Open Access Article
Open Access ArticleAccessing biominerals from by-products wasted by the seafood processing industry
Sarah
Boudreau
a,
Edmond
Lam
 b and
Francesca M.
Kerton
b and
Francesca M.
Kerton
 *a
*a
aDepartment of Chemistry, Memorial University of Newfoundland, St. John's, NL, Canada. E-mail: fkerton@mun.ca
bGreen Chemistry Institute, American Chemical Society, Washington, DC, USA
First published on 10th October 2025
Abstract
Due to the global population's significant growth, the demand for seafood has increased exponentially over the last century. This has led to the generation of large amounts of inedible by-products from the seafood processing industry from a multitude of marine organisms, including finfish, mollusks, and crustaceans. In this review, the potential extraction and application of biominerals from waste bones and shells originating from the seafood processing industry are discussed. Existing reviews on fishery by-products often highlight only the potential of organic by-products and disregard the biominerals present. Shells always contain calcium carbonate, CaCO3, in the form of either calcite or aragonite and bones are primarily made of hydroxyapatite, Ca10(PO4)6(OH)2. The conditions used in different extraction processes to isolate the mentioned biominerals and the resulting products are compared. Furthermore, we highlight the sustainability impact of sourcing natural biominerals for global applications (e.g., limestone industry) as this would prevent further environmental risks caused by improper disposal. Bio-derived minerals have also been used in other applications, such as environmental remediation and biomedicine. For calcium carbonate, the use of raw shells, their transformation to calcium oxide, CaO, and the production of precipitated calcium carbonate are presented. Next, we present in detail the isolation of hydroxyapatite from fish bones and scales, its transformation to other biphasic calcium phosphates, and its applications. The review concludes with a discussion on the strengths, weaknesses, opportunities, and threats to accessing biominerals wasted by the seafood processing industry.
Sustainability spotlightSeafood is a global requirement as an important source of protein as the human population grows significantly (UN SDG2). Currently, the seafood processing industry relies on landfill and/or ocean disposal to manage the generated by-products, but this had led to greenhouse gas emissions (UN SDG13), groundwater contamination (UN SDG6 and UN SDG15), eutrophication, and acidification (UN SDG14). The biominerals present offer the potential for the seafood processing industry to create other higher-value products for consumers, a step towards achieving a circular economy (UN SDG8, UN SDG9, and UN SDG12). Sustainable processes exist in the literature to isolate biominerals from bones and shells of aquatic organisms (UN SDG11) which have then been used in multiple applications, including biomedicine (UN SDG3), environmental remediation, and catalysis. |
1. Introduction
Seafood is a necessary source of protein globally and its production has increased significantly in the last century to meet the demands of the growing human population.1,2 For example, in 1961 a person would consume on average 8.96 kg of seafood,3 but that has increased to 20.25 kg in 2020.3 Therefore it is not surprising that global seafood production has increased by nearly 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000% from 1961 (1.96 million tonnes) to 2020 (214 million tonnes).4 The countries that consume the most fish are those that do not necessarily have access to food from land-based livestock. The Maldives consumes the greatest amount with 87.30 kg per person per year,3 followed by Iceland, Macao, and Kiribati.3 Despite not being the largest consumers of fish, developing countries in Africa and Asia have experienced the greatest increase in seafood consumption. For example, Niger has seen the largest overall increase among all countries with an increase of over 3000% in seafood consumption while Rwanda, Nepal, and Iran have also seen significant increases of ∼2400–2600%.3
000% from 1961 (1.96 million tonnes) to 2020 (214 million tonnes).4 The countries that consume the most fish are those that do not necessarily have access to food from land-based livestock. The Maldives consumes the greatest amount with 87.30 kg per person per year,3 followed by Iceland, Macao, and Kiribati.3 Despite not being the largest consumers of fish, developing countries in Africa and Asia have experienced the greatest increase in seafood consumption. For example, Niger has seen the largest overall increase among all countries with an increase of over 3000% in seafood consumption while Rwanda, Nepal, and Iran have also seen significant increases of ∼2400–2600%.3
Technological advances have contributed to the increased production of seafood. Historically, the only method of supplying fish and shellfish was by harvesting seafood directly from the ocean, from the place in which it was naturally grown, otherwise known as “capture fisheries”.3,5 However, since the 1980s, the global production from industrial capture fisheries has remained relatively stable at 90–95 million tonnes annually,5 and is limited by the ecosystems and legislation to control over-fishing. In many regions, the quantity of fish caught by industrial vessels has been declining due to geopolitical and/or commercial dynamics. Furthermore enormous progress has been achieved in aquaculture over recent decades.6,7 According to the Food and Agriculture Organization (FAO) of the United Nations (UN), aquaculture is defined as “the farming of aquatic organisms including fish, mollusks, crustaceans, and aquatic plants”.6,8 It was developed as a method to continue producing seafood products while alleviating some pressure on capture fisheries. In fact, aquaculture produced 123 million tonnes of seafood in 2020, surpassing quantities harvested via capture fisheries for the first time.4 Finfish species represent the majority of aquaculture production and account for 46.9% of processing, followed by algae (28.6%), mollusks (14.5%), crustaceans (9.2%), and other aquatic animals (0.9%).9 Aquatic organisms that are considered as the major aquaculture species by the FAO are listed in Table 1.
| Group | Species | World production (103 t, 2020) | % of group |
|---|---|---|---|
| Finfish | Grass carp (Ctenopharyngodon idellus) | 5791.5 | 10.1% |
| Silver carp (Hypophthalmichthys molitrix) | 4896.6 | 8.5% | |
| Nile tilapia (Oreochromis niloticus) | 4514.6 | 7.9% | |
| Common carp (Cyprinus carpio) | 4236.3 | 7.4% | |
| Catla (Catla catla) | 3540.3 | 6.2% | |
| Bighead carp (Hypophthalmichthys nobilis) | 3187.2 | 5.5% | |
| Carassius spp. | 2748.6 | 4.8% | |
| Atlantic salmon (Salmo salar) | 2719.6 | 4.7% | |
| Striped catfish (Pangasianodon hypophthalmus) | 2520.4 | 4.4% | |
| Roho labeo (Labeo rohita) | 2484.8 | 4.3% | |
| Clarias catfish (Clarias spp.) | 1249.0 | 2.2% | |
| Milkfish (Chanos chanos) | 1167.8 | 2.0% | |
| Tilapias nei (Oreochromis spp.) | 1069.9 | 1.9% | |
| Rainbow trout (Oncorhynchus mykiss) | 959.6 | 1.7% | |
| Wuchang bream (Megalobrama amblycephala) | 781.7 | 1.4% | |
| Black carp (Mylopharyngodon piceus) | 695.5 | 1.2% | |
| Largemouth black bass (Micropterus salmoides) | 621.3 | 1.1% | |
| Mullets nei, (Mugilidae) | 291.2 | 0.5% | |
| Gilthead seabream (Sparus aurata) | 282.1 | 0.5% | |
| Large yellow croaker (Larimichthys croceus) | 254.1 | 0.4% | |
| European seabass (Dicentrarchus labrax) | 243.9 | 0.4% | |
| Groupers nei (Ephinephelus spp.) | 226.2 | 0.4% | |
| Coho (silver) salmon (Oncorhynchus kisutch) | 221.8 | 0.4% | |
| Japanese seabass (Lateolabrax japonicus) | 196.9 | 0.3% | |
| Pompano (Trachinotus ovatus) | 160.0 | 0.3% | |
| Japanese amberjack (Seriola quinqueradiata) | 137.1 | 0.2% | |
| Barramundi (Giant seaperch, Lates calcarifer) | 105.8 | 0.2% | |
| Red drum (Scianops ocellatus) | 84.3 | 0.1% | |
| Subtotal of major species |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 388.1 388.1
|
79.0% | |
| Subtotal of other species |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 072.8 072.8
|
21.0% | |
| Total |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 461.1 461.1
|
100.0% | |
| Algae | Japanese kelp (Laminaria japonica) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 469.8 469.8 |
35.5% |
| Eucheuma seaweed (Eucheuma spp.) | 8129.4 | 23.2% | |
| Gracilaria seaweed (Gracilaria spp.) | 5180.4 | 14.8% | |
| Wakame (Undaria pinnatifida) | 2810.6 | 8.0% | |
| Nori (Porphyra spp.) | 2220.2 | 6.3% | |
| Elkhorn sea moss (Kappaphycus alvarezii) | 1604.1 | 4.6% | |
| Fusiform sargassum (Sargassum fusiforme) | 292.9 | 0.8% | |
| Spiny eucheuma (Eucheuma denticulatum) | 154.1 | 0.4% | |
| Subtotal of major species |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 861.5 861.5
|
93.7% | |
| Subtotal of other species | 2216.0 | 6.3% | |
| Total |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 077.6 077.6
|
100% | |
| Mollusks | Cupped oyster (Crassostrea spp.) | 5540.3 | 30.7% |
| Japanese carpet shell (Ruditapes philippinarum) | 4266.2 | 24.0% | |
| Scallops nei (Pectinidae) | 1746.4 | 9.8% | |
| Sea mussel (Mytilidae) | 1108.3 | 6.2% | |
| Constricted tagelus (Sinonovacula constricta) | 860.3 | 4.8% | |
| Pacific cupper oyster (Magallana gigas) | 610.3 | 3.4% | |
| Blood cockle (Anadara granosa) | 457.9 | 2.6% | |
| Chilean mussel (Mytilus chilensis) | 399.1 | 2.2% | |
| Subtotal of major species |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 898.6 898.6
|
84.0% | |
| Subtotal of other species | 2843.6 | 16.0% | |
| Total |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 742.2 742.2
|
100% | |
| Crustaceans | Whiteleg shrimp (Penaeus vannamei) | 5812.2 | 51.7% |
| Red swamp crawfish (Procambarus clarkii) | 2469.0 | 22.0% | |
| Chinese mitten crab (Eriocheir sinensis) | 775.9 | 6.9% | |
| Giant tiger prawn (Penaeus monodon) | 717.1 | 6.4% | |
| Giant river prawn (Macrobrachium rosenbergii) | 294.0 | 2.6% | |
| Indo-Pacific swamp crab (Scylla serrata) | 248.8 | 2.2% | |
| Oriental river prawn (Macrobrachium nipponense) | 228.8 | 2.0% | |
| Green mud crab (Scylla paramamosain) | 159.4 | 1.4% | |
| Subtotal of major species |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 705.3 705.3
|
95.3% | |
| Subtotal of other species | 531.8 | 4.7% | |
| Total |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 237.0 237.0
|
100% | |
| Other | Chinese softshell turtle (Trionyx sinensis) | 334.3 | 31.5% |
| Japanese sea cucumber (Apostichopus japonicus) | 201.5 | 19.0% | |
| Frog (Rana spp.) | 147.8 | 13.9% | |
| Edible red jellyfish (Rhopilema esculentum) | 90.4 | 8.5% | |
| River and lake turtle (Testudinata) | 49.3 | 4.6% | |
| Subtotal of major species | 823.3 | 77.5% | |
| Subtotal of other species | 239.0 | 22.5% | |
| Total | 1062.3 | 100% |
The seafood processing industries are critical for global food security, but face sustainability challenges. Industrial capture fisheries rely on fishing vessels that emit significant quantities of CO2 (ref. 10) and can result in species depletion through overfishing. The industrial capture fish industry also causes food loss, waste, and ocean pollution during harvesting because management often encourages disposal of fish at sea (especially bycatch i.e. capture of unintended species),5 and fishing gear can be abandoned, lost, or discarded.5 Abandoned fishing gear has numerous environmental consequences including the introduction of microplastics and toxins into feed webs, habitat degradation, and the spread of invasive alien species.11 While aquaculture was developed to overcome some of these challenges, it has also led to unintended problems that have been amplified with significant growth in this sector.† A lot of seafood is wasted due to poor harvesting practices that contribute to diseases that can cause entire pens of fish to die.5,12 Some of the fish can potentially escape the pens and interact with local species, altering the environment and also affecting the genetics of wild fish that are protected as species at risk. If some of the escaped fish are sick, they can spread disease,3 further affecting the local environment. Additionally, some companies have taken advantage of aquaculture and have attempted to breed too many fish in one area, thus creating anoxic zones and affecting local wildlife. Aquaculture has the potential to contribute to many of the UN's Sustainable Development Goals if care is taken to ensure economic, environmental, and social viability.7
Another source of waste that has yet to be mentioned is the processing of aquatic organisms post-harvest.13 In general, processing involves chemical and mechanical operations required to transform and also preserve seafood. The first step typically involves mechanically (frequently manually) removing the entrails (e.g., gutting). Most of the time, processing takes place in a factory environment which can often be labor intensive and/or highly automated. The main objective of fish processing plants is to provide fish for human consumption and the by-products (frames, skins, viscera) are sometimes processed into secondary low-profit products such as fertilizer and less frequently into higher value nutritional supplements. Processing plants situated in remote areas face challenges regarding management of by-products because alternative uses are not economically viable nor accessible. While landfills remain the most common method of disposal, remote areas are sometimes issued permits for disposal at sea when waste cannot be feasibly sent for reprocessing or to landfills.14 In emerging economic regions of the world and in places based on subsistence/traditional fisheries, disposal of waste at sea is most common.
Relying on landfills and oceans for the disposal of seafood discards has environmental consequences. According to the FAO, food loss and waste account for 8–10% of global greenhouse gas (GHG) emissions.15 Landfills have a significant global warming potential (GWP) because the organic content is decomposed by bacteria through aerobic and anaerobic digestion, releasing carbon dioxide (CO2), methane (CH4), sulfur dioxide (SO2), and nitrogen oxides (NOx).16 Discards, often containing P and N, are dumped in the ocean causing eutrophication due to the abundance of nutrients present.17,18 Eutrophication creates algae blooms that are potentially toxic, disrupt the ecosystem, taint drinking water, and eventually result in anoxic zones.17–19 Furthermore, once these bacteria die, they release more CO2 and contribute to ocean acidification.20 The ocean's decrease in pH will eventually be crucial for any capture fishery or aquaculture practice at sea because it slows the growth of fish and shellfish, thus reducing catch and harvest size.20 Due to their contribution to global warming, seafood discards should be valorized rather than disposed of and the fishing industry should strive towards a more circular economy.21 For example, Iceland has been making significant strides in utilizing every aspect of fish towards creating higher-value products. In 2021, 80% of all Icelandic raw materials from fish were valorized as either food or other consumer products.22 Within Iceland, the 100% Fish Project seeks a wide range of products that could be created using every part of the fish (Fig. 1).22–24 Other than the edible seafood products, items such as cosmetics, medicinal products, and even leather have been prepared from wasted by-products that would have historically been unsustainably wasted.22–24
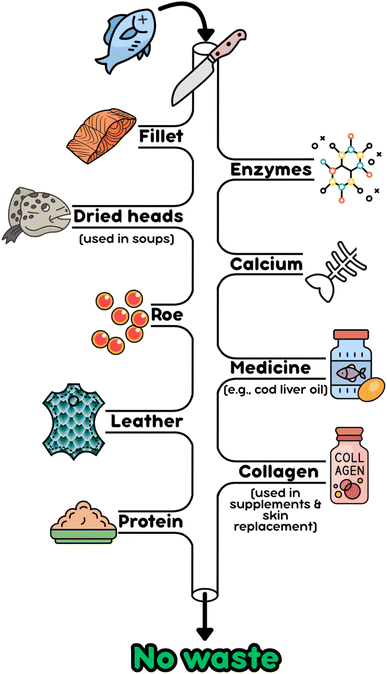 | ||
| Fig. 1 Schematic representation of different value streams achieved by the Iceland Ocean Cluster's 100% Fish Project. Prepared with Canva. | ||
Fish and shellfish waste offer the potential to extract many high-value compounds. Typically, researchers focus on extracting organic compounds and biopolymers from seafood waste. Fish waste has been processed into various products including collagen, non-collagenous proteins, omega-3 fatty acids, and biogas. Marine collagen is of particular interest among researchers because it is highly abundant not only in fish and sharks, but also in by-catch organisms (e.g., jellyfish and sea stars – previously known as starfish) too. Collagen has been extracted from seafood waste with several processes such as chemical hydrolysis, enzymatic treatment, and fermentation using proteolytic bacteria.25 This biogenic collagen has been used in several biomedical and pharmaceutical applications because it is biocompatible and biodegradable, and it has also been used as a food additive and incorporated into packaging materials.25
Other biologically active compounds, including various fatty acids, have been isolated from fish by-products. For example, Dave et al. investigated fatty acid yields in fish oil extracted from Atlantic salmon (Salmo salar) using enzymes and the extracted compounds included saturated fatty acids, monosaturated fatty acids, polyunsaturated fatty acids, omega-3 fatty acids, and omega-6 fatty acids.26 Other sustainable methods for accessing omega-3 polyunsaturated acids from waste have been reported including extraction with supercritical CO2.27 This oil can either be used for nutritional supplementation, or it can be used as a starting material that is further reacted to create a product with other value-added applications. This has been achieved by Laprise et al. by epoxidizing waste-derived fish oil followed by reaction with CO2 to yield an oil rich in cyclic carbonate groups, which could then be converted to a non-isocyanate polyurethane material.28 Often, a sludge remains after the oil extraction process and is viewed as a by-product,26 but it also has potential value. Paone et al. described their concept of an anchovy (Engraulis spp.) biorefinery and in their attempts to create a fully circular process, the remaining sludge after extraction was used for the production of biogas through anaerobic digestion.29
Crustacean shells are also useful materials because they consist of ∼40% protein, ∼20–25% chitin, ∼5–10% lipids, and pigments.30 Chitin, a linear polysaccharide of N-acetyl-D-glucosamine, is a particularly valuable compound. While it only has a few applications as a raw material because of its hydrophobicity, it is widely used in its deacetylated form, chitosan.31 Because chitosan is more soluble in acidic and aqueous environments, it has been used in water treatment, nutraceuticals, biomedicine, and cosmetics.32 The standard protocol to extract chitin from crustacean shells is energy- and chemically intensive, relying on hydrochloric acid (HCl) and sodium hydroxide (NaOH) for the elimination of minerals and proteins.32 However, Burke and Kerton have recently investigated green extraction methods for the sequential extraction of carotenoid pigments, protein, and chitin from snow crab (Chionoecetes opilio) shells.33 Astaxanthin was extracted with vegetable oil, a protein powder was yielded using citric acid, and chitin was recovered using enzymes.33 Other methods that do not rely on acids and bases to extract chitin from crustacean shells include ionic liquids,34 deep eutectic solvents (DESs),35 supercritical fluids,36 and mechanochemistry.37
The isolation of biominerals has been less prevalent compared to organic compounds despite being present in large quantities. For example, mussel shells are made of approximately 95% calcium carbonate (CaCO3) and fish bones consist of 60% hydroxyapatite (HAP). In this review, the two biominerals most abundant in seafood waste, CaCO3 and HAP, will be discussed. Marine-derived CaCO3 is compared to mined limestone and exists as several polymorphs. In Section 2, CaCO3 polymorphs present in shells are compared between different species of bivalves, crustaceans, and gastropods. We also touch on the transformation of biogenic CaCO3 to CaO and its further processing to precipitated calcium carbonate (PCC) or HAP. In Section 3, HAP isolated from fish is compared to stoichiometric HAP and its potential benefits for biomedical applications are discussed. Finally, different isolation methods such as calcination and alkaline deproteinization are discussed for obtaining HAP by treating fish backbones and scales. Articles describing processes to isolate CaCO3 or HAP from waste biomass, but with limited characterization details on the resulting minerals are omitted from this review.
2. Calcium carbonate, CaCO3
It is estimated that the average American uses 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lbs of new minerals annually.38 Among these minerals, limestone makes up 71% of all crushed stone produced by the United States.39 To be officially considered as limestone, the rock must contain >50% CaCO3.40 Limestone can be classified as impure, low purity, medium purity, high purity, and very high purity depending on the CaCO3 content. Rocks with <85% CaCO3 are considered impure limestone while very high purity limestone contains >98.5% CaCO3.40 While limestone itself has several applications (e.g., filler in cements, concretes, plastics, paints, rubbers, and chalk),41 it is often converted to lime, CaO.
000 lbs of new minerals annually.38 Among these minerals, limestone makes up 71% of all crushed stone produced by the United States.39 To be officially considered as limestone, the rock must contain >50% CaCO3.40 Limestone can be classified as impure, low purity, medium purity, high purity, and very high purity depending on the CaCO3 content. Rocks with <85% CaCO3 are considered impure limestone while very high purity limestone contains >98.5% CaCO3.40 While limestone itself has several applications (e.g., filler in cements, concretes, plastics, paints, rubbers, and chalk),41 it is often converted to lime, CaO.
Lime is one of the most important materials globally because it is necessary for several sectors, including steel, construction, agriculture, environmental remediation, and chemical industries.42 For example, in 2023, 88 million tons of Portland cement were produced in the United States,43 accounting for 95% of hydraulic cement production.44 Portland cement is made of 60.2–68.7% CaO45 and therefore its production is very important industrially across the world. The transformation of CaCO3 to CaO is carried out via calcination in kilns at very high temperatures of 700–900 °C.46–48
Other products that can be formed from limestone: CaO, otherwise known as lime (or quicklime), can be prepared through calcination as described above. Hydrated lime (or slaked lime), Ca(OH)2, is easily prepared from lime by reacting it with water.47 PCC is CaCO3 that has been artificially prepared by reacting CO2 with a slurry of hydrated lime to produce CaCO3 purer than limestone.49 Synthesizing PCC also ensures that the CaCO3 product has a definite morphology, size, and structure that may be required for specific applications.49 For example, PCC is preferred for pharmaceutical purposes because of its high purity that comes at a relatively high cost.40
The limestone and lime industries are major contributors to GHG emissions, especially CO2. According to the International Energy Agency (IEA), cement production is responsible for 7% of global anthropogenic CO2 emissions and is the third-largest industrial energy consumer.50 This trend is expected to rise with the growing global population, infrastructure development, and urbanisation patterns. By 2050, a 12% increase in cement production is expected. To limit the Earth's rising temperature to 2 °C, CO2 emissions from cement manufacturing must decrease by an estimated 24%.50 It is difficult to decarbonize the cement industry because of its inherent nature, although strides towards reducing the CO2 emissions have been made utilizing clinker replacement, fuel alternatives, and carbon capture technologies.46 The conversion of CaCO3 to CaO will continue to be unsustainable because it is energetically demanding and releases CO2 from both the fuel and decomposition process. Despite the difficulty in decarbonizing the limestone industry, other researchers have expressed optimism. Rissman et al. suggested that accepting and adopting new cement chemistries and alternative materials could help achieve a 50% reduction of industrial emissions by 2050.51
Another unsustainable aspect of the cement industry is obtaining limestone by mining in open quarries and blasting techniques. These methods are environmentally harmful and hazardous to the employees tasked with mining the mineral.52 Issues that arise from mining limestone include groundwater contamination, high energy usage that generally comes from fossil fuels, creation of sinkholes, reduced biodiversity, and dust-related hazards.53 In 2021, Stepkin et al. published a population health risk assessment involving communities near an industrial site for ace quarrying. They detected several pollutants in the atmosphere that have concerning toxicological characteristics, including a (1) carcinogenic effect, (2) embryotropic action, (3) gonadotropic action, (4) teratogenic action, and/or (5) a mutagenic effect.54 To overcome the environmental and health risks associated with quarrying, CaCO3 can be prepared through synthetic processes to limit environmental impact, such as precipitation/crystallization from mixing calcium and carbonate salts, but the calcium salts likely originate from the mined/quarried limestone as a feedstock.55 A more sustainable way to obtain CaCO3 is by isolating it from calcifiers: animals that rely on CaCO3 to form their shells.56 After all, most carbonate deposits that are commercially viable were created by the sedimentation of carbonate-secreting organisms many years ago.57 This method reduces the amount of waste shells being disposed of by the fish processing industry and could thus reduce CO2 emissions, acidification, and eutrophication.58 Overall, extracting CaCO3 from waste shellfish could help meet demands more effectively and result in an ocean-based circular economy.59–61 Furthermore, bio-derived CaCO3 is better than limestone-based CaCO3 for applications that involve human use (e.g., CaCO3 tablets).62 An example of this is Biocoral®, a CaCO3-based coral material used as a substitute for bone grafts.63
CaCO3 from shellfish exists as one or a combination of its three polymorphs: calcite, aragonite, and vaterite.64 Calcite particles are found in various morphologies; it is the most thermodynamically stable polymorph and the most abundant polymorph,65 and it has a trigonal structure with the most common form being a rhombohedron.66,67 Aragonite particles can be needle-like66 or plate-like nacre,68 and occur in an orthorhombic system.67 Vaterite particles are typically spherical66 and belong to the hexagonal crystal system,69 but there is ongoing discussion on the space group of vaterite, with several additional structural models being proposed such as monoclinic.69 Calcite is the main polymorph of CaCO3 found in limestone; therefore for specific applications it may be required to separate it from the other polymorphs present. Aragonite and vaterite are metastable polymorphs of CaCO3 that readily transform to calcite,65,66,70 especially vaterite which recrystallizes to calcite when it is dissolved in water.64 CaCO3 from shellfish waste is present in the form of calcite and/or aragonite depending on the species (Table 2), but vaterite can also be synthesized based on shell treatment (Table 5).71–73
| Category | Species | Polymorphs in raw shells | Reference |
|---|---|---|---|
| a Abbreviations – n.s., non-specified. | |||
| Bivalves | Blue mussel (Mytilus edulis) | 75% calcite & 25% aragonite | Murphy68,79 |
| Blue mussel (Mytilus edulis) | 68% calcite & 32% aragonite | Cardoso78 | |
| Sururu mussel (Mytella falcata) | 94% aragonite & 6% calcite | Cardoso78 | |
| Sururu mussel (Mytella falcata) | Mostly aragonite, little calcite | Araújo80 | |
| Green mussel (Perna viridis) | 100% aragonite | Ismail73 | |
| Mediterranean mussel (Mytilus galloprovincialis) | Calcite & aragonite | Nam81 | |
| West Indian pointed venus clam (Anomalocardia brasiliana) | 100% aragonite | Cardoso78 | |
| Spiny fileclam (Lima lima) | Aragonite | Anand82 | |
| Brown clam (n.s.a) | Calcite & aragonite | Aguila-Almanza83 | |
| Grey clam (n.s.a) | Calcite & aragonite | Aguila-Almanza83 | |
| Ark clam (Scarpharca subcrenata) | Aragonite | Nam81 | |
| Manila clam (Ruditapes philippinarum) | Mostly aragonite, little calcite | Nam81 | |
| Venus clam (Meretrix petechialis) | Mostly aragonite, little calcite | Nam81 | |
| True oyster (Ostreidae) | 90% calcite & 10% aragonite | Ramakrishna84 | |
| Pacific oyster (Crassostrea gigas) | Calcite & aragonite | Nam81 | |
| Oyster (n.s.a) | Calcite | Aguila-Almanza83 | |
| Oyster (n.s.a) | Calcite | Nguyen Quang & Ta Hong85 | |
| Yesso scallop (Patinopecten yessoensis) | Calcite & aragonite | Nam81 | |
| Pen shell (Atrina pectinate) | Calcite & aragonite | Nam81 | |
| Egg cockle (Fulvia mutica) | Aragonite | Nam81 | |
| Crustaceans | Blue crab (Callinectes sapidus) | Calcite | Ogresta86 |
| Mediterranean green crab (Carcinus aestuarii) | Calcite | Ogresta86 | |
| Mangrove crab (Ucides cordatus) | Calcite | Cardoso78 | |
| Mangrove crab (Ucides cordatus) | Calcite | Araujo80 | |
| Flower crab (Portunus pelagicus) | Calcite | Bayuseno73 | |
| Gastropods | Sea snail (Loittiodea) | Aragonite | Anand82 |
| Netted olive sea snail (Oliva reticularis) | Aragonite | Anand82 | |
Shellfish are made of two main groups of organisms: mollusks and crustaceans. Researchers have investigated waste shells from both groups as potential feedstocks for CaCO3. The main difference between crustacean and mollusk shells is that while mollusk shells are made of ∼95% CaCO3, crustacean shells are more complex (e.g., crab shells are composed of ∼20–50% CaCO3, Fig. 2).74 Therefore, as a raw material, mollusk shells are exploited more than crab shells as a source of CaCO3. However, some applications require treating the shells with calcination, thus decomposing the protein (and also chitin in crustaceans) in the process. In the next section, we will discuss using raw shells as a source of different CaCO3 polymorphs and subsequently transforming them to CaO and PCC. It should be noted that seashells often contain Mg, Mn, Zn, Y, Cu, Sr, Ba, and Pb that can significantly influence the performance of materials.75
 | ||
| Fig. 2 Natural sources of CaCO3 include limestone and calcifiers (e.g., crustaceans & mollusks). Prepared with Canva. | ||
2.1. Raw shells
Raw shells from mollusks are made almost entirely of CaCO3 in the form of calcite and aragonite. However, to utilize the shells, the organic residues must be removed; otherwise they will eventually decompose and result in a stench that is unwanted by seafood processing industries and surrounding communities. Herein, organic residues refer to all organic-containing material, including collagen. The process of cleaning waste shells is rarely discussed in the literature; however Murphy et al. optimized two enzymatic treatments to decompose the organic residues from raw and cooked blue mussel (Mytilus edulis) shells by using 1.0–2.0 μL g−1 Multifect PR 6L for 4 h at 55 °C and 6.0 μL g−1 Multifect 7L for 10 h at 25 °C, respectively.76 A more recent preparation techniques used to isolate CaCO3 from shells uses 55% v/v NaClO instead of enzymes to degrade the organic residues; however this process is less sustainable than using enzymes.77 To isolate CaCO3 from natural sources, calcination is typically avoided because it converts CaCO3 to CaO at 800–900 °C.78 Examples of CaCO3 polymorphs that have been found in shells from bivalves, crustaceans, and gastropods are summarized in Table 2.Species that evolved within the same subclass have similar properties and contain the same CaCO3 polymorph or a mixture of polymoprhs.81 For example, bivalves belonging to the Heterodonata and Pteriomorphia subclasses are almost entirely made of aragonite. Species belonging to the Heterodonata subclass include mud-dwelling clams (Meretrix petechialis), Manila clams (Ruditapes philippinarum), and cockles (Fulvia mutica) while lischke (Scarpharca subcrenata) belongs to the Pteriomorphia subclass. The Eupteriomorphia subclass of bivalves includes species that have shells containing either calcite alone or a mixture of calcite and aragonite. The Ostreoida subclass includes species that have only calcite in their shells, such as Pacific oysters (Crassostrea gigas) and yesso scallops (Patinopecten yessoensis). A combination of calcite and aragonite is found in the shells of species from the Pterioda (e.g., pen shells, Atrina pectinata) and Mytiloida (e.g., Mediterranean mussels, Mytilus galloprovincialis) orders of the subclass Eupteriomorphia; however the calcite content consistently greater than that of aragonite.
Mussel shells are the most studied source of CaCO3 described in the literature. Murphy et al. characterized the mussel shells cleaned enzymatically and showed that calcite and aragonite were present by XRD analysis.68,77,79 Since that study, it has been consistently observed that blue mussel shells in particular contain a mixture of calcite and aragonite.78 The same trend can be applied to the shells from sururu mussels, but in this case there is significantly more aragonite than calcite.78 Interestingly, green mussel shells were observed by XRD to be composed entirely of aragonite,87 which may be a geographical evolutionary trait.
Clam and oyster shells have also been investigated as potential feedstocks of CaCO3, although they are less prevalent. Águila-Almanza et al. observed that clams were made of calcite and aragonite; however they did not specify the specific species of clams studied but rather categorized them based on their shell colour.83 Cardoso et al. studied the shell composition of Anomalocardia brasiliana by XRD and reported that aragonite was the only polymorph present.78 Oyster shells have been shown by multiple studies to consist mostly of calcite.81,83–85
| Shell | Replacement | Suggested replacement level reported in the literature | Parameter(s) studied | Reference |
|---|---|---|---|---|
| Oyster | Fine aggregate | <20% | • Compressive strength | Yang89 |
| Oyster | Fine aggregate | <50% | • Compressive strength | Eo & Yi90 |
| Scallop | Fine aggregate | <40% | • Workability | Varhen91 |
| • Mechanical properties | ||||
| Scallop | Aggregate | 20–60% | • Compressive strength | Cuadrado-Rica92 |
| • Durability | ||||
| Cockle | Cement | <15% | • Porosity | Othman93 |
| Cockle | Fine aggregate | 20–30% | • Compressive strength | Muthusamy94 |
In another study by Lertwattanaruk et al., Portland cement incorporated with seashells from four species demonstrated comparable properties to those of typical plastering and masonry construction.95 The shells were processed using wet ball milling and had average particle sizes of 13.56–29.87 μm, similar to that of Portland cement (22.82 μm). While the water requirements and setting time were improved by increasing the proportion of seashells incorporated (5–20 wt%), it should be noted that the compressive strength of mortar decreased, but the shell-based mortar continued to have adequate compressive strengths that are still higher than those needed for plastering purposes. Interestingly, the specific properties of shell-based cements were species-dependent. For example, short-necked clams (Leukoma staminea) and oysters decreased drying shrinkage of mortars while green mussels and cockles resulted in the opposite effect. According to the authors, mortars prepared with high volumes of short-necked clams were the best in terms of overall performance because of its relatively low mixing water requirement, good compressive strength, lower drying shrinkage, decrease in thermal conductivity, and increased setting time.
Outside of the cement industry, shell-derived CaCO3 has been explored for other applications like catalysis. For example, Pasa and co-workers prepared a CaCO3 catalyst from shellfish waste for biocrude production from sugarcane bagasse and ethanol.80 Carapaces from mangrove crab and sururu mussels were crushed and sieved before being used as-is for catalysis. After 30 min at 300 °C and 10 bar N2, biocrudes were prepared from sugarcane bagasse in high yields (72–83%). The shellfish catalysts increased the yields slightly; however there was a greater impact on the product's composition. When anhydrous ethanol was used in the presence of the catalyst, a biocrude rich in furans was observed, while hydrous ethanol instead produced ethyl esters.
Another application for shells involves dye adsorption and oil recovery. In 2020, following their work on enzymatically processing blue mussel shells,76 Murphy et al. discovered that they can create an absorbent material from the shells termed “soft calcite” (SC).79 Unlike calcite that is hard and ordered, SC has a nest-like morphology able to adsorb dyes from aqueous solutions and absorb crude oil from seawater. As mentioned previously, mussel shells are made of calcite and aragonite; however they are always present as two distinct layers. After heating the shells to 220 °C for 48 h, the outer prismatic layer (calcite) and inner nacreous layer (aragonite) can be separated mechanically. The calcite layer then undergoes two subsequent treatments with 5% acetic acid to yield SC. This novel material has a sponge-like morphology when moist, and when dried it has a cotton candy texture. It was demonstrated that SC can adsorb 1–24 wt% of common dyes (e.g., crystal violet) and exhibits a significant absorption capacity of 977 g oil/g SC ± 84 g for crude oil with good recyclability over ten reuse cycles. A more detailed study on the adsorption of methylene blue and safranin-O using the same materials was recently published where adsorption capacities of 1.81 and 1.51 mg g−1, respectively, were achieved.96 Since these studies, calcite from blue mussel shells has been investigated for other uses including the production of calcium acetate that can be used as a de-icer97 or as a replacement for calcium chloride to form hydrogels.98 Other researchers have since used mussel shells to adsorb other industrial dyes, including Eosin Y.77
Shells have also been explored as a possible source of CaCO3 nanoparticles that can carry out drug delivery applications. In 2021, Ibiyeye and Zuki used cockle-shell-derived aragonite CaCO3 nanoparticles to successfully co-deliver doxorubicin and thymoquinone to treat breast cancer.99 The CaCO3 nanoparticles were synthesized by simply grinding raw shells, adding SB-12, and then rolled on a roller mill for five days.100 The drug-loaded aragonite nanoparticles were able to successfully destroy breast cancer stem cells and also suppress their properties. The authors describe that this combinational therapy could be useful as a potential curative strategy to manage metastasis and breast cancer recurrence.99
2.2. Shells as a precursor to CaO
Rather than quarrying limestone and transforming it to lime, CaCO3 from shells can serve as the precursor material. The most prevalent method described in the literature to transform shells from bivalves, crustaceans, and gastropods to CaO is by calcination at temperatures above 800 °C.78,82,101–104 CaCO3 decomposes and releases CO2 at 800 °C, resulting in CaO as the final product. A few authors, including Dampang et al., use NaOH as a pre-treatment before calcination to remove organic residues;101 however this step is probably unnecessary as proteins would decompose during calcination. A list of calcination conditions and properties of the CaO obtained are described in Table 4.| Species | Calcination conditions | CaO properties | Reference |
|---|---|---|---|
| a Abbreviations – n.s., none specified. | |||
| Blue mussel (Mytilus edilus) | 900 °C for 3 h | Irregular morphology, diameter <5 μm | Cardoso78 |
| Sururu mussel (Mytella falcata) | 900 °C for 3 h | Irregular morphology, diameter <2 μm | Cardoso78 |
| Mussel (n.s.a) | 950 °C for 4 h | Irregular morphology, diameter <10 μm | Nasir & Hazri102 |
| West Indian pointed venus clam (Anomalocardia brasiliana) | 900 °C for 3 h | Irregular morphology, diameter >5 μm | Cardoso78 |
| Spiny fileclam (Lima lima) | 900 °C for 4 h | Spherical nanoparticles, diameter: 17–31 nm | Anand82 |
| Mangrove crab (Ucides cordatus) | 800 °C for 2 h | Agglomerated microstructures with cauliflower morphology | Rezende103 |
| Mangrove crab (Ucides cordatus) | 900 °C for 3 h | Honeycomb morphology, diameter <5 μm | Cardoso78 |
| Flower crab (Portunus pelagicus) | 1000 °C for 5 h | n.s.a | Wibisono104 |
| Sea snail (Loittiodea) | 900 °C for 4 h | Spherical nanoparticles, diameter: 20–34 nm | Anand82 |
| Netted olive sea snail (Oliva reticularis) | 900 °C for 4 h | Spherical nanoparticles, diameter: 5–28 nm | Anand82 |
| Seashell (n.s.a) | 800–1000 °C for 2–4 h | Agglomerated irregular nanoparticles | Dampang101 |
Shell-derived CaO has been investigated as a catalyst in the literature,107 especially for the production of biodiesel. For example, shells from sururu, blue mussels, mangrove crab, and West Indian pointed venus clam were used to create CaO catalysts.78 The catalyst was studied for the production of fatty acid methyl esters (FAMEs) by transesterification of soybean oil with yields of 79–94% achieved after 3.5 h. The transformation of CaCO3 to CaO was achieved by calcining selected shells at 900 °C and pre-activating it with methanol for 1 h before reactions. Significantly different results were achieved depending on the shell which the CaO was derived. CaO from sururu mussels had a FAME yield of 89% after 1 h, while the other catalysts had less than a 50% yield in the same time period. The sururu CaO was investigated further, producing FAME yields of 91% after 3 h following four consecutive reuse cycles. While this is lower than the 96% FAME required to meet biodiesel production, it is a step towards using recycled waste materials for more sustainable biodiesel.
Pal and co-workers prepared a CaO catalyst using unspecified “used mollusk shells” as the starting material.108 Interestingly, rather than just using calcination to transform CaCO3 to CaO, the authors used a sol–gel method to produce CaCl2 before hydrolyzing it to Ca(OH)2 and then finally calcining it at 850 °C to yield CaO. The resulting product had a very distinct cubic morphology with an average particle size distribution of 341 nm. This catalyst was studied for the adsorption of a neutral red dye and the transesterification of cotton seed oil and methanol to biodiesel. When compared to commercial CaO, the mollusk-derived catalyst had better removal efficiency after 140 min (49 vs. 78%). Similar results were observed for transesterification reactions as mollusk-derived CaO had a maximum 98% biodiesel yield compared to commercial CaO which reached 79% with the same catalyst loading after 4.5 h of reaction time.
Biodiesel has also been produced from castor oil and methanol using a shell-based CaO catalyst doped with praseodymium.109 Similarly to Cardoso et al.,78 mussel shells were calcined at 950 °C for 4 h to yield CaO.109 This was doped using praseodymium nitrate hexahydrate, Pr(NO3)3·6H2O and further calcined at 700 °C to create 3–11 wt% Pr-CaO catalysts. A maximum FAME yield of 87.42% was achieved using a 7 wt% Pr-CaO catalyst with a 2.5 wt% catalyst loading and methanol to oil ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 65 °C for 4 h. It should be noted that compared to other methods discussed previously,78,108 the reusability of this catalyst decreased significantly to 52.46% after four cycles because of the reduced number of active sites.109 While doping CaO with praseodymium improved the FAME yield by 7% when compared to using only calcined mussel shells, it has a negative impact on the catalyst's reusability and therefore may be impractical industrially.
1 at 65 °C for 4 h. It should be noted that compared to other methods discussed previously,78,108 the reusability of this catalyst decreased significantly to 52.46% after four cycles because of the reduced number of active sites.109 While doping CaO with praseodymium improved the FAME yield by 7% when compared to using only calcined mussel shells, it has a negative impact on the catalyst's reusability and therefore may be impractical industrially.
Biodiesel production by transesterification of oils is not the only reaction studied using shell-derived CaO catalysts. In 2023, Eddy et al. used CaO from mangrove oyster (Crassostrea gasar) shells as a photocatalyst for the degradation of procaine penicillin in aqueous solutions.110 The catalyst was prepared following a similar sol–gel procedure to that of Thakur et al.108 The resulting CaO nanoparticles were observed by TEM to be spherical, with an average diameter of 50 nm, but SEM showed that the size was relatively uneven and some particles were >100 μm.110 The photocatalyst had an average band gap of 4.46 eV and an absorption maximum at 280 nm, and it successfully degraded procaine penicillin under sunlight. A maximum of 97% degradation was realized experimentally after a 2 h reaction at a pH of 12 with a drug concentration of 0.0009 M. The catalyst could be reused five times while retaining efficiency above 86%. In a similar study, bromocresol green dye was degraded with efficiencies between 68 and 89% using the same CaO catalyst from oyster shells.111
2.3. Transforming shells to precipitated CaCO3
Precipitated CaCO3 (PCC) is a purified and refined version of CaCO3 that has been synthesized commercially since 1841.112 A typical process to make PCC using limestone involves (i) mining and crushing limestone, (ii) calcination to form lime, (iii) addition of water to form calcium hydroxide, Ca(OH)2, (iv) combining Ca(OH)2 with CO2 to form CaCO3, and (v) drying the slurry to yield PCC (eqn (1)).113 Other reactions that produce PCC include the lime soda process (eqn (2)) and the calcium chloride process (eqn (3)).112| Ca(OH)2 + CO2 ↔ CaCO3 + H2O | (1) |
| Ca(OH)2 + Na2CO3 ↔ CaCO3 + 2NaOH | (2) |
| CaCl2 + Na2CO2 ↔ CaCO3 + 2NaCl | (3) |
CaCO3 produced via PCC processes is purer than limestone and a specific polymorph formation can be achieved – including vaterite.70 Limestone often contains impurities such as silica and clay (Si, Al, Fe, and Mg)114 that are avoided by using it as a precursor rather than a direct source of CaCO3. Meanwhile, CaCO3 must have a purity of >99% to be considered PCC.112 Furthermore, the average size and distribution of particles formed by crushing limestone are much larger than PCC where nanoparticles can be synthesized.70 For specific applications, such as filler pigments, PCC must be <2 μm.112 This makes PCC superior to limestone for the preparation of impact resistant plastics and oil absorption.
As mentioned in Section 2.2, shells can also act as a source of CaO by calcining them at high temperatures to decompose CaCO3. Therefore, they can be used as a precursor to synthesize PCC instead of relying on mined limestone. First, the shells are calcined at 800–1000 °C for several hours to yield CaO.71–73,84,115 After calcination, CaO can be treated with HNO3 before being injected with CO2.71,72 For example, Prihanto et al. reported treating CaO with HNO3 to form an acidic solution of Ca2+ ions, and the pH was then increased using NH4OH, and the desired PCC product was formed by CO2 injection into the Ca(NO3)2 solution.71 Other methods report that CaO becomes hydrated to form Ca(OH)2 during storage by absorbing water,73 or the CaO is directly treated with water.84
One of the main advantages of using PCC rather than CaCO3 is that the polymorph formation can be controlled. Naturally-derived CaCO3 obtained in bulk is sometimes observed as mixture of calcite and aragonite particles with a wide particle size distribution of microparticles and nanoparticles. However, by modifying the conditions of the carbonation reaction to form PCC from Ca(OH)2, the polymorph content can be tailored. Ramakrishna et al. studied the favourable formation of aragonite by optimizing the reaction conditions, such as the presence of MgCl2, the temperature, and the time.84 When using less than 0.1 M MgCl2, calcite makes up 80% of the PCC formed; however at 0.2 M, Mg2+ ions start to incorporate into the Ca2+ ion sites of the calcite lattice to form magnesium calcite. At higher concentrations, 0.3 M, aragonite makes up 90% of the product, and at 0.6 M it makes up 100% of the PCC formed. The optimal temperature for the carbonation reaction was determined to be 80 °C by XRD as aragonite was the only polymorph present while calcite remained in small quantities at 60 and 70 °C. Finally, a reaction time of 3 h produced solely aragonite while 2.5 and 2 h resulted in a product containing calcite and unreacted Ca(OH)2. Irfa'i et al. also investigated polymorph formation based on the duration of treatment of Ca(OH)2 from green mussels with 0.6 M MgCl2 prior to CO2 injection.115 Stirring for 0.5 h resulted solely in calcite formation; at 1–1.5 h aragonite began to precipitate, and brucite (Mg(OH)2) began to form when reacted for 2 h. Some examples of PCC processes and the resulting polymorphs formed are summarized in Table 5.
| Species | PCC process | Polymorph(s) | Reference |
|---|---|---|---|
| a Abbreviations – PCC, precipitated calcium carbonate. | |||
| Green mussel (Perna viridis) | (1) Calcined at 900 °C for 5 h | 55.20% vaterite | Prihanto71 |
| (2) 2 M HNO3 | 44.80% calcite | ||
| (3) pH adjusted to 11 with NH4OH | |||
| (4) Injected with CO2 (0.25 L min−1) until pH reached 7 | |||
| (5) Filtered and dried | |||
| Green mussel (Perna viridis) | (1) Calcined at 800–900 °C for 5 h | 91.26–92.16% vaterite | Ismail72 |
| (2) 2 M HNO3 at 60 °C for 30 min | 7.84–8.74% calcite | ||
| (3) pH adjusted to 12 with NH4OH | |||
| (4) Injected with CO2 | |||
| (5) Washed until pH reached 7 | |||
| (6) Calcined at 800–900 °C for 5 h | |||
| Green mussel (Perna viridis) | (1) Calcined at 900 °C for 5 h | 0.5 h stirring: 100% calcite | Irfa'i115 |
| (2) Absorbed moisture during storage to form Ca(OH)2 | 1 h stirring: 67.1% calcite and 32.9% aragonite | ||
| (3) 0.6 M MgCl2 for 0.5–2 h | 1.5 h stirring: 75.4% calcite and 24.6% aragonite | ||
| (4) Injected with CO2 (50 mL min−1) until pH reached 7 | 2 h stirring: 60.6% calcite, 10.0% aragonite, and 29.4% brucite | ||
| (5) Filtered and dried | |||
| True oyster (Ostreidae) | (1) Calcined at 1000 °C for 2 h | Aragonite | Ramakrishna84 |
| (2) Hydrated with H2O (40 g L−1) at 80 °C for 1 h | |||
| (3) 0.6 MgCl2 at 80 °C for 3 h | |||
| (4) Injected with CO2 (50 mL min−1) | |||
| (5) Filtered and dried | |||
| Flower crab (Portunus pelagicus) | (1) Calcined at 900 °C for 5 h | Mostly vaterite | Bayuseno73 |
| (2) 2 M HNO3 at 60 °C for 30 min | Aragonite | ||
| (3) Washed until pH reached 7 | Very low amount of calcite | ||
| (4) Injected with CO2 | |||
| (5) Filtered and dried | |||
3. Hydroxyapatite (HAP), Ca10(PO4)6(OH)2
HAP is an inorganic mineral that makes up 60% of human bones116 and has been used extensively in biomedicine because of its biocompatibility,117 non-toxicity,118 and osteoconductivity.119 Some examples include its incorporation into drug carriers,120 surface coatings,121 anti-tumour drugs,122,123 and composites.120 HAP has also been applied in dentistry for enamel remineralization and tooth restoration123,124 because it makes up 70–80% of human dentin and enamel.125 Beyond biomedicine, HAP has also been used for the bioremediation of organic and inorganic pollutants from wastewater,126 catalysis,127 and energy storage materials.128HAP can be synthesized through a variety of methods that can be divided into three categories: dry, wet, and high temperature.129 Solid state and mechanochemical routes are examples of dry methods in which the precursors are mixed in a dry form to synthesize HAP. Wet methods, including chemical precipitation, hydrothermal, and hydrolysis reactions are useful for controlling the morphology and size of the produced particles. Pyrolysis and combustion, high-temperature methods, are more rarely reported for HAP synthesis because they can result in the production of secondary aggregates, such as β-tricalcium phosphate (β-TCP).
Biogenic HAP and stoichiometric HAP do not have identical properties because of the presence of impurities. While stoichiometric HAP has the formula Ca10(PO4)6(OH)2 with a Ca/P ratio of 1.67, biogenic HAP, (Ca10−x(PO4)6−x(CO3)x (OH or ½CO3)2−x); 0 ≤ x ≤ 2, has a Ca/P ratio below 1.67 because of the presence of carbonate impurities and calcium and hydroxyl deficiencies (Fig. 4).130 Other impurities that have been found in biogenic HAP include the following metals: Na, Mg, Cr, Co, Cu, and Sr.131 Biogenic HAP also differs by having a lower degree of crystallinity, thus making it a more amorphous material. Stoichiometric HAP continues to be used for biomedical applications although biogenic HAP may prove to be superior because it is more biomimetic compared to the stoichiometric HAP. Stoichiometric HAP lacks the required carbonate substitutions that are critical for bones' growth, strength, and physiology. It is also a challenge to synthesize biomimetic HAP through a bottom–up process because the surface of biological apatite is rarely smooth and it contains many defects that affect crystallinity, crystal morphology, solubility, and thermal stability. Furthermore, some biomedical applications require HAP with low crystallinity, such as cell bone regeneration because it is more biosorbable.132
 | ||
| Fig. 4 Structures of stoichiometric HAP, Ca10(PO4)6(OH)2 (left) and biogenic HAP, Ca10−x(PO4)6−x(CO3)x (OH or ½CO3)2−x 0 ≤ x ≤ 2 (right, green). | ||
Instead of synthesizing biomimetic HAP, this material can be obtained by isolating biogenic HAP from by-products of the seafood processing industry. Shells can be utilized as calcium precursors for HAP synthesis through bottom–up processes by converting the CaCO3 in shells to CaO through calcination. The CaO can then be converted to HAP by reaction with a phosphate precursor (e.g., H3PO4 and NH4H2PO4).133 Cuttlefish bones, which are made of CaCO3, have also been investigated to synthesize HAP by replacing the carbonate with a phosphate to preserve the porous CaCO3 structure (note: despite the name, cuttlefish are mollusks).134 Fish bones remain the most extensively researched source of HAP, followed by scales. Several forms of fish-based biomass can be exploited to directly isolate HAP, primarily bones, scales, and teeth.
3.1. Bones
As animal species evolved from primitive organisms, the existing exoskeletons based on CaCO3 were replaced with apatite-containing endoskeletons.135 HAP has been isolated from the bones of many species of fish, ranging from sardines to tuna, and there are no obvious differences in HAP content reported between species. There are three broad processes that have been reported in the literature to remove the organic residues from the discards to yield clean bones as a source of HAP: calcination, alkaline/acidic deproteinization (AAD), and enzyme hydrolysis (Fig. 5).| Species | Optimal treatment | Ca/P | Crystallinity | Size | Morphology | Reference |
|---|---|---|---|---|---|---|
| a Abbreviations – BCP, biphasic calcium phosphate; n.s., non-specified. b Crystallinity calculated using an unspecified method. c Crystallinity calculated using software. d Crystallinity calculated by comparing the intensity of the largest peak and the valley between the largest peak and first peak on the right (eqn (S1)). e Crystallinity calculated by comparing the sum of peaks and the area between the peaks and background (eqn (S2)). | ||||||
| Black scabbardfish (Aphanopus carbo) | (1) Ground with a hand mill | 1.47–1.54 | n.s.a | <100 nm | Isotropic | Ideia139 |
| (2) Calcined at 400–1000 °C for 4 h | ||||||
| Milkfish (Chanos chanos) | (1) Crushed using a ball mill | 1.66 | 98.3% | n.s.a | n.s.a | Permatasari140 |
| (2) Calcined at 1000 °C for 4 h | ||||||
| Belida (Chitala lopis) | (1) Crushed to fine powder | n.s.a | 26.8% | <20 μm | Rough and irregular | Lestari143 |
| (2) Carbonized at 500 °C for 2 h | ||||||
| Walking catfish (Clarias batrachus) | (1) Crushed using as ball mill | 1.68 | 95.0% | n.s.a | n.s.a | Permatasari140 |
| (2) Calcined at 1000 °C for 4 h | ||||||
| Tambaqui (Colossoma macropopum) | (1) Briefly boiled | n.s.a | High | 481.1–721.7 nm (average 602.3 nm)/561.7–666.6 nm (average 620.2 nm) | Agglomerates of spherical/cubical particles | Renda144 |
| (2) Ground with a mortar | ||||||
| (3) Calcined at 600/900 °C for 2 h | ||||||
| Common carp (Cyprinus carpio) | (1) Boiled for 2 h (2×) | 1.53–1.73 | High | <1 μm | Agglomerates of uniform polyhedral grains | Maidaniuc136 |
| (2) 500 °C for 2 h | ||||||
| (3) Calcined at 800–1200 °C for 2–6 h | ||||||
| (4) Ground with a mortar | ||||||
| Mackerel scad (Decapterus macarellus) | (1) Boiled for several hours | n.s.a | High | 100–500 μm | Spherical microstructures | Ishak145 |
| (2) Ground with a grinder | ||||||
| (3) Calcined at 900 °C for 5 h | ||||||
| South Asian carp (Gibelion catla) | (1) Manually blended | n.s.a | Polycrystalline | 200–260 nm | Hexagonal particles | Sathiyavimal146 |
| (2) Boiled for 8 h | ||||||
| (3) 900 °C for 4 h | ||||||
| (4) Ground with a mortar | ||||||
| Asian stinging catfish (Heteropneustes fossilis) | (1) Boiled for 1.5 h | 2.82 | High | <1 μm | Agglomerated spherical particles | Moulick147 |
| (2) Crushed into pieces | ||||||
| (3) Calcined at 900 °C for 2 h | ||||||
| Surma (Katsuwonus pelamis) | (1) Boiled | 3.16 | 92% | <1 μm | Agglomerated spherical particles | Mamun148 |
| (2) Crushed | ||||||
| (3) Calcined at 900 °C for 2 h | ||||||
| Nile tilapia (Oreochromis niloticus) | (1) Calcined at 900 °C for 8 h | 1.66 | n.s.a | 650–2000 nm (average 1200 nm) | Agglomerates of irregular and rounded particles | da Cruz160 |
| (2) Ball-milled for 8 h | ||||||
| Nile tilapia (Oreochromis niloticus) | (1) Boiled | 1.64 | Low | 10–25 μm | Irregular-shaped particles | Khamkongkaeo132 |
| (2) Calcined at 800 °C for 5 h | ||||||
| (3) Ground with a herb grinder for 10 min | ||||||
| Nile tilapia (Oreochromis niloticus) | (1) Boiled | 1.85–1.93 | 39–99% | 1–2 μm | Irregular particles that become bigger with temperature | Modolon150 |
| (2) Crushed in a knife-mill | ||||||
| (3) Ball-milled | ||||||
| (4) Calcined at 600–1200 °C for 2 h | ||||||
| (5) Ball-milled for 1–3 h with isopropyl alcohol | ||||||
| Nile tilapia (Oreochromis niloticus) | (1) Boiled | 2.99 | 94% | <1 μm | Agglomerated spherical particles | Mamun148 |
| (2) Crushed into small pieces | ||||||
| (3) Calcined at 900 °C for 2 h | ||||||
| Black tilapia (Oreochromis placidus) | (1) Boiled | n.s.a | High | 0.5–4 μm (average 1.2 μm) | Plate-like structure | Hubadillah153 |
| (2) Calcined at 800 °C | ||||||
| Pama croaker (Otolithoides pama) | (1) Boiled for 1.5 h | 2.82 | n.s.a | <1 μm | Agglomerated spherical particles | Prosad Moulick147 |
| (2) Crushed into pieces | ||||||
| (3) Calcined at 900 °C for 2 h | ||||||
| Perch (Perca sp.) | (1) Boiled for 8 h | ∼1.67 | High | 50–80 nm | Agglomerated spherical particles | Naga161 |
| (2) Calcined at 900 °C for 2 h | ||||||
| (3) Ground with a mortar | ||||||
| Round sardinella (Sardinella aurita) | (1) Calcined at 300–900 °C for 1 h | 1.54–1.60 | <5–60% | 50–100 nm (600 °C); 0.2–2 μm (900 °C) | Round particles (600 °C); regular and coarse rods (900 °C) | Carella152 |
| (2) Ground and sieved (50 μm) | ||||||
| Atlantic salmon (Salmo salar) | (1) Immersed in NaOH for 24 h | 1.57 | High | <5 μm | Agglomerated spherical particles | Popescu-Pelin142 |
| (2) Calcined at 850 °C for 4 h | ||||||
| (3) Crushed with a mortar | ||||||
| (4) Ball-milled for 4 h | ||||||
| Rabbitfish (Siganus sp.) | (1) Boiled | 1.696–1.876 | n.s.a | 1–5 μm | Agglomerated irregular particles | Fendi141 |
| (2) Boiled in water & acetone for 1 h | ||||||
| (3) Ground with a mortar into 200-mesh powder | ||||||
| (4) Annealed at 800–1000 °C for 2 h | ||||||
| (5) Ball-milled for 30 min | ||||||
| Catfish (Siluriformes sp.) | (1) Boiled for 4 h | 1.58 | 99% | <200 μm | Irregular flower-like microstructures with petal-shaped flakes | Akpan162 |
| (2) Sintered at 900 °C for 2 h | ||||||
| (3) Ground with a mortar and sieved (300 μm) | ||||||
| Gilt-head bream (Sparus aurata) | (1) Immersed in NaOH for 24 h | 1.63 | High | <5 μm | Agglomerated spherical particles | Popescu-Pelin142 |
| (2) Calcined at 850 °C for 4 h | ||||||
| (3) Crushed with a mortar | ||||||
| (4) Ball-milled for 4 h | ||||||
| Spanish mackerel (Tenggiri scomberomorini) | (1) Crushed to fine powder | n.s.a | 25.6%c | <20 μm | Rough and irregular particles | Lestari143 |
| (2) Carbonized at 500 °C for 2 h | ||||||
| Houndfish (Tylosurus crocodilus) | (1) Crushed with a ball mill | 1.62 | 67.2% | n.s.a | n.s.a | Permatasari140 |
| (2) Calcined at 1000 °C for 4 h | ||||||
The choice of temperature has an impact on the HAP-containing material produced. In most studies reported to date, a range of temperatures up to 1200 °C have been explored. For example, Pal et al. studied the impact of different temperatures to treat barramundi (Lates calcarifer) waste by heating it to 200, 400, 800, 1,000, and 1200 °C with a heating ramp of 5 °C min−1 and holding it isothermally for 1 h.149 By XRD analysis, it was observed that the crystallinity of the product increases depending on the chosen temperature. The samples treated at 200 and 400 °C were amorphous; however those treated at 800 °C had increased crystallinity of 80.8%. The crystallinity further increased to 89.2 and 92.9% when heated to 1000 and 1200 °C, respectively. A similar trend was observed for crystallite size, which increased from 5 nm when treated at 200 °C to 63 nm at 1200 °C. Similar results were achieved by Modolon et al. when they calcined tilapia (Oreochromis niloticus) bones at 600, 900, and 1200 °C for 2 h, observing crystallinities of 39.5, 98.3, and 99.6% and crystallite sizes of 14.4, 85.8, and 95.0 nm, respectively.150
At higher calcination temperatures, biphasic calcium phosphates (BCPs) are formed from the decomposition of HAP, including β-TCP and/or α-tricalcium phosphate (α-TCP).151 This was reported by Castilho and co-workers after calcining black scabbardfish (Aphanopus carbo) bones at 400, 600, 800, and 1000 °C for 4 h, resulting in samples consisting of 0, 17.5, 29.7, and 40.1% β-TCP, respectively.139 The formation of β-TCP is inconsistent across species. For example, Permatasari et al. observed that houndfish (Tylosurus crocodilus) bones calcined at 1000 °C for 4 h formed β-TCP while milkfish (Chanos chanos) and walking catfish (Clarias batrachus) did not.140 This ultimately resulted in houndfish having much lower crystallinity (67.2%) compared to milkfish (98.3%) and walking catfish (95.0%).
The choice of calcination temperature also impacts size and morphology of BCP particles. In general, particles created at lower temperatures are smaller but less regular than those prepared at higher temperatures. For example, Carella et al. calcined round sardinella (Sardinella aurita) for 1 h at 300, 600, and 900 °C.152 The sample calcined at 300 °C had an aligned arrangement of char that remained from the incomplete combustion of organic matter, but the size of individual particles could not be established. At 600 °C, the particles were agglomerated and spherical with a diameter of 50–100 nm. When the temperature increased to 900 °C, the particle size increased drastically to 0.20–2.0 μm with rod shape geometry. Interestingly, the morphology of BCP particles from fish bones differs across studies. Mamun et al. calcined tilapia and surma (Katsuwonus pelamis) at 900 °C for 2 h and both species resulted in agglomerated spherical particles with diameters less than 1 μm.148 Adam and co-workers reported that black tilapia (Oreochromis placidus) waste produced crystal plate-like particles that are 0.5–4 μm in diameter.153 Finally, Renda et al. observed that calcining tambaqui (Colossoma macropopum) at 900 °C for 2 h yielded cubic particles with an average diameter of 620.2 nm.144 More details regarding calcination procedures, including the Ca/P ratio, crystallinity, and size of yielded particles, are summarized in Table 6. Processes reported without sufficient characterization information have been omitted from the table.137,138,154–159
Autoclaving and calcination are other treatment steps that are used in conjunction with AAD to ensure thorough removal of organic residues. Triwitono and co-workers treated fish bones from six species of fish by autoclaving them at 121 °C for 3 h before using 1 N HCl and 1 N NaOH.165 Meanwhile, Hariani et al. calcined milkfish (Chanidae sp.) at 750 °C for 3 h after using 0.1 N HCl and 50% NaOH.166 The method reported by Idowu et al. is quite different from others: Atlantic salmon bones were immersed in 2 M NaOH and soaked in hexane before being treated with 2.5% NaClO and finally calcined at 900 °C for 6–9 h.167
AAD often yields a more amorphous product, whereas calcination increases crystallinity. For example, Horta et al. extracted HAP from tambaqui bones with 1% NaOH and calcined a portion of these bones at 800 °C.168 They determined that the alkaline treatment decomposed a significant amount of organic material, but calcination was required to increase the crystallinity significantly. Based on SEM observations, samples not exposed to heat were made of agglomerated, irregularly shaped nanoparticles while calcination yielded larger, more defined particles. As with calcination, the HAP particles tend to agglomerate regardless of the method of preparation and it is often difficult to compare results since some studies report the size of individual nanoparticles while others describe the size of agglomerates. Deproteinization treatments involving acids and/or bases are summarized in Table 7 but reports that did not include sufficient characterization data have been omitted.164,169
| Species | Optimal treatment | Ca/P | Crystallinity | Size | Morphology | Reference |
|---|---|---|---|---|---|---|
| a Abbreviations – AAD, alkaline and/or acidic deproteinization; n.s., none specified; Ave., average. b Crystallinity calculated using software. | ||||||
| Milkfish (Channidae sp.) | (1) Crushed by fast milling | 1.65 | n.s.a | <3 μm | Irregular and agglomerated particles | Hariani166 |
| (2) Sieved through a 200-mesh sieve | ||||||
| (3) Washed with 0.1 N HCl | ||||||
| (4) Treated with 50% NaOH for 5 h at 60 °C | ||||||
| (5) Calcined at 750 °C for 3 h | ||||||
| Tambaqui (Colossoma macropomum) | (1) Boiled in water for 1 h | 1.67 | n.s.a | <500 nm | Defined morphology with a faceted structure | Horta168 |
| (2) Treated with 1% NaOH at 90 °C for 5 h | ||||||
| (3) Crushed with a mortar | ||||||
| (4) Calcined at 800 °C | ||||||
| Grouper (Epinephelus sp.) | (1) Boiled in water for 1 h | 1.44 | 69.8% | Ave. 281.4 nm | Agglomerates of asymmetrical particles | Kusumawati165 |
| (2) Autoclaved at 121 °C for 3 h | ||||||
| (3) Crushed with a mortar | ||||||
| (4) Soaked in 1 N HCl for 1 h | ||||||
| (5) Centrifuged for 15 min | ||||||
| (6) Treated with 1 N NaOH for 1 h at 100 °C (3×) | ||||||
| (7) Neutralized with 1 N HCl and centrifuged | ||||||
| (8) Refined with a disc mill for 1 min | ||||||
| (9) Sieved through a 200-mesh sieve | ||||||
| Barramundi (Lates calcarifer) | (1) Boiled in water for 1 h | 1.845 | High | Ave. 39.42 nm long, 17.15 nm wide | Spherical and rectangular particles | Le Ho170 |
| (2) Boiled in 1% NaOH | ||||||
| (3) Calcined at 650 °C for 4 h | ||||||
| (4) Crushed with a mortar | ||||||
| Snapper (Lutjanus sp.) | (1) Boiled in water for 1 h | 1.38 | 71.2% | Ave. 254.7 nm | Agglomerates of asymmetrical particles | Kusumawati165 |
| (2) Autoclaved at 121 °C for 3 h | ||||||
| (3) Crushed with a mortar | ||||||
| (4) Soaked in 1 N HCl for 1 h | ||||||
| (5) Centrifuged for 15 min | ||||||
| (6) Treated with 1 N NaOH for 1 h at 100 °C (3×) | ||||||
| (7) Neutralized with 1 N HCl and centrifuged | ||||||
| (8) Refined with disc mill for 1 min | ||||||
| (9) Sieved through a 200-mesh sieve | ||||||
| Whitemouth croaker (Micropogonias furnieri) | (1) Washed with 1 N NaOH for 24 h | 1.40 | High | >10 μm | Agglomerates of asymmetrical particles | Yamamura171 |
| (2) Washed in water overnight | ||||||
| (3) Treated with 30% H2O2 for 24 h | ||||||
| (4) Calcined at 800 °C for 5 h | ||||||
| (5) Milled | ||||||
| Tilapia (Oreochromis niloticus) | (1) Boiled in water for 1 h | 1.61 | 70.2% | Ave. 87.4 nm | Agglomerates of asymmetrical particles | Kusumawati165 |
| (2) Autoclaved at 121 °C for 3 h | ||||||
| (3) Crushed with mortar | ||||||
| (4) Soaked in 1 N HCl for 1 h | ||||||
| (5) Centrifuged for 15 min | ||||||
| (6) Treated with 1 N NaOH for 1 h at 100 °C (3×) | ||||||
| (7) Neutralized with 1 N HCl and centrifuged | ||||||
| (8) Refined with disc mill for 1 min | ||||||
| (9) Sieved through a 200-mesh sieve | ||||||
| Catfish (Pangasius sp.) | (1) Boiled in water for 1 h | 1.43 | 72.2% | Ave. 239.9 nm | Agglomerates of asymmetrical particles | Kusumawati165 |
| (2) Autoclaved at 121 °C for 3 h | ||||||
| (3) Crushed with amortar | ||||||
| (4) Soaked in 1 N HCl for 1 h | ||||||
| (5) Centrifuged for 15 min | ||||||
| (6) Treated with 1 N NaOH for 1 h at 100 °C (3×) | ||||||
| (7) Neutralized with 1 N HCl and centrifuged | ||||||
| (8) Refined with a disc mill for 1 min | ||||||
| (9) Sieved through a 200-mesh sieve | ||||||
| Atlantic salmon (Salmo salar) | (1) Boiled in water for 1 h | 1.63 | High | Ave. 122 nm | Agglomerates of grain-like particles | Bas172 |
| (2) Treated with 1% NaOH | ||||||
| (3) Calcined at 800 °C for 3 h | ||||||
| (4) Ground in a centrifugal ball mill | ||||||
| (5) Sieved through a 63 μm sieve | ||||||
| Atlantic salmon (Salmo salar) | (1) Immersed in 2 M NaOH at 50 °C for 2 h | 1.66 | High | 22.2–27.5 μm | Nanoparticles agglomerates into large clusters | Idowu167 |
| (2) Ground with a crushing mill until particles were 3–4 mm long | ||||||
| (3) Treated with hexanes for 1 h at 25 °C | ||||||
| (4) Bleached with 2.5% NaClO for 30 min | ||||||
| (5) Bleached with 2.5% H2O2 for 1 h | ||||||
| (6) Milled with a planetary ball mill for 2.5 h | ||||||
| (7) Sieved with a 75 mm sieve | ||||||
| (8) Calcined at 900 °C for 6–9 h | ||||||
| (9) Milled with a planetary mill | ||||||
| Indian oil sardine (Sardinella longiceps) | (1) Boiled at 200 °C | n.s. | Low | <5 μm | Agglomerates of asymmetrical particles | Surya163 |
| (2) Boiled with 2% NaOH and acetone for 1 h | ||||||
| (3) Grinded with a mortar | ||||||
| (4) Treated with 5% NaOH at 70 °C for 5 h | ||||||
| (5) Precipitants treated with 50% NaOH at 100 °C for 1 h | ||||||
| (6) Sieved | ||||||
| Kingfish mackerel (Scomberomorus sp.) | (1) Boiled in water for 1 h | 1.69 | 64.9% | Ave. 105.2 nm | Agglomerates of asymmetrical particles | Kusumawati165 |
| (2) Autoclaved at 121 °C for 3 h | ||||||
| (3) Crushed with a mortar | ||||||
| (4) Soaked in 1 N HCl for 1 h | ||||||
| (5) Centrifuged for 15 min | ||||||
| (6) Treated with 1 N NaOH for 1 h at 100 °C (3×) | ||||||
| (7) Neutralized with 1 N HCl and centrifuged | ||||||
| (8) Refined with a disc mill for 1 min | ||||||
| (9) Sieved through a 200-mesh sieve | ||||||
| Tuna (Thunnus sp.) | (1) Boiled in water for 1 h | 1.36 | 64.3% | Ave. 150.2 nm | Agglomerates of asymmetrical particles | Kusumawati165 |
| (2) Autoclaved at 121 °C for 3 h | ||||||
| (3) Crushed with a mortar | ||||||
| (4) Soaked in 1 N HCl for 1 h | ||||||
| (5) Centrifuged for 15 min | ||||||
| (6) Treated with 1 N NaOH for 1 h at 100 °C (3×) | ||||||
| (7) Neutralized with 1 N HCl and centrifuged | ||||||
| (8) Refined with a disc mill for 1 min | ||||||
| (9) Sieved through a 200-mesh sieve | ||||||
To achieve a product with higher purity, Pou and co-workers calcined fish bones from horse mackerel (Trachurus trachurus), scorpionfish (Scorpaena scrofa), and Atlantic salmon at 750–950 °C for 10 h after treating them with 1% Alcalase for 4 h at 64.2 °C.176 As expected, the extracted HAP is more crystalline and has no traces of organic residues (e.g., lipids and proteins) compared to that obtained by Boudreau et al. because of the calcination step.174–176 The samples calcined at 950 °C were more crystalline than those calcined at 750 °C, but higher amounts of β-TCP were also detected. Similar results were achieved by enzymatically treating catfish (Pangasius hypophthalmus) waste with Alcalase and calcination for 3 h at 500–900 °C.177 As the temperature increased, partial decomposition of HAP to β-TCP also increased.
Enzymes have the potential to create a more circular economy for the seafood processing industry. As mentioned above, not only are enzymes considered green, but every aspect of the fish waste could be utilized. While the focus of this review is on the inorganic fraction of the by-products, the protein hydrolysate remaining after enzymatic treatment contains beneficial amino acids that could be incorporated into fertilizers.178–180
3.2. Scales
Researchers have successfully isolated HAP from waste fish scales despite having lower inorganic content (38–46%) compared to bones. While many follow processes previously reported for the treatment of bones (i.e., calcination, AAD, and enzymes), other novel methods have also been used such as ultrasonic-assisted extraction (UAE) and treatment with DES. Among these methods, alkaline deproteinization is the most common treatment, followed by calcination, UAE, and finally DES. The isolated HAP is often observed as agglomerates of particles with sizes ranging from 5 nm to 167 μm. A detailed list of these methods is summarized in Table 8 but reports that did not provide sufficient characterization information are omitted.181–184| Method | Species | Optimal treatment | Ca/P | Crystallinity | Particle size & morphology | Reference |
|---|---|---|---|---|---|---|
| a Abbreviations – AAD, alkaline and/or acid deproteinization; UAE, ultrasound assisted extraction; DES, deep eutectic solvent; BCP, biphasic calcium phosphate; n.s., none specified. b Crystallinity calculated by comparing the area of crystalline peaks to the total area of amorphous and crystalline peaks (eqn (S3)). c Crystallinity calculated using software. | ||||||
| AADa | White seabass (Astractoscion nobilis) | (1) Immersed in 0.1 M HCl for 1 h | 2.01 | 80.99% | Size: 100–500 nm (mean: 172.9 nm) | Injorhor185 |
| (2) Treated with 5% NaOH at 60 °C for 3 h | ||||||
| (3) Treated with 50% NaOH at 80 °C for 3 h | ||||||
| Catla (Catla catla) | (1) Soaked in 83 mL of 1 N HCl for 30 min | 1.66 | n.s.a | Size: <1 μm | Buraiki186 | |
| (2) pH raised to 11 with 6 N NaOH | Morphology: agglomerates of needles | |||||
| (3) Vacuum filtered | ||||||
| (4) Boiled in a bag in a water bath for 30 min | ||||||
| (5) Frozen at −80 °C overnight | ||||||
| (6) Incubated in a hot air oven at 160 °C overnight | ||||||
| (7) Calcined at 800 °C for 2 h | ||||||
| Mullya garra (Garra mullya) | (1) Immersed in 500 mL 0.1 M HCl | 1.67 | High | Size: 20–40 nm long and 5–20 nm wide | Eswaran187 | |
| (2) 250 mL 5% NaOH added and stirred at 70 °C for 7 h | Morphology: interconnected nanostructured rods | |||||
| (3) Dried precipitates crushed using a mortar and pestle | ||||||
| (4) Stirred in 100 mL 50% NaOH at 100 °C for 2 h | ||||||
| Rohu (Labeo rohita) | (1) 100 g immersed in 0.6% KOH (×2) | 1.52–1.82 | n.s.a | Size: 1–1000 μm, mean of 155–167 μm | Sarkar and Das188 | |
| (2) Calcined at 550–1000 °C for 3 h | Morphology: surface layer with bony ridges of HAP in concentric rings & an inner fibrous layer of mainly collagen | |||||
| Asian sea bass (Lates calcarifer) | (1) Stirred in 1 L of 0.25 M HCl for 2 h | 1.67 | n.s.a | Size: 50–150 nm | Wu189 | |
| (2) Calcined at 900 °C for 3 h | Morphology: spherical agglomerates | |||||
| (3) Ground and sieved through a cloth mesh | ||||||
| Nile tilapia (Oreochromis niloticus) | (1) Soaked in 83 mL of 1 N HCl for 30 min | 2.14 | n.s.a | Size: <1 μm | Buraiki186 | |
| (2) pH raised to 11 with 6 N NaOH | Morphology: spherical agglomerates | |||||
| (3) Vacuum filtered | ||||||
| (4) Boiled in a bag in a water bath for 30 min | ||||||
| (5) Frozen at −80 °C overnight | ||||||
| (6) Incubated in a hot air oven at 160 °C overnight | ||||||
| (7) Calcined at 800 °C for 2 h | ||||||
| Nile tilapia (Oreochromis niloticus) | (1) Ground into a fine powder | 2.25 | Low | Size: 250–2500 nm | Rashad190 | |
| (2) Stirred in 1 L 0.75 M NaOH at 60 °C for 5 min | Morphology: dense agglomerated soft morphology with high surface roughness | |||||
| (3) Solid portion added to 1.3 M HCl at 60 °C for 30 min | ||||||
| (4) 5 M NaOH added dropwise | ||||||
| (5) Ground | ||||||
| Black tilapia (Oreochromis placidus) | (1) Immersed in 0.1 M HCl for 12 h | 1.75 | Low | Size: 50–60 mm wide, 30–200 nm long | Selimin191 | |
| (2) Stirred in 5% NaOH at 70 °C for 5 h | Morphology: irregular rod shape and crystal size | |||||
| (3) Treated with 50% NaOH at 100 °C for 1 h | ||||||
| Sardine (Sardinella longiceps) | (1) Treated with 1 N HCl | n.s.a | High | Size: <1 μm | Ashwitha192 | |
| (2) Treated with 1 N NaOH | Morphology: spherical agglomerates | |||||
| (3) Calcined at 600–1000 °C | ||||||
| n.s.a | (1) Soaked in 40% NaOH solution | 1.18 | High | Size: <5 μm | Wang193 | |
| (2) Ground | Morphology: irregular and uneven-sized blocks | |||||
| (3) Calcined at 800 °C for 2 h | ||||||
| Calcination | Nile tilapia (Oreochromis niloticus) | (1) Calcined at 450–600 °C for 5 h | 3.06–3.17 | n.s.a | Size: very small | Sittitut194 |
| (2) Collected on a 0.5-mm sieve | ||||||
| UAEa | Pirarucu (Arapaima gigas) | (1) Calcined at 700 °C for 2 h | 1.58 | 48% | Size: n.s.a | de Amorim195 |
| (2) Sieved (325 mesh) | ||||||
| (3) UAEa for 30 min | Morphology: n.s.a | |||||
| (4) Matured for 24 h | ||||||
| Nile tilapia (Oreochromis niloticus) | (1) UAEa with 0.8 M HCl 45 min at 60 °C | 1.68 | n.s.a | Size: 22.8 nm long and 8.6 nm wide | Sricharoen196 | |
| (2) Added NaOH to increase pH to 12 | ||||||
| (3) Sonicated for 30 min | Morphology: rice-shaped agglomerates | |||||
| (4) Ground with a mortar and pestle | ||||||
| DESa | Bighead carp (Aristichthys nobilis) | (1) Ground and sieved (80 mesh) | 1.73 | 43.1% | Size: 0.3–95 μm (median diameter 13.98 μm) | Liu197 |
(2) Treated with DESa (choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol = 1 glycerol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 70 °C for 2.5 h 2) at 70 °C for 2.5 h |
||||||
| (3) Centrifuged for 7 min | Morphology: irregular and agglomerated morphology | |||||
| (4) Purified by stirring with 5% NaOH for 5 h at 70 °C | ||||||
| Crucian carp (Carassius carassius) | (1) Ground and sieved (80 mesh) | 1.78 | Low | Size: 0.235–117.1 μm (median diameter: 12.71 μm) | Liu198 | |
(2) Treated with DESa (choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-butanediol = 1 1,4-butanediol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) at 65 °C for 2 h 15) at 65 °C for 2 h |
||||||
| (3) Centrifuged | Morphology: irregular and agglomerated morphology | |||||
| (4) Purified by stirring with 5% NaOH for 5 h at 70 °C | ||||||
In 2022, Rattanakam and co-workers published a study on the dissolution performance of carbon/HAP nanocomposites from tilapia scales.194 Instead of calcining, they pyrolyzed the discards at 450, 500, 550, and 600 °C for 5 h. The final material prepared was ultimately collected on a 0.5-mm sieve prior to analysis. The Ca/P ratio of each product was significantly higher (2.43–2.50) than those observed from other thermal treatments, but this can be attributed to the fact this was a pyrolysis treatment in the presence of nitrogen rather than calcination. Without oxygen, biochar was produced from the decomposition of protein, and therefore the carbon content was high (22.6–25.6 wt%). Broad and low intensity peaks were observed in the XRD diffractograms, indicating that the products were amorphous. The authors highlighted that the crystal size of the samples did not increase proportionally with temperature, unlike calcination, because the carbon in organic residues linked to HAP could block its nucleation sites, preventing particle growth and recrystallization. The authors later noted that distinguishing between carbon and HAP particles during TEM analysis was not possible because of severe aggregation. Therefore, while pyrolysis may not increase the size of particles yielded, it does not prevent agglomeration.
The morphology of HAP particles extracted from natural sources is still not fully understood as the same technique can provide particles with different shapes. For example, Eswaran et al.187 and Injorhor et al.185 followed a method very similar to that reported by Kongsri et al.200 HAP from the Nile tilapia scales was in the form of hexagonal crystals with an average size of 20 nm. While the size is similar to those of other HAP particles prepared,200 scales from mullya garra were rod-like187 and white seabass scales had irregular morphology.185 While Eswaran et al. describe grinding their scales with a mortar and pestle during their multi-step alkaline treatment,187 the other methods do not mention any further treatment prior to characterization.185,200 More research into the choice of mechanochemical processing method (e.g., mortar and pestle, ball-milling, and planetary mill) could provide further insight into whether it has an impact on particle morphology.
Other researchers have used AAD as a pre-treatment before calcination. Compared to fish bones that are often directly calcined without the presence of acid or base, it is much more common to deproteinize fish scales prior to thermal treatment. These methods often use dilute base rather than concentrated base because calcination will ultimately remove a significant quantity of residual protein. Like fish bones, HAP particles from scales after calcination are larger than those not exposed to high temperatures.182,188
Compared to calcination and AAD, UAE remains relatively uncommon. In 2020, Sricharoen et al. applied UAE for the extraction of HAP from fish scales.196 They performed UAE on Nile tilapia scales by immersing them in 0.2–1.2 M HCl and using ultrasonic power of 0.1–0.4 kW at 30–60 °C for 15–90 min. Based on results from ICP-AES, calcium and phosphorus extraction was optimized using 0.8 M HCl and 0.4 kW of ultrasonic power at 60 °C for 45 min. Furthermore, the pH of the solution was adjusted between 8 and 12 with NaOH, followed by sonication at 0.4 kW at room temperature for 30 min. The material was then dried, and ground with a mortar and pestle. As the pH increased, the Ca/P ratio also increased from 1.44 to 1.68 which was considered ideal since it was closest to the stoichiometric ratio of HAP. Furthermore, HAP prepared at pH 12 was the most crystalline, and rice-shaped nanoparticles (22.8 nm long and 8.6 nm in diameter) were observed while particles synthesized at lower pH had more irregular and overlapping shapes.
In the same year, Chen and co-workers also isolated HAP from Mozambique tilapia (Oreochromis mossambicus) scales using UAE; however they also used calcination and AAD as additional processing steps.208 The scales were first immersed in 1 N HCl for 24 h, 1 N NaOH for 24 h, boiled at 80 °C in water for 20 min, and dried. Next, the sample was sonicated in 50% (v/v) alcohol and calcined at 1000 °C before being ground with a mortar and pestle, and passed through a 200-mesh sieve. Without calcination, the scale powder showed broad peaks in the observed XRD pattern while the calcined product matched the diffractogram of a HAP standard. The mean diameter of particles was 5.96 μm, ranging from 4.2 to 7.8 μm, values that are significantly larger than those reported by Sricharoen et al.196 These values are also higher than those observed after their initial extraction technique (not incorporating UAE) where sizes ranged from 500 nm–1.5 μm.208 This suggests that UAE might provide enough energy to grow HAP particles, similar to calcination, as similar results were observed during Boudreau et al.'s treatment on bones – larger particle sizes after prolonged US treatment.175
That being said, the opposite effect has also been observed. For example, de Amorim et al. also used calcination with UAE, and the heat treatment was performed before sonication.195 Scales from pirarucu (Arapaima gigas) were calcined at 700 °C for 2 h and sieved through a 325-mesh sieve. While a portion of this was doped with niobium ions during UAE, some scales were not in order to see whether doping was successful. These scales were sonicated in ethanol at 20 kHz for 30 min with an amplitude of 70% and dried prior to analysis. Unlike Chen and co-workers,208 the crystallite size decreased from 128.5 to 52.3 nm from the added UAE treatment after calcination. Furthermore, the crystallinity increased from 34 to 48%, suggesting that UAE successfully removed residual protein remaining after calcining for 2 h.
Liu et al. have investigated different DESs for the extraction of HAP from scales of bighead carp (Aristichthys nobilis)197 in 2020 and crucian carp (Carassius carassius) in 2021.198 In both cases, the scales were ground and passed through a 80 mesh sieve before being treated with DES.197,198 For their initial study on bighead carp, three DESs were investigated – choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1
glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride
2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid (2
citric acid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and choline chloride
1), and choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (1
acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).197 The DES that was selected as the best extraction medium was choline chloride
2).197 The DES that was selected as the best extraction medium was choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1
glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) because it had high HAP solubility, moderate viscosity, and excellent stability, and it did not decompose HAP. An optimal extraction rate of 47.67% was achieved by treating scales at 70 °C in DES for 2.5 h with a solid
2) because it had high HAP solubility, moderate viscosity, and excellent stability, and it did not decompose HAP. An optimal extraction rate of 47.67% was achieved by treating scales at 70 °C in DES for 2.5 h with a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 g g−1. The particles were observed to have a high median particle size of 13.98 μm and tended to agglomerate, as observed in all other methods described. Because this process does not involve high temperatures, the product was amorphous and the relative crystallinity of the extracted HAP was 43.13%. Liu et al. screened other DESs for HAP extraction from crucian carp scales, including choline chloride
15 g g−1. The particles were observed to have a high median particle size of 13.98 μm and tended to agglomerate, as observed in all other methods described. Because this process does not involve high temperatures, the product was amorphous and the relative crystallinity of the extracted HAP was 43.13%. Liu et al. screened other DESs for HAP extraction from crucian carp scales, including choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1
glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride
2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) triethylene glycol (1
triethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), choline chloride
4), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycol (1
glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and choline chloride
2), and choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-butanediol (1
1,4-butanediol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).198 In this case, choline chloride
2).198 In this case, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-butanediol (1
1,4-butanediol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was chosen since it had the best extraction rate of 40.58%. This is interesting because this extraction rate is lower than that in their initial study where choline chloride
2) was chosen since it had the best extraction rate of 40.58%. This is interesting because this extraction rate is lower than that in their initial study where choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1
glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was the best.197 The crystallinity and median particle size diameter (12.71 μm) of crucian carp were similar to those of bighead carp.197,198
2) was the best.197 The crystallinity and median particle size diameter (12.71 μm) of crucian carp were similar to those of bighead carp.197,198
3.3. Applications of HAP and BCPs
HAP from fish bones and scales has also been shown to have enough mechanical strength for biomedical ceramics.162 In fact, Akpan et al.162 observed that BCPs from bones experienced a Vickers hardness value of 0.48 GPA which is within the range reported for human femoral cortical bone. The particles also had a fracture toughness of 5.72 MPa m1/2 while maintaining a stable phase at 900 °C and a low brittleness index value of 0.084. Aiza Jaafar et al. created a high-density polyethylene (HDPE) composite incorporating BCPs obtained from the thermal degradation of fish scales at 1200 °C.183 The size of resulting BCP particles was tailored by using spray drying and some were additionally treated with 3-methacryloxypropyltrimethoxysilane (MPTMS, C10H20O5Si) to study its effect on the mechanical properties of composites. Young's modulus of HDPE was enhanced by 29.4% by adding 30 wt% HAP treated with MPTMS to the composite, reaching 1272 MPa, with a tensile strength of 28.26 MPa and a flexural modulus of 796 MPa. Furthermore the composite was shown by in vitro analyses to be non-toxic with potential to be used towards biomedical applications.
BCP materials derived from fish bones have been explored in other biomedical applications. For example, there have been several reports using BCPs from fish bones in sunscreen as an alternative to UV filters associated with health risks.218 Adamiano et al. isolated BCPs from Atlantic salmon bones by calcining them at 800 °C and doped this material with Zn or Mn to increase sun protection factor (SPF)-boosting abilities.219 Interestingly, the undoped BCP material was the most effective at increasing SPF. This demonstrates that bio-derived BCPs can be used to decrease the concentration of UV filters needed in sunscreens while maintaining high SPF values.
Table 9 lists several other example studies using BCPs sourced from fish bones and scales for biomedical purposes. Based on these reports, there is great potential for use of waste-derived HAP in bioceramics and implants, contributing towards a more sustainable industry and circular economy.
| Material | Preparation | Characteristics | Reference |
|---|---|---|---|
| a Abbreviations – HAP, hydroxyapatite; BCP, biphasic calcium phosphates; HDPE, high-density polyethylene; MPTMS, 3-methacryloxypropyltrimethoxysilane; CMC, carboxymethylcellulose; SA, sodium alginate; SBF, simulated body fluid; PLD, pulsed laser deposition; DMEM, Dulbecco's Modified Eagle medium; LDH, lactate dehydrogenase. b Haemolytic activity <5% is labelled as non-haemolytic (non-toxic); haemolytic activity >5% is labelled as haemolytic (toxic). c Cell viability must decrease >30% to be considered cytotoxic. | |||
| HAPa nanoparticles | (1) Alkaline hydrolysis (silver carp bones) | • Retains organic moieties to assist in better osseoinduction | Acharya216 |
| • Ca/P: 1.65 | |||
| • Haemolytic activity 2.24b–3.35% at 1–5 mg mL−1 | |||
| • 91% cell viability at 500 μL g−1 | |||
| • Significant cell proliferation of 20% at 250 μg mL−1 | |||
| BCPa nanoparticles | (1) Calcination (silver carp bones) | • Removal of organic content and more crystalline | Acharya216 |
| • Ca/P: 1.45 | |||
| • Haemolytic activity 3.94b–9.22% at 1–5 mg mL−1 | |||
| • 86% cell viability at 500 μL g−1 | |||
| • Cell proliferation of 15% at 250 μg mL−1 | |||
| HDPEa-BCPa composite | (1) Calcination (tilapia scales) | • Density: 1.17 g cm−3 | Aiza Jaafar183 |
| (2) Spray-dried | • Melting point of 138.4 °C | ||
| (3) 30 wt% HAPa mixed with HDPEa in extruder | • Tensile strength: 28.26 MPa | ||
| (4) Surface treated with MPTMSa | • Young's modulus: 1272 MPA | ||
| • Elongation at break: 43.6% | |||
| • Flexural strength: 21.4 MPa | |||
| • Flexural modulus: 796 MPa | |||
| • Impact strength: 46.90 kJ m−2 | |||
| • 98.51% cell viability at 200 mg mL−1 | |||
| BCPa bioceramics | (1) Calcination (salmon bones) | • Density: 2.96 g cm−3 | Bas172 |
| (2) Compacted and sintered | • Elastic modulus: 633 MPa | ||
| • Ca/P: 1.67 | |||
| • No cytotoxic effect compared to the control | |||
| • Suitable cytocompatibility | |||
| • No cell proliferation after 3 days | |||
| HAPa particles | (1) Alkaline hydrolysis (arowana scales) | • Forms a bone-like apatite layer after immersed in McCoy medium for 3 days, characterizing its bioactivity | Horta182 |
| (2) Calcination | • 102.8% cell viability after 48 h | ||
| BCPa bioceramics | (1) Calcination (tilapia bones) | • Ca/P: 1.64 | Khamkongkaeo132 |
| (2) Compacted and sintered | • Relative density: 90.92–96.43% | ||
| • Vickers hardness: 5.77 GPa | |||
| • Compressive stress: 89.16 MPa | |||
| • Young's modulus: 13.88 GPa | |||
| HAPa scaffold | (1) Calcination (tuna bones) | • Ca/P: 1.87 | Mondal131 |
| (2) Mixed with 2% starch, compacted, and sintered | • Nontoxic behavior at concentrations <300 μg mL−1 | ||
| • Enhanced cell proliferation over control | |||
| PLAa-HAPa filament | (1) Alkaline hydrolysis (sea bass scales) | • Ca/P: 1.67 | Wu189 |
| (2) Mixed with PLAa and eggshells (CaO) in extruder | • Young's modulus: 3.52–3.95 GPa | ||
| (3) Pressed into membranes | • Tensile strength: 43.1–50.9 MPa | ||
| • Elongation at failure: 2.8–4.8% | |||
| • Decreased water resistance | |||
| • Cell viability was not different from control group at 18 days | |||
| • Significantly improved cell viability compared to PLA at 2 days | |||
| • Enhanced cell adhesion | |||
| • Good cytocompatibility | |||
| • Scavenging rate: 5–30% | |||
| • Generated inhibition zones for E. coli and S. aureus | |||
| HAPa nanoparticles | (1) Alkaline hydrolysis (Indian oil sardine bones) | • Cell proliferation 141% at 100 μg mL−1 | Surya163 |
| • Loss of contact with neighbouring cells at 250 μg mL−1, suggesting higher concentrations cannot support osteoblastic growth | |||
| • Good reposition of calcium in MG-63 cells at 7 days, which may be important for bone mineral density | |||
| • Appropriate for cellular activities | |||
| HAPa-CMCa/SAa coating on Ti6Al4V alloy | (1) Calcination (rohu bones) | • Induces growth of apatite on composite when immersed in SBFa | Sridevi157 |
| (2) HAPa added to CMCa in ethanol/water for 12 h | • Biomineralization evident | ||
| (3) SAa added and pH adjusted to 7 | • Microhardness value: 190 Hv | ||
| (4) Ti6Al4V added by electrophoretic deposition | • Enhanced antibacterial activity against S. aureus | ||
| • Lower antibacterial resistance against E. coli | |||
| • Cell viability up to 87% | |||
| • No significant toxicity | |||
| BCPa coating | (1) Alkaline hydrolysis (sea bream & salmon bones) | • Ca/P of targets: 1.57–1.63 | Popescu-Pelin142 |
| (2) Calcination | • Ca/P of films: 1.47–1.50 | ||
| (3) Pressed and sintered | • Pull-off bonding strength sea bream: 49 MPa | ||
| (4) Coatings prepared by PLDa | • Pull-off bonding strength salmon: 33 MPa | ||
| • Mass gain of salmon after 7 days in DMEMa: 3% | |||
| • Mass gain of sea bream after 7 days in DMEMa: 9% | |||
| • Good biocompatibility | |||
| • LDHa release salmon after 24 h: 97% of control | |||
| • LDHa release sea bream after 24 h: 94% of control | |||
| • Significantly increased anti-biofilm performances | |||
Several industrial dyes have been removed from aqueous solutions using materials created with fish-derived HAP. For example, Mamum et al. recently prepared a photocatalyst from calcined tilapia and surma bones for the degradation of Congo red dye,148 an environmental pollutant. In a similar study, pama croaker and Asian stinging catfish were used to create the same photocatalyst, achieving degradation rates of 82% and 73%, respectively, towards Congo red.147 That being said, much higher degradation rates of up to 97% were reported by Biswas and co-workers using raw, pulverized silver carp bones.220 This is interesting because the product contains significantly higher quantities of organic impurities compared to other photocatalysts due to the lack of heat or alkaline treatment. Congo red has anionic sites from sulfonate groups, and other anionic dyes have been studied (e.g., acid blue 185),221 while neutral and cationic dyes have also been successfully treated with fish bones such as methyl green,222 crystal violet,146,223 brilliant green,224 and methylene blue.225 (Table 10).
| Preparation | Dye | Optimal conditions | Degradation/adsorption | Reference |
|---|---|---|---|---|
| a Abbreviations – HAP, hydroxyapatite. b Reaction time for optimal dye degradation/adsorption. c Dye concentration for optimal dye degradation/adsorption. d Volume of dye solution for optimal dye degradation/adsorption. e Adsorbent mass/concentration for optimal dye degradation/adsorption. f pH for optimal dye degradation/adsorption. g Temperature for optimal dye degradation/adsorption. | ||||
| Calcined tilapia & surma bones | Congo red | 240 min,b 20 ppm,c 40 mL,d 0.1e g | 65% | Mamun148 |
| Pulverized silver carp bones | Congo red | 240 min,b 200–300 ppm,c 100 mL,d 100–700 mg,e pH 2f | 91–97% | Parvin220 |
| Calcined shing and poa bones | Congo red | 180 min,b 10 ppm,c 0.08e mg | 73–82% | Prosad Moulick147 |
| Pulverized fish bones treated with Al2O3 | Methyl green | 60 min,b 80 mg L−1,c 0.1 g,e pH 10.64,f 25g °C | 92% | Al-Kazragi222 |
| Calcined of catla bones | Congo red & crystal violet | 75 min,b 50 mg L−1,c 10 mge | Congo red: 87% | Sathiyavimal146 |
| Crystal violet: 77% | ||||
| Pulverized fish bones doped with copper and calcined | Crystal violet | 35 min,b 20 mg L−1,c 2 g L−1,e pH 10,f 50g °C | 98% | Mejbar223 |
| Bleached and calcined fish bones | Brilliant green | 20 min,b 50 mg L−1,c 1 g L−1,e pH 12f | 49.1 mg g−1 | Miyah224 |
| Calcined fish bones | Methylene blue | 10 min,b 100 mg L−1,c 250 mg,e pH 6.9f, 30g °C | >90%, 56.49 mg g−1 | Nurhadi225 |
| Calcined catfish bones mixed with chitosan | Methylene blue & methylene orange | Methylene blue: 60 min,b 35 mg L−1,c 100 mL,d 0.1 g,e pH 8;f methylene orange: 180 min,b 35 mg L−1,c 100 mL,d 0.1 g,e pH 6f | Methylene blue: 84.89 mg g−1 Methylene orange: 44.05 mg g−1 | Trung226 |
| Rohu bones treated with NaOH | Methylene blue | 12 min,b 50 mg L−1,c pH 5,f 34.85g °C | 96.1%, 666.67 mg g−1 | Swamiappan227 |
| Rohu bones treated with NaOH | Melioderm HF brown G | 120 min,b 200 ppm,c 2 g L−1,e pH 2,f 24.85g °C | 98.33% | Hossain228 |
Other pollutants besides dyes have been treated with HAP derived from fish discards. Synthetic HAP has been studied widely for the removal of a broad range of of heavy metals.229,230 There have been several studies using bio-sourced HAP to remediate Cd2+,231,232 Pb2+,232–237 Co2+,144 As3+,153 Zn2+,237 and Cu2+232,238 (Table 11). For example, Cd2+ and Pb2+ have been successfully adsorbed from water by Rashed et al. using calcined fish bones.239 A 99% removal rate of Cd2+ and Pb2+ was achieved by treating wastewater with 0.1 g HAP for 30 min at ∼55 °C with a metal concentration of 10 ppm.239 Renda et al. discovered that HAP could also be used for the adsorption and desorption of Co2+ ions from aqueous solutions.144 Co2+ is a controversial heavy metal because of its effect on human health, but it is also necessary in small quantities for certain bodily functions (e.g., vitamin B12, essential coenzyme for cell mitosis, metabolism, and N2 fixation) and industrial applications (e.g., mining, electronics, and electroplating). The desorbed Co2+ was then observed to have beneficial effects towards seed germination and root elongation.
| BCP source | Additives | Metal(s) | Optimal conditions | Removal rate/adsorption | Reference |
|---|---|---|---|---|---|
| a Abbreviations – BCP, biphasic calcium phosphate; HAP, hydroxyapatite; NA, not applicable. b Reaction time. c Heavy metal concentration. d Adsorbent mass/concentration. e pH for optimal dye degradation/adsorption. f Temperature for optimal degradation/adsorption. | |||||
| Fish bones | Chitosan, Fe3O4 | Cd2+ | 3 min,b 200 mg L−1,c 0.05 g,d pH 5.4,e 25f °C | 25.134 mg g−1 | Yang231 |
| Fish bones | NAa | Pb2+ | 672 h,b 10d wt% | 94–96% | Nag235 |
| Fish scales | Polylactic acid | Pb2+, Cd2+ | 12 h,b 5–100 mg mL−1,c 15d wt% | Pb2+: 112.6 mg g−1 | Fijoł236 |
| Cu2+: 360.5 mg g−1 | |||||
| Fish bones | NAa | Pb2+, Zn2+ | 672 hb | Pb2+: 86.39% | Saffarzadeh237 |
| Zn2+: 63% | |||||
| Fish bones | NAa | Co2+ | 400 min,b 10–1000 mg L−1,c 0.001 g mL−1d | 52 mg g−1 | Renda144 |
| Fish bones | NAa | As3+ | 40 minb | 1.4 mg g−1 | Hubadillah153 |
| Fish scales | MgCl2 | Cu2+, Cd2+, Pb2+ | 180 min,b 20–800 mg L−1,c 1 mg mL−1,d pH 3–7e | Cu2+: 84.2% | Qi232 |
| Cd2+: 74.2% | |||||
| Pb2+: 53.7% | |||||
| Fish bones | Graphene oxide, chitosan | Cu2+ | 120 min,b 100–1000 mg L−1,c 1 mg mL−1,d pH 5e | 256.41 mg g−1 | Hoa238 |
4. Conclusions
There has been a significant increase in fish production over the last few decades to meet the demands of a growing human population. This has led to an abundance of seafood processing byproducts being disposed of unsustainably, contributing to greenhouse gas emissions, eutrophication, and ocean acidification. There have been several global examples that demonstrate that it is possible to process each portion of fish to higher value products instead of just focusing on the edible portion as being of sole value. Iceland's 100% Fish Project has created many different products from fish including fish leather. Norway has also been making great progress towards whole fish utilization. However, these examples face regulatory challenges.22–24 While successes have been achieved with new products based on marine collagen and materials from fish skin, there are, as far as we are aware, no commercial processes focused on the inorganic components present in seafood waste.In this review, we have summarized the possibilities of isolating valuable biominerals from fish inedible discards. In general, it is more advantageous to source calcium-based materials naturally instead of producing them synthetically for sustainability and applicability purposes. Valorizing by-products from the seafood processing industry offers additional streams for financial gain, and prevents significant food waste from ending up in landfills and/or the ocean. This would limit GHG emissions, eutrophication, groundwater contaminations, and odor nuisances caused by the wasted discards. Specifically, this topic tackles many of the UN SDGs, including UN SDG2: Zero Hunger, UN SDG3: Good Health & Well-Being, UN SDG8: Decent Work and Economic Growth, UN SDG9: Industry, Innovation and Infrastructure, UN SDG11: Sustainable Cities and Communities, UN SDG12: Responsible Consumption and Production, UN SDG13: Climate Action, and UN SDG14: Life Below Water. While synthetic CaCO3 and HAP continue to be used more prominently than biogenic materials for most applications, the biomimetic properties of natural HAP and CaCO3 could make it more advantageous for biomedical purposes (e.g., enhanced biomineralization).
Shells from bivalves, crustaceans, and gastropods have the potential to be used as a feedstock for CaCO3 which can then be converted to other value-added products, including CaO and PCC. An interesting area of research that could be investigated is the tailored morphology of polymorphs. While specific polymorphs have been achieved through PCC processes, calcite itself has a range of morphologies that could be explored for biomedical purposes. Khanjani et al. recently explored the factors that influence the morphology of microbially induced PCC, discovering that Ca2+ and functional groups present have an impact on the resulting polymorph and shape.240 While this study primarily focused on vaterite and calcite's spherical shape, it would be interesting to study other possible morphologies.
HAP has been isolated from fish bones and scales using a range of techniques including calcination, alkaline/acidic deproteinization, enzymatic hydrolysis, ultrasound assisted extraction, and the use of deep eutectic solvents. This bio-derived HAP product has been explored for various applications such as incorporation into biomedical composites and the treatment of heavy metals and industrial dyes. These processes have the potential to mitigate the large number of byproducts wasted in landfills and the ocean, although there are some drawbacks. While some reports have focused specifically on making their treatment sustainable and scalable (e.g., enzymes), many of the processes described are industrially inapplicable in seafood processing plants, requiring high temperatures for calcination and the use of hazardous chemicals such as concentrated base (e.g., KOH 50 wt%) for deproteinization. There are also inconsistencies in particle size and morphology of products originating from the same species of fish. One of the main drawbacks of studying biomass-derived products is the development of bacteria, especially in samples that contain trace amounts of residual protein. In fact, we have observed bacteria in TEM images of some of our own HAP nanoparticle solutions from fish bone that were left at room temperature (Fig. 6). For future studies, it would be interesting to develop a low temperature method without the use of acids or bases that also prevents the growth of potentially harmful bacteria. This would ensure that CaCO3 and HAP can be produced sustainably from seafood discards while also being suitable for biomedical purposes. Reduction in endotoxin contamination has been important in advancing the use of organic products such as chitosan from seafood waste streams.241 As the global seafood industry continues to shift from wild catch to aquaculture practices, several threats have emerged. Some of these include the spread of diseases between wild fish and farmed fish and the risk of rising ocean temperatures from climate change affecting harvested species. By completely valorizing every aspect of caught marine organisms, industries would be able to continue making a profit despite challenges with the edible portion. Therefore, the fish itself would remain useful and valuable even if not being considered seafood. For food production, it is important to adapt to climate change and consider indoor recirculating aquaculture systems instead of open ocean systems to mitigate the variations in water temperature and composition.
Author contributions
Conceptualization, F. M. K. and S. B.; writing – original draft preparation, S. B.; writing – review and editing, S. B., E. L., and F. M. K.; supervision, E. L. and F. M. K.; funding acquisition, E. L. and F. M. K.Conflicts of interest
There are no conflicts to declare.Data availability
There is limited supporting data for this review article. The instrumental conditions for Fig. 6 are described in Boudreau et al.'s article in RSC Sustainability (2025) and in the supplementary information file (SI) for this review. Supplementary information is available. See DOI: https://doi.org/10.1039/d5su00527b.Acknowledgements
We thank the NRC Ocean program, OGEN (OCN-110-4), OFI, NSERC of Canada, Memorial University of Newfoundland (MUN), and Dr Liqin Chen for funding.References
- A. Estim, R. Shapawi, S. R. M. Shaleh, C. Fui-Fui and S. Mustafa, in SDGs in the Asia and Pacific Region, Springer, Cham, 2024, pp. 415–444 Search PubMed.
- J. R. Stevens, R. W. Newton, M. Tlusty and D. C. Little, Mar. Pol., 2018, 90, 115–124 CrossRef.
- Fish and seafood consumption per capita, 2020, https://ourworldindata.org/grapher/fish-and-seafood-consumption-per-capita?tab=table%26time=earliest, accessed August 14, 2025.
- Fish and overfishing, https://ourworldindata.org/fish-and-overfishing, accessed August 14, 2025.
- Capture fisheries production, https://www.fao.org/3/cc0461en/online/sofia/2022/capture-fisheries-production.html, accessed August 14, 2025.
- S. Idowu, R. Schmidpeter, N. Capaldi, L. Zu, M. D. Baldo and R. Abreu, Encyclopedia of Sustainable Management, Springer Nature, 2023 Search PubMed.
- FAO leads global efforts to strengthen aquaculture for food and sustainable development, https://www.fao.org/newsroom/detail/fao-leads-global-efforts-to-strengthen-aquaculture-for-food-and-sustainable-development/en, accessed August 14, 2025.
- Aquaculture – Fisheries and Aquaculture, https://www.fao.org/fishery/en/topic/16064, accessed August 14, 2025.
- Aquaculture production, https://www.fao.org/3/cc0461en/online/sofia/2022/aquaculture-production.html, accessed August 14, 2025.
- K. Greer, D. Zeller, J. Woroniak, A. Coulter, M. Winchester, M. L. D. Palomares and D. Pauly, Mar. Policy, 2019, 107, 103382 CrossRef.
- E. Gilman, M. Musyl, P. Suuronen, M. Chaloupka, S. Gorgin, J. Wilson and B. Kuczenski, Sci. Rep., 2021, 11, 7195 CrossRef.
- Aquaculture|Food Loss and Waste in Fish Value Chains|Food and Agriculture Organization of the United Nations, https://www.fao.org/flw-in-fish-value-chains/value-chain/aquaculture/en/, accessed August 14, 2025.
- Processing & Storage|Food Loss and Waste in Fish Value Chains|Food and Agriculture Organization of the United Nations, https://www.fao.org/flw-in-fish-value-chains/value-chain/processing-storage/en/, accessed August 14, 2025.
- K. Mazik, D. Burdon and M. Elliott, Seafood-waste Disposal at Sea – a Scientific review., Institute of Estuarine & Coastal Studies, University of Hull, Hull, UK, 2005 Search PubMed.
- Tackling food loss and waste, https://www.fao.org/newsroom/detail/FAO-UNEP-agriculture-environment-food-loss-waste-day-2022/en, accessed August 14, 2025.
- J. W. Levis and M. A. Barlaz, Environ. Sci. Technol., 2011, 45, 7438–7444 CrossRef PubMed.
- A. A. Ansari and S. S. Gill, Eutrophication: Causes, Consequences and Control: Volume 2, Springer Science & Business Media, 2013 Search PubMed.
- M. F. Chislock, E. Doster, R. A. Zitomer and A. E. Wilson, Nat. Educ. Knowl., 2013, 4, 10 Search PubMed.
- Eutrophication, https://www.nature.com/scitable/knowledge/library/eutrophication-causes-consequences-and-controls-in-aquatic-102364466/, accessed August 14, 2025.
- N. O. and A. A. US Department of Commerce, What is eutrophication?, https://oceanservice.noaa.gov/facts/eutrophication.html, accessed August 14, 2025.
- Z. Fadeeva and R. Van Berkel, in Sustainable Food Value Chain Development, Springer, Singapore, 2023, pp. 61–86 Search PubMed.
- A. V. Strand, S. Mehta, M. S. Myhre, G. Ólafsdóttir and N. M. Saviolidis, Resour. Environ. Sustain., 2024, 16, 100157 Search PubMed.
- 100% Fish, https://sjavarklasinn.is/en/iceland-ocean-cluster/100-fish/, accessed August 14, 2025.
- D. C. Finger, G. Saevarsdottir, H. G. Svavarsson, B. Björnsdóttir, S. Arason and L. Böhme, Circ. Econ. Sustain., 2021, 1, 525–543 CrossRef.
- D. Coppola, M. Oliviero, G. A. Vitale, C. Lauritano, I. D'Ambra, S. Iannace and D. de Pascale, Mar. Drugs, 2020, 18, 214 CrossRef.
- D. Dave, V. vasudevan ramakrishnan, J. Pohling, S. Cheema, S. Trenholm, H. Burke and W. Murphy, J. Food Process. Technol., 2014, 5, 1000401 Search PubMed.
- R. Melgosa, M. T. Sanz and S. Beltrán, J. Supercrit. Fluids, 2021, 169, 105121 CrossRef.
- C. M. Laprise, K. A. Hawboldt, F. M. Kerton and C. M. Kozak, Macromol. Rapid Commun., 2021, 42, 2000339 CrossRef PubMed.
- E. Paone, F. Fazzino, D. M. Pizzone, A. Scurria, M. Pagliaro, R. Ciriminna and P. S. Calabrò, Sustainability, 2021, 13, 2428 CrossRef.
- N. Yan and X. Chen, Nature, 2015, 524, 155–157 CrossRef PubMed.
- T. Jin, T. Liu, E. Lam and A. Moores, Nanoscale Horiz., 2021, 6, 505–542 RSC.
- J. L. Vidal, T. Jin, E. Lam, F. Kerton and A. Moores, Curr. Res. Green Sustainable Chem., 2022, 5, 100330 CrossRef.
- H. J. Burke and F. Kerton, Mar. Drugs, 2023, 21, 366 CrossRef.
- J. L. Shamshina and P. Berton, Front. Bioeng. Biotechnol., 2020, 8, 00011 CrossRef PubMed.
- M. Khajavian, V. Vatanpour, R. Castro-Muñoz and G. Boczkaj, Carbohydr. Polym., 2022, 275, 118702 CrossRef.
- P. A. Aneesh, R. Anandan, L. R. G. Kumar, K. K. Ajeeshkumar, K. A. Kumar and S. Mathew, Biomass Convers. Biorefinery, 2023, 13, 205–214 CrossRef.
- F. Hajiali, J. Vidal, T. Jin, L. C. de la Garza, M. Santos, G. Yang and A. Moores, ACS Sustainable Chem. Eng., 2022, 10, 11348–11357 CrossRef.
- Mining facts – Teaching activities, https://www.dnr.state.mn.us/education/teachers/activities/soudan_mine/miningfacts.html, accessed August 14, 2025.
- W. H. Langer, in Encyclopedia of Materials: Science and Technology, ed. K. H. J. Buschow, R. W. Cahn, M. C. Flemings, B. Ilschner, E. J. Kramer, S. Mahajan and P. Veyssière, Elsevier, Oxford, 2001, pp. 1537–1545 Search PubMed.
- Calcium Carbonate: from the Cretaceous Period into the 21st Century, ed. F. W. Tegethoff, J. Rohleder and E. Kroker, Birkhäuser Verlag, Basel; Boston, English edn, 2001 Search PubMed.
- D. Cree and A. Rutter, ACS Sustainable Chem. Eng., 2015, 3, 941–949 CrossRef.
- A. Dowling, J. O'Dwyer and C. C. Adley, J. Clean. Prod., 2015, 92, 13–22 CrossRef.
- U. S. G. Survey, Mineral Commodity Summaries 2024, U.S. Geological Survey, 2024 Search PubMed.
- O. US EPA, AP 42, 5th edn, ch. 11, vol. 1, https://www.epa.gov/air-emissions-factors-and-quantification/ap-42-fifth-edition-volume-i-chapter-11-mineral-products-0, accessed August 14, 2025 Search PubMed.
- J. M. Paris, J. G. Roessler, C. C. Ferraro, H. D. DeFord and T. G. Townsend, J. Clean. Prod., 2016, 121, 1–18 CrossRef.
- K. M. Ripley, F. H. Saadi and Z. L. Burke, RSC Sustain., 2025, 3, 255–263 RSC.
- M. Simoni, M. D. Wilkes, S. Brown, J. L. Provis, H. Kinoshita and T. Hanein, Renew. Sustain. Energy Rev., 2022, 168, 112765 CrossRef.
- C. Rodriguez-Navarro, E. Ruiz-Agudo, A. Luque, A. B. Rodriguez-Navarro and M. Ortega- Huertas, Am. Mineral., 2009, 94, 578–593 CrossRef.
- O. A. Jimoh, A. K. Shah, H. B. Hussin and A. E. Temitope, Carbonates Evaporites, 2018, 33, 331–346 CrossRef.
- International Energy Agency, Technology Roadmap – Low-Carbon Transition in the Cement Industry, 2018 Search PubMed.
- J. Rissman, C. Bataille, E. Masanet, N. Aden, W. R. Morrow, N. Zhou, N. Elliott, R. Dell, N. Heeren, B. Huckestein, J. Cresko, S. A. Miller, J. Roy, P. Fennell, B. Cremmins, T. Koch Blank, D. Hone, E. D. Williams, S. de la Rue du Can, B. Sisson, M. Williams, J. Katzenberger, D. Burtraw, G. Sethi, H. Ping, D. Danielson, H. Lu, T. Lorber, J. Dinkel and J. Helseth, Appl. Energy, 2020, 266, 114848 CrossRef CAS.
- S. Kittipongvises, Environ. Clim. Technol., 2017, 20, 67–83 CAS.
- G. M. Naja, R. Rivero, S. E. Davis and T. Van Lent, Water, Air, Soil Pollut., 2011, 217, 95–104 CrossRef CAS.
- Y. I. Stepkin, T. I. Prozhorina, S. A. Kurolap, O. V. Klepikov and A. S. Boeva, J. Phys.: Conf. Ser., 2021, 1889, 032028 CrossRef CAS.
- Y.-Q. Niu, J.-H. Liu, C. Aymonier, S. Fermani, D. Kralj, G. Falini and C.-H. Zhou, Chem. Soc. Rev., 2022, 51, 7883–7943 RSC.
- M. H. Azarian and W. Sutapun, Front. Mater., 2022, 9, 1024977 CrossRef.
- J. A. H. Oates, Lime and Limestone: Chemistry and Technology, Production and Uses, John Wiley & Sons, 2008 Search PubMed.
- F. M. Kerton, RSC Sustain., 2023, 1, 401–403 RSC.
- N. Vieira Veríssimo, C. Ussemane Mussagy, A. Alves Oshiro, C. M. Nóbrega Mendonça, V. de Carvalho Santos-Ebinuma, A. Pessoa, R. P. de Souza Oliveira and J. F. Brandão Pereira, Green Chem., 2021, 23, 9377–9400 RSC.
- F. M. Kerton, Y. Liu, K. W. Omari and K. Hawboldt, Green Chem., 2013, 15, 860–871 RSC.
- M. A. Yusoff, P. Mohammadi, F. Ahmad, N. A. Sanusi, H. Hosseinzadeh-Bandbafha, H. Vatanparast, M. Aghbashlo and M. Tabatabaei, Sci. Total Environ., 2024, 952, 175810 CrossRef PubMed.
- A. Singh, N. Kelkar, K. Natarajan and S. Selvaraj, Environ. Sci. Pollut. Res., 2021, 28, 46985–46998 CrossRef PubMed.
- F. Soost, Chirurg, 1996, 67, 1193–1196 CrossRef PubMed.
- M. Ni and B. D. Ratner, Surf. Interface Anal., 2008, 40, 1356–1361 CrossRef.
- R. A. Boulos, F. Zhang, E. S. Tjandra, A. D. Martin, D. Spagnoli and C. L. Raston, Sci. Rep., 2014, 4, 3616 CrossRef.
- R. Febrida, S. Setianto, E. Herda, A. Cahyanto and I. M. Joni, Heliyon, 2021, 7, e08344 CrossRef.
- W. L. Bragg, Proc. R. Soc. London, Ser. A, 1997, 105, 16–39 Search PubMed.
- J. N. Murphy, C. M. Schneider, L. K. Mailänder, Q. Lepillet, K. Hawboldt and F. M. Kerton, Green Chem., 2019, 21, 3920–3929 RSC.
- A. G. Christy, Cryst. Growth Des., 2017, 17, 3567–3578 CrossRef.
- F. Liendo, M. Arduino, F. A. Deorsola and S. Bensaid, Powder Technol., 2022, 398, 117050 CrossRef.
- A. Prihanto, S. Muryanto, R. Ismail, J. Jamari and A. P. Bayuseno, Environ. Technol., 2024, 45, 235–245 CrossRef.
- R. Ismail, D. F. Fitriyana, Y. I. Santosa, S. Nugroho, A. J. Hakim, M. S. Al Mulqi, J. Jamari and A. P. Bayuseno, J. Cryst. Growth, 2021, 572, 126282 CrossRef.
- A. P. Bayuseno, A. I. Prasetya, R. Ismail, B. Setiyana and J. Jamari, Rasayan J. Chem., 2022, 15, 523–528 CrossRef.
- F. M. Kerton and N. Yan, Fuels, Chemicals and Materials from the Oceans and Aquatic Sources, John Wiley & Sons, 2017 Search PubMed.
- J.-A. Barrat, L. Chauvaud, F. Olivier, P. Poitevin and M.-L. Rouget, Chem. Geol., 2023, 638, 121695 CrossRef.
- J. N. Murphy, K. Hawboldt and F. M. Kerton, Green Chem., 2018, 20, 2913–2920 RSC.
- C. Triunfo, S. Gärtner, C. Marchini, S. Fermani, G. Maoloni, S. Goffredo, J. G. Morales, H. Cölfen and G. Falini, ACS Omega, 2022, 7, 43992–43999 CrossRef PubMed.
- C. C. Cardoso, A. S. Cavalcanti, R. O. Silva, S. Alves Junior, F. P. de Sousa, V. M. D. Pasa, S. Arias and J. G. A. Pacheco, J. Braz. Chem. Soc., 2020, 31, 756–767 Search PubMed.
- J. N. Murphy, C. M. Schneider, K. Hawboldt and F. M. Kerton, Matter, 2020, 3, 2029–2041 CrossRef.
- M. F. R. S. Araújo, P. C. Lima, C. C. Cardoso and V. M. D. Pasa, J. Clean. Prod., 2020, 277, 123709 CrossRef.
- K.-W. Nam, S.-W. Lee, J.-H. Song, H.-D. Jeung and K.-I. Park, Korean J. Malacol., 2015, 31, 279–283 CrossRef.
- K. Anand, M. Reshma, M. Kannan, M. Selvan, S. Chaturvedi, A. Shalan and K. Govindaraju, J. Nanostruct. Chem., 2021, 11, 409–422 CrossRef.
- E. Águila-Almanza, H. Hernández-Cocoletzi, E. Rubio-Rosas, M. Calleja-González, H. R. Lim, K. S. Khoo, V. Singh, J. C. Maldonado-Montiel and P. L. Show, Chemosphere, 2022, 288, 132550 CrossRef PubMed.
- C. Ramakrishna, T. Thenepalli, C. Han and J.-W. Ahn, Korean J. Chem. Eng., 2017, 34, 225–230 CrossRef.
- B. Nguyen Quang and D. Ta Hong, J. Chem., 2022, 2022, e3821717 Search PubMed.
- L. Ogresta, F. Nekvapil, T. Tămaş, L. Barbu-Tudoran, M. Suciu, R. Hirian, M. Aluaş, G. Lazar, E. Levei, B. Glamuzina and S. C. Pinzaru, ACS Omega, 2021, 6, 27773–27780 CrossRef PubMed.
- R. Ismail, T. Cionita, W. L. Shing, D. F. Fitriyana, J. P. Siregar, A. P. Bayuseno, F. W. Nugraha, R. C. Muhamadin, R. Junid and N. A. Endot, Materials, 2022, 15, 5712 CrossRef.
- K. H. Mo, U. J. Alengaram, M. Z. Jumaat, S. C. Lee, W. I. Goh and C. W. Yuen, Constr. Build. Mater., 2018, 162, 751–764 CrossRef CAS.
- E.-I. Yang, S.-T. Yi and Y.-M. Leem, Cem. Concr. Res., 2005, 35, 2175–2182 CrossRef CAS.
- S.-H. Eo and S.-T. Yi, Mag. Concr. Res., 2015, 67, 833–842 CrossRef.
- C. Varhen, S. Carrillo and G. Ruiz, Constr. Build. Mater., 2017, 136, 533–540 CrossRef CAS.
- H. Cuadrado-Rica, N. Sebaibi, M. Boutouil and B. Boudart, Mater. Struct., 2016, 49, 1805–1816 CrossRef CAS.
- N. H. Othman, B. H. A. Bakar, M. M. Don and M. A. M. Johari, J. Civ. Eng., 2013, 25, 201–211 Search PubMed.
- K. Muthusamy, S. M. A. Nasir and A. M. Budiea, Concr. Res. Lett., 2016, 7, 132–137 CAS.
- P. Lertwattanaruk, N. Makul and C. Siripattarapravat, J. Environ. Manag., 2012, 111, 133–141 CrossRef PubMed.
- S. Christian-Robinson and F. M. Kerton, Pure Appl. Chem., 2024, 96, 1247–1255 CrossRef CAS.
- J. N. Murphy, M. A. Morgan, S. Christian-Robinson, M. M. Fitzgerald and F. M. Kerton, Can. J. Chem., 2025, 1–10 CAS.
- M. M. Fitzgerald, M. A. Morgan and F. M. Kerton, J. Sustain. Circ. NOW, 2025, 02, a24874285 Search PubMed.
- K. M. Ibiyeye and A. B. Z. Zuki, Int. J. Mol. Sci., 2020, 21, 1900 CrossRef CAS.
- K. M. Ibiyeye, A. B. Z. Zuki, N. Nurdin and M. Ajat, Nanosci. Nanotechnol.–Asia, 2020, 10, 518–533 CrossRef CAS.
- S. Dampang, E. Purwanti, F. Destyorini, S. B. K. B. Kurniawan, S. R. S. Abdullah and M. F. Imron, J. Econ. Eng., 2021, 22, 221–228 Search PubMed.
- N. F. Nasir and M. M. Hazri, Res. Prog. Mech. Manuf. Eng., 2020, 1, 44–55 Search PubMed.
- M. V. S. Rezende, U. C. Pereira, Y. R. R. S. Rezende, I. S. Carvalho, W. S. Silveira, D. O. Junot, R. S. Silva, C. X. Resende and N. S. Ferreira, Optik, 2021, 235, 166636 CrossRef CAS.
- Y. Wibisono, S. R. Ummah, M. B. Hermanto, G. Djoyowasito and A. Noviyanto, Res. Eng., 2024, 21, 101781 CAS.
- J. H. Seo, S. M. Park, B. J. Yang and J. G. Jang, Materials, 2019, 12, 1322 CrossRef CAS.
- W. Kurdowski, in Cement and Concrete Chemistry, Springer, Dordrecht, 2014, pp. 21–127 Search PubMed.
- A. Hart, Waste Manag. Res., 2020, 38, 514–527 CrossRef.
- S. Thakur, S. Singh and B. Pal, Fuel Process. Technol., 2021, 213, 106707 CrossRef CAS.
- S. Gohar Khan, M. Hassan, M. Anwar, Zeshan, U. Masood Khan and C. Zhao, Fuel, 2022, 330, 125480 CrossRef CAS.
- N. O. Eddy, A. O. Odiongenyi, R. Garg, R. A. Ukpe, R. Garg, A. E. Nemr, C. M. Ngwu and I. J. Okop, Environ. Sci. Pollut. Res., 2023, 30, 64036–64057 CrossRef CAS.
- E. C. Ogoko, H. I. Kelle, O. Akintola and N. O. Eddy, Biomass Convers. Biorefinery, 2024, 14, 14859–14875 CrossRef CAS.
- N. Erdogan and H. A. Eken, Physicochem. Probl. Miner. Process., 2017, 53, 57–68 CAS.
- Precipitated Calcium Carbonate, https://www.lime.org/resource/other-uses-of-lime/, accessed August 14, 2025.
- M. Eriksson, K. Sandström, M. Carlborg and M. Broström, Minerals, 2024, 14, 244 CrossRef CAS.
- M. A. Irfa'i, A. Prihanto, S. Muryanto, J. Jamari, R. Ismail and A. P. Bayuseno, IOP Conf. Ser. Earth Environ. Sci., 2022, 1098, 012021 CrossRef.
- X. Feng, Curr. Chem. Biol., 2009, 3, 189–196 CAS.
- R.-M. Kavasi, C. C. Coelho, V. Platania, P. A. Quadros and M. Chatzinikolaidou, Nanomaterials, 2021, 11, 1152 CrossRef CAS.
- P. Dan, V. Sundararajan, H. Ganeshkumar, B. Gnanabarathi, A. K. Subramanian, G. D. Venkatasubu, S. Ichihara, G. Ichihara and S. Sheik Mohideen, Appl. Surf. Sci., 2019, 484, 568–577 CrossRef CAS.
- K. Kuroda and M. Okido, Bioinorgan. Chem. Appl., 2012, 2012, 730693 Search PubMed.
- S.-M. Huang, S.-M. Liu, C.-L. Ko and W.-C. Chen, Polymers, 2022, 14, 976 CrossRef CAS PubMed.
- S. Awasthi, S. Kumar Pandey, E. Arunan and C. Srivastava, J. Mater. Chem. B, 2021, 9, 228–249 RSC.
- Y. Yang, J. Yang, N. Zhu, H. Qiu, W. Feng, Y. Chen, X. Chen, Y. Chen, W. Zheng, M. Liang, T. Lin, J. Yu and Z. Guo, J. Nanobiotechnol., 2023, 21, 470 CrossRef CAS.
- M. Du, J. Chen, K. Liu, H. Xing and C. Song, Compos. B Eng., 2021, 215, 108790 CrossRef CAS.
- S. Balhuc, R. Campian, A. Labunet, M. Negucioiu, S. Buduru and A. Kui, Crystals, 2021, 11, 674 CrossRef.
- T. U. Habibah, D. V. Amlani and M. Brizuela, in StatPearls, StatPearls Publishing, Treasure Island (FL), 2024 Search PubMed.
- M. Ibrahim, M. Labaki, J.-M. Giraudon and J.-F. Lamonier, J. Hazard. Mater., 2020, 383, 121139 CrossRef.
- A. Fihri, C. Len, R. S. Varma and A. Solhy, Coord. Chem. Rev., 2017, 347, 48–76 CrossRef.
- A. Das and D. Pamu, Mater. Sci. Eng., C, 2019, 101, 539–563 CrossRef PubMed.
- N. A. S. Mohd Pu'ad, R. H. Abdul Haq, H. Mohd Noh, H. Z. Abdullah, M. I. Idris and T. C. Lee, Mater. Today Proc., 2020, 29, 233–239 CrossRef.
- J. M. Delgado-López and M. Iafisco, Apatite: Synthesis, Structural Characterization, and Biomedical Applications, Nova Science Publishers, Inc, New York, 2014 Search PubMed.
- S. Mondal, G. Hoang, P. Manivasagan, M. S. Moorthy, H. H. Kim, T. T. Vy Phan and J. Oh, Mater. Chem. Phys., 2019, 228, 344–356 CrossRef.
- A. Khamkongkaeo, T. Boonchuduang, W. Klysubun, P. Amonpattaratkit, H. Chunate, N. Tuchinda, A. Pimsawat, S. Daengsakul, P. Suksangrat, W. Sailuam, D. Vongpramate, A. Bootchanont and B. Lohwongwatana, Ceram. Int., 2021, 47, 34575–34584 CrossRef.
- K. A. Zakaria, N. I. Yatim, N. Ali and H. Rastegari, Environ. Sci. Pollut. Res., 2022, 29, 46471–46486 CrossRef PubMed.
- M. Greiner, L. Férnandez-Díaz, E. Griesshaber, M. N. Zenkert, X. Yin, A. Ziegler, S. Veintemillas- Verdaguer and W. W. Schmahl, Minerals, 2018, 8, 315 CrossRef.
- J. A. Ruben and A. A. Bennett, Evolution, 1987, 41, 1187–1197 CrossRef PubMed.
- A. Maidaniuc, F. Miculescu, R. C. Ciocoiu, T. M. Butte, I. Pasuk, G. E. Stan, S. I. Voicu and L. T. Ciocan, Ceram. Int., 2020, 46, 10159–10171 CrossRef.
- M. R. Hasan, N. S. M. Yasin, M. S. M. Ghazali and N. F. Mohtar, J. Sustain. Sci. Manag., 2020, 15, 9–21 CrossRef.
- M. R. Hasan, M. S. M. Ghazali and N. F. Mohtar, J. Mech. Eng. Sci., 2021, 15, 7792–7806 Search PubMed.
- P. Ideia, L. Degli Esposti, C. C. Miguel, A. Adamiano, M. Iafisco and P. C. Castilho, Int. J. Appl. Ceram. Technol., 2021, 18, 235–243 CrossRef.
- H. A. Permatasari, R. Wati, R. M. Anggraini, A. Almukarramah and Y. Yusuf, Key Eng. Mater., 2020, 840, 318–323 Search PubMed.
- F. Fendi, B. Abdullah, S. Suryani, I. Raya and D. Tahir, IOP Conf. Ser. Earth Environ. Sci., 2023, 1230, 012042 CrossRef.
- G. Popescu-Pelin, C. Ristoscu, L. Duta, I. Pasuk, G. E. Stan, M. S. Stan, M. Popa, M. C. Chifiriuc, C. Hapenciuc, F. N. Oktar, A. Nicarel and I. N. Mihailescu, Mar. Drugs, 2020, 18, 623 CrossRef CAS PubMed.
- S. Lestari, M. Nurhadi, R. K. Wardani, E. Saputro, R. Pujisupiati, N. S. Muskita, N. Fortuna, A. S. Purwandari, F. Aryani, S. Y. Lai and H. Nur, Bull. Chem. React. Eng. Catal., 2022, 17, 565–576 CrossRef CAS.
- C. G. Renda, T. M. D. O. Ruellas, J. O. D. Malafatti, C. S. S. Araújo, G. L. da Silva, B. A. M. Figueira, S. Quaranta and E. C. Paris, Physchem, 2023, 3, 34–60 CrossRef CAS.
- A. Q. Ishak, N. A. N. Ali, A. M. S. Nurhaziqah and H. Salleh, Solid State Phenom., 2020, 307, 339–344 Search PubMed.
- S. Sathiyavimal, S. Vasantharaj, M. Shanmugavel, E. Manikandan, P. Nguyen-Tri, K. Brindhadevi and A. Pugazhendhi, Prog. Org. Coating, 2020, 148, 105890 CrossRef CAS.
- S. Prosad Moulick, Md. Sahadat Hossain, Md. Zia Uddin Al Mamun, F. Jahan, Md. Farid Ahmed, R. A. Sathee, Md. Sujan Hossen, Md. Ashraful Alam, Md. Sha Alam and F. Islam, Results Eng., 2023, 20, 101418 CrossRef CAS.
- M. Z. U. A. Mamun, M. S. Hossain, S. P. Moulick, M. Begum, R. A. Sathee, M. S. Hossen, F. Jahan, M. M. Rashid, F. Islam, R. H. Bhuiyan and M. S. Alam, Heliyon, 2023, 9, e18012 CrossRef PubMed.
- A. Pal, S. Paul, A. R. Choudhury, V. K. Balla, M. Das and A. Sinha, Mater. Lett., 2017, 203, 89–92 CrossRef CAS.
- H. B. Modolon, J. Inocente, A. M. Bernardin, O. R. Klegues Montedo and S. Arcaro, Ceram. Int., 2021, 47, 27685–27693 CrossRef CAS.
- P. Satish, A. Salian, K. Hadagalli and S. Mandal, Adv. Appl. Ceram., 2023, 122, 69–78 CrossRef CAS.
- F. Carella, M. Seck, L. D. Esposti, H. Diadiou, A. Maienza, S. Baronti, P. Vignaroli, F. P. Vaccari, M. Iafisco and A. Adamiano, J. Environ. Chem. Eng., 2021, 9, 104815 CrossRef CAS.
- S. K. Hubadillah, M. R. Jamalludin, M. H. D. Othman and M. R. Adam, Mater. Today Proc., 2023 DOI:10.1016/j.matpr.2022.12.232.
- R. M. Anggraini, T. Restianingsih, F. Deswardani, Y. Fendriani and R. A. P. Purba, J. Phys., 2023, 9, 49–54 Search PubMed.
- A. Pramono, F. Sulaiman, S. Suryana, A. Alfirano and A. Milandia, Mater. Sci. Forum, 2020, 988, 182–191 Search PubMed.
- A. D. Wuntu, D. M. H. Mantiri, J. J. H. Paulus and H. F. Aritonang, AACL Bioflux, 2021, 14, 612–619 Search PubMed.
- S. Sridevi, S. Sutha, L. Kavitha and D. Gopi, Mater. Chem. Phys., 2020, 254, 123455 CrossRef CAS.
- T. P. Boaventura, A. M. Peres, V. S. B. Gil, C. S. B. Gil, R. L. Oréfice and R. K. Luz, Quim. Nova, 2020, 43, 168–174 Search PubMed.
- G. Dorcioman, V. Grumezescu, G. E. Stan, M. C. Chifiriuc, G. P. Gradisteanu, F. Miculescu, E. Matei, G. Popescu-Pelin, I. Zgura, V. Craciun, F. N. Oktar and L. Duta, Pharmaceutics, 2023, 15, 1294 CrossRef.
- J. A. da Cruz, W. R. Weinand, A. M. Neto, R. S. Palácios, A. J. M. Sales, P. R. Prezas, M. M. Costa and M. P. F. Graça, JOM, 2020, 72, 1435–1442 CrossRef.
- S. M. Naga, H. F. El-Maghraby, E. M. Mahmoud, M. S. Talaat and A. M. Ibrhim, Ceram. Int., 2015, 41, 15010–15016 CrossRef.
- E. S. Akpan, M. Dauda, L. S. Kuburi, D. O. Obada and D. Dodoo-Arhin, Res. Phys., 2020, 17, 103051 Search PubMed.
- P. Surya, A. Nithin, A. Sundaramanickam and M. Sathish, J. Mech. Behav. Biomed. Mater., 2021, 119, 104501 CrossRef PubMed.
- M. Nag, A. Saffarzadeh, T. Nomichi, T. Shimaoka and H. Nakayama, Waste Manag., 2020, 118, 281–290 CrossRef PubMed.
- P. Kusumawati, P. Triwitono, S. Anggrahini and Y. Pranoto, Squal. Bull. Mar. Fish. Postharvest Biotechnol., 2022, 17, 1–12 CrossRef.
- P. L. Hariani, A. Rachmat, M. Said and S. Salni, Indones. J. Chem., 2021, 21, 1471–1483 CrossRef.
- A. T. Idowu, S. Benjakul, S. Sinthusamran, T. Sae-leaw, N. Suzuki, Y. Kitani and P. Sookchoo, Appl. Sci., 2020, 10, 4141 CrossRef.
- M. K. dos, S. Horta, C. Westin, D. N. da Rocha, J. B. de Campos, R. F. M. de Souza, M. S. Aguilar and F. J. Moura, Mater. Res., 2023, 26, e20220466 CrossRef.
- A. F. Ahamed, M. Manimohan and N. Kalaivasan, J. Inorg. Organomet. Polym., 2022, 32, 3902–3922 CrossRef.
- K. H. Le Ho, V. H. Dao, X. K. Pham, P. A. Nguyen, B. V. Phan, T. T. Doan and T. H. Lam, Reg. Stud. Mar. Sci., 2022, 55, 102560 Search PubMed.
- H. Yamamura, V. H. P. da Silva, P. L. M. Ruiz, V. Ussui, D. R. R. Lazar, A. C. M. Renno and D. A. Ribeiro, J. Mech. Behav. Biomed. Mater., 2018, 80, 137–142 CrossRef PubMed.
- M. Bas, S. Daglilar, N. Kuskonmaz, C. Kalkandelen, G. Erdemir, S. E. Kuruca, D. Tulyaganov, T. Yoshioka, O. Gunduz, D. Ficai and A. Ficai, Int. J. Mol. Sci., 2020, 21, 8082 CrossRef PubMed.
- M. Fitri, S. U. Tartar, S. Nurmiah and I. Syukroni, Asian Food Sci. J., 2022, 21, 78–85 CrossRef.
- S. Boudreau, S. Hrapovic, Y. Liu, A. C. W. Leung, E. Lam and F. M. Kerton, RSC Sustain., 2023, 1, 1554–1564 RSC.
- S. Boudreau, S. Hrapovic, E. McIsaac, E. Lam, F. Berrué and F. M. Kerton, RSC Sustain., 2025, 3, 2325–2332 RSC.
- M. Fernández-Arias, I. Álvarez-Olcina, P. Malvido-Fresnillo, J. A. Vázquez, M. Boutinguiza, R. Comesaña and J. Pou, Appl. Sci., 2021, 11, 3387 CrossRef.
- P. V. Nam, N. Van Hoa, T. T. L. Anh and T. S. Trung, Waste Biomass Valorization, 2020, 11, 4195–4206 CrossRef CAS.
- Home, https://nlmarineorganics.com/, accessed August 14, 2025.
- J. C. García-Santiago, C. J. Lozano Cavazos, J. A. González-Fuentes, A. Zermeño-González, E. Rascón Alvarado, A. Rojas Duarte, P. Preciado-Rangel, E. Troyo-Diéguez, F. M. Peña Ramos, L. A. Valdez-Aguilar, D. Alvarado-Camarillo and J. A. Hernández Maruri, Biol. Agric. Hortic., 2021, 37, 107–124 CrossRef.
- W. Sun, M. H. Shahrajabian, Y. Kuang and N. Wang, Plants, 2024, 13, 210 CrossRef CAS.
- D.-T. Van-Pham, V. V. Phat, N. H. Chiem, T. T. B. Quyen, N. T. N. Mai, D. H. Giao, T. N. Don and D. V. H. Thien, Environ. Nat. Resour. J., 2022, 20, 323–329 Search PubMed.
- M. K. dos, S. Horta, F. J. Moura, M. S. Aguilar, C. B. Westin, D. Navarro da Rocha and J. B. de Campos, Int. J. Appl. Ceram. Technol., 2021, 18, 1930–1937 CrossRef.
- C. N. Aiza Jaafar, I. Zainol, M. I. Izyan Khairani and T. T. Dele-Afolabi, Polymers, 2022, 14, 251 CrossRef CAS.
- B.-S. Liaw, T.-T. Chang, H.-K. Chang, W.-K. Liu and P.-Y. Chen, J. Hazard. Mater., 2020, 382, 121082 CrossRef CAS PubMed.
- P. Injorhor, T. Trongsatitkul, J. Wittayakun, C. Ruksakulpiwat and Y. Ruksakulpiwat, Polymers, 2022, 14, 4158 CrossRef CAS.
- N. S. S. A. Buraiki, B. Ali Albadri, S. Alsheriqi, B. Alshabibi, S. Al-Mammari, S. Premkumar, M. K. Sah and M. S. Sudhakar, Mater. Today Proc., 2020, 27, 2609–2616 CrossRef CAS.
- M. Eswaran, S. Swamiappan, B. Chokkiah, R. Dhanusuraman, S. Bharathkumar and V. K. Ponnusamy, Mater. Lett., 2021, 302, 130341 CrossRef CAS.
- C. Sarkar and M. Das, Indian J. Nat. Prod. Res., 2021, 12, 359–368 CAS.
- C.-S. Wu, S.-S. Wang, D.-Y. Wu and W.-L. Shih, Addit. Manuf., 2021, 46, 102169 CAS.
- R. T. Rashad, Acta Sci. Malays., 2023, 7, 54–59 CrossRef.
- M. A. Selimin, A. F. A. Latif, C. W. Lee, M. S. Muhamad, H. Basri and T. C. Lee, Mater. Today Proc., 2022, 57, 1142–1146 CrossRef.
- A. Ashwitha, K. Thamizharasan and P. Bhatt, SN Appl. Sci., 2020, 2, 1228 CrossRef.
- H. Wang, M. Qian, D. Zhang, Q. Wu and Z. Zhao, Water, 2023, 15, 1274 CrossRef.
- U. Sittitut, J. Jettanasen, S. Supothina and R. Rattanakam, Inorganics, 2022, 10, 242 CrossRef.
- M. O. de Amorim, J. C. C. S. Júnior, Y. L. Ruiz and J. C. S. Andrade, Cerâmica, 2021, 67, 23–27 CrossRef.
- P. Sricharoen, N. Limchoowong, P. Nuengmatcha and S. Chanthai, Ultrason. Sonochem., 2020, 63, 104966 CrossRef PubMed.
- Y. Liu, J. Li, D. Wang, F. Yang, L. Zhang, S. Ji and S. Wang, J. Food Sci., 2020, 85, 150–156 CrossRef PubMed.
- Y. Liu, M. Liu, S. Ji, L. Zhang, W. Cao, H. Wang and S. Wang, Ceram. Int., 2021, 47, 9366–9372 CrossRef.
- O. Grahl-Nielsen and K. A. Glover, Mar. Biol., 2010, 157, 1567–1576 CrossRef.
- S. Kongsri, K. Janpradit, K. Buapa, S. Techawongstien and S. Chanthai, Chem. Eng. J., 2013, 215–216, 522–532 CrossRef.
- I. M. Savic and I. M. Savic Gajic, J. Food Sci. Technol., 2020, 57, 2809–2818 CrossRef PubMed.
- M. M. Kininge and P. R. Gogate, Ultrason. Sonochem., 2022, 82, 105870 CrossRef PubMed.
- M. Mofijur, F. Kusumo, I. M. R. Fattah, H. M. Mahmudul, M. G. Rasul, A. H. Shamsuddin and T. M. I. Mahlia, Energies, 2020, 13, 1770 CrossRef.
- E. Calcio Gaudino, G. Grillo, S. Tabasso, L. Stevanato, G. Cravotto, K. Marjamaa, V. Pihlajaniemi, A. Koivula, N. Aro, J. Uusitalo, J. Ropponen, L. Kuutti, P. Kivinen, H. Kanerva, A. Arshanitsa, L. Jashina, V. Jurkjane, A. Andersone, T. Dreyer and G. Schories, J. Clean. Prod., 2022, 380, 134897 CrossRef.
- L. Liu, J. Li, J. Tian, Z. Zhou, J. Gao, L. Qin and J. Qiu, Sustain. Chem. Pharm., 2024, 41, 101718 CrossRef.
- Q. Wang, L. Niu, J. Jiao, N. Guo, Y. Zang, Q. Gai and Y. Fu, Biores. Technol., 2017, 244, 969–974 CrossRef.
- E. M. M. Flores, G. Cravotto, C. A. Bizzi, D. Santos and G. D. Iop, Ultrason. Sonochem., 2021, 72, 105455 CrossRef CAS.
- W.-K. Liu, B.-S. Liaw, H.-K. Chang, Y.-F. Wang and P.-Y. Chen, JOM, 2017, 69, 713–718 CrossRef CAS.
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS.
- J. Afonso, A. Mezzetta, I. M. Marrucho and L. Guazzelli, Green Chem., 2023, 25, 59–105 RSC.
- K. L. Hernández-Ruiz, J. López-Cervantes, D. I. Sánchez-Machado, M. D. R. Martínez-Macias, M. A. Correa-Murrieta and A. Sanches-Silva, Sustain. Chem. Pharm., 2022, 28, 100726 CrossRef.
- L. Degli Esposti, A. C. Ionescu, S. Gandolfi, N. Ilie, A. Adamiano, E. Brambilla and M. Iafisco, Dent. Mater., 2024, 40, 593–607 CrossRef CAS.
- M. Boutinguiza, J. Pou, R. Comesaña, F. Lusquiños, A. de Carlos and B. León, Mater. Sci. Eng., C, 2012, 32, 478–486 CrossRef CAS.
- L. Zhang, C. Zhang, R. Zhang, D. Jiang, Q. Zhu and S. Wang, Mater. Lett., 2019, 236, 680–682 CrossRef CAS.
- P. Acharya, M. Kupendra, A. Fasim, K. S. Anantharaju, N. Kottam, V. K. Murthy and S. S. More, Biotechnol. Lett., 2022, 44, 1175–1188 CrossRef CAS.
- P. Shi, M. Liu, F. Fan, C. Yu, W. Lu and M. Du, Mater. Sci. Eng., C, 2018, 90, 706–712 CrossRef CAS PubMed.
- S. Righi, E. Prato, G. Magnani, V. Lama, F. Biandolino, I. Parlapiano, F. Carella, M. Iafisco and A. Adamiano, Sci. Total Environ., 2023, 862, 160751 CrossRef CAS PubMed.
- A. Adamiano, F. Carella, L. Degli Esposti, C. Piccirillo and M. Iafisco, ACS Biomater. Sci. Eng., 2022, 8, 4987–4995 CrossRef CAS PubMed.
- S. Parvin, Md. M. Hussain, F. Akter and B. K. Biswas, J. Chem., 2021, 2021, 9535644 Search PubMed.
- G. Falini, M. L. Basile, S. Gandolfi, F. Carella, G. Guarini, L. D. Esposti, M. Iafisco and A. Adamiano, Ceram. Int., 2023, 49, 243–252 CrossRef CAS.
- M. A. U. R. Al-Kazragi and D. T. A. Al-Heetimi, J. Phys.: Conf. Ser., 2021, 1879, 022073 CrossRef CAS.
- F. Mejbar, Y. Miyah, A. Lahrichi, S. Ssouni, A. Khalil, L. Nahali, M. Benjelloun, G. E. Mouhri and F. Zerrouq, Moroccan J. Chem., 2021, 9, 434–445 CAS.
- Y. Miyah, M. Benjelloun, R. Salim, L. Nahali, F. Mejbar, A. Lahrichi, S. Iaich and F. Zerrouq, J. Mol. Liq., 2022, 362, 119739 CrossRef CAS.
- M. Nurhadi, R. Kusumawardani, W. Wirhanuddin, R. Gunawan and H. Nur, Bull. Chem. React. Eng. Catal., 2019, 14, 660–671 CrossRef.
- T. S. Trung, N. C. Minh, H. N. Cuong, P. T. D. Phuong, P. A. Dat, P. V. Nam and N. V. Hoa, J. Sci. Adv. Mater. Devices, 2022, 7, 100485 CrossRef.
- S. Swamiappan, S. Ganesan, V. Sekar, S. Devaraj, A. Subramanian, V. K. Ponnusamy and P. Kathirvel, Int. J. Appl. Ceram. Technol., 2021, 18, 902–912 CrossRef.
- Md. A. Hossain, P. I. Turzo, Md. S. R. Shakil and F. Tuj-Zohra, Water Pract. Technol., 2023, 18, 2277–2295 CrossRef.
- A. Nayak and B. Bhushan, Mater. Today Proc., 2021, 46, 11029–11034 CrossRef.
- F. H. Jaffar, M. H. D. Othman, N. J. Ismail, M. H. Puteh, T. A. Kurniawan, S. Abu Bakar and H. Abdullah, J. Taiwan Inst. Chem. Eng., 2024, 164, 105668 CrossRef.
- W. Yang, W. Luo, T. Sun, Y. Xu and Y. Sun, Int. J. Environ. Res. Public Health, 2022, 19, 1260 CrossRef PubMed.
- X. Qi, H. Yin, M. Zhu, X. Yu, P. Shao and Z. Dang, Chemosphere, 2022, 294, 133733 CrossRef PubMed.
- S. Mostofa, S. A. Jahan, B. Saha, N. Sharmin and S. Ahmed, Environ. Nanotechnol. Monit. Manag., 2022, 18, 100738 Search PubMed.
- H. M. Tauqeer, Z. Basharat, P. M. Adnan Ramzani, M. Farhad, K. Lewińska, V. Turan, A. Karczewska, S. A. Khan, G. Faran and M. Iqbal, Environ. Pollut., 2022, 313, 120064 CrossRef PubMed.
- M. Nag, A. Saffarzadeh, T. Shimaoka and H. Nakayama, Environ. Sci. Technol., 2022, 5, 137–147 Search PubMed.
- N. Fijoł, H. Nasser Abdelhamid, B. Pillai, S. A. Hall, N. Thomas and A. P. Mathew, RSC Adv., 2021, 11, 32408–32418 RSC.
- A. Saffarzadeh, M. Nag, T. Nomichi, T. Shimaoka, H. Nakayama and T. Komiya, in Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions, ed. M. Ksibi, A. Ghorbal, S. Chakraborty, H. I. Chaminé, M. Barbieri, G. Guerriero, O. Hentati, A. Negm, A. Lehmann, J. Römbke, A. Costa Duarte, E. Xoplaki, N. Khélifi, G. Colinet, J. Miguel Dias, I. Gargouri, E. D. Van Hullebusch, B. Sánchez Cabrero, S. Ferlisi, C. Tizaoui, A. Kallel, S. Rtimi, S. Panda, P. Michaud, J. N. Sahu, M. Seffen and V. Naddeo, Springer Int. Publ., Cham, 2nd edn, 2021, pp. 789–794 Search PubMed.
- N. V. Hoa, N. C. Minh, H. N. Cuong, P. A. Dat, P. V. Nam, P. H. T. Viet, P. T. D. Phuong and T. S. Trung, Molecules, 2021, 26, 6127 CrossRef PubMed.
- M. N. Rashed, A. A. E. Gad and N. M. Fathy, Biomass Convers. Biorefinery, 2024, 14, 31703–31720 CrossRef.
- M. Khanjani, D. J. Westenberg, A. Kumar and H. Ma, ACS Omega, 2021, 6, 11988–12003 CrossRef PubMed.
- M. Ghattas, A. Chevrier, D. Wang, M.-G. Alameh and M. Lavertu, Carbohydr. Polym. Technol. Appl., 2025, 10, 100797 Search PubMed.
Footnote |
| † It is thought that the origins of aquaculture date back to 3000 BC via the construction of artificial fish ponds in China. |
| This journal is © The Royal Society of Chemistry 2026 |