Electrolyte additives in Li-ion batteries: from mechanisms to application
Runze
Zhang†
a,
Yinglei
Wu†
 *a,
Guangfu
Ge
ab,
Jinxuan
Liu
a,
Jihu
Wang
a,
Sirui
Wang
c and
Zhongyi
He
*a,
Guangfu
Ge
ab,
Jinxuan
Liu
a,
Jihu
Wang
a,
Sirui
Wang
c and
Zhongyi
He
 d
d
aSchool of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, Shanghai 201620, China. E-mail: wuyl@sues.edu.cn
bFaculty of Engineering Technology, University of Twente, The Netherlands
cSchool Affiliated to Shanghai Jiao Tong University, Shanghai 200240, China
dSchool of Materials Science and Engineering, East China Jiaotong University, Nanchang 330013, China
First published on 8th December 2025
Abstract
This article systematically reviews the mechanisms, classifications, and applications of electrolyte additives for lithium-ion batteries. The addition of trace amounts of additives can significantly enhance battery performance, with common types including film-forming agents, flame retardants, acid scavengers, overcharge protectants, and multifunctional composite additives. They play a key role in building a stable SEI/CEI layer at the electrode/electrolyte interface, removing harmful substances (such as HF), regulating the solvation structure of lithium-ions, enhancing thermal stability, and inhibiting dendrite growth. The article discusses in detail the additives containing elements such as boron, phosphorus, sulfur, fluorine, and nitrogen, as well as their synergistic effects. The article also explores emerging directions such as ionic liquids, multifunctional molecules, nanomaterials, polymers, and bio-based additives, and points out the challenges currently faced by additive technologies, including compatibility, mechanism complexity, and long-term effectiveness. It also looks forward to the development prospects of rational design and collaborative strategies for high-voltage, high-energy-density, and solid-state batteries.
1 Introduction
Lithium-ion batteries (LIBs), as efficient energy storage devices, have been widely used in portable electronic devices, electric vehicles, and grid energy storage fields. Their performance core depends on the physical and chemical properties of the internal key components – the electrolyte and the electrode/electrolyte interface behavior.1,2 The current traditional electrolyte system is mainly composed of LiPF6 dissolved in carbonate solvents (such as EC, DMC, DEC). Although it has high ionic conductivity and a moderate electrochemical stability window, it has a series of inherent defects that limit the further improvement of battery performance. These defects mainly include the instability of the solid-electrolyte interphase (SEI) and cathode-electrolyte interphase (CEI), which are prone to rupture and reconstruction during cycling, leading to continuous consumption of active lithium and impedance increase.3 Meanwhile, side reactions occur frequently inside the battery, such as the decomposition of electrolyte solvents, gas generation (for example, the formation of CO2 and olefins), and the dissolution of transition metal ions (especially nickel in nickel-rich cathodes).4 In addition, there is a risk of thermal runaway. This is because the commonly used LiPF6 salt is prone to decomposition at high temperatures or in the presence of trace amounts of water, generating highly corrosive substances such as HF and PF5, which in turn catalyze the decomposition of the electrolyte and corrode the electrode material. Finally, the performance of batteries under extreme conditions also has shortcomings, such as the obstruction of ion migration in low-temperature environments, the intensification of polarization during high-rate charging and discharging, and the acceleration of side reactions at high temperatures, etc.1,5To overcome the above limitations, electrolyte additives have become a key strategy for improving the energy density, cycle life, and safety of lithium batteries due to their ability to significantly improve the overall performance of batteries with trace amounts (usually less than 5 wt%). The core function of additives is to precisely regulate the electrolyte and interface properties. According to functional classification, additives mainly include film-forming agents, overcharge protectors, flame-retardants, and lithium salt stabilizers.6 Its core function includes constructing a stable SEI on the anode surface, such as fluoroethylene carbonate (FEC), which forms inorganic components rich in LiF through preferential reduction, effectively inhibiting lithium dendrite growth and reducing loss of active lithium.7,8 On the cathode side, additives such as boronic acid esters (such as TMB) catalyze decomposition to form a dense CEI film, which blocks the leaching of transition metals and alleviates electrolyte oxidation and decomposition under high voltage.7,9 Specifically, film-forming additives such as FEC and VC enhance cycling stability by regulating the composition and mechanical properties of SEI/CEI,10 and by constructing stable SEI or CEI at the electrode/electrolyte interface, they suppress side reactions and optimize ion transport behavior.6 Additives can also remove harmful substances, such as capturing HF and inhibiting LiPF6 hydrolysis, thereby reducing transition metal dissolution (such as boronic acid ester additives).5,11 In addition, additives can also regulate the solvation structure, change the coordination environment of Li+, and help improve the ion migration ability of batteries at low temperatures and stability at high temperatures.12 Regarding safety, functional additives effectively alleviate the risk of battery thermal runaway by inhibiting electrolyte combustion chain reactions or enhancing thermal stability.13 For example, flame-retardant additives (such as organic phosphides) reduce the flammability of electrolytes through gas-phase free radical quenching mechanisms,14 phosphorus-containing flame-retardants (such as organic phosphate esters) reduce the flammability of electrolytes through free radical quenching mechanisms,13 and fluorinated solvents broaden the operating temperature range of batteries by increasing the decomposition temperature.13
The mechanism of action of additives is closely related to their molecular structure.15 Taking ionic liquid additives as an example, the introduction of functional groups (such as ether or cyanide groups) at C-2 can regulate the dissociation degree and viscosity of ion pairs, thereby affecting ion conductivity and interfacial impedance. The introduction of electron-donating ether groups at the C-2 position raises the LUMO energy level of the imidazolium cation, thereby enhancing its reductive stability. This is consistent with the general principle that a higher-lying LUMO corresponds to higher reductive stability, as it makes the cation less susceptible to accepting an electron.15 In addition, molecular engineering strategies optimize the solvation structure of lithium ions and induce uniform lithium deposition by designing additives containing polar functional groups such as cyanide and sulfonyl groups, thereby inhibiting dendrite growth.16 Ionic conductive additives (such as LiNO3) enhance the coulombic efficiency (CE) of lithium sulfur batteries by promoting Li+ migration and suppressing polysulfide shuttle effects.10 With the development of high-energy density battery systems, the design of new multifunctional additives needs to take into account interface stability, ion migration number improvement, and high-voltage compatibility.17 For example, the synergistic effect of fluorine/boron additives can form a passivation layer rich in LiF Li3N at the electrode interface, achieving an electrostatic shielding effect and significantly improving the cycling stability of the 4.4 V high-voltage cathode.17
Electrolyte additives have become the core technology path for unlocking high-energy density, long-cycle life, and wide temperature range lithium-ion batteries by precisely regulating interface chemistry and bulk electrolyte properties. The current research challenge lies in the insufficient long-term effectiveness of additives under extreme conditions, such as low temperature and high-rate, as well as the unclear synergistic mechanism of multifunctional additives.18 This article aims to review the mechanism of action, performance gains, and cutting-edge progress, providing a theoretical framework and material strategy for the design of next-generation LIB electrolytes. Fig. 1 presents the classification system of the additives involved in this article. The following text will analyze the effects and internal mechanisms of each type of additive in detail according to this classification.
 | ||
| Fig. 1 The classifications of additives based on their structures, functions, and material properties in this article. | ||
2 Additives based on the elements
2.1 Boron-containing
Boron-based electrolyte additives, such as lithium bis oxalate borate (LiBOB) and lithium difluorooxalate borate (LiDFOB), significantly enhance the high-temperature stability and cycling performance of lithium-ion batteries through a multifunctional film-forming mechanism. LiBOB undergoes a reduction reaction on the electrode surface, forming a stable SEI rich in borates, oxalates, and carboxylates, effectively inhibiting the detachment of graphite structures and reducing the dissolution of transition metals such as Mn.19 The mechanism of action of LiBOB specifically includes three aspects. Firstly, the boric acid or borate produced by its decomposition can react with the HF produced by the hydrolysis of LiPF6, generating thermodynamically more stable LiBF4 and LiDFOB, thereby clearing HF and inhibiting its corrosion on electrodes.19 Secondly, electron-deficient boron atoms are prone to pair with electron-rich species such as PF6− and F−, inhibiting the decomposition of anions.19 Thirdly, the boron (B) and oxygen (O) rich environment formed on the surface of the graphite anode helps to reduce the kinetic potential barrier for lithium-ion transport and improve ion conductivity.19As an important boron-based additive, LiDFOB's molecular structure can be regarded as half LiBOB and half LiBF4, thus combining the film-forming properties of LiBOB with the low-temperature performance advantages of LiBF4.19 On the surface of high-voltage cathodes (such as LiNi0.5Mn1.5O4), LiDFOB can oxidize to form an inorganic enrichment layer, effectively suppressing local stress and enhancing the kinetics of charge transfer.19 In silicon-based electrode applications, adding 1% LiDFOB can increase the content of oxalate and lithium fluorophosphate in the SEI, reduce the generation of LiF, and significantly improve the cycling performance of silicon-based full cells at 45 °C.20 In terms of performance comparison, there are some key differences between LiBOB and LiDFOB. LiBOB has lower solubility in organic solvents and is sensitive to impurities, while LiDFOB exhibits higher solubility and conductivity in linear carbonate solvents.13 In terms of film-forming properties, LiDFOB decomposition can generate 5–10 nm nanostructured LiF, forming a uniform diffusion gradient. In contrast, LiBOB/LiBF4 mixed electrolytes generate large particles of LiF larger than 300 nm, resulting in uneven SEI formation.21 In terms of high-temperature performance, LiNi1−xyMxNyO2/graphite batteries based on LiDFOB exhibit excellent stability, with a capacity retention rate of up to 90% after 200 cycles at 60 °C, and good rate performance in the current density range of 0.5–10 mA.22
Zhuang et al.23 investigated the mechanism of action of boron-based additive tris(trimethylsilyl)borate (TMSB) in lithium selenium batteries. As shown in Fig. 2a, TMSB adsorbs F− and PFx− anions in the electrolyte through its electron-deficient boron atoms, forming polyanion groups that inhibit the formation of insulating LiF at the CEI, thereby reducing interface impedance and increasing lithium-ion diffusion rate. This mechanism significantly enhances electrode conductivity and cycling stability, resulting in a 130% increase in capacity of the battery containing 1 wt% TMSB after 500 cycles.23 Dong et al.27 explored the dual role of lithium difluorooxalate borate (LiDFOB) as a boron-containing additive in high-nickel NMC811‖graphite lithium-ion batteries. LiDFOB can preferentially oxidize and reduce on the surfaces of the cathodes and anodes, forming a stable interface layer (CEI/SEI) rich in boron, fluorine and carbonate compounds, effectively inhibiting the decomposition of the electrolyte and the dissolution of transition metals (Fig. 2b). This significantly enhances the battery's cycle stability and capacity retention rate at high cut-off voltages (4.4 V) (still maintaining 83.1% after 200 cycles). The figure shows its molecular orbital energy levels, film-forming characteristics, electrochemical performance, and interface component analysis.27Fig. 2c illustrates the action mechanism of tri(2-cyanoethyl)borate (TCEB) as a boron-based additive in the high-voltage lithium battery. Its electron-deficient boron atoms can combine with electrolyte by-products (such as F− in HF), while the cyano (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) stabilizes highly oxidized transition metal ions (such as Ni4+) through coordination, and cooperates to form a stable CEI, which inhibits the oxidative decomposition of electrolyte and electrode structure degradation, thus improving the cycle stability of nickel cobalt lithium manganate (SC-NCM)‖lithium metal battery at 4.7 V.25 Karkar et al.24 investigated a novel polymeric borate ester (PBE) electrolyte additive for enhancing the interfacial stability and cycling performance of silicon/graphite (Si–C) composite anodes in lithium-ion batteries. Boron-containing polymers were synthesized through a one-step condensation reaction, and the structure was confirmed by nuclear magnetic resonance and infrared spectroscopy, represented by glycol-based PBE (PBE-DG). Electrochemical tests indicated that the capacity retention rate of the electrolyte using 2% PBE-DG in the half-cell reached 75% after 50 cycles, which was significantly higher than 37% with the fluoroethylene carbonate (FEC) additive. Analysis of the electrode surface through SEM and XPS revealed that PBE promoted the formation of a stable SEI layer rich in inorganic substances such as LiF and low in organic substances, effectively inhibiting electrode degradation and enhancing battery performance (Fig. 2d).24 Song et al.28 investigated the reaction mechanism and film-forming behavior of boron-based high-voltage electrolyte additives (represented by TMB and TCEB) in lithium-ion batteries by combining first-principles calculations with experiments. The results show that these boron-containing additives can preferentially oxidize and undergo nucleophilic reactions with fluorides (such as F−, PF6−, HF, PF5) to form species containing B–F and B–O, thereby participating in the construction of a stable cathode-electrolyte interface (CEI) film, inhibiting electrolyte decomposition and electrode corrosion. The research also found that although the electron-absorbing CN group was introduced into TCEB, its reaction site and energy barrier were similar to those of TMB, indicating that molecular structure modification did not affect its reaction pathway (Fig. 2e). This work provides a theoretical basis for the design of electrolyte additives for high-voltage lithium-ion batteries.28 Qin et al.29 proposed a LiDFOB–TMSB dual additive electrolyte strategy for improving the performance of lithium metal batteries. As shown in Fig. 2f, DFT calculations reveal that TMSB has the highest binding energy with PF6− (−0.31 eV), forming the TMSB–PF6− complex and inhibiting PF6− migration. Fig. 2f also shows that the composite has the highest HOMO value (−5.09 eV), preferentially forming a robust CEI rich in F, Si, and B through cathodic oxidation decomposition, while LiDFOB has the lowest LUMO value (−1.79 eV), preferentially forming an SEI through anodic reduction, synergistically suppressing lithium dendrite growth, electrolyte oxidation, and cathodic structure collapse, thereby improving Coulomb efficiency and cycling stability.29 Sun et al.30 suggested using a boron-containing compound, DPD-F, as an electrolyte additive to preferentially oxidize and decompose the LiCoO2 cathode through its electronic defect character, creating a stable CEI rich in LiF (lower layer) and B–O polymer (upper layer). Fig. 2g illustrates the mechanism pathway of DPD-F additive oxidation to generate CEI. This interface effectively suppresses the dissolution of transition metal cobalt and electrolyte decomposition under high voltage (4.6 V) and high temperature (70 °C), significantly improving the cycling performance of lithium batteries.30
N) stabilizes highly oxidized transition metal ions (such as Ni4+) through coordination, and cooperates to form a stable CEI, which inhibits the oxidative decomposition of electrolyte and electrode structure degradation, thus improving the cycle stability of nickel cobalt lithium manganate (SC-NCM)‖lithium metal battery at 4.7 V.25 Karkar et al.24 investigated a novel polymeric borate ester (PBE) electrolyte additive for enhancing the interfacial stability and cycling performance of silicon/graphite (Si–C) composite anodes in lithium-ion batteries. Boron-containing polymers were synthesized through a one-step condensation reaction, and the structure was confirmed by nuclear magnetic resonance and infrared spectroscopy, represented by glycol-based PBE (PBE-DG). Electrochemical tests indicated that the capacity retention rate of the electrolyte using 2% PBE-DG in the half-cell reached 75% after 50 cycles, which was significantly higher than 37% with the fluoroethylene carbonate (FEC) additive. Analysis of the electrode surface through SEM and XPS revealed that PBE promoted the formation of a stable SEI layer rich in inorganic substances such as LiF and low in organic substances, effectively inhibiting electrode degradation and enhancing battery performance (Fig. 2d).24 Song et al.28 investigated the reaction mechanism and film-forming behavior of boron-based high-voltage electrolyte additives (represented by TMB and TCEB) in lithium-ion batteries by combining first-principles calculations with experiments. The results show that these boron-containing additives can preferentially oxidize and undergo nucleophilic reactions with fluorides (such as F−, PF6−, HF, PF5) to form species containing B–F and B–O, thereby participating in the construction of a stable cathode-electrolyte interface (CEI) film, inhibiting electrolyte decomposition and electrode corrosion. The research also found that although the electron-absorbing CN group was introduced into TCEB, its reaction site and energy barrier were similar to those of TMB, indicating that molecular structure modification did not affect its reaction pathway (Fig. 2e). This work provides a theoretical basis for the design of electrolyte additives for high-voltage lithium-ion batteries.28 Qin et al.29 proposed a LiDFOB–TMSB dual additive electrolyte strategy for improving the performance of lithium metal batteries. As shown in Fig. 2f, DFT calculations reveal that TMSB has the highest binding energy with PF6− (−0.31 eV), forming the TMSB–PF6− complex and inhibiting PF6− migration. Fig. 2f also shows that the composite has the highest HOMO value (−5.09 eV), preferentially forming a robust CEI rich in F, Si, and B through cathodic oxidation decomposition, while LiDFOB has the lowest LUMO value (−1.79 eV), preferentially forming an SEI through anodic reduction, synergistically suppressing lithium dendrite growth, electrolyte oxidation, and cathodic structure collapse, thereby improving Coulomb efficiency and cycling stability.29 Sun et al.30 suggested using a boron-containing compound, DPD-F, as an electrolyte additive to preferentially oxidize and decompose the LiCoO2 cathode through its electronic defect character, creating a stable CEI rich in LiF (lower layer) and B–O polymer (upper layer). Fig. 2g illustrates the mechanism pathway of DPD-F additive oxidation to generate CEI. This interface effectively suppresses the dissolution of transition metal cobalt and electrolyte decomposition under high voltage (4.6 V) and high temperature (70 °C), significantly improving the cycling performance of lithium batteries.30
 | ||
| Fig. 2 (a) (top) The formation of a thick insulation layer on the surface of the electrode without TMSB additive; (below) the electrode surface containing TMSB maintains a clean thin-layer.23 Copyright 2021 Elsevier Ltd. (b) LiDFOB additive suppresses electrolyte decomposition and metal leaching by preferentially forming a stable interface layer rich in boron/fluorine at the cathode and anodes, significantly improving the cycling stability and high-temperature performance of NMC811‖graphite lithium batteries.27 Copyright 2019 American Chemical Society. (c) The electrochemical oxidation decomposition mechanism of TCEB additives reveals the process of boron atoms and cyanide groups synergistically stabilizing the electrode interface.25 Copyright 2022 Elsevier B.V. (d) The rich LiF inorganic SEI formed by PBE-DG inhibits electrolyte decomposition.24 Copyright 2022 American Chemical Society. (e) After oxidation of boron-based additives, boron atoms exhibit high positivity and are attacked by fluoride nucleophiles to form B–F/B–O species, forming a stable CEI that inhibits electrolyte decomposition and enhances the performance of 4.6 V high-voltage LiCoO2 batteries.28 Copyright 2023 American Chemical Society. (f) The highest binding energy (−0.31 eV) between TMSB and PF6− promotes the formation of the complex. The highest HOMO value (−5.09 eV) of TMSB–PF6− and the lowest LUMO value (−1.79 eV) of LiDFOB guide the preferential decomposition position.29 Copyright 2024 Elsevier B.V. (g) The mechanism pathway of DPD-F additive oxidation to form CEI.30 Copyright 2023 American Chemical Society. | ||
2.2 Phosphorus-containing
Phosphide additives are widely studied flame-retardant categories in lithium-ion battery electrolytes, mainly including alkyl phosphate and aryl phosphate compounds, such as trimethyl phosphate (TMP) and triethyl phosphate (TEP). These compounds are liquid at room temperature, soluble in organic solvents, and exert flame-retardant effects through gas-phase free radical capture mechanisms. Under high temperature conditions, phosphides (such as TMP) undergo thermal decomposition to generate phosphorus-containing free radicals (such as and
and 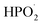 ), which can effectively capture hydrogen free radicals (H˙) and hydroxyl free radicals (HO˙) in the combustion chain reaction, thereby terminating the exothermic reaction and suppressing electrolyte combustion.13
), which can effectively capture hydrogen free radicals (H˙) and hydroxyl free radicals (HO˙) in the combustion chain reaction, thereby terminating the exothermic reaction and suppressing electrolyte combustion.13
However, traditional phosphide additives have significant limitations. To make the electrolyte completely nonflammable, it is necessary to add TMP with high content (>12 mol%), but excessive addition will damage the SEI of the graphite anode, leading to deterioration of the battery cycle performance.31 For example, although TEP has similar flame-retardant efficiency to TMP, it is still difficult to maintain long-term cycling stability at high volume contents, mainly due to its lower reduction and decomposition activity and difficulty in co-embedding with Li+ in graphite anodes.32
Fig. 3a shows the mechanism of action of phosphorus-containing additive, tris(2,2,2-trifluoroethyl)phosphite (TTFP). In high-voltage lithium-ion batteries, TTFP acts as an “oxygen scavenger” that preferentially oxidizes on the surface of the LiMn2O4 cathode, capturing active oxygen radicals released by the lattice under high voltage, inhibiting manganese dissolution and electrolyte oxidation. Meanwhile, the synergistic fluorinated vinyl carbonate (FEC) forms a stable SEI on the anode, thereby achieving dual protection of the cathode and anode at a high voltage of 4.7 V and improving cycling stability.33 Han et al.34 investigated the mechanism of action of phosphorus-based additive LiDFBP (lithium difluorobis oxalate phosphate) in lithium-rich cathode lithium batteries. As shown in Fig. 3b, LiDFBP forms a uniform and ion-conductive SEI on the cathode surface through preferential oxidation. This SEI effectively suppresses electrolyte decomposition under high voltage, alleviates voltage decay caused by layered spinel phase transition, and improves rate performance (20C discharge capacity reaches 116 mAh g−1), while reducing the generation of by-product LiF, thereby significantly improving cycle stability (100-cycle efficiency > 99.5%) and high-temperature performance.34 Chen et al.35 synthesized the lithium salt additive LiOTFP (lithium tetrafluorooxalate phosphate) through molecular design, and simultaneously optimized the interface stability between the high-nickel cathode (NCM) and the silicon-based anode by taking advantage of its phosphorus-based molecular properties. As shown in Fig. 3c (left), LiOTFP constructs a matching double interface layer rich in LiF, Li3PO4, and phosphorus-containing polymers (CEI/SEI, Fig. 3e) through a preferential REDOX reaction (Fig. 3c (right) shows that its HOMO/LUMO energy level is conducive to decomposition at the cathodes/anodes). Enhance the mechanical strength of the interface and inhibit the dissolution/volume expansion of transition metals. Even more encouraging is that the proposed strategy enables the 4.4 V 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cylindrical battery (5 Ah) to exhibit excellent cycling stability under practical conditions (with a capacity retention rate of 92.9% after 300 cycles).35 Guéguen et al.36 compared the working mechanisms of two phosphorus-based additives, trimethylsilylphosphate (TMSPi) and trimethylsilylphosphate (TMSPa), in carbonate electrolytes for lithium-ion batteries. Research has shown that both mainly improve battery performance through chemical cleaning rather than film formation protection. TMSPa preferentially reacts with HF to generate Me3SiF (Fig. 3d, right), while TMSPi directly reacts with LiPF6 due to the high activity of P(III) to generate Li phosphate and Me3SiF (Fig. 3d, left). Both significantly inhibited the generation of POF3 by reducing the acidity of the electrolyte (by about 2/3), thereby delaying the decomposition of LiPF6, and increasing impedance, and prolonging the battery life.36 Cheon et al.37 added phosphorus-containing flame-retardant DMMP to PEO-based solid polymer electrolytes, which not only improved ion conductivity and lithium ion migration number, but also significantly enhanced flame-retardant performance. Fig. 3f shows the mechanism by which PO˙ radicals generated by Dimethyl methylphosphonate (DMMP) decomposition capture active radicals and interrupt the combustion chain reaction, effectively suppressing electrolyte combustion and improving the safety of lithium batteries.37 Lu et al.38 investigated the application of phosphorus-containing additive triallyl phosphate (TAP) in high-voltage LiNi0.5Mn1.5O4/graphite batteries. Through electrochemical tests, spectroscopic analysis, and theoretical calculations, it was found that TAP can be preferentially oxidized at the cathode to form a low-molecular-weight polymer layer (Fig. 3g), effectively inhibiting the oxidation of the electrolyte and the dissolution of the electrode, and enhancing the cycling stability of the battery.38 Peebles et al.39 investigated the mechanism of action of phosphorus-containing additive tris(trimethylsilyl)phosphate (TMSPi) in lithium batteries. Research has found that TMSPi, after being chemically “pickled” (aged) in the electrolyte, decomposes to produce the key product PF2OSiMe3, which is anchored to the active sites on the cathode surface to form a protective ligand layer (Fig. 3h), blocking solvent contact with catalytic centers, thereby inhibiting electrolyte oxidation and transition metal leaching, ultimately alleviating battery impedance rise and capacity degradation.39
700 cylindrical battery (5 Ah) to exhibit excellent cycling stability under practical conditions (with a capacity retention rate of 92.9% after 300 cycles).35 Guéguen et al.36 compared the working mechanisms of two phosphorus-based additives, trimethylsilylphosphate (TMSPi) and trimethylsilylphosphate (TMSPa), in carbonate electrolytes for lithium-ion batteries. Research has shown that both mainly improve battery performance through chemical cleaning rather than film formation protection. TMSPa preferentially reacts with HF to generate Me3SiF (Fig. 3d, right), while TMSPi directly reacts with LiPF6 due to the high activity of P(III) to generate Li phosphate and Me3SiF (Fig. 3d, left). Both significantly inhibited the generation of POF3 by reducing the acidity of the electrolyte (by about 2/3), thereby delaying the decomposition of LiPF6, and increasing impedance, and prolonging the battery life.36 Cheon et al.37 added phosphorus-containing flame-retardant DMMP to PEO-based solid polymer electrolytes, which not only improved ion conductivity and lithium ion migration number, but also significantly enhanced flame-retardant performance. Fig. 3f shows the mechanism by which PO˙ radicals generated by Dimethyl methylphosphonate (DMMP) decomposition capture active radicals and interrupt the combustion chain reaction, effectively suppressing electrolyte combustion and improving the safety of lithium batteries.37 Lu et al.38 investigated the application of phosphorus-containing additive triallyl phosphate (TAP) in high-voltage LiNi0.5Mn1.5O4/graphite batteries. Through electrochemical tests, spectroscopic analysis, and theoretical calculations, it was found that TAP can be preferentially oxidized at the cathode to form a low-molecular-weight polymer layer (Fig. 3g), effectively inhibiting the oxidation of the electrolyte and the dissolution of the electrode, and enhancing the cycling stability of the battery.38 Peebles et al.39 investigated the mechanism of action of phosphorus-containing additive tris(trimethylsilyl)phosphate (TMSPi) in lithium batteries. Research has found that TMSPi, after being chemically “pickled” (aged) in the electrolyte, decomposes to produce the key product PF2OSiMe3, which is anchored to the active sites on the cathode surface to form a protective ligand layer (Fig. 3h), blocking solvent contact with catalytic centers, thereby inhibiting electrolyte oxidation and transition metal leaching, ultimately alleviating battery impedance rise and capacity degradation.39
 | ||
| Fig. 3 (a) The high-voltage stabilization mechanism of the TTFP additive in removing cathode reactive oxygen species and synergistically protecting the anode interface with FEC.33 Copyright 2022 Elsevier B.V. (b) Schematic diagram of the protective mechanism of LiDFBP-derived SEI on the lithium-rich cathode.34 Copyright 2017 Wiley-VCH Verlag GmbH & Co. (c) (left) Schematic diagram of LiOTFP molecular structure and its dual interface stability mechanism. (right) Comparison of HOMO/LUMO energy levels of electrolyte components and OTFP anions.35 (d) Schematic diagram comparing the mechanisms of TMSPi reacting with LiPF6 and TMSPa reacting with HF to generate Me3SiF.36 Copyright 2019 American Chemical Society. (e) Phosphorus-containing LiPF4C2O4 additive synergistically enhances the interface stability and cycle life of NCM/SiOx batteries.35 (c and e) Copyright 2024 American Chemical Society. (f) The phosphorus-containing additive DMMP decomposes during combustion to produce PO˙ radicals, which capture H˙ and HO˙ radicals to form HPO and HPO2, thereby inhibiting the chain combustion reaction.37 Copyright 2024 American Chemical Society. (g) Triallyl phosphate ester (TAP) is used as an electrolyte additive to preferentially oxidize the cathode to form a polymer deposition layer, which enhances the performance of LNMO/graphite batteries and inhibits electrolyte decomposition.38 Copyright 2022 American Chemical Society. (h) TMSPi additive undergoes electrolyte chemical aging to generate PF2OSiMe3, which forms a protective layer by anchoring the active sites on the cathode surface to suppress side reactions and alleviate the performance degradation of lithium batteries.39 Copyright 2018 American Chemical Society. | ||
2.3 Sulfur-containing
Sulfide additives significantly enhance the high voltage performance and cycling stability of batteries by preferentially oxidizing on the electrode surface to form a stable interfacial layer. For example, sulfur-containing additives such as 1,3,2-dioxathiocyclopentane-2,2-dioxide (DTD), propane sulfone lactone (PES), and ethylene sulfonate ester can preferentially oxidize and decompose under high-pressure conditions (>4.4 V vs. Li/Li+) over carbonate solvents, creating a CEI rich in sulfur oxides. This interface layer effectively suppresses the continuous oxidation and decomposition of the electrolyte, reduces the dissolution of transition metals, and lowers the interface impedance, thereby improving the cycling stability of the NCM ternary cathode.40Dimethyl sulfoxide (DMS) plays an important role as a sulfur-containing additive in optimizing the low-temperature performance of batteries. It enhances the low-temperature diffusion kinetics of lithium ions by reducing the activation energy of the SEI. Experiments have shown that adding 0.5 wt% DMS electrolyte can maintain 86% room temperature capacity of graphite/NCM523 batteries at −20 °C, while significantly reducing charge transfer impedance (by 61%).41
The synergistic effect of sulfur-containing additives and inorganic salts can effectively construct a high-pressure stable interface. For example, when lithium sulfide (Li2S) is compounded with acrylonitrile (AN), Li2S preferentially oxidizes on the surface of the NCM523 cathode to form stable sulfonate-based compounds, while AN inhibits solvent decomposition. This synergistic effect increases the capacity retention rate of the battery to 83% (51.2% for blank electrolyte) after 200 cycles at a high voltage of 4.5 V.42
In the lithium sulfur battery system, sulfide additives suppress the shuttle effect of polysulfides by changing the redox pathway. For example, PhSeH reacts with elemental sulfur to form phenylselenide sulfide, forming a new highly reversible redox pathway that enables the battery to maintain a capacity retention rate of 92.86% after 200 cycles, while reducing discharge overpotential.43
Lin et al.44 studied the mechanism of sulfur-containing additive PDTD in lithium batteries and found that PDTD preferentially reduces the graphite anode compared to electrolyte solvents (Fig. 4a), forming a stable SEI film rich in thiooxides (such as Li2SO3), effectively inhibiting electrolyte decomposition, transition metal dissolution, and lithium dendrite formation, thereby improving the first coulombic efficiency (87.8% vs. baseline 83.7%) and 500 cycle capacity retention rate (83.7% vs. 75.7%) of graphite/NMC622 batteries.44 Xiong et al.45 found that in the high-voltage NCM523 cathode system of lithium batteries, trace sulfur additives (0.1 mg mL−1) preferentially oxidize to create a thin and dense CEI layer on the cathode surface (as shown in Fig. 4b), effectively suppressing the decomposition of carbonate electrolyte and optimizing the interface lithium-ion transport kinetics. This mechanism significantly improved the cycling stability of the battery at a high voltage of 4.5 V (the capacity retention rate of the half-cell increased from 61.2% to 82.0% after 200 cycles) and the rate performance (the capacity increased by 30% after 5C), and its feasibility was verified in the entire battery. Excessive sulfur additives can lead to a thick and deteriorated layer.45Fig. 4c specifically illustrates the oxidative polymerization mechanism of diphenyl disulfide (DPDS) as a cathode additive. DPDS preferentially oxidizes under high pressure to generate phenylthio radicals, which then polymerize into a conductive polymer layer covering the cathode active sites, inhibiting transition metal dissolution and enhancing interfacial ion conductivity, thereby improving the cycling performance of high-voltage batteries.46 Huang et al.47 investigated the formation of a CEI protective layer with high ionic conductivity on the surface of the LiNi0.5Mn1.5O4 (LNMO) cathode by the sulfur-based additive methylphenylsulfone (MPS) through preferred oxidation. Meanwhile, Fig. 4d shows that its molecules block the HF generation path by generating a sulfur-containing Lewis base that combines with PF5, thereby effectively inhibiting the dissolution of transition metals and the decomposition of electrolytes, and significantly enhancing the cycle stability (capacity retention rate of 89.8% at 400 cycles) and rate performance (110.2 mAh g−1 at 5C) of 5 V high-voltage lithium batteries.47
 | ||
| Fig. 4 (a) Schematic diagram of the mechanism by which the PDTD additive improves battery performance by optimizing the electrode/electrolyte interface.44 Copyright 2018 American Chemical Society. (b) The schematic diagram of the effect of sulfur additive concentration on the formation of CEI shows that 0.1 mg per mL sulfur forms the optimal thin-layer structure.45 Copyright 2021 American Chemical Society. (c) Schematic diagram of the reaction mechanism of diphenyl disulfide (DPDS) oxidizing and polymerizing on the cathode surface to form a conductive protective layer.46 Copyright 2021 John Wiley & Sons Australia, Ltd. (d) Schematic diagram of the reaction pathway between the Lewis base generated by the decomposition of MPS additive molecules and the binding of PF5.47 Copyright 2021 American Chemical Society. | ||
2.4 Fluorine-containing
Fluorinated additives significantly improve the performance of lithium-ion batteries by removing harmful species and stabilizing the electrode/electrolyte interface. Tri(2,2,2-trifluoroethyl)phosphite (TTFP) is a typical representative, and its phosphorus atom (P(III)) provides lone pair electrons to form a complex with the strong Lewis acid PF5 (such as LiPF6 + TTFP → LiPF6·TTFP), effectively inhibiting the hydrolysis of LiPF6 and the generation of HF, thereby enhancing the thermal stability of the electrolyte (no color change after two weeks of storage at 60 °C).48 TTFP undergoes a deoxygenation reaction at high voltage (4.6 V vs. Li/Li+), generating stable tris(2,2,2-trifluoroethyl)phosphate (TTFP). Some products further react with solvents to create bis(2,2,2-trifluoroethyl)phosphate, which binds to the cathode surface through P–O-TM bonds to suppress structural damage.49TTFP has both flame-retardant and interface control functions in lithium sulfur batteries. Its decomposition products promote dense lithium deposition, while the formed LiF-rich SEI reduces the risk of lithium dendrite growth.50 The screening of fluorinated phosphate ester additives requires three elements: (1) the oxidation potential is lower than that of ethylene carbonate (VC); (2) the reduction potential is lower than that of ethylene carbonate (EC); (3) the reactivity with HF/LiF is close to or better than that of tris(trimethylsilyl)phosphite (TMSP). For instance, tris(1,1,1,3,3,3-hexafluoro-2-propyl)phosphate can serve as a promising additive for high-performance cathode electrolyte interphase (CEI) formation, owing to its high fluorine content, which fulfills the aforementioned requirements.51,52 Fluorine-containing additives also enhance electrode stability by forming a LiF-rich interface layer. For instance, tris(trimethylsilyl)phosphite (TMSPi) reacts with HF (P(OSiCH3)3 + HF → P(OSiF)3), and the product further reacts with LiF to form fluorosilicates, effectively removing HF from the electrolyte and reducing the dissolution of transition metals.53 The optimized addition amount of TTFP (1–5 wt%) can significantly enhance the battery cycle performance. For instance, the capacity retention rate of LiMn2O4/graphite batteries at 4.7 V high voltage is improved.33,54
Fig. 5a shows the effect of fluorine-containing additive LiPO2F2 as a functional additive. It effectively inhibits the structural degradation of the NCM523 cathode and the growth of lithium dendrites by forming a CEI rich in Li2CO3 and P–O species on the cathode (NCM523) surface, and constructing an SEI of LiF, Li2O, and P–O species on the lithium anode surface. This mechanism enables the Li‖NCM523 battery to exhibit excellent cycling stability and rate capability (with a capacity retention rate of 72.5%) in the extreme temperature range of −40 to 90 °C. Fluorinated additives, by introducing an inorganic-enriched interface layer, not only enhance the stability of the electrode/electrolyte interface but also reduce the energy barrier for lithium ion transport, thereby solving the contradiction between kinetic limitations at low temperatures and electrolyte decomposition at high temperatures.55 Zhao et al.56 studied the stabilizing effect of a fluorinated electrolyte (FEC/EMC/TFA solvent + LiDFOB additive) on 4.6 V high-voltage graphite/NCM811 lithium batteries. As shown in Fig. 5b, the fluorinated electrolyte can form a stable electrode electrolyte interface rich in inorganic substances (LiF/Li2CO3/LixPOyFz, etc.) on the cathode and anode surfaces. The LiDFOB additive preferentially decomposes to form B/F compounds, which work together with the fluorinated solvent to form a dense interface layer with high ionic conductivity, effectively suppressing electrolyte oxidation decomposition and transition metal leaching, thereby improving battery cycle stability and rate performance.56 An et al.57 studied the mechanism of fluoride additives PFPA and FEC in lithium batteries. Fig. 5c (top) shows that the symmetrical molecular structure of PFPA contains 10 fluorine atoms and has a low HOMO energy level, enhancing the high-pressure stability of the electrolyte. Fig. 5c (bottom) illustrates that the instability of the SEI layer without additives leads to lithium dendrites and short circuits. Additives rapidly form a thin and uniform SEI layer rich in fluorine and organic matter, which suppresses dendrites at the cathode and anode, achieving 20C ultra-fast charging and 400 long-cycles.57 Tu et al.58 investigated the application of fluorine-containing additive difluoroethylene carbonate (DFEC) in high-voltage lithium metal batteries. By preferentially decomposing it to form a solid electrolyte interface (SEI) rich in LiF and a cathode electrolyte interface (CEI), it effectively inhibited lithium dendrite growth and transition metal dissolution (Fig. 5d). DFEC exhibits a lower LUMO energy level and higher oxidation stability, making it suitable for 4.5 V high-voltage systems. Experiments show that the full battery capacity retention rate of Li/NCM811 with 5% DFEC added reaches 75% after 200 cycles, which is superior to that with FEC additive.58 Chen et al.59 optimized the graphite anode interface and improved the rate performance of lithium batteries by using a novel fluorinated additive 1H,1H,2H,2H-perfluorooctyltrimethoxysilane (FTMS). The mechanism of action is shown in Fig. 5e. FTMS preferentially adsorbs and reduces on the graphite surface, breaking the Si–C bond to generate fluorinated organic anions and silicon oxygen radicals, forming fluorinated organic lithium salts (improving SEI's ion conductivity and flexibility) and silicon-based polymers (enhancing SEI's stability), respectively, to jointly construct a highly robust SEI, accelerating lithium ion transport and suppressing electrolyte decomposition, increasing the capacity to 229 mAh g−1 at 2C rate.59
 | ||
| Fig. 5 (a) Fluorination additive enhances electrode stability and reduces lithium ion transport energy barrier by constructing an inorganic interface layer, thereby improving the cycling performance of the battery at −40 to 90 °C.55 Copyright 2024 Elsevier B.V. (b) Schematic diagram of the mechanism by which fluorinated electrolyte forms an inorganic-rich electrode interface on the surface of the graphite anode and the NCM811 cathode at 4.6 V.56 Copyright 2022 Elsevier B.V. (c) (above) DFT calculation results of HOMO and LUMO energy levels of EC, EMC solvent molecules, and FEC, PFPA additives. (below) Schematic diagram of lithium dendrite growth and battery short circuit under reference electrolyte.57 Copyright 2023 Wiley-VCH GmbH. (d) The mechanism diagram shows DFEC enabling a LiF-rich SEI to suppress dendrites at the anode and a thin CEI to inhibit transition metal dissolution at the cathode.58 Copyright 2023 American Chemical Society. (e) Schematic diagram of the SEI construction mechanism of fluorinated organic lithium salts and silicon polymers formed by FTMS molecular reduction fracture.59 Copyright 2023 American Chemical Society. | ||
2.5 Nitrogen-containing additive
Nitrogen-containing additives significantly enhance the overall performance of lithium-ion batteries by optimizing the electrode/electrolyte interface chemistry and suppressing side reactions. It has been found that the synergistic effect of nitrogen-containing phosphate esters and traditional carbonate additives can improve the formation of SEI and CEI, enhance coulombic efficiency, and suppress impedance growth during cycling. This synergistic effect originates from the preferential decomposition of nitrogen-containing additives to form a nitrogen-rich interface layer, where components such as Li3N and LiNxOy can enhance the interface ion conductivity and stabilize the lithium metal anode, especially in solid-state batteries, which can effectively suppress dendrite growth and achieve uniform lithium deposition/stripping.60 Nitrogen-containing compounds, such as 1,3-dimethyl-1H-imidazole-2(3H)-one (DMIO), preferentially oxidize at the high-voltage cathode surface and undergo reduction polymerization at the anode surface. The decomposition products can generate nitrogen-containing inorganic compounds (such as Li3N), promoting the formation of SEI and CEI rich in inorganic substances, effectively suppressing side reactions at the electrode/electrolyte interface. In addition, the lone pair electrons of nitrogen atoms can coordinate to capture the acidic by-products (such as PF5 and HF) produced by the decomposition of LiPF6, delaying the hydrolysis of LiPF6 and interface parasitic reactions, thereby improving the cycling stability of high-voltage lithium metal batteries.61 Cyanosiloxane additives, such as TDSTCN, form a CEI layer rich in cyano groups on the surface of the cathode, chelating transition metal ions (such as Ni2+, Co3+) and inhibiting their dissolution. Meanwhile, it can also eliminate HF and inhibit graphite exfoliation.62 Methylacrylamide generates a Li3N interface layer through a three-step decomposition mechanism. The uniform CEI formed in LiNi0.5Mn1.5O4 cells results in a capacity retention rate of 81.2% after 200 cycles at 4.9 V high-voltage, which is 26% higher than that of the base electrolyte.63 In high-voltage cathode systems (such as LiNi0.5Mn1.5O4), nitrogen-containing additives like LiNO3 are electrochemically reduced to produce products such as Li3N and LiNO2, forming an interface layer with high ionic conductivity. Toe-sims analysis indicated that the LiNO3 modified separator formed nitrogen-containing species (with a relative nitrogen content of up to 2.9 ± 0.1%) on the surface of the copper electrode, including reduction products such as NO2− and NO−, significantly reducing interfacial polarization and enhancing cycling stability.64 In silicon-based anodes, LiNO3 induces the formation of a LiF-rich SEI and inhibits salt decomposition and reduces transition metal dissolution by altering the LiPF6 solvation structure.65The molecular structure design further expands the functionality of nitrogen-containing additives. For example, N,N′-carbonyldiimidazole (CDI) is preferentially reduced at the graphite anode due to its low LUMO energy level (−1.58 eV vs. Li/Li+), forming a dense SEI rich in nitrogen species, while promoting the decomposition of PF6− into LiF and enhancing interfacial stability. The electrolyte containing CDI increased the capacity retention of Gr‖LiFePO4 pouch batteries by 18% after 1000 cycles at 45 °C.66 Pyrazine additives, such as 2-fluoropyrazine (2-FP), regulate the composition of CEI through molecular design. The nitrogen atom in the pyrazine ring cooperates with the fluorine atom to create a dense and uniform interface film on the surface of the NCM90 cathode, which inhibits the oxidative decomposition of the electrolyte. The electrolyte containing 0.2% 2-FP resulted in a capacity retention rate of 82.1% for NCM90/graphite full cell after 200 cycles at 4.5 V high voltage, which was significantly improved compared to the untreated system (72.3%).67 Benzotriazole derivatives, such as benzotriazole sulfobetaine, function as multifunctional additives. The sulfonic acid group enhances Li+ dissociation through high dipole moment, while the free nitrogen atom acts as a Lewis base to form a stable complex with PF5, inhibiting electrolyte decomposition. This type of additive can significantly reduce interfacial impedance, improve the coulombic efficiency (over 99%), and cycling reversibility of graphite anodes.68
Fig. 6a shows the key mechanism of action of the nitrogen-containing additive TPPO (Triphenylphosphine Oxide) in lithium batteries. TPPO inhibits lithium dendrite growth and achieves uniform lithium deposition by promoting the dissolution of LiNO3 to form a Li3N-rich SEI. Meanwhile, TPPO builds a stable CEI layer in the cathode oxidation and chelates PF5 to reduce HF generation, thereby enhancing the cycling stability of the battery over a wide temperature range (−15 to 70 °C).55 Nitrogen-based electrolyte additives (such as LiNO3, hexane trimethylnitrile, etc.) can enhance the high voltage stability and cycle life of lithium-ion batteries by promoting stable SEI/CEI formation, inhibiting dendrite growth, and removing harmful by-products (such as HF). Fig. 6b compares the electrochemical performance advantages of nitrogen-containing additive electrolytes, such as higher conductivity and lower SEI's resistance. Fig. 6b also shows the molecular structures of three typical nitrogen-based additives, elucidating their chemical design basis.60 Nitrogen-containing additives act as redox shuttle agents (Fig. 6c), consuming excess current through reversible electron transfer reactions (such as TEMPO oxidation to TEMPO+) under overcharge conditions, stabilizing the cathode potential at a specific voltage (3.5–3.8 V vs. Li+/Li), thereby suppressing electrolyte decomposition and thermal runaway, and improving battery safety. The mechanism relies on the nitrogen atoms in the molecular structure providing redox-active centers, but requires a balance between high oxidation potential and electrochemical stability.69Fig. 6d shows that the existence of lone pair electrons of nitrogen atoms in the isocyanate group of the nitrogen-containing additive, TDI, inhibits the formation of HF and H2O, thus forming a thin and dense CEI rich in nitrogen.70Fig. 6e shows the dual action mechanism of the nitrogen-based additive LiHMDS (lithium hexamethyldisilazane). Firstly, it preferentially oxidizes on the surface of the NMC811 cathode to generate free radical anions, and then captures the protons of the carbonate solvent and polymerizes to form a stable CEI. At the same time, its strong alkalinity can efficiently remove HF and H2O impurities in the electrolyte, significantly inhibiting the dissolution of transition metals and widening the voltage window to 4.5 V.70Fig. 6f shows that nitride additives attack Si–N bonds through Lewis basic nitrogen atoms, reacting chemically with H2O/HF in the electrolyte, effectively inhibiting LiPF6 hydrolysis and HF generation, thereby improving the high-temperature stability and cycle life of lithium batteries.71 It can be seen that nitrogen-containing additives mainly improve battery performance through the following mechanisms: (1) forming nitrogen-containing compounds (such as nitrides) to enhance the stability and protective effect of SEI/CEI; (2) using the Lewis base properties of nitrogen atoms to neutralize acidic byproducts produced by electrolyte decomposition (such as PF5, HF); (3) enhancing the stability of the electrode/electrolyte interface through the synergistic effect of specific functional groups, such as cyanide and sulfonic acid groups. These mechanisms provide effective electrolyte engineering solutions for the development of high-voltage, high-nickel cathode and lithium metal anode systems.
 | ||
| Fig. 6 (a) Schematic diagram and experimental results comparison of the mechanism of nitrogen-containing additive TPPO regulating lithium deposition morphology and interface chemistry.55 Copyright 2024 Elsevier B.V. (b) Comparison of electrochemical performance (conductivity, SEI's resistance, cycling stability) of nitrogen-containing additive electrolytes, as well as the molecular structures of LiNO3, N-phenylmaleimide, and butanitrile.60 Copyright 2024 Springer Nature. (c) Chemical structures of six nitrogen-containing redox shuttle additives, including TEMPO, 4-cyano-TEMPO, MPT, EPT, 3-chloro-EPT, and IPT.69 Copyright 2021 Wiley-VCH GmbH. (d) Schematic diagram of the benefits of nitrogen-containing additive TDI in improving the electrochemical performance of NMC cathodes.70 (e) Schematic diagram of the oxidative polymerization mechanism of nitrogen-based additive LiHMDS on the cathode surface of NMC811 and the pathway for removing HF/H2O impurities.70 (d and e) Copyright 2025 Wiley-VCH GmbH. (f) Nitride additives capture H2O and HF in electrolytes through chemical bond-breaking mechanisms.71 Copyright 2024 Wiley-VCH GmbH. | ||
2.6 Other salts/compounds
Salt additives containing cesium (Cs+) or rubidium (Rb+), such as CsPF6 and RbPF6, regulate lithium deposition behavior through a self-healing electrostatic shielding (SHES) mechanism. At appropriate concentrations, Cs+ or Rb+ accumulates at the tip of the lithium metal anode, forming a local electrostatic repulsion layer that forces Li+ to deposit uniformly in adjacent areas, thereby inhibiting dendrite growth and improving cycling stability.72Lithium nitrate (LiNO3) is a key additive for improving the performance of lithium metal batteries, but its solubility is low in carbonate-based electrolytes. The dissolution of LiNO3 can be promoted and the solvation structure of Li+ can be altered by introducing carrier solvents (such as sulfones) or co-additives (such as tris (pentafluorophenyl) borane, TPFPB). It can promote the dissolution of LiNO3 and alter the solvation structure of Li+. Among them, NO3− enters the Li+ primary solvation sheath layer, preferentially reducing to generate SEI rich in Li3N, enhancing interfacial ion conductivity and suppressing side reactions.73 In addition, the dual salt additive strategy (such as LiTFA/LiNO3 combination) optimizes the solvation structure through synergistic effects: TFA− (trifluoroacetate) and NO3− both strongly coordinate with Li+ (peak radial distribution function at 1.65 Å), reducing the coordination number of carbonate solvents, lowering the desolvation energy barrier, and improving the mechanical stability and electrochemical window of SEI/CEI.74
Magnesium salts (such as Mg(NO3)2) alter the Li+ solvation environment through cation displacement. The coordination ability between Mg2+ and solvent (DOL/DME) is stronger than that of Li+, reducing the number of free solvent molecules and promoting the entry of NO3− into the solvation sheath of Li+. This structure induces the formation of a dense SEI rich in LiF/Li3N, enhancing the interfacial kinetic stability.75
Fig. 7a systematically presents the multi-functional roles of different elements in battery components (such as additives, electrodes, and electrolytes) in the form of a periodic table, highlighting how the synergy of elements addresses the core challenges of high-energy-density batteries by regulating interfacial chemistry and ion transport.70 Huang et al.76 found that introducing a trace amount of KI (0.01 M) as an additive into ether-based (LiTFSI/DOL-DME) and ester-based (LiPF6/EC-DMC) electrolytes could significantly enhance the performance of lithium metal batteries. Its mechanism of action involves two aspects (Fig. 7b). Firstly, K+ forms an electrostatic shielding layer at the lithium deposition tip, guiding the uniform deposition of lithium ions and suppressing dendrites. The second is the I−/I3− REDOX reaction to the dead lithium produced during the reversible repair cycle. This additive enables the coulombic efficiency of the Cu-mesh@Ag‖Li half-cell to reach up to 98.8% after 200 cycles and significantly enhances the cycle stability of the Li/LiFePO4 and Li/NCM811 full cells.76 Zhang et al.77 found that adding multivalent calcium salt (Ca(TFSI)2) to the silicon anode of lithium batteries can significantly improve the lifespan of the electrolyte (GenFC). The mechanism is as shown in Fig. 7c, calcium ions react with fluoride ions in the electrolyte to form a nanocrystalline CaF2 layer (approximately 100 nm thick), which tightly covers silicon particles and effectively blocks electrolyte side reactions. The conventional electrolyte (GenF) forms a porous organic–inorganic mixed SEI, while the magnesium salt additive (GenFM) mainly forms a thinner MgF2 layer, accompanied by Mg embedded in the silicon matrix. Both have weaker protective effects than the CaF2 layer.77 Ding et al.78 proposed a bifunctional selenium-substituted polymer additive, PTA-Se (Fig. 7d), to enhance the performance of lithium sulfur batteries through a synergistic curing and catalytic mechanism at the cathode. On the cathode side, selenium atoms accelerate the conversion kinetics of polysulfides and solidify in situ into macromolecular polymers, suppressing shuttle effects. On the anode side, they promote the formation of a stable SEI rich in LiF/SeO3−, achieving uniform lithium deposition (symmetrical battery life >4500 h).78 Fu et al.79 investigated the application of potassium selenocyanate (KSeCN) as a bifunctive electrolyte additive in high-voltage lithium//cobalt oxide lithium (Li‖LiCoO2) batteries. Through the synergistic effect of its –Se and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups, KSeCN constructed stable solid-state electrolyte interface (SEI) and cathode electrolyte interface (CEI) films on the surfaces of lithium metal anodes and LiCoO2 cathodes, respectively (Fig. 7e), effectively inhibiting lithium dendrite growth and cathode structural degradation. The cycle performance of the battery at a high voltage of 4.6 V has been significantly improved.79
N groups, KSeCN constructed stable solid-state electrolyte interface (SEI) and cathode electrolyte interface (CEI) films on the surfaces of lithium metal anodes and LiCoO2 cathodes, respectively (Fig. 7e), effectively inhibiting lithium dendrite growth and cathode structural degradation. The cycle performance of the battery at a high voltage of 4.6 V has been significantly improved.79
 | ||
Fig. 7 (a) The periodic table of elements and their functional roles used as electrolyte additives for lithium batteries.70 Copyright 2021 Wiley-VCH GmbH. (b) A schematic diagram comparing the mechanism of lithium deposition behavior in basic electrolytes and KI-containing electrolytes is shown on the right, where K+ electrostatic shielding and iodine oxidation–reduction synergistically suppress dendrites and repair dead lithium.76 Copyright 2022 Elsevier B.V. (c) Compare the differences in the structure and composition of the SEI formed on the surface of the silicon anode by three electrolytes (GenF, GenFM, GenFC).77 Copyright 2021 Wiley-VCH GmbH. (d) Schematic illustration of the dual-functional PTA-Se additive synchronously regulating the polysulfide conversion pathway in the sulfur cathode and SEI formation on the lithium anode.78 Copyright 2024 Elsevier B.V. (e) Schematic diagram of the mechanism of KSeCN stabilizing the interface between the lithium anode and the LCO cathode through the synergy of –Se/–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups.79 Copyright 2022 American Chemical Society. N groups.79 Copyright 2022 American Chemical Society. | ||
3 Functional additives
3.1 SEI/CEI forming additives
Organic electrolyte additives form stable SEI or CEI on the electrode surface through preferential reduction or oxidation, significantly improving the cycling stability and interface ion transport efficiency of lithium-ion batteries. According to the chemical structure, it can be mainly divided into the following categories. In terms of carbon-based additives, ethylene carbonate (EC) and fluorinated ethylene carbonate (FEC) are the most widely used. VC, as an anodic protective agent, creates an SEI rich in polycarbonate on the surface of graphite, inhibiting solvent co-embedding and graphite stripping, while improving the first cycle coulombic efficiency.19 FEC is particularly important for silicon-based anodes, as its reduction products (such as LiF) form a dense inorganic layer, effectively alleviating the volume expansion of the silicon and improving low-temperature performance. However, excessive FEC (>5 wt%) may lead to capacity attenuation due to increased interface impedance. Sulfur-based additives are represented by ethylene sulfate (DTD) and 1,3-propanesulfonic acid lactone. DTD triggers a reduction reaction through electronegative sulfur atoms, generating a stable CEI of sulfur-containing oxides (such as Li2SO3 and ROSO2Li) at the cathode interface, inhibiting transition metal dissolution and enhancing oxidation stability (up to 6 V vs. Li+/Li).19 In high-temperature (55 °C) cycles, the synergistic use of DTD and propanesultone (PES) can reduce gas production and capacity degradation.80 Nitrogen-based additive succinic acid optimizes SEI/CEI performance by enhancing interfacial stability and ionic conductivity. Its cyanide functional group promotes the formation of a high ion conductivity interfacial layer, which is suitable for high-pressure lithium cobalt oxide (LCO) systems.19 In S/N heterocyclic additives, DTD inhibits electrode pulverization in silicon/carbon composite anodes by forming a thin SEI containing sulfates.20 Tris(trimethylsilane)phosphate (TTSPi) alleviates LiPF6 hydrolysis by clearing HF and water, while creating a protective CEI on the surface of high-nickel cathodes (such as NMC811).81Regarding the mechanism of action, taking DTD as an example, its reduction pathway consists of three steps: the first step is to lose electrons and form a divalent anion. The second step is to induce S–O bond reduction through the metastable ring structure. The third step is to generate inorganic Li2SO3 components as efficient passivators to suppress the continuous decomposition of electrolytes.19 This type of additive provides a key solution for high-voltage, wide temperature range batteries by regulating the interfacial chemical composition and mechanical strength.
Chen et al.59 reported a novel electrolyte additive, FTMS (1H,1H,2H,2H-perfluorooctane trimethoxysilane), for constructing a solid electrolyte interface film (SEI) with high stability and high mechanical strength on the surface of graphite anodes. Research shows that adding 2% FTMS can significantly enhance the cycle stability and rate performance of lithium-ion batteries. The capacity retention rate increases from 77.6% to 91.2% (0.5C, 100 weeks), and the capacity at 2C rises from 86 to 229 mAh g−1. Through various characterization methods (such as XPS, TEM, TOF-SIMS, etc.) combined with theoretical calculations, it was confirmed that FTMS preferentially reduces to form fluorinated organolithium salts and silicon-containing polymers on the graphite surface, jointly constituting a robust SEI layer with both high ionic conductivity and flexibility, effectively promoting lithium ion migration and suppressing side reactions (Fig. 8a).59 Kim et al.5 elucidated the mechanism by which additives enhance the performance of lithium batteries by forming a stable SEI/CEI, removing harmful species such as HF, and inhibiting gas production. Fig. 8b specifically reveals the mechanism of fluorinated ethylene carbonate (FEC) on the surface of silicon-based anodes. Its reduction decomposition forms a composite SEI containing LiF, LixSiOy, and insoluble polycarbonate/polyolefin. Compared with traditional EC-based electrolytes, it can effectively buffer the volume expansion of silicon and maintain interface integrity, thereby significantly improving cycling stability.5 Li et al.82 systematically investigated the effect of using fluoroethylene carbonate (FEC) as an electrolyte additive on the formation of the solid electrolyte interface (SEI) on silicon–carbon composite anodes through various characterization methods such as solid-state nuclear magnetic resonance (ssNMR), X-ray photoelectron spectroscopy (XPS), and X-ray light emission electron microscopy (X-PEEM). The results show that FEC promotes the formation of a dense and stable SEI layer (Fig. 8c), which is rich in LiF and can effectively inhibit the hydrolysis and side reactions of LiPF6, reduce the continuous decomposition of the electrolyte, and alleviate the volume expansion and breakage of silicon particles, thereby significantly improving the cycling stability of the battery.82 Zheng et al.83 proposed phenyl vinyl sulfone (PVS) as an electrolyte additive to construct a protective CEI on the surface of lithium-rich layered cathodes (Li(Li0.2Mn0.54Ni0.13Co0.13)O2) through its special molecular structure (double bond preferential oxidation, benzene ring enhanced chemical stability, sulfur improved ionic conductivity). Therefore, it significantly enhances the cycle stability (with a volume retention rate of 80% after 240 cycles). The SEM/TEM results confirmed (Fig. 8d) that when PVS was not added, the cathode particles were covered by uneven deposits and their size was reduced, while the electrolyte containing PVS maintained the original morphology of the particles and formed a uniform thin CEI, effectively inhibiting the decomposition of the electrolyte and the dissolution of the cathode.83
 | ||
| Fig. 8 (a) The addition of 2% fluorosilane (FTMS) to construct a fluorinated silicon interface significantly improves the rate performance of graphite anodes (2C capacity increased from 86 to 229 mAh g−1), solving the problem of slow kinetics.59 Copyright 2023 American Chemical Society. (b) Fluorinated ethylene carbonate forms a stable solid electrolyte interphase mechanism containing LiF and polymer on the surface of silicon-based anodes.5 Copyright 2020 American Chemical Society. (c) FEC additives form a dense LiF SEI, which inhibits silicon cracking and blocks the migration of LiPF6 hydrolysis products, improving the cycling stability of lithium batteries.82 Copyright 2019 American Chemical Society. (d) After cycling in the PVS electrolyte, the cathode particles maintained a complete morphology and uniform CEI, while the blank group showed corrosive deposition and size shrinkage.83 Copyright 2016 American Chemical Society. | ||
3.2 Overcharge protection additives
Overcharge protection additives are key components for improving the safety of lithium-ion batteries, mainly divided into two categories. Redox shuttles and electropolymerized additives. Redox shuttle agents undergo reversible redox reactions between the cathode and anode, consuming overcharging current and suppressing the continuous rise of voltage. Typical shuttle agents include ferrocene derivatives, dihydrophenazine systems, dimethoxybenzene derivatives, 2,5-di-tert-butyl-1,4-dimethoxybenzene (DDB) derivatives, 2,2,6,6-tetramethylpiperidine nitrogen oxides (TEMPO) and their derivatives, triphenylamine derivatives, etc.84,85 These additives must meet three core standards: (1) the oxidation potential should be slightly higher than the charging cut-off potential of the cathode, but not exceed the electrochemical window of the electrolyte; (2) the redox mechanism is highly reversible; (3) form stable free radical cations and consume overcharge current through diffusion.69Electropolymerization additives undergo electrochemical polymerization at high potentials, forming an insulating polymer layer on the surface of the cathode or separator, thereby blocking ion transport or triggering internal short circuits to terminate charging. For example, biphenyl, xylene, cyclohexylbenzene, and their derivatives release gas to activate current interruption devices during overcharging, while pyrrole, thiophene, and substituted aromatic compounds polymerize on the cathode surface to create a protective layer.86 The limitation of such additives is that the polymerization process is irreversible and may cause an increase in internal pressure of the battery due to the formation of a polymer layer.87 In recent years, new shuttle agents such as 1,4-bis[bis(1-methylethyl)phosphonyl]-2,5-dimethoxybenzene (BPDB) and tetraethyl-2,5-di-tert-butyl-1,4-phenylene diphosphate (TEDBPDP) have been developed to meet the demand for high-voltage cathode materials (such as >4.5 V), with oxidation potentials of 4.5 V and 4.75 V (vs. Li/Li+), respectively, significantly improving the overcharge tolerance of high-voltage batteries.84 In addition, UV-Vis spectroscopy and electron paramagnetic resonance techniques can be used to quickly evaluate the stability of shuttle free radical cations, providing an efficient method for screening high-performance additives.88
Fig. 9a visually illustrates the core mechanism of redox shuttle agents in overcharge protection. When the battery is overcharged, the additive molecules (RS) are oxidized to free radical cations (RS+) at the cathode interface, which inhibits the oxidative decomposition of the electrolyte. RS+ then diffuses to the anode and is reduced to the original molecule (RS), forming a continuous oxidation diffusion reduction cycle. This mechanism limits the cathode potential to the characteristic oxidation potential of the additive (such as about 4.0 V in the LiFePO4 system) by consuming overcharge current, thereby avoiding electrode structure collapse, dendrite growth, and thermal runaway. Taking graphite anode and carbon-coated lithium iron phosphate cathode as examples, the key role of the dynamic cycling of shuttle agent molecules between the electrodes in maintaining the voltage safety window is emphasized in Fig. 9a.69Fig. 9b (left) shows that the bifunctional additive PFPTFBB exhibits a highly reversible oxidation–reduction peak at 4.43 V (vs. Li/Li+) on a platinum electrode (conventional electrolyte), significantly higher than conventional shuttle agents such as DDB (3.9 V), meeting the overcharge protection requirements for high-voltage cathodes (>4.2 V) and avoiding electrolyte decomposition. Fig. 9b (right) shows that the NCA/graphite full cell containing 5 wt% PFPTFBB maintains stable capacity after 170 cycles under C/5, 55 °C, and 100% overcharge conditions. Its boronic ester group (anion receptor) enhances interfacial stability, inhibits high-temperature side reactions, and provides long-term high-pressure overcharge protection.69 Weng et al.89 developed a novel overcharge protection additive, TDTN (Fig. 9c), whose axisymmetric rigid skeleton locks methoxy based on the aromatic ring plane in the oxidized state, forming a high energy barrier (>0.13 eV) to maintain symmetric configuration A. This configuration evenly distributes positive charges between two methoxy groups (each + 0.22 eV), inhibits demethylation reactions, and significantly improves the stability of free radical cations. Compared to the charge localized asymmetric configuration B (single methoxy + 0.44), TDTN achieves over 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100% overcharge cycles (C/2 rate) in LFP/LTO batteries, with a solubility of 0.4 M, providing a new strategy for the design of highly stable electrolyte additives.89 Choi et al.90 found that Redox Shuttles, as electrolyte additives, can prevent overcharging of lithium batteries. The mechanism is that additive molecules oxidize at the cathode to form free radical cations (S+), which migrate to the anode through the electrolyte and are reduced (S+ + e− → S). Through reversible cycling, external charges are transferred to the interior of the battery, avoiding excessive lithium deintercalation at the electrode. Fig. 9d shows the electrochemical behavior of the first lithium insertion process of graphite/Li half-cells in organic electrolytes containing ionic liquids (IL) through the differential capacity (dQ/dV) curve, revealing the reduction stability of IL at the anode interface.90 Casselman et al.91 investigated the failure mechanism of phenothiazine based redox shuttle agents in lithium-ion batteries. Fig. 9e shows its overcharge protection mechanism. Neutral molecules are oxidized to free radical cations at the cathode, diffuse to the anode for reduction and regeneration, and form a cycle to limit voltage (black path). Meanwhile, Fig. 9e reveals that free radical cations decompose through fragmentation, nucleophilic attack, or polymerization pathways (red pathway), leading to additive failure. Experimental results have shown that the substituent structure directly affects the stability of C–N bonds (such as easy removal of alkyl groups) or reduction sensitivity (such as C–Br bond cleavage), providing a key basis for designing long-lasting electrolyte additives.91 Krauss et al.92 used a four-electrode device to elucidate the selective passivation mechanism of electrolyte decomposition and overcharge protection additives (ferrocene ions, Fc+) in the SEI of lithium batteries at near working potential (0.8 V vs. Li+/Li). Fig. 9f reveals the key mechanism: in the early stage of SEI formation (high porosity), Fc+ diffuses to the electrode-SEI interface and is rapidly reduced. In the long-term formation of dense SEI, electrons penetrate the SEI and dominate the reaction, causing the Fc+ reduction site to transfer to the SEI-electrolyte interface (Scenario B). This dynamic transformation explains the differential passivation of SEI on electrolyte decomposition (electron transport limitation) and Fc+ reduction (molecular transport limitation), providing a theoretical basis for optimizing additive design.92 Feng et al.93 investigated a novel bifunctional electrolyte additive, FPPN (pentafluorophenoxy cyclotriphosphazene), for overcharge protection in 5 V-class high-voltage lithium-ion batteries. Fig. 9g demonstrates by cyclic voltammetry (CV) that FPPN undergoes electropolymerization (oxidation peak) at 5.05 V (vs. Li/Li+), which is higher than the charging cut-off voltage (4.8 V) of the 5 V cathode (such as LiNi0.5Mn1.5O4), but lower than the decomposition potential of the carbonate electrolyte (5.3 V). When overcharged, FPPN polymerizes on the surface of the cathode to form a protective layer, consuming the overcharge current and generating H2 to trigger the safety valve, while reducing the flammability of the electrolyte (SET test), without affecting the normal cycle performance of the battery.93
100% overcharge cycles (C/2 rate) in LFP/LTO batteries, with a solubility of 0.4 M, providing a new strategy for the design of highly stable electrolyte additives.89 Choi et al.90 found that Redox Shuttles, as electrolyte additives, can prevent overcharging of lithium batteries. The mechanism is that additive molecules oxidize at the cathode to form free radical cations (S+), which migrate to the anode through the electrolyte and are reduced (S+ + e− → S). Through reversible cycling, external charges are transferred to the interior of the battery, avoiding excessive lithium deintercalation at the electrode. Fig. 9d shows the electrochemical behavior of the first lithium insertion process of graphite/Li half-cells in organic electrolytes containing ionic liquids (IL) through the differential capacity (dQ/dV) curve, revealing the reduction stability of IL at the anode interface.90 Casselman et al.91 investigated the failure mechanism of phenothiazine based redox shuttle agents in lithium-ion batteries. Fig. 9e shows its overcharge protection mechanism. Neutral molecules are oxidized to free radical cations at the cathode, diffuse to the anode for reduction and regeneration, and form a cycle to limit voltage (black path). Meanwhile, Fig. 9e reveals that free radical cations decompose through fragmentation, nucleophilic attack, or polymerization pathways (red pathway), leading to additive failure. Experimental results have shown that the substituent structure directly affects the stability of C–N bonds (such as easy removal of alkyl groups) or reduction sensitivity (such as C–Br bond cleavage), providing a key basis for designing long-lasting electrolyte additives.91 Krauss et al.92 used a four-electrode device to elucidate the selective passivation mechanism of electrolyte decomposition and overcharge protection additives (ferrocene ions, Fc+) in the SEI of lithium batteries at near working potential (0.8 V vs. Li+/Li). Fig. 9f reveals the key mechanism: in the early stage of SEI formation (high porosity), Fc+ diffuses to the electrode-SEI interface and is rapidly reduced. In the long-term formation of dense SEI, electrons penetrate the SEI and dominate the reaction, causing the Fc+ reduction site to transfer to the SEI-electrolyte interface (Scenario B). This dynamic transformation explains the differential passivation of SEI on electrolyte decomposition (electron transport limitation) and Fc+ reduction (molecular transport limitation), providing a theoretical basis for optimizing additive design.92 Feng et al.93 investigated a novel bifunctional electrolyte additive, FPPN (pentafluorophenoxy cyclotriphosphazene), for overcharge protection in 5 V-class high-voltage lithium-ion batteries. Fig. 9g demonstrates by cyclic voltammetry (CV) that FPPN undergoes electropolymerization (oxidation peak) at 5.05 V (vs. Li/Li+), which is higher than the charging cut-off voltage (4.8 V) of the 5 V cathode (such as LiNi0.5Mn1.5O4), but lower than the decomposition potential of the carbonate electrolyte (5.3 V). When overcharged, FPPN polymerizes on the surface of the cathode to form a protective layer, consuming the overcharge current and generating H2 to trigger the safety valve, while reducing the flammability of the electrolyte (SET test), without affecting the normal cycle performance of the battery.93
 | ||
Fig. 9 (a) Schematic diagram of the mechanism by which the redox shuttle consumes excess current through the oxidation–diffusion–reduction cycle during overcharging.69 (b) (left) Cyclic voltammetry diagram of highly reversible REDOX characteristics of PFPTFBB at 4.43 V; (right) stability of 170 overcharge cycles of LiNi0.8Co0.15Al0.05O2/graphite battery containing PFPTFBB at 55 °C.69 (a and b) Copyright 2021 Wiley-VCH GmbH. (c) (left) The rotational potential energy curve and energy barrier difference of methoxy groups between TDTN neutral molecules and free radical cations; (right) structure, charge distribution, and relative energy of TDTN+ symmetric configuration A and asymmetric configuration B.89 Copyright 2016 Wiley-VCH GmbH. (d) The differential capacity (dQ/dV) and voltage relationship during the first lithium insertion process of graphite/Li half-cells containing DMC/ionic liquid (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7).90 Copyright 2012 Wiley-VCH GmbH. (e) The normal overcharge protection cycle (black pathway) and potential decomposition pathway (red pathway) of redox shuttle agents.91 Copyright 2015 The Royal Society of Chemistry. (f) Schematic diagram of the concentration gradient and dominant reduction mechanism of organic carbonate molecules (M), electrons (e−), and ferrocene ions (Fc+) in the early stage (high porosity) and long-term stage (dense) of SEI formation.92 Copyright 2024 American Chemical Society. (g) Comparison of CV curves of LiNi0.5Mn1.5O4 cathodes with/without FPPN.93 Copyright 2016 The Royal Society of Chemistry. 7).90 Copyright 2012 Wiley-VCH GmbH. (e) The normal overcharge protection cycle (black pathway) and potential decomposition pathway (red pathway) of redox shuttle agents.91 Copyright 2015 The Royal Society of Chemistry. (f) Schematic diagram of the concentration gradient and dominant reduction mechanism of organic carbonate molecules (M), electrons (e−), and ferrocene ions (Fc+) in the early stage (high porosity) and long-term stage (dense) of SEI formation.92 Copyright 2024 American Chemical Society. (g) Comparison of CV curves of LiNi0.5Mn1.5O4 cathodes with/without FPPN.93 Copyright 2016 The Royal Society of Chemistry. | ||
3.3 Flame-retardants
Organophosphorus compounds are the most commonly used flame-retardants in lithium-ion battery electrolytes, mainly including phosphate esters, phosphonates, hypophosphite esters, and cyclic phosphazenes. Its mechanism of action is to thermally decompose at high temperatures to produce phosphorus-based radicals (such as PO˙, HPO˙), which can capture hydrogen radicals (H˙) and hydroxyl radicals (OH˙) in the combustion chain reaction, terminate the exothermic reaction, and thus suppress electrolyte combustion.94 Phosphate ester flame-retardants usually require a high addition amount (>15 wt%) to achieve non-flammability of the electrolyte. However, this high addition amount can seriously damage the SEI of the graphite anode, leading to a decrease in the cycling stability of the battery. For example, although electrolytes based on triethyl phosphate (TEP) can improve safety, they can hinder the formation of protective SEI on graphite surfaces, resulting in rapid capacity degradation of batteries.95 Fluorinated phosphate ester flame-retardants (such as TFP, BMP) not only improve flame-retardant efficiency by replacing hydrogen atoms in molecules with fluorine atoms (because fluorine radicals can also quench hydrogen radicals), but also improve compatibility with anodes. For example, bis(2,2,2-trifluoroethyl)methyl phosphate can significantly reduce the flammability of the electrolyte at a relatively low addition of 10 wt%, and can form a stable SEI on the surface of the graphite anode.68 Fluorinated cyclophosphazene flame-retardants (such as PFPN, FPPN) exhibit superior flame-retardant efficiency, with only 5 wt% added to achieve a non-flammable state of the electrolyte. This efficiency stems from the synergistic effect between fluorine, nitrogen, and phosphorus atoms in its molecular structure. Meanwhile, due to its low addition amount, it usually does not cause significant damage to the conductivity of the electrolyte or the overall performance of the battery.96Researchers have proposed optimization strategies to address the issue of poor compatibility between phosphorus-containing flame-retardants (especially phosphate esters) and graphite anodes. One strategy is to use a high-concentration electrolyte design, such as dissolving 2.2 M lithium difluorosulfonyl imide (LiFSI) in TEP solvent. The high concentration of lithium salt increases the ratio of salt to solvent, causing strong complexation between TEP molecules and Li+ ions, thereby inhibiting the reduction and decomposition of TEP on the surface of the graphite anode and promoting the formation of a stable SEI.97 Another strategy is to introduce composite functional additives, such as fluorinated vinyl carbonate (FEC). FEC can preferentially decompose phosphate esters in phosphate-based electrolytes, acting as an effective film-forming agent to alleviate side reactions caused by phosphate esters at the anode interface and improve compatibility.98 The latest research progress shows that fluorinated ring phosphorus nitrile flame retardants (such as FPPN) not only have high flame retardant efficiency, but also exhibit excellent compatibility with high-voltage cathode materials (such as spinel-structured LiNi0.5Mn1.5O4). After 250 cycles at a high cut-off voltage of 4.5 V, the battery using FPPN electrolyte can still maintain 82.6% of its initial capacity. Meanwhile, the electrolyte itself is non-flammable, significantly enhancing the safety of high-voltage lithium-ion batteries.99
Jiang et al.100 developed a new electrolyte using diethyl ethylphosphonic acid ester (DEEP) as a flame-retardant solvent, lithium difluorooxalate borate (LiODFB) as a lithium salt, and fluorinated vinyl carbonate (FEC) as a co-solvent. Through the synergistic effect of LiODFB and FEC, the safety and electrochemical performance of lithium batteries were significantly improved. As shown in Fig. 10a (left), the 1.3 M LiODFB/DEEP 30% FEC electrolyte is completely non-flammable during combustion testing, while traditional carbonate electrolytes are flammable. The heat flow curve in Fig. 10a (right) further confirms that the peak temperature of LiODFB decomposition (240 °C) is higher than that of traditional LiPF6 (196 °C), and the high thermal stability of DEEP (221 °C) and FEC (259 °C) jointly suppress thermal runaway, effectively reducing the heat release of NCM811 cathode thermal decomposition.100 Hyeong Jun Cheon et al.37 significantly improved the safety and electrochemical performance of lithium-ion batteries by introducing the liquid flame retardant DMMP as a bifunctional additive into the PEO-based solid polymer electrolyte. DMMP exerts its flame-retardant effect by capturing active free radicals (such as H˙ and HO˙) during the combustion process. Fig. 10b visually compares the self-extinguishing phenomenon of the electrolyte containing DMMP upon contact with a flame. In addition, DMMP also plays a plasticizing role, promoting the dissociation of lithium salts and improving the migration of lithium ions, thereby enhancing ionic conductivity and cycling stability.37Fig. 10c shows the molecular structure of the phosphorus–silicon bifunctional additive DETSP and its mechanism of action in lithium batteries. The phosphorus group reduces the flammability of the electrolyte, and the silicon group synergistically creates a stable CEI.101Fig. 10d shows the mechanism of action of flame-retardant additive D3F in lithium batteries. In the early stage of thermal runaway, lithium graphite (LiCx) triggers the ring-opening polymerization of D3F, forming a highly thermally stable poly-D3F, targeted repair of damaged SEI, isolating LiCx from reacting with electrolyte. At the same time, fluorine radicals are released to quench the combustible hydrogen radicals, inhibiting the later combustion, thereby significantly enhancing the thermal safety of the battery (the self-heat release temperature rises from 159.6 °C to 300.5 °C).102Fig. 10e illustrates the temperature-sensitive mechanism of the thermal-responsive polymer PBMA in the ionic liquid electrolyte – high temperature triggers the sol–gel phase transition to block the ion conduction path and achieve battery self-protection.13 The mechanism of action of flame-retardant additives includes the following. Phosphorus-based additives (such as TMP) release  free radicals to capture H˙ and terminate the chain reaction, halogen-based additives decompose and absorb heat and isolate oxygen, while composite additives (such as phosphorus fluorine) synergistically improve flame-retardant efficiency and electrochemical stability. Fig. 10f shows a protective mechanism of an intelligent thermal-responsive electrolyte. A polymer protective layer is formed on the surface of the lithium anode at room temperature, which rapidly solidifies when the temperature exceeds 130 °C, physically blocking the risk of internal short circuit.13
free radicals to capture H˙ and terminate the chain reaction, halogen-based additives decompose and absorb heat and isolate oxygen, while composite additives (such as phosphorus fluorine) synergistically improve flame-retardant efficiency and electrochemical stability. Fig. 10f shows a protective mechanism of an intelligent thermal-responsive electrolyte. A polymer protective layer is formed on the surface of the lithium anode at room temperature, which rapidly solidifies when the temperature exceeds 130 °C, physically blocking the risk of internal short circuit.13
 | ||
| Fig. 10 (a) (left) The 1.3 M LiODFB/DEEP 30% FEC electrolyte is completely non-flammable, while the traditional carbonate electrolyte is highly flammable; (right) the decomposition peak temperatures of LiODFB, DEEP, and FEC are 240 °C, 221 °C, and 259 °C, respectively, all of which are superior to the thermal stability of traditional electrolytic salts.100 Copyright 2023 Elsevier B.V. (b) The electrolyte containing DMMP self-extinguishes when exposed to fire, while the blank sample continues to burn.37 Copyright 2024 American Chemical Society. (c) Schematic diagram of DETSP molecular structure and its phosphorus–silicon synergistic flame-retardant and interface stability mechanism in batteries.101 Copyright 2024 Elsevier B.V. (d) The schematic diagram of the triple function of early suppression of battery thermal runaway through the formation of thermally stable SEI, targeted repair of lithium graphite surface, and quenching of hydrogen free radicals by D3F.102 Copyright 2023 Wiley-VCH GmbH. (e) The temperature-sensitive phase transition mechanism and corresponding battery structure diagram of thermally responsive polymer PBMA in ionic liquid electrolytes.13 (f) The protective layer formed by thermal-responsive electrolyte on the lithium anode (at room temperature) and its high-temperature curing mechanism (130 °C).13 (e and f) Copyright 2020 American Chemical Society. | ||
3.4 Acid scavengers
The core function of acid scavengers/stabilizers is to capture corrosive substances present in the electrolyte, especially hydrofluoric acid (HF) and water (H2O), to reduce their corrosion on electrode materials (especially cathodes), thereby improving the cycling stability and lifespan of the battery. This type of additive mainly eliminates HF through chemical reactions, inhibiting the dissolution of transition metals, the destruction of the cathode structure, and the excessive generation of harmful by-products caused by it.Silicon-containing organic compounds represented by tris(trimethylsilyl)borate (TMSB) and tris(trimethylsilyl)phosphate (TMSP) are highly efficient acid scavengers. They react with HF through trimethylsilyl groups (–Si(CH3)3) to generate volatile trimethylfluorosilane (Me3SiF), effectively reducing the acidity of the electrolyte. The specific reaction is (CH3)3SiOR + HF → (CH3)3SiF + HOR (R represents boronic acid or phosphate groups). This process directly consumes HF in the electrolyte, suppresses the dissolution and damage to Ni/Co/Mn elements in layered transition metal oxide cathodes (such as NCM, NCA) by HF, and reduces the deposition of high impedance LiF at the electrode interface. Experimental results have shown that the addition of TMSP significantly reduces the precipitation of POF3 gas, while the generation of Me3SiF is detected, indicating that its chemical scavenging effect is the main mechanism for improving battery life, rather than a simple interface passivation layer effect.36,103 In the high-voltage lithium cobalt oxide (LiCoO2, LCO) system, TMSB as an additive has been proven to effectively inhibit the formation of HF. Its oxidation and decomposition products participate in the construction of the CEI. Meanwhile, its –Si(CH3)3 groups react with HF to form (CH3)3SiF and dimethyldifluorosilane, significantly reducing the dissolution of transition metals. This helps to form a thinner interfacial film with lower LiF content on the surface of the LiNi0.8Co0.15Al0.05O2 (NCA) cathode, enhancing structural stability and inhibiting electrolyte decomposition. Adding 1% TMSB electrolyte enabled the capacity retention rate of the NCA cathode to reach 86.0% after 200 cycles at a 1C rate, which was much higher than the 67.2% of the base electrolyte.104
In addition to TMSB and TMSP, other organic molecules containing silicon or phosphorus have also been shown to have acid-scavenging functions, such as hexamethylphosphoramide, tris-2,2,2-trifluoroethyl phosphate (TTFP), dimethoxydimethylsilane (DODSi), diethylphenylphosphine, N,N-diethylaminotrimethylsilane, and p-toluenesulfonyl isocyanate. These compounds can directly react with PF5, HF, F−, and PF6− in the electrolyte, inhibiting the generation of LiF and Li2CO3, and reducing the leaching of metal elements.68 Kim et al.105 investigated the application of lithium bis(trimethylsilyl)phosphate (LiTMSP) as a novel bifunctive additive in high-voltage LiNi1.5Mn0.5O4/graphite lithium-ion batteries. This additive can not only remove hydrofluoric acid (HF) from the electrolyte and reduce the dissolution of transition metals, but also form a more stable passivation film on the surface of the graphite electrode, inhibiting the further decomposition of the electrolyte, thereby improving the cycling performance and rate performance of the battery at high temperatures (Fig. 11a). The reaction of LiTMSP with HF to form TMS-F was confirmed by nuclear magnetic resonance spectroscopy. XPS and electrochemical tests also supported its excellent film-forming ability and interface stability.105 Zheng et al.106 investigated the role of the multifunctional electrolyte additive N,O-di(trimethylsilyl)acetamide (BSA) in stabilizing the interface of the high-nickel lithium-ion battery cathode material LiNi0. Youdaoplaceholder0 Co0.1Mn0.1O2 (NCM811). BSA effectively removes HF and H2O from the electrolyte through Si–N and Si–O bonds in its molecules, reduces LiPF6 hydrolysis, and forms a uniform and strong CEI film on the cathode surface through electrochemical oxidation (Fig. 11b), significantly improving the capacity retention rate of the battery at high rates and long cycles, and inhibiting the dissolution of transition metals. Improved the electrochemical performance of the battery.106Fig. 11c illustrates the mechanism of borate additives (such as LiBOB) as acid scavengers. The borate radicals generated by their decomposition effectively remove corrosive HF from the electrolyte by forming B–F species (such as BF3 or BF4−), suppressing the dissolution of transition metals and structural degradation of cathode materials under high voltage, thereby improving the cycling stability of lithium batteries.107 Liu et al.81 found that TMSPi, as an electrolyte additive in NMC811/Si-Gr lithium batteries, mainly plays a dual role. Firstly, it acts as an acid scavenger (HF scavenger), capturing HF generated by the hydrolysis of LiPF6 by breaking the O–Si bond, inhibiting the dissolution of transition metals at the cathode, and the fluorination of silicon particles at the anode (formation of SiO4F5). The second is to remove the residual Li2CO3 layer on the surface of the cathode, eliminate the charging overpotential it triggers, and convert the phase transition reaction into a solid solution mechanism (Fig. 11d). These effects significantly enhance the battery cycle stability by forming a stable CEI/SEI rich in phosphate.81 Wotango et al.108 studied 1-(trimethylsilyl)imidazole (1-TMSI) as a novel acid scavenger additive, which captures trace amounts of water and HF in the electrolyte through the Lewis basicity of its N–Si bond, effectively inhibiting the decomposition of LiPF6 and the generation of insulating LiF and other by-products. Fig. 11e shows that ΔGHF = −16.9 kcal mol−1 is thermodynamically more favorable and effectively inhibits the LiPF6 hydrolysis chain reaction.108Fig. 11f shows that the additive forms a thinner and more ion-conductive SEI on the surface of MCMB electrodes, reducing interfacial impedance and significantly improving the cycling capacity retention of lithium batteries (the cumulative irreversible capacity decreased from 17.1% to 11.0% after 40 cycles).108
 | ||
Fig. 11 (a) LiTMSP additive significantly improves the high-temperature cycle and magnification performance of high-voltage lithium battery by capturing HF to inhibit metal dissolution and constructing a low resistance interface film.105 Copyright 2021 American Chemical Society. (b) BSA, as an acid scavenger, removes HF/H2O through Si–N/Si–O bonds to inhibit hydrolysis, while C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds preferentially oxidize to form a stable CEI, significantly improving the high-rate long-cycle performance of NCM811.106 Copyright 2020 American Chemical Society. (c) Schematic diagram of the mechanism of using borate additives to remove HF.107 Copyright 2024 Elsevier B.V. (d) Schematic diagram of the synergistic interface protection mechanism of TMSPi additive in removing Li2CO3 residue and HF at the cathode and forming P–O–Si-based SEI at the anode.81 Copyright 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (e) Comparison of Gibbs free energy calculations for the reaction between 1-TMSI and HF/H2O.108 (f) Comparison of SEI formation mechanisms on the MCMB electrode surface between traditional electrolyte (left) and electrolyte containing 1-TMSI (right).108 (e and f) Copyright 2016 American Chemical Society. N bonds preferentially oxidize to form a stable CEI, significantly improving the high-rate long-cycle performance of NCM811.106 Copyright 2020 American Chemical Society. (c) Schematic diagram of the mechanism of using borate additives to remove HF.107 Copyright 2024 Elsevier B.V. (d) Schematic diagram of the synergistic interface protection mechanism of TMSPi additive in removing Li2CO3 residue and HF at the cathode and forming P–O–Si-based SEI at the anode.81 Copyright 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (e) Comparison of Gibbs free energy calculations for the reaction between 1-TMSI and HF/H2O.108 (f) Comparison of SEI formation mechanisms on the MCMB electrode surface between traditional electrolyte (left) and electrolyte containing 1-TMSI (right).108 (e and f) Copyright 2016 American Chemical Society. | ||
3.5 LiPF6 stabilizers
LiPF6 is the most commonly used electrolyte salt for lithium-ion batteries, but its insufficient thermal stability is a key factor leading to battery performance degradation and safety risks. LiPF6 is prone to decomposition at high temperatures or in the presence of trace amounts of water (e.g., LiPF6 → LiF + PF5), and the generated PF5 will further catalyze the decomposition reaction of organic solvents (such as carbonates) and produce corrosive HF gas, accelerating electrode/electrolyte interface side reactions.109 Stabilizers such as pyrazine derivatives play a crucial role by inhibiting the thermal decomposition pathway of LiPF6. Unsaturated additives (such as acrylic esters) can preferentially bind with PF5 to form stable compounds, blocking its catalytic chain reaction of solvent decomposition and playing a role in removing acidic impurities. Density functional theory (DFT) calculations indicate that the binding energy between such additives and PF5 is significantly lower than that of solvents such as ethylene carbonate (EC) (e.g., −0.42 eV vs. −0.16 eV), making it thermodynamically easier to capture PF5.109 After being stored at 45 °C for 3 months, the UV-visible spectrum of the electrolyte with added pyrazine derivatives showed a significantly lower degree of discoloration compared to the base electrolyte, indicating that the stabilizer effectively delayed the oxidative decomposition of the electrolyte.12Wang et al.110 reported that 1,4-phenylene diisocyanate (PPDI), as a multi-functional electrolyte additive, inhibits the hydrolysis of LiPF6 by removing H2O/HF, and forms a stable high-conductivity film on the surfaces of the cathode and anode, significantly improving the high and low temperature cycling and storage performance of NCM622‖graphite batteries. Fig. 12a reveals the three-step exothermic reaction mechanism of PPDI scavenging H2O through DFT calculations. H2O undergoes nucleophilic addition to form phenylenediamine, decarboxylation to form phenylenediamine, and finally polymerizes with PPDI to form a urea derivative, whose by-products participate in the construction of the electrode protective layer.110 Ma et al.111 reported an ionic liquid additive IL-AC (1-methyl-1-butylpyrrolidinium-acetate) that enhances the stability of lithium batteries in LiPF6-based electrolytes containing trace amounts of water (2500 ppm) through a dual functional mechanism. As shown in Fig. 12b, IL-AC captures H2O through the coulombic action of the cation (Py14+) and the hydrogen bond of the anion (CH3COO−), inhibiting the hydrolysis of LiPF6 to generate HF and phosphate by-products. Its anion reacts with HF (CH3COO− + HF → CH3COOH + F−) to remove corrosive HF, protect the electrode interface, and reduce the dissolution of transition metals, enabling the Li‖NCM811 battery to maintain a capacity of 153.7 mAh g−1 after 300 cycles.111 Jia et al.112 developed a bifunctional insoluble ligand Li3BPy based on bipyridine as an electrolyte additive for lithium batteries. It can stabilize LiPF6 through Lewis alkalinity (inhibiting its decomposition to produce harmful products such as HF) and chelate transition metal ions to prevent their migration. The DFT calculation in Fig. 12c confirms that Li3BPy forms a complex with the decomposition product PF5 of LiPF6 (PF5 is located at the center of ligand symmetry), with a binding energy of −3.86 kJ mol−1, significantly reducing the reaction activity of PF5, thereby slowing down electrolyte degradation and electrode interface side reactions, and improving the cycling stability of the battery at 60 °C.112 He et al.113 developed a multi-functional electrolyte additive PHIS (2-phenyl-1H-imidazole-1-sulfonate), which significantly improved the high-temperature performance of NCM811/graphite battery by eliminating H2O/HF (inhibiting LiPF6 hydrolysis) and building a stable electrode interface film. The 19F NMR spectrum in Fig. 12d shows that the HF peak (−185 ppm) of the PHIS-containing electrolyte almost disappears after storage at 60 °C, while the HF content of the blank electrolyte increases sharply, confirming that PHIS effectively blocks the acid production pathway of LiPF6 decomposition.113
 | ||
| Fig. 12 (a) Transition state geometry and energy changes of the reaction pathway between PPDI and H2O.110 Copyright 2020 Elsevier B.V. (b) (above) The stepwise hydrolysis pathway of LiPF6 and the H2O capture/HF clearance mechanism of IL-AC. (below) Schematic diagram of the comparison between IL-AC and baseline electrolyte on LiPF6 hydrolysis, HF corrosion, and interfacial stability.111 Copyright 2022 American Chemical Society. (c) The geometric structure of the Li3BPy-PF5 complex optimized by DFT, with PF5 located at the center of ligand symmetry, has a calculated binding energy of −3.86 kJ mol−1.112 Copyright 2019 American Chemical Society. (d) The 19F NMR spectrum containing PHIS electrolyte shows no characteristic HF peak at −185 ppm, while a significant HF peak appears in the blank group.113 Copyright 2023 Elsevier B.V. | ||
4 Emerging additives and composite strategies
4.1 Ionic liquid (IL) additives
Ionic liquid additives have become key materials for improving the performance of lithium-ion batteries due to their unique physical and chemical properties. Their cations mainly include imidazolium, pyrrolidinium, and quaternary ammonium salts, while anions are mostly bis (trifluoromethanesulfonyl)imide (TFSI−), bis (fluorosulfonyl)imide (FSI−), tetrafluoroboric acid (BF4−), and hexafluorophosphate (PF6−).13 These additives significantly improve the thermal stability, flame retardancy, and dendrite suppression ability of batteries by optimizing the electrode/electrolyte interface behavior.13In terms of performance enhancement, ionic liquid additives provide multiple advantages. Ionic liquids have low volatility and non-flammability, with a thermal decomposition temperature of up to 300–400 °C, much higher than traditional carbonate electrolytes (about 150 °C), which can effectively suppress battery thermal runaway.69 In terms of dendrite inhibition mechanism, anions such as FSI can decompose on the surface of lithium metal to form an SEI rich in LiF. This layer has high interfacial energy and high lithium-ion diffusion coefficient, which can homogenize lithium deposition and inhibit dendrite growth.114 The electrochemical stability window of pyrrolidinium-based ionic liquids (such as PYR14-TFSI) exceeds 5.5 V (vs. Li+/Li) and is compatible with high-voltage cathodes (such as LiNi0.5Mn1.5O4), reducing electrolyte oxidation and decomposition.115
However, ionic liquid additives face three major challenges. Adding IL significantly increases the viscosity of the electrolyte (such as the viscosity of pure [Pyr14][TFSI] reached 40 cP), reduces the ionic conductivity (about 0.56 mS cm−1), and affects the low-temperature and rate performance.116 The reduction potential of imidazolium cations is relatively high (about 1 V vs. Li+/Li), making them prone to reduction and decomposition at the anode, resulting in a decrease in coulombic efficiency. Although quaternary ammonium salts have good compatibility, they have higher viscosity.13 In addition, the synthesis and purification process of IL is complex, and the price is much higher than traditional additives such as FEC and VC, which restricts its large-scale application.117 Recent research has overcome the above limitations through functional design. For example, ether-modified pyrrolidinium IL (such as PZ2o2-2-FSI) introduces methoxy groups to reduce viscosity (32.0 cP), while increasing ionic conductivity (13.0 mS cm−1) and thermal stability (Td = 159 °C).118 The mixed electrolyte strategy, such as mixing IL (e.g., PYR14TFSI) with a low-viscosity solvent (diethyl carbonate, DEC) in a mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, combined with 3 M LiPF6, can enable LiNi0.5Mn1.5O4/Li batteries to maintain a capacity of 140 mAh g−1 at room temperature, with a capacity retention rate of 97% after 300 cycles.119
1, combined with 3 M LiPF6, can enable LiNi0.5Mn1.5O4/Li batteries to maintain a capacity of 140 mAh g−1 at room temperature, with a capacity retention rate of 97% after 300 cycles.119
Chatterjee et al.120 developed a thiourea-based ionic liquid (IL) as an electrolyte additive, which enhances the safety and cycling performance of lithium batteries by preferentially decomposing to form a stable SEI. The impedance spectrum in Fig. 13a confirms that after 100 cycles, the electrolyte resistance (Re) and SEI resistance (RSEI) of the IL-containing battery significantly decrease, suppressing electrode degradation and improving Coulomb efficiency. DFT calculations reveal that its HOMO/LUMO energy levels contribute to sacrificial protection mechanisms. Fig. 13b shows that the electrochemical window of the electrolyte widened to −0.101 to +6.679 V (vs. Li/Li+) after adding IL, confirming its high voltage stability.120Fig. 13c (left) illustrates the dual functional mechanism of ionic liquid (IL) additives. Pyr (12)+ cations guide the uniform deposition of lithium ions through the electrostatic shielding effect, while FSI− anions reduce to form a rigid SEI rich in LiF, inhibiting dendrite growth. Fig. 13c (right) shows the self-assembly of a novel symmetric structure IL at the tip of a lithium dendrite, which synergistically optimizes the stability of the electrode interface.121 Cai et al.122 used computer-assisted ionic liquid design to screen ionic liquids containing vinyl and amide groups, [VAIM][TFSA] as electrolyte additives for lithium batteries. Its amide group can stabilize LiPF6 and inhibit the generation of water and HF. Ethylene promotes film formation on the surface of the LTO anode. Experiments have shown that the addition of 0.5 wt% significantly reduces the content of HF and moisture in the electrolyte. After 300 cycles, the capacity retention rate of LTO/Li batteries reaches 99.24% (Fig. 13d), and the impedance is the lowest, effectively improving the cycling stability and rate performance of the battery.122Fig. 13e (left) shows the molecular structure of imidazole-based ionic liquids, whose low volatility and high thermal stability can suppress electrolyte combustion. Fig. 13e (right) compares the ion conductivity of different P(VDF-co-HFP) – imidazole-based electrolytes, demonstrating that increasing the IL ratio can improve conductivity but reduce the lithium-ion migration number. It is necessary to balance safety and electrochemical performance (for example, the electrolyte containing 50% IL has a conductivity of 6.9 × 10−4 S cm−1 and a migration number of 0.15).13 Nirmala et al.123 synthesized imidazolium-based double cationic ionic liquid [C6(mim)2][TFSI]2 as an electrolyte additive for lithium batteries (Fig. 13f), which improved ion conductivity by reducing viscosity (362 cP) and optimizing the anion/cation structure (reaching 1.12 × 10−3 S cm−1 at 30 °C). The mechanism is that the double cation structure reduces ion cluster aggregation, promotes Li+ migration (activation energy 17.41 kJ mol−1), and forms a stable SEI. The electrochemical window reaches 5.3 V, which is suitable for high-voltage cathodes. The Li/LiFePO4 battery maintains a capacity of 133 mAh g−1 (98.8% coulombic efficiency) after 100 cycles at 0.1C.123 Wang et al.124 used imidazole-based ionic liquid Im1(8)PF6 as an electrolyte additive, and significantly improved the performance of lithium metal batteries by reducing it below 0.9 V (vs. Li+/Li) to form an SEI rich in Li3N (Fig. 13g). This SEI has high ion conductivity (Li3N: 6 × 10−3 S cm−1), induces uniform and dense deposition of lithium, achieves a stable coulombic efficiency of 97.4% (250 cycles) in Li‖Cu batteries, a symmetric polarization voltage lower than 25 mV for Li‖Li batteries, and a capacity retention rate of 97.8% (570 cycles) for Li‖LiFePO4 batteries, demonstrating its effective suppression of dendrites and improvement of interfacial stability.124
 | ||
| Fig. 13 (a) The electrolyte resistance (Re) and SEI resistance (RSEI) of the battery containing IL are significantly lower than those of the battery without IL after 100 cycles.120 (b) The electrolyte containing IL additive (red curve) widens the electrochemical window to −0.101 to +6.679 V vs. Li/Li+ compared to the base electrolyte (black curve).120 (a and b) Copyright 2020 American Chemical Society. (c) (left) IL additives stabilize the lithium metal interface through electrostatic shielding and the SEI layer; (right) a symmetric ionic liquid (IL) structure self-assembles into a lithium-repellent layer that effectively suppresses dendrite growth.121 (d) The [VAIM][TFSA] additive containing vinylamide groups inhibits HF/moisture and promotes LTO film formation, maintaining a battery capacity of 99.24% after 300 cycles.122 Copyright 2022 American Chemical Society. (e) (left) Molecular structure of imidazole ionic liquids. (right) Comparison of ionic conductivity of different P(VDF-co-HFP) – imidazole electrolytes.13 Copyright 2020 American Chemical Society. (f) Double cation ionic liquid additives enhance the ion conductivity and cycling stability of lithium batteries.123 Copyright 2022 American Chemical Society. (g) Imidazole-based ionic liquid additives are used to construct a Li3N SEI, suppress dendrites, and enhance the interfacial stability and cycling performance of lithium metal batteries.124 Copyright 2022 American Chemical Society. | ||
4.2 Single-molecule with multi-functional additives
The development of single-molecule multifunctional additives aims to integrate multiple functional groups, such as film-forming, flame-retardant, and stabilization, into a single molecular structure through molecular design, thereby synergistically improving the comprehensive performance of lithium-ion batteries (such as cycle stability, safety, and interface stability), while avoiding compatibility issues that may arise from the combination of multiple single functional additives. Phosphoronitrile compounds such as hexafluorocyclotriphosphazene, ethoxypentafluorocyclotriphosphazene (PFPN), and phenoxypentafluorocyclotriphosphazene (FPPN) are typical representatives of single-molecule multifunctional additives. This type of additive not only has excellent flame-retardant properties, but the P–N bonds and fluorine atoms in its molecular structure also endow it with film-forming ability. For instance, FPPN can preferentially oxidize and decompose on the surface of high-voltage cathodes (such as LiNi0.5Mn1.5O4), forming a CEI rich in linear polymers, polycyclic polymers, LiF, Li3PO4, and ROPO3Li, effectively inhibiting the decomposition of the electrolyte and the dissolution of transition metals. It also significantly reduces the flammability of the electrolyte.125 In addition, by introducing alkenyl groups (such as butenoxy groups) into the cyclotriphosphazene molecule, its compatibility with the silicon–carbon (Si–G) anode can be further optimized, achieving a synergic improvement in cycling performance and safety in 4.45 V LiCoO2/Si-G pouch cells.96Sulfur-containing compounds (such as sulfonic acid esters) are another important multifunctional additive design platform. For example, N,O-bis (trimethylsilyl)acetamide (BSA) molecules contain Si–N, Si–O, and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds, serving as both HF scavengers and interface modifiers. The Si–N and Si–O bonds can effectively capture HF and H2O in the electrolyte, while the C
N bonds, serving as both HF scavengers and interface modifiers. The Si–N and Si–O bonds can effectively capture HF and H2O in the electrolyte, while the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond participates in the construction of a stable CEI on the surface of the nickel cobalt manganese (NCM) cathode, significantly suppressing transition metal dissolution and electrolyte oxidation decomposition, and improving the performance of NCM811‖graphite full cell over a wide temperature range.106 Phenyl-4-fluorobenzenesulfonate forms a uniform and dense SEI on the surface of graphite anode through the synergistic effect of –OSH2– and –F groups, effectively blocking the erosion of LiPF6 and inhibiting the continuous decomposition of electrolyte, thereby improving the cycling stability of NCM811/graphite battery.126
N bond participates in the construction of a stable CEI on the surface of the nickel cobalt manganese (NCM) cathode, significantly suppressing transition metal dissolution and electrolyte oxidation decomposition, and improving the performance of NCM811‖graphite full cell over a wide temperature range.106 Phenyl-4-fluorobenzenesulfonate forms a uniform and dense SEI on the surface of graphite anode through the synergistic effect of –OSH2– and –F groups, effectively blocking the erosion of LiPF6 and inhibiting the continuous decomposition of electrolyte, thereby improving the cycling stability of NCM811/graphite battery.126
To balance flame retardancy and electrochemical performance, researchers have proposed improvement strategies through molecular structure optimization. Fluorination modification strategy replaces hydrogen atoms in phosphides with fluorine atoms, which can improve flame-retardant efficiency and interface compatibility. For example, fluorinated cyclotriphosphazene (such as (ethoxy) pentafluorocyclotriphosphazene (PFPN)) can achieve electrolyte non-flammability at low addition levels (5 wt%), while reducing negative effects on ion conductivity and battery performance. The synergistic effect of fluorine with phosphorus and nitrogen atoms significantly enhances flame-retardant efficiency.96 Multi-functional molecular design strategies have developed molecules that combine flame retardancy and interface stability, such as 1-diphenylphosphoryloxy-4-methylbenzene, which not only achieves flame retardancy through phosphorus groups, but also forms redox pairs in its phenyl derivative structure during overcharging, providing overcharge protection.19
The core of molecular design strategies lies in the rational combination of functional groups and the optimization of spatial configuration. Introducing unsaturated bonds (such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) can enhance the oxidation/reduction activity of molecules, enabling them to decompose preferentially in the base electrolyte and participate in film formation. For instance, the allyl groups in triallyl phosphate (TAP) can be oxidized and reduced, respectively, on the surfaces of the high-nickel cathode (LiNi0.8Co0.1Mn0.1O2) and the graphite anode, forming CEI and SEI rich in organophosphorus components, which simultaneously stabilize the interface between the cathode and anodes and suppress gas production.38 Regulating the steric hindrance effect is achieved by introducing large steric hindrance groups to influence the polymerization behavior and the flexibility of the SEI. Research shows that attaching large-volume functional groups (such as phosphonitrile groups) to the core of N-carboxylic anhydride (NCA) can optimize the mechanical flexibility and ion transport capacity of the SEI, thereby enhancing the cycling performance of LiNi0.8Co0.1Mn0.1O2‖Si/graphite batteries. The effect depends on the size of the functional groups.127 The synergy of complex elements (such as P/N/F/Si) refers to the integration of multiple active elements (such as P/N in phosphoronitrile, F in fluorinated carbonates, Si in silanes) within a single molecule, which can simultaneously achieve flame retardancy, HF removal, and in situ generation of interfacial lithiation products (such as LiF, Li3PO4). Form an electrode interface layer with both high ionic conductivity and mechanical stability.128 Compared with single-function additives or multi-additive compound schemes, single-molecule multifunctional additives simplify the electrolyte formula through intramolecular synergistic effects and reduce possible negative interactions among additives, representing the cutting-edge direction of electrolyte design for high-performance and high-safety lithium-ion batteries.125
C) can enhance the oxidation/reduction activity of molecules, enabling them to decompose preferentially in the base electrolyte and participate in film formation. For instance, the allyl groups in triallyl phosphate (TAP) can be oxidized and reduced, respectively, on the surfaces of the high-nickel cathode (LiNi0.8Co0.1Mn0.1O2) and the graphite anode, forming CEI and SEI rich in organophosphorus components, which simultaneously stabilize the interface between the cathode and anodes and suppress gas production.38 Regulating the steric hindrance effect is achieved by introducing large steric hindrance groups to influence the polymerization behavior and the flexibility of the SEI. Research shows that attaching large-volume functional groups (such as phosphonitrile groups) to the core of N-carboxylic anhydride (NCA) can optimize the mechanical flexibility and ion transport capacity of the SEI, thereby enhancing the cycling performance of LiNi0.8Co0.1Mn0.1O2‖Si/graphite batteries. The effect depends on the size of the functional groups.127 The synergy of complex elements (such as P/N/F/Si) refers to the integration of multiple active elements (such as P/N in phosphoronitrile, F in fluorinated carbonates, Si in silanes) within a single molecule, which can simultaneously achieve flame retardancy, HF removal, and in situ generation of interfacial lithiation products (such as LiF, Li3PO4). Form an electrode interface layer with both high ionic conductivity and mechanical stability.128 Compared with single-function additives or multi-additive compound schemes, single-molecule multifunctional additives simplify the electrolyte formula through intramolecular synergistic effects and reduce possible negative interactions among additives, representing the cutting-edge direction of electrolyte design for high-performance and high-safety lithium-ion batteries.125
Moon et al.129 reported a multifunctional electrolyte additive, 4-(allyloxy)phenylfluorosulfonate (APFS), which was used in conjunction with vinyl carbonate (VC) to construct an SEI containing LiF and elastic polymer species on a silicon–carbon composite anode (SiG–C). At the same time, a thermally stable CEI containing S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/S–F species was formed on the surface of a nickel-rich cathode (NCM811) (as shown in Fig. 14a). This additive adapts to the volume change of silicon through free radical copolymerization reaction, inhibits nickel leaching, and passivates PF5 to reduce HF generation, resulting in a capacity retention rate of 72.5% for SiG–C/NCM811 full cell after 300 cycles at 45 °C.129 Chen et al.130 proposed a novel multifunctional electrolyte additive SOFPB (5-(tert-butyldimethylsiloxy)-2-fluorophenylboronic acid pinal ester), whose molecular design strategy (Fig. 14b) achieves synergistic effects by integrating boron, fluorine, phenyl, and silicon oxygen groups. The boron/silicon oxygen group enhances the lithium-ion conductivity and elasticity of CEI/SEI, the phenyl group enhances mechanical/thermal stability, and the fluorine group enhances oxidative stability. This additive preferentially decomposes the electrolyte, forming a highly conductive lithium-ion, strong, and temperature-stable electrode interface, significantly improving the cycling performance of NCM622/graphite lithium batteries at extreme temperatures of −30 °C to 55 °C (such as a 2.6-fold increase in capacity retention at −30 °C).130 Pham et al.26 reported the mechanism of action of multifunctional electrolyte additive AEDB (2-aminoethyldiphenylboronic acid ester) in a high-voltage nickel-enriched cathode (NCM851005) full cell. Fig. 14c compares the performance improvement of AEDB through a dual interface stabilization mechanism. It preferentially oxidizes to form a thin protective film on the cathode, suppressing electrolyte decomposition, transition metal leaching, and crack propagation. Simultaneously, it prioritizes the construction of a stable SEI on the graphite anode to reduce side reactions. This additive also removes harmful species such as HF through boron atoms, significantly inhibiting cross-talk between cathode and anode, resulting in a capacity retention rate of 88% for the entire battery after 100 cycles at a high voltage of 4.4 V.26 Zhu et al.131 reported a novel multifunctional electrolyte additive, diethyl(thiophene-2-yl methyl)phosphinate (DTYP), whose molecular structure (Fig. 14d) integrates thiophene and phosphate ester groups. Through preferential oxidation, an ion-conducting protective layer is formed on the surface of the high-voltage cathode (LiNi0.5Mn1.5O4), which inhibits the decomposition of the electrolyte. Meanwhile, the complexation of phosphate groups with PF5 enhances thermal stability (the initial decomposition temperature rises from 193 °C to 223 °C), and the phosphorus component captures free radicals to achieve flame retardancy (the self-extinguishing time is reduced from 88 seconds to 77 seconds). Adding 0.5% DTYP increased the capacity retention rate of lithium batteries at 60 °C after 280 cycles from 18% to 85%.131
O/S–F species was formed on the surface of a nickel-rich cathode (NCM811) (as shown in Fig. 14a). This additive adapts to the volume change of silicon through free radical copolymerization reaction, inhibits nickel leaching, and passivates PF5 to reduce HF generation, resulting in a capacity retention rate of 72.5% for SiG–C/NCM811 full cell after 300 cycles at 45 °C.129 Chen et al.130 proposed a novel multifunctional electrolyte additive SOFPB (5-(tert-butyldimethylsiloxy)-2-fluorophenylboronic acid pinal ester), whose molecular design strategy (Fig. 14b) achieves synergistic effects by integrating boron, fluorine, phenyl, and silicon oxygen groups. The boron/silicon oxygen group enhances the lithium-ion conductivity and elasticity of CEI/SEI, the phenyl group enhances mechanical/thermal stability, and the fluorine group enhances oxidative stability. This additive preferentially decomposes the electrolyte, forming a highly conductive lithium-ion, strong, and temperature-stable electrode interface, significantly improving the cycling performance of NCM622/graphite lithium batteries at extreme temperatures of −30 °C to 55 °C (such as a 2.6-fold increase in capacity retention at −30 °C).130 Pham et al.26 reported the mechanism of action of multifunctional electrolyte additive AEDB (2-aminoethyldiphenylboronic acid ester) in a high-voltage nickel-enriched cathode (NCM851005) full cell. Fig. 14c compares the performance improvement of AEDB through a dual interface stabilization mechanism. It preferentially oxidizes to form a thin protective film on the cathode, suppressing electrolyte decomposition, transition metal leaching, and crack propagation. Simultaneously, it prioritizes the construction of a stable SEI on the graphite anode to reduce side reactions. This additive also removes harmful species such as HF through boron atoms, significantly inhibiting cross-talk between cathode and anode, resulting in a capacity retention rate of 88% for the entire battery after 100 cycles at a high voltage of 4.4 V.26 Zhu et al.131 reported a novel multifunctional electrolyte additive, diethyl(thiophene-2-yl methyl)phosphinate (DTYP), whose molecular structure (Fig. 14d) integrates thiophene and phosphate ester groups. Through preferential oxidation, an ion-conducting protective layer is formed on the surface of the high-voltage cathode (LiNi0.5Mn1.5O4), which inhibits the decomposition of the electrolyte. Meanwhile, the complexation of phosphate groups with PF5 enhances thermal stability (the initial decomposition temperature rises from 193 °C to 223 °C), and the phosphorus component captures free radicals to achieve flame retardancy (the self-extinguishing time is reduced from 88 seconds to 77 seconds). Adding 0.5% DTYP increased the capacity retention rate of lithium batteries at 60 °C after 280 cycles from 18% to 85%.131
 | ||
| Fig. 14 (a) APFS additive forms an elastic SEI on the silicon carbon anode, a stable CEI on the nickel-rich cathode, and passivates PF5 in the electrolyte.129 Copyright 2023 Wiley-VCH GmbH. (b) The molecular structure design strategy of SOFPB additives integrates boron, fluorine, phenyl, and silicon oxygen groups to achieve multifunctional synergistic effects.130 Copyright 2024 Elsevier B.V. (c) Comparative schematic diagram of interface protection and cross reaction inhibition mechanism between AEDB additive (b) and base electrolyte (a) in high-voltage full cell.26 Copyright 2021 Wiley-VCH GmbH. (d) DTYP molecular structure and its three functional mechanisms (film formation/thermal stability/flame retardancy).131 Copyright 2018 Royal Society of Chemistry. | ||
4.3 Nanomaterial additives
Nanomaterials, as electrolyte additives, can significantly enhance the performance of lithium-ion batteries due to their unique physical and chemical properties, such as high specific surface area, controllable pore structure, and abundant surface functional groups. According to the material type, these additives are mainly divided into three categories: oxide nanoparticles, metal/covalent organic frameworks, and carbon nanomaterials.Oxide nanoparticles, such as Al2O3, SiO2, and TiO2, mainly optimize battery performance by removing harmful impurities from the electrolyte, modifying the electrode/electrolyte interface, and providing mechanical reinforcement. Studies have shown that adding SiO2 nanoparticles to polypropylene oxide (PPO) based solid electrolytes can reduce the crystallinity of the electrolyte, improve its room temperature ionic conductivity (up to 6.67 × 10−4 S cm−1), and thermal stability (over 200 °C).132
Metal organic frameworks (MOFs) and covalent organic frameworks (COFs) utilize their highly ordered pore structure and large specific surface area to selectively screen ions, adsorb impurities, and fix electrolyte molecules in electrolytes. Cationic COFs are incorporated into polyethylene oxide (PEO) based electrolytes, and their pore structure provides a fast transport path for lithium ions. At the same time, their surface cations can promote lithium salt dissociation, significantly improving the rate performance and cycling stability of all-solid-state batteries based on LiFePO4/Li.133 Functionalized Zr-based metal organic frameworks (MOFs) serve as sulfur cathode additives, effectively suppressing the shuttle effect of polysulfides through chemical adsorption and improving the capacity retention of lithium sulfur batteries.134 COFs materials play the role of ion conductors in PEO composite electrolytes, and their ordered skeleton structure helps to reduce the proportion of PEO crystalline regions, resulting in an ion conductivity of 1.5 × 10−4 S cm−1 at 40 °C for the composite electrolyte.135
Carbon nanomaterials, such as carbon nanotubes (CNTs) and graphene oxide (GO), mainly improve electrode reaction kinetics by enhancing the conductivity and mechanical properties of the electrode/electrolyte interface.136 After lithium functionalization of graphene oxide (GO) and the addition of PVDF-HFP polymer electrolyte, the crystallinity of the electrolyte can be reduced and its lithium-ion conductivity can be enhanced, enabling the capacity retention rate of the 0.2 Ah lithium metal pouch battery to reach 78.4% after 150 cycles.137
Chu et al.138 proposed a novel strategy of using metal–organic framework (MOF) nanoparticles as electrolyte additives to suppress the growth of lithium dendrites in lithium metal batteries and enhance cycle stability. The electroplating/stripping behavior of lithium anodes was significantly improved by introducing MOF particles such as UiO-66, HKUST-1, and MIL-101-NH2 into the carbonate electrolyte. Among them, the zirconium-based MOF (UUO-66) additive demonstrated the best performance, enabling a stable cycle of over 1400 hours (Fig. 15a), reducing the nucleation overpotential, and promoting the formation of LiF-rich SE. This study systematically verified the potential of MOF additives in enhancing the cycle life and safety of lithium metal batteries.138 Liang et al.137 developed a nano additive based on lithium grafted graphene oxide, which enhances the lithium-ion conductivity of polymer electrolytes (PVDF-HFP/LiTFSI) by covalently modifying lithium adenosine diphosphate (7.59 × 10−4 S cm−1 at 80 °C). The mechanism involves reducing the crystallinity of the polymer and accelerating the transition of Li+ at the interface between functionalized GO and polymer chains. This artificial interface is applied to lithium metal soft pack batteries (Li/NCM811), and after 150 cycles under strict practical conditions, the capacity retention rate reaches 78.4% (Fig. 15b), significantly inhibiting dendrite growth and “dead lithium” formation, providing a new strategy for the design of high-stability lithium battery electrolytes.137 Yan et al.139 used metal organic framework (MOF) nanocapsules to encapsulate insoluble lithium nitrate and potassium tetraborate electrolyte additives (Fig. 15c). Through the continuous release of MOF, lithium nitrate formed a nitrogen rich SEI on the lithium anode to inhibit dendrite growth, while potassium tetraborate constructed a boron rich CEI on the high-voltage cathode to reduce transition metal dissolution, thereby synergistically improving the cycling stability of lithium metal batteries (80.1% capacity retention rate after 200 cycles at 4.4 V).139 Li et al.140 investigated a highly ordered porous nanoadditive based on SSZ-13 for enhancing the performance of polyethylene oxide (PEO)-based solid electrolytes (Fig. 15d). The results in the figure show that this additive significantly enhances the lithium-ion conductivity (up to 4.43 × 10−5 S cm−1 at 20 °C) through the nanopore adsorption effect, broadens the electrochemical window to 4.7 V, and strengthens the interface stability with lithium metal, effectively inhibiting dendrite growth. The LiFePO4 all-solid-state battery based on this electrolyte exhibits excellent rate performance and cycle stability.140 Yadav et al.141 first applied boron nitride nanotubes (BNNTs) as a multifunctional electrolyte additive to lithium-ion batteries. The mechanism is shown in Fig. 15e. BNNTs are dispersed in the electrolyte (1 M LiPF6/EC:DMC), promoting lithium-ion desolvation through electron-deficient boron sites and utilizing their tubular structure to provide efficient ion transport channels. The 0.9 wt% BNNT additive increased the ion conductivity by 30% (up to 0.87 mS cm−1), increased the lithium-ion migration number to 0.73, and achieved a high capacity of 153 mAh g−1 (1C) and a coulombic efficiency of 99.6% (10C) after 500 cycles in NCM622//graphite full cell, significantly better than the untreated system.141
 | ||
| Fig. 15 (a) MOF additives (such as UiO-66) achieve stable cycling of the lithium anode for 1400 hours, suppress dendrites and polarization, and improve battery performance.138 Copyright 2018 American Chemical Society. (b) Lithium grafted graphene oxide additives enhance the conductivity of polymer electrolytes, achieving a 78.4% capacity retention rate for 150 cycles in soft pack batteries.137 Copyright 2021 American Chemical Society. (c) A schematic diagram of MOF nanocapsules continuously releasing borate/nitrate additives and constructing stable SEI and CEI on the surfaces of the lithium anode and high-voltage cathode, respectively.139 Copyright 2024 Elsevier B.V. (d) SSZ-13 porous additive enhances the ion conductivity of PEO electrolyte to 1.91 × 10−3 S cm−1 (60 °C) through nanopore adsorption, broadens the voltage window to 4.7 V, strengthens the stability of the lithium anode, and achieves a high-rate all-solid-state battery.140 Copyright 2018 American Chemical Society. (e) Schematic diagram of NCM622 cathode/separator/graphite anode full battery structure, in which BNNT-dispersed electrolyte is used as an ion transport enhancement medium.141 Copyright 2018 American Chemical Society. | ||
4.4 Polymer additives
Polymer additives in gel electrolytes are mainly used to build quasi-solid electrolyte systems to improve battery safety and interface stability. For example, polyvinylidene fluoride co-hexafluoropropylene (PVDF-HFP) is widely used as the base matrix of gel polymer electrolyte because of its semi-crystallization characteristics, high dielectric constant, porous network structure, and excellent chemical and thermal stability.142 When this type of copolymer is combined with ionic liquids (IL), it can significantly improve ion conductivity and achieve higher charge and discharge efficiency at lower IL loads. The assembled lithium metal battery can operate stably at high coulombic efficiency (98%).142PVDF-HFP can also be blended with polyethylene oxide (PEO) as a cathode electrolyte interlayer to enhance antioxidant capacity and broaden the electrochemical stability window, thereby improving cycling stability.143 In addition, PVDF-HFP, as a polymer host, can be used to load functional additives (such as Li2BPy), forming a porous structure through phase transition technology to achieve uniform distribution of additives, thereby improving the interfacial performance and electrochemical stability of electrolytes.112
In solid-state batteries, polymer gel electrolytes (such as PEO and PVDF-HFP-based systems) have attracted attention due to their non-flammability and good mechanical toughness, but their low bulk ionic conductivity and interface SEI problems remain to be solved.144 Introducing polymer additives to regulate interface structure and ion transport behavior is one of the effective strategies for improving the performance of quasi-solid-state electrolytes.
Qi et al.145 proposed a novel multifunctional nanoporous polymer additive, PIM-1 (Fig. 16a), to replace traditional PVDF binders. PIM-1 forms a three-dimensional nanoporous structure (specific surface area of 591 m2 g−1) through its helical rigid aromatic chains, and can achieve excellent performance in lithium secondary batteries (NCM811, LFP, etc.) with only a small amount of addition (0.05–1 wt%). The mechanism includes: (1) uniformly dispersing conductive carbon black through point to surface contact to enhance electronic conductivity; (2) strong adsorption of lithium ions (adsorption energy −0.44 eV), promoting desolvation and inhibiting electrolyte decomposition; (3) forming a stable CEI rich in inorganic substances (such as LiF) to enhance cycling stability (with a capacity retention rate of 92% after 100 cycles). Fig. 16b reveals through simulation that PIM-1 has more lithium-ion adsorption sites and stronger binding energy compared to PVDF, effectively regulating the solvation structure of lithium ions. This design overcomes the problems of poor ion/electron conductivity and high usage (>2%) of PVDF, providing a new strategy for lithium battery electrode processing.145 Guan et al.146 proposed a PVDF-based polymer electrolyte additive AMPS (Fig. 16c), which significantly improved ion conductivity (2.2 × 10−4 S cm−1) and inhibited lithium dendrite growth by reducing PVDF crystallinity and forming a LiF/Li2Sx/Li2SO3/Li3N enrichment interface. FTIR spectra indicate that AMPS monomers polymerize into PAMPS during the preparation process, and their hydrogen bonding fixes TFSI− anions, increasing the Li+ migration number to 0.49 and enhancing the cycling stability of solid-state lithium batteries.146 Shin et al.147 developed a composite solid electrolyte based on a PAES-g-PEG matrix and POSS-PEG nanofiller. Fig. 16d reveals the mechanism of action of POSS-PEG. Its rigid Si–O–Si skeleton selectively adsorbs lithium ions through dipole ion interactions, while the PEG segments grafted on the surface provide ion transport channels, significantly increasing the lithium-ion migration number (0.687) and conductivity (2.78 mS cm−1). This electrolyte combines high mechanical strength (3.7 MPa) and thermal stability (220 °C), effectively suppressing lithium dendrites and achieving a capacity retention rate of 85.9% after 200 cycles at 0.2C for lithium sulfur batteries.147 Li et al.148 prepared a porous PVDF-based gel polymer electrolyte (PGPE) containing star-shaped polymers (with linear PEG arms and cyclic PEG cores) by the phase separation method (Fig. 16e) for lithium-ion batteries. When the additive content was 20 wt%, the membrane porosity was the largest and evenly distributed, significantly enhancing the absorption capacity of the electrolyte and the ionic conductivity (up to 1.27 mS cm−1 at 30 °C). The gelling agent MDBS was further introduced to form GPGPE, which effectively suppressed the leakage of the electrolyte and achieved a higher electrical conductivity (6.02 mS cm−1) at a high temperature (80 °C). The capacity retention rate of the assembled LiFePO4 battery was 86.2% after 50 cycles, indicating that this star-shaped polymer additive can significantly enhance the battery's cycle performance and safety.148 Xu et al.132 studied the preparation of cross-linked polypropylene oxide (PPO) solid electrolyte films through additive modification. Cellulose skeleton was introduced to enhance mechanical strength, peg400 plasticizer improved segment mobility (Fig. 16f), and ZrO2 filler promoted lithium salt dissociation and broadened the electrochemical window. The room temperature ionic conductivity of the composite electrolyte reaches 6.67 × 10−4 S cm−1, with thermal stability >200 °C. The assembled LiFePO4 battery has stable cycling for 600 times, with high safety and electrochemical performance, making it suitable for solid-state lithium batteries.132
 | ||
| Fig. 16 (a) Synthesis pathway of PIM-1 (TTSBI and TFTPN condensation) and the three-dimensional nanopore structure constructed by its helical rigid molecular chain.145 (b) Simulation of lithium-ion adsorption sites for PIM-1, PVDF, and electrolyte solvents; PIM-1 regulates the solvation structure of lithium ions through strong adsorption, promoting the desolvation process.145 (a and b) Copyright 2022 Royal Society of Chemistry. (c) AMPS promotes Li+ conduction and forms a stable SEI through hydrogen bonding.146 Copyright 2022 Royal Society of Chemistry. (d) Schematic diagram of the dipole interaction between POSS-PEG nanofillers and lithium ions through Si–O–Si bonds and the promotion of lithium-ion transport by PEG segments.147 Copyright 2024 Royal Society of Chemistry. (e) Schematic diagram of star-shaped polymer additives forming highly conductive PGPE in PVDF.148 Copyright 2020 American Chemical Society. (f) Schematic diagram of the synthesis of cross-linked PPO electrolyte integrating cellulose skeleton, peg400 plasticizer, and ZrO2 filler.132 Copyright 2021 American Chemical Society. | ||
4.5 Bio-derived additives
Biobased additives have become a sustainable solution for improving the performance of lithium-ion batteries due to their renewability, low environmental load, and unique functional characteristics. Chitin, as a natural biopolymer, is mainly derived from squid, diatom extracellular fibers, etc. Its structure consists of a mixture of two parallel chains and one antiparallel chain, which is closely related to its performance in energy applications. Chitosan-based composite materials can serve as an environmentally friendly and low-cost alternative to traditional synthetic energy materials, especially due to their overall net cationic charge, low cost, ease of processing, and high availability, showing broad prospects in the energy field.149 Notably, the production cost of chitin is inherently low as it valorizes waste streams from the seafood industry, presenting a clear economic advantage over petroleum-derived synthetic additives, which are subject to volatile fossil fuel prices.Lignin, as the second-largest biomass resource in the plant kingdom, is a biopolymer with a three-dimensional network structure, formed by three phenylpropane units connected by ether bonds and carbon bonds. Its annual output reaches 50 million tons, with a wide range of sources (20–30% from forestry biomass, 10–20% from agricultural residues). This abundant availability as a by-product of the pulp and paper industry makes it a very low-cost raw material, significantly cheaper than many conventional synthetic additives. In electrochemical applications, lignin derivatives can serve as dopants for conductive polymers such as PEDOT. For example, lignosulfonate, as a negatively charged polyelectrolyte, can promote reversible redox reactions through counter ion exchange with PEDOT, resulting in a charge storage performance improvement of over 60%. In addition, phenolic acids derived from lignin can also serve as polymer dopants, significantly enhancing charge storage performance. Among them, the guaiacol unit is more capable of enhancing charge storage capacity than the eugenol unit.150
Mesoporous carbon materials derived from polysaccharides (such as Starbon). Electrode performance can be optimized through pore regulation. This type of material has a large pore volume (up to 0.91 cm3 g−1) and mesoporous structure, providing efficient transport pathways for lithium ions and electrons while promoting contact between the electrolyte and the active material surface. Its fibrous morphology helps to connect active material particles, significantly improving the electrochemical performance of Li4Ti5O12 (LTO) and TiO2-based electrodes, and becoming an ideal carbon additive for lithium-ion batteries due to its sustainability and environmental characteristics.151 The production of such polysaccharide-derived carbons often utilizes biomass waste and involves less energy-intensive processes compared to the synthesis of conventional carbon additives (e.g., carbon black or carbon nanotubes), offering a promising route for cost-effective and sustainable battery manufacturing. Biomaterials, such as cellulose nanofibers, play an important role in inhibiting electrode dissolution, buffering volume changes, and enhancing interfacial stability through their physical/chemical/environmental properties, such as high specific surface area and thermal stability, providing eco-friendly solutions for high-energy density batteries.152
Fig. 17a shows the process of preparing sustainable lithium battery separators by mixing cellulose slurry with biobased sodium alginate (SA) and flame-retardant through suspension, filtration, hot pressing, and drying processes. The biomass membrane enhances ion conductivity and suppresses polysulfide diffusion through SA, while flame-retardant improves thermal safety, thereby improving the cycling stability and environmental compatibility of lithium batteries.153 According to Fig. 17b, loofah anion exchange fiber is applied as a biomass additive in PEO-based electrolytes. Its surface quaternary ammonium cation promotes LiTFSI dissociation by interacting with TFSI− anion, while the surface –OH group forms a hydrogen bond network with PEO oxygen atoms, thereby improving ion conductivity and mechanical strength, and assisting in the development of safe lithium metal batteries.149Fig. 17c illustrates the mechanism of action of bio-based additive cellulose nanocrystals (CNC) in structural battery electrolytes. When the CNC content is low, the monomers are randomly dispersed. When the CNC content is high, the reaction-induced phase transition forms a multi-channel ion transport path. This design utilizes the physical cross-linking of CNC to enhance mechanical strength, and surface functional groups promote lithium salt dissociation, resulting in a 300% increase in ion conductivity (up to 1.1 × 10−3 S cm−1), effectively suppressing dendrite growth in lithium batteries and achieving safe high-energy density energy storage.149Fig. 17d shows the preparation process of the whole alginate-based energy storage device with kelp as a raw material. The alginate is carbonized and activated to form a porous carbon electrode, while the alginate gel acts as both electrolyte and diaphragm, realizing the integration of electrode–electrolyte diaphragm. This bio-based additive strongly binds to the active substance through carboxyl groups, inhibits electrode dissolution, and enhances ion conductivity, solving the environmental toxicity problem of traditional PVDF adhesives and providing a highly sustainable solution for lithium batteries.153
 | ||
| Fig. 17 (a) Schematic diagram of the wet forming process of a cellulose-based lithium battery separator containing sodium alginate and flame-retardant.153 (b) Schematic diagram of the preparation process of modified PEO electrolyte with loofah complex anion exchange fiber.149 (c) The influence of cellulose nanocrystal content on the conduction pathway of lithium ions in structured battery electrolytes and the schematic diagram of the multi-channel formation mechanism.149 (b and c) Copyright 2023 Royal Society of Chemistry. (d) Schematic diagram of the process of preparing a full component biomass energy storage device based on kelp-derived alginate, covering carbon electrode synthesis, gel electrolyte, and diaphragm integration.153 (a and d) Copyright 2020 Wiley-VCH Verlag GmbH & Co. KGaA. | ||
4.6 Synergistic additives
In the field of lithium-ion batteries, the synergistic/co-additive effect optimizes the electrode/electrolyte interface behavior through the combination of multiple additives, demonstrating significant performance improvement advantages. Research has demonstrated that incorporating a duplex additive of 2% vinylene carbonate (VC) and 1% tris(trimethylsilyl)phosphite (TMSP) into an EC/DMC electrolyte significantly enhances the cycling stability of Li-SPAN batteries. The synergistic mechanism arises from their cooperative action at the Li-metal anode interface: VC forms a flexible polymeric SEI through ring-opening polymerization, while TMSP decomposes to contribute robust inorganic components such as Li–P–O and Li–Si–O species. Together, they form a hybrid SEI layer that is both ionically conductive and mechanically strong, which effectively suppresses lithium dendrite growth and reduces parasitic electrolyte decomposition. This collaborative interface stabilization enables the cell to maintain a high reversible capacity of 1385.6 mAh g−1 after 500 cycles at 1.0C, far exceeding the performance obtained with either additive alone.50The combination of lithium difluorooxalate borate (LiDFOB) salt and FEC additive can synergistically enhance the interfacial stability of silicon-based anodes. This combination optimizes the mechanical stability and ionic conductivity of the SEI by forming inorganic components rich in LiF and a flexible organic polymer network on the anode surface. The formed SEI can effectively suppress the volume expansion effect of silicon-based anodes during cycling and reduce the loss of active lithium.154
Tri(trimethylsilyl)phosphate ester (TMSP) and propane sulfonate lactone (PCS), as dual functional additives, can preferentially decompose at the cathode and anode interface, achieving synergistic protection of the electrode. TMSP mainly acts on high-voltage cathodes (such as NCM811), stabilizing the cathode structure by removing harmful HF from the electrolyte and modifying the CEI layer. PCS mainly decomposes on the surface of the anode (such as MCMB), forming a sulfur-containing passivation layer (such as ROSO2Li, Li2SO3). This division of labor and collaboration mechanism synergistically improves the cycling performance of the battery over a wide temperature range (−60 to 50 °C).155
The combination of fluorinated vinyl carbonate (FEC) and lithium difluorophosphate (LiDFP) additives can effectively suppress the toxic by-products produced by the thermal decomposition of LiDFP. Although LiDFP can improve the high-voltage performance of batteries, its thermal decomposition may produce toxic substances such as oligofluorophosphate esters (Oligo OFPs). The addition of FEC can suppress the generation of these toxic by-products. Therefore, the combined application of the two not only retains the advantage of LiDFP in improving high-voltage performance but also significantly improves the environmental compatibility of the battery through the toxicity suppression effect of FEC.156
An et al.57 constructed a robust and uniform solid electrolyte interface film (SEI) in a 4.8 V high-voltage manganese-rich lithium-rich cathode/lithium metal battery by introducing trace amounts of pentafluoropropionic anhydride (PFPA) and fluoroethylene carbonate (FEC) as synergistic additives. Fig. 18a (top) shows the lithium dendrite growth and short circuit caused by unstable SEI when using the base electrolyte, as well as the dendrite-free safe cycling achieved by the uniform SEI formed after using PFPA + FEC. Fig. 18a (bottom) indicates that this additive combination still maintains high capacity at a high rate of 20 °C, significantly enhancing the rate performance and cycle stability of the battery.57Fig. 18b visually illustrates the collaborative mechanism between DTD and LiDFP through a schematic diagram. In the basic electrolyte (left), the loose SEI continues to deteriorate due to side reactions, leading to LiPF6 decomposition and gas production. In the DTD + LiDFP system (right), the Li2SO4 generated by DTD decomposition envelops the intermediate layer Li2CO3, blocking acid erosion. At the same time, LiDFP recrystallizes to form LiF/LixPOyFz, jointly constructing a dense and conductive SEI. This model clarifies that additives suppress interface side reactions through a synergistic pathway of “sulfide barrier + phosphate passivation”, providing a theoretical basis for the design of fast charging batteries.157Fig. 18c reveals the synergistic reduction mechanism of APFS and VC through dQ/dV curves. At the SiG–C anode interface, the disappearance of the reduction peak of APFS at 1.1 V and the reduction peak of VC indicates that the two undergo co-decomposition to form an elastic SEI containing LiF and copolymer, which significantly improves the high-temperature cycling performance of lithium batteries (72.5% capacity retention rate). The mechanism is attributed to free radical copolymerization and interface stabilization.129Fig. 18d illustrates the synergistic mechanism of various electrolyte additives (such as VC, LiPO2F2, etc.) during the formation of SEI and CEI through a reaction mechanism diagram. For example, VC protects the anode by forming a stable polycarbonate layer, while LiPO2F2 stabilizes the cathode interface by removing acidic byproducts such as HF. This synergistic effect significantly improves the cycling stability and thermal safety of lithium batteries in high and low temperature environments, providing a theoretical basis for the design of multifunctional additives.80 Duan et al.158 improved the performance of high-voltage lithium batteries through the synergistic effect of LiDFP (inorganic) and HTN (organic) dual additives. LiDFP preferentially reduces at the anode due to its lower LUMO, forming a stable SEI to suppress the expansion of the silicon carbon anode (Fig. 18e). HTN enhances the antioxidant property of the electrolyte due to its lower HOMO. Its cyano group (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) removes trace amounts of water/HF and inhibits cathode dissolution by complexing nickel/manganese ions. The two worked together to optimize the interface stability, enabling the capacity retention rate of the Si@C/LNMO full battery to reach 91.57% after 150 cycles (34.58% without additives), and the coulombic efficiency to reach 99.75%.158Fig. 18f shows the synergistic mechanism of LiTFA-LiNO3 double salt additives. LiTFA preferentially captures moisture to inhibit LiPF6 hydrolysis, enhance electrolyte moisture resistance, and promote LiNO3 dissolution. During charging, NO3− accumulates at the cathode interface, forming a double layer rich in Li+, solvent coordinated, and thermodynamically stable, optimizing interface dynamics and thermodynamic compatibility, significantly improving the cycling stability and rate performance of high-voltage lithium metal batteries (such as 4.4 V-NCM622).74
N) removes trace amounts of water/HF and inhibits cathode dissolution by complexing nickel/manganese ions. The two worked together to optimize the interface stability, enabling the capacity retention rate of the Si@C/LNMO full battery to reach 91.57% after 150 cycles (34.58% without additives), and the coulombic efficiency to reach 99.75%.158Fig. 18f shows the synergistic mechanism of LiTFA-LiNO3 double salt additives. LiTFA preferentially captures moisture to inhibit LiPF6 hydrolysis, enhance electrolyte moisture resistance, and promote LiNO3 dissolution. During charging, NO3− accumulates at the cathode interface, forming a double layer rich in Li+, solvent coordinated, and thermodynamically stable, optimizing interface dynamics and thermodynamic compatibility, significantly improving the cycling stability and rate performance of high-voltage lithium metal batteries (such as 4.4 V-NCM622).74
 | ||
| Fig. 18 (a) PFPA + FEC additives form a uniform SEI to achieve dendrite-free lithium metal batteries, and exhibit high performance at rates ranging from 0.1C to 20C.57 Copyright 2023 Wiley-VCH GmbH. (b) DTD and LiDFP collaborate to construct a dense SEI consisting of a sulfide barrier (Li2SO4 encapsulating Li2CO3) and phosphate passivation (LiF/LixPOyFz), which suppresses side reactions and enhances interface stability.157 Copyright 2022 Wiley-VCH GmbH. (c) The synergistic reduction characteristic peaks of APFS and VC in SiG–C/Li half-cells.129 Copyright 2023 Wiley-VCH GmbH. (d) VC and LiPO2F2 and other additives build a protective SEI/CEI on the electrode surface through preferential oxidation/reduction to synergistically inhibit the side reaction of electrolyte decomposition (such as ester exchange), thus significantly improving the mechanism of lithium battery performance at high and low temperatures.80 Copyright 2020 WILEY-VCH Verlag GmbH. (e) Schematic diagram of the synergistic mechanism between LiDFP and HTN additives at the cathodes and anodes.158 Copyright 2022 American Chemical Society. (f) Schematic diagram of the mechanism by which LiTFA–LiNO3 double salt additive enhances electrolyte performance through water capture and interface double-layer reconstruction.74 Copyright 2024 Wiley-VCH GmbH. | ||
Electrolyte additives, as a key component for enhancing the performance of lithium-ion batteries, can significantly improve the stability of the electrode/electrolyte interface, inhibit side reactions, enhance safety, and provide a wide-temperature range performance by introducing trace amounts. According to their functions and chemical structures, additives are mainly classified into various types such as film-forming agents, flame retardants, acid removers, and overcharge protectants, covering multiple elemental base compounds including boron, phosphorus, sulfur, fluorine, and nitrogen. They synergistically enhance the overall performance of the battery through mechanisms such as preferentially oxidizing/reducing to form a stable SEI/CEI layer, eliminating harmful species, and regulating the solvation structure. Table 1 provides a detailed classification, functional attributes, and mechanism overview to systematically summarize the characteristics and action mechanisms of various current additives.
| Classification | Name/structure | Main functions | Mechanisms | Ref. |
|---|---|---|---|---|
| B-containing | B(OSi(CH3)3)3 | Clear HF and form CEI | Reacting with HF to inhibit the leaching of transition metals | 19 and 159 |
| B-containing | C6H5B(OH)2 | Dual function interface modification | Low LUMO reduction, high HOMO oxidation | 160 |
| B-containing | B(OCH3)3 | Reacts with fluorine to generate B–F and participates in CEI | Nucleophilic reaction generates B–F interface, self-aggregation | 28 |
| B-containing | Diborate ester | High voltage interface stabilizer | Prioritize oxidation to form a film, adjust Li+ solvation | 161 |
| B-containing | C18BF15 | Promote LiNO3 dissolution and interface regulation | Lewis acid mediated solvation promotes anionic SEI | 162 |
| P-containing | P(OSi(CH3)3)3 | Clear HF and stabilize the interface | Reacting with HF to reduce LiF, form SEI | 104, 159 and 163 |
| P-containing | C9H9NO2S | High voltage cathode interface stability | Oxidation film formation after complexation with PF5 | 164 |
| P-containing | (C2H5O)3P | Cathodic protection, flame-retardant | Priority oxidation to passivation layer, low Li+ conductivity | 165 |
| P-containing | (CF3CH2O)3P | Cathodic protection, HF removal | Prioritize oxidation to form a stable interface, fluorine enhances oxidation stability | 165 |
| P-containing | C18F15P | High voltage cathode interface stability | Prioritize oxidation to form a protective layer | 115 |
| P-containing | CH3P(OC6H5)2 | High-pressure and high temperature enhancement | Interacting with TM to form CEI, clearing reactive oxygen species | 166 |
| S-containing | C2H6OS | Improve low-temperature performance | Form low impedance SEI to enhance Li+ transmission | 167 |
| S-containing | R–SO2–R′ | High anode stability | It has antioxidant properties but lacks film-forming ability | 159 |
| S-containing | C3H8O2S | High voltage electrolyte solvent | High oxidation stability ∼5.9 V | 115 |
| S-containing | C6H6Se | Change the sulfur pathway to improve cycle reversibility | Generate PhSeSSePh and change the redox path | 43 |
| S-containing | C7H13NO4S | Improve ion conductivity and Li+ migration number | Reduce PVDF crystallization and fix anions | 146 |
| S-containing | C2H4O6S2 | Protect the cathode under high voltage | Oxidation decomposition forms CEI, inhibiting decomposition | 168 |
| S-containing | C3H6O4S | Build a sulfur rich CEI/SEI to stabilize the interface | Oxidation-/reduction-triggered sulfur-rich interfacial layer formation | 159 and 169 |
| S-containing | C2F6LiNO4S2 | Improve oxidation stability | Form a stable CEI containing F/S | 169 |
| S-containing | C3H6O3S (hexacyclic sulfonate) | Cathode interface stabilizer | Reduce to generate RSO3Li and inhibit EC reduction | 170 |
| F-containing | 2-Fluoropyrazine | Formation and stability of cathode CEI | Ring opening polymerization forms dense CEI | 67 |
| N-containing | C5F5N | Solvation adjustment, rich LiF/Li3N interface | Prioritize restoring participation in CEI/SEI and regulating Li+ sheath | 171 |
| N-containing | C6H12LiN4O3Si | Anodic protection, SEI modification | Decompose nitrogen-containing species to enhance SEI conductivity | 172 |
| Salts/other compounds | Li2CO3 | Interface stability, Li+ conductor | Weaken the oxidation of Ni4+ and stabilize the interface | 159 |
| Salts/other compounds | LiB(C2O4)2 | Multi-functional film-forming agent | Forming a boron rich oxygen interface to inhibit decomposition | 173 |
| Salts/other compounds | LiBF2C2O4 | Enhance high voltage stability | Cathode forms inorganic CEI, anode has low impedance SEI | 20 and 173 |
| Salts/other compounds | LiBF4 | High temperature stability, improved low-temperature performance | Form a passivation layer to prevent aluminum corrosion | 13 and 173 |
| Salts/other compounds | CsGeI3 | Inhibit shuttle, reduce PEO crystallization, and improve conductivity | Adsorption of polysulfides, reduction of crystallization, generation of Cs/Ge/I/F-rich SEI | 174 |
| Salts/other compounds | CsPF6, RbPF6 | Inhibition of lithium dendrite growth | SHES mechanism guides the uniform deposition of lithium | 72 |
| SEI/CEI forming | PMSL | Form insoluble CEI, clear acid | C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C polymerization forms 3D CEI, capturing PF5/HF C polymerization forms 3D CEI, capturing PF5/HF |
109 |
| SEI/CEI forming | AMSL | Forming CEI, clearing acid | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C polymerization, forming soluble CEI C polymerization, forming soluble CEI |
109 |
| SEI/CEI forming | TMSL | Forming CEI, clearing acid | Si–O bond breaking generates Si–F, weak polymerization | 109 |
| SEI/CEI forming | BSA | HF removal, stable CEI, synergistic film formation | Si–N/Si–O clear HF, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N oxidative polymerization N oxidative polymerization |
106 |
| SEI/CEI forming | C12H10 | SEI/CEI forming agent | Oxidation polymerization forms an insulating layer | 115 |
| SEI/CEI forming | C3H2O3 | Anode SEI film-forming agent | Priority reduction to generate poly (VC) and CO2 | 170 and 175 |
| SEI/CEI forming | C3H3FO3 | SEI film formation, LiF precursor | Low LUMO reduction, generating LiF | 20, 159 and 175–177 |
| SEI/CEI forming | C9H10O2S | Enhance the interface stability of silicon-based electrodes | Prioritize reduction to dense SEI and suppress side reactions | 178 |
| Overcharge protection | NC(CH2)4CN | High voltage cosolvent | High oxidation stability ∼6.9 V, inhibits Al corrosion | 115 |
| Overcharge protection | C12H16 | Overcharge protection | Electropolymerization to increase resistance or short circuit | 68 |
| Overcharge protection | C12H10 | Overcharge protection | Electropolymerization consumes current and generates H2 to trigger voltage release | 179 and 180 |
| Overcharge protection | BPDB, TEDBPDP | High voltage overcharge protection | High oxidation potential 4.5–4.75 V | 84 |
| Flame-retardants | I2 | Inhibit high temperature expansion and temperature response | High temperature desorption, reversible redox, triggering self release | 181 |
| Flame-retardants | N3P3F5(OC2H5) | Efficient flame-retardant, CEI forming, high-voltage compatible | P, N, F synergistic flame-retardant, gas phase capture | 96, 182 and 183 |
| Flame-retardants | N3P3F5(OC6H5) | Efficient flame-retardant, thermally stable, electrode passivation | Gas phase free radical capture, electrode surface passivation | 184 |
| Flame-retardants | Fluorinated PNC with olefin groups | Flame-retardant, compatible with silicon anode | Olefins form SEI, phosphazene flame-retardant | 96 |
| Flame-retardants | (CF3CH2O)3PO | Flame-retardant and interface stability | Fluorine enhances reduction stability, phosphorus free radicals capture H˙ | 69, 95 and 182 |
| Flame-retardants | (C2H5O)3PO4 | Flame-retardant, electrolyte solvent | Gasification decomposes phosphorus free radicals and terminates combustion | 13, 32 and 175 |
| Flame-retardants | (CH3O)3PO4 | Flame-retardant | Thermal decomposition generates free radicals, interrupting combustion | 13 and 32 |
| Flame-retardants | CH3P(O)(OCH2CF3)2 | Flame-retardant, suitable for a wide temperature range | Release PO˙ free radicals and quench H˙/OH˙ | 182 |
| Flame-retardants | (C6H5O)3PO | Flame-retardant, thermal runaway suppression | Decompose to generate H3PO4, catalyze dehydration to carbon | 13 |
| Flame-retardants | C8H17OP(O)(OC6H5)2 | Flame-retardant and improved cycling stability | Electrochemical stability, compatible with graphite to form SEI | 13 |
| Acid scavengers | DSON | Acid removal and CEI/SEI formation | Prioritize oxidation to CEI, amine capture of H+ | 185 |
| Acid scavengers | Al2O3, MgO, CaCO3 | Neutralize HF and stabilize the cathode | Neutralize HF and reduce corrosion | 68 |
| Acid scavengers | (Me3SiO)3P | Multi-functional stabilizer | Eliminate HF/H2O and oxidize to CEI | 20 |
| Acid scavengers | Bis(trimethylsilyl)carbodiimide | Cathode protection, HF clearing | Oxidation into Si/N film, N captures H+/HF | 186 |
| LiPF6 stabilizers | P(OC2H4CF3)3 | HF cleared, PF5 stable | P(III) complex PF5, inhibit LiPF6 hydrolysis | 48, 49 and 182 |
| IL | PYR1(12)-FSI | Suppress dendrites and stabilize interfaces | Cationic electrostatic shielding, POM core rich in Li+ | 187 |
| IL | [C9(mim)2][TFSI]2 | High thermal stability >400 °C, high conductivity | Double cation structure enhances thermal stability and migration | 123 |
| IL | EMIBF4, BMIBF4 | High voltage electrolyte solvent | Form a passivation layer to increase the reduction potential | 159 |
| IL | BMP-TFSI, MPPpTFSI | High voltage electrolyte solvent | Wide window >5.5 V, forming a stable interface layer | 159 |
| IL | Py14-TFSI | High voltage electrolyte/cosolvent | Wide electrochemical window >5.5 V, good thermal stability | 115 |
| IL | EMIm-TFSI | Non-combustible cosolvents enhance safety | High thermal stability, low vapor pressure, diluted flammable | 188 |
| IL | Pyr14-TFSI | Non-combustible co-solvents enhance safety and thermal stability | Wide window, miscible with carbonate, reduces evaporation | 188 |
| IL | N5555-TFSI | Expand the window and suppress decomposition | High oxidation stability, reducing side reactions | 13 |
| IL | [C2OCH3mim][TFSI] | Reduce viscosity, improve conductivity, and enhance wetting | Ether based melting point viscosity reduction | 118 |
| Multi-functional | DMIO | Dual function interface protector | Generates SEI/CEI rich in inorganic | 61 |
| Multi-functional | lacOCA | Enhance SEI stability and interface protection | FEC induces LiF, LiDFOB enriches LiF layer | 189 |
| Multi-functional | C11H25O6PSi | Dual function of flame-retardant and film-forming | Phosphorus flame-retardant, silicon film-forming, synergistic stable interface | 101 |
| Multi-functional | N3P3F5(OC6H5) | SEI enhancement, electrolyte stability | Ring opening polymerization forms N/P-rich SEI | 128 |
| Nanomaterial | TiO2, SiO2, Al2O3 | Improve ion conductivity | Filler enhanced amorphous region | 190 |
| Polymer | CPs-BT COF | Enhance strength and provide ion channels | Reduce PEO crystallinity and enhance interface stability | 135 |
| Polymer | Se–S/Se–Se-containing polymer | Cathode/anode dual function protection | Curing polysulfides, catalytic conversion, promoting CEI/SEI | 78 |
| Bio-derivative | Bio-MOF-100 | Inhibit shuttle and dendrite formation | Ordered channel selective adsorption of Li+, catalyzing polysulfides | 191 |
| Bio-derivative | Biological MOF materials (such as bio-MOF-100) | Synchronize inhibition of shuttle and dendrite | Anionic framework selectively absorbs Li+, promoting uniform transport | 153 |
| Bio-derivative | Lignin derived carbon | Sustainable carbon source for electrodes | Carbonization forms a conductive network, enhancing performance | 153 and 192 |
| Bio-derivative | Starch derived carbon | Improve conductivity and ion transport | Large pore volume, optimized transmission path | 151 and 153 |
| Bio-derivative | Chitosan-based materials | Alternative synthetic materials, environmentally friendly | Net cationic charge promotes ion conduction | 149 and 153 |
| Bio-derivative | Sulfonated phenolic polymers | Improve charge storage and enhance electrode stability | Anti ion exchange promotes oxidation–reduction | 153 |
| Synergistic additive | PES + DTD/MMDS | High temperature optimization, gas suppression | PES stabilizes high temperature and forms a stable interface when used in combination | 80 |
| Synergistic additive | VC + TMSP | Improve cycle stability and maintain capacity | VC forms SEI, TMSP builds cathode film | 50 |
| Synergistic additive | TMSP + PCS | Dual function interface protection, clear HF | TMSP cathode film forms clear HF, PCS anode forms SEI | 166 |
| Synergistic additive | LiBOB + LiBF4 | Wide temperature range performance optimization | LiBF4 conducts electricity at low temperatures, while LiBOB forms a film at high temperatures | 55 |
| Synergistic additive | LiDFOB + FEC | Collaboratively build SEI/CEI | LiDFOB supplies boron and fluorine, and FEC increases LiF | 173 |
5 Challenges and outlook
Electrolyte additive technology still faces multiple compatibility issues, including interactions between additives (additive–additive conflicts), compatibility between additives and electrode materials (additive–electrode conflicts), and chemical incompatibility between additives and bulk electrolytes (additive–bulk electrolyte conflicts), which may lead to decreased interfacial stability or intensified side reactions. In terms of concentration optimization and side effects, the optimal concentration of additives needs to balance their effectiveness and negative effects, including increased electrolyte viscosity, loss of conductivity, and overgrowth of the SEI caused by excessive addition. For example, although high-concentration additives can improve performance, they may exacerbate polarization effects. The complexity of the mechanism is also a key bottleneck. The mechanism of action of additives often involves a multi-path interwoven reaction network (such as electrochemical decomposition, interface film formation, and ion solvent structure adjustment), and there are still difficulties in in situ characterization and basic theoretical understanding, which hinder directional design. Long-term stability assessment urgently needs to be strengthened, and existing research is mostly based on short-term testing, lacking validation of attenuation data under ultra-long cycles (>1000 times) and actual operating conditions. In addition, high-performance additives typically contain precious metals or toxic elements (such as fluorine and boron compounds), which result in high synthesis costs, difficulty in large-scale production, and insufficient consideration of environmental sustainability (such as biodegradability).76The development of new-generation battery-specific additives has become a key direction for enhancing battery performance. For high-voltage lithium-ion batteries (operating voltage higher than 4.5 V vs. Li+/Li), it is necessary to develop additives with strong antioxidant capabilities, such as sulfones, nitriles, and ionic liquid hybrids, to effectively inhibit the oxidation and decomposition of the electrolyte at high voltages. For lithium metal batteries (LMBs), the research focus should be on additives that inhibit lithium dendrite growth, and in combination with CEI stabilizers to enhance the uniformity of lithium deposition and interface stability. In silicon-based and alloy anode systems, it is necessary to design additives that can adapt to drastic volume changes, such as developing new binder–additive combinations that surpass traditional FEC, thereby constructing an ultra-stable SEI. The development of solid-state batteries (SSBs) urgently needs to address the issue of high solid–solid interface impedance. Therefore, interface compatibilizers, plasticizers, and mixed electrolyte formulations should be developed to improve ion transport performance.
In terms of additive design methods, it is necessary to integrate high-throughput computing driven by artificial intelligence and machine learning, such as molecular dynamics simulation and density functional theory, to achieve rational design and performance prediction. Through multifunctional molecular engineering, composite functional additives with film formation, flame retardancy, and conductivity improvement can be developed, thereby simplifying the electrolyte formula. In terms of characterization, advanced techniques such as in situ TEM, XRD, XPS, Raman, SIMS, NMR, and EQCM should be adopted to monitor the dynamic behavior of additives at the electrode interface in real time, and a standardized test protocol should be established to systematically evaluate their safety and long-cycle performance, accelerating the industrial transformation process.193 Additives are the core elements for achieving high energy density, fast charging, long life, and safety of lithium-ion batteries, and their commercial application depends on the continuous optimization of the formula. Under the current dual constraints of cost and environment, the development of low-cost and environmentally friendly additives (such as fluorine-free or low-toxicity compounds) has also become an important trend to meet the requirements of sustainable manufacturing. In addition, all parties involved in industry, academia, and research need to enhance collaboration, focus on the large-scale production of additives and their integration technology with electrolyte systems, and shorten the cycle from research and development to the market.
Overall, additives play an irreplaceable role in aspects such as the energy/power density, cycle life, safety, and temperature adaptability of batteries. Future research needs to further deepen the understanding of interface mechanisms, combine multi-scale simulation and in situ characterization to reveal the dynamic processes of additives in complex interfaces, and accelerate the development of dedicated additives for high-voltage lithium-ion batteries, lithium metal batteries, and solid-state batteries to break through the existing interface bottlenecks. At the same time, it is necessary to overcome the problem of large-scale production of high-cost additives and establish a standardized and systematic long-term stability evaluation system. As additive technology gradually evolves from single-function to multi-function and from experience-based trial and error to rational design, the performance boundaries of lithium-ion batteries will continue to expand, thereby better meeting various high-demand application scenarios.
Conflicts of interest
There are no conflicts of interest to declare.Abbreviations
| AEDB | 2-Aminoethyldiphenylboronic acid ester |
| AMPS | 2-Acrylamido-2-methylpropanesulfonic acid |
| AN | Acrylonitrile |
| APFS | 4-(Allyloxy)phenylfluorosulfonate |
| BMP | Boron fluoride |
| BNNT | Boron nitride nanotube |
| BPDB | 1,4-Bis[bis(1-methylethyl)phosphonyl]-2,5-dimethoxybenzene |
| BSA | Bis(trimethylsilyl)acetamide |
| CDI | N,N′-Carbonyldiimidazole |
| CEI | Cathode–electrolyte interphase |
| CNC | Cellulose nanocrystals |
| COF | Covalent organic framework |
| CPE | Composite electrolyte |
| CV | Cyclic voltammetry |
| DDB | Diaminodiphenylamine |
| DEC | Diethyl carbonate |
| DEEP | Diethyl ethylphosphonate |
| DETSP | Phosphorus–silicon bifunctional additive |
| DFEC | Difluoroethylene carbonate |
| DFT | Density functional theory |
| DMC | Dimethyl carbonate |
| DME | Dimethoxyethane |
| DMIO | 1,3-Dimethyl-1H-imidazole-2(3H)-one |
| DMMP | Dimethyl methylphosphonate |
| DMS | Dimethyl sulfoxide |
| DOL | 1,3-Dioxolane |
| DPDS | Diphenyl disulfide |
| DTD | 1,3,2-Dioxathiocyclopentane-2,2-dioxide |
| DTYP | Diethyl(thiophene-2-yl methyl)phosphinate |
| EC | Ethylene carbonate |
| EMC | Ethyl methyl carbonate |
| EPT | Ethyl phosphonic trichloride |
| EQCM | Electrochemical quartz crystal microbalance |
| FEC | Fluoroethylene carbonate |
| FP | Fluoropyrazine |
| FPPN | Pentafluorophenoxy cyclotriphosphazene |
| FSI | Bis(fluorosulfonyl)imide |
| FTIR | Fourier transform infrared spectroscopy |
| FTMS | Perfluorooctyltrimethoxysilane |
| GO | Graphene oxide |
| GPGPE | Glyme-based polymer gel electrolyte |
| HFP | Hexafluorophosphate |
| HOMO | Highest occupied molecular orbital |
| IL | Ionic liquid |
| LCO | Lithium cobalt oxide |
| LFP | Lithium iron phosphate |
| LIB | Lithium-ion battery |
| LNMO | Lithium nickel manganese oxide |
| LTO | Lithium titanate |
| LUMO | Lowest unoccupied molecular orbital |
| MCMB | Mesocarbon microbeads |
| MDBS | p,p′-Dibenzylidene sorbitol |
| MOF | Metal–organic framework |
| MPS | Methylphenylsulfone |
| MPT | Methyl propionate |
| NCA | Lithium nickel cobalt aluminum oxide |
| NCM | Lithium nickel manganese cobalt oxide |
| NMR | Nuclear magnetic resonance |
| PAES | Poly(arylene ether sulfone) |
| PAMPS | Poly(2-acrylamido-2-methylpropanesulfonic acid) |
| PBE-DG | Polymer borate ester based on diethylene glycol |
| PBE-DG | polymer borate ester based on diethylene glyco |
| PBMA | Poly(butyl methacrylate) |
| PCS | Propane sulfonate lactone |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEG | Polyethylene glycol |
| PEO | Polyethylene oxide |
| PES | Polyether sulfone |
| PFPN | Poly(pentafluorophenyl)phosphazene |
| PGPE | Polymer gel polymer electrolyte |
| PHIS | 2-Phenyl-1H-imidazole-1-sulfonate |
| POM | Polyoxometalate |
| POSS | Polyhedral oligomeric silsesquioxane |
| PPDI | Phenylene diisocyanate |
| PPO | Poly(phenylene oxide) |
| PVDF | Poly(vinylidene fluoride) |
| PVS | Poly(vinylsulfone) |
| REDOX | Reduction–oxidation |
| SA | Sodium alginate |
| SEI | Solid-electrolyte interphase |
| SEM | Scanning electron microscopy |
| SHES | Self-healing electrostatic shielding |
| SIMS | Secondary ion mass spectrometry |
| SPAN | Sulfurized polyacrylonitrile |
| TAP | Triallyl phosphate |
| TCEB | Tri(2-cyanoethyl)borate |
| TEDBPDP | Tetraethyl dibromophosphate |
| TEM | Transmission electron microscopy |
| TEMPO | 2,2,6,6-Tetramethylpiperidine-1-oxyl |
| TEP | Triethyl phosphate |
| TFA | Trifluoroacetic acid |
| TFP | Trifluorophosphate |
| TFSA | Trifluoromethanesulfonamide |
| TFSI | Bis(trifluoromethanesulfonyl)imide |
| TM | Transition metal |
| TMB | Trimethyl borate |
| TMP | Trimethyl phosphate |
| TMSB | Tris(trimethylsilyl)borate |
| TMSI | Tri(methylsilyl)imidazole |
| TMSP | Tris(trimethylsilyl)phosphite |
| TPPO | Triphenylphosphine oxide |
| TTFP | Tris(2,2,2-trifluoroethyl)phosphate |
| UV | Ultraviolet |
| VC | Vinylene carbonate |
| VDF | Vinylidene fluoride |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
The authors would like to acknowledge the financial support from the National Natural Science Foundation of China (NSFC: 22462009), Natural Science Foundation of Jiangxi Province (20224ACB204014, 20232ACB204001).References
- P. Dai, X. Kong, H. Yang, S. Kuang, J. Zeng and J. Zhao, ACS Appl. Mater. Interfaces, 2022, 14, 39927–39938 CrossRef CAS PubMed.
- Q. Huang, Y. Wu, Z. He, S. Wang, J. Zhu and X. Zhuang, Energy Technol., 2024, 13, 2401420 CrossRef.
- Y. Wu, G. Ge, S. Wang, L. Xiong and Z. He, Mater. Today, 2025, 54, 102124 CAS.
- Y. Wu, G. Ge, S. Wang, J. Zhu and X. Zhuang, J. Mater. Chem. A, 2025 10.1039/D5TA07277H.
- K. Kim, H. Ma, S. Park and N. Choi, ACS Energy Lett., 2020, 5, 1537–1553 CrossRef CAS.
- S. Wilken, P. Johansson and P. Jacobsson, Additives in Organic Electrolytes for Lithium Batteries, in Lithium Batteries, ed. B. Scrosati, K. M. Abraham, W. Van Schalkwijk and J. Hassoun, 2013 Search PubMed.
- Q. Liu, G. Yang, S. Li, S. Zhang, R. Chen, Z. Wang and L. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 21459–21466 CrossRef CAS PubMed.
- C. Wang, Y. Wu, S. Wang, J. Zhu and X. Zhuang, J. Energy Storage, 2025, 130, 117454 CrossRef.
- Y. Wu, R. Zhang, Q. Huang, J. Wang, K. Jiang, Z. Chen, J. Zhu and X. Zhuang, Chem. Commun., 2024, 60, 13578–13581 RSC.
- M. Huang, T. Hu, Y. Zhang, Z. Zhang, J. Yu and Z. Yang, ACS Appl. Mater. Interfaces, 2022, 14, 17959–17967 CrossRef CAS PubMed.
- D. Ouyang, K. Wang, J. Guan and Z. Wang, J. Power Sources, 2024, 607, 234550 CrossRef CAS.
- Y. Zhang, Y. Lu, J. Jin, M. Wu, H. Yuan, S. Zhang, K. Davey, Z. Guo and Z. Wen, Adv. Mater., 2023, 36, 2308484 CrossRef PubMed.
- X. Tian, Y. Yi, B. Fang, P. Yang, T. Wang, P. Liu, L. Qu, M. Li and S. Zhang, Chem. Mater., 2020, 32, 9821–9848 CrossRef CAS.
- N. von Aspern, D. Diddens, T. Kobayashi, M. Börner, O. Stubbmann-Kazakova, V. Kozel, G. Röschenthaler, J. Smiatek, M. Winter and I. Cekic-Laskovic, ACS Appl. Mater. Interfaces, 2019, 11, 16605–16618 CrossRef CAS PubMed.
- C. Liao, N. Shao, K. S. Han, X. Sun, D. Jiang, E. W. Hagaman and S. Dai, Phys. Chem. Chem. Phys., 2011, 13, 21503 RSC.
- C. Wang, M. Zhou, L. Zhou, L. Chang, J. He, W. Zhao, J. Sun and Q. Wang, Green Chem., 2025, 27, 8613–8624 RSC.
- T. Li, K. Chen, B. Yang, K. Li, B. Li, M. He, L. Yang, A. Hu and J. Long, Chem. Sci., 2024, 15, 12108–12117 RSC.
- D. Luo, M. Li, Y. Zheng, Q. Ma, R. Gao, Z. Zhang, H. Dou, G. Wen, L. Shui, A. Yu, X. Wang and Z. Chen, Adv. Sci., 2021, 8, 2101051 CrossRef CAS PubMed.
- S. Bolloju, N. Vangapally, Y. Elias, S. Luski, N. Wu and D. Aurbach, Prog. Mater. Sci., 2025, 147, 101349 CrossRef CAS.
- C. Wölke, B. A. Sadeghi, G. G. Eshetu, E. Figgemeier, M. Winter and I. Cekic-Laskovic, Adv. Mater. Interfaces, 2022, 9, 2101898 CrossRef.
- M. Yeddala, L. Rynearson, B. L. Lucht and L. Lucht, ACS Energy Lett., 2023, 8, 4782–4793 CrossRef CAS.
- L. Ma, J. Tan, Y. Wang, Z. Liu, Y. Yang, T. Gray, X. Zhang, M. Ye and J. Shen, Adv. Energy Mater., 2023, 13, 2300042 CrossRef CAS.
- Z. Zhuang, X. Dai, W. Dong, L. Jiang, L. Wang, C. Li, J. Yang, L. Wu, Z. Hu, J. Liu, L. Chen, Y. Li and B. Su, Electrochim. Acta, 2021, 393, 139042 CrossRef CAS.
- Z. Karkar, M. S. E. Houache, S. Niketic, C. Yim and Y. Abu-Lebdeh, ACS Appl. Energy Mater., 2022, 5, 14081–14091 CrossRef CAS.
- F. Liu, Z. Zhang, Z. Yu, X. Fan, M. Yi, M. Bai, Y. Song, Q. Mao, B. Hong, Z. Zhang and Y. Lai, Chem. Eng. J., 2022, 434, 134745 CrossRef CAS.
- H. Q. Pham, M. T. Nguyen, M. Tarik, M. El Kazzi and S. Trabesinger, ChemSusChem, 2021, 14, 2461–2474 CrossRef CAS PubMed.
- Q. Dong, F. Guo, Z. Cheng, Y. Mao, R. Huang, F. Li, H. Dong, Q. Zhang, W. Li, H. Chen, Z. Luo, Y. Shen, X. Wu and L. Chen, ACS Appl. Energy Mater., 2020, 3, 695–704 CrossRef CAS.
- Y. Song, Q. Mao, Q. Li, Z. Huang, Y. Wan, B. Hong and Q. Zhong, ACS Appl. Energy Mater., 2023, 6, 4271–4282 CrossRef CAS.
- Z. Qin, Y. Gao, F. Wang, W. Fang, T. Zhang, Y. Zhong, H. Liu, N. Zhang, X. Yuan and G. Chen, J. Power Sources, 2024, 614, 235035 CrossRef CAS.
- Z. Sun, F. Li, J. Ding, Z. Lin, M. Xu, M. Zhu and J. Liu, ACS Energy Lett., 2023, 8, 2478–2487 CrossRef CAS.
- C. Cao, Y. Zhong and Z. Shao, Chin. J. Chem., 2023, 41, 1119–1141 CrossRef CAS.
- D. Zhu, Y. Ren, Y. Yu, L. Zhang, Y. Wan, H. Wang, W. Gao, B. Han and L. Zhang, ChemElectroChem, 2023, 10, e202300009 CrossRef CAS.
- Z. Iskandar Radzi, K. Helmy Arifin, M. Zieauddin Kufian, V. Balakrishnan, S. Rohani Sheikh Raihan, N. Abd Rahim and R. Subramaniam, J. Electroanal. Chem., 2022, 920, 116623 CrossRef CAS.
- J. Han, I. Park, J. Cha, S. Park, S. Park, S. Myeong, W. Cho, S. Kim, S. Y. Hong, J. Cho and N. Choi, ChemElectroChem, 2016, 4, 56–65 CrossRef.
- S. Chen, G. Zheng, X. Yao, J. Xiao, W. Zhao, K. Li, J. Fang, Z. Jiang, Y. Huang, Y. Ji, K. Yang, Z. Yin, M. Zhang, F. Pan and L. Yang, ACS Nano, 2024, 18, 6600–6611 CrossRef CAS PubMed.
- A. Guéguen, C. Bolli, M. A. Mendez and E. J. Berg, ACS Appl. Energy Mater., 2019, 3, 290–299 CrossRef.
- H. J. Cheon, T. T. Vu, M. Woo, Y. Kim, H. Choi, J. Kim, Y. Choi, Y. A. Kim, J. W. Yun, Y. Ko and M. Chang, ACS Sustainable Chem. Eng., 2024, 12, 2686–2699 CrossRef CAS.
- D. Lu, J. He, Y. Qiu, J. Zhu, M. Zhang and Y. Cai, ACS Appl. Energy Mater., 2022, 5, 13600–13609 CrossRef CAS.
- C. Peebles, J. Garcia, A. P. Tornheim, R. Sahore, J. Bareño, C. Liao, I. A. Shkrob, H. H. Iddir and D. P. Abraham, J. Phys. Chem. C, 2018, 122, 9811–9824 CrossRef CAS.
- X. Wang, Z. Gu, W. Li, X. Zhao, J. Guo, K. Du, X. Luo and X. Wu, Chem.–Asian J., 2020, 15, 2803–2814 CrossRef CAS PubMed.
- R. Guo, Y. Che, G. Lan, J. Lan, J. Li, L. Xing, K. Xu, W. Fan, L. Yu and W. Li, ACS Appl. Mater. Interfaces, 2019, 11, 38285–38293 CrossRef CAS PubMed.
- X. Shi, T. Zheng, J. Xiong, B. Zhu, Y. Cheng and Y. Xia, ACS Appl. Mater. Interfaces, 2021, 13, 57107–57117 CrossRef CAS PubMed.
- J. Sun, K. Zhang, Y. Fu and W. Guo, Nano Res., 2022, 16, 3814–3822 CrossRef.
- Y. Lin, M. Xu, S. Wu, Y. Tian, Z. Cao, L. Xing and W. Li, ACS Appl. Mater. Interfaces, 2018, 10, 16400–16409 CrossRef CAS PubMed.
- J. Xiong, T. Zheng, Y.-J. Cheng, J. Sun, R. Cao and Y. Xia, ACS Appl. Mater. Interfaces, 2021, 13, 18648–18657 CrossRef CAS PubMed.
- B. Tong, Z. Song, H. Wan, W. Feng, M. Armand, J. Liu, H. Zhang and Z. Zhou, InfoMat, 2021, 3, 1364–1392 CrossRef CAS.
- Y. Huang, Y. Li, C. Tan, Z. Huang, Q. Pan, Y. Chu, F. Zheng, H. Wang and Q. Li, ACS Appl. Energy Mater., 2022, 5, 639–647 CrossRef CAS.
- J. Han, K. Kim, Y. Lee and N. Choi, Adv. Mater., 2018, 31, 1804822 CrossRef PubMed.
- J. Liu, S. He, S. Liu, S. Wang and J. Zhang, J. Mater. Chem. A, 2022, 10, 22929–22954 RSC.
- W. Chen, B. Li, C. Zhao, M. Zhao, T. Yuan, R. Sun, J. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2020, 59, 10732–10745 CrossRef CAS PubMed.
- Y. Han, J. Yoo and T. Yim, RSC Adv., 2017, 7, 20049–20056 RSC.
- Y. Han, J. Yoo and T. Yim, J. Mater. Chem. A, 2015, 3, 10900–10909 RSC.
- A. K. Haridas, Q. A. Nguyen, T. Terlier, R. Blaser and S. L. Biswal, ACS Appl. Mater. Interfaces, 2021, 13, 2662–2673 CrossRef CAS PubMed.
- J. Han, M. Jeong, K. Kim, C. Park, C. H. Sung, D. W. Bak, K. H. Kim, K. Jeong and N. Choi, J. Power Sources, 2020, 446, 227366 CrossRef CAS.
- X. Yang, P. Li, C. Guo, W. Yang, N. Zhou, X. Huang and Y. Yang, J. Power Sources, 2024, 624, 235563 CrossRef CAS.
- Q. Zhao, Y. Wu, Z. Yang, D. Song, X. Sun, C. Wang, L. Yang, Y. Zhang, J. Gao, T. Ohsaka, F. Matsumoto and J. Wu, Chem. Eng. J., 2022, 440, 135939 CrossRef CAS.
- K. An, M. J. Joo, Y. H. T. Tran, S. Kwak, H. G. Kim, C. S. Jin, J. Suk, Y. Kang, Y. J. Park and S. Song, Adv. Funct. Mater., 2023, 33, 2301755 CrossRef CAS.
- H. Tu, S. Li, C. Liu, Z. Luo, L. Ni, Y. Zhang, W. Deng, G. Zou, L. Zhou, H. Hou and X. Ji, ACS Appl. Mater. Interfaces, 2023, 15, 53533–53539 CrossRef CAS PubMed.
- Q. Chen, M. Chen, Z. Xie, K. Zhou, T. Chen, S. Luo, S. Chen, R. Li, X. Li, M. Xu and W. Li, J. Phys. Chem. Lett., 2023, 14, 10863–10869 CrossRef CAS PubMed.
- B. K, M. Jareer, G. L. Sagar, P. Mukesh, A. Amudha, D. Mandal, H. S. Nagaraja and S. Shahgaldi, J. Phys. Chem. C, 2025, 129, 11221–11251 CAS.
- J. Wang, T. Ou, L. Gao, L. Zeng, H. Sun, Y. Hu, X. Pei and Y. Tan, ACS Nano, 2024, 18, 5930–5939 Search PubMed.
- T. Dong, S. Zhang, Z. Ren, L. Huang, G. Xu, T. Liu, S. Wang and G. Cui, Adv. Sci., 2023, 11, 2305753 CrossRef PubMed.
- T. Ren, S. Liu, T. He, S. Zhong, H. Wan, Q. Liu, Y. Zhang and J. Liu, J. Phys. Chem. Lett., 2025, 16, 6402–6409 CrossRef CAS PubMed.
- S. Stuckenberg, M. M. Bela, C. Lechtenfeld, M. Mense, V. Küpers, T. T. K. Ingber, M. Winter and M. C. Stan, Small, 2023, 20, 2305203 CrossRef PubMed.
- A. Gupta, Z. Yang, S. Trask, I. Bloom and C. Johnson, J. Electrochem. Soc., 2023, 170, 010504 CrossRef CAS.
- Z. Wu, J. Duan, C. Sun, J. Zheng, D. Sun and M. Hong, ACS Appl. Mater. Interfaces, 2025, 17, 35372–35381 CrossRef CAS PubMed.
- X. Zhang, G. Liu, K. Zhou, Y. Cheng, T. Jiao, L. Huang, Y. Zou, M. Wang, Y. Yang and J. Zheng, J. Colloid Interface Sci., 2022, 618, 431–441 CrossRef CAS PubMed.
- L. Liu, M. Li, L. Chu, B. Jiang, R. Lin, X. Zhu and G. Cao, Prog. Mater. Sci., 2020, 111, 100655 CrossRef CAS.
- Z. Chen, Y. Chao, W. Li, G. G. Wallace, T. Bussell, J. Ding and C. Wang, Adv. Sci., 2021, 8, 2003694 CrossRef CAS PubMed.
- C. Senthil, A. Subramani, R. K. Gupta and Z. Sofer, Small, 2025, 21, 2504276 CrossRef CAS PubMed.
- H. Du, Y. Wang, Y. Kang, Y. Zhao, Y. Tian, X. Wang, Y. Tan, Z. Liang, J. Wozny, T. Li, D. Ren, L. Wang, X. He, P. Xiao, E. Mao, N. Tavajohi, F. Kang and B. Li, Adv. Mater., 2024, 36, 2401482 CrossRef CAS PubMed.
- Y. Yu, Y. Liu and J. Xie, ACS Appl. Mater. Interfaces, 2020, 13, 18–33 Search PubMed.
- N. Piao, S. Liu, B. Zhang, X. Ji, X. Fan, L. Wang, P.-F. Wang, T. Jin, S.-C. Liou, H. Yang, J. Jiang, K. Xu, M. A. Schroeder, X. He and C. Wang, ACS Energy Lett., 2021, 6, 1839–1848 CrossRef CAS.
- Z. Wen, W. Fang, F. Wang, H. Kang, S. Zhao, S. Guo and G. Chen, Angew. Chem., Int. Ed., 2024, 63, e202314876 Search PubMed.
- J. Tan, X. Li, Z. Fang and J. Shen, ACS Appl. Mater. Interfaces, 2023, 15, 17893–17903 Search PubMed.
- D. Huang, C. Zeng, M. Liu, X. Chen, Y. Li, S. Hu, Q. Pan, F. Zheng, Q. Li and H. Wang, Chem. Eng. J., 2023, 454, 140395 CrossRef CAS.
- Y. Zhang, X. Li, E. Sivonxay, J. Wen, K. A. Persson, J. T. Vaughey, B. Key and F. Dogan, Adv. Energy Mater., 2021, 11, 2101820 CrossRef CAS.
- Y. Ding, X. Li, Y. Chen, Y. Pi, J. Yu, L. Yuan and F. Wang, Chem. Eng. J., 2024, 482, 148803 CrossRef CAS.
- A. Fu, J. Lin, Z. Zhang, C. Xu, Y. Zou, C. Liu, P. Yan, D.-Y. Wu, Y. Yang and J. Zheng, ACS Energy Lett., 2022, 7, 1364–1373 CrossRef CAS.
- J. Hou, M. Yang, D. Wang and J. Zhang, Adv. Energy Mater., 2020, 10, 1904152 CrossRef CAS.
- H. Liu, A. J. Naylor, A. S. Menon, W. R. Brant, K. Edström and R. Younesi, Adv. Mater. Interfaces, 2020, 7, 2000277 CrossRef CAS.
- Q. Li, X. Liu, X. Han, Y. Xiang, G. Zhong, J. Wang, B. Zheng, J. Zhou and Y. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 14066–14075 CrossRef CAS PubMed.
- X. Zheng, X. Wang, X. Cai, L. Xing, M. Xu, Y. Liao, X. Li and W. Li, ACS Appl. Mater. Interfaces, 2016, 8, 30116–30125 CrossRef CAS PubMed.
- L. Wen, J. Liang, J. Chen, Z. Chu, H. Cheng and F. Li, Small Methods, 2019, 3, 1900323 CrossRef CAS.
- J. Liu, Y. Wu, Z. Chen, Z. Xu, J. Zhu and X. Zhuang, Mater. Chem. Front., 2025, 9, 1971–1996 RSC.
- S. Rana, R. Kumar and R. S. Bharj, Chem. Eng. J., 2023, 463, 142336 CrossRef CAS.
- D. Gao, J. B. Xu, M. Lin, Q. Xu, C. F. Ma and H. F. Xiang, RSC Adv., 2015, 5, 17566–17571 RSC.
- S. A. Odom, S. Ergun, P. P. Poudel and S. R. Parkin, Energy Environ. Sci., 2014, 7, 760–767 RSC.
- W. Weng, J. Huang, I. A. Shkrob, L. Zhang and Z. Zhang, Adv. Energy Mater., 2016, 6, 1600795 CrossRef.
- N. Choi, Z. Chen, S. A. Freunberger, X. Ji, Y. Sun, K. Amine, G. Yushin, L. F. Nazar, J. Cho and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 9994–10024 CrossRef CAS PubMed.
- M. D. Casselman, A. P. Kaur, K. A. Narayana, C. F. Elliott, C. Risko and S. A. Odom, Phys. Chem. Chem. Phys., 2015, 17, 6905–6912 RSC.
- F. T. Krauss, I. Pantenburg, V. Lehmann, M. Stich, J. O. Weiershäuser, A. Bund and B. Roling, J. Am. Chem. Soc., 2024, 146, 19009–19018 CrossRef CAS PubMed.
- J. Feng, X. Gao, L. Ci and S. Xiong, RSC Adv., 2016, 6, 7224–7228 RSC.
- C. Kim, D. Shin, K. Kim, K. Shin, J. Woo, S. Kim, S. Y. Hong and N. Choi, ChemElectroChem, 2016, 3, 913–921 CrossRef CAS.
- Y. Gu, S. Fang, L. Yang and S.-i. Hirano, ACS Appl. Energy Mater., 2021, 4, 4919–4927 CrossRef CAS.
- T. Lyu, T. Ding, Z. Wang, G. Chen, S. Zhao, Z. Wang and S. Fang, J. Electroanal. Chem., 2024, 971, 118606 CrossRef CAS.
- X. Zhang, X. Lin, P. Xu, R. Yuan, D. Gupta, R. Rupp, G. Barozzino-Consiglio, H. Xu, Q. Dong and A. Vlad, J. Power Sources, 2022, 541, 231644 CrossRef CAS.
- Y. Sun, P. Shi, H. Xiang, X. Liang and Y. Yu, Small, 2019, 15, 1805479 CrossRef PubMed.
- L. Chen, Q. Nian, D. Ruan, J. Fan, Y. Li, S. Chen, L. Tan, X. Luo, Z. Cui, Y. Cheng, C. Li and X. Ren, Chem. Sci., 2023, 14, 1184–1193 RSC.
- L. Jiang, Y. Cheng, S. Wang, Y. Cheng, K. Jin, J. Sun, M. Winter, I. Cekic-Laskovic and Q. Wang, J. Power Sources, 2023, 570, 233051 CrossRef CAS.
- M. Liu, Q. Liu, Y. Quan, J. Yu, G. Wu, X. Wang and Y. Wang, Chin. Chem. Lett., 2024, 35, 109123 CrossRef CAS.
- Y. Wu, Z. Zeng, S. Lei, M. Liu, W. Zhong, M. Qin, S. Cheng and J. Xie, Angew. Chem., 2023, 135, e202217774 CrossRef.
- C. Wang, Y. Wu, S. Wang, E. van der Heide and X. Zhuang, Mater. Res. Bull., 2025, 181, 113078 CrossRef CAS.
- F. Zou, J. Wang, X. Zheng, X. Hu, J. Wang and M. Wang, Electrochim. Acta, 2022, 428, 140958 CrossRef CAS.
- J. Kim, V. A. K. Adiraju, N. Rodrigo, J. Hoffmann, M. Payne and B. L. Lucht, ACS Appl. Mater. Interfaces, 2021, 13, 22351–22360 CrossRef CAS PubMed.
- Y. Zheng, N. Xu, S. Chen, Y. Liao, G. Zhong, Z. Zhang and Y. Yang, ACS Appl. Energy Mater., 2020, 3, 2837–2845 CrossRef CAS.
- S. Dai, W. Fang, T. Wang, Y. Gao, T. Zhang, Z. Qin, G. Chen and X. Zhou, Chem. Eng. J., 2024, 500, 157269 CrossRef CAS.
- A. S. Wotango, W.-N. Su, E. G. Leggesse, A. M. Haregewoin, M.-H. Lin, T. A. Zegeye, J.-H. Cheng and B.-J. Hwang, ACS Appl. Mater. Interfaces, 2017, 9, 2410–2420 CrossRef CAS PubMed.
- Y. Chen, Q. He, Y. Mo, W. Zhou, Y. Zhao, N. Piao, C. Liu, P. Xiao, H. Liu, B. Li, S. Chen, L. Wang, X. He, L. Xing and J. Liu, Adv. Energy Mater., 2022, 12, 2201631 CrossRef CAS.
- W. Wang, T. Yang, S. Li, J. Lu, X. Zhao, W. Fan, C. Fan, X. Zuo, S. Tie and J. Nan, J. Power Sources, 2021, 483, 229172 CrossRef CAS.
- X. Ma, J. Yu, Q. Dong, X. Zou, L. Zheng, Y. Hu, Y. Shen, L. Chen and F. Yan, ACS Appl. Mater. Interfaces, 2022, 14, 41103–41113 CrossRef CAS PubMed.
- H. Jia, B. Billmann, H. Onishi, J. Smiatek, S. Roeser, S. Wiemers-Meyer, R. Wagner, M. Winter and I. Cekic-Laskovic, Chem. Mater., 2019, 31, 4025–4033 CrossRef CAS.
- X. He, Y. Li, W. Wang, X. Zeng, H. Hu, H. Li, W. Fan, C. Fan, J. Cai, Z. Ma and J. Nan, J. Energy Chem., 2023, 80, 10–22 CrossRef CAS.
- H. Zhang, G. G. Eshetu, X. Judez, C. Li, L. M. Rodriguez-Martínez and M. Armand, Angew. Chem., Int. Ed., 2018, 57, 15002–15027 CrossRef CAS PubMed.
- S. Tan, Y. J. Ji, Z. R. Zhang and Y. Yang, ChemPhysChem, 2014, 15, 1956–1969 CrossRef CAS PubMed.
- S. Moharana, G. West, A. S. Menon, W. L. da Silva, M. Walker and M. J. Loveridge, ACS Appl. Mater. Interfaces, 2023, 15, 50185–50195 CrossRef CAS PubMed.
- X. Chen, Y. Gong, X. Li, F. Zhan, X. Liu and J. Ma, Miner. Metall., 2022, 30, 1–13 Search PubMed.
- F. Hu and T. Song, RSC Adv., 2017, 7, 54203–54212 RSC.
- Z. Zou, H. Xu, H. Zhang, Y. Tang and G. Cui, ACS Appl. Mater. Interfaces, 2020, 12, 21368–21385 CrossRef CAS PubMed.
- K. Chatterjee, A. D. Pathak, K. K. Sahu and A. K. Singh, ACS Omega, 2020, 5, 16681–16689 CrossRef CAS PubMed.
- X. Ma, J. Yu, Y. Hu, J. Texter and F. Yan, Ind. Chem. Mater., 2023, 1, 39–59 RSC.
- Y. Cai, Y. Chen, H. Zhang, N. von Solms, G. M. Kontogeorgis, J. M. Woodley and K. Thomsen, J. Phys. Chem. C, 2022, 126, 11498–11509 CrossRef CAS.
- T. C. Nirmale, N. D. Khupse, R. S. Kalubarme, M. V. Kulkarni, A. J. Varma and B. B. Kale, ACS Sustainable Chem. Eng., 2022, 10, 8297–8304 CrossRef CAS.
- W. Wang, W. Ma, Q. Yang, Z. Lin, J. Tang, M. Wang, Y. He, C. Fan and K. Sun, Ind. Eng. Chem. Res., 2022, 61, 10883–10890 CrossRef CAS.
- W. Wang, H. Hu, X. Zeng, W. Fan, T. Yang, X. Zhao, C. Fan, X. Zuo and J. Nan, ACS Appl. Energy Mater., 2021, 4, 7101–7111 CrossRef CAS.
- J. Lu, X. Xu, W. Fan, Y. Xin, W. Wang, C. Fan, P. Cheng, J. Zhao, J. Liu and Y. Huo, ACS Appl. Energy Mater., 2022, 5, 6324–6334 CrossRef CAS.
- L. Quach, E. Adhitama, V. Göldner, A. Das, F. Demelash, M. Winter, U. Karst, T. Placke and F. Glorius, ACS Appl. Energy Mater., 2023, 6, 9837–9850 CrossRef CAS.
- A. Ghaur, C. Peschel, I. Dienwiebel, L. Haneke, L. Du, L. Profanter, A. Gomez-Martin, M. Winter, S. Nowak and T. Placke, Adv. Energy Mater., 2023, 13, 2203503 CrossRef CAS.
- H. Moon, H. Nam, M. P. Kim, S. Lee, H. Kim, M. H. Jeon, Y. Lee, K. Kim, J. Chun, S. K. Kwak, S. Y. Hong and N. Choi, Adv. Funct. Mater., 2023, 33, 2303029 CrossRef CAS.
- Y. Chen, Z. Xie, G. Lan, Y. Liu, J. He, Z. Li, C. Wu, L. Xing and W. Li, Chem. Eng. J., 2024, 500, 157218 CrossRef CAS.
- Y. Zhu, X. Luo, H. Zhi, Y. Liao, L. Xing, M. Xu, X. Liu, K. Xu and W. Li, J. Mater. Chem. A, 2018, 6, 10990–11004 RSC.
- H. Xu, M. Jing, Z. Huang, J. Li, T. Wang, W. Yuan, B. Ju and X. Shen, ACS Appl. Polym. Mater., 2021, 3, 6539–6547 CrossRef CAS.
- J. Chen and S. Han, Chem. Eng. J., 2023, 470, 144150 CrossRef CAS.
- T. Luo, Y. Wang, B. Elander, M. Goldstein, Y. Mu, J. Wilkes, M. Fahrenbruch, J. Lee, T. Li, J. L. Bao, U. Mohanty and D. Wang, Adv. Mater., 2023, 36, 2306239 CrossRef PubMed.
- J. Chen, J. Zhang, X. Wang, N. Fu and Z. Yang, Electrochim. Acta, 2023, 449, 142267 CrossRef CAS.
- G. Ge, Y. Wu, E. van der Heide, Z. Chen, J. Zhu and X. Zhuang, Energy Storage, 2024, 90, 111900 CrossRef.
- J. Liang, W. Deng, X. Zhou, S. Liang, Z. Hu, B. He, G. Shao and Z. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 29500–29510 CrossRef CAS PubMed.
- F. Chu, J. Hu, C. Wu, Z. Yao, J. Tian, Z. Li and C. Li, ACS Appl. Mater. Interfaces, 2018, 11, 3869–3879 CrossRef PubMed.
- J. Yan, J. Li, W. Fang, Y. Gao, Z. Qin, M. Sun, Y. Zhang, N. Zhang, X. Liu and G. Chen, Chem. Eng. J., 2024, 494, 153104 CrossRef CAS.
- W. Li, S. Zhang, B. Wang, S. Gu, D. Xu, J. Wang, C. Chen and Z. Wen, ACS Appl. Mater. Interfaces, 2018, 10, 23874–23882 CrossRef CAS PubMed.
- D. Yadav, J.-H. Jung, Y. Lee, T. Y.-S. Kim, E. Park, K.-I. Choi, J. Cha, W.-J. Song, J.-H. Choi, S. Doo and J. Kim, ACS Mater. Lett., 2023, 5, 2648–2655 CrossRef CAS.
- S. Chaudhari, P. Nandi and C. Subramaniam, Nanoscale, 2025, 17, 13121–13144 RSC.
- L. Zhao, Y. Zhong, C. Cao, T. Tang and Z. Shao, Nano–Micro Lett., 2024, 16, 124 CrossRef CAS PubMed.
- M. Zhu, J. Cheng, Q. Wang, M. Zhu, Y. Lin, C. Lian and H. Liu, Ind. Eng. Chem. Res., 2025, 64, 9396–9405 CrossRef CAS.
- R. Qi, C. Yang, L. Ma, X. Fan, Q. Wu, C. Wang, Y. Cheng, K. Guo, Y. Gao and Y. Xia, J. Mater. Chem. A, 2022, 10, 8047–8058 RSC.
- S. Guan, K. Wen, Y. Liang, C. Xue, S. Liu, J. Yu, Z. Zhang, X. Wu, H. Yuan, Z. Lin, H. Yu, L. Li and C. Nan, J. Mater. Chem. A, 2022, 10, 24269–24279 RSC.
- Y. Shin, A. Le Mong, C. Nguyen Thi Linh and D. Kim, J. Mater. Chem. A, 2024, 12, 13980–13993 RSC.
- S. Li, Z. Xiao, K. Guo, H. Gan, J. Wang, Y. Zhang, L. Yu and Z. Xue, Langmuir, 2020, 36, 9616–9625 CrossRef CAS PubMed.
- O. Sheng, C. Jin, T. Yang, Z. Ju, J. Luo and X. Tao, Energy Environ. Sci., 2023, 16, 2804–2824 RSC.
- W. Zheng, Z. Huang, C. Shi, Y. Deng, Z. Wen, Z. Li, H. Chen, Z. Chen, L. Huang and S. Sun, ChemSusChem, 2023, 16, e202202252 CrossRef CAS PubMed.
- S. Kim, A. M. Escamilla-Pérez, M. De bruyn, J. G. Alauzun, N. Louvain, N. Brun, D. Macquarrie, L. Stievano, B. Boury, L. Monconduit and P. H. Mutin, J. Mater. Chem. A, 2017, 5, 24380–24387 RSC.
- S. Kim, J. Yang, D. Liu, Y. Huang and Y. Lee, Adv. Funct. Mater., 2020, 31, 2008354 CrossRef.
- C. Liedel, ChemSusChem, 2020, 13, 2110–2141 CrossRef CAS PubMed.
- D. Hubble, D. E. Brown, Y. Zhao, C. Fang, J. Lau, B. D. McCloskey and G. Liu, Energy Environ. Sci., 2022, 15, 550–578 RSC.
- K. Zhang, X. Zhang, W. He, W. Xu, G. Xu, X. Yi, X. Yang and J. Zhu, J. Mater. Chem. A, 2019, 7, 9890–9902 RSC.
- M. Kubot, L. Frankenstein, E. Muschiol, S. Klein, M. Esselen, M. Winter, S. Nowak and J. Kasnatscheew, ChemSusChem, 2023, 16, e202202189 CrossRef CAS PubMed.
- Y. Hu, Z. Zhang and H. Wang, ChemistrySelect, 2022, 7, e202200740 CrossRef CAS.
- K. Duan, J. Ning, L. Zhou, S. Wang, Q. Wang, J. Liu and Z. Guo, ACS Appl. Mater. Interfaces, 2022, 14, 10447–10456 CrossRef CAS PubMed.
- Y. Zhu and T. Yi, Ionics, 2016, 22, 1759–1774 CrossRef CAS.
- S. Luo, C. Ge, L. Ou, F. Zeng, Y. Wang and H. Lu, Electrochim. Acta, 2024, 498, 144684 CrossRef CAS.
- Y. Xiao, X. Shi, T. Zheng, Y. Yue, S. Shi, Y. Cheng and Y. Xia, ACS Appl. Energy Mater., 2023, 6, 4817–4824 CrossRef CAS.
- S. Li, W. Zhang, Q. Wu, L. Fan, X. Wang, X. Wang, Z. Shen, Y. He and Y. Lu, Angew. Chem., 2020, 132, 15045–15051 CrossRef.
- A. Jamal, G. D. Salian, A. Mathew, W. Wahyudi, R. P. Carvalho, R. Gond, S. K. Heiskanen, D. Brandell and R. Younesi, ChemElectroChem, 2023, 10, e202300139 CrossRef CAS.
- J. Zhu, M. Zhang, Y. Gai, R. Zeng, Y. Cai and D. Lu, J. Power Sources, 2023, 576, 233227 CrossRef CAS.
- M. He, C. Su, C. Peebles, Z. Feng, J. G. Connell, C. Liao, Y. Wang, I. A. Shkrob and Z. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 11450–11458 CrossRef CAS PubMed.
- H. Zhao, X. Yu, J. Li, B. Li, H. Shao, L. Li and Y. Deng, J. Mater. Chem. A, 2019, 7, 8700–8722 RSC.
- C. Zhang, S. Huo, B. Su, C. Bi, C. Zhang and W. Xue, J. Power Sources, 2024, 606, 234559 CrossRef CAS.
- Y. Cui, C. Yang, Z. Zhuang, M. Wang and Q. Zhuang, J. Inorg. Organomet. Polym. Mater., 2017, 28, 731–737 CrossRef.
- S. H. Kim, N. Park, W. Bo Lee and J. H. Park, Small, 2023, 20, 2309160 CrossRef PubMed.
- B. Zhang, M. Metzger, S. Solchenbach, M. Payne, S. Meini, H. A. Gasteiger, A. Garsuch and B. L. Lucht, J. Phys. Chem. C, 2015, 119, 11337–11348 CrossRef CAS.
- P. Li, Z. Cheng, J. Liu, L. Che, Y. Zhou, E. Xu, X. Tian and Z. Yuan, Small, 2023, 19, 2303149 CrossRef CAS PubMed.
- V. A. K. Adiraju, O. B. Chae, J. R. Robinson and B. L. Lucht, ACS Energy Lett., 2023, 8, 2440–2446 CrossRef CAS.
- G. Hernández, R. Mogensen, R. Younesi and J. Mindemark, Batteries Supercaps, 2022, 5, e202100373 CrossRef.
- P. Wang, Y. Zhao, S. Tian, Z. Shen and T. Yi, Energy Fuels, 2023, 37, 15198–15205 CrossRef CAS.
- L. Jiang, C. Liang, H. Li, Q. Wang and J. Sun, ACS Appl. Energy Mater., 2020, 3, 1719–1729 CrossRef CAS.
- Y. Xu, T. Ding, D. Sun, X. Ji and X. Zhou, Adv. Funct. Mater., 2022, 33, 2211290 CrossRef.
- Y. Liu, C. Zhao, J. Du, X. Zhang, A. Chen and Q. Zhang, Small, 2022, 19, 2205315 CrossRef PubMed.
- G. Liu, J. Gao, M. Xia, Y. Cheng, M. Wang, W. Hong, Y. Yang and J. Zheng, ACS Appl. Mater. Interfaces, 2022, 14, 38281–38290 CrossRef CAS PubMed.
- J. Feng, L. Ci and S. Xiong, RSC Adv., 2015, 5, 96649–96652 RSC.
- G. G. Eshetu, M. Martinez-Ibañez, E. Sánchez-Diez, I. Gracia, C. Li, L. M. Rodriguez-Martinez, T. Rojo, H. Zhang and M. Armand, Chem.–Asian J., 2018, 13, 2770–2780 CrossRef CAS PubMed.
- S. Park, H. J. Kim, J. Jeon, Y. Choi, J. Cho and H. Lee, ChemElectroChem, 2016, 3, 1915–1921 CrossRef CAS.
- T. Dagger, B. R. Rad, F. M. Schappacher and M. Winter, Energy Technol., 2018, 6, 2011–2022 CrossRef CAS.
- H. Pan, Y. Gu, T. Lyu, Z. Wang, G. Wang, Y. Zhang, M. Xue and S. Fang, ACS Appl. Energy Mater., 2023, 6, 1955–1964 CrossRef CAS.
- T. Dagger, V. Meier, S. Hildebrand, D. Brüggemann, M. Winter and F. M. Schappacher, Energy Technol., 2018, 6, 2001–2010 CrossRef.
- X. Luo, S. Jiang, X. Yan, C. Chen, S. Liu, S. Zhan and L. Zhang, Ionics, 2022, 29, 87–96 CrossRef.
- J. Lan, Q. Zheng, H. Zhou, J. Li, L. Xing, K. Xu, W. Fan, L. Yu and W. Li, ACS Appl. Mater. Interfaces, 2019, 11, 28841–28850 CrossRef CAS PubMed.
- J. Meng, M. Lei, C. Lai, Q. Wu, Y. Liu and C. Li, Angew. Chem., Int. Ed., 2021, 133, 23444–23454 CrossRef.
- I. Cekic-Laskovic, N. von Aspern, L. Imholt, S. Kaymaksiz, K. Oldiges, B. R. Rad and M. Winter, Top. Curr. Chem., 2017, 375, 37 CrossRef PubMed.
- R. Nölle, J.-P. Schmiegel, M. Winter and T. Placke, Chem. Mater., 2020, 32, 173–185 CrossRef.
- S. Hosseini, S. Masoudi Soltani and Y. Li, Chem. Eng. J., 2021, 408, 127241 CrossRef CAS.
- W. Yao, J. Xu, L. Ma, X. Lu, D. Luo, J. Qian, L. Zhan, I. Manke, C. Yang, P. Adelhelm and R. Chen, Adv. Mater., 2023, 35, 2212116 CrossRef CAS PubMed.
- X. Wu, J. Jiang, C. Wang, J. Liu, Y. Pu, A. Ragauskas, S. Li and B. Yang, Biofuels, Bioprod. Biorefin., 2020, 14, 650–672 CrossRef CAS.
- R. Zhang, Y. Wu, Z. Chen, Y. Wang, J. Zhu and X. Zhuang, J. Mater. Chem. A, 2023, 11, 19195–19209 RSC.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |
