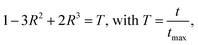Open Access Article
Open Access ArticlePhysical effects of hydrogel coatings on seed germination
Tori Melise
Phillips
 a,
Joshua
Green
a,
Alvaro
Sanz-Saez
a,
Joshua
Green
a,
Alvaro
Sanz-Saez
 b and
Jean-François
Louf
b and
Jean-François
Louf
 *a
*a
aDepartment of Chemical Engineering, Auburn University, Auburn, Alabama 36849, USA. E-mail: jlouf@auburn.edu
bDepartment of Crop, Soil, and Environmental Sciences, Auburn University, Auburn, Alabama 36849, USA
First published on 22nd December 2025
Abstract
Hydrogel coatings are increasingly applied to seeds to enhance hydration and support germination; however, their outcomes remain inconsistent, and the underlying physical mechanisms remain unclear. Here, we dissect the hydrogel–seed interface as a soft material system, isolating how water imbibition, mechanical confinement, and oxygen permeability govern germination dynamics. Using artificial and natural seeds, we show that water uptake follows classical Lucas–Washburn dynamics and is not impeded by the hydrogel coating. Instead, germination delays arise from two key physical effects: the mechanical stiffness of the coating and its restriction of gas exchange. In Petri dishes, soft coatings accelerate germination, suggesting minimal resistance to radicle emergence; however, this advantage disappears in soil, where all coatings delay germination regardless of stiffness. Controlled-pressure experiments in transparent soil rule out mechanical load as the dominant factor. Instead, selectively exposing the hilum and micropyle—critical sites for gas exchange—restores germination timing. These findings demonstrate that hydrogel-coated seeds are primarily limited by oxygen diffusion, not water transport, revealing how soft material interfaces modulate biological function. This work provides design principles for soft coatings that balance hydration, mechanical compliance, and gas permeability in bio-integrated systems.
1. Introduction
Soft interfaces that mediate fluid transport and mechanical interactions are central to many systems in soft matter physics. Hydrogels, in particular, are widely used in applications ranging from tissue engineering and drug delivery to filtration and biosensing, where their ability to simultaneously deform, swell, and control mass transfer gives rise to tunable, multifunctional behavior.1–6 In these contexts, the interplay between porosity, elasticity, and permeability defines how molecules and fluids move across the material interface.While extensive work has been done on hydrogel interfaces in biomedical7–9 and synthetic environments,10,11 relatively little attention has been given to soft interfaces that interact dynamically with whole living organisms, especially in early developmental stages. One underexplored but scientifically rich example is the interface between a hydrogel coating and a germinating seed. This system naturally couples capillary transport, mechanical confinement, and gas diffusion in a confined geometry, and provides a biologically meaningful platform for investigating the physicochemical principles that govern hydration and growth under soft constraints.
Seeds are inherently multiscale systems where germination involves tightly regulated uptake of water,13,14 mechanical rupture of protective layers,15,16 and aerobic respiration.17,18 These processes unfold sequentially and are sensitive to the properties of the surrounding environment.19–21 Any external coating that modifies the local transport of water or oxygen, or that introduces mechanical resistance, has the potential to alter the timing and success of germination. Despite the complexity of this interface, seed–hydrogel interactions have received relatively little mechanistic study in the context of soft matter.
In parallel, hydrogel-based seed coatings have become increasingly common in agricultural practice, where they are used to retain moisture, mitigate desiccation stress, and deliver nutrients or microbial additives.12,22–24 Inspired by mucilage-producing seeds such as chia and basil, these coatings are promoted as a means to enhance crop establishment, particularly under challenging environmental conditions.22,23,25–27 However, their effects remain inconsistent: in some cases, coated seeds germinate faster than uncoated controls,12,28–32 in others, germination is delayed or entirely suppressed.32 These contradictory outcomes have typically been attributed to environmental variables, but a lack of physical insight into the coating–seed interface has hindered rational design.
Here, we investigate hydrogel-coated seeds as a soft matter system by dissecting the coupled roles of water uptake, mechanical confinement, and oxygen diffusion in determining germination behavior. Using a combination of artificial and natural seed models, transparent soil platforms, and analytical modeling, we show that water uptake is not impaired by the coating and follows classical Lucas–Washburn dynamics. We demonstrate that the stiffness of the hydrogel affects radicle emergence in hydrated, unconfined environments, but that these effects are outweighed in soil by limitations in gas exchange. Most notably, we find that oxygen availability at the seed surface—especially near the hilum and micropyle—is the dominant factor determining germination timing under hydrogel coatings.
Together, these results position the seed–hydrogel interface as a robust and generalizable model for studying transport and mechanics in soft porous systems. By linking materials design to biological function, this work opens new directions in soft matter research with applications in agriculture, biotechnology, and bioinspired interface engineering.
2. Results
2.1. Hydrogel coating does not inhibit water uptake
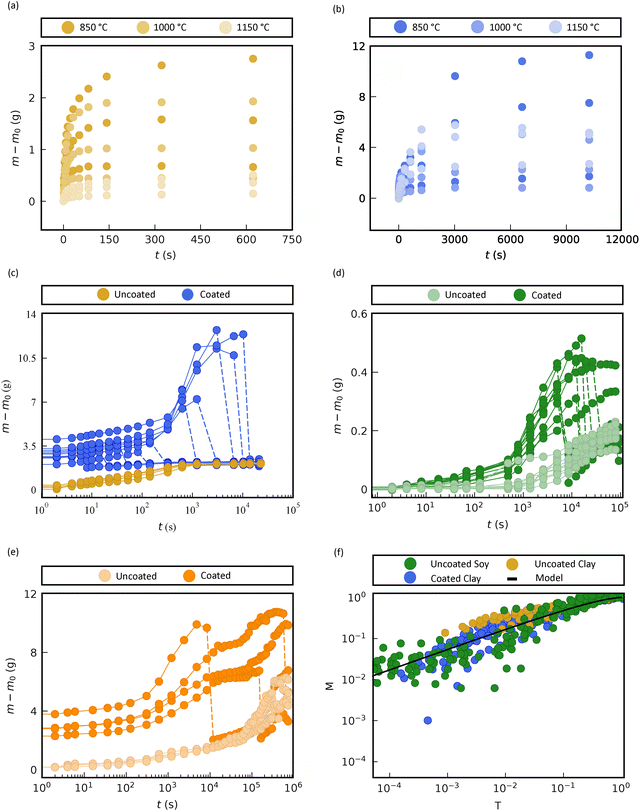
To test whether hydrogel coatings interfere with seed hydration, we first quantified water uptake during imbibition in artificial and natural seeds with and without coatings. Artificial seeds were used to simplify transport processes and enhance experimental repeatability. We fabricated them using smooth terracotta earthenware clay (Red Rock Red Smooth, Rocky Mountain Clay), molded into spheres of three diameters—14 mm, 22 mm, and 27 mm—and fired at 850 °C, 1000 °C, or 1150 °C. Firing temperature was selected to tune the internal porosity, with higher temperatures reducing water uptake due to sintering.33The hydrogel coating was made from 2% w/v sodium alginate, crosslinked in a 4% w/v CaCl2 solution. Both solutions were degassed twice (3 min each) to remove bubbles before coating. Each seed was dipped in alginate for 5 seconds, transferred to CaCl2 for 30 seconds, and then oven-dried at 80 °C for 20 minutes. For imbibition trials, seeds were submerged in degassed water and removed at regular time intervals. Excess water was blotted off, and mass was measured using a high-precision balance (Ohaus Pioneer PX523/E). Seed mass was also measured before and after coating to ensure consistent coating application across all samples. The mass was tracked until saturation (Fig. 1).
 | ||
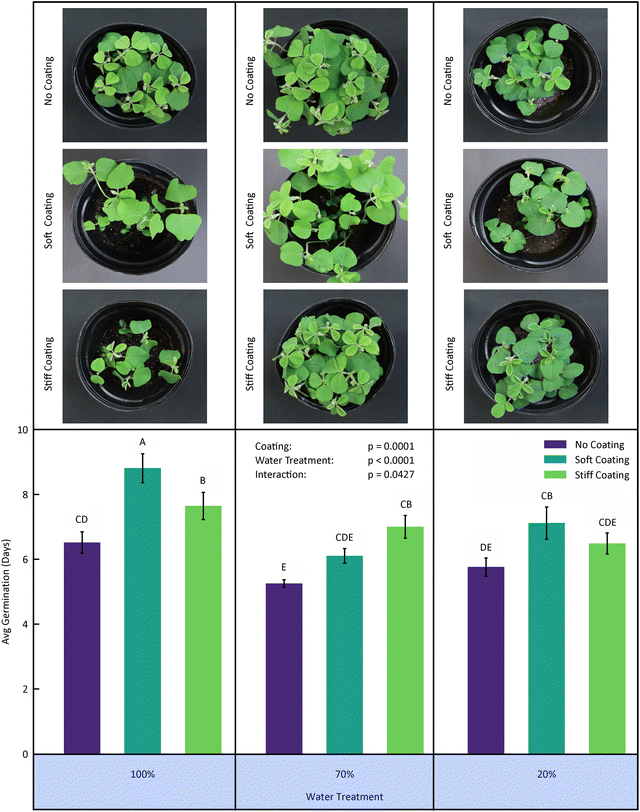
| Fig. 1 Inspiration and motivation for hydrogel seed coatings. (a) Natural mucilage layer surrounding a chia seed upon hydration, which helps retain water in arid environments. Photo credit: Jean-François Louf, Auburn University. (b) Laboratory-applied hydrogel coating on a soybean seed, mimicking mucilage function. Photo credit: Tori Phillips, Auburn University. (c) and (d) Images of potted soybeans Adapted with permission from Zvinavashe et al.12 comparing soybean growth outcomes for uncoated (c) versus hydrogel-coated seeds (d). The coated seeds included additives such as plant growth-promoting rhizobacteria (PGPRs), highlighting both the promise and complexity of seed coating technologies in agriculture. | ||
For all samples, mass increased rapidly before leveling off (Fig. 2a and b). Seeds fired at 850 °C absorbed the most water, consistent with their higher porosity (∼12.4%), while those fired at 1150 °C absorbed the least (∼4.0%). Coated seeds showed a higher total mass gain due to hydrogel swelling; the coating absorbed approximately 200% of its dry mass in water over the course of the experiment, resulting in visible swelling by the end of imbibition. To isolate the contribution of the seed alone, we removed the hydrogel coating at fixed intervals during imbibition by peeling it off the surface of artificial clay seeds. For soybean and mango seeds, the coating detached spontaneously as swelling progressed. In all cases, the resulting de-coated seeds followed the same imbibition curve as uncoated controls (Fig. 2c–e), indicating that the hydrogel does not impede water access to the seed interior.
2.2. Seed hydration follows Lucas–Washburn dynamics
To interpret the dynamics of water uptake during seed imbibition, we modeled the process as capillary-driven flow into a porous sphere—a geometry that approximates the artificial clay seeds used in our experiments and provides a first-order description of the early hydration behavior of natural seeds prior to swelling.The flow into the dry porous medium is driven by capillary pressure and resisted by viscous drag, consistent with Darcy's law. We adopt the analytical framework derived in Louf et al. (2018),14 where the pressure profile within the spherical medium is solved under the assumptions of radial symmetry, constant permeability, incompressibility, and negligible swelling.
The characteristic time to fully saturate a sphere of radius a is
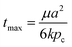 | (1) |
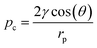 , the capillary pressure with γ the surface tension of water, θ the contact angle between water and the pore wall, and rp the effective pore radius.
, the capillary pressure with γ the surface tension of water, θ the contact angle between water and the pore wall, and rp the effective pore radius.
In our system, although the seed–hydrogel structure comprises two porous domains in series, we modeled it using a single effective permeability. This simplification is justified by the thinness and high initial permeability of the hydrogel relative to the seed, and by experimental observations showing that coated and uncoated seeds follow nearly identical imbibition dynamics (see Fig. 2). When the hydrogel was removed mid-imbibition, the mass gain curve continued unchanged, supporting that the coating does not dominate the resistance to water uptake under our experimental conditions. In systems where both layers contribute significantly to resistance, bilayer modeling frameworks (e.g., Reyssat et al. 2009)34 may be required.
The position of the wetting front rf(t), normalized by the sphere radius a, is defined as 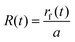 . The normalized front position obeys the implicit equation:
. The normalized front position obeys the implicit equation:
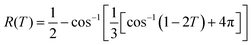 | (2) |
The mass of absorbed water is directly related to the position of the front. Assuming a uniform porosity ε and fluid density ρ, the total mass gain can be written as:
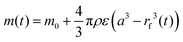 | (3) |
Defining the dimensionless mass uptake as 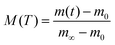 , we obtain:
, we obtain:
| M(T) = 1 − R3(T) | (4) |
Experimental data for artificial seeds of varying porosity and size, both coated and uncoated, collapsed onto this theoretical prediction (Fig. 2f). Although the hydrogel coating swells during imbibition, its expansion occurs after the initial rapid uptake phase and does not appreciably alter the imbibition rate of the seed itself. This is supported by the collapse of mass uptake curves between coated and uncoated seeds, and by trials in which the coating was removed mid-experiment. Minor deviations in the early stages of the coated trials may arise from transient swelling of the hydrogel layer or measurement lag, but the agreement at later times confirms that imbibition is dominated by the seed's porous structure. This model also captured the behavior of natural soybean, validating the generality of the approach.
These results demonstrate that hydrogel coatings do not impede water access to the seed interior. Instead, imbibition remains governed by intrinsic porous properties and follows classical capillary dynamics.
2.3. Hydrogel stiffness alters germination
Following the observation that hydrogel coatings do not restrict water uptake, we next investigated whether their mechanical properties influence the timing of germination. One plausible mechanism is that stiffer coatings physically constrain the seed, limiting the ability of the emerging radicle to rupture the surrounding matrix. Such mechanical confinement has been shown to delay germination in compacted soils35–38 and inhibit hydrogel swelling in Louf et al.,39 suggesting that material stiffness may play a similar role in engineered coatings.To test this, we synthesized coatings with two levels of stiffness by varying the crosslinking density of the alginate hydrogel. All coatings were made from 2% w/v sodium alginate, but the crosslinking bath contained either 0.5% or 4% w/v CaCl2, yielding a soft and stiff gel, respectively. Using indentation tests and dynamic mechanical analysis, we quantified the elastic modulus of the two formulations. The soft hydrogel exhibited an average Young's modulus of 11.8 ± 1.8 kPa (SE; n = 4; 95% CI 6.1–17.5), while the stiff hydrogel reached 824.0 ± 57.9 kPa (SE; n = 4; 95% CI 639.8–1008.2)—spanning nearly two orders of magnitude in resistance to deformation.
To isolate mechanical effects from environmental factors, we germinated coated soybean seeds in closed Petri dishes on sterile filter paper. Each dish contained five seeds of identical treatment placed equidistantly, and seven replicate dishes were prepared for each condition: uncoated, soft-coated, and stiff-coated. The plates were incubated at 23 °C in the dark and misted daily to maintain uniform hydration. Germination was defined by radicle emergence and recorded photographically each day over the course of one week (Fig. 3a).
As shown in Fig. 3b, seeds coated with the soft hydrogel germinated significantly earlier than all other groups, typically by day 3. Uncoated seeds followed at day 4, and stiff-coated seeds lagged behind, germinating on average by day 5. This trend is consistent with the hypothesis that the coating exerts a mechanical barrier to growth, and that lower stiffness facilitates earlier rupture and radicle protrusion. Notably, the uncoated seeds did not germinate faster than the soft-coated seeds, suggesting that soft hydrogels may not impose any appreciable mechanical resistance under these conditions—and may even provide a microenvironment conducive to radicle emergence.
These findings demonstrate that the elastic modulus of the hydrogel coating is a key determinant of germination timing in a hydrated, air-rich, mechanical stress-free environment. The confinement due to the coating limits the onset of germination in stiff-coated seeds.
2.4. Hydrogel coatings delay germination in soil across all moisture levels
While soft hydrogel coatings accelerated germination in Petri dishes, their performance in soil—a more heterogeneous and confined environment—was less favorable. In soil, hydrogel swelling may be restricted by compaction,39 and gas diffusion is often limited, especially under high water content.40,41 These factors could jointly impact the coating's interaction with the seed and its microenvironment.To investigate how soil moisture affects hydrogel-coated seed germination, we tested three saturation levels: 20%, 70%, and 100%. For each combination of coating type (uncoated, soft-coated, stiff-coated) and saturation, four replicate pots were prepared per treatment, each containing 15 soybean seeds. Germination was scored on the day of shoot emergence from soil. Because shoot emergence lags behind radicle emergence and varies by environment, comparisons across assay types (e.g., Petri dish vs. soil) are based on relative differences among treatments rather than absolute timing.
Across all conditions, hydrogel-coated seeds germinated more slowly than uncoated seeds (Fig. 4). The effect of both coating type (p = 0.0001) and soil saturation (p < 0.0001) was statistically significant. At 100% saturation, the germination delay was most pronounced, likely due to reduced gas exchange and inhibited coating expansion. At 70% saturation, germination times were shorter overall, and differences between coating types narrowed. At 20% saturation, uncoated seeds again germinated the fastest, but interestingly, stiff-coated seeds outperformed soft-coated ones by a small margin.
These results suggest that in soil, hydrogel coatings introduce new physical constraints beyond those observed in hydrated laboratory conditions. Restricted swelling may hinder radicle emergence, while reduced porosity in water-saturated soils could exacerbate oxygen limitation. The consistent delay across moisture levels—particularly under full saturation—raises the possibility that hypoxia plays a key role in slowing germination. We tested this hypothesis directly in subsequent experiments. Together, these findings underscore the importance of evaluating hydrogel performance in realistic environmental conditions. Soil structure, water availability, and coating mechanics all interact to determine germination outcomes.
2.5. External pressure does not significantly alter germination in coated seeds
The reversal of trends between Petri dish and soil germination prompted us to investigate the role of mechanical pressure in modulating the behavior of hydrogel-coated seeds. In Petri dishes, soft-coated seeds germinated more quickly than uncoated controls, but this advantage was lost or reversed in soil. One plausible explanation is that soil imposes a mechanical constraint on hydrogel expansion, particularly under high compaction or saturation, thereby delaying radicle emergence. To directly isolate the effect of external mechanical pressure, we developed a system to apply controlled loads in a transparent and well-characterized medium.We constructed vertical columns filled with transparent soil, which allows visual access to germinating seeds while approximating the mechanical environment of real soil (Fig. 5a). Seeds were embedded at fixed depths within these columns. To impose additional vertical stress, we used a known mass m atop a piston of area a, thereby generating a load 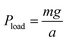 at the soil surface.39 Combined with the hydrostatic pressure of the overlying material Psoil = ρgh, the total pressure experienced by each seed at depth h was given by:
at the soil surface.39 Combined with the hydrostatic pressure of the overlying material Psoil = ρgh, the total pressure experienced by each seed at depth h was given by:
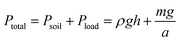 | (5) |
We focused this experiment on soft-coated and uncoated seeds, excluding stiff-coated seeds since their germination was already significantly delayed in both environments. Germination was scored by radicle emergence. Across all applied pressures, soft-coated seeds germinated more slowly than uncoated controls (p = 0.0226), consistent with our earlier observations. However, the application of external pressure had no statistically significant effect on germination time (p = 0.8781), and no interaction was detected between pressure and coating condition (p = 0.9914) (Fig. 5b).
These results suggest that vertical mechanical loading alone is not sufficient to account for the germination delay observed in soil. The fact that external pressure—applied in a reproducible and well-controlled manner—did not significantly alter germination timing indicates that other environmental factors, such as oxygen availability or radial constraint, may play a more dominant role in vivo. This conclusion is reinforced by the observation that even low-pressure transparent media can still induce delays in coated seeds, hinting at more complex interactions between gas diffusion, hydration dynamics, and the local microenvironment.
2.6. Oxygen restriction by hydrogel coating delays germination
Previous experiments showed that hydrogel-coated seeds germinate more slowly in soil, particularly under saturated conditions. Mechanical explanations such as external pressure or constrained swelling appeared insufficient to account for these delays. Instead, the consistent underperformance of fully coated seeds suggested that restricted oxygen availability might be a dominant limiting factor. Germination in terrestrial plants is well known to depend on aerobic respiration, and reduced oxygen levels are associated with delayed or failed emergence.21,44,45 Supporting this, previous studies observed that a hydro-absorber coating reduced embryonic oxygen concentration by approximately 60% compared to uncoated seeds.46,47 To directly test whether hydrogel coatings impede gas exchange at the seed surface, we designed an experiment in which key anatomical features were deliberately left uncoated.We prepared a set of soybean seeds with partial hydrogel coatings, intentionally leaving the hilum and micropyle regions uncoated (Fig. 6a). The hilum marks the point where the seed was attached to the pod and includes the micropyle, a small pore through which both water and gases are known to enter.48,49 These sites are recognized as critical for respiration during germination. For comparison, we included two additional groups: seeds that were fully coated and those that were left uncoated.
All seeds were planted in soil under identical conditions, and germination was scored based on shoot emergence. The experimental setup for each treatment group—uncoated, partially coated, and fully coated—is shown in Fig. 6b, illustrating the consistent planting depth and environmental control. As shown in Fig. 6c, fully coated seeds exhibited a clear delay in germination relative to both partially coated and uncoated controls. Strikingly, partially coated seeds germinated at the same time as uncoated seeds, with no statistically significant difference between the two groups (p = 0.1426), despite the majority of their surface being covered in hydrogel.
These findings indicate that the location of the coating, rather than its presence alone, governs its impact on germination. Covering the hilum and micropyle appears to inhibit seed respiration by creating a local diffusion barrier to oxygen. When these sites are left exposed, the seed resumes its typical germination schedule, regardless of whether the rest of the surface is coated. This experiment provides direct evidence that oxygen availability at specific surface features—and not water uptake or mechanical resistance—is the critical factor limiting germination in hydrogel-coated seeds.
3. Conclusion
Seed coatings are widely used in agriculture to enhance germination outcomes and protect seedlings during early development; however, their physical effects on seed physiology remain poorly understood. Here, we investigated how hydrogel coatings alter germination dynamics by disentangling three major physical processes: water imbibition, mechanical confinement, and oxygen diffusion. Across a series of well-controlled experiments, we show that the timing of germination in hydrogel-coated seeds is not limited by hydration or mechanical confinement, but rather by the availability of oxygen at the seed surface.Initial imbibition experiments using artificial and natural seeds revealed that water uptake proceeds according to classical porous media flow laws. Mass gain followed the predictions of Lucas–Washburn and Darcy-based models for spherical porous bodies, and hydrogel coatings had no measurable effect on the imbibition kinetics. These findings refute a common assumption in the literature that delays in coated seeds arise from water availability.31,32,50
We then demonstrated that the mechanical stiffness of the coating modulates the onset of germination in hydrated environments. In Petri dishes, soft hydrogel-coated seeds germinated earlier than uncoated seeds, while stiff-coated seeds were delayed. These results implicate coating compliance as a critical parameter governing the physical barrier to radicle emergence. However, in soil, a more complex environment where mechanical loading, gas exchange, and moisture distribution are coupled, soft-coated seeds germinated more slowly than uncoated controls under all water saturations. This reversal in behavior suggested that environmental factors beyond bulk stiffness must be at play.
To isolate the role of mechanical stress, we applied external loads to hydrogel-coated seeds embedded in transparent soil columns, enabling direct visualization and quantification of germination under controlled compressive forces. The applied pressures, which ranged from 180 to 3995 Pa, exceeded the estimated overburden pressure that would be exerted by the soil alone (<2 kPa). Yet, across this range, we observed no statistically significant changes in germination timing, suggesting that external mechanical load is not the primary driver of the delays observed in soil. While previous studies have reported that substantially higher pressures, on the order of 0.15 to 0.87 MPa, can inhibit germination,51,52 our results indicate that moderate compression levels, comparable to realistic field conditions, are insufficient to explain the inhibitory effect of hydrogel coatings. Together, these results suggest that under realistic field conditions, mechanical confinement alone is insufficient to explain the observed delays in germination.
To directly assess whether gas diffusion through the coating was a limiting factor, we engineered partially coated seeds in which the hilum and micropyle, known anatomical sites for gas exchange,48,49 were intentionally left uncoated. These seeds germinated at the same time as uncoated controls, while fully coated seeds remained delayed. This single-variable experiment provides direct causal evidence that localized hydrogel coverage over respiration-critical features impairs gas exchange and delays germination. While previous studies (e.g., Gorim et al. 2017)47 measured reduced internal oxygen concentrations in thickly coated cereal seeds, they did not isolate anatomical effects or decouple hydration and diffusion. In contrast, our approach demonstrates that targeted oxygen restriction, rather than hydration or mechanical pressure, is the dominant limiting factor in germination under thin hydrogel coatings.
The contrasting behavior observed between Petri dish and soil germination highlights how the dominant physical constraint shifts with environmental conditions. In Petri dishes, seeds are surrounded by air and maintained under constant hydration, providing abundant oxygen and minimal external mechanical resistance. Under these conditions, the soft hydrogel coating may enhance surface wetting and water retention while offering negligible resistance to radicle emergence, resulting in earlier germination compared to uncoated seeds. In soil, however, oxygen availability is substantially lower, particularly under high water saturation, and the same coating that aids hydration becomes a barrier to gas exchange. A simple Fickian estimate indicates that oxygen flux through a 1 mm alginate hydrogel layer is approximately 1.5 × 10−5 mol m−2 s−1 in air but decreases by roughly one order of magnitude in partially saturated soil and by nearly two orders in fully saturated soil. These differences align with the observed transition from accelerated germination in Petri dishes to delayed germination in soil, confirming that oxygen diffusion, not mechanical confinement or hydration, is the rate-limiting process in hydrogel-coated seeds.
Together, these findings suggest that oxygen permeability, not water availability, is the limiting factor for germination under hydrogel coatings. This insight has important implications for the design of next-generation seed technologies, particularly for applications involving encapsulated bioactives, such as plant growth-promoting rhizobacteria (PGPRs).12,53,54 Hydrogels remain a promising platform for supporting microbial viability,55,56 stabilizing biostimulants,57–59 and enabling moisture retention in dry environments.24,60,61 However, if the coating interferes with respiration during the early stages of seedling development, its benefits may be offset by delayed emergence and reduced vigor.
While our work focused on coating stiffness and coverage, additional hydrogel properties such as porosity, adhesion, and thickness are also likely to influence seed–environment interactions. Prior studies in tissue engineering have shown that oxygen diffusion decreases with increasing polymer density,62–65 while larger pores and interconnectivity enhance gas transport at the cost of structural integrity.65–67 Similarly, hydrogel–seed adhesion affects water contact and may vary with seed type or surface chemistry.68 In addition to stiffness and surface coverage, coating thickness may influence germination by altering the permeability of the seed–environment interface. Prior studies show that thicker coatings reduce oxygen diffusion and can delay or restore germination, depending on species-specific tolerance to hypoxia. In our system, we expect thicker coatings would likely amplify germination delays in soil due to reduced gas exchange, highlighting coating thickness as a key parameter for future optimization.46 These factors warrant further investigation to decouple their effects on hydration from those on aeration.
Seed-specific traits also play a role in coating performance. For example, some cereals, such as barley and rye, exhibit greater tolerance to hypoxia than wheat and may rely on anaerobic pathways or alternative respiratory enzymes during germination.46 Moreover, anatomical features such as seed coat porosity, micropyle size, and tissue arrangement can alter both water14,69 and gas transport.70–73 These differences must be taken into account when translating coating strategies across different plant species.
In conclusion, our study demonstrates that the primary limitation imposed by hydrogel coatings on germination is not a lack of water, but rather a lack of air. This mechanistic shift, from hydrodynamics to gas diffusion, reframes how seed coatings should be engineered and evaluated. More broadly, this work shows how a soft, porous biological interface (the seed) responds to encapsulating hydrogel layers that modulate both hydration and gas exchange. The framework developed here offers generalizable insights into soft–rigid systems where confined transport and mechanical resistance interact, including hydrogel sensors, drug-release capsules, and bioencapsulation platforms.
4. Experimental materials and methods
4.1. Artificial seed fabrication and characterization
To create a model system that isolates physical processes governing imbibition, we fabricated artificial seeds from porous ceramic materials. Smooth terracotta clay (Red Rock Red Smooth, Rocky Mountain Clay, USA) was selected for its tunable microstructure and ease of shaping. Samples were molded into spheres of three nominal diameters, 14 mm, 22 mm, and 27 mm, and subjected to controlled firing protocols to modulate pore size and porosity.Clay spheres were air-dried for 24 hours and then kiln-fired using a five-hour ramp schedule to target peak temperatures of 850 °C, 1000 °C, or 1150 °C. The heating rate was fixed across all samples to avoid ramp-induced variation in porosity, based on prior findings.33 After firing, samples were cooled gradually in the closed furnace to minimize cracking.
Pore size distribution was measured using nitrogen adsorption with the Barrett–Joyner–Halenda (BJH) method. BET surface area analysis revealed a progressive reduction in porosity with increasing kiln temperature. Above 1075 °C, the BET signal was insufficient to resolve pore characteristics, indicating very low surface area. Nonetheless, trends were consistent with literature: pore volume decreased, while average pore radius increased modestly with temperature (from 8.5 nm at 850 °C to 9.9 nm at 1150 °C).
To complement these measurements, we estimated porosity via gravimetric imbibition. For each sample, dry mass mdry and fully saturated mass mwet were recorded, and porosity ϕ was computed as:
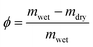 | (6) |
This analysis confirmed that porosity declined from 12.41% at 850 °C to 3.98% at 1150 °C. These results guided the selection of clay parameters for imbibition modeling and enabled systematic tuning of the artificial seed structure to approximate plant tissue behavior (Fig. 7).
4.2. Oxygen diffusion flux in the hydrogel coating
To estimate the diffusion flux of oxygen through the hydrogel coating, we use Fick's law of diffusion under the assumption of one-dimensional steady-state transport through a slab: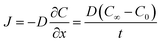 | (7) |
Here, J is the diffusion flux of oxygen J (mol m−2 s−1) D the diffusion coefficient of the hydrogel, C∞ (mol m−3) is the oxygen concentration in the surroundings, C0 is the oxygen concentration at the hydrogel–seed interface (assumed to be zero under the perfect sink approximation), and t is the thickness of the hydrogel layer (1 mm). For alginate hydrogel, D ≈ 1.75 × 10−9 m2 s−1.74
In Petri dish conditions, C∞ is taken as the atmospheric oxygen concentration, 8.6 mol m−3, yielding a diffusion flux of J = 1.51 × 10−5 mol m−2 s−1. For seeds sown in 100% and 70% saturated soil, we assume they are surrounded by water, and use the dissolved oxygen concentration in water, 0.27 mol m−3, resulting in a diffusion flux of J = 4.73 × 10−7 mol m−2 s−1. In 20% saturated soil and in transparent soil, the pore space is approximately 30% air-filled, so we take C∞ = 2.58 mol m−3, giving a diffusion flux of J = 4.52 × 10−6 mol m−2 s−1.
4.3. Soil hydration control and germination assays
To assess how water availability interacts with hydrogel coating properties, we conducted a factorial germination experiment across three soil moisture levels and three coating types. Nine experimental conditions were tested in total, each with four replicates of 15 soybean seeds.All trials were conducted in 3.8 L plastic pots with drainage holes. Soil was composed of Pro-Mix BX general-use media (75–80% peat moss). For each pot, dry mass of the soil was determined by oven-drying a reference set of samples at 60 °C for 5 days. Saturated mass was measured after full hydration followed by drainage equilibration. These endpoints enabled precise interpolation of intermediate moisture levels. Each pot's target water content (20%, 70%, or 100%) was maintained by daily weighing and water adjustment.
To ensure germination was triggered even in the driest treatment, pots assigned to 20% saturation were initially watered to 40% on day 0 and brought down gradually thereafter. Germination was scored daily as the first visible emergence of the seedling shoot from the soil surface. The number of germinated seeds per pot was recorded across a one-week window. Because shoot emergence lags behind radicle emergence, it is important to note that in our transparent soil system, we measured an average delay of 2.4 ± 0.3 days between radicle and shoot emergence for seeds sown at a depth of 1.84 cm.
4.4. Transparent soil preparation
To visually monitor seed behavior under applied pressure and to reproduce soil-like mechanical conditions in a transparent medium, we synthesized hydrogel-based soil beads adapted from the formulation described by Ma et al.75 A 1.2% polymer solution was prepared by dissolving phytagel and alginate in water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mass ratio. The solution was heated to 90 °C with intermittent stirring until fully dissolved, then cooled to room temperature under constant mixing.
4 mass ratio. The solution was heated to 90 °C with intermittent stirring until fully dissolved, then cooled to room temperature under constant mixing.
Using a syringe pump, the polymer solution was dispensed dropwise into a 2% CaCl2 bath, where it crosslinked into semi-rigid hydrogel spheres. Beads were rinsed to remove residual ions, stored submerged in deionized water, and kept at 8 °C until use. This granular medium mimicked the mechanical impedance of wet soil while enabling direct imaging of seeds and surrounding structures during germination.
Author contributions
Tori Phillips: data curation, software, formal analysis, investigation, visualization, validation, and writing (original draft). Joshua Green: data curation. Alvaro Sanz-Saez: methodology, investigation, and writing (review and editing). Jean-François Louf: conceptualization, supervision, funding acquisition, investigation, methodology, project administration, and writing (review and editing).Conflicts of interest
There are no conflicts to declare.Data availability
Data for this article are available at https://osf.io/srgz9/?view_only=befd94809ab94cb2aee58e44055c3818 and https://osf.io/9nwam/?view_only=ca60ed1b722d44c0b7f496cdb3fe0dbb.Acknowledgements
We thank Thorsten Knappenberger for his insightful suggestion to avoid coating the seed embryo, which helped shape the experimental design. We are also grateful to Morteza Taghavi for training and guidance on using the BET surface area analyzer, and to Jessica Armstrong for coordinating access to the growth chamber and organizing planting facilities, which were essential to the success of the germination trials. We gratefully acknowledge support from the Alabama Soybean Producers (Grant No. G00015315).References
- R. Narayanaswamy and V. P. Torchilin, Hydrogels and Their Applications in Targeted Drug Delivery, Molecules, 2019, 24(3), 603 CrossRef.
- S. Chaudhary and E. Chakraborty, Hydrogel based tissue engineering and its future applications in personalized disease modeling and regenerative therapy, Beni-Suef Univ. J. Basic Appl. Sci., 2022, 11(1), 3 Search PubMed.
- L. Alsaka, L. Alsaka, A. Altaee, S. J. Zaidi, J. Zhou and T. Kazwini, A Review of Hydrogel Application in Wastewater Purification, Separations, 2025, 12, 51 CrossRef CAS.
- O. Lieleg and K. Ribbeck, Biological hydrogels as selective diffusion barriers, Trends Cell Biol., 2011, 21, 543–551 Search PubMed.
- J. Tavakoli and Y. Tang, Hydrogel Based Sensors for Biomedical Applications: An Updated Review, Polymers, 2017, 9(8), 364 CrossRef.
- A. Herrmann, R. Haag and U. Schedler, Hydrogels and Their Role in Biosensing Applications, Adv. Healthcare Mater., 2021, 10, 2100062 Search PubMed.
- A. Ullah, D. Y. Kim, S. I. Lim and H.-R. Lim, Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human–Machine Integration, Gels, 2025, 11, 232 Search PubMed.
- H. Tang, Y. Li, S. Liao, H. Liu, Y. Qiao and J. Zhou, Multifunctional Conductive Hydrogel Interface for Bioelectronic Recording and Stimulation, Adv. Healthcare Mater., 2024, 13, 2400562 Search PubMed.
- H. Yuk, J. Wu and X. Zhao, Hydrogel interfaces for merging humans and machines, Nat. Rev. Mater., 2022, 7, 935–952 CrossRef CAS.
- T. Takahashi, N. Karasawa and K. Sano, Gel-Gel Interface Engineering for the Synthesis of Anisotropic Hydrogels with Designable Polymer Orientations, Adv. Mater., 2025, 2505268 Search PubMed.
- J. Liu, S. Qu, Z. Suo and W. Yang, Functional hydrogel coatings, Natl. Sci. Rev., 2021, 8, nwaa254 CrossRef CAS.
- A. T. Zvinavashe, J. Laurent and M. Mhada, Programmable design of seed coating function induces water-stress tolerance in semi-arid regions, Nat. Food, 2021, 2, 485–493 CrossRef.
- A. C. Leopold and C. W. Vertucci, Moisture as a Regulator of Physiological Reaction in Seeds. in Seed Moisture, ed. P. C. Stanwood and M. B. McDonald, 1989, pp. 93–115 Search PubMed.
- J.-F. Louf, Y. Zheng, A. Kumar, T. Bohr, C. Gundlach, J. Harholt, H. F. Poulsen and K. H. Jensen, Imbibition in plant seeds, Phys. Rev. E, 2018, 98, 042403 CrossRef CAS.
- M. A. Farooq, W. Ma, S. Shen and A. Gu, Underlying Biochemical and Molecular Mechanisms for Seed Germination, Int. J. Mol. Sci., 2022, 23(15), 8502 CrossRef CAS.
- G. Carrera-Castaño, J. Calleja-Cabrera, M. Pernas, L. Gómez and L. Oñate-Sánchez, An Updated Overview on the Regulation of Seed Germination, Plants, 2020, 9(6), 703 CrossRef PubMed.
- L. W. Woodstock, Berlin, Heidelberg, Relationships Between Seed Respiration During Imbibition and Subsequent Seedling Growth in Zea mays L., Quant. Biol. Metab., 1967, 42(8), 1071–1076 CAS.
- H. El-Maarouf-Bouteau, The Seed and the Metabolism Regulation, Biology, 2022, 11(2), 168 CrossRef CAS PubMed.
- A. Hadas and D. Russo, Water Uptake by Seeds as Affected by Water Stress, Capillary Conductivity, and Seed-Soil Water Contact. II. Analysis of Experimental Data, Agron. J., 1974, 66, 647–652 CrossRef.
- S. Benvenuti and M. Mazzoncini, Soil Physics Involvement in the Germination Ecology of Buried Weed Seeds, Plants, 2018, 8(1), 7 CrossRef.
- F. Corbineau, Oxygen, a key signalling factor in the control of seed germination and dormancy, Seed Sci. Res., 2022, 32, 126–136 CrossRef CAS.
- D. Skrzypczak, Ł. Jarzembowski, G. Izydorczyk, K. Mikula, V. Hoppe, M. K. Anna, N. Pudełko-Malik, P. Młynarz, K. Chojnacka and A. Witek-Krowiak, Hydrogel alginate seed coating as an innovative method for delivering nutrients at the early stages of plant growth, Polymers, 2021, 13(23), 4233 CrossRef CAS PubMed.
- R. Abdukerim, L. Li, J.-H. Li, S. Xiang, Y.-X. Shi, X.-W. Xie, A. L. Chai, T.-F. Fan and B.-J. Li, Coating seeds with biocontrol bacteria-loaded sodium alginate/pectin hydrogel enhances the survival of bacteria and control efficacy against soil-borne vegetable diseases, Int. J. Biol. Macromol., 2024, 279, 135317 CrossRef CAS PubMed.
- K. Ali, Z. Asad, G. H. D. Agbna, A. Saud, A. Khan and S. J. Zaidi, Progress and Innovations in Hydrogels for Sustainable Agriculture, Agronomy, 2024, 14, 2815 CrossRef CAS.
- B. Tursynova, T. Zharkynbek, R. Mangazbayeva, N. Mukhamadiyev, R. Koizhaiganova, G. Mengdibayeva, A. Ten, B. Yermukhambetova, G. Mun and V. Yu, Application of Gellan Hydrogel and Kaz-6 in Wheat Seed Coating for Improved Productivity and Environmental Resilience, Polymers, 2025, 17, 1330 CrossRef CAS PubMed.
- S. Mandal, H. Chi, R. E. Moss, P. Dhital, E. O. Babatunde, R. Gurav and S. Hwang, Seed gum-based polysaccharides hydrogels for sustainable agriculture: A review, Int. J. Biol. Macromol., 2024, 263, 130339 CrossRef CAS PubMed.
- W. Zhang, S. Li, X. Liu, H. Zhang, R. Wang, X. Mu and Z. Lei, Preparation of nutrient hydrogels with core-shell structure for seed germination and seedling growth in high temperature saline environment, Int. J. Biol. Macromol., 2024, 283, 137626 Search PubMed.
- M. A. Ovalesha, B. Yadav and P. K. Rai, Effects of polymer seed coating and seed treatment on plant growth, seed yield and quality of Cowpea (Vigna unguiculata), J. Pharmacogn. Phytochem., 2017, 6, 106–109 Search PubMed.
- S. T. Dexter and T. Miyamoto, Acceleration of water uptake and germination of sugar beet seed balls by surface coatings of hydrophilic colloids, Agron. J., 1959, 51, 388–389 Search PubMed.
- W. Abobatta, Impact of hydrogel polymer in agricultural sector, Adv. Agric. Environ. Sci. Open Access, 2018, 1(2), 59–64 Search PubMed.
- V. Pathak and K. P. R. Ambrose, Starch-based biodegradable hydrogel as seed coating for corn to improve early growth under water shortage, J. Appl. Polym. Sci., 2020, 137, 48523 Search PubMed.
- M. J. Mangold and R. L. Shelley, Effects of soil texture, watering frequency, and a hydrogel on the emergence and survival of coated and uncoated crested wheatgrass seeds, Ecol. Restor., 2007, 25, 6–11 Search PubMed.
- M. J. Salar-García and I. Ieropoulos, Optimisation of the internal structure of ceramic membranes for electricity production in urine-fed microbial fuel cells, J. Power Sources, 2020, 451, 227741 CrossRef PubMed.
- M. Reyssat, L. Sangne, E. van Nierop and H. Stone, Imbibition in layered systems of packed beads, Europhys. Lett., 2009, 86(5), 56002 Search PubMed.
- T. T. Kozlowski, Soil Compaction and Growth of Woody Plants, Scand. J. For. Res., 1999, 14, 596–619 CrossRef.
- B. J. Atwell, The effect of soil compaction on wheat during early tillering, New Phytol., 1990, 115, 29–35 Search PubMed.
- J. Shierlaw and A. M. Alston, Effect of soil compaction on root growth and uptake of phosphorus, Plant Soil, 1984, 77, 15–28 CrossRef CAS.
- J. Masaka and N. Khumbula, The Effect of Soil Compaction Levels on Germination and Biometric Characteristics of Coffee (Coffee arabica) Seedlings in the Nursery, Int. J. Agric. Res., 2007, 2, 581–589 CrossRef.
- J.-F. Louf, B. N. Lu, G. M. O’Connell, J. H. Cho and S. S. Datta, Under pressure: Hydrogel swelling in a granular medium, Sci. Adv., 2021, 7, eabd2711 Search PubMed.
- M. M. T. Lakshani, T. K. K. Chamindu Deepagoda, S. Hamamoto, B. Elberling, W. Fu, T. Yang, J. Fan, X. Ma, T. Clough, K. M. Smits, T. G. Parameswaran, G. L. Sivakumar Babu and H. Chanakya, A new exponential model for predicting soil gas diffusivity with varying degree of saturation, Vadose Zone J., 2023, 22, e20236 CrossRef.
- A. Herrera, Responses to flooding of plant water relations and leaf gas exchange in tropical tolerant trees of a black-water wetland, Front. Plant Sci., 2013, 4, 106 CAS.
- A. G. Bengough, B. M. McKenzie, P. D. Hallett and T. A. Valentine, Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits, J. Exp. Bot., 2011, 62, 59–68 CrossRef CAS PubMed.
- H. Tomobe, S. Tsugawa, Y. Yoshida, T. Arita, A. Y.-L. Tsai, M. Kubo, T. Demura and S. Sawa, A mechanical theory of competition between plant root growth and soil pressure reveals a potential mechanism of root penetration, Sci. Rep., 2023, 13, 7473 CrossRef CAS.
- U. Ladror, R. L. Dyck and M. J. Silbernagel, Effects of Oxygen and Temperature During Imbibition on Seeds of Two Bean Lines at Two Moisture Levels, J. Am. Soc. Hortic. Sci., 1986, 111, 572–577 Search PubMed.
- K. J. Bradford, D. Côme and F. Corbineau, Quantifying the oxygen sensitivity of seed germination using a population-based threshold model, Seed Sci. Res., 2007, 17, 33–43 CAS.
- L. Gorim and F. Asch, Effects of Composition and Share of Seed Coatings on the Mobilization Efficiency of Cereal Seeds During Germination, J. Agron. Crop Sci., 2012, 198, 81–91 CrossRef.
- L. Gorim and F. Asch, Seed Coating Increases Seed Moisture Uptake and Restricts Embryonic Oxygen Availability in Germinating Cereal Seeds, Biology, 2017, 6(2), 31 CrossRef PubMed.
- L. C. Purcell, M. Salmeron and L. Ashlock, Soybean Growth and Development, in Arkansas Soybean Production Handbook, University of Arkansas Division of Agriculture, Research & Extension, Fayetteville, AR, 2014, pp. 1–2 Search PubMed.
- E. Wiraguna, The role of micropyle for grass pea germination, Arab Gulf J. Sci. Res., 2024, 42, 722–729 Search PubMed.
- L.-Q. Su, J.-G. Li, H. Xue and X.-F. Wang, Super absorbent polymer seed coatings promote seed germination and seedling growth of Caragana korshinskii in drought, J. Zhejiang Univ., Sci., B, 2017, 18, 696–706 Search PubMed.
- N. P. Capobiango, L. Junio da Silva, R. B. A. Fernandes, R. A. da Silva Júnior, L. H. Barcellos Júnior, A. Dantas de Medeiros and W. M. Lopes, A Proposal for Evaluation of Seedling Emergence and Growth under Mechanical Impedance, Commun. Soil Sci. Plant Anal., 2022, 53, 2374–2387 CrossRef CAS.
- W. R. Whalley, L. J. Clark, W. E. Finch-Savage and R. E. Cope, The impact of mechanical impedance on the emergence of carrot and onion seedlings, Plant Soil, 2004, 265, 315–323 CrossRef CAS.
- S. Mousa, R. Nyaruaba, H. Yang and H. Wei, Engineering seed microenvironment with embedded bacteriophages and plant growth promoting rhizobacteria, BMC Microbiol., 2024, 24, 503 CrossRef CAS.
- M. Y. Adoko, A. D. P. Noumavo, N. A. Agbodjato, O. Amogou, H. A. Salami, R. M. Aguégué, N. Adjovi Ahoyo, A. Adjanohoun and L. Baba-Moussa, Effect of the application or coating of PGPR-based biostimulant on the growth, yield and nutritional status of maize in Benin, Front. Plant Sci., 2022, 13, 1064710 Search PubMed.
- R. A. Davis, K. K. Mafune and M. K. H. Winkler, Biodegradable hydrogels and microbial consortia as a treatment for soil dysbiosis, Front. Microbiol., 2025, 16, 1565940 Search PubMed.
- X. Wang, Z. Yang, Q. Zeng, X. Wang, S. Liu, E. Wang, Y. Wu, Y. Zeng, M. He, Y. Wang, G. Shen, X. Jing, R. Ping, X. Zhang and B. Chen, Chitosan hydrogel microspheres loaded with Bacillus subtilis promote plant growth and reduce chromium uptake, Int. J. Biol. Macromol., 2025, 286, 138401 Search PubMed.
- M. M. Ghobashy, M. A. Amin, A. E. Mustafa, M. A. El-diehy, B. K. El-Damhougy and N. Nady, Synthesis and application of a multifunctional poly (vinyl pyrrolidone)-based superabsorbent hydrogel for controlled fertilizer release and enhanced water retention in drought-stressed Pisum sativum plants, Sci. Rep., 2024, 14, 27734 CrossRef CAS.
- O. D. Abdul Sattar, R. M. Khalid and S. F. M. Yusoff, Eco-friendly natural rubber-based hydrogel loaded with nano-fertilizer as soil conditioner and improved plant growth, Int. J. Biol. Macromol., 2024, 280, 135555 CrossRef CAS.
- A. El Idrissi, F. Tayi, O. Dardari, Y. Essamlali, I. Jioui, I. Ayouch, A. Akil, G. Achagri, K. Dänoun, O. Amadine and M. Zahouily, Urea-rich sodium alginate-based hydrogel fertilizer as a water reservoir and slow-release N carrier for tomato cultivation under different water-deficit levels, Int. J. Biol. Macromol., 2024, 272, 132814 CrossRef CAS.
- I. Singh, R. R. Verma and T. K. Srivastava, Growth, Yield, Irrigation Water Use Efficiency, Juice Quality and Economics of Sugarcane in Pusa Hydrogel Application Under Different Irrigation Scheduling, Sugar Tech., 2018, 20, 29–35 CrossRef.
- T. A. Adjuik, S. E. Nokes, M. D. Montross and O. Wendroth, The Impacts of Bio-Based and Synthetic Hydrogels on Soil Hydraulic Properties: A Review, Polymers, 2022, 14(21), 4721 Search PubMed.
- L. Figueiredo, R. Pace, C. D'Arros, G. Réthoré, J. Guicheux, C. Le Visage and P. Weiss, Assessing glucose and oxygen diffusion in hydrogels for the rational design of 3D stem cell scaffolds in regenerative medicine, J. Tissue Eng. Regener. Med., 2018, 12, 1238–1246 CrossRef CAS.
- L.-K. Ju and C. S. Ho, The measurement of oxygen diffusion coefficients in polymeric solutions, Chem. Eng. Sci., 1986, 41, 579–589 CrossRef CAS.
- C. S. Ho, The anomaly of oxygen diffusion in aqueous xanthan solutions, Biotechnol. Bioeng., 1988, 32, 8–17 CrossRef CAS PubMed.
- F. Dehghani and N. Annabi, Engineering porous scaffolds using gas-based techniques, Curr. Opin. Biotechnol, 2011, 22, 661–666 Search PubMed.
- L. R. Madden, D. J. Mortisen, E. M. Sussman, S. K. Dupras, J. A. Fugate, J. L. Cuy, K. D. Hauch, M. A. Laflamme, C. E. Murry and B. D. Ratner, Proangiogenic scaffolds as functional templates for cardiac tissue engineering, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 15211–15216 Search PubMed.
- S. M. Mavris and L. M. Hansens, Optimization of Oxygen Delivery Within Hydrogel, J. Biomech. Eng., 2021, 143(10), 101004 CrossRef.
- Z. Zhou, J. Lei and Z. Liu, Effect of water content on physical adhesion of polyacrylamide hydrogels, Polymer, 2022, 246, 124730 Search PubMed.
- D. B. Egli and D. M. TeKrony, Species differences in seed water status during seed maturation and germination, Seed Sci. Res., 1997, 7, 3–12 Search PubMed.
- L. Borisjuk and H. Rolletschek, The oxygen status of the developing seed, New Phytol., 2009, 182, 17–30 Search PubMed.
- Y. Tang, F. Gao, S. Guo and F. Li, Effects of hypobaria and hypoxia on seed germination of six plant species, Life Sci. Space Res., 2014, 3, 24–31 Search PubMed.
- N. Budko, A. Corbetta, B. van Duijn, S. C. Hille, O. Krehel, V. Rottschäfer, L. Wiegman and D. Zhelyazov, Oxygen transport and consumption in germinating seeds. Proceedings of the 90th European Study Group Mathematics in Industry (SWI 2013, Leiden, The Netherlands, January 28-February 1, 2013), 2013, pp. 5–30.
- N. C. Garwood and J. R. B. Lighton, Physiological ecology of seed respiration in some tropical species, New Phytol., 1990, 115, 549–558 Search PubMed.
- A. C. Hulst, H. J. H. Hens, R. M. Buitelaar and J. Tramper, Determination of the effective diffusion coefficient of oxygen in gel materials in relation to gel concentration, Biotechnol. Tech., 1989, 3, 199–204 Search PubMed.
- L. Ma, Y. Shi, O. Siemianowski, B. Yuan, T. K. Egner, S. V. Mirnezami, K. R. Lind, B. Ganapathysubramanian, V. Venditti and L. Cademartiri, Hydrogel-based transparent soils for root phenotyping in vivo, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11063–11068 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |