MPs | HDPE: 86.6; PS: 67 | High sludge, pH reduction | 277 and 278 |
| Ferric chloride | 50–100 | HDPE, PS | PS: 48 | Corrosive, heavy metal risk, sludge management | 277 and 278 |
| PAC | 0.4 mmol L−1 | PS, PP, PVC, PA, PE, PU | PS: 97 | Cost, residual Al, pH sensitivity | 278 |
| PAC + chitosan | — | PET | PET: up to 90; improved for others | Cost, limited scale, stability issues | 279 |
| Chitosan | 10–40 | PET | PET: up to 90 | Higher cost, limited scale, pH sensitive | 279 |
| Moringa oleifera seed extract (MOCP) | 100–150 | HDPE, PE, pristine & weathered MPs | PE, HDPE: 70–87 | Variable quality, shelf life, less effective for some MPs | 277, 278 and 280 |
| Benincasa hispida extract | 100 | HDPE, general MPs | HDPE: 83.7 | — | 277 |
| Protein-coated sand (f-sand) | — | PE (weathered) | 60 | Lower efficiency, charge reversal issues | 278 and 280 |
6. Adsorption mechanism
Adsorption mechanisms are complex and depend on several factors, including the properties of the MPs, the adsorbent material, and surrounding environmental conditions.281–283 Different MP types have varying chemical compositions and surface properties, which can affect their affinity for pollutants like 4-nonylphenol.284 Additionally, the surface area,284 pore size,285 surface charge,286 and chemical functional groups of the adsorbent material determine its capacity to bind MPs. For example, the linear mycelium of Ganoderma lucidum shows high adsorption efficiency due to its morphology, achieving equilibrium adsorption capacities of 102.92 mg g−1 for polyethylene, 156.39 mg g−1 for polypropylene, and 311.76 mg g−1 for polystyrene.287 Narwal et al.282 showed that interactions between π electrons in aromatic rings can improve if both the MP and adsorbent have aromatic moieties. Another study by Yuan et al.288 showed that 3D RGO has adsorption capacity of polystyrene MPs, showing a distinct porous spatial structure beneficial for adsorption. As shown in Fig. 19, various physicochemical interactions contribute to the adsorption of contaminants by MPs.289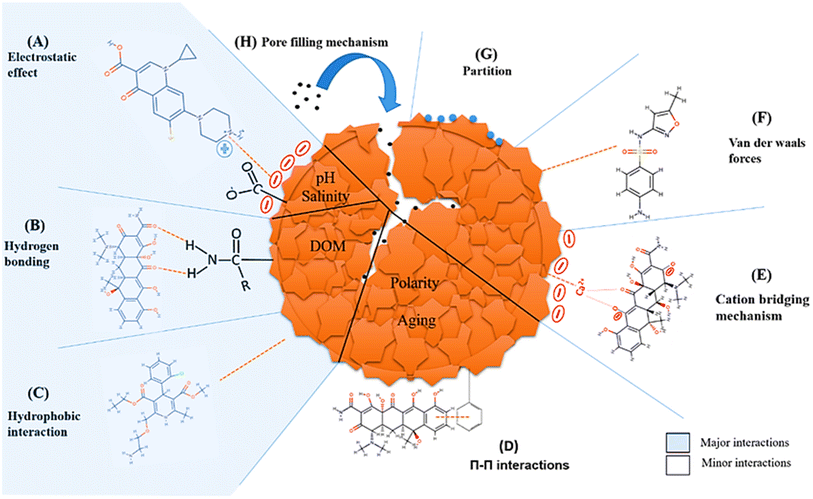 | ||
| Fig. 19 Illustration of interactions involved in the adsorption of pollutants onto MP surfaces, including electrostatic forces, hydrogen bonding, hydrophobic interactions, π–π stacking, cation bridging, van der Waals forces, partitioning, and pore-filling. Published under the CC-BY License.289 Copyright 2022, The authors. Springer Nature Switzerland AG. | ||
To understand the interaction between absorbers and absorbents, adsorption isotherm models are important. They provide a mathematical description of the adsorption process, essential for predicting the behavior of adsorption systems. The Langmuir isotherm is one of the models that assume monolayer adsorption onto a homogeneous surface with identical adsorption sites and no interaction between adsorbed molecules.290–292 The Langmuir model is expresses as:
Several studies have utilized the Langmuir isotherm model for adsorption processes. For example, the adsorption of Cd and landfill leachate on wood-derived biochar was analyzed, and the Langmuir model was one of the isotherms applied.293 Similarly, the adsorption of nitrate onto solid olive mill residues was examined using the Langmuir isotherm, yielding the equation y = 0.007x + 0.4576 with R2 = 0.9787, indicating a good fit.294 In another study, the adsorption of benzene onto grass-derived biochar was modeled using the Langmuir isotherm, resulting in KL = 0.008, qm = 238.9, and R2 = 0.934.292 Another mathematical model is the Freundlich isotherm, which is an empirical model that describes adsorption on heterogeneous surfaces, where adsorption energy varies across different sites.290–292 The Freundlich model is expressed as:
| qe = KFCe1/n |
Zand and Abyaneh293 found that the adsorption of Cd from landfill leachate and the adsorption of nitrate onto solid olive mill residues fit the experimental data with the equation y = 0.3888x + 2.5552 and R2 = 0.9816.294 The adsorption of benzene onto grass-derived biochar was also modeled using the Freundlich isotherm, resulting in parameters KF = 8.065, n = 1.778, and R2 = 0.963.292 While Langmuir and Freundlich isotherms are commonly used, other models such as the Temkin, Redlich–Peterson, Sips, Toth, Dubinin–Radushkevich, and Koble–Corrigan isotherms can also be employed to describe adsorption processes, especially when the assumptions of Langmuir and Freundlich models are not fully met.291,292,295–297
7. Synergistic application of NC and biopolymers
Biopolymers combined with NC form composite materials with enhanced properties for MP removal.298 For instance, the integration of NC and chitosan results in synergistic effects, increasing overall adsorption capacity and selectivity for MPs.299 Wu et al.300 developed a sustainable, environmentally adaptable adsorbent through the supramolecular self-assembly of chitin and cellulose. This biomass-based fibrous framework exhibited excellent adsorption performance across various MP types. Similarly, Mok et al.301 demonstrated that reusable polyvinyl alcohol/chitin/NC biopolymer composite films, crosslinked with maleic acid, effectively removed both MPs and methylene blue dye. Another renewable and abundant biopolymer, starch, when combined with NC, shows improved mechanical properties and enhanced MP capture and retention capabilities.302 Additionally, composite aerogels made from activated carbon and NC blended with cross-linked biopolymers such as hydroxypropyl methylcellulose and chitosan have been evaluated for their ethylene gas adsorption capacity, as illustrated in Fig. 20.303 Although this study focused on gas removal, the materials' high porosity and surface area suggest strong potential for MP adsorption applications as well.298 The synergistic application of NC and biopolymers is commonly used for those MPs that cannot be eliminated through the sole biopolymer or NC. Fig. 21 shows the removal efficiency of NC, biopolymer, and synergistic materials.304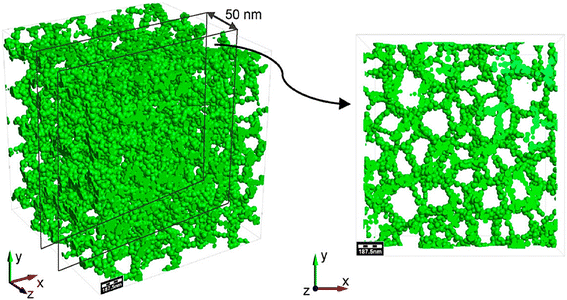 | ||
| Fig. 20 Aerogel structure comprises atom or nanoparticle clusters forming a solid network (green), with interstitial spaces representing its pores. Published under the CC-BY License.303 Copyright 2018, The authors. Published by MDPI. | ||
 | ||
| Fig. 21 Removal efficiency comparison across NC, biopolymer, and synergistic materials (created with MS Excel). | ||
Despite the advantages, NC and biopolymers face compatibility challenges. Many biopolymers are hydrophilic, while some NC modifications can introduce hydrophobic characteristics, leading to poor compatibility.179 Poor interfacial bonding can degrade the mechanical properties of the composite material, reducing its overall effectiveness in MP removal.305 Additionally, NC has a tendency to aggregate due to strong hydrogen bonding between individual fibers.306 Uniform dispersion on the NC matrix is important for achieving optimal functional properties.307 Incomplete dispersion can lead to stress concentration points and reduced performance of the composite material.308,309
8. Sustainability of NC and biopolymer-based treatment systems
The advancement of renewable, sustainable, biodegradable, environmentally sound, safe, and sustainable materials depends on the fabrication of NC-based products.124 NC can be created from bacteria, plants, or biomass using relatively straightforward, scalable, and effective isolation methods. The most common nanostructured component found in wood, cotton, hemp, flax, and other plant-based materials is cellulose, which is biodegradable and non-toxic. One can extract NC from renewable resources via mechanical, chemical, enzymatic, or a combination of these methods. Source, isolation method, and possible surface changes all affect the characteristics of NC.310 Ali et al.311 demonstrated that materials based on NC work well as adsorbents for pollutants in water. By enriching the surface of materials like NC, water contaminants can be eliminated. Water treatment methods, especially adsorption, are required to meet the growing demand for clean water and to mitigate pollution caused by bottled water waste. However, adopting the right adsorbent is an important issue for water treatment since it necessitates striking a careful balance between natural components, high removal effectiveness, ease of separation from treated water, environmental friendliness, and economic viability. As a result, there is a lot of interest in creating innovative adsorbent films, such as bacterial NC-clay film, for use in water treatment.312 NC is a prime contender to enable novel, sustainable, and affordable water purification technologies because of its special physicochemical characteristics, which include high surface area, several functions, nontoxic and biodegradable characteristics, and scaffolding stability and versatility.313 Academic research discussing the cutting-edge advantages of using NC-based membrane filtration processes has grown exponentially, reflecting the predicted success of NCs in the field of improving water quality through membrane filtration processes.314 This move to a bio-based solutions solve our worldwide plastic pollution problem and offers a chance for a circular economy, which would decrease reliance on fossil fuels and create a cleaner environment.315In the case of biopolymers, the most prevalent kind of biobased polymer, polyhydroxyalkanoate (PHA), is made by a variety of microorganisms and acts as a carbon and energy storage medium.316 PHA is a viable substitute for non-biodegradable petro-based plastics due to its biodegradability and advantageous material properties.317 Prior research explored the ability of synthetic biomaterials to absorb or immobilize pollutants, as well as their adsorption capabilities for eliminating a variety of impurities and enhancing water quality.318
However, carbon footprint analysis plays a crucial role in assessing the overall environmental impact of NC and biopolymer production and application.319,320 This analysis involves quantifying greenhouse gas emissions throughout the entire life cycle, from raw material extraction to end-of-life disposal.321,322 In this context, particular attention should be given to electricity and chemical consumption, which are critical sub-aspects influencing carbon footprint growth.319 To enhance sustainability, the choice of disposal methods for NC and biopolymer-based products is also significant.323 Therefore, sustainable end-of-life management should align with circular economy principles, emphasizing the safe and economical recycling and reuse of composite plastics.324 Additionally, employing energy-efficient manufacturing processes, such as chemo- and bio-mechanical methods, can substantially reduce the energy requirements for NC production.325
Moreover, several Life Cycle Assessment (LCA) studies have compared synthetic polymers with biopolymer/NC-based materials to evaluate environmental trade-offs.148 For instance, the LCA of garbage bags made from polyethylene (PE), biomass polyethylene (Bio-PE), and poly(butylene adipate-co-terephthalate)–starch blends (PBAT/starch) illustrates the potential benefits of bioplastics in waste management scenarios.326 Supporting this, a review of LCA studies on NC-based adsorbents highlights their effectiveness in environmental remediation applications.327 Furthermore, an LCA conducted by Hervy et al.328 confirms that NC materials typically exhibit a lower environmental footprint, particularly concerning resource consumption.
9. Regulatory framework and policies
Mitigating MP contamination in emerging nations requires an understanding of the laws and policies governing plastic waste in the coastal environment.329 Many countries implemented laws and regulations to reduce plastic pollution or are in the process of engaging in stakeholder discussions, as demonstrated in Table 7. These laws and regulations differ in their approach (i.e., traditional viewpoint (top-down) methods, market-oriented approaches, and voluntary efforts) because African nations have different customs for the consumption, manufacture, and disposal of plastics.330 Aquatic systems were mostly exposed to MPs from WWTPs. However, a sustainable governance framework—such as the one proposed by the European Parliament in its review of the Urban Waste Water Treatment Directive (TA/2019/0071)—is useful to ensure the removal of MPs at WWTPs. This framework also calls for the adoption of multiple technologies along the value chain.331 To control the growth of oyster aquaculture, the Chinese Taipei Shallow Sea Oyster Aquaculture Management Autonomous Regulations have been implemented. Specific clauses of the regulation encourage oyster farmers to use non-styrofoam buoys by providing subsidies, even though its main purpose is to handle the oyster aquaculture zoning system. The government took this step to stop styrofoam buoys from shedding MPs.332 A regional action plan was set up by HELCOM in 2015 to address marine litter and secondary MPs by 2025. By enhancing stormwater management and increasing the number of WWTPs, assessing primary sources and legal mechanisms to address them, promoting MP-free formulae, putting certification programs (like EU Ecolabel and Blue Angel) into place, encouraging no littering policies, supplanting primary MPs in personal care products, and increasing public awareness, this plan suggests ways to combat primary MPs.333 To lessen the negative environmental effects of plastic waste, the European Union has established in place an extensive set of policies.334 The European Union has banned single-use plastics and set broadened manufacturer responsibility and recycling standards for its member states.335 In Vietnam, several measures accompanied by particular laws are used to reduce the diffusion of MPs into the aquatic environment, particularly the riverine environment.336| Plastic category | Policy | Country | Goal |
|---|---|---|---|
| Aquatic MPs | Microbead-free waters act 2015 | USA | • Ban the production and sale of wash-off cosmetic products |
| Plastics | The break free from plastic pollution act 2023 | • Shift the financial responsibility of plastic waste management to producers of plastics | |
| • Ban single use of plastic products | |||
| • Prohibit the export of plastic waste | |||
| Aquatic MPs | Circular economy law (waste prevention and management) 2018 | France | • Ban cosmetic products containing plastic particles |
| Microfibers, microbeads | Draft law on combating plastic pollution (adopted 2022) | • Regulate the loss and leakage of industrial granules, prohibit intentional usage of microbeads in detergent, and provide impact assessment of plastic fibers on the textile industry | |
| Larger plastics | Single-use plastics prohibition regulations (2022) | Canada | • Prohibit the manufacture, importation, and distribution of single-use plastic products |
| Aquatic MPs | Microbeads in toiletries regulations (2017) | • Reduce the amount of plastic microbeads entering Canadian freshwater and marine environments | |
| Larger plastics | Plastic bag control and management regulations (2018) | Kenya | • Reduce usage, manufacture, and importation of plastic bags |
| The wildlife conservation and management act 2020 | • Ban single-use plastic products | ||
| Aquatic MPs | The plastic reduction and circular economy act 2021 | Australia | • Ban the distribution of wash-off personal care products |
| Aquatic MPs | Waste minimization act through waste minimization (microbeads) regulations 2017 | New Zealand | • Prohibit plastic beads as an ingredient in personal care products |
| Larger plastics, aquatic MPs | Environmental permitting regulations 2018 | UK | • Ban cosmetics and cleaning products containing microbeads |
| • Charge levies on single-use carrier bags | |||
| • Ban single-use plastics | |||
| Aquatic MPs | The environmental protection (microbeads) (Northern Ireland) regulations 2018 | Northern Ireland | • Prohibit the use of plastic beads |
| Aquatic MPs | 2019 industrial catalogue | China | • Ban the production and sale of cosmetics containing microbeads |
| Aquatic MPs | The single-use plastics directive 2019 | EU | • Target eradicating the 10 most common single-use plastics found on the beaches and seas in Europe |
| Aquatic plastics | Clean up | The ocean cleanup | • Developing technologies to reduce plastics in the ocean by 90% by 2040 |
| Aquatic MPs, larger plastics | Thailand ministry of public health (2019) through roadmap on plastic waste management (2018–2030) | Thailand | • Ban the production, sale, and distribution of cosmetics with microbeads as an ingredient |
| • Ban single use of plastics | |||
| Plastics waste | Environmental management act (the commodities act decree) | The Netherlands | • Control packaging and consumer products |
| • Regulate single-use plastics | |||
| Aquatic MPs | The microbeads (prohibition) act 2019 | Ireland | • Ban the use of plastic beads in households and industrial cleaning products |
| Larger plastics | Plastic waste management (amendment) 2022 | India | • Phase out single-use plastics |
| Larger plastics | The Germane ordinance on single-use plastics 2021 | Germany | • Reduce the impact of plastic waste on the environment |
| • Ban some single-use plastic products | |||
| Larger plastics | The national environmental management waste act 2008 (amended 2014) through national waste management strategy 2020 | South Africa | • Reduce production of single-use plastics destroying the marine environment |
| Larger plastics | Tax/levies on single-use plastics | Wales, Ireland, Scotland | • Discourage the single use of plastic products to reduce waste |
| Larger plastics | The single-use foodware and litter reduction ordinance (2022) | Berkeley, California | • Reduce plastic waste in the environment |
In addition to legislative measures, voluntary ecolabeling schemes such as the EU Ecolabel and Blue Angel play a significant role in promoting environmentally responsible technologies. These certifications assess products based on their entire life cycle—from raw material sourcing to end-of-life disposal.338 NC and biopolymer-based MP removal systems align with the criteria of these ecolabels, as they are derived from renewable resources, are biodegradable, and pose minimal toxicity risks to ecosystems.339,340 Also, these materials ensure that they break down naturally in the environment, minimizing long-term pollution.339 NC and biopolymers are generally less toxic compared to synthetic materials.341 Their production can also be optimized to reduce energy use and waste generation,342 thereby supporting circular economy goals.343 Importantly, alignment with such ecolabels may unlock policy-driven incentives, including subsidies, grants, or preferential procurement in public and private sectors. For example, ecolabel-certified technologies can benefit from EU-level funding schemes (e.g., Horizon Europe, LIFE Programme) and national green procurement strategies, encouraging broader adoption of natural MP removal methods.344,345
10. Conclusion and future outlook
Microplastics (MPs) are commonly found in water, posing risks to the environment and human health, which makes it crucial to find practical and sustainable methods for their removal. This review highlights the key role that natural compounds (NCs) and biopolymers can play in water treatment due to their unique properties. NC has a large surface area that effectively captures MPs and is biodegradable, making it an eco-friendly choice compared to synthetic materials. It works by sticking to, filtering, and clumping together pollutants. Biopolymers also help gather and separate MPs. When NC and biopolymers are used together, they often enhance the effectiveness of each other, resulting in better removal of MPs than when used separately. Even with these improvements, there are still significant areas of research that need to be addressed before these materials can be effectively utilized in real-life situations. First, improving the production, surface treatment, and blending of nanocomposites and biopolymers is essential to ensure strong performance across different types of MPs, particularly those with varying shapes and chemical compositions. It is essential to modify the surface properties of these materials to enhance their interactions with specific substances. In addition, limited research has been conducted on their long-term environmental behavior and potential ecological effects after use. Comprehensive assessments of their entire life cycle and environmental impact are necessary to ensure safe and sustainable large-scale application.There is a significant gap in research regarding the identification, quantification, and understanding of nanoplastics. These tiny plastic particles can enter into our bodies and may be harmful. Unlike larger MPs, nanoplastics are more challenging to study because they are small, contain a mixture of materials, and their signals can be weak and difficult to detect. Some methods, like Raman spectroscopy and nanoscale FTIR, can help, but they need to be improved and made more sensitive. Clear guidelines are needed to ensure that results can be replicated and compared across different studies. Additionally, developing improved tools for imaging and analyzing nanoplastics in complex environments is crucial for accurately tracking these particles. Many studies to date have focused on specific areas of the environment, such as oceans, rivers, land, or the air, without adequately considering how MPs and nanoplastics move between these areas. Future research should adopt a broader perspective to understand how these particles travel and transform throughout the environment. This means investigating how MPs move from wastewater systems to soil when sludge is applied, from surface waters to living organisms, and through the interactions between air, soil, and water under different environmental conditions. It is also important to conduct experiments that consider various factors, such as pH, salt levels, organic matter, and temperature, to understand how these factors influence the removal of MPs and their behavior. Studies in regions with poorly managed plastic waste are critical, as these areas are major spots for MP pollution. Conducting pilot and large-scale studies is essential to bridge the gap between laboratory findings and real-world applications. Such studies should involve collaboration among researchers, industry professionals, policymakers, and local communities to jointly identify problems, design treatment systems, and share knowledge. Working together in this way not only makes the technology more relevant but also helps the community accept it and ensures it aligns with policies. It is also essential to test these treatment systems on a larger scale with various types of wastewaters, including those from cities and industries, to determine if they are practical and cost-effective.
The use of nanocellulose and biopolymer technologies in wastewater treatment plants presents exciting possibilities; however, careful consideration is required regarding their integration with existing systems. There are challenges, including the risk of clogging, increased operational complexity, and managing or recycling materials. A modular approach, which may include additional filtration steps utilizing these new materials, could provide flexibility while ensuring the primary treatment processes run smoothly. Future research should focus on developing scalable production methods, enhancing material durability under real wastewater conditions, and implementing long-term performance monitoring. Additionally, emerging technologies such as machine learning could enhance traditional treatment processes, potentially optimizing the management of wastewater through the use of these advanced materials.
The implementation of new ideas in real-world settings requires addressing several challenges, including financial constraints, technological limitations, and regulatory hurdles. Some of these challenges involve the high costs of producing bioplastics, differences in the sources of materials such as biomass, energy-intensive processing steps, and the lack of clear safety standards for waste materials. Policy-driven plans and cross-sector collaboration are essential to address these issues. Increasing production by utilizing eco-friendly chemistry, leveraging agricultural waste as raw materials, and exploring methods to reduce energy consumption in production can help lower costs and make processes more sustainable. At the same time, regulatory agencies should create clear guidelines to assess the safety and effectiveness of bioplastics and related technologies in environmental applications. Nanocellulose and biopolymers hold great potential for removing microplastics; however, effective utilization requires focused attention on several key areas. This includes improving materials for enhanced performance, developing more effective tools for detecting tiny plastics, and conducting real-world pilot studies to assess their effectiveness. It is also necessary to implement innovative monitoring systems that provide quick feedback during use. On the policy front, reducing microplastics can be achieved by linking wastewater management with solid waste processes, ensuring high standards in recycling, regulating new sources of microplastics, and raising public awareness. Addressing these challenges will be essential for making these solutions practical and environmentally friendly in tackling microplastic pollution in our water systems.
Author contributions
Sayam conceived the study, developed the methodology, wrote the original draft, created visualizations, and performed proofreading; Tarikul Islam conceived the study, developed the methodology, contributed to writing, editing, and reviewing the manuscript; conducted the investigation; prepared visualizations; supervised the project; curated data; validated results; and provided resources; Tasnim Hanan Tusti contributed to writing the original draft, prepared visualizations, edited the manuscript, curated data, and conducted formal analysis; Joyjit Ghosh contributed to writing and editing the manuscript and assisted with the literature review.Conflicts of interest
The authors declare that they have no conflict of interest.Data availability
All data related to the research are included in the manuscript.Acknowledgements
The authors declare that they did not receive any internal or external funding for the research.References
- M. Cole, P. Lindeque, C. Halsband and T. S. Galloway, Microplastics as contaminants in the marine environment: A review, Mar. Pollut. Bull., 2011, 62, 2588–2597, DOI:10.1016/j.marpolbul.2011.09.025.
- A. Rafey and F. Z. Siddiqui, A review of plastic waste management in India – challenges and opportunities, Int. J. Environ. Anal. Chem., 2023, 103, 3971–3987, DOI:10.1080/03067319.2021.1917560.
- J. Ghosh, N. S. Rupanty, T. R. Asif, T. Noor, T. Islam and V. Reukov, Advancing biomedical applications: integrating textile innovations with tissue engineering, Biomed. Mater., 2025, 20, 042002, DOI:10.1088/1748-605X/adda81.
- F. J. G. S. Rima, Setting the Facts Straight on Plastics, 2020, https://www.weforum.org/agenda/2019/10/plastics-what-are-they-explainer/ Search PubMed.
- J. Sun, X. Dai, Q. Wang, M. C. M. van Loosdrecht and B.-J. Ni, Microplastics in wastewater treatment plants: Detection, occurrence and removal, Water Res., 2019, 152, 21–37, DOI:10.1016/j.watres.2018.12.050.
- J. G. B. Derraik, The pollution of the marine environment by plastic debris: a review, Mar. Pollut. Bull., 2002, 44, 842–852, DOI:10.1016/S0025-326X(02)00220-5.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2025, 3, e1700782, DOI:10.1126/sciadv.1700782.
- Carbiolice, The history of plastic in 15 key dates, 2023, https://www.carbiolice.com/en/news/the-history-of-plastic-in-15-key-dates-2/.
- D. Terry Townsend, Chair, Natural Fibres and the World Economy July 2019, 2019, https://dnfi.org/natural-fibres-and-the-world-economy-july-2019.
- K. I. G. H. Beverley and L. Kirsi, Microplastic pollution from textiles: a literature review, consumption research norway -Oslo and akershus university college of applied Sciences, 2018, https://oda.oslomet.no/oda-xmlui/handle/20.500.12199/5360.
- R. M. A. Freitas and G. Zhang, Water footprint assessment of polyester and viscose, https://waterfootprint.org/media/downloads/WFA_Polyester_and__Viscose_2017.pdf.
- E. Commision, Single-use plastics, https://environment.ec.europa.eu/topics/plastics/single-use-plastics_en.
- S. Spary, National recycling week 2019: how many times can one plastic bottle be recycled?, 2019, https://www.huffingtonpost.co.uk/entry/how-many-times-can-one-plastic-bottle-be-recycled_uk_5bc9b98be4b0d38b58771df3.
- D. K. A. Barnes, F. Galgani, R. C. Thompson and M. Barlaz, Accumulation and fragmentation of plastic debris in global environments, Philos. Trans. R. Soc., B, 2009, 364, 1985–1998, DOI:10.1098/rstb.2008.0205.
- D. Neves, P. Sobral, J. L. Ferreira and T. Pereira, Ingestion of microplastics by commercial fish off the Portuguese coast, Mar. Pollut. Bull., 2015, 101, 119–126, DOI:10.1016/j.marpolbul.2015.11.008.
- L. Parker, Plastic trash flowing into the seas will nearly triple by 2040 without drastic action, 2020, https://www.nationalgeographic.com/science/article/plastic-trash-in-seas-will-nearly-triple-by-2040-if-nothing-done.
- M. Ferreira, J. Thompson, A. Paris, D. Rohindra and C. Rico, Presence of microplastics in water, sediments and fish species in an urban coastal environment of Fiji, a Pacific small island developing state, Mar. Pollut. Bull., 2020, 153, 110991, DOI:10.1016/j.marpolbul.2020.110991.
- C. M. Free, O. P. Jensen, S. A. Mason, M. Eriksen, N. J. Williamson and B. Boldgiv, High-levels of microplastic pollution in a large, remote, mountain lake, Mar. Pollut. Bull., 2014, 85, 156–163, DOI:10.1016/j.marpolbul.2014.06.001.
- S. A. Mason, D. Garneau, R. Sutton, Y. Chu, K. Ehmann, J. Barnes, P. Fink, D. Papazissimos and D. L. Rogers, Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent, Environ. Pollut., 2016, 218, 1045–1054, DOI:10.1016/j.envpol.2016.08.056.
- S. L. Wright, R. C. Thompson and T. S. Galloway, The physical impacts of microplastics on marine organisms: A review, Environ. Pollut., 2013, 178, 483–492, DOI:10.1016/j.envpol.2013.02.031.
- G. Gatidou, O. S. Arvaniti and A. S. Stasinakis, Review on the occurrence and fate of microplastics in Sewage Treatment Plants, J. Hazard. Mater., 2019, 367, 504–512, DOI:10.1016/j.jhazmat.2018.12.081.
- F. Bessa, P. Barría, J. M. Neto, J. P. G. L. Frias, V. Otero, P. Sobral and J. C. Marques, Occurrence of microplastics in commercial fish from a natural estuarine environment, Mar. Pollut. Bull., 2018, 128, 575–584, DOI:10.1016/j.marpolbul.2018.01.044.
- A. L. Andrady, The plastic in microplastics: A review, Mar. Pollut. Bull., 2017, 119, 12–22, DOI:10.1016/j.marpolbul.2017.01.082.
- C. M. Kershaw and P. J. Rochman, Sources, fate and effects of microplastics in the marine environment: part 2 of a global assessment, https://agris.fao.org/search/en/providers/122621/records/6473afaf13d110e4e7a9371b.
- M. R. Gregory, Environmental implications of plastic debris in marine settings—entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions, Philos. Trans. R. Soc., B, 2009, 364, 2013–2025, DOI:10.1098/rstb.2008.0265.
- Z. L. R. Botterell, N. Beaumont, T. Dorrington, M. Steinke, R. C. Thompson and P. K. Lindeque, Bioavailability and effects of microplastics on marine zooplankton: A review, Environ. Pollut., 2019, 245, 98–110, DOI:10.1016/j.envpol.2018.10.065.
- J. Michels, A. Stippkugel, M. Lenz, K. Wirtz and A. Engel, Rapid aggregation of biofilm-covered microplastics with marine biogenic particles, Philos. Trans. R. Soc., B, 2018, 285, 20181203, DOI:10.1098/rspb.2018.1203.
- X. Liu, M.-C. Li, Y. Lu, Z. Li, C. Liu, Z. Liu, C. Mei and Q. Wu, Cellulose nanofiber-coated delignified wood as an efficient filter for microplastic removal, Prog. Nat. Sci.:Mater. Int., 2024, 34, 162–171, DOI:10.1016/j.pnsc.2024.02.010.
- C. E. Enyoh, A. Devi, T. O. Maduka, L. Tyagi, S. Rana, I. S. Akuwudike and Q. Wang, A Review of Materials for the Removal of Micro- and Nanoplastics from Different Environments, Micro, 2025, 5, 17, DOI:10.3390/micro5020017.
- K. S. Randhawa, Microplastics Removal by Coagulation: Cutting-Edge Coagulants and Coagulation Processes, Pigment Resin Technol, 2024, DOI:10.1108/PRT-07-2024-0070.
- Y. Zhang, A. Diehl, A. Lewandowski, K. Gopalakrishnan and T. Baker, Removal efficiency of micro- and nanoplastics (180 nm–125 µm) during drinking water treatment, Sci. Total Environ., 2020, 720, 137383, DOI:10.1016/j.scitotenv.2020.137383.
- M. Faria, C. Cunha, M. Gomes, I. Mendonça, M. Kaufmann, A. Ferreira and N. Cordeiro, Bacterial cellulose biopolymers: The sustainable solution to water-polluting microplastics, Water Res., 2022, 222, 118952, DOI:10.1016/j.watres.2022.118952.
- R. T. Varghese, R. M. Cherian, T. Antony, A. Tharayil, H. Das, H. Kargarzadeh, C. J. Chirayil and S. Thomas, A review on the best bioadsorbent membrane- nanocellulose for effective removal of pollutants from aqueous solutions, Carbohydr. Polym. Technol. Appl., 2022, 3, 100209, DOI:10.1016/j.carpta.2022.100209.
- K. Christina, K. Subbiah, P. Arulraj, S. K. Krishnan and P. Sathishkumar, A sustainable and eco-friendly approach for environmental and energy management using biopolymers chitosan, lignin and cellulose — A review, Int. J. Biol. Macromol., 2024, 257, 128550, DOI:10.1016/j.ijbiomac.2023.128550.
- A. Tshikovhi, S. B. Mishra and A. K. Mishra, Nanocellulose-based composites for the removal of contaminants from wastewater, Int. J. Biol. Macromol., 2020, 152, 616–632, DOI:10.1016/j.ijbiomac.2020.02.221.
- J. Ghosh, N. S. Rupanty, T. Noor, T. R. Asif, T. Islam and V. Reukov, Functional coatings for textiles: advancements in flame resistance, antimicrobial defense, and self-cleaning performance, RSC Adv., 2025, 15, 10984–11022, 10.1039/D5RA01429H.
- A. Jha and A. Kumar, Biobased technologies for the efficient extraction of biopolymers from waste biomass, Bioprocess Biosyst. Eng., 2019, 42, 1893–1901, DOI:10.1007/s00449-019-02199-2.
- K. Shanmugam, Methods for Fabrication of Freestanding Nanocellulose Film as a Sustainable Material for Packaging and Biomedical Applications: A Review, Soc. Sci. Res., 2024, 6, 29–40, DOI:10.26689/ssr.v6i3.6350.
- T. Shahnaz, V. Vishnu Priyan, A. Jayakumar and S. Narayanasamy, Magnetic nanocellulose from Cyperus rotundas grass in the absorptive removal of rare earth element cerium (III): Toxicity studies and interpretation, Chemosphere, 2022, 287, 131912, DOI:10.1016/j.chemosphere.2021.131912.
- A. S. Norfarhana, R. A. Ilyas and N. Ngadi, A review of nanocellulose adsorptive membrane as multifunctional wastewater treatment, Carbohydr. Polym., 2022, 291, 119563, DOI:10.1016/j.carbpol.2022.119563.
- A. A. Babaei, N. Reshadatian and R. Feizi, A state of the art-mini review on the sources, contamination, analysis, and consequences of microplastics in water, Results Eng., 2024, 23, 102827, DOI:10.1016/j.rineng.2024.102827.
- S. Sharma, A. Bhardwaj, M. Thakur and A. Saini, Understanding microplastic pollution of marine ecosystem: a review, Environ. Sci. Pollut. Res., 2023, 31, 41402–41445, DOI:10.1007/s11356-023-28314-1.
- J. Ghosh, M. R. Repon, N. S. Rupanty, T. R. Asif, M. I. Tamjid and V. Reukov, Chemical Valorization of Textile Waste: Advancing Sustainable Recycling for a Circular Economy, ACS Omega, 2025, 10, 11697–11722, DOI:10.1021/acsomega.4c10616.
- L. An, Q. Liu, Y. Deng, W. Wu, Y. Gao and W. Ling, Sources of Microplastic in the Environment, in Microplastics in Terrestrial Environments, 2020, pp. 143–159, DOI:10.1007/698_2020_449.
- E. S. Okeke, K. I. Chukwudozie, C. I. Addey, J. O. Okoro, T. P. Chidike Ezeorba, E. O. Atakpa, C. O. Okoye and C. O. Nwuche, Micro and nanoplastics ravaging our agroecosystem: A review of occurrence, fate, ecological impacts, detection, remediation, and prospects, Heliyon, 2023, 9, e13296, DOI:10.1016/j.heliyon.2023.e13296.
- R. Ramasamy, T. A. Aragaw and R. Balasaraswathi Subramanian, Wastewater treatment plant effluent and microfiber pollution: focus on industry-specific wastewater, Environ. Sci. Pollut. Res., 2022, 29, 51211–51233, DOI:10.1007/s11356-022-20930-7.
- M. Prabhakaran, T. G. Sunitha, K. Omine and V. Sivasankar, Microfibers release during washing and, removal by tamarind fruit shell carbon filters: Subsequent utilization in crystal violet dye removal from water, Process Saf. Environ. Prot., 2025, 193, 132–147, DOI:10.1016/j.psep.2024.11.011.
- H. Kye, J. Kim, S. Ju, J. Lee, C. Lim and Y. Yoon, Microplastics in water systems: A review of their impacts on the environment and their potential hazards, Heliyon, 2023, 9, e14359, DOI:10.1016/j.heliyon.2023.e14359.
- A. O. C. Iroegbu, R. E. Sadiku, S. S. Ray and Y. Hamam, Plastics in municipal drinking water and wastewater treatment plant effluents: challenges and opportunities for South Africa—a review, Environ. Sci. Pollut. Res., 2020, 27, 12953–12966, DOI:10.1007/s11356-020-08194-5.
- I. Sheriff, M. S. Yusoff, T. S. B. A. Manan and M. Koroma, Microplastics in manure: Sources, analytical methods, toxicodynamic, and toxicokinetic endpoints in livestock and poultry, Environ. Adv., 2023, 12, 100372, DOI:10.1016/j.envadv.2023.100372.
- J. Ghosh, M. R. Repon, A. D. Pranta, N. S. Rupanty, F. Khan and T. Noor, Bioactive component integrated textiles: A promising source of medicine and healthcare, J. Eng. Fibers Fabr., 2025, 20, 1–44, DOI:10.1177/15589250241308561.
- J.-H. Lee, S.-J. Cheon, C.-S. Kim, S.-H. Joo, K.-I. Choi, D.-H. Jeong, S.-H. Lee and J.-K. Yoon, Nationwide evaluation of microplastic properties in municipal wastewater treatment plants in South Korea, Environ. Pollut., 2024, 358, 124433, DOI:10.1016/j.envpol.2024.124433.
- M. Nivetha and D. Mathiyazhagan, Microplastic Pollution In Fresh Water, Int. J. Sci. Res. Eng. Manag., 2023, 07, 1–11, DOI:10.55041/IJSREM25960.
- J. Sun, Z. Peng, Z.-R. Zhu, W. Fu, X. Dai and B.-J. Ni, The atmospheric microplastics deposition contributes to microplastic pollution in urban waters, Water Res., 2022, 225, 119116, DOI:10.1016/j.watres.2022.119116.
- C. N. Reddy, P. Kallem, K. V. S. S. N. Mounika, A. Muqeet, J. C. J. Raj, C. V. S. Aishwarya, R. K. Gupta, V. Polisetti, B. Mishra, R. Yadavalli, S. K. Mandal, M. S. Hedenqvist and F. Banat, Review of microplastic degradation: Understanding metagenomic approaches for microplastic degrading organisms, Polym. Test., 2023, 128, 108223, DOI:10.1016/j.polymertesting.2023.108223.
- C. Chen, J. Deng, Q. Zhang and J. Cai, Microplastic pollution and characteristics in the surface waters of the middle and lower reaches of the Han River along Hubei Province, China, Int. J. Environ. Sci. Technol., 2023, 20, 10205–10216, DOI:10.1007/s13762-022-04559-0.
- H. Deng, R. Wei, W. Luo, L. Hu, B. Li, Y. Di and H. Shi, Microplastic pollution in water and sediment in a textile industrial area, Environ. Pollut., 2020, 258, 113658, DOI:10.1016/j.envpol.2019.113658.
- Y. Y. Tsang, C. W. Mak, C. Liebich, S. W. Lam, E. T.-P. Sze and K. M. Chan, Microplastic pollution in the marine waters and sediments of Hong Kong, Mar. Pollut. Bull., 2017, 115, 20–28, DOI:10.1016/j.marpolbul.2016.11.003.
- W. M. Conard, K. E. O'Reilly, C. Hartlage and G. A. Lamberti, Widespread microplastic pollution in Indiana, <scp>USA</scp> , rivers, River Res. Appl., 2023, 39, 2092–2101, DOI:10.1002/rra.4204.
- L. S. Fendall and M. A. Sewell, Contributing to marine pollution by washing your face: Microplastics in facial cleansers, Mar. Pollut. Bull., 2009, 58, 1225–1228, DOI:10.1016/j.marpolbul.2009.04.025.
- G. Zhou, Q. Wang, J. Li, Q. Li, H. Xu, Q. Ye, Y. Wang, S. Shu and J. Zhang, Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism, Sci. Total Environ., 2021, 752, 141837, DOI:10.1016/j.scitotenv.2020.141837.
- L. Deng, L. Cai, F. Sun, G. Li and Y. Che, Public attitudes towards microplastics: Perceptions, behaviors and policy implications, Resour. Conserv. Recycl., 2020, 163, 105096, DOI:10.1016/j.resconrec.2020.105096.
- S. Feng, H. Lu and Y. Liu, The occurrence of microplastics in farmland and grassland soils in the Qinghai-Tibet plateau: Different land use and mulching time in facility agriculture, Environ. Pollut., 2021, 279, 116939, DOI:10.1016/j.envpol.2021.116939.
- P. J. Anderson, S. Warrack, V. Langen, J. K. Challis, M. L. Hanson and M. D. Rennie, Microplastic contamination in Lake Winnipeg, Canada, Environ. Pollut., 2017, 225, 223–231, DOI:10.1016/j.envpol.2017.02.072.
- X.-T. Bui, T.-D.-H. Vo, P.-T. Nguyen, V.-T. Nguyen, T.-S. Dao and P.-D. Nguyen, Microplastics pollution in wastewater: Characteristics, occurrence and removal technologies, Environ. Technol. Innov., 2020, 19, 101013, DOI:10.1016/j.eti.2020.101013.
- P. G. Ryan, A Brief History of Marine Litter Research, in Mar. Anthropog. Litter, Springer International Publishing, Cham, 2015, pp. 1–25, DOI:10.1007/978-3-319-16510-3_1.
- K. Zhang, A. H. Hamidian, A. Tubić, Y. Zhang, J. K. H. Fang, C. Wu and P. K. S. Lam, Understanding plastic degradation and microplastic formation in the environment: A review, Environ. Pollut., 2021, 274, 116554, DOI:10.1016/j.envpol.2021.116554.
- S. Sharma, V. Sharma and S. Chatterjee, Microplastics in the Mediterranean Sea: Sources, Pollution Intensity, Sea Health, and Regulatory Policies, Front. Mar. Sci., 2021, 8, 634934, DOI:10.3389/fmars.2021.634934.
- T. van Emmerik, T.-C. Kieu-Le, M. Loozen, K. van Oeveren, E. Strady, X.-T. Bui, M. Egger, J. Gasperi, L. Lebreton, P.-D. Nguyen, A. Schwarz, B. Slat and B. Tassin, A Methodology to Characterize Riverine Macroplastic Emission Into the Ocean, Front. Mar. Sci., 2018, 5, 372, DOI:10.3389/fmars.2018.00372.
- J. Čulin and T. Bielić, Plastic Pollution from Ships, J. Marit. Transp. Sci., 2016, 51, 57–66, DOI:10.18048/2016.51.04.
- C. M. Rochman, Microplastics research—from sink to source, Science, 2018, 360, 28–29, DOI:10.1126/science.aar7734.
- K. Lei, F. Qiao, Q. Liu, Z. Wei, H. Qi, S. Cui, X. Yue, Y. Deng and L. An, Microplastics releasing from personal care and cosmetic products in China, Mar. Pollut. Bull., 2017, 123, 122–126, DOI:10.1016/j.marpolbul.2017.09.016.
- S. Ziajahromi, D. Drapper, A. Hornbuckle, L. Rintoul and F. D. L. Leusch, Microplastic pollution in a stormwater floating treatment wetland: Detection of tyre particles in sediment, Sci. Total Environ., 2020, 713, 136356, DOI:10.1016/j.scitotenv.2019.136356.
- P. K. Cheung and L. Fok, Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China, Water Res., 2017, 122, 53–61, DOI:10.1016/j.watres.2017.05.053.
- R. Z. Habib, M. M. Salim Abdoon, R. M. Al Meqbaali, F. Ghebremedhin, M. Elkashlan, W. F. Kittaneh, N. Cherupurakal, A.-H. I. Mourad, T. Thiemann and R. Al Kindi, Analysis of microbeads in cosmetic products in the United Arab Emirates, Environ. Pollut., 2020, 258, 113831, DOI:10.1016/j.envpol.2019.113831.
- L. Hou, D. Kumar, C. G. Yoo, I. Gitsov and E. L.-W. Majumder, Conversion and removal strategies for microplastics in wastewater treatment plants and landfills, Chem. Eng. J., 2021, 406, 126715, DOI:10.1016/j.cej.2020.126715.
- S. He, M. Jia, Y. Xiang, B. Song, W. Xiong, J. Cao, H. Peng, Y. Yang, W. Wang, Z. Yang and G. Zeng, Biofilm on microplastics in aqueous environment: Physicochemical properties and environmental implications, J. Hazard. Mater., 2022, 424, 127286, DOI:10.1016/j.jhazmat.2021.127286.
- D. Huang, J. Tao, M. Cheng, R. Deng, S. Chen, L. Yin and R. Li, Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures, J. Hazard. Mater., 2021, 407, 124399, DOI:10.1016/j.jhazmat.2020.124399.
- A. Cózar, M. Sanz-Martín, E. Martí, J. I. González-Gordillo, B. Ubeda, J. Á. Gálvez, X. Irigoien and C. M. Duarte, Plastic Accumulation in the Mediterranean Sea, PLoS One, 2015, 10, e0121762, DOI:10.1371/journal.pone.0121762.
- Y. Xiang, L. Jiang, Y. Zhou, Z. Luo, D. Zhi, J. Yang and S. S. Lam, Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments, J. Hazard. Mater., 2022, 422, 126843, DOI:10.1016/j.jhazmat.2021.126843.
- D. Schymanski, C. Goldbeck, H.-U. Humpf and P. Fürst, Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water, Water Res., 2018, 129, 154–162, DOI:10.1016/j.watres.2017.11.011.
- C. Alomar, F. Estarellas and S. Deudero, Microplastics in the Mediterranean Sea: Deposition in coastal shallow sediments, spatial variation and preferential grain size, Mar. Environ. Res., 2016, 115, 1–10, DOI:10.1016/j.marenvres.2016.01.005.
- S. Karbalaei, A. Golieskardi, H. B. Hamzah, S. Abdulwahid, P. Hanachi, T. R. Walker and A. Karami, Abundance and characteristics of microplastics in commercial marine fish from Malaysia, Mar. Pollut. Bull., 2019, 148, 5–15, DOI:10.1016/j.marpolbul.2019.07.072.
- O. OFOEGBU, P. O. ONUWA, H. I. Kelle, S. U. ONOGWU, O. J. AJAKAYE, J. A. LULLAH-DEH, J. EGWUMAH and T. P. UNONGUL, The Environmental and Health Implications of Microplastics on Human and Aquatic Life, Int. J. Res. Innov. Appl. Sci., 2024, IX, 367–377, DOI:10.51584/IJRIAS.2024.909031.
- S. Du, R. Zhu, Y. Cai, N. Xu, P.-S. Yap, Y. Zhang, Y. He and Y. Zhang, Environmental fate and impacts of microplastics in aquatic ecosystems: a review, RSC Adv., 2021, 11, 15762–15784, 10.1039/D1RA00880C.
- M. Saisanthosh Vamshi Harsha, P. Abhiram Siva Prasad and D. Bhanu Prakash, Theoretical Review on Microplastic Pollution: A Multifaceted Threat to Marine Ecosystems, Human Health, and Environment, IgMin Res., 2024, 2, 460–468, DOI:10.61927/igmin203.
- G. G. N. Thushari and J. D. M. Senevirathna, Plastic pollution in the marine environment, Heliyon, 2020, 6, e04709, DOI:10.1016/j.heliyon.2020.e04709.
- P. Sethia, D. Nandhini and S. Amutha, Effects of marine microplastic on marine life and the food webs – A detailed review, Mar. Ecol., 2024, 45, 1–14, DOI:10.1111/maec.12819.
- E. Moto, M. Hossein, R. Bakari, A. S. Mateso, J. R. Selemani, S. Nkrumah, A. Ripanda, M. J. Rwiza, E. C. Nyanza and R. L. Machunda, Ecological consequences of microplastic pollution in sub-Saharan Africa aquatic ecosystems: An implication to environmental health, HydroResearch, 2024, 7, 39–54, DOI:10.1016/j.hydres.2023.11.003.
- S. Singh, S. Chakma, B. Alawa, M. Kalyanasundaram and V. Diwan, Identification, characterization, and implications of microplastics in soil – A case study of Bhopal, central India, J. Hazard. Mater. Adv., 2023, 9, 100225, DOI:10.1016/j.hazadv.2022.100225.
- S. Saud, A. Yang, Z. Jiang, D. Ning and S. Fahad, New insights in to the environmental behavior and ecological toxicity of microplastics, J. Hazard. Mater. Adv., 2023, 10, 100298, DOI:10.1016/j.hazadv.2023.100298.
- A. Parsaeimehr, C. M. Miller and G. Ozbay, Microplastics and their interactions with microbiota, Heliyon, 2023, 9, e15104, DOI:10.1016/j.heliyon.2023.e15104.
- L. Zhu, W. Pan, X. Zhao, F. Wang, C. Wang, Y. Kang, Z. Dong, B. Shao, F. Wu and L. AN, Insights into human exposure to microplastics through drinking water: Current state of the science, Crit. Rev. Environ. Control, 2024, 54, 1875–1901, DOI:10.1080/10643389.2024.2371622.
- B. E. Oßmann, Microplastics in drinking water? Present state of knowledge and open questions, Curr. Opin. Food Sci., 2021, 41, 44–51, DOI:10.1016/j.cofs.2021.02.011.
- S. Akbulut, P. K. Akman, F. Tornuk and H. Yetim, Microplastic Release from Single-Use Plastic Beverage Cups, Foods, 2024, 13, 1564, DOI:10.3390/foods13101564.
- I. Semmouri, M. Vercauteren, E. Van Acker, E. Pequeur, J. Asselman and C. Janssen, Presence of microplastics in drinking water from different freshwater sources in Flanders (Belgium), an urbanized region in Europe, Int. J. Food Contam., 2022, 9, 6, DOI:10.1186/s40550-022-00091-8.
- G. Zhou, Q. Wu, X.-F. Wei, C. Chen, J. Ma, J. C. Crittenden and B. Liu, Tracing microplastics in rural drinking water in Chongqing, China: Their presence and pathways from source to tap, J. Hazard. Mater., 2023, 459, 132206, DOI:10.1016/j.jhazmat.2023.132206.
- L. N. Meshram and K. J. Mhatre, Microplastics: Impacts on Environment and Human Health Hazards, Uttar Pradesh, J. Zool., 2024, 45, 22–33, DOI:10.56557/upjoz/2024/v45i43902.
- Y. Yue, X. Guo, Z. Wang, L. Gan, X. Dong, M. Zhang, H. Jiang, M. An and J. Shao, Alleviating effect of EMs on oxidative stress and inflammation of Micropterus salmoides after microplastics exposure, Aquacult. Int., 2024, 32, 3719–3732, DOI:10.1007/s10499-023-01347-6.
- N. Sharma, V. Kumar, S. Vimal, M. Umesh, P. Chakraborty, T. Basheer, S. Sarojini, P. Sharma, R. Pasrija and D. Barcelo, Microplastic residues in clinical samples: A retrospection on sources, entry routes, detection methods and human toxicity, TrAC, Trends Anal. Chem., 2024, 173, 117618, DOI:10.1016/j.trac.2024.117618.
- V. and S. K. Singh, Microplastics in Freshwater Ecosystems: Sources, Transport and Ecotoxicological Impacts on Aquatic Life and Human Health, Environ. Ecol., 2025, 43, 289–295, DOI:10.60151/envec/DEVO7788.
- Y. Ma and X. You, Microplastics in freshwater ecosystems: A significant force of disrupting health and altering trophic transfer patterns by reduced assimilation efficiency of aquatic organisms, Aquaculture, 2025, 594, 741463, DOI:10.1016/j.aquaculture.2024.741463.
- C. G. Eze, C. E. Nwankwo, S. Dey, S. Sundaramurthy and E. S. Okeke, Food chain microplastics contamination and impact on human health: a review, Environ. Chem. Lett., 2024, 22, 1889–1927, DOI:10.1007/s10311-024-01734-2.
- M. Banaee, A. Gholamhosseini, A. Sureda, S. Soltanian, M. S. Fereidouni and A. T. A. Ibrahim, Effects of microplastic exposure on the blood biochemical parameters in the pond turtle (Emys orbicularis), Environ. Sci. Pollut. Res., 2021, 28, 9221–9234, DOI:10.1007/s11356-020-11419-2.
- S. Messinetti, S. Mercurio, G. Scarì, A. Pennati and R. Pennati, Ingested microscopic plastics translocate from the gut cavity of juveniles of the ascidian Ciona intestinalis, Eur. Zool. J., 2019, 86, 189–195, DOI:10.1080/24750263.2019.1616837.
- Y. Yu, H. Chen, X. Hua, Y. Dang, Y. Han, Z. Yu, X. Chen, P. Ding and H. Li, Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans, Sci. Total Environ., 2020, 726, 138679, DOI:10.1016/j.scitotenv.2020.138679.
- H. K. A. Lo and K. Y. K. Chan, Negative effects of microplastic exposure on growth and development of Crepidula onyx, Environ. Pollut., 2018, 233, 588–595, DOI:10.1016/j.envpol.2017.10.095.
- M. S. Hossain, F. Sobhan, M. N. Uddin, S. M. Sharifuzzaman, S. R. Chowdhury, S. Sarker and M. S. N. Chowdhury, Microplastics in fishes from the Northern Bay of Bengal, Sci. Total Environ., 2019, 690, 821–830, DOI:10.1016/j.scitotenv.2019.07.065.
- L. Lei, S. Wu, S. Lu, M. Liu, Y. Song, Z. Fu, H. Shi, K. M. Raley-Susman and D. He, Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans, Sci. Total Environ., 2018, 619–620, 1–8, DOI:10.1016/j.scitotenv.2017.11.103.
- C. G. Eze, C. E. Nwankwo, S. Dey, S. Sundaramurthy and E. S. Okeke, Food chain microplastics contamination and impact on human health: a review, Environ. Chem. Lett., 2024, 22, 1889–1927, DOI:10.1007/s10311-024-01734-2.
- J. C. da Silva Antunes, P. Sobral, V. Branco and M. Martins, Uncovering layer by layer the risk of nanoplastics to the environment and human health, J. Toxicol. Environ. Health, Part B, 2025, 28, 63–121, DOI:10.1080/10937404.2024.2424156.
- S. Kauts, S. Shabir, S. Yousuf, Y. Mishra, R. Bhardwaj, A. A. Milibari, S. K. Singh and M. P. Singh, The evidence of in-vivo and in-vitro studies on microplastic and nano plastic toxicity in mammals: A possible threat for an upcoming generation?, Phys. Chem. Earth, 2023, 132, 103511, DOI:10.1016/j.pce.2023.103511.
- E. A. Christopher, Y. Christopher-de Vries, A. Devadoss, L. D. B. Mandemaker, J. van Boxel, H. M. Copsey, H. M. Dusza, J. Legler, F. Meirer, J. Muncke, T. S. Nawrot, N. D. Saenen, B. M. Scholz-Böttcher, L. Tran, B. M. Weckhuysen, R. Zou, L. Zimmermann, K. S. Galea, R. Vermeulen and M. S. P. Boyles, Impacts of micro- and nanoplastics on early-life health: a roadmap towards risk assessment, Microplast. Nanoplast., 2024, 4, 13, DOI:10.1186/s43591-024-00089-3.
- S. Haldar, Y. Muralidaran, D. Míguez, S. I. Mulla and P. Mishra, Eco-toxicity of nano-plastics and its implication on human metabolism: Current and future perspective, Sci. Total Environ., 2023, 861, 160571, DOI:10.1016/j.scitotenv.2022.160571.
- W. Liu, H. Liao, M. Wei, M. Junaid, G. Chen and J. Wang, Biological uptake, distribution and toxicity of micro(nano)plastics in the aquatic biota: A special emphasis on size-dependent impacts, TrAC, Trends Anal. Chem., 2024, 170, 117477, DOI:10.1016/j.trac.2023.117477.
- S.-Y. Lee, J. An and J.-H. Kwon, Sequential quantification of number and mass of microplastics in municipal wastewater using Fourier-transform infrared spectroscopy and pyrolysis gas chromatography-mass spectrometry, Environ. Pollut., 2023, 336, 122452, DOI:10.1016/j.envpol.2023.122452.
- M. S. M. Al-Azzawi, M. Funck, M. Kunaschk, E. Von der Esch, O. Jacob, K. P. Freier, T. C. Schmidt, M. Elsner, N. P. Ivleva, J. Tuerk, O. Knoop and J. E. Drewes, Microplastic sampling from wastewater treatment plant effluents: Best-practices and synergies between thermoanalytical and spectroscopic analysis, Water Res., 2022, 219, 118549, DOI:10.1016/j.watres.2022.118549.
- E. Özgenç, Advanced analytical techniques for assessing and detecting microplastic pollution in water and wastewater systems, Environ. Qual. Manag., 2024, 34, 1–14, DOI:10.1002/tqem.22217.
- L. Hildebrandt, D. M. Mitrano, T. Zimmermann and D. Pröfrock, A Nanoplastic Sampling and Enrichment Approach by Continuous Flow Centrifugation, Front. Environ. Sci., 2020, 8, 89, DOI:10.3389/fenvs.2020.00089.
- J. Reineccius, J. Bresien and J. J. Waniek, Separation of microplastics from mass-limited samples by an effective adsorption technique, Sci. Total Environ., 2021, 788, 147881, DOI:10.1016/j.scitotenv.2021.147881.
- D. Iqbal, Y. Zhao, R. Zhao, S. J. Russell and X. Ning, A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment, Polymers, 2022, 14, 2343, DOI:10.3390/polym14122343.
- B. Yuan, L. Li, V. Murugadoss, S. Vupputuri, J. Wang, N. Alikhani and Z. Guo, Nanocellulose-based composite materials for wastewater treatment and waste-oil remediation, ES Food Agrofor., 2020, 1, 41–52, DOI:10.30919/esfaf0004.
- P. Phanthong, P. Reubroycharoen, X. Hao, G. Xu, A. Abudula and G. Guan, Nanocellulose: Extraction and application, Carbon Resour. Convers., 2018, 1, 32–43, DOI:10.1016/j.crcon.2018.05.004.
- T. Abitbol, A. Rivkin, Y. Cao, Y. Nevo, E. Abraham, T. Ben-Shalom, S. Lapidot and O. Shoseyov, Nanocellulose, a tiny fiber with huge applications, Curr. Opin. Biotechnol., 2016, 39, 76–88, DOI:10.1016/j.copbio.2016.01.002.
- O. Nechyporchuk, M. N. Belgacem and J. Bras, Production of cellulose nanofibrils: A review of recent advances, Ind. Crops Prod., 2016, 93, 2–25, DOI:10.1016/j.indcrop.2016.02.016.
- A. F. Jozala, L. C. de Lencastre-Novaes, A. M. Lopes, V. de Carvalho Santos-Ebinuma, P. G. Mazzola, A. Pessoa-Jr, D. Grotto, M. Gerenutti and M. V. Chaud, Bacterial nanocellulose production and application: a 10-year overview, Appl. Microbiol. Biotechnol., 2016, 100, 2063–2072, DOI:10.1007/s00253-015-7243-4.
- H. Kargarzadeh, M. Ioelovich, I. Ahmad, S. Thomas and A. Dufresne, Methods for Extraction of Nanocellulose from Various Sources, in Handb. Nanocellulose Cellul. Nanocomposites, Wiley, 2017, pp. 1–49, DOI:10.1002/9783527689972.ch1.
- A. Okiyama, H. Shirae, H. Kano and S. Yamanaka, Bacterial cellulose I. Two-stage fermentation process for cellulose production by Acetobacter aceti, Food Hydrocolloids, 1992, 6, 471–477, DOI:10.1016/S0268-005X(09)80032-5.
- T. T. T. Ho, T. Zimmermann, R. Hauert and W. Caseri, Preparation and characterization of cationic nanofibrillated cellulose from etherification and high-shear disintegration processes, Cellulose, 2011, 18, 1391–1406, DOI:10.1007/s10570-011-9591-2.
- Y. Chen, Y. He, D. Fan, Y. Han, G. Li and S. Wang, An Efficient Method for Cellulose Nanofibrils Length Shearing via Environmentally Friendly Mixed Cellulase Pretreatment, J. Nanomater., 2017, 2017, 1–12, DOI:10.1155/2017/1591504.
- J. Zhang, T. J. Elder, Y. Pu and A. J. Ragauskas, Facile synthesis of spherical cellulose nanoparticles, Carbohydr. Polym., 2007, 69, 607–611, DOI:10.1016/j.carbpol.2007.01.019.
- J. Dai, M. Chae, D. Beyene, C. Danumah, F. Tosto and D. C. Bressler, Co-Production of Cellulose Nanocrystals and Fermentable Sugars Assisted by Endoglucanase Treatment of Wood Pulp, Materials, 2018, 11, 1645, DOI:10.3390/ma11091645.
- F. G. Torres, O. P. Troncoso, D. Lopez, C. Grande and C. M. Gomez, Reversible stress softening and stress recovery of cellulose networks, Soft Matter, 2009, 5, 4185, 10.1039/b900441f.
- N. Mittal, F. Ansari, K. Gowda.V, C. Brouzet, P. Chen, P. T. Larsson, S. V. Roth, F. Lundell, L. Wågberg, N. A. Kotov and L. D. Söderberg, Multiscale Control of Nanocellulose Assembly: Transferring Remarkable Nanoscale Fibril Mechanics to Macroscale Fibers, ACS Nano, 2018, 12, 6378–6388, DOI:10.1021/acsnano.8b01084.
- K. Rodríguez, J. Sundberg, P. Gatenholm and S. Renneckar, Electrospun nanofibrous cellulose scaffolds with controlled microarchitecture, Carbohydr. Polym., 2014, 100, 143–149, DOI:10.1016/j.carbpol.2012.12.037.
- A. Rege, I. Preibisch, M. Schestakow, K. Ganesan, P. Gurikov, B. Milow, I. Smirnova and M. Itskov, Correlating Synthesis Parameters to Morphological Entities: Predictive Modeling of Biopolymer Aerogels, Materials, 2018, 11, 1670, DOI:10.3390/ma11091670.
- A. Salama, R. Abouzeid, W. S. Leong, J. Jeevanandam, P. Samyn, A. Dufresne, M. Bechelany and A. Barhoum, Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration, Nanomaterials, 2021, 11, 3008, DOI:10.3390/nano11113008.
- Q. Ji, C. Zhou, Z. Li, I. D. Boateng and X. Liu, Is nanocellulose a good substitute for non-renewable raw materials? A comprehensive review of the state of the art, preparations,and industrial applications, Ind. Crops Prod., 2023, 202, 117093, DOI:10.1016/j.indcrop.2023.117093.
- V. M. Serrano-Martínez, H. Pérez-Aguilar, M. P. Carbonell-Blasco, A. García-García, F. Arán-Ais and E. Orgilés-Calpena, Effect of Acid Hydrolysis Conditions on the Extraction of Cellulose Nanocrystals, Polymers, 2025, 17, 1313, DOI:10.3390/polym17101313.
- Z.-Z. Chen, S. Si, Z.-H. Cai, W.-J. Jiang, Y.-N. Liu and D. Zhao, Application of metal-organic skeletons and cellulose composites in nanomedicine, Cellulose, 2023, 30, 9955–9972, DOI:10.1007/s10570-023-05523-y.
- W. Zhu, Y. Zhang, X. Wang, Y. Wu, M. Han, J. You, C. Jia and J. Kim, Aerogel nanoarchitectonics based on cellulose nanocrystals and nanofibers from eucalyptus pulp: preparation and comparative study, Cellulose, 2022, 29, 817–833, DOI:10.1007/s10570-021-04370-z.
- M. Raza and B. Abu-Jdayil, Cellulose nanocrystals from lignocellulosic feedstock: a review of production technology and surface chemistry modification, Cellulose, 2022, 29, 685–722, DOI:10.1007/s10570-021-04371-y.
- M. E. Lamm, K. Li, D. Ker, X. Zhao, H. E. Hinton, K. Copenhaver, H. Tekinalp and S. Ozcan, Exploiting chitosan to improve the interface of nanocellulose reinforced polymer composites, Cellulose, 2022, 29, 3859–3870, DOI:10.1007/s10570-021-04327-2.
- D. Burhani, V. S. D. Voet, R. Folkersma, D. Maniar and K. Loos, Potential of Nanocellulose for Microplastic removal: Perspective and challenges, Tetrahedron Green Chem., 2024, 3, 100045, DOI:10.1016/j.tgchem.2024.100045.
- I. Leppänen, T. Lappalainen, T. Lohtander, C. Jonkergouw, S. Arola and T. Tammelin, Capturing colloidal nano- and microplastics with plant-based nanocellulose networks, Nat. Commun., 2022, 13, 1814, DOI:10.1038/s41467-022-29446-7.
- T. Yu, X. Huang, X. F. Zhang, K. Li, S. P. Liu, N. Dai, K. Zhang, Y. X. Zhang and H. Li, A review of nanomaterials with excellent purification potential for the removal of micro- and nanoplastics from liquid, DeCarbon, 2024, 5, 100064, DOI:10.1016/j.decarb.2024.100064.
- S. Liu and J. Wang, Exploring the Potential of Cellulose Benzoate Adsorbents Modified with Carbon Nanotubes and Magnetic Carbon Nanotubes for Microplastic Removal from Water, Chem. Eng. J., 2023 DOI:10.2139/ssrn.4427621.
- Z. Jiang, X. Wang, H. Zhao, Z. Yang, J. Zhou, X. Sun, H. Yang, C. Wang and S. Huan, Micro/nano-plastic removal from wastewater using cellulose membrane: Performance and life cycle assessment, Sep. Purif. Technol., 2023, 317, 123925, DOI:10.1016/j.seppur.2023.123925.
- L. Wang, H. Wang, Q. Huang, C. Yang, L. Wang, Z. Lou, Q. Zhou, T. Wang and C. Ning, Microplastics in Landfill Leachate: A Comprehensive Review on Characteristics, Detection, and Their Fates during Advanced Oxidation Processes, Water, 2023, 15, 252, DOI:10.3390/w15020252.
- J. Yang, M. Monnot, Y. Sun, L. Asia, P. Wong-Wah-Chung, P. Doumenq and P. Moulin, Microplastics in different water samples (seawater, freshwater, and wastewater): Methodology approach for characterization using micro-FTIR spectroscopy, Water Res., 2023, 232, 119711, DOI:10.1016/j.watres.2023.119711.
- M. Funck, A. Yildirim, C. Nickel, J. Schram, T. C. Schmidt and J. Tuerk, Identification of microplastics in wastewater after cascade filtration using Pyrolysis-GC–MS, MethodsX, 2020, 7, 100778, DOI:10.1016/j.mex.2019.100778.
- P. Sharma, P. Sharma and K. Abhishek, Sampling, separation, and characterization methodology for quantification of microplastic from the environment, J. Hazard. Mater. Adv., 2024, 14, 100416, DOI:10.1016/j.hazadv.2024.100416.
- C. Thomas, T. Spatayeva, D. Yu, A. Loh, U. H. Yim and J.-Y. Yoon, A comparison of current analytical methods for detecting particulate matter and micro/nanoplastics, Appl. Phys. Rev., 2024, 11(1) DOI:10.1063/5.0153106.
- Q. Xue, Y. Dong, F. Lu, H. Yang and G. Yu, ELM combined with differential Raman spectroscopy for the detection of microplastics in organisms, Spectrochim. Acta, Part A, 2024, 312, 124039, DOI:10.1016/j.saa.2024.124039.
- T. Dey, Microplastic pollutant detection by Surface Enhanced Raman Spectroscopy (SERS): a mini-review, Nanotechnol. Environ. Eng., 2023, 8, 41–48, DOI:10.1007/s41204-022-00223-7.
- F. Ö. Sefiloglu, C. N. Stratmann, M. Brits, M. J. M. van Velzen, Q. Groenewoud, A. D. Vethaak, R. Dris, J. Gasperi and M. H. Lamoree, Comparative microplastic analysis in urban waters using µ-FTIR and Py-GC-MS: A case study in Amsterdam, Environ. Pollut., 2024, 351, 124088, DOI:10.1016/j.envpol.2024.124088.
- A. Dehaut, L. Hermabessiere and G. Duflos, Microplastics Detection Using Pyrolysis-GC/MS-Based Methods, in Handb. Microplastics Environ., Springer International Publishing, Cham, 2022, pp. 141–175, DOI:10.1007/978-3-030-39041-9_27.
- N. Choran and B. Örmeci, Micro-flow imaging for in-situ and real-time enumeration and identification of microplastics in water, Front. Water, 2023, 5, 1148379, DOI:10.3389/frwa.2023.1148379.
- S. Primpke, M. Fischer, C. Lorenz, G. Gerdts and B. M. Scholz-Böttcher, Comparison of pyrolysis gas chromatography/mass spectrometry and hyperspectral FTIR imaging spectroscopy for the analysis of microplastics, Anal. Bioanal. Chem., 2020, 412, 8283–8298, DOI:10.1007/s00216-020-02979-w.
- J. Zhuang, N. Rong, X. Wang, C. Chen and Z. Xu, Adsorption of small size microplastics based on cellulose nanofiber aerogel modified by quaternary ammonium salt in water, Sep. Purif. Technol., 2022, 293, 121133, DOI:10.1016/j.seppur.2022.121133.
- L. Valencia, R. Handa, S. Monti, A. B. Jasso-Salcedo, D. Georgouvelas, I. Magaña, R. Díaz de León, K. P. Velikov, A. P. Mathew and S. Kumar, On the mineralization of nanocellulose to produce functional hybrid materials, J. Mater. Chem. A, 2022, 10, 9248–9276, 10.1039/D2TA00457G.
- B. Xu, X. Hu, S. Lu, T. Wang, Z. Chen, C. Bai, T. Ma and Y. Song, 3D printing of cellulose nanocrystal-based Pickering foams for removing microplastics, Sep. Purif. Technol., 2024, 339, 126642, DOI:10.1016/j.seppur.2024.126642.
- S. Sonkaria, K.-H. Ryu, V. Khare and H.-J. Kim, Packaging Applications of Biodegradable Nanocellulose Composites, in Handb. Biopolym., Springer Nature Singapore, Singapore, 2023, pp. 1–26, DOI:10.1007/978-981-16-6603-2_38-1.
- L. Hossain, R. M. B. Ledesma, J. Tanner and G. Garnier, Effect of crosslinking on nanocellulose superabsorbent biodegradability, Carbohydr. Polym. Technol. Appl., 2022, 3, 100199, DOI:10.1016/j.carpta.2022.100199.
- B. P. Frank, D. P. Durkin, E. R. Caudill, L. Zhu, D. H. White, M. L. Curry, J. A. Pedersen and D. H. Fairbrother, Impact of Silanization on the Structure, Dispersion Properties, and Biodegradability of Nanocellulose as a Nanocomposite Filler, ACS Appl. Nano Mater., 2018, 1, 7025–7038, DOI:10.1021/acsanm.8b01819.
- J. Yin, G. Huang, C. An, P. Zhang, X. Xin and R. Feng, Exploration of nanocellulose washing agent for the green remediation of phenanthrene-contaminated soil, J. Hazard. Mater., 2021, 403, 123861, DOI:10.1016/j.jhazmat.2020.123861.
- J. L. Sanchez-Salvador, H. Xu, A. Balea, A. Blanco and C. Negro, Enhancement of the production of TEMPO-mediated oxidation cellulose nanofibrils by kneading, Int. J. Biol. Macromol., 2024, 261, 129612, DOI:10.1016/j.ijbiomac.2024.129612.
- M. N. F. Norrrahim, N. A. Mohd Kasim, V. F. Knight, K. K. Ong, S. A. Mohd Noor, N. Abdul Halim, N. A. Ahmad Shah, S. H. Jamal, N. Janudin, M. S. M. Misenan, M. Z. Ahmad, M. H. Yaacob and W. M. Z. Wan Yunus, Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes, Polymers, 2021, 13, 3249, DOI:10.3390/polym13193249.
- A. James, M. R. Rahman, K. A. Mohamad Said, D. Kanakaraju, A. Z. Sueraya, K. K. Kuok, M. K. Bin Bakri and M. M. Rahman, A review of nanocellulose modification and compatibility barrier for various applications, J. Thermoplast. Compos. Mater., 2024, 37, 2149–2199, DOI:10.1177/08927057231205451.
- J. Peng, H. Zhang, X. Li, S. Fang, M. Duan, L. Wan and H. Zuo, Effect of concentration and functional group of cellulose nanocrystals on the rheological and filtration properties of water-based drilling fluids at various temperatures, Cellulose, 2024, 31, 5151–5169, DOI:10.1007/s10570-024-05890-0.
- P. Pande, Design of an Efficient Model for Microplastic Removal in Wastewater using Advanced Filtration, Nanotechnology, and Bioremediation, Commun, Appl. Nonlinear Anal., 2024, 32, 125–138, DOI:10.52783/cana.v32.2744.
- M. Guo, R. Noori and S. Abolfathi, Microplastics in freshwater systems: Dynamic behaviour and transport processes, Resour. Conserv. Recycl., 2024, 205, 107578, DOI:10.1016/j.resconrec.2024.107578.
- E. Wiśniowska, K. Moraczewska-Majkut, W. Nocoń and A. Popenda, Microplastics removal from natural surface water by coagulation process, Desalin. Water Treat., 2024, 319, 100462, DOI:10.1016/j.dwt.2024.100462.
- A. Singh, J. G. Vijayan and K. G. Moodley, Surface Functionalizations of Nanocellulose for Wastewater Treatment, in Handb. Nanocelluloses, Springer International Publishing, Cham, 2021, pp. 1–48, DOI:10.1007/978-3-030-62976-2_49-1.
- M. Ghasemlou, F. Daver, E. P. Ivanova, Y. Habibi and B. Adhikari, Surface modifications of nanocellulose: From synthesis to high-performance nanocomposites, Prog. Polym. Sci., 2021, 119, 101418, DOI:10.1016/j.progpolymsci.2021.101418.
- Y. Zhang, Y. Zhang, W. Xu, H. Wu, Y. Shao, X. Han, M. Zhou, P. Gu and Z. Li, Preparation methods of cellulose nanocrystal and its application in treatment of environmental pollution: A mini-review, Colloid Interface Sci. Commun., 2023, 53, 100707, DOI:10.1016/j.colcom.2023.100707.
- T. Kopač, M. Krajnc and A. Ručigaj, A rheological study of cationic micro- and nanofibrillated cellulose: quaternization reaction optimization and fibril characteristic effects, Cellulose, 2022, 29, 1435–1450, DOI:10.1007/s10570-021-04365-w.
- Q. Wang, X. Feng and X. Liu, Functionalization of nanocellulose using atom transfer radical polymerization and applications: a review, Cellulose, 2023, 30, 8495–8537, DOI:10.1007/s10570-023-05403-5.
- H. Jiao, J. Sun, Y. Shi, X. Lu, S. S. Ali, Y. Fu, H. Zhang, Y. Li, Q. Wang, M. Zhou and J. Liu, Recent advances in strategies of nanocellulose surface and/or interface engineering for potential biomedical applications as well as its ongoing challenges: a review, Cellulose, 2023, 30, 6741–6771, DOI:10.1007/s10570-023-05302-9.
- E. Megiel, Surface modification using TEMPO and its derivatives, Adv. Colloid Interface Sci., 2017, 250, 158–184, DOI:10.1016/j.cis.2017.08.008.
- K. Yamato, Y. Yoshida, Y. Kumamoto and A. Isogai, Surface modification of TEMPO-oxidized cellulose nanofibers, and properties of their acrylate and epoxy resin composite films, Cellulose, 2022, 29, 2839–2853, DOI:10.1007/s10570-021-04131-y.
- H. Chen, J. Jiang, S. Zhang, J. Yu, L. Liu and Y. Fan, Surface modification of cellulose originated from different plant sources through TEMPO/Laccase/O2 oxidation, Ind. Crops Prod., 2022, 176, 114295, DOI:10.1016/j.indcrop.2021.114295.
- R. P. Gandhiraman*, C. Volcke, V. Gubala, C. Doyle, L. Basabe-Desmonts, C. Dotzler, M. F. Toney, M. Iacono, R. I. Nooney, S. Daniels, B. James and D. E. Williams, High efficiency amine functionalization of cycloolefin polymer surfaces for biodiagnostics, J. Mater. Chem., 2010, 20, 4116, 10.1039/b925737c.
- N. I. Khan, S. Halder, N. Talukdar, S. Das and M. S. Goyat, Surface oxidized/silanized graphite nanoplatelets for reinforcing an epoxy matrix, Mater. Chem. Phys., 2021, 258, 123851, DOI:10.1016/j.matchemphys.2020.123851.
- Y. S. Kim and G. S. An, Optimizing Amine Functionalization of Maghemite Nanoparticles Through Controlled Hydroxylation and Silica Interlayer Engineering, Processes, 2025, 13, 1575, DOI:10.3390/pr13051575.
- A. Benkaddour, C. Rusin, E. C. Demir, C. Ayranci and M. McDermott, Cationic surface functionalization of cellulose nanocrystals and its effect on the mechanical properties of polyamide 6 thin films, Cellulose, 2023, 30, 7653–7665, DOI:10.1007/s10570-023-05313-6.
- W. Wang, C. Xue and X. Mao, Chitosan: Structural modification, biological activity and application, Int. J. Biol. Macromol., 2020, 164, 4532–4546, DOI:10.1016/j.ijbiomac.2020.09.042.
- S. Kim, Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities, Int. J. Polym. Sci., 2018, 2018, 1–13, DOI:10.1155/2018/1708172.
- I. Aranaz, M. Mengibar, R. Harris, I. Panos, B. Miralles, N. Acosta, G. Galed and A. Heras, Functional Characterization of Chitin and Chitosan, Curr. Chem. Biol., 2009, 3, 203–230, DOI:10.2174/2212796810903020203.
- X. Zhang, Y. Zhai, Z. Wang, Y. Zhou, C. Huang, L. Zhao and C. Ma, Effect of Microplastic Ingredient on the Removal of Microplastics by Calcium Alginate Flocculation, Chem. Eng. J., 2024, 23 DOI:10.2139/ssrn.4892747.
- F. Lotfigolsefidi, M. Davoudi, M. Sarkhosh and Z. Bonyadi, Removal of microplastics by algal biomass from aqueous solutions: performance, optimization, and modeling, Sci. Rep., 2025, 15, 501, DOI:10.1038/s41598-024-84114-8.
- H.-Y. Wang and Y.-R. Cheng, Highly Effective Removal of Microplastics by Microalgae Scenedesmus Abundans, SSRN Electron. J., 2021, 15, 501, DOI:10.2139/ssrn.3976141.
- R. Ma, Y. Feng, J. Yu, X. Zhao, Y. Du and X. Zhang, Ultralight sponge made from sodium alginate with processability and stability for efficient removal of microplastics, Environ. Sci. Pollut. Res., 2023, 30, 104135–104147, DOI:10.1007/s11356-023-29740-x.
- W. Gao, A. Mo, J. Jiang, Y. Liang, X. Cao and D. He, Removal of microplastics from water by coagulation of cationic-modified starch: An environmentally friendly solution, Sci. Total Environ., 2023, 904, 166787, DOI:10.1016/j.scitotenv.2023.166787.
- P. Hu, K. Su, Y. Sun, P. Li, J. Cai and H. Yang, Efficient removal of nano- and micro- sized plastics using a starch-based coagulant in conjunction with polysilicic acid, Sci. Total Environ., 2022, 850, 157829, DOI:10.1016/j.scitotenv.2022.157829.
- E+ELeader, A Sustainable Solution for Eliminating Microplastics and Nanoplastics: The Starch-Gelatin Sponge, 2025, https://www.environmentenergyleader.com/stories/a-sustainable-solution-for-eliminating-microplastics-and-nanoplastics-the-starch-gelatin-sponge,5007.
- M. T. Khan, M. Ahmad, M. F. Hossain, A. Nawab, I. Ahmad, K. Ahmad and S. Panyametheekul, Microplastic removal by coagulation: a review of optimizing the reaction conditions and mechanisms, Water Emerging Contam. Nanoplast., 2023, 2, 22, DOI:10.20517/wecn.2023.39.
- P. Li, J. Zhang, Y. Shen, X. Feng, W. Jia, M. Liu and S. Zhao, Efficient, quick, and low-carbon removal mechanism of microplastics based on integrated gel coagulation-spontaneous flotation process, Water Res., 2024, 259, 121906, DOI:10.1016/j.watres.2024.121906.
- I. Găgeanu, F. Carvalheiro, A. Ekielski and L. C. Duarte, Lignin Utilization For The Removal Of Microplastic Particles From Water, INMATEH – Agric. Eng., 2023, 511–521, DOI:10.35633/inmateh-71-44.
- V. P. Austeja Burbulyte and I. Uoginte, Study of different parameters impact to microplastic Removal from water using lignin-Magnetite nanosorbent, chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj, https://openreadings.eu/wp-content/latex/17060950419fbe7022/abstract.pdf.
- X.-H. Li, X. Qi, D. Nie, Y.-L. Leng and X.-H. Cai, Lignin/Polyvinyl Alcohol Hydrogel for Efficient Removal of Microplastics, SSRN, 2025 DOI:10.2139/ssrn.5160373.
- Y. Jiao, S. Wang, B. Sun, Y. Han, Z. Zhang, X. Shen and Z. Li, Adsorption efficiency and in-situ catalytic thermal degradation behaviour of microplastics from water over Fe-modified lignin-based magnetic biochar, Sep. Purif. Technol., 2025, 353, 128468, DOI:10.1016/j.seppur.2024.128468.
- L. Yu, L. Jiang, S. Wang, M. Sun, D. Li and G. Du, Pectin microgel particles as high adsorption rate material for methylene blue: Performance, equilibrium, kinetic, mechanism and regeneration studies, Int. J. Biol. Macromol., 2018, 112, 383–389, DOI:10.1016/j.ijbiomac.2018.01.193.
- U. Anand, S. Dey, E. Bontempi, S. Ducoli, A. D. Vethaak, A. Dey and S. Federici, Biotechnological methods to remove microplastics: a review, Environ. Chem. Lett., 2023, 21, 1787–1810, DOI:10.1007/s10311-022-01552-4.
- H. Qiu, J. Yan, G. Lan, Y. Liu, X. Song, W. Peng and Y. Cui, Removal of Cu 2+ from wastewater by modified xanthan gum (XG) with ethylenediamine (EDA), RSC Adv., 2016, 6, 83226–83233, 10.1039/C6RA11423G.
- ACS Chemistry Life, The carrageenans, 2023, https://www.acs.org/molecule-of-the-week/archive/c/carrageenans.html.
- J. Hale, J. Gerhäuser, V. Gaukel and D. Wefers, Commercially available carrageenans show broad variation in their structure, composition, and functionality, Eur. Food Res. Technol., 2024, 250, 2989–3003, DOI:10.1007/s00217-024-04605-w.
- J. A. Kazlauskaite, L. Ivanauskas and J. Bernatoniene, Cyclodextrin-Assisted Extraction Method as a Green Alternative to Increase the Isoflavone Yield from Trifolium pratensis L. Extract, Pharmaceutics, 2021, 13, 620, DOI:10.3390/pharmaceutics13050620.
- M. Q. Farooq, V. R. Zeger and J. L. Anderson, Comparing the extraction performance of cyclodextrin-containing supramolecular deep eutectic solvents versus conventional deep eutectic solvents by headspace single drop microextraction, J. Chromatogr. A, 2021, 1658, 462588, DOI:10.1016/j.chroma.2021.462588.
- Y. Wu, C. Ye, F. Liu, X. Gu, L. Yu, X. Shi, Y. Du, M. Ding, C. Chen and H. Deng, Highly Efficient, Recyclable Microplastic Adsorption Enabled by Chitin Hydrogen Bond Network Rearrangement, Adv. Funct. Mater., 2024, 34 DOI:10.1002/adfm.202311075.
- A. Karrat, A. Lamaoui, A. Amine, J. M. Palacios-Santander and L. Cubillana-Aguilera, Applications of Chitosan in Molecularly and Ion Imprinted Polymers, Chem. Afr., 2020, 3, 513–533, DOI:10.1007/s42250-020-00177-w.
- A. Balakrishnan, S. Appunni, M. Chinthala, M. M. Jacob, D.-V. N. Vo, S. S. Reddy and E. S. Kunnel, Chitosan-based beads as sustainable adsorbents for wastewater remediation: a review, Environ. Chem. Lett., 2023, 21, 1881–1905, DOI:10.1007/s10311-023-01563-9.
- J. Desbrières and E. Guibal, Chitosan for wastewater treatment, Polym. Int., 2018, 67, 7–14, DOI:10.1002/pi.5464.
- A. Aouadi, D. H. Saoud, A. Rebiai, S. E. Laouini, A. Achouri, A. Zaater, F. Alharthi, A. Bouafia, H. A. Mohammed, G. G. Hasan and J. A. A. Abdullah, Valorizing shrimp shell chitosan: a versatile biomaterial for fabricating effective antibacterial and antioxidant silver nanoparticles, J. Sol-Gel Sci. Technol., 2024, 112, 752–767, DOI:10.1007/s10971-024-06578-4.
- N. A. I. Muddin, M. M. Badsha, M. A. Arafath, Z. M. A. Merican and M. S. Hossain, Magnetic chitosan nanoparticles as a potential bio-sorbent for the removal of Cr(VI) from wastewater: Synthesis, environmental impact and challenges, Desalin. Water Treat., 2024, 319, 100449, DOI:10.1016/j.dwt.2024.100449.
- E. Lichtfouse, N. Morin-Crini, M. Fourmentin, H. Zemmouri, I. O. do Carmo Nascimento, L. M. Queiroz, M. Y. M. Tadza, L. A. Picos-Corrales, H. Pei, L. D. Wilson and G. Crini, Chitosan for direct bioflocculation of wastewater, Environ. Chem. Lett., 2019, 17, 1603–1621, DOI:10.1007/s10311-019-00900-1.
- S. X. Chin, C. H. Chia, N. H. A. Rosli and C. Wongchoosuk, Porous Chitosan Adsorbents for Wastewater Treatment, in Mech. Phys. Porous Mater., Apple Academic Press, New York, 2024, pp. 3–34, DOI:10.1201/9781003414469-2.
- M. T. Nazari, J. F. Freitag, V. A. F. Cavanhi and L. M. Colla, Microalgae harvesting by fungal-assisted bioflocculation, Rev. Environ. Sci. Bio/Technol., 2020, 19, 369–388, DOI:10.1007/s11157-020-09528-y.
- C. Cook and V. G. Gude, Characteristics of Chitosan Nanoparticles for Water and Wastewater Treatment, in Adv. Nanomater. Water Eng. Treat. Hydraul., 2017, pp. 223–261, DOI:10.4018/978-1-5225-2136-5.ch009.
- U. John Sunday, N. Nweke Chinenyenwa, O. Nwachukwu Josiah and O. Olufemi Gideon, Fabrication and Optimization of Alginate Membranes for Improved Wastewater Treatment, Arch. Case Reports, 2025, vol. 9, pp. 026–043, DOI:10.29328/journal.acr.1001125.
- X. Zhang, Y. Zhai, Z. Wang, Y. Zhou, C. Huang, L. Zhao and C. Ma, Effect of microplastic ingredient on the removal of microplastics by calcium alginate flocculation, Chem. Eng. J., 2024, 498, 155701, DOI:10.1016/j.cej.2024.155701.
- B. Wang, Y. Wan, Y. Zheng, X. Lee, T. Liu, Z. Yu, J. Huang, Y. S. Ok, J. Chen and B. Gao, Alginate-based composites for environmental applications: a critical review, Crit. Rev. Environ. Control, 2019, 49, 318–356, DOI:10.1080/10643389.2018.1547621.
- M. K. Rasmussen, J. Gold, M. W. Kaiser, J. Moritz, N. Räty, S. B. Rønning, T. Ryynänen, S. Skrivergaard, A. Ström, M. Therkildsen, H. L. Tuomisto and J. F. Young, Critical review of cultivated meat from a Nordic perspective, Trends Food Sci. Technol., 2024, 144, 104336, DOI:10.1016/j.tifs.2024.104336.
- M. Pagliaro, R. Ciriminna and S. M. Morozova, Sustainable optics? A critical insight into biopolymer-enabled optics, Tetrahedron Green Chem., 2023, 1, 100005, DOI:10.1016/j.tgchem.2023.100005.
- A. S. Mahmoud, M. S. Mahmoud, N. Y. Mohamed and M. K. Mostafa, Advanced Wastewater Treatment by Using Entrapped nZVI into Alginate (Ag) Biopolymer: Adsorption Isotherm, Kinetic Models, and Statistical Analysis, Key Eng. Mater., 2022, 921, 147–160, DOI:10.4028/p-xygkon.
- M. B. Ayoub Grouli, Y. Bachra, F. Damiri and V. U. Pandit, Removal of Pollutants from Wastewater using Fe-Doped Hydroxyapatite Encapsulated with Alginate, Biointerface Res. Appl. Chem., 2023, 13, 438, DOI:10.33263/BRIAC135.438.
- M. M. Jayakody, M. P. G. Vanniarachchy and I. Wijesekara, Seaweed derived alginate, agar, and carrageenan based edible coatings and films for the food industry: a review, J. Food Meas. Charact., 2022, 16, 1195–1227, DOI:10.1007/s11694-021-01277-y.
- O. Peter Adigwe, H. O. Egharevba and M. Ochubiojo Emeje, Starch: A Veritable Natural Polymer for Economic Revolution, in Starch - Evol. Recent Adv., 2022, DOI:10.5772/intechopen.102941.
- M. A. Dircio-Morales, H. A. Fonseca-Florido, G. Velazquez, C. A. Ávila-Orta, L. F. Ramos-De Valle, F. Hernández-Gámez, J. E. Rivera-Salinas and F. Soriano-Corral, Relationship among Extrusion Conditions, Cell Morphology, and Properties of Starch-Based Foams—A Review, Starch, 2023, 75, 2200103, DOI:10.1002/star.202200103.
- S. Tang, L. Gao, T. Zhao and A. Tian, Enhancing the removal efficiency of microplastics in drinking water treatment, J. Water Process Eng., 2024, 57, 104630, DOI:10.1016/j.jwpe.2023.104630.
- M. F. Mohd Amin, S. G. J. Heijman and L. C. Rietveld, Clay–starch combination for micropollutants removal from wastewater treatment plant effluent, Water Sci. Technol., 2016, 73, 1719–1727, DOI:10.2166/wst.2016.001.
- J. Fu, N. Liu, Y. Peng, G. Wang, X. Wang, Q. Wang, M. Lv and L. Chen, An ultra-light sustainable sponge for elimination of microplastics and nanoplastics, J. Hazard. Mater., 2023, 456, 131685, DOI:10.1016/j.jhazmat.2023.131685.
- N. K. Othman, E. M. Salleh, Z. Dasuki and A. M. Lazim, Dimethyl Sulfoxide-Treated Starch of Dioescorea hispida as a Green Corrosion Inhibitor for Low Carbon Steel in Sodium Chloride Medium, in Corros. Inhib. Princ. Recent Appl., InTech, 2018, DOI:10.5772/intechopen.73552.
- S. Manewal, J. Patadiya, B. Kandasubramanian and P. Mahajan-Tatpate, Additive manufactured membranes of polylactic acid for effluent treatment, Polym. Technol. Mater., 2023, 62, 1587–1609, DOI:10.1080/25740881.2023.2222825.
- R. M. Rasal, A. V. Janorkar and D. E. Hirt, Poly(lactic acid) modifications, Prog. Polym. Sci., 2010, 35, 338–356, DOI:10.1016/j.progpolymsci.2009.12.003.
- N. Shekhar and A. Mondal, Synthesis, properties, environmental degradation, processing, and applications of Polylactic Acid (PLA): an overview, Polym. Bull., 2024, 81, 11421–11457, DOI:10.1007/s00289-024-05252-7.
- B. Das, N. Soundararajan, S. P. Kashyap, J. Jang, C. T. Wang, A. R. Katha and V. Katiyar, Bioaugmented polyaniline decorated polylactic acid nanofiber electrode by electrospinning technique for real wastewater-fed MFC application, Int. J. Energy Res., 2022, 46, 3588–3601, DOI:10.1002/er.7407.
- J. Goodsel and S. Madbouly, Biodegradable polylactic acid (PLA), Phys. Sci. Rev., 2023, 8, 869–894, DOI:10.1515/psr-2020-0072.
- E. Petinakis, L. Yu, G. Simon and K. De, Natural Fibre Bio-Composites Incorporating Poly(Lactic Acid), in Fiber Reinf. Polym. - Technol. Appl. Concr. Repair, InTech, 2013, DOI:10.5772/52253.
- M. A. Sayam, M. Al-amin, R. Zhou and A. Al Mamun, A BRIEFREVIEW OF 3D PRINTING TECHNOLOGIES AND MATERIALS USED IN SMART TEXTILES, Tekst. Muhendis, 2025, 32, 305–318, DOI:10.7216/teksmuh.1599672.
- H. Khalil, V. S. Wadi, H. M. Hegab, L. Nassar, V. Naddeo, A. F. Yousef, F. Banat and S. W. Hasan, High-performance f-GO/MWCNTs-COOH nanohybrid-based polylactic acid mixed matrix membrane for wastewater treatment, J. Water Process Eng., 2023, 53, 103784, DOI:10.1016/j.jwpe.2023.103784.
- L. Nassar, V. S. Wadi, H. M. Hegab, H. Khalil, F. Banat, V. Naddeo and S. W. Hasan, Sustainable and green polylactic acid-based membrane embedded with self-assembled positively charged f-MWCNTs/GO nanohybrids for the removal of nutrients from wastewater, npj Clean Water, 2022, 5, 57, DOI:10.1038/s41545-022-00206-w.
- C. Cunha, L. Silva, J. Paulo, M. Faria, N. Nogueira and N. Cordeiro, Microalgal-based biopolymer for nano- and microplastic removal: a possible biosolution for wastewater treatment, Environ. Pollut., 2020, 263, 114385, DOI:10.1016/j.envpol.2020.114385.
- N. Jagadeesh and B. Sundaram, Adsorption of Pollutants from Wastewater by Biochar: A Review, J. Hazard. Mater. Adv., 2023, 9, 100226, DOI:10.1016/j.hazadv.2022.100226.
- M. T. Sturm, H. Horn and K. Schuhen, Removal of Microplastics from Waters through Agglomeration-Fixation Using Organosilanes—Effects of Polymer Types, Water Composition and Temperature, Water, 2021, 13, 675, DOI:10.3390/w13050675.
- Z. Wang, M. Sedighi and A. Lea-Langton, Filtration of microplastic spheres by biochar: removal efficiency and immobilisation mechanisms, Water Res., 2020, 184, 116165, DOI:10.1016/j.watres.2020.116165.
- D. Qian, J. Zhao, W. Zhai, J. Tang and J. Zhang, Acrylamide Cross-Linked Psyllium Polysaccharide with Improved Flocculation Performance for the Removal of Microplastics from Water, ChemistrySelect, 2024, 9, e202401084, DOI:10.1002/slct.202401084.
- F. Wang, Y. Ma, Y. Liu, Z. Cui, X. Ying, F. Zhang and R. J. Linhardt, A simple strategy for the separation and purification of water-soluble polysaccharides from the fresh Spirulina platensis, Sep. Sci. Technol., 2017, 52, 456–466, DOI:10.1080/01496395.2016.1244549.
- B. Liu, J. Ma, T. Li, P. Li, D. Yan, J. Zhu and X. Zhang, Advances in the Preparation, Structure and Bioactivity of Polysaccharides from Lycium ruthenicum Murr.: A Review, Foods, 2024, 13, 1995, DOI:10.3390/foods13131995.
- N. Fattahi, T. Fattahi, M. Kashif, A. Ramazani and W.-K. Jung, Lignin: A valuable and promising bio-based absorbent for dye removal applications, Int. J. Biol. Macromol., 2024, 276, 133763, DOI:10.1016/j.ijbiomac.2024.133763.
- P. Santander, B. Butter, E. Oyarce, M. Yáñez, L.-P. Xiao and J. Sánchez, Lignin-based adsorbent materials for metal ion removal from wastewater: A review, Ind. Crops Prod., 2021, 167, 113510, DOI:10.1016/j.indcrop.2021.113510.
- A. A. Pam, G. A. Obiyenwa, C. N. Abah, A. A. Adeyi, A. W. Ojoniko, E. I. Ibrahim and B. O. Teslim, Preparation of Citrate Porous Activated Carbon and Its Application in Adsorption Modeling of Zn(II) from Aqueous Environment, Chem. Afr., 2024, 7, 2049–2059, DOI:10.1007/s42250-024-00886-6.
- A. Almahri, M. Morad, M. M. Aljohani, N. M. Alatawi, F. A. Saad, H. M. Abumelha, M. G. El-Desouky and A. A. El-Bindary, Atrazine reclamation from an aqueous environment using a ruthenium-based metal-organic framework, Process Saf. Environ. Prot., 2023, 177, 52–68, DOI:10.1016/j.psep.2023.06.091.
- Y. Wang, Q. Wang, S. Sabaghi, A. Kaboli, F. Soltani, K. Kang, C. Kongvarhodom and P. Fatehi, Dual lignin-derived polymeric system for peptone removal from simulated wastewater, Environ. Pollut., 2024, 343, 123142, DOI:10.1016/j.envpol.2023.123142.
- C. Moore, W. Gao and P. Fatehi, Cationic Lignin Polymers as Flocculant for Municipal Wastewater, Polymers, 2021, 13, 3871, DOI:10.3390/polym13223871.
- E. Mutegoa, Efficient techniques and practices for wastewater treatment: an update, Discover Water, 2024, 4, 69, DOI:10.1007/s43832-024-00131-8.
- S. Song, Q. Li, G. Leslie and Y. Shen, Water treatment methods in heavy metals removal during photovoltaic modules recycling: a review, Resour. Conserv. Recycl., 2024, 208, 107701, DOI:10.1016/j.resconrec.2024.107701.
- R. Kaur, S. Sandhu, A. K. Pujari, S. Paul and J. Bhaumik, Lignin-Derived Metal Oxide Nanoadsorbents for Wastewater Remediation, ChemistrySelect, 2024, 9, e202402934, DOI:10.1002/slct.202402934.
- Y. Yu, X. Zhou, Y. Jiang, Y. Ji, X. Fang and Y. Zhang, Synthesis of eco-friendly lignin-betaine and its application for dye wastewater treatment, Ind. Crops Prod., 2023, 192, 116014, DOI:10.1016/j.indcrop.2022.116014.
- W. Tang, H. Li, L. Fei, B. Wei, T. Zhou and H. Zhang, The removal of microplastics from water by coagulation: A comprehensive review, Sci. Total Environ., 2022, 851, 158224, DOI:10.1016/j.scitotenv.2022.158224.
- M. Lapointe, J. M. Farner, L. M. Hernandez and N. Tufenkji, Understanding and Improving Microplastic Removal during Water Treatment: Impact of Coagulation and Flocculation, Environ. Sci. Technol., 2020, 54, 8719–8727, DOI:10.1021/acs.est.0c00712.
- Q. Ali, M. A. Zia, M. Kamran, M. Shabaan, U. Zulfiqar, M. Ahmad, R. Iqbal and M. F. Maqsood, Nanoremediation for heavy metal contamination: A review, Hybrid, Adv, 2023, 4, 100091, DOI:10.1016/j.hybadv.2023.100091.
- I. M. Chathuranika, E. Sachinthanie, P. Zam, M. B. Gunathilake, D. Denkar, N. Muttil, A. Abeynayaka, K. Kantamaneni and U. Rathnayake, Assessing the water quality and status of water resources in urban and rural areas of Bhutan, J. Hazard. Mater. Adv., 2023, 12, 100377, DOI:10.1016/j.hazadv.2023.100377.
- B. Ma, W. Xue, C. Hu, H. Liu, J. Qu and L. Li, Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment, Chem. Eng. J., 2019, 359, 159–167, DOI:10.1016/j.cej.2018.11.155.
- H. Zhang, H. Deng, H. Ou, R. Liu, Y. Chen, X. Wu and J. Fu, Optimizing Microplastic Removal Through Coagulation-Sedimentation with Permanganate Pre-oxidation and Pre-chlorination, Water, Air, Soil Pollut., 2024, 235, 86, DOI:10.1007/s11270-024-06895-y.
- P. Maćczak, H. Kaczmarek and M. Ziegler-Borowska, Application of Chitosan and Its Derivatives as Bioflocculants for Iron and Turbidity Removal from Filter Backwash Water, Water, 2023, 15, 2913, DOI:10.3390/w15162913.
- B. Li, J. Zhao, W. Ge, W. Li and H. Yuan, Coagulation-flocculation performance and floc properties for microplastics removal by magnesium hydroxide and PAM, J. Environ. Chem. Eng., 2022, 10, 107263, DOI:10.1016/j.jece.2022.107263.
- M. M. A. Awan, T. Malkoske, H. Almuhtaram and R. C. Andrews, Microplastic removal in batch and dynamic coagulation-flocculation-sedimentation systems is controlled by floc size, Sci. Total Environ., 2024, 909, 168631, DOI:10.1016/j.scitotenv.2023.168631.
- A. M. Elgarahy, M. G. Eloffy, A. K. Priya, V. Yogeshwaran, K. Z. Elwakeel, Z. Yang and E. A. Lopez-Maldonado, A review on the synergistic efficacy of sonication-assisted water treatment process with special attention given to microplastics, Chem. Eng. Res. Des., 2024, 206, 524–552, DOI:10.1016/j.cherd.2024.05.027.
- Y. Cui, W. Yuan and J. Cheng, Understanding pH and Ionic Strength Effects on Aluminum Sulfate-Induced Microalgae Flocculation, Appl. Biochem. Biotechnol., 2014, 173, 1692–1702, DOI:10.1007/s12010-014-0957-4.
- S. B. Ummalyma, A. K. Mathew, A. Pandey and R. K. Sukumaran, RETRACTED: Harvesting of microalgal biomass: Efficient method for flocculation through pH modulation, Bioresour. Technol., 2016, 213, 216–221, DOI:10.1016/j.biortech.2016.03.114.
- B. Cao, B. Gao, C. Xu, Y. Fu and X. Liu, Effects of pH on coagulation behavior and floc properties in Yellow River water treatment using ferric based coagulants, Chin. Sci. Bull., 2010, 55, 1382–1387, DOI:10.1007/s11434-010-0087-5.
- A. E. Metaxas, V. Panwar, R. L. Olson and C. S. Dutcher, Ionic strength and polyelectrolyte molecular weight effects on floc formation and growth in Taylor–Couette flows, Soft Matter, 2021, 17, 1246–1257, 10.1039/D0SM01517B.
- X. Li, Y. Cheng, C. Yi, Y. Hua, C. Yang and S. Cui, Effect of ionic strength on the heat-induced soy protein aggregation and the phase separation of soy protein aggregate/dextran mixtures, Food Hydrocolloids, 2009, 23, 1015–1023, DOI:10.1016/j.foodhyd.2008.07.024.
- N. Wilkinson, A. Metaxas, E. Brichetto, S. Wickramaratne, T. M. Reineke and C. S. Dutcher, Ionic strength dependence of aggregate size and morphology on polymer-clay flocculation, Colloids Surf., A, 2017, 529, 1037–1046, DOI:10.1016/j.colsurfa.2017.06.085.
- Y. X. Zhao, H. K. Shon, S. Phuntsho and B. Y. Gao, Removal of natural organic matter by titanium tetrachloride: The effect of total hardness and ionic strength, J. Environ. Manage., 2014, 134, 20–29, DOI:10.1016/j.jenvman.2014.01.002.
- P. Agarwal, S. Prakash and G. Saini, Natural coagulants (Moringa oleifera and Benincasa hispida) based removal of microplastics, Clean, Water, 2024, 1, 100010, DOI:10.1016/j.clwat.2024.100010.
- C. Li, R. Busquets and L. C. Campos, Enhancing microplastic removal from natural water using coagulant aids, Chemosphere, 2024, 364, 143145, DOI:10.1016/j.chemosphere.2024.143145.
- L. Huang, W. He, Y. Zhang, X. Wang, K. Wu, Z. Yang and J. Zhang, Chitosan enhances poly aluminum chloride flocculation system removal of microplastics: Effective, stable, and pollution free, J. Water Process Eng., 2023, 54, 103929, DOI:10.1016/j.jwpe.2023.103929.
- C. Panigrahi, S. Kamal, J. Qin, S. Ziemann, E. Rahman, M. House, C. Dutcher and B. Xiong, Removal of Pristine and UV-Weathered Microplastics from Water: Moringa oleifera Seed Protein as a Natural Coagulant, Environ. Eng. Sci., 2024, 41, 477–489, DOI:10.1089/ees.2024.0135.
- M. Zhao, L. Huang, S. R. B. Arulmani, J. Yan, L. Wu, T. Wu, H. Zhang and T. Xiao, Adsorption of Different Pollutants by Using Microplastic with Different Influencing Factors and Mechanisms in Wastewater: A Review, Nanomaterials, 2022, 12, 2256, DOI:10.3390/nano12132256.
- N. Narwal, M. A. Kakakhel, D. Katyal, S. Yadav, P. K. Rose, E. R. Rene, M. R. J. Rakib, K. S. Khoo and N. Kataria, Interactions Between Microplastic and Heavy Metals in the Aquatic Environment: Implications for Toxicity and Mitigation Strategies, Water, Air, Soil Pollut., 2024, 235, 567, DOI:10.1007/s11270-024-07343-7.
- C. Geng, Y. Gao, H. Zhang, D. Xue, H. Shan, B. Wang, X. Wang and J. Zhao, Microplastic migration in porous media at various scales: a review, Environ. Chem. Lett., 2024, 22, 691–713, DOI:10.1007/s10311-023-01688-x.
- R. A. Funari Junior, L. M. Frescura, B. B. de Menezes and M. B. da Rosa, 4-Nonylphenol adsorption, environmental impact and remediation: a review, Environ. Chem. Lett., 2025, 23, 241–269, DOI:10.1007/s10311-024-01788-2.
- H. Li, M. Tang, J. Wang, L. Dong, L. Wang, Q. Liu, Q. Huang and S. Lu, Theoretical and experimental investigation on rapid and efficient adsorption characteristics of microplastics by magnetic sponge carbon, Sci. Total Environ., 2023, 897, 165404, DOI:10.1016/j.scitotenv.2023.165404.
- X. Fu, S. Zhang, X. Zhang, Y. Zhang, B. Li, K. Jin, X. Feng, J. Hong, X. Huang, H. Cao, Q. Yuan, P. Ai, H. Yu and Q. Li, Sustainable Microplastic Remediation with Record Capacity Unleashed via Surface Engineering of Natural Fungal Mycelium Framework, Adv. Funct. Mater., 2023, 33, 2212570, DOI:10.1002/adfm.202212570.
- L. Song, Y. Liu, S. Xiao, Y. Li, H. Yu, Y. Zeng and X. Han, Exploring the adsorption potential of different Ganoderma lucidum mycelium morphologies for microplastic removal, Colloids Surf., A, 2025, 720, 137173, DOI:10.1016/j.colsurfa.2025.137173.
- F. Yuan, L. Yue, H. Zhao and H. Wu, Study on the adsorption of polystyrene microplastics by three-dimensional reduced graphene oxide, Water Sci. Technol., 2020, 81, 2163–2175, DOI:10.2166/wst.2020.269.
- R. Upadhyay, S. Singh and G. Kaur, Sorption of pharmaceuticals over microplastics' surfaces: interaction mechanisms and governing factors, Environ. Monit. Assess., 2022, 194, 803, DOI:10.1007/s10661-022-10475-0.
- M. Tang, C. Wu, J. Liu, G. Li, Y. Wang, Y. Zhao and G. Zhang, Effective adsorption of Rhodamine B by N/O Co-doped AC@CNTs composites prepared through catalytic coal pyrolysis, Surf. Interfaces, 2024, 46, 103972, DOI:10.1016/j.surfin.2024.103972.
- R. Alrowais, M. T. Bashir, A. A. Khan, M. Bashir, I. Abbas and M. M. Abdel Daiem, Adsorption and Kinetics Modelling for Chromium (Cr6+) Uptake from Contaminated Water by Quaternized Date Palm Waste, Water, 2024, 16, 294, DOI:10.3390/w16020294.
- Y. Na, S. H. Weon, G.-W. Lee, H. J. Kim, S. H. Lee, Y.-H. Kim, J. E. Kim, G. Kang, S. Park and Y.-K. Choi, Evaluation of Benzene Adsorption onto Grass-Derived Biochar and Comparison of Adsorption Capacity via RSM (Response Surface Methodology), J. Compos. Sci., 2024, 8, 132, DOI:10.3390/jcs8040132.
- A. D. Zand and M. R. Abyaneh, Adsorption of Cadmium from Landfill Leachate on Wood-Derived Biochar: Non-linear Regression Analysis, Environ. Processes, 2020, 7, 1129–1150, DOI:10.1007/s40710-020-00461-4.
- J. M. Angosto, J. M. Obón, M. J. Roca, M. Alacid, J. A. Fernández-López and A. Promising, Highly Effective Nitrate Sorbent Derived from Solid Olive Mill Residues, Agronomy, 2023, 13, 1325, DOI:10.3390/agronomy13051325.
- T. Chenet, M. Mancinelli, E. Sarti, V. Costa, C. D'Anna, A. Martucci and L. Pasti, Competitive Adsorption of 4-Hydroxybenzaldehyde and Toluene onto High Silica Zeolites, Environ. Processes, 2024, 11, 49, DOI:10.1007/s40710-024-00726-2.
- N. A. Rosli, M. A. Ahmad and T. U. Noh, Nature's waste turned savior: Optimizing pineapple peel–based activated carbon for effective Remazol Brilliant Violet dye adsorption using response surface methodology, Inorg. Chem. Commun., 2023, 153, 110844, DOI:10.1016/j.inoche.2023.110844.
- M. Michalska, P. Pietrzyk-Thel, K. Sobczak, M. Janssen and A. Jain, Carbon framework modification; an interesting strategy to improve the energy storage and dye adsorption, Energy Adv., 2024, 3, 1354–1366, 10.1039/D4YA00159A.
- Asadullah, K. Ngiwngam, J. Han, P. Rachtanapun, R. Auras, T. Karbowiak, D. Noiwan, M. Thongngam and W. Tongdeesoontorn, Creation of Composite Aerogels Consisting of Activated Carbon and Nanocellulose Blended with Cross-Linked Biopolymers: Application as Ethylene Scavengers, Polymers, 2024, 16, 3081, DOI:10.3390/polym16213081.
- M. Bassyouni, M. S. Zoromba, M. H. Abdel-Aziz and I. Mosly, Extraction of Nanocellulose for Eco-Friendly Biocomposite Adsorbent for Wastewater Treatment, Polymers, 2022, 14, 1852, DOI:10.3390/polym14091852.
- Y. Wu, S. Chen, J. Wu, F. Liu, C. Chen, B. Ding, X. Zhou and H. Deng, Revivable self-assembled supramolecular biomass fibrous framework for efficient microplastic removal, Sci. Adv., 2024, 10, eadn8662, DOI:10.1126/sciadv.adn8662.
- C. F. Mok, Y. C. Ching, N. A. A. Osman, F. Muhamad, N. D. Hai, J. H. Choo and C. R. Hassan, Adsorbents for removal of cationic dye: nanocellulose reinforced biopolymer composites, J. Polym. Res., 2020, 27, 373, DOI:10.1007/s10965-020-02347-3.
- Z. Hoek, E. L. du Toit, D. Niemand, J. Wesley-Smith and W. W. Focke, Dried nanocellulose/xanthan as reinforcing fillers in thermoplastic starch, Cellulose, 2024, 31, 6733–6746, DOI:10.1007/s10570-024-06006-4.
- H. Ma, X. Zheng, X. Luo, Y. Yi and F. Yang, Simulation and Analysis of Mechanical Properties of Silica Aerogels: From Rationalization to Prediction, Materials, 2018, 11, 214, DOI:10.3390/ma11020214.
- K. Mohanrasu, A. C. Manivannan, H. J. R. Rengarajan, R. Kandaiah, A. Ravindran, L. Panneerselvan, T. Palanisami and C. I. Sathish, Eco-friendly biopolymers and composites: a sustainable development of adsorbents for the removal of pollutants from wastewater, npj Mater. Sustain., 2025, 3, 13, DOI:10.1038/s44296-025-00057-9.
- P. Feng, J. He, S. Peng, C. Gao, Z. Zhao, S. Xiong and C. Shuai, Characterizations and interfacial reinforcement mechanisms of multicomponent biopolymer based scaffold, Mater. Sci. Eng., C, 2019, 100, 809–825, DOI:10.1016/j.msec.2019.03.030.
- S. Wang, L. Zhang, K. A. Khan, M. W. Ullah, Y. Fan and Z. Wang, Deep eutectic solvents as a ‘multifunctional operating platform’ for nanocellulose production and modification: A review on sustainable composite advancements, Mater. Sci. Eng., R, 2025, 165, 101025, DOI:10.1016/j.mser.2025.101025.
- A. R. Lokanathan, E. Kontturi, M. B. Linder, O. J. Rojas, O. Ikkala and A. H. Gröschel, Nanocellulose-Based Materials in Supramolecular Chemistry, in Compr. Supramol. Chem. II, Elsevier, 2017, pp. 351–364, DOI:10.1016/B978-0-12-409547-2.12531-4.
- Y. Uetsuji, S. Higuchi, K. Murayama and K. Aoki, Interfacial adhesion of a grafted polymer on a cellulose surface: a first-principles study, J. Mater. Sci., 2021, 56, 3589–3599, DOI:10.1007/s10853-020-05463-z.
- L. Chen, L. Yu, L. Qi, S. J. Eichhorn, A. Isogai, E. Lizundia, J. Y. Zhu and C. Chen, Cellulose nanocomposites by supramolecular chemistry engineering, Nat. Rev. Mater., 2025, 10, 728–749, DOI:10.1038/s41578-025-00810-5.
- B. Thomas, M. C. Raj, A. K. B, R. M. H, J. Joy, A. Moores, G. L. Drisko and C. Sanchez, Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications, Chem. Rev., 2018, 118, 11575–11625, DOI:10.1021/acs.chemrev.7b00627.
- I. Ali and V. K. Gupta, Advances in water treatment by adsorption technology, Nat. Protoc., 2006, 1, 2661–2667, DOI:10.1038/nprot.2006.370.
- A. Maged, O. E. A. Al-Hagar, S. Ahmed Abu El-Magd, S. Kharbish, A. Bhatnagar and D. Abol-Fotouh, Bacterial nanocellulose-clay film as an eco-friendly sorbent for superior pollutants removal from aqueous solutions, Environ. Res., 2024, 257, 119231, DOI:10.1016/j.envres.2024.119231.
- R. Das, T. Lindström, P. R. Sharma, K. Chi and B. S. Hsiao, Nanocellulose for Sustainable Water Purification, Chem. Rev., 2022, 122, 8936–9031, DOI:10.1021/acs.chemrev.1c00683.
- S. Mbakop, L. N. Nthunya and M. S. Onyango, Recent Advances in the Synthesis of Nanocellulose Functionalized–Hybrid Membranes and Application in Water Quality Improvement, Processes, 2021, 9, 611, DOI:10.3390/pr9040611.
- J. Rajvanshi, M. Sogani, A. Kumar and S. Arora, Biomaterials: A Sustainable Solution for a Circular Economy, in RAiSE-2023, MDPI, Basel Switzerland, 2023, p. 133, DOI:10.3390/engproc2023059133.
- K. Mohanrasu, A. C. Manivannan, H. J. R. Rengarajan, R. Kandaiah, A. Ravindran, L. Panneerselvan, T. Palanisami and C. I. Sathish, Eco-friendly biopolymers and composites: a sustainable development of adsorbents for the removal of pollutants from wastewater, npj Mater. Sustain., 2025, 3, 13, DOI:10.1038/s44296-025-00057-9.
- G. Mannina, D. Presti, G. Montiel-Jarillo, J. Carrera and M. E. Suárez-Ojeda, Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review, Bioresour. Technol., 2020, 297, 122478, DOI:10.1016/j.biortech.2019.122478.
- M. B. Ahmed, J. L. Zhou, H. H. Ngo, W. Guo and M. Chen, Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater, Bioresour. Technol., 2016, 214, 836–851, DOI:10.1016/j.biortech.2016.05.057.
- S. Qu, Y. Hu, R. Wei, K. Yu, Z. Liu, Q. Zhou, C. Wang and L. Zhang, Carbon Footprint Drivers in China’s Municipal Wastewater Treatment Plants and Mitigation Opportunities through Electricity and Chemical Efficiency, Engineering, 2025, 50, 106–116, DOI:10.1016/j.eng.2024.01.021.
- Y. Wang, W. Gao, L. Lv, X. Ma, Z. Ren, L. Sun, X. Liu, P. Wang, Z. Sun, Y. Tian and G. Zhang, Comprehensive carbon footprint analysis of wastewater treatment: A case study of modified cyclic activated sludge technology for low carbon source urban wastewater treatment, Sci. Total Environ., 2024, 923, 171550, DOI:10.1016/j.scitotenv.2024.171550.
- L. Chen, T. Bi, E. Lizundia, A. Liu, L. Qi, Y. Ma, J. Huang, Z. Lu, L. Yu, H. Deng and C. Chen, Biomass waste-assisted micro(nano)plastics capture, utilization, and storage for sustainable water remediation, Innov, 2024, 5, 100655, DOI:10.1016/j.xinn.2024.100655.
- Q. Li, S. McGinnis, C. Sydnor, A. Wong and S. Renneckar, Nanocellulose Life Cycle Assessment, ACS Sustain. Chem. Eng., 2013, 1, 919–928, DOI:10.1021/sc4000225.
- E. Aigaje, A. Riofrio and H. Baykara, Processing, Properties, Modifications, and Environmental Impact of Nanocellulose/Biopolymer Composites: A Review, Polymers, 2023, 15, 1219, DOI:10.3390/polym15051219.
- F. Golbabaei, J. Bunker, T. Yigitcanlar, D. Baker, A. Mirhashemi and A. Paz, Sustainable end-of-life management of abandoned and derelict vessels through the lens of circular economy, J. Cleaner Prod., 2024, 474, 143559, DOI:10.1016/j.jclepro.2024.143559.
- A. K. Bharimalla, S. P. Deshmukh, P. G. Patil and N. Vigneshwaran, Energy Efficient Manufacturing of Nanocellulose by Chemo- and Bio-Mechanical Processes: A Review, World J. Nano Sci. Eng., 2015, 05, 204–212, DOI:10.4236/wjnse.2015.54021.
- W. Saibuatrong, N. Cheroennet and U. Suwanmanee, Life cycle assessment focusing on the waste management of conventional and bio-based garbage bags, J. Cleaner Prod., 2017, 158, 319–334, DOI:10.1016/j.jclepro.2017.05.006.
- B. S. Ramadan, Y. G. Wibowo, D. Anwar and A. T. Maryani, A Review of Life Cycle Assessment of Nanomaterials-Based Adsorbent for Environmental Remediation, Global NEST J., 2024, 26, 1–18, DOI:10.30955/gnj.06216.
- M. Hervy, S. Evangelisti, P. Lettieri and K.-Y. Lee, Life cycle assessment of nanocellulose-reinforced advanced fibre composites, Compos. Sci. Technol., 2015, 118, 154–162, DOI:10.1016/j.compscitech.2015.08.024.
- E. O. Akindele, S. M. Ehlers and J. H. E. Koop, Freshwater insects of different feeding guilds ingest microplastics in two Gulf of Guinea tributaries in Nigeria, Environ. Sci. Pollut. Res., 2020, 27, 33373–33379, DOI:10.1007/s11356-020-08763-8.
- G. G. Deme, D. Ewusi-Mensah, O. A. Olagbaju, E. S. Okeke, C. O. Okoye, E. C. Odii, O. Ejeromedoghene, E. Igun, J. O. Onyekwere, O. K. Oderinde and E. Sanganyado, Macro problems from microplastics: Toward a sustainable policy framework for managing microplastic waste in Africa, Sci. Total Environ., 2022, 804, 150170, DOI:10.1016/j.scitotenv.2021.150170.
- D. Sol, A. Laca, A. Laca and M. Díaz, Approaching the environmental problem of microplastics: Importance of WWTP treatments, Sci. Total Environ., 2020, 740, 140016, DOI:10.1016/j.scitotenv.2020.140016.
- A.-P. E. Cooperation, Microplastics in Coastal Aquaculture Systems: Development of Regulatory Frameworks, Practices and Mitigation Efforts in APEC Economies, 2023. https://prod-statistics.apec.org/publications/2023/06/microplastics-in-coastal-aquaculture-systems-development-of-regulatory-frameworks-practices-and-mitigation-efforts-in-apec-economies Search PubMed.
- HELCOM, Baltic Sea Action Plan, 2021. https://helcom.fi/baltic-sea-action-plan/ Search PubMed.
- F. Liu, Y. Wu, M. Long, Y. Ma, M. Zheng, S. Cao, S. Chen, Y. Du, C. Chen and H. Deng, Activating Adsorption Sites of Waste Crayfish Shells via Chemical Decalcification for Efficient Capturing of Nanoplastics, ACS Nano, 2024, 18, 15779–15789, DOI:10.1021/acsnano.4c02511.
- E. Commission, Plastics Strategy, 2023, https://environment.ec.europa.eu/strategy/plastics-strategy_en Search PubMed.
- E. Strady, T.-C. Kieu-le, T.-N.-S. Truong, P.-D. Nguyen, N.-B. Pham and Y. Inamura, Riverine Microplastic Pollution in Vietnam: A Review of Current Scientific Knowledge and Legal Policies, Appl. Environ. Res., 2023, 45, 014, DOI:10.35762/AER.2023014.
- A. Adelugba and C. Emenike, Comparative Review of Instrumental Techniques and Methods for the Analysis of Microplastics in Agricultural Matrices, Microplastics, 2023, 3, 1–24, DOI:10.3390/microplastics3010001.
- J. Niskanen, J. Anshelm and D. McLaren, Local conflicts and national consensus: The strange case of circular economy in Sweden, J. Cleaner Prod., 2020, 261, 121117, DOI:10.1016/j.jclepro.2020.121117.
- N. T. Tung, T. T. Y Nhi, T. D. Cong, T. T. Thanh Hop and D. T. Mai, Nanocellulose as promising reinforcement materials for biopolymer nanocomposites: a review, Vietnam J. Sci. Technol., 2024, 62, 197–221, DOI:10.15625/2525-2518/18831.
- K. Shanmugam, Nanocellulose as Sustainable Eco-friendly Nanomaterials: Production, Characterization, and Applications, Dermatological Heal., 2024, 2, 8–33, DOI:10.26689/dh.v2i3.7611.
- P. Saraswat, S. Singh, M. Prasad, R. Misra, V. D. Rajput and R. Ranjan, Applications of bio-based nanomaterials in environment and agriculture: A review on recent progresses, Hybrid Adv, 2023, 4, 100097, DOI:10.1016/j.hybadv.2023.100097.
- M. M. Ansari, Y. Heo, K. Do, M. Ghosh and Y.-O. Son, Nanocellulose derived from agricultural biowaste by-products–Sustainable synthesis, biocompatibility, biomedical applications, and future perspectives: A review, Carbohydr. Polym. Technol. Appl., 2024, 8, 100529, DOI:10.1016/j.carpta.2024.100529.
- K. Shanmugam, P. Dhanasekaran and V. Santhosh, Utilization of Nanocellulose as an Eco-friendly Sustainable Nanomaterial for Potential Pathway to Circular Economy and Sustainability, Soc. Sci. Res., 2024, 6, 10–21, DOI:10.26689/ssr.v6i10.8165.
- T. Islam, M. R. Repon, M. A. Alim, T. Islam and S. Shukhratov, Detection and Characterisation Techniques for Microfiber in Wastewater, in Microfiber Pollut., CRC Press, Boca Raton, 2024, pp. 63–81, DOI:10.1201/9781003529903-4.
- T. Islam, F. Mushtari, M. R. Repon and M. H. Rahaman, Marine Microfiber Pollution, in Microfiber Pollut., CRC Press, Boca Raton, 2024, pp. 102–123, DOI:10.1201/9781003529903-6.
Footnotes |
| † Py-GC/MS: a thermoanalytical method that thermally decomposes polymers into fragments for identification via gas chromatography and mass spectrometry. |
| ‡ MFI: an optical technique for real-time, in situ analysis of suspended microplastic particles in liquids. |
| This journal is © The Royal Society of Chemistry 2026 |