 Open Access Article
Open Access ArticleMechanistic insights and catalyst design for the selective hydrogenolysis of cellulose to C2–C3 alcohols
Yuandong
Cui
a,
Dandan
Wang
a,
Haoxi
Ben
a,
Xiong
Su
 *b,
Xiaoli
Yang
*a and
Yanqiang
Huang
*b,
Xiaoli
Yang
*a and
Yanqiang
Huang
 b
b
aCollege of Textiles and Clothing, State Key Laboratory of Bio-Fibers and Eco-textiles, Qingdao University, Qingdao 266071, P. R. China. E-mail: xlyang@qdu.edu.cn
bState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: suxiong@dicp.ac.cn
First published on 30th January 2026
Abstract
Catalytic hydrogenolysis of cellulose into low-carbon alcohols offers a promising route toward sustainable chemical manufacture and carbon-neutral energy systems. Recent advances in aqueous-phase catalysis have progressively clarified the reaction network encompassing cellulose depolymerization, glucose isomerization, retro-aldol C–C scission, and the hydrogenation of key carbonyl intermediates. This review integrates these mechanistic insights with catalyst and process design, highlighting how noble-metal and non-noble bifunctional systems leverage acid–metal cooperation, redox flexibility, and spatial confinement to orchestrate glycosidic, C–O, and C–C bond activation with increasing precision. Kinetic and engineering studies further reveal how reactor configuration, operating conditions, and feedstock pretreatment shape conversion efficiency and carbon utilization in both batch and continuous modes. Emerging opportunities, including single-atom catalysts that maximize atom efficiency and enable precise site control, defect-engineered oxides, machine-learning-assisted discovery for accelerated catalyst optimization, and integrated reaction-separation platforms, hold considerable promise for enabling cost-effective, scalable, and recyclable catalytic systems. Together, these advances establish a coherent framework linking fundamental chemistry with reactor-level engineering, laying the groundwork for practical one-step aqueous-phase routes to bio-derived low-carbon alcohols.
1 Introduction
The global energy system is undergoing an unprecedented transition driven by the pursuit of carbon neutrality and sustainable growth.1 Decades of dependence on fossil fuels have powered industrialization but also intensified greenhouse-gas emissions, resource depletion, and environmental degradation.2–4 These intertwined challenges have spurred the search for renewable carbon resources capable of supporting a circular and low-carbon chemical economy. Among them, biomass stands out as the only renewable reservoir of organic carbon,5 offering an essential foundation for the production of green fuels and value-added chemicals (Fig. 1). It is projected that the global biomass market size will grow from USD 79.26 billion in 2025 to USD 157.38 billion in 2035.6 However, the utilization rate of cellulosic resources available for industrial processes remains relatively low at present. The contradiction between such enormous resource potential and the currently limited industrial conversion efficiency highlights that the value-added utilization of biomass has become a pivotal pathway to achieve energy security and environmental sustainability.7,8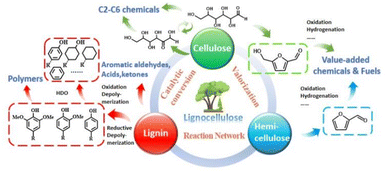 | ||
| Fig. 1 Catalytic conversion network for value-added utilization of lignocellulosic biomass. Reproduced with permission.5 Copyright 2023, the Royal Society of Chemistry. | ||
Cellulose, the most abundant polysaccharide in nature, is a major structural component of lignocellulosic biomass derived from agricultural residues, woody materials, and non-food herbaceous plants. Composed of D-glucose units connected through β-1,4-glycosidic bonds, cellulose forms a rigid crystalline network that provides structural strength to biomass yet poses intrinsic challenges to its chemical transformation.9–12 Unlike starch or sugar-based feedstocks, cellulose conversion avoids competition with food resources and thus enables large-scale deployment without disrupting the food-energy-environment nexus. Its selective transformation into low-carbon alcohols—such as ethanol (EtOH), ethylene glycol (EG), and 1,2-propylene glycol (1,2-PG)—represents a promising route to bridge renewable carbon with existing chemical and fuel infrastructures.13–15 These polyfunctional alcohols serve as key intermediates for polymers, solvents, food and pharmaceutical products, and fine chemicals, with their global market scale continuously expanding.16 Consequently, the direct catalytic conversion of abundant cellulose resources into these high-value chemicals holds significant strategic and economic importance.
Multiple strategies have been developed to convert cellulose into low-carbon alcohols, including biological fermentation,17 acid hydrolysis coupled with fermentation,18 and thermochemical processes such as gasification-synthesis.19 Despite their maturity, biological routes suffer from low carbon efficiency, high enzyme cost, and complex pretreatment requirements,17,20,21 while gasification demands high temperature, high pressure, and substantial capital investment.22 In contrast, catalytic hydrogenolysis has emerged as a more direct and atom-efficient approach capable of simultaneously cleaving C–C and C–O bonds and hydrogenating intermediates under comparatively mild aqueous conditions.23–25 This thermochemical pathway offers high atom economy, controllable selectivity, and potential scalability, making it a cornerstone technology for sustainable biomass valorization.
During the past two decades, significant progress has been made in elucidating and optimizing the aqueous-phase hydrogenolysis of cellulose. The development of bifunctional catalysts integrating metallic and acidic sites, such as W-, Ni-, Ru-, and Pt-based systems,26–30 has markedly improved reaction efficiency and product selectivity. Mechanistic studies have further revealed that cellulose depolymerization, glucose isomerization, retro-aldol condensation (RAC), and hydrogenation occur through interconnected steps requiring precise spatial and electronic coordination between active sites.31–34 In particular, the cooperative interplay between acid and metal centers, exemplified in catalysts such as Pt–WOx, Ni–WOx, and PdZn-zeolite frameworks, determines the dominant reaction pathway and selectivity toward EG, 1,2-PG, ethanol, or polyols. These mechanistic insights have inspired the design of new generations of spatially confined, electronically modulated catalysts featuring enzyme-like efficiency and stability under hydrothermal conditions.
In parallel, advances in reactor design and process intensification have bridged the gap between molecular reactivity and engineering scalability. The transition from batch to semi-continuous and continuous-flow systems has provided deeper kinetic understanding and improved control over intermediate concentration, hydrogen supply, and mass transport—key factors for achieving high selectivity and yield at scale.31,33 Furthermore, optimized feedstock pretreatment and hybrid catalytic systems have extended the applicability of aqueous-phase hydrogenolysis from pure cellulose to raw lignocellulosic biomass, addressing issues associated with lignin, waxes, and mineral impurities.34
This review provides a comprehensive yet critical assessment of recent developments in the aqueous-phase catalytic hydrogenolysis of cellulose into low-carbon alcohols. Beginning with mechanistic insights that define the fundamental reaction network, it examines how these principles guide the rational design of catalytic systems—from noble and non-noble metals to tungsten-based multifunctional catalysts. Subsequent sections explore product-oriented reaction pathways and structure–selectivity relationships for ethanol, EG, 1,2-PG, and polyols, followed by a discussion of reactor engineering, feedstock utilization, and techno-economic considerations. The review concludes with emerging challenges and opportunities, emphasizing the integration of molecular catalysis, materials innovation, and process engineering as the foundation for scalable, sustainable biomass-to-alcohol conversion.
2 Mechanistic insights
Mechanistic understanding constitutes the cornerstone of rational catalyst design and process optimization in biomass conversion. In the case of cellulose hydrogenolysis, elucidating how macromolecular activation, C–C and C–O bond cleavage, and hydrogenation steps interconnect is essential for achieving selective transformation into low-carbon alcohols. The overall reaction does not proceed through a single step but rather a cascade of hydrolysis, isomerization, RAC, hydrogenation, and dehydration, each governed by distinct active sites and kinetic regimes. Over the past decade, research in this field has shifted from empirical catalyst screening toward an integrated, molecular-level comprehension of how catalyst structure and reaction environment jointly dictate product distribution. The following discussion traces this mechanistic evolution—from the organization of the overall reaction network and its elementary steps, through the kinetic and molecular evidence supporting these pathways, to the emerging understanding of how catalyst structure modulates C–C and C–O bond scission to control product selectivity.2.1 Reaction framework
The crystalline microfibrils of cellulose are stabilized by dense intra- and intermolecular hydrogen bonding,35 which endows them with exceptional rigidity and chemical resistance. These structural features originating from the β-(1,4)-linked D-glucopyranose framework pose formidable challenges for activation and depolymerization. Early attempts to transform cellulose into low-carbon alcohols, dating back to the 1930s,36,37 were limited by poor substrate accessibility and minimal control over the complex reaction sequence, typically yielding <40% of target products.37–39 A breakthrough occurred in 2008, when Zhang and co-workers achieved a 61% yield of EG from cellulose using a Ni–W2C/AC catalyst.40 This so-called “direct lignocellulose-to-ethylene glycol” (DLEG) strategy41 demonstrated the feasibility of one-step catalytic hydrogenolysis, stimulating extensive mechanistic exploration into C–C/C–O bond cleavage and product selectivity.42–44Cellulose hydrogenolysis proceeds through a sequence of interconnected elementary transformations that collectively govern product distribution. Conceptually, the process can be viewed as a tandem sequence integrating hydrolysis of β-1,4-glycosidic bonds with hydrogen-assisted deoxygenation reactions.45 Hydrolysis generates glucose monomers, which subsequently undergo isomerization, RAC, hydrogenation, and dehydration to yield smaller oxygenates and polyols. Because these steps exhibit distinct kinetic and thermodynamic characteristics,46–49 efficient conversion demands catalysts combining metal and acid functionalities in a finely tuned balance. Acid sites catalyze glycosidic-bond cleavage and sugar RAC or isomerization, whereas metal sites facilitate selective hydrogenation and C–C bond activation. Only when these functions operate synergistically can the network progress in a concerted manner, avoiding over-hydrogenation of intermediates or uncontrolled degradation—a dynamic equilibrium that ultimately shapes the selectivity landscape.50,51
Within this intricate network (Fig. 2), multiple pathways coexist for the generation of low-carbon alcohols. The primary EG pathway involves cellulose hydrolysis to glucose, followed by RAC of glucose into erythrose and glycolaldehyde, which is rapidly hydrogenated to EG. Under more reductive environments, EG can undergo further hydrogenative dehydration to ethanol, indicating a shared upstream chemistry in which EG and ethanol diverge primarily in the degree of reduction. In contrast, 1,2-PG originates from an alternative route—glucose isomerizes to fructose, which then undergoes RAC to form C3 intermediates such as glyceraldehyde and dihydroxyacetone; these are subsequently hydrogenated to 1,2-PG. Because this route requires additional rearrangements and hydrogen-transfer steps, its overall carbon efficiency is typically lower than that of EG formation.
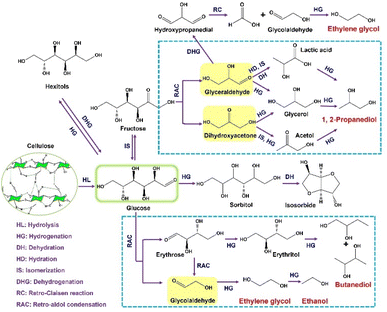 | ||
| Fig. 2 Possible reaction pathways involved in catalytic conversion of cellulosic biomass to low-carbon alcohols. | ||
Besides RAC, alternative cleavage modes, such as retro-Claisen (RC) condensations, can contribute to EG formation by converting C3 intermediates into glycolaldehyde under alkaline or bifunctional catalytic conditions.52,53 Although this route enhances EG selectivity, it may compromise 1,2-PG yields. Excessive hydrogenation activity can further redirect intermediates toward hexitols such as sorbitol or mannitol. These polyols can undergo partial dehydrogenation to regenerate ketose species (e.g., fructose), which re-enter the RAC cycle.54–56 These forward and backward transformations underscore a dynamic interplay between hydrogenation and bond-scission reactions that governs overall product distribution. Excessive hydrogenation yields unreactive polyols, while insufficient reduction promotes over-fragmentation and carbon loss.
Mechanistic studies consistently demonstrate that selectivity toward EG or 1,2-PG is dictated by the relative kinetics of hydrogenation versus RAC. This kinetic balance is highly sensitive to the ratio and spatial proximity of metal and acid sites within the catalyst.57,58 Metal-dominant catalysts accelerate hydrogenation but risk over-reduction, whereas acid-rich systems promote C–C cleavage but can induce dehydration and condensation side reactions.59,60 Bifunctional catalysts with optimized metal–acid synergy, such as W-based oxides or Ru/Ni-supported systems, achieve concurrent activation and stabilization of intermediates, maximizing diol selectivity. The mechanistic principle of balanced bifunctionality thus provides the conceptual foundation for rational catalyst design, integrating hydrolysis, C–C scission, and hydrogenation within a unified catalytic cycle. These mechanistic hypotheses form the conceptual basis for the kinetic and molecular-level investigations discussed below.
Overall, the hydrogenolysis of cellulose can be rationalized through three recurring elementary motifs: (i) RAC of glucose-type intermediates yielding C2–C4 fragments (EG pathway); (ii) RAC of fructose-type intermediates generating C3 species (1,2-PG pathway); and (iii) direct hydrogenation of erythrose-like intermediates producing polyols. These routes frequently operate simultaneously, and their relative importance is determined by the catalyst structure and reaction environment. Understanding and orchestrating these competing elementary steps is fundamental to developing highly selective hydrogenolysis systems for the sustainable production of low-carbon alcohols.
2.2 Mechanistic and kinetic evidence
Mechanistic and kinetic investigations have provided the quantitative foundation for understanding the multistep cascade governing cellulose hydrogenolysis to low-carbon alcohols. As discussed above, the transformation involves sequential hydrolysis, RAC, and hydrogenation, accompanied by numerous parallel and competing reactions that jointly determine selectivity.42 Over the past decade, this complex network has been systematically dissected through kinetic modeling, isotopic tracing, and reactor-scale analyses, revealing how intrinsic kinetics, surface adsorption, and process dynamics interconnect to shape EG and 1,2-PG yields.The one-pot conversion of cellulose to EG follows a characteristic three-step sequence: hydrolysis of β-1,4-glycosidic bonds to release glucose; RAC of glucose to glycolaldehyde catalyzed by Lewis acid sites;61 and hydrogenation of glycolaldehyde to EG over metallic sites. However, this idealized sequence is complicated by the high reactivity of intermediates such as glucose, fructose, and glycolaldehyde, which can undergo isomerization, condensation, or over-hydrogenation.48,49 Glucose may isomerize to fructose or hydrogenate to hexitols (e.g., sorbitol, mannitol), competing with the RAC pathway. Fructose-derived C3 intermediates—glyceraldehyde and dihydroxyacetone—yield 1,2-PG upon hydrogenation,48–50 while reactive aldehydes like glycolaldehyde and glyceraldehyde condense to form higher oxygenates, complicating separation.42,50 These side reactions necessitate precise control of catalyst functionality and operating conditions to couple bond cleavage with hydrogenation while suppressing condensation.
Quantitative kinetic elucidation of these processes was pioneered by Zhang and co-workers,32 who modeled glucose RAC over ammonium metatungstate (AMT) catalysts. Three consecutive reactions were identified: (R1) glucose RAC to erythrose and glycolaldehyde, (R2) erythrose RAC to glycolaldehyde, and (R3) self-condensation of glycolaldehyde to by-products.62,63 The first step (R1) followed pseudo-first-order kinetics with an apparent activation energy of 141–149 kJ mol−1, whereas R2 and R3 exhibited higher reaction orders (1.7 and 2.5) but lower activation energies (79.9 and 52.7 kJ mol−1). These data indicate that condensation reactions are highly sensitive to glycolaldehyde concentration, emphasizing the need to maintain low steady-state intermediate levels.
Further refinement incorporated hydrogenation into the kinetic framework. Using an AMT-4% Ru/AC bifunctional catalyst, Zhang's group33 identified hydrogenation of glucose to hexitols (R4) and of glycolaldehyde to EG (R5). Reaction R4 exhibited a first-order rate dependence64 with an activation energy of 38 kJ mol−1—about one-fourth that of RAC (R1). This disparity explains the observed trade-off between hexitol and EG selectivity with temperature.65 As temperature increases, the endothermic RAC reaction accelerates faster than hydrogenation, improving EG yield but reducing sorbitol formation. Conversely, at lower temperatures, rapid hydrogenation locks glucose into inert polyols, halting further bond cleavage. Moreover, the condensation rate (R3, 2.5-order) increases more sharply with glycolaldehyde concentration than hydrogenation (R5, ∼1.1-order), making intermediate levels control critical. In semi-continuous reactors, lowering the glucose feed rate from 10 to 2 mL min−1 increased EG yield from 17.7% to over 50%,33 highlighting the importance of maintaining kinetic balance among sequential steps. The kinetic hierarchy—RAC slower than hydrogenation in low temperature but faster than condensation in low intermediate levels—thus defines the narrow temperature–concentration window for selective EG formation.
A Langmuir–Hinshelwood–Hougen–Watson (LHHW) model further clarified the surface reaction mechanisms of glucose and glycolaldehyde on Ru catalysts.31 Under 373–403 K and 6 MPa H2, the apparent activation energies for glycolaldehyde and glucose hydrogenation were 42.6 and 49.6 kJ mol−1, respectively. However, the pre-exponential factor for glycolaldehyde was nearly four times larger, resulting in a significantly faster reaction rate. The addition of AMT modified the adsorption behavior. Strong AMT–Ru interactions (KAMT ≈ 103 to 104 × KG) and formation of AMT-glucose complexes suppressed glucose hydrogenation by an order of magnitude, while the smaller glycolaldehyde was minimally affected. This competitive adsorption effect underpins the “self-selective hydrogenation” behavior observed in bifunctional catalysts, where acid–metal proximity dynamically regulates surface coverage and reaction order. Such kinetic self-regulation provides a molecular explanation for the dual-site synergy central to cellulose hydrogenolysis.
Building on intrinsic kinetics, Zhang et al.66 developed a macroscopic model integrating six elementary steps—RAC, hydrogenation, thermal degradation, condensation, and EG gasification. The model quantitatively reproduced the yields of EG, hexitols, and gaseous products as functions of temperature, glucose concentration, and feed rate, and accurately predicted that low feed rates favor high EG yield, temperature elevation shifts selectivity from hexitols to EG, and excessive temperature and extremely low feed rate can trigger EG gasification. The strong agreement between simulation and experiment validated the kinetic scheme and provided a design basis for continuous hydrogenolysis reactors, enabling industrially relevant scale-up of cellulose-to-EG processes using high-solid slurries67 or dual-stage systems coupling saccharification and hydrogenation.68
The hierarchy of activation energies explains why cellulose hydrogenolysis requires elevated temperatures. Hydrolysis of β-1,4-glycosidic bonds presents a high barrier (120–180 kJ mol−1),69,70 reinforced by a crystalline hydrogen-bonding network, while RAC of glucose to glycolaldehyde demands ∼140 kJ mol−1,71,72 and hydrogenation of sugars and intermediates proceeds more readily (∼50 kJ mol−1). Thus, reactions typically operate above 453 K.24,25 Lowering the activation barriers of hydrolysis and RAC remains crucial for mild-temperature catalysis. Shuai et al.73 developed a cellulase–mimetic sulfonated chloromethyl polystyrene resin (CP–SO3H) catalyst, reducing the hydrolysis activation energy from 170 kJ mol−1 (H2SO4 catalysis) to 83 kJ mol−1via cooperative –Cl/–SO3H interactions. Sulfonated carbon catalysts containing phenolic hydroxyl and carboxyl groups achieved hydrolysis activation energies around 110 kJ mol−1,74 highlighting the importance of hydrogen-bond disruption. However, analogous reductions in RAC activation energy have yet to be achieved, and current W-based Lewis acid systems remain dependent on temperatures above 453 K.
The convergence of kinetic modeling, spectroscopic evidence, and reactor validation has transformed cellulose hydrogenolysis from empirical observation into a quantitatively predictive science. These findings elucidate not only the relative energy barriers among hydrolysis, RAC, and hydrogenation but also how catalyst structure governs this energetics via site balance and adsorption dynamics. Looking forward, biomimetic strategies inspired by enzyme catalysis—constructing multifunctional active centers that mimic the cooperative binding and cleavage of cellulases and aldolases—represent a promising direction. Hybrid catalysts combining Sn–W acid pairs or metal–enzyme interfaces could reduce the activation barriers of both hydrolysis and C–C scission, enabling efficient diol production under mild conditions. These mechanistic foundations underpin the selectivity-control strategies discussed in the following section.
2.3 Selectivity control in C–C and C–O bonds cleavage
Building upon the mechanistic and kinetic insights discussed above, product selectivity in cellulose hydrogenolysis fundamentally depends on the interplay between C–C and C–O bond transformations within the reaction network. The cascade proceeds through cellulose depolymerization, glucose and aldose/ketose intermediates, α-hydroxy aldehydes or ketones, and finally to alcohols such as ethanol, EG, and 1,2-PG. Each step competes dynamically with parallel processes, including isomerization, RAC, hydrogenation, dehydration, and over-hydrogenolysis, making product distribution highly sensitive to catalyst composition, active-site balance, and reaction microenvironment.Hydrolysis synchronization further governs this balance. Moderate hydrolysis that releases glucose at rates matching RAC and hydrogenation minimizes transient accumulation and polymerization. Zhao et al.33 reported that lowering glucose feed rate from 10 to 0.67 mL min−1 increased EG yield from 17.7% to 53.4%, due to second-order glycolaldehyde condensation versus first-order hydrogenation kinetics. Operational variables such as temperature, hydrogen pressure, and residence time also critically influence selectivity. Raising temperature (453–518 K) enhances EG formation (from 14.6% to 60.0%) at the expense of hexitols (from 62.4% to 6.8%),33 while both excessive and insufficient hydrogen pressure induce undesired condensation or over-hydrogenation.75 Similarly, prolonged reaction time promotes re-hydrogenation and dehydration cycles, generating heavy polyols and humins.76 Industrially, 4–6 MPa H2 and 3–4 h residence achieve optimal trade-offs.
Solvent effects further tune selectivity by modulating hydrogen bonding and acid–metal balance. Methanol, for instance, weakens hydrogen-bonding networks and stabilizes α-hydroxy aldehydes,77 shifting products toward EG and its monoethers, whereas water favors isosorbide formation.57,78 Controlled oxidative atmospheres also provide selectivity tuning via redox-depth control, as Mo-based catalysts yield glycolic acid intermediates under O2 that can be hydrogenated to EG.79
Beyond the aforementioned operational and solvent parameters, the selective control of the glucose RAC pathway is rooted in the microscopic realm of catalyst design and process kinetics. The initial C–C bond cleavage site in the glucose RAC reaction—either between C2–C3 or C3–C4—constitutes a critical branch point that determines the ultimate product distribution. This selectivity is governed primarily by the local active microenvironment of the catalyst. For instance, in tungsten-based catalysts,32,33 the valence state and coordination structure of the W species modulate the surface acidity/redox properties, which in turn influence the adsorption mode of glucose and the activation of specific C–C bonds. When the catalytic sites favor preferential cleavage of the C2–C3 bond and stabilization of the glycolaldehyde intermediate, the reaction pathway is directed toward EG; conversely, cleavage at the C3–C4 bond promotes the formation of erythrose and subsequent C4 products. Therefore, achieving directed pathway control fundamentally relies on atomic-level catalyst design to tailor the electronic and geometric structure of the active sites.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 but fell to 31.8% at Ni/W = 1
5 but fell to 31.8% at Ni/W = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where hexitol formation dominates.43,91 Similarly, Ni–Cu/WO3 catalysts display compositional dependence. Increasing Cu shifts selectivity from EG to 1,2-PG and 1,2-butanediol (1,2-BDO).92 In Pt-SnOx/Al2O3 systems, increasing the Sn/Pt ratio suppresses over-hydrogenation, steering products toward C2–C3 alcohols.83
1, where hexitol formation dominates.43,91 Similarly, Ni–Cu/WO3 catalysts display compositional dependence. Increasing Cu shifts selectivity from EG to 1,2-PG and 1,2-butanediol (1,2-BDO).92 In Pt-SnOx/Al2O3 systems, increasing the Sn/Pt ratio suppresses over-hydrogenation, steering products toward C2–C3 alcohols.83
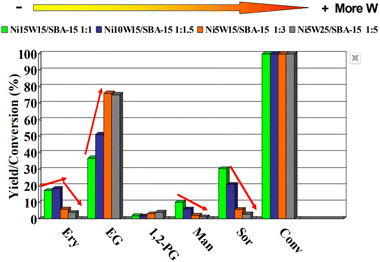 | ||
| Fig. 3 The variation of product distribution on the Ni–W/SBA-15 bimetallic catalyst with the Ni/W mass ratio. Conv, Ery, EG, 1,2-PG, Mann, and Sor represent the cellulose conversion rate and the yields of erythritol, ethylene glycol, 1,2-propanediol, mannitol, and sorbitol, respectively. Reproduced with permission.43 Copyright 2014, Elsevier. | ||
At the metal–support interface, tungsten dispersion critically affects selectivity. Highly dispersed WO4 units and oligomeric WOx clusters in Pd–WOx/Al2O3 provide abundant Lewis acid sites, promoting glucose–fructose isomerization and enhancing 1,2-PG selectivity (∼60.8%),93 while poorly dispersed crystalline WO3 phases reduce acid site density, weakening RAC-isomerization coupling and shifting selectivity toward EG (∼45%).94 Such dispersion-dependent tuning exemplifies how atomic-level control over acid–metal proximity dictates C2/C3 product balance.
Collectively, these findings demonstrate that selectivity in cellulose hydrogenolysis arises from multiscale coupling—from electronic-level metal–acid interactions to reactor-scale concentration dynamics. Rational tuning of active-site composition, dispersion, and reaction microenvironment enables the precise steering of C–C and C–O bond cleavage, establishing a mechanistic platform for the sustainable synthesis of value-added, low-carbon alcohols. Nevertheless, maintaining long-term metal–acid synergy and structural integrity under continuous operation remains a key challenge for future development.
3 Catalyst design concepts
Building upon the mechanistic landscape outlined in Section 2, the following discussion focuses on how these principles are translated into concrete catalyst architectures. The central challenge in cellulose hydrogenolysis is not merely identifying active metals or acids, but engineering spatial and electronic cooperation among functional sites so that hydrolysis, C–C cleavage, and hydrogenation proceed in a controlled, mutually reinforcing sequence. Catalysts capable of achieving this synchronization combine hydrogenation-active metals with acid or redox functionalities that promote glycosidic bond activation and retro-aldol scission. The decisive factors are the density, proximity, and electronic alignment of these sites, which together determine whether reaction intermediates are channelled toward desired diols or diverted into condensation and over-hydrogenation.The sections below examine how these mechanistic requirements are embodied across catalyst families—from noble metals that offer controlled hydrogen activation to base-metal systems that integrate bifunctional chemistry at lower cost. Tungsten-containing oxides, phosphates, and mixed-metal supports are discussed not as a separate class but as acidic and electronic modifiers that mediate metal–acid synergy. Finally, we consider structural strategies—confinement, interface engineering—that bridge intrinsic reactivity and reactor-scale performance.
3.1 Design principles derived from mechanistic insights
The mechanistic framework provides three practical descriptors that guide catalyst design for selective cellulose hydrogenolysis. First, site coordination and spatial intimacy govern reaction sequencing. Instead of simply co-locating metal and acid functions, modern catalysts aim to tune their distance and coupling strength so that reactive intermediates can migrate rapidly from cleavage to hydrogenation sites. Atomic-scale proximity, as achieved in Ru–W71 or Ni–W40 nanocomposites, minimizes the lifetime of unstable species such as glycolaldehyde, thus suppressing polymerisation without over-reducing glucose. Second, electronic modulation translates mechanistic selectivity into tunable reactivity. Interfacial charge redistribution between metal and oxide components controls hydrogen adsorption and carbonyl activation. This concept underpins systems such as Pt–SnOx83 and Ni–W,40 where tailored electronic interaction balances hydrogenation power with cleavage activity, ensuring kinetic alignment across the cascade. Third, mesoscale architecture provides the physical environment for sustaining these interactions. Hierarchical porosity, oxide confinement, and hydrophilic–hydrophobic interfaces regulate molecular transport and stabilize transition states. By manipulating local polarity and solvent accessibility, the same active composition can exhibit markedly different selectivity toward EG or 1,2-PG.Together, these design parameters—site coordination, electronic modulation, and mesoscale environment—offer a transferable framework that links molecular understanding to catalyst engineering. They set the stage for analyzing how different metal systems realize these principles in practice.
3.2 Noble metal catalysts
Noble metals, particularly Ru, Pd, and Pt, represent the benchmark hydrogenation components for aqueous-phase cellulose hydrogenolysis, combining high intrinsic H2 activation ability with tunable chemoselectivity toward C–O and C–C bond cleavage. Their function is primarily associated with the activation of molecular hydrogen and the selective hydrogenation of reactive intermediates such as glycolaldehyde and glyceraldehyde, which would otherwise undergo condensation in aqueous media. When coupled with acid or oxophilic promoters, these metals enable efficient cascade conversion of cellulose to low-carbon alcohols under comparatively mild conditions.Cellulose hydrogenolysis. Early demonstrations of direct cellulose hydrogenolysis highlighted the feasibility of one-pot hydrolytic hydrogenation under hydrothermal conditions. Fukuoka and Dhepe first achieved 31% hexitol yield (25% sorbitol, 6% mannitol) with Pt/γ-Al2O3 at 190 °C.25 Building on this concept, Luo et al.24 showed that Ru/C at 245 °C enabled hydrolysis through proton generation from subcritical water, yielding ∼30% sorbitol. These studies established the conceptual basis for green cellulose valorization but also revealed the necessity of bifunctional catalysts capable of balancing hydrolysis, RAC, and hydrogenation steps.
Integration of Ru with tungstate or acidic components has proven crucial for orchestrating these elementary reactions, as shown in Table 1. Fu et al.95 prepared Ru–WOx/HZSM-5, producing 42.3% ethanol at 235 °C after 24 h, attributed to highly dispersed Ru3W17 alloy species and the moderate acid sites with HZSM-5, which together balanced C–O and C–C scission. Comparative evaluations by Li et al.96 among (Pd, Pt, Rh, Ru, Ir)/WO3 on rectangular WO3 nanosheets identified 1% Ru/WO3 as the most active, yielding 76.3% EG at 240 °C, 4 MPa H2 within 2 h. The high activity was ascribed to interfacial W5+/RuOxδ+ sites that simultaneously promoted RAC and hydrogenation. Similarly, Wiesfeld et al.97 found 2% Ru/tungstate delivering 61% EG at 225 °C, 4.5 MPa H2, and 1 h, confirming the universality of Ru–W cooperative effects.
| Entry | Catalyst | T (°C) | P (MPa, H2) | T (h) | Yield (C mol%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Ru/C | 245 | 6.0 | 0.1 | Sorbitol 30 | 24 |
| 2 | Ru–W/AC | 245 | 6.0 | 0.5 | EG 61.7 | 71 |
| 3 | 50% WO3/Al2O3 + Ru/C | 245 | 6.0 | 0.5 | EG 16.6 | 94 |
| 1,2-PG 30.7 | ||||||
| 4 | 5Ru–25WOx/HZSM-5 | 235 | 3.0 | 24.0 | Ethanol 42.3 | 95 |
| 5 | 1% Ru/WO3 | 240 | 4.0 | 2.0 | EG 76.3 | 96 |
| 6 | 2% Ru/tungstate | 225 | 4.5 | 1.0 | EG 61.0 | 97 |
| 7 | Ru/W/AC | 220 | 6.5 | 3.0 | EG 34.2 | 98 |
| Sorbitol 18.5 | ||||||
| 8 | 0.8% Ru–30% W/CNT | 205 | 5.0 | 3.0 | EG 40.0 | 99 |
| 9 | Ru/CNTHNO3 + W/CNT | 205 | 5.0 | 5.0 | EG 41.0 | 100 |
| 10 | Ru/CGHNO3 + W/CG | 205 | 5.0 | 5.0 | EG 48.4 | 101 |
| 11 | 5% Ru–30% W18O49/graphene | 245 | 6.0 | 1.0 | EG 62.5 | 103 |
| 12 | Ru/AC–SO3H | 165 | 5.0 | 24.0 | Sorbitol 71 | 104 |
| 13 | 2 wt% Ru2P/C–SO3H | 200 | 3.0 | 2.0 | Sorbitol 64 | 105 |
| 14 | 3 wt% Ru/AC–SO3H | 180 | 2.0 | 24.0 | Hexitols 44.5 | 106 |
| 15 | Ru/Al2O3 | 190 | 5.0 | 24.0 | Sorbitol 53.2 | 108 |
| 16 | Ru/NbOPO4 | 170 | 4.0 | 24.0 | Sorbitol 50–60 | 109 |
| 17 | 3 wt% Ru/BEA zeolite | 180 | 1.6 | 3.0 | Sorbitol 72.8 | 110 |
| 18 | Ru/MCM-48 | 200 | 5.0 | 0.1 | Hexitols 48.5 | 111 |
| 19 | 1% Ru/MN-270 | 245 | 6.0 | 0.1 | Hexitols 50 | 112 |
| 20 | 1% Ru/Cs3HSiW12O40 | 180 | 5.0 | 3.0 | Sorbitol 59 | 113 |
| 21 | Ru/AC + H2WO4 | 245 | 6.0 | 0.5 | EG 54.4 | 114 |
| 22 | Ru/AC + WO3/ZrO2 | 215 | 5.2 | 1.5 | EG 52.9 | 115 |
| 23 | Ru/SBA-15 + H3PW12O40 | 245 | 5.0 | 4.0 | EG 55.5 | 119 |
| 24 | Ru/C + H3PW12O40/ZrO2 | 220 | 5.0 | 5.0 | EG 40.0 | 120 |
| Hexitols 26.2 | ||||||
| 25 | Ru/C + H4[Si(W3O10)4] | 160 | 5.0 | 7.0 | C yield 90 | 121 |
| 26 | Ru/C + 0.10 M HCl | 215 | 6.0 | 0.5 | Isosorbide 49 | 122 |
| 27 | Ru/CNT + H3PO4 | 185 | 5.0 | 24.0 | Sorbitol 69 | 123 |
| 28 | Ru/H-USY + HCl | 190 | 5.0 | 24.0 | Hexitols 90 | 124 |
| 29 | Ru/C + H4SiW12O40 | 180 | 5.0 | 24.0 | Hexitols 86 | 125 |
| 30 | Ru/C–H4SiW12O40 | 210 | 5.0 | 1.0 | Isosorbide 50–63 | 126 |
| 31 | Ru/NC@void@MC–SO3H | 230 | 6.0 | 5.0 | 1,2-PG 38.0 | 127 |
| 32 | 1% Ru/h-WO3 | 240 | 4.0 | 2.0 | EG 77.5 | 133 |
| 33 | Ru/CNT | 205 | 5.0 | 5.0 | Sorbitol 60 | 134 |
| 34 | Ru/AC | 205 | 5.0 | 5.0 | Sorbitol 80 | 135 |
| 35 | Ru–Ni/AC or Ru–Ni/CNT | 205 | 5.0 | 5.0 | Sorbitol 50–70 | 136 |
| 36 | Ru–N/AC-1.3 | 200 | 3.0 | 2.0 | Sorbitol 82 | 139 |
| 37 | Ru–Fe3O4–SiO2 | 255 | 6.0 | 0.8 | EG 19.0 | 143 |
| 1,2-PG 20.0 | ||||||
| 38 | Fe3O4@SiO2/10% Ru–20% WOx | 245 | 5.0 | 2.0 | 1,2-PG 32.4 | 144 |
| 39 | 3 wt% Ru/C + 6 wt% WO3/C | 205 | 6.0 | 0.5 | EG 14.6 | 145 |
| 1,2-PG 3.7 | ||||||
| 40 | Ru/CNT | 170; 205 | 5.0 | 2.0; 4.0 | Sorbitol 75 | 146 |
| Xylitol 77 | ||||||
| 41 | Ru/AG-CNT | 205 | 5.0 | 3.0 | Sorbitol 64.1 | 147 |
| 42 | Ru/CNT | 205 | 5.0 | 5.0 | Sorbitol 50 | 148 |
Zheng et al.71 systematically compared Pd–W/AC, Pt–W/AC, Ru–W/AC, and Ir–W/AC under identical conditions, revealing that Ru–W/AC afforded the highest EG selectivity (61.7%) and emphasizing the critical role of Ru–W synergy. Fabičovicová et al.98 demonstrated that Ru/W/AC converted microcrystalline cellulose with 84% polyol yield and raw biomass (pine, birch, eucalyptus), confirming scalability. Ribeiro et al.99 achieved 40% EG at 205 °C, 5 MPa H2 with 0.8% Ru–30% W/CNT, outperforming monometallic counterparts and their physical mixtures. HNO3-oxygenated CNT surfaces enhanced hydrolysis and suppressed glucose isomerization.100 Replacing common CNTs with hydrothermal glucose-derived carbon supports (CG and CG-CNT) delivered 48% EG yield at lower cost.101 Graphene-based composites further improved performance via extended π networks and functional group cooperation.102 Carboxyl (–COOH) sites promoted hydrolysis, while hydroxyl (–OH) groups stabilized glycosidic oxygens. Zhang et al.103 reported 5% Ru–30% W18O49/graphene achieving 62.5% EG at 245 °C and 6 MPa H2 within 1 h, attributed to Ru cluster-W18O49 nanowire synergy and interfacial charge transfer.
Acidic and heteroatom-functionalized supports have also proven effective for stabilizing Ru nanoparticles and modulating acidity. Ru/AC–SO3H retained ∼71% sorbitol yield after five cycles,104 while Ru2P/C–SO3H achieved 64% sorbitol via moderated H adsorption.105 Ru loading optimization (3 wt%) on sulfonated carbon maximized hexitol yield (44.5%) at 94.8% cellulose conversion.106,107 Diverse acidic supports, including Ru/Al2O3,108 Ru/NbOPO4,109 Ru/BEA zeolite,110 Ru/MCM-48,111 Ru/MN-270 (highly cross-linked polystyrene),112 Ru/Cs3HSiW12O40,113 exhibited 50–70% sorbitol selectivity with excellent hydrothermal stability. In temperature-responsive phase-transfer systems, Ru/AC combined with H2WO4 achieved 54.4% EG yield with negligible loss over 20 cycles, as H2WO4 dissolved and reprecipitated reversibly.114 Likewise, Ru/C coupled with WO3/ZrO2 exhibited enhanced activity (52.9% EG at 215 °C, 5.2 MPa H2) due to surface W5+–OH species acting as active C–C scission centers.115
The introduction of additional acid components can further lower the activation barrier for cellulose hydrolysis by protonating glycosidic oxygens.116 Heteropoly acids (HPAs) have been particularly effective as co-catalysts, providing strong Brønsted acidity and facile regeneration.117,118 For instance, Ru/SBA-15 coupled with H3PW12O40 achieved 55.5% EG selectivity,119 while Ru/C + HPA/ZrO2 yielded 40% EG and 26.2% hexitols after ball-milling pretreatment of cellulose.120 Palkovits et al.121 reported that H4[Si(W3O10)4] and Ru/C achieved >81% cellulose conversion and 65% sugar–alcohol yield from spruce wood, demonstrating excellent activity and recyclability. Acid-assisted systems such as Ru/C + 0.10 M HCl,122 Ru/CNT + H3PO4,123 and Ru/H-USY + HCl124 also afforded 49.5% isosorbide, 69% sorbitol, and ∼90% hexitols yield, respectively. HPAs offer the added benefits of high selectivity, low corrosion, and easy recovery,125 affording >50% isosorbide from cellulose and 63% from wheat-straw pulp.126
Nano-structural and compositional engineering have provided further means of optimizing activity and selectivity. Yang et al.127 developed a yolk–shell Ru/NC@void@MC-SO3H catalyst featuring 1.4 nm Ru clusters confined within sulfonated mesoporous carbon. This catalyst yielded 38% 1,2-PG and productivity of 342.86 mol h−1 gRu−1, with –SO3H groups facilitating hydrolysis, Ru–Nx pairs promoting isomerization and RAC, and Ru0 sites hydrogenating intermediates—an elegant example of spatially cooperative catalysis. Crystal-phase engineering of WO3 also governs activity.128–131 Hexagonal (h-WO3) exposing (100) planes and retaining structural H2O forms HxWO3·H2O132 acid centers that enhance both hydrolysis and RAC, giving 77.5% EG at 240 °C, 4 MPa H2 and 2.0 h.133
Mechanical and interfacial engineering have also advanced sorbitol productivity. Ball-milling Ru/CNT or Ru/AC with cellulose enhanced contact and crystallinity reduction, increasing sorbitol selectivity to ∼80%.134,135 Incorporation of Ni produced Ru–Ni bimetallic catalysts that exhibited electronic synergy and additional hydrogenation functionality, affording 50–70% sorbitol depending on Ni content.136 N-doped carbon supports further enhanced Ru dispersion and modulated adsorption properties.137,138 Ru–N/AC-1.3 produced 82% sorbitol, with DFT indicating that pyridinic–N modulates Ru adsorption energy, balancing glucose activation and product desorption.139
To facilitate catalyst recovery and long-term stability, magnetic Ru systems have been developed. Lai et al.140 reported a magnetic sulfonated mesoporous SiO2 catalyst for the hydrolysis of biomass to glucose. Fe3O4@C–SO3H core–shell systems combined acidity and magnetic recoverability.141 Ru–Fe3O4–SiO2 achieved 19% EG and 20% 1,2-PG selectivities at 255 °C, where Fe3O4 modulated the Ru0/Ru4+ ratio.142,143 Lv et al.144 optimized Ru–WOx ratios on magnetic Fe3O4@SiO2 supports, obtaining 32.4% 1,2-PG yield. In dual-bed configurations, spatial separation of cleavage and hydrogenation zones (50% WO3/Al2O3 and Ru/C) afforded 16.6% EG and 30.7% 1,2-PG at 245 °C, 6 MPa H2 and 0.5 h,94 while significant variations in the distribution of diol products over Ru/C and WO3/C.145
Monosaccharides and polyols hydrogenolysis. As the monomeric unit of cellulose, glucose and its isomer fructose represent central intermediates linking polysaccharide and polyol hydrogenolysis. Their aqueous-phase transformation bypasses solid-state hydrolysis, offering higher conversion efficiency under mild conditions.149,150 Among various systems, Ru–W systems again dominate owing to robust metal–acid cooperation that regulates RAC versus hydrogenation. Zhang et al.151 found that tungstate species dramatically enhanced C–C cleavage, following the order H2WO4 > HPW > WO3 > AMT > HSiW. PEG-modified Ru–W catalysts yielded 35.2% EG, 27.1% 1,2-PG, and 31.1% 1,2-BDO in continuous operation,152 while tuning W oxidation states (W4+vs. W5+) shifted selectivity from mixed diols (87%) to EG (56%)153 and 1,2-BDO (72.1%).154 Magnetic Fe3O4@SiO2/10% Ru-20% WOx produced 30.8% 1,2-PG;144 5% Ru/C + ZnO gave 38% PG at 180 °C and 0.4 MPa H2;155 and RuSn/AC with balanced alloy–oxide interfaces afforded 25% 1,2-PG and 26.9% EG.156 These findings demonstrate that Ru–W interfacial redox state and secondary oxide modifiers determine branching between C2 and C3 products. In addition, extensive screening of Ru catalysts157–160 and their loading on carbon,161,162 SiO2,163 Al2O3,164 NiO–TiO2,165,166 zeolites,167–169 and ordered mesoporous silicas149 shows that activity and stability are governed by metal–support intimacy and surface acidity. Bimetallic RuNi/MCM-48 (Ru/Ni = 0.45)168 and 1% Ru/HY169 afford >98% sorbitol selectivity with good reusability, while sol–gel (RuO2)0.038·(SiO2)0.962149 and polymer-encapsulated Ru/ASMA@AC170 exhibit increasing activity and stability, and the latter can steady 99.7% glucose conversion and 93% sorbitol selectivity over multiple cycles. The decisive stability factor is the strength and homogeneity of Ru–support interactions engineered during synthesis.
Fructose, more reactive than glucose, provides a direct entry to C3 polyols via RAC reaction to glyceraldehyde/dihydroxyacetone and subsequent hydrogenation.171,172 Under AMT-4% Ru/AC, 37.9% 1,2-PG was obtained at 240 °C, 5 MPa H2;33 Fe3O4@SiO2/10% Ru–20% WOx yielded 33.4% 1,2-PG;144 3 wt% Ru/C + WO3 gave 47.9% 1,2-PG at 205 °C, 6 MPa H2;94 and Ru–WOx/hydroxyapatite (HAP) produced 91.3% 1,2-PG at 180 °C, 1 MPa H2,172 attributed to highly dispersed Ru and WOx on weakly basic sites that facilitate RAC and suppress humin formation. These consistent results emphasize that fine control of acidity and metal dispersion governs whether glucose or fructose routes dominate diol selectivity.
Beyond monosaccharides, Ru-based catalysts are also highly effective for the hydrogenolysis of sugar alcohols such as sorbitol and xylitol—representative intermediates bridging carbohydrate conversion and polyol production. These reactions typically proceed through sequential dehydrogenation, RAC, and hydrogenation steps (Fig. 4).173 On Ru surfaces, sorbitol dehydrogenation initiates at the C(5)–H bond,173 followed by retro-aldol cleavage at either the C3–C4 or C2–C3 position to yield 1,2-PG or EG, respectively. The product ratio depends sensitively on catalyst basicity and the electronic state of Ru.174 Mechanistic studies have confirmed that C–C bond scission predominantly follows the RAC pathway under both neutral and alkaline conditions, with moderate basicity accelerating the rate and shifting selectivity toward diols.175,176 Zhou et al.177 achieved 58.6% diol selectivity at 220 °C, 8 MPa H2 with 3% Ru/CNF (carbon nanofiber), while Ru–CNF/graphite-felt hybrids gave 79.1% toward EG, 1,2-PG, and glycerol.178 Continuous trickle-bed tests179 showed that optimizing mass transfer and catalyst-layer thickness maximized diol selectivity.
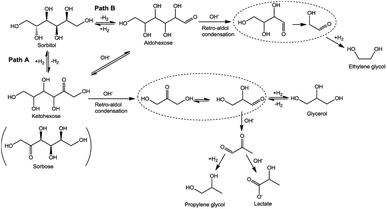 | ||
| Fig. 4 The proposed reaction pathway for the hydrogenolysis of sorbitol to produce C2 and C3 products in the presence of alkaline and metal catalysts. Reproduced with permission.173 Copyright 2016, the Royal Society of Chemistry. | ||
In alkaline media, Ru catalysts supported on basic oxides or co-promoted by Ca(OH)2 exhibit enhanced stabilization of enolate intermediates and improved C–C bond cleavage activity. For instance, Ru0.25WOx/CNTs with Ca(OH)2 delivered 60.2% combined EG with 1,2-PG at 205 °C,180 and surface functional groups of CNT enhanced Ru dispersion and adjusted acidity/basicity.181 Similarly, bifunctional acid–metal systems such as Ru/Al2O3 have delivered superior diol selectivity due to moderate acidity, appropriate Ru/Al ratios, and partially oxidized Ru species.182 Mechanistically, Ru initiates dehydrogenation and C–C cleavage, while basic or acidic co-catalysts stabilize reactive intermediates and regulate electron density—together dictating the EG versus 1,2-PG selectivity landscape.
Across cellulose, monosaccharide, and polyol transformations, Ru-based catalysts display a unifying mechanistic motif. Cooperative interaction between metal and acid/base sites governs the sequence of bond-cleavage and hydrogenation steps leading to low-carbon polyols. W-containing supports and Brønsted or Lewis acids synchronize RAC and hydrogenation, alloying and redox tuning modulate electron density at Ru sites, and structural confinement ensures stability under hydrothermal conditions. Despite significant advances, challenges remain in correlating Ru oxidation dynamics with turnover frequencies and in designing scalable catalysts that maintain metal–acid/base balance during long-term operation. Future progress will hinge on integrating operando spectroscopy and kinetic modeling to link interfacial structure with product selectivity, translating the mechanistic foundation of Ru-based systems into predictive catalyst design principles for sustainable polyol production.
| Entry | Catalyst | T (°C) | P (MPa, H2) | T (h) | Yield (C mol%) | Ref. |
|---|---|---|---|---|---|---|
| a Entries 1–9 used cellulose as the feedstock, entries 10–13 used glucose or fructose as the feedstock, and entries 14 and 15 used sorbitol as the feedstock. | ||||||
| 1 | Pd–Cu–WOx/SiO2 | 300 | 4.0 | 10.0 | Ethanol 42.5 | 27 |
| 2 | Pd/Fe3O4 | 240 | 0.5 N2 | 12.0 | Ethanol 51.0 | 183 |
| 3 | 2Pd–W2C/C | 220 | 4.0 | 2.5 | EG 58.2 | 184 |
| 4 | 3Pd/WOx | 230 | 5.0 | 4.0 | EG 61.2 | 185 |
| Ethanol 17.3 | ||||||
| 5 | Pd/o-WO3 | 245 | 5.5 | 4.0 | EG 64.8 | 186 |
| Ethanol 16.1 | ||||||
| 6 | Pd–Fe/CNTs (Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe = 1 Fe = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
240 | 6.0 | 2.0 | 1,2-PG 20.0 | 187 |
| Hexitols ∼18.0 | ||||||
| 7 | Pd@W/Al–MSiO2 | 240 | 4.0 | 2.0 | EG 56.5 | 188 |
| 8 | Pd@Al–SiO2 | 245 | 4.5 | 4.0 | Ethanol 59.3 | 189 |
| 9 | PdZn@silicalite-1 | 245 | 4.5 | 4.0 | Ethanol 69.2 | 190 |
| 10 | Pd–WOx/Al2O3 | 180 | 4.0 | WHSV 0.48 h−1 | 1,2-PG | 93 |
| 56.1 and 62.2 | ||||||
| 11 | Pd@WOx–MSiO2 | 200 | 5.0 | 2.0 | EG 59.4 | 191 |
| 12 | Pd@Al–MSiO2 | 200 | 5.0 | 3.0 | 1,2-PG 45.2 | 192 |
| 13 | Pd/C–Zn/ZnO | 250 | / | 0.5 | 1,2-PG 33.3 | 197 |
| 14 | Pd/ZrO2 + ZnO + Mg3AlOx | 220 | 5.0 | 1.5 | EG | 198 |
| 1,2-PG 54.6 | ||||||
| 15 | Pd–Cu/ZrO2 + La(OH)3 | 220 | 5.0 | 4.0 | EG 15.9 | 199 |
| 1,2-PG 37.0 | ||||||
Cellulose hydrogenolysis. Early studies confirmed the viability of Pd as the hydrogenation component in bifunctional cellulose hydrogenolysis. Chu et al.27 developed a multifunctional Pd–Cu–WOx/SiO2 catalyst that converted corn stover-derived cellulose to ethanol in a one-pot process, yielding 42.5C % ethanol at 300 °C and 4 MPa H2. The synergy among Pd, Cu, and WOx enabled sequential hydrolysis, RAC, and hydrogenation under relatively mild hydrothermal conditions. Gumina et al.183 further demonstrated Pd/Fe3O4 catalysis without external hydrogen, attaining 51% ethanol selectivity after 12 h through in situ H2 generation via water dissociation. A Pd–W2C/C composite yielded 58.2% EG at 220 °C and 4 MPa H2, demonstrating the cooperative hydrogenation-RAC function of Pd and W2C.184
Particle-size and morphological effects further modulate electronic structure and metal–acid synergy. Jia et al.185 synthesized Pd/WOx catalysts with particle sizes from 1.81 to 4.46 nm and discovered a volcano-type relationship, with ∼3.0 nm Pd particles affording the highest total C2 alcohol yield (78.5%, including 61.2% EG and 17.3% ethanol at 230 °C and 5 MPa H2). Smaller clusters exhibited abundant oxygen vacancies but insufficient hydrogenation activity, while larger Pd domains lost metal–acid synergy. Morphological control of WO3 further optimized this balance. Pd supported on orthorhombic WO3 (Pd/o-WO3) produced 64.8% EG and 16.1% ethanol at 245 °C and 5.5 MPa H2, due to the abundance of crystalline defects attributable to the formation of W5+–OH species as well as the Pd–O(H)–W linkages that enhanced both acidity and hydrogenation.186
Bimetallic and nanostructured Pd systems further extend catalytic versatility. Pd–Fe/CNTs catalysts exhibited 55% total polyols and ∼20% 1,2-PG at 240 °C and 6 MPa H2, where charge transfer from Fe to Pd downshifted the Pd d-band center, suppressing over-hydrogenation and improving C–C cleavage selectivity.187 Core-shell Pd@W/Al-MSiO2 nanostructures achieved 96.1% cellulose conversion and 56.5% EG selectivity, with the W component leading to the formation of extra-framework Al species and increasing acidity, while the mesoporous shell prevented Pd sintering, retaining 48.5% EG selectivity after five cycles.188 Similarly, encapsulated Pd@Al–SiO2 catalyst featuring Lewis-acidic silicate shells afforded 59.3% ethanol selectivity,189 whereas confined PdZn@Silicalite-1 structures reached 69.2% ethanol selectivity.190 Collectively, these results establish that despite its weaker intrinsic hydrogenolysis activity relative to Ru, Pd can achieve comparable diol yields when integrated with well-defined supports and finely tuned acid–metal interfaces.
Monosaccharides and polyols hydrogenolysis. Monosaccharides such as glucose and fructose serve as crucial intermediates bridging cellulose depolymerization and polyol hydrogenolysis. Their aqueous-phase conversion provides mechanistic insight into C–C scission and hydrogenation. Xin et al.191 prepared core–shell Pd@WOx–MSiO2 nanostructures and demonstrated that Pd@1.5% WOx–MSiO2 exhibited 59.4% EG selectivity at 200 °C and 5 MPa H2. In this system, W5+-derived oxygen vacancies facilitated glucose retro-aldol cleavage, followed by hydrogenation on Pd sites. Lv et al.192 tuned the acidity of Pd@Al–MSiO2 by varying Al content. Tetra-coordinated AlO4 sites introduced strong Lewis acidity, promoting glucose isomerization to fructose. Pd@Al3–MSiO2 achieved 95.4% glucose conversion and 45.2% 1,2-PG yield at 200 °C and 5 MPa H2. Such spatially integrated Lewis-acid and Pd-metal sites effectively couple isomerization, RAC, and hydrogenation.
Support composition also dictates the acid–metal balance and, consequently, product distribution. Liu et al.93 studied Pd-WOx/Al2O3 catalysts with varying WOx loadings and found that isolated WO4 and oligomeric WOx species, together with AlO4 sites, provided abundant Lewis acidity, favoring glucose–fructose isomerization and subsequent RAC to C3 intermediates. These intermediates were then hydrogenated on Pd sites to form 1,2-PG, achieving 92.2% conversion and 56.1% yield. The synergistic role of Al2O3 was further supported by the studies,193,194 which established that WOx/Al2O3 interactions lead to two-dimensional oxide overlayers whose acidity can be finely tuned by surface W density.195,196 Comparative experiments confirmed that fructose conversion over Pd–WOx (5%)/Al2O3 was more selective toward 1,2-PG (62.2% at 180 °C and 4 MPa H2) than glucose under identical conditions,93 consistent with fructose's intrinsically lower isomerization barrier. Using Pd@Al3–MSiO2, fructose conversion achieved 47.4% 1,2-PG yield.192
Alternative hydrogen sources have also been explored. Wang et al.197 employed Pd/C–Zn/ZnO systems, where Zn promoted water dissociation to supply reactive hydrogen and simultaneously generated ZnO that enhanced glucose isomerization and fructose RAC, yielding 33.3% 1,2-PG at 250 °C within 0.5 h. These findings highlight that Pd-based Lewis-acid systems can finely direct the isomerization–cleavage–hydrogenation cascade toward C3 products under moderate conditions.
Beyond monosaccharides, Pd catalysts are also active in sugar–alcohol hydrogenolysis. Jia et al.198 employed a Pd/ZrO2 + ZnO hybrid system, forming in situ PdZn alloys through physical mixing for the hydrogenolysis of sorbitol using Mg3AlOx as a solid base. At 220 °C and 5 MPa H2, EG and 1,2-PG yields reached 54.6%. Activity and selectivity were strongly dependent on the amounts of ZnO and the solid base, which controlled the extent of alloy formation and modulated competition between base-catalyzed and metal-catalyzed steps. In a related study,199 a bimetallic Pd–Cu/ZrO2 catalyst in the presence of La(OH)3 achieved 15.9% EG and 37.0% 1,2-PG at 220 °C and 5 MPa H2. Electronic interaction between Pd and Cu inhibited excessive hydrogenation and maintained high stability during recycling. These findings collectively demonstrate that the introduction of secondary metals and solid bases enables Pd catalysts to efficiently mediate polyol hydrogenolysis by balancing metallic and basic functionalities.
Overall, Pd-based catalysts unite structural stability with adjustable hydrogenation strength and finely tunable acidity, providing a versatile platform for selective C–C and C–O bond activation. Their catalytic behavior depends critically on particle size and morphology (governing Pd0/Pd2+ balance), interfacial acid–metal structure (particularly at Pd–WOx or Pd–AlO4 junctions), and synergistic promotion by secondary metals or bases (Cu, Zn, Mg3AlOx, La(OH)3). Properly engineered Pd systems rival Ru catalysts in selectivity toward ethanol, EG, and 1,2-PG, yet offer superior recyclability and hydrothermal robustness, positioning them as promising candidates for continuous, scalable polyol production in aqueous-phase biomass conversion.
Cellulose hydrogenolysis. Early studies demonstrated that Pt supported on reducible oxides can drive direct cellulose hydrogenolysis through cooperative acid–metal catalysis. Zhang and co-workers first reported Mo/Pt/WOx catalysts for one-pot cellulose conversion to ethanol under hydrothermal conditions, achieving 43.2% ethanol selectivity.26 The OxMo–Pt–WOx interface created highly active sites for C–O bond cleavage in EG intermediates, facilitating subsequent hydrogenation. Similarly, Song et al.28 found that H2WO4–Pt/ZrO2 produced 32% ethanol under aqueous medium, where H2WO4 promoted glucose fragmentation via selective C–C cleavage and Pt/ZrO2—featuring a balanced Pt0/Pt2+ ratio—enabled controlled hydrogenolysis. Wu et al.30 further demonstrated that combining Pt/WOx with hollow Pt@HZSM-5 generated 54.4% yield of ethanol, underscoring the importance of spatial integration between Lewis acid and metallic sites in cascade cellulose hydrogenolysis.
Strong metal–support interactions play a defining role in regulating Pt catalytic behavior. Deng et al.83 examined Pt-SnOx/Al2O3 catalysts with different Sn/Pt ratios and observed a volcano-shaped dependence of selectivity on composition. At low Sn/Pt ratios (0.1–1.0), electron transfer from SnOx to Pt enhanced hydrogenation, favoring hexitol formation (82.7% at Sn/Pt = 0.5). Higher ratios (>1.5) promoted C2–C3 diols such as EG and 1,2-PG, as detached Sn(OH)2 species provided Lewis acidity for glucose isomerization and RAC reaction. Yang et al.200 achieved 34.3% EG and 37.1% 1,2-PG (73.9% total alcohol yield) using Pt/CNT catalysts at 240 °C and 2 MPa H2, while Wang et al.201 synthesized Pt/RGO via microwave-assisted reduction to produce 58.9% sorbitol yield, attributed to optimized 3.6 nm Pt particles and efficient hydrogen spillover across the reduced graphene network. These examples illustrate how tuning oxidation state, support conductivity, and interfacial chemistry governs product selectivity and catalyst longevity.
Beyond oxide and carbon supports, hybrid systems combining homogeneous and heterogeneous functions have been developed to improve activity and stability. Girard et al.202 employed CeCl3·7H2O as a co-catalyst with Pt/BaZrO3, achieving 40.9% total yield of EG and PG, though Ba leaching and coking limited recyclability. Gu et al.203 introduced a self-alkaline Pt/SiO2@Mg(OH)2 system, where Mg(OH)2 acted as an intrinsic base to drive glucose isomerization and RAC. The catalyst produced 53.8% 1,2-PG at 180 °C and 6 MPa H2 within 4 h, benefiting from synergistic effects between basic and metallic functions. These findings demonstrate the potential of integrating acid–base components within Pt frameworks to regulate the reaction microenvironment and sustain selectivity.
Monosaccharides and polyols hydrogenolysis. Pt-based catalysts are also highly active in the hydrogenolysis and hydrodeoxygenation of monosaccharides and sugar alcohols, particularly sorbitol. Huber and co-workers55 identified key intermediates in sorbitol hydrodeoxygenation over Pt/SiO2–Al2O3, showing that C–C bond cleavage (via RAC and decarbonylation) occurred predominantly on acid sites, while C–O scission was catalyzed on Pt sites. The reaction followed dual pathways: intramolecular dehydration-cyclization forming cyclic C6 species and RAC yielding C3 polyols such as glycerol and 1,2-PG. Duprez et al.204 systematically investigated the effect of the aqueous medium on Pt/SiO2–Al2O3 catalysts under hydrothermal conditions (225 °C, 2.5 MPa, H2O), revealing that prolonged exposure altered both metal dispersion and support acidity. Subsequent work205 demonstrated that tuning the metal/acid ratio directly influenced selectivity. Higher Pt loadings favored C–C cleavage and hydrogenation, while excessive acidity led to over-dehydration.
To improve stability and tunability, bifunctional Pt/ZrO2 and TiO2–WOx systems were developed.206,207 The ZrO2 phase imparted hydrothermal robustness and strong metal anchoring, whereas the TiO2–WOx phase offered adjustable Brønsted acidity. The optimized 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 ratio of Pt/ZrO2 to TiO2–WOx achieved the highest liquid alcohol yield and prolonged stability, exemplifying the principle of modular acid–metal integration for biomass conversion.
8 ratio of Pt/ZrO2 to TiO2–WOx achieved the highest liquid alcohol yield and prolonged stability, exemplifying the principle of modular acid–metal integration for biomass conversion.
Overall, Pt-based catalysts present a versatile platform for selective hydrogenolysis and hydrodeoxygenation, capable of achieving both mild-condition C–C/C–O cleavage and stable long-term operation. Compared with Ru and Pd systems, Pt exhibits stronger C–O activation and greater thermal resilience but typically requires cooperative promoters, such as SnOx, WOx, or Mg(OH)2, to balance hydrogenation and acid functions. Rational tuning of Pt dispersion, oxidation state, and support reducibility enables precise control over reaction pathways, directing selectivity between C2–C3 diols and C6 polyols. These insights establish Pt-based materials as key bifunctional catalysts bridging high selectivity with robustness, advancing sustainable catalytic strategies for cellulose-derived platform molecules.
3.3 Non-noble catalysts
Non-noble metal catalysts are increasingly pursued as cost-effective, scalable alternatives to noble metals for aqueous-phase hydrogenolysis of cellulose and its derivatives. Their earth abundance, tunable redox and acid–base chemistries, and intrinsic H2-activation capability make them attractive for large-scale biomass valorization. Moreover, tungsten-containing oxides and tungstate species have emerged as indispensable promoters, supplying Lewis and Brønsted acidity, oxygen-vacancy redox sites, and strong electronic coupling to transition metal phases. Rather than being treated as an independent class, W-based components are best regarded as cross-cutting modifiers that complement both noble and non-noble hydrogenation centers, establishing bifunctional catalytic ensembles that can orchestrate hydrolysis, RAC, and hydrogenation steps in a concerted fashion. Nevertheless, the practical implementation of non-noble systems faces persistent challenges, including particle sintering, metal leaching, and dependence on soluble acid/base additives, which continue to limit their recyclability and process compatibility. Recent developments have therefore focused on robust bifunctional architectures capable of integrating metallic, acidic, and basic functionalities within stable composite frameworks, allowing selective C–C and C–O bond scission without the need for corrosive cocatalysts.Cellulose hydrogenolysis. Ni remains the primary non-noble metal for hydrogenolysis due to its high intrinsic hydrogenation activity and low cost. Early work established that metallic Ni alone is insufficient for glycosidic hydrolysis or RAC, but appropriately engineered Ni systems can achieve competitive hexitol and polyol yields. High-loading catalysts such as 70 wt% Ni/Ketjen black provided 67% hexitol yield with notable sintering resistance,208 while Ni/CNF prepared by chemical vapor deposition reached 92% cellulose conversion and 50% sorbitol selectivity.209 Further studies revealed that an appropriate balance between metallic active sites and acidic functional groups on Ni/CNF is crucial. A 7.5 wt% Ni/CNF catalyst achieved a sorbitol yield of 76% at 93% cellulose conversion.210 Notably, bare Ni is unable to promote glycosidic hydrolysis or RAC efficiently, necessitating the incorporation of basic or oxophilic promoters. La2O3-modified Ni catalysts exemplify this approach. The basic La phase enhances Ni dispersion, stabilizes active sites, and enables sustained conversion, delivering high EG/1,2-PG yields over multiple cycles.211 Basic ZnO provides similar functionality by promoting glucose–fructose isomerization and thereby directing the pathway toward C3 products.212 Sn promoters modulate hydrogenation strength and C–C scission selectivity. Sun et al.84 reported that metallic Sn in 20% Ni/AC favored EG formation (57.6%), whereas SnO promoted 1,2-PG (32.2%). Xiao et al.85 developed 10% Ni–15% Sn/SBA-15, where mixed-valence SnOx species provided Lewis acid and redox sites, yielding 55.4% EG and 11.8% 1,2-PG at 245 °C and 5 MPa H2. Phosphide systems (Ni2P/AC, Ni2P/SiO2) introduce additional bifunctionality, improving dispersion and stability under hydrothermal conditions.213–215
Cu and Co catalysts provide complementary acid–base and redox environments that allow fine control over isomerization and C–C cleavage. Xiao et al.86 reported that CuCr(4) with Ca(OH)2 achieved 42.6% 1,2-PG and 31.6% EG at 245 °C, 6 MPa H2, where Ca(OH)2 promoted glucose isomerization and RAC, outperforming other bases due to the optimal ionic radius and charge density of Ca2+.87,88 Li et al.216 demonstrated that 10% Co/CeOx produced 55.2% EG and 33.9% 1,2-PG at 245 °C, with interfacial Con+–Ox–Ce3+ pairs acting as cooperative acid–base sites, suppressing humin formation.
Advanced alloying extends this functionality further. Pang et al.69 achieved full cellulose conversion and 58% hexitol yield with bimetallic Ni–Rh/MC and Ni–Ir/MC. Zhang et al.217 synthesized a cost-effective Ni4.63Cu1.00Al1.82Fe0.79 catalyst, achieving 68.07% sorbitol yield under 488 K and 4.0 MPa H2 for 3.0 h with the assistance of 0.08 wt% H3PO4. Zeolite-supported Ni integrates acidity and confinement, enabling hexitol yield of 58.1% over 17% Ni/ZSM-5 (ref. 218) and 76.9% with NiPt/ZSM-5 while preserving stability over cycles.219 These examples highlight the versatility of transition-metal frameworks for tuning hydrolysis–hydrogenation cooperativity.
Monosaccharides and polyols hydrogenolysis. The hydrogenolysis of glucose and fructose offers a mechanistically simplified platform to probe C–C and C–O activation, avoiding the β-1,4-glycosidic hydrolysis barrier. The reaction proceeds via glucose–fructose isomerization, RAC to C2–C3 carbonyl intermediates, and hydrogenation to polyols. Non-noble Ni-, Cu-, and Co-based catalysts have provided critical insight into how electronic structure and surface chemistry dictate product selectivity.
Kirali et al.220 prepared Ni–Mo/MC catalysts for converting aqueous glucose, where Ni facilitated the dispersion of Mo and partial reduction of Mo6+ to Mo4+/Mo5+, generating Lewis acidic centers that assisted RAC, affording 63.2% EG at 200 °C and 4 MPa H2. Basic supports such as MgO and ZnO enhance glucose–fructose isomerization, a key upstream step controlling whether C2 or C3 pathways dominate, and enable selective formation of 1,2-PG.221 A weakly basic 10% Co/CeOx system for fructose conversion achieved a 50.8% yield of 1,2-PG.216 B2O3-modified Cu/Al2O3 for glucose hydrogenolysis showed 49.5% 1,2-PG selectivity,222 while La2O2CO3-modified Cu/Al2O3 obtained 32% 1,2-PG yield.223 Xiao et al.86 found that Ca(OH)2 combined with CuCr(4) boosted glucose to 1,2-PG of 52.8% yield, with Ca2+ stabilizing cyclic transition states during glucose–fructose isomerization. These studies collectively demonstrate that coupling redox-active promoters (Mo, Cr, Sn) with hydrogenation metals (Ni, Cu) enhances RAC-hydrogenation synergy, while basic supports (MgO, ZnO, CeOx, La2O2CO3) favor isomerization and suppress condensation. Fine-tuning the acid–base environment through B2O3 or alkaline additives governs EG vs. 1,2-PG selectivity.
Sorbitol hydrogenolysis, as a representative reaction for polyol conversion, proceeds through dehydrogenation to ketose/aldose intermediates, RAC to C2–C3 carbonyls, and hydrogenation to EG and 1,2-PG. The sequence requires intimate cooperation among metal, acid, and base sites, making it an instructive platform for evaluating bifunctional non-noble catalysts. Ye et al.224 employed a 20% Ni–0.5% Ce/Al2O3 catalyst with Ca(OH)2 promoter, achieving >90% sorbitol conversion and 55–60% diol (EG and 1,2-PG) yield at 240 °C and 7 MPa H2 over 12 h. Ce enhanced Ni dispersion and introduced moderate basicity, favoring RAC-hydrogenation coupling. Zhou et al.225 prepared ordered mesoporous M–NiCeAl via evaporation-induced self-assembly (EISA), affording 58.1% combined diol yield under 220 °C, 6 MPa H2 with added Ca(OH)2. Zhang et al.54 found that 8% Ni–2% Re/C produced 15.8% EG and 31.0% 1,2-PG at 250 °C in Ba(OH)2 medium, with Re preventing Ni sintering and enhancing selective C–C cleavage.
Zeolite-mediated confinement alters selectivity. Sivasanker et al.226,227 revealed that Ni–NaY selectively converts sorbitol to 1,2-PG, whereas Pt–NaY favors glycerol. Activity trends Ni > Pt > Ru on NaY zeolites, correlating with sorbitol adsorption strength on Ni(111) surfaces that facilitates C–C activation. 6% Ni/FA catalysts using fly-ash supports achieved 65% conversion and 37% 1,2-PG selectivity at 200 °C and 6 MPa H2 within 12 h,228 while 2% Ni2P/AC in aqueous Ba(OH)2 yielded 17% EG and 29% 1,2-PG under 200 °C, 4 MPa H2, 0.75 h.229
To avoid soluble bases, solid-base catalysts have emerged as a major advance. Cu/CaO–Al2O3230 contained dual CaxCuyAlzOp (dehydrogenation and isomerization) and CuAl2O4 (hydrogenation) domains, achieving high diol yields without alkali. 6% Ni–SrHAP–R231 exploited the alkalinity of Sr-hydroxyapatite nanorods to achieve ≈60% diol selectivity. Ni–MgO catalysts232 displayed performance correlated with basicity and the active metal sites, and 3 Ni–7 MgO achieved 80.8% total selectivity (EG, 1,2-PG, and glycerol) at 200 °C and 4 MPa H2. Across Ni–, Cu–, and Co–MgO series,233 activity decreased as Ni > Co > Cu, emphasizing the role of basicity and metal–support interaction in C–C cleavage control.
Composite oxides and rare-earth promoters further enhance stability and dispersion. Ni/Mg1.29Al0.06O1.38 catalysts derived from layered double hydroxides (LDHs) achieved 97% sorbitol conversion with tunable selectivity governed by surface area and alkalinity.234 Subsequently, the Ni3.6Mg2.4Al2(OH)16CO3–SF catalyst obtained by changing the preparation method exhibited higher activity and stability in the conversion of sorbitol.235 Ni/La2O3/ZrO2 systems236,237 exhibited strong Ni2+–O–La3+ interactions improving dispersion and basicity. 10% Ni/10% La2O3/ZrO2 achieved complete sorbitol conversion and >48% polyol yield at 240 °C under 4.0 MPa H2.236 Moreover, 10% Ni/5% La2O3/ZrO2 reached 96.8% sorbitol conversion and 74.8% polyol selectivity at 220 °C and 4 MPa H2.237 The cooperative tuning of metal–support interactions, intrinsic basicity, and promoter chemistry in these systems demonstrates how non-noble catalysts can effectively orchestrate dehydrogenation, isomerization, and C–C/C–O bond cleavage in aqueous hydrogenolysis, setting the foundation for the tungsten-assisted systems discussed in the following section.
Across cellulose, monosaccharide, and polyol hydrogenolysis, transition-metal catalysts reveal a unifying design philosophy: the catalytic landscape is governed by the interplay between metallic hydrogenation sites and acid–base or redox co-functions that control isomerization and C–C bond cleavage. Nickel-based catalysts serve as the primary platform, yet Cu and Co analogues offer complementary electronic and acid–base environments. Alloying promoter incorporation and structural confinement within oxides or zeolites enable fine control over reactivity and stability. By integrating these cooperative functionalities, non-noble transition-metal systems can rival noble metals in efficiency and selectivity, offering a sustainable foundation for next-generation hydrogenolysis catalysts.
Cellulose hydrogenolysis. Under aqueous conditions, tungsten species such as WOx, WCx, H2WO4, and heteropoly acids are partially reduced to hydrogen tungsten bronzes (HxWO3), which act as true active intermediates.42,238 These bronzes generate protons that provide Brønsted acidity for cellulose hydrolysis, while reduced W centers offer Lewis acid sites for C–C bond cleavage. Liu et al.239 confirmed the reversible transformation of WO3 → H0.23WO3 → H0.33WO3, establishing a proton-coupled redox mechanism that accounts for the dynamic heterogeneous-homogeneous nature of tungsten catalysis. Kinetic analyses of tungstic acids240 identified H3O40PW12 as the most active hydrolysis catalyst, while isotopic-labeling and DFT calculations241 confirmed that β-position C–C scission proceeds via tridentate coordination of sugars to W–O–W bridges, forming glycolaldehyde, glyceraldehyde, and dihydroxyacetone as primary intermediates. These features establish WOx/HxWO3 as redox-active acid frameworks ideally suited for depolymerization and RAC initiation.
Beyond oxides, tungsten carbides (W2C/WCx) represent a second, highly effective class of tungsten-based catalysts distinguished by their Pt-like electronic structure, high resistance to hydrothermal leaching, and strong W–C bonding. These properties allow WCx to maintain reduced W centers and surface oxygen vacancies under reaction conditions, making them powerful platforms for C–C/C–O bond activation. Ni–W carbide systems consistently deliver state-of-the-art EG yields, as shown in Table 3. A (2% Ni–30% W2C)/AC-973 catalyst achieved complete cellulose conversion and 61% EG yield at 245 °C and 6 MPa H2.40 Post-impregnation treatment minimized W2C sintering, yielding a highly dispersed 10% Ni-(30% W2C/AC) catalyst with 73% EG yield.242 Similar strategies extended to raw lignocellulose, where Ni–W2C/AC achieved 38.5% 1,2-PG from Jerusalem artichoke tuber (JAT)243 and 75.6% total diols from untreated woody biomass.244 Mechanistic analyses245,246 attributed the high EG selectivity to Ni–W2C interfacial synergy, which facilitated hydrogen spillover and moderated EG adsorption to prevent over-hydrogenolysis. Wu et al.247 synthesized 9% Ni–13.5% W-graphitic carbon (GC850), WOx → W → WCx evolution with increasing temperature and W loading, delivered 69% EG at 230 °C, 5 MPa H2. Zhang et al.248 dispersed WCx on mesoporous carbon, reaching 73% EG. The MC support enhances WCx dispersion and pore transport, maximizing activity.
| Entry | Catalyst | T (oC) | P (MPa, H2) | T (h) | Yield (C mol%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | (2% Ni–30%W2C)/AC-973 | 245 | 6.0 | 0.5 | EG 61.0 | 40 |
| 1,2-PG 7.6 | ||||||
| 2 | 10% Ni–(30% W2C/AC) | 245 | 6.0 | 0.5 | EG 73.0 | 242 |
| 1,2-PG 8.5 | ||||||
| 3 | 4% Ni–20% W2C/AC | 245 | 6.0 | 1.3 | EG 14.1 | 243 |
| 1,2-PG 38.5 | ||||||
| 4 | 4% Ni–30% W2C/AC | 235 | 6.0 | 4.0 | EG 52.7 | 244 |
| 1,2-PG 11.9 | ||||||
| 1,2-BG 5.1 | ||||||
| 5 | 2% Ni–W2C/AC-973 | 245 | 6.0 | 0.5 | EG 61.0 | 245 |
| 1,2-PG 7.6 | ||||||
| 6 | 9% Ni–13.5% W–GC850 | 230 | 5.0 | 2.0 | EG 69.2 | 247 |
| 7 | WCx/MC | 245 | 6.0 | 0.5 | EG 72.9 | 248 |
| 1,2-PG 5.1 | ||||||
| 8 | 2% Ni–20% WP/AC | 245 | 6.0 | 0.5 | EG 46.0 | 249 |
| 9 | Raney Ni + H2WO4 | 245 | 6.0 | 0.5 | EG 65.0 | 250 |
| 10 | Raney Ni + WO3 | 245 | 6.0 | 2.0 | EG 37.6 | 251 |
| 1,2-PG 6.3 | ||||||
| 11 | 5% Ni–15% W/SBA-15 | 245 | 6.0 | 0.5 | EG 76.1 | 71 |
| 1,2-PG 3.2 | ||||||
| 12 | 3% Ni–15% WO3/SBA-15 | 230 | 6.0 | 6.0 | EG 70.7 | 252 |
| 13 | 10% Ni–20% W/SBA-15 | 245 | 5.0 | 2.0 | EG 64.9 | 253 |
| 14 | Ni/W/SiO2–Al2O3 | 245 | 6.0 | 2.0 | EG 23.3 | 254 |
| 1,2-PG 5.1 | ||||||
| 15 | 7Ni–20W–ZnO/Beta | 245 | 6.0 | 0.5 | 1,2-PG 35.8 | 255 |
| 16 | 10% Ni–15% W/MOR | 240 | 5.0 | 2.0 | EG 52.3 | 256 |
| 17 | 15% Ni–20% W/SiO2 | 240 | 5.0 | 2.0 | EG 61.3 | 257 |
| 18 | 15% Ni–20% W/SiO2-EEG | 240 | 5.0 | 2.0 | EG 63.3 | 258 |
| 19 | 15% Ni–20% W/SiO2–OH | 240 | 5.0 | 2.0 | EG 63.1 | 259 |
| 20 | 15% W–5% Ni/MSM | 245 | 6.0 | 2.0 | EG 27.9 | 260 |
| 1,2-PG 13.9 | ||||||
| 21 | 3% Ni–15% WO3–3% Al-TUD-1 (zeolite) | 230 | 4.0 | 1.5 | EG 76.0 | 261 |
| 22 | 5% Al–8% Ni–25% W/NaZSM-5 | 220 | 7.0 | 6.0 | EG 89.0 | 262 |
| 23 | NiWB/CNTs | 250 | 6.0 | 2.0 | EG 57.7 | 263 |
| 24 | Ni–W/MIL-125(Ti) | 245 | 4.0 | 2.0 | EG 68.7 | 264 |
| 1,2-PG 6.5 | ||||||
| 25 | 5Ni–15W–15Cu/MgAl2O4 | 245 | 3.0 | 2.0 | EG 52.8 | 265 |
| 26 | 30% Cu–30% WOx/AC + Ni/AC | 245 | 4.0 | 2.0 | EG 71.0 | 266 |
| 27 | 30% Cu–30% WOx–10% Ni/AC | 245 | 4.0 | 2.0 | EG 58.0 | |
| 28 | Ni6Cu1.5/WO3 | 245 | 5.5 | 4.0 | EG 58.9 | 267 |
| 29 | Ni–W@C700 | 240 | 5.0 | 1.0 | EG 60.1 | 268 |
| 30 | Ni–W/SiO2@CxNy | 240 | 5.0 | 2.0 | EG 48.3 | 269 |
Beyond carbides, tungsten phosphides (WP) provide strong W–P bonding and enhanced electronic density at W sites, creating redox-active surfaces that cooperate effectively with hydrogenation metals. Zhao et al.249 obtained 46% EG yield using 2% Ni–20% WP/AC at 245 °C, 6 MPa H2, and 0.5 h, highlighting the synergistic effect between Ni and WP. Tai et al.250 achieved 65% EG yield and excellent recyclability over H2WO4–Raney Ni, outperforming H4SiW12O40, H3PW12O40, and WO3 analogues. Similar WO3–Raney Ni composites converted JAT to EG with 41.4% yield,251 demonstrating the adaptability of W-based systems for diverse carbohydrate substrates.
Support architecture critically governs tungsten dispersion, acidity, and metal–acid proximity. Zheng et al.71 found that 5% Ni–15% W/SBA-15 delivered 76.1% EG yield, whereas Cao et al.252 linked strong NiO–WO3 coupling in 3% Ni–15% WO3/SBA-15 to the formation of WO3−x species responsible for selective C–C cleavage (70.7% EG). Adjusting the impregnation solution pH controlled dispersion and acidity. The 10% Ni–20% W/SBA-15 sample prepared at pH = 1 achieved 64.9% EG yield.253 Baek et al.254 correlated polyol selectivity in (Ni, Cu, Fe, Co)/W/SiO2–Al2O3 catalysts with total surface acidity, with Al-rich Ni/W/SiO2–Al2O3 (Al/(Al + Si) = 0.6) showing optimal performance. Silica- and zeolite-based supports enable in situ alloying and controlled reduction. For example, the 7 Ni–20 W–ZnO/Beta catalyst achieved 1,2-PG yield of 35.8%,255 and self-reducing 10% Ni–15% W/MOR yielded 52.3% EG,256 while 15% Ni–20% W/SiO2 nanospheres reached 61–63% EG when chelating agents stabilized Ni species and Ni–W alloys.257–259 Unique meso-structure silica microspheres further enhanced metal loading and mass transfer, yielding 82.2% total diols.260
Heteroatom incorporation and additional metallic or metalloid elements further improve activity. Al-doped Ni–W systems, such as 3 Ni–15 WO3–3 Al-TUD-1,261 5% Al–8% Ni–25% W/NaZSM-5,262 achieved up to 76% and 89% EG yield, respectively, where Al-induced acid sites promoted depolymerization, W5+ vacancies acted as Lewis sites for RAC, and Ni completed hydrogenation. Amorphous NiWB/CNTs,263 Ni–W/MIL-125(Ti),264 5 Ni–15 W–15 Cu/MgAl2O4,265 30% Cu–30% WOx/AC combined with Ni/AC,266 and NiCu/WO3267 further highlight the importance of tuning metal–support interfaces and oxygen vacancy concentrations to control C–C cleavage selectivity.
Despite their outstanding activity, tungsten-based catalysts can deactivate through leaching, sintering, or phase dissolution under hydrothermal conditions. Stabilization strategies address these challenges. Ti–O–W coordination in Ni–W/MIL-125(Ti) prevented tungsten loss and maintained high activity over seven cycles,264 while 30% Cu–30% WOx/AC + Ni/AC dual-bed systems minimized coke formation by coupling vacancy generation and glycolaldehyde hydrogenation.266 Carbon encapsulation has also proven effective—Ni-W@C700 achieved 60.1% EG yield with minimal deactivation,268 and C,N-modified Ni–W/SiO2@CxNy269 stabilized metal clusters via Ni–N–N coordination, imparting excellent hydrothermal durability.
These results establish tungsten-based materials as multifunctional platforms uniting hydrolysis, RAC, and hydrogenation within a single redox-flexible framework. Their reversible formation of protonated bronzes (HxWO3) allows dynamic acid modulation, while coupling with hydrogenation metals such as Ni or Cu enhances reduction capability.
Monosaccharides and polyols hydrogenolysis. Glucose hydrogenolysis provides a direct window into W-mediated RAC chemistry, allowing decoupling of glycosidic hydrolysis from C–C/C–O activation. Tungsten-based catalysts drive this transformation via an isomerization–RAC–hydrogenation cascade modulated by W oxidation state and metal coupling. Zhang et al.32 quantified glucose conversion kinetics using AMT, showing that elevated temperature favored glycolaldehyde formation and validated the kinetic model for concentration–time (C–t) profiles. Ooms et al.270 extended this concept to concentrated feeds, obtaining 66% EG yield over 2% Ni–30% W2C/AC-973, while fine control of temperature, pressure, and feed rate enhanced overall efficiency.
Support and metal–acid balance strongly influence glucose fragmentation pathways. Ni-WO3/SBA-15 catalysts with variable W/Ni ratios revealed that higher ratios favored RAC–hydrogenation pathways to EG, while lower ratios promoted direct hydrogenation to sorbitol.271 The optimized 5% Ni–15% WO3/SBA-15 catalyst yielded 41.5% EG at 175 °C, 6 MPa H2, and 1.3 h. The catalytic performance correlated with structure of tungsten compounds and dispersion, following WO3 < WO3/SBA-15 < AMT.272 Liu et al.273 tuned acid–metal cooperation via Cu-WOx/Al2O3 catalysts, showing that isolated WO4 species combined with tetrahedrally coordinated Al generated Lewis acid sites for glucose–fructose isomerization, while Cu sites catalyzed RAC and hydrogenation to yield 55.4% 1,2-PG at 180 °C, 4 MPa H2. These results show that controlling W local coordination and redox state provides a powerful handle for steering C2vs. C3 polyol distributions.
The W-mediated framework extends naturally to polyol hydrogenolysis, where C–C cleavage depends on stabilizing transient aldose/ketose intermediates and accelerating retro-aldol steps. Tungsten's ability to dynamically interconvert Brønsted and Lewis acid sites facilitates rapid dehydrogenation–cleavage sequences. Metals subsequently hydrogenate the carbonyl intermediates, suppressing recombination or humin formation. This cooperative acid–redox–metal functionality enables tungsten catalysts to couple dehydrogenation, β-C–C scission, and hydrogenation with a degree of selectivity unmatched by other non-noble oxides.
Across cellulose, glucose, and polyol hydrogenolysis, tungsten-based catalysts exhibit a unifying mechanistic paradigm rooted in reversible proton-coupled redox chemistry and the coexistence of Brønsted and Lewis acid sites. Collectively, these studies establish that tungsten species—whether present as oxides, carbides, or phosphides—operate as adaptive redox-acid frameworks that synergize with hydrogenation metals to drive highly selective C–C/C–O activation. Through tunable valence states, proton-coupled redox chemistry, and interface engineering, W-based catalysts unify hydrolysis, RAC, and hydrogenation in a single multifunctional platform. Rational control of tungsten dispersion, support architecture, and acid–metal balance defines a powerful design paradigm for efficient, stable, and recyclable systems in cellulose and sugar hydrogenolysis.
3.4 Advanced catalyst architectures
Despite significant progress in noble- and non-noble-metal catalysts, conventional supported systems still face intrinsic limitations in cellulose hydrogenolysis. Metal nanoparticles and acid/base promoters are typically dispersed randomly on oxide or carbon supports, preventing efficient cooperation between hydrolysis, isomerization, C–C/C–O scission, and hydrogenation sites. Under hydrothermal conditions, these catalysts additionally suffer from sintering, leaching, and structural degradation,274 which hinder sustained activity and precise control of reaction pathways.To address these constraints, recent research has moved toward advanced catalyst architectures that combine spatial confinement, electronic modulation, and intentionally organized multifunctional sites within well-defined nanostructures. These systems aim to achieve the threefold objective of (i) maximizing proximity and synergy between hydrogenation and acid–base sites, (ii) stabilizing reactive metal species against thermal and chemical degradation, and (iii) selectively channeling reaction intermediates along preferred pathways toward C2–C3 diols and low-carbon alcohols. Such designs draw direct inspiration from enzymatic catalysis, wherein confined microenvironments and cooperative active sites orchestrate complex multi-step transformations with high precision and minimal energy dissipation. In the context of biomass valorization, this paradigm has led to the development of diverse nanoscale configurations—including yolk–shell,127 core–shell,191,192 mesoporous,189 and zeolite-confined catalysts190—that deliver superior activity, selectivity, and stability compared with conventional supported systems. The following subsections summarize the underlying principles and representative advances of confined catalysts for biomass hydrogenolysis.
Physical stabilization and size confinement. Confinement physically restricts the mobility and coalescence of nanoparticles, preventing sintering and preserving uniform dispersion even under harsh hydrothermal conditions.277 The resulting small, well-dispersed particles ensure consistent exposure of active sites and maintain catalytic stability. Since metal particle size strongly influences hydrogenation and hydrogenolysis selectivity, precise control enabled by confinement directly improves catalytic precision.278
Electronic environment modulation. Beyond physical stabilization, confinement significantly modifies the electronic state of metal centers through metal–support interactions. For instance, encapsulating Ru or Pt nanoparticles within zeolitic frameworks can lead to electron-enriched metal centers via charge donation from the surrounding lattice.279 Such tuning of electron density facilitates the activation of H2 and optimizes adsorption of oxygenated intermediates, enhancing C–O and C–C bond hydrogenation steps.280
Intermediate enrichment and shape selectivity. The confined nano-spaces can locally enrich reactants and intermediates by concentration or adsorption effects, thereby accelerating reaction kinetics. Moreover, the geometric constraints of pores or cavities induce shape-selective catalysis, preferentially stabilizing intermediates of specific configurations and suppressing undesired side reactions.281–283 This combination of molecular sieving and intermediate control is particularly advantageous for the selective hydrogenolysis of carbohydrates to low-carbon alcohols.
A representative example is the yolk–shell catalyst Ru/NC@void@MC–SO3H designed by Yang et al.127 In this architecture, Ru clusters are confined in the inner core, while sulfonic acid groups are grafted on the mesoporous carbon shell (Fig. 5a–c). The hierarchical structure achieved a 38.0% yield of 1,2-PG with a productivity of 342.86 mol h−1 gRu−1. Hydrolysis occurred on sulfonic acid sites, glucose isomerization and fructose RAC proceeded on Ru–Nx acid–base pairs, and final hydrogenation was catalyzed by metallic Ru, illustrating seamless integration of multi-step reactions within a confined domain.
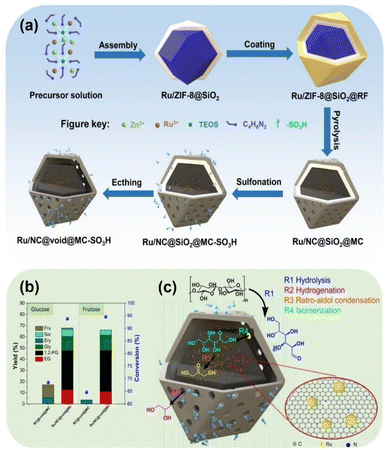 | ||
| Fig. 5 (a) Preparation process of Ru/NC@void@MC–SO3H catalyst; (b) performance evaluation of glucose and fructose on NC@void@MC and Ru/NC@void@MC; (c) possible reaction mechanism of cellulose generating 1,2-PG on Ru/NC@void@MC–SO3H. Reproduced with permission.127 Copyright 2023, Elsevier. | ||
Liu et al.188 reported a Pd@W/Al–MSiO2 yolk–shell nanosphere (YSNS) catalyst for cellulose hydrogenolysis to EG. The porous shell protected Pd nanoparticles from sintering and leaching, maintaining 48.5% EG selectivity after five cycles. The confined environment also strengthened W–Al synergy. Extra-framework Al enhanced acidity, while W dispersion improved, affording 56.5% EG selectivity at 96.1% cellulose conversion. Further development of Pd@WOx–MSiO2191 (Fig. 6) revealed that oxygen vacancies on W5+ sites facilitated C–C bond cleavage, while adjacent Pd sites catalyzed hydrogenation, yielding 59.4% EG.
 | ||
| Fig. 6 Schematic representation of the synthesis of Pd@WOx–MSiO2 YSNSs. Reproduced with permission.191 Copyright 2021, American Chemical Society. | ||
By systematically tuning shell acidity, Pd@Al–MSiO2 catalysts with varying Al content were optimized (Fig. 7).192 Tetrahedrally coordinated AlO4 units introduced abundant Lewis acid sites essential for glucose–fructose isomerization. The optimized Pd@Al3–MSiO2 catalyst achieved 95.4% glucose conversion and 45.2% 1,2-PG yield, confirming that controlled acid–metal proximity governs product distribution. Likewise, Pd@Al–SiO2 catalysts189 prepared by a one-pot sol–gel method achieved 59.3% ethanol selectivity and a total carbon yield of >95% under 245 °C and 4.5 MPa H2 (Fig. 8), with Al incorporation promoting Pd–O–Si(Al) linkages and Pd0/Pdδ+ bifunctional ensembles for synchronized cascade reactions. The spatial distribution of active sites ensures the sequential exposure of substrates to acid and metal sites, effectively regulating reaction order and suppressing side reactions.
 | ||
| Fig. 7 The possible reaction route of glucose hydrogenolysis on Pd@Al–MSiO2 YSNSs. Reproduced with permission.192 Copyright 2020, Elsevier. | ||
 | ||
| Fig. 8 Schematic representation of cascade steps and active site locations of cellulose hydrogenation to ethanol over Pd@Al3–SiO2 catalyst. Reproduced with permission.189 Copyright 2025, Elsevier. | ||
These results collectively demonstrate that spatial confinement coupled with precisely engineered multi-site integration provides kinetic and thermodynamic control in complex biomass hydrogenolysis pathways.
The controlled interplay between zeolitic acidity and confined metal centers has been systematically demonstrated by Yu, Xiao, and co-workers, who developed a series of in situ encapsulated metal@zeolite catalysts exhibiting enzyme-like cooperativity in hydrogenation and oxidation reactions.284,287,291 Their studies revealed that the co-confined Brønsted and Lewis sites create a microenvironment capable of orchestrating multistep transformations with high precision.292 For example, Weckhuysen et al. encapsulated ∼1 nm Ru clusters within La-modified Y zeolite,293 the restricted proximity between Ru and acid sites enabled solvent-free conversion of ethyl levulinate to valerate biofuels. Similarly, Cho et al.294 reported a “nest effect” in Pt@H-ZSM-5, where Brønsted acid–metal cooperation facilitated a five-step cascade from furfural to valeric acid and ethyl valerate. These studies collectively highlight how zeolite confinement strategies can orchestrate complex multi-step transformations with high efficiency and selectivity.
The catalytic performance of metal@zeolite systems is governed by their acid–metal balance and nanoscale spatial distribution.278 In Ru/H-ZSM-5-catalyzed hydrodeoxygenation of levulinic acid, dominant hydrogenation yields γ-valerolactone, whereas stronger acidity drives further conversion to valeric acid.58 Rational tuning of acid–metal ratios during synthesis thus becomes essential for optimizing selectivity. Moreover, as emphasized by de Jongh and other researchers,288–290 nanoscale site separation governs electronic coupling between metal and acid centers, which in turn tunes adsorption and activation energies.280
Building on these principles, our group designed PdxZny@S-1 zeolite catalysts via a ligand-protection and direct hydrogen-reduction strategy.190 The PdZn alloy clusters and adjacent Pdδ+–O(H)–Si coordination within the S-1 zeolite respectively act as hydrogenation and acid sites, forming bifunctional active sites that significantly enhanced the hydrogenolysis of cellulose to ethanol (Fig. 9). This architecture modulated Pd's electronic structure and acid density, achieving 69.2% ethanol selectivity and 97.4% total carbon yield from cellulose, and surpassing most reported heterogeneous systems.
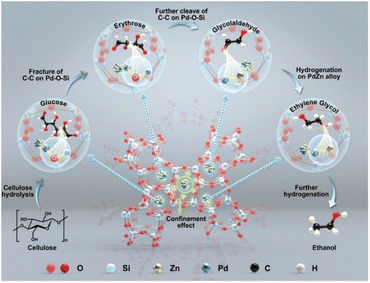 | ||
| Fig. 9 Presumptive reaction mechanism for the cellulose hydrogenolysis to ethanol over PdZn0.5@S-1 catalyst. Reproduced with permission.190 Copyright 2025, Wiley-VCH. | ||
Across yolk–shell, core–shell, mesoporous, and zeolite-confined catalysts, three unifying principles emerge: (i) nanoscale confinement prevents sintering, maintains metal dispersion, and enables size-dependent selectivity; (ii) electronic coupling between confined metals and supports tailors adsorption and activation energies for optimized hydrogenation and C–C cleavage; and (iii) spatial integration of multifunctional sites minimizes diffusion losses and promotes synchronized cascade reactions. These spatially engineered architectures establish a robust and sustainable platform for the selective conversion of lignocellulosic biomass into low-carbon alcohols and other renewable chemicals, bridging fundamental catalytic principles with practical, high-efficiency biomass valorization.
In summary, although the aforementioned advanced architectures exhibit material diversity, their design adheres to transferable universal principles. Spatial confinement, as a physical stabilization strategy, can effectively prevent the sintering and leaching of various active components under hydrothermal conditions. Electronic modulation, achieved through well-designed metal–support interfacial interactions, provides a general means to regulate the electron density of different active centers and optimize their adsorption and activation behaviors toward reactants. The nanoscale integration of multiple active sites serves as a universal blueprint for constructing efficient tandem catalytic reactions. Its core lies in the precise control of distances between acid, base, and metal sites to ensure the directed and rapid transfer of intermediates. Together, these principles form the architectural foundation for the rational design of high-performance cellulose hydrogenolysis catalysts that transcend specific materials.
Although remarkable progress has been made, key challenges remain in quantitatively correlating site proximity (e.g., optimal site spacing and acid/metal ratio windows) with turnover-determining steps, understanding dynamic restructuring under hydrothermal conditions, and scaling deterministic confinement. Future progress will rely on operando spectroscopy, microkinetic modeling, and adaptive supports capable of on-demand tuning of metal–acid interactions.
4 Product-oriented pathways and selectivity principles
The catalytic conversion of cellulose into well-defined alcohols represents a critical step toward the sustainable utilization of lignocellulosic biomass. While mechanistic studies elucidate how individual bond-breaking and hydrogenation steps proceed, and catalyst design focuses on optimizing active-site structures, product-oriented strategies integrate both dimensions—linking reaction pathways to the architecture and electronic properties of the catalyst.Selectivity in hydrogenolysis arises not from a single active function but from the cooperative synchronization of acid, base, and metal sites that govern hydrolysis, isomerization, C–C/C–O bond cleavage, and hydrogenation in sequence. The distribution of products, ranging from C2 (EG, ethanol) to C3 (1,2-PG) and C6 (polyols), is determined by how these functions interact dynamically within the catalytic microenvironment. Building upon the mechanistic insights outlined in Section 2 and the catalyst architectures described in Section 3, this chapter examines how the interplay between electronic structure, acid–base balance, and spatial organization directs selectivity toward specific products, using ethanol, EG, 1,2-PG, and polyols as representative cases.
4.1 Ethanol system
Ethanol, a key platform molecule for both renewable fuels and fine chemicals,295 serves as a benchmark for C2-selective hydrogenolysis. Its formation from cellulose proceeds through sequential hydrolysis, RAC, and hydrogenolysis steps, where C–O bond cleavage and subsequent hydrogenation of C2 intermediates define the selectivity window. Achieving high ethanol yields requires precise coordination of acid and metal functionalities to steer the reaction toward C2 termination rather than over-fragmentation or polymerization.As shown in Table 4, tungsten-containing catalysts have emerged as the most effective systems for ethanol-oriented hydrogenolysis. In Mo/Pt/WOx catalysts, interfacial OxMo–Pt–WOx ensembles promote C–O bond scission in EG intermediates, achieving 43.2% ethanol selectivity under hydrothermal conditions.26 Similarly, Pd–Cu–WOx/SiO2 catalysts enable one-pot cellulose conversion to ethanol with over 42% yield, benefiting from cooperative effects between redox-active WOx and bimetallic hydrogenation centers.27 In both systems, dynamic electron transfer between metal and oxide components stabilizes W5+ species and generates acid sites in situ, facilitating selective C–O cleavage without over-hydrogenation.
| Catalyst | Solvent | T (°C) | P (MPa) | T (h) | Yield (C mol%) | Ref. |
|---|---|---|---|---|---|---|
| Ni–W + Cu/SiO2 | Tetrahydrofuran | 280 | 3.0H2 | — | 29.0 | 296 |
| Pt–Cu/SiO2 | Tetrahydrofuran | 230 | 3.0H2 | — | 44.0 | 297 |
| Mo/Pt/WOx | H2O | 245 | 6.0H2 | 2.0 | 43.2 | 26 |
| Pd–Cu–WOx/SiO2 | H2O | 300 | 4.0H2 | 10.0 | 42.5 | 27 |
| H2WO4 + Pt/ZrO2 | H2O | 250 | 4.0H2 | 5.0 | 32.0 | 28 |
| H3PO4 + Ni@C-700 | H2O | 200 | 5.5H2 | 3.0 | 69.1 | 29 |
| 5Ru–25WOx/HZSM-5 | H2O | 235 | 3.0H2 | 24.0 | 42.3 | 95 |
| Pt/WOx + Pt@HZSM-5 | H2O | 245 | 4.0H2 | 4.0 | 54.4 | 30 |
| Pd/Fe3O4 | H2O | 240 | 0.5N2 | 12.0 | 51.0 | 183 |
| Pd@Al–SiO2 | H2O | 245 | 4.5H2 | 4.0 | 59.3 | 189 |
| PdZn@silicalite-1 | H2O | 245 | 4.5H2 | 4.0 | 69.2 | 190 |
Protonic acid–metal composite systems provide an alternative route for ethanol synthesis. In H2WO4–Pt/ZrO2 catalysts, the balanced Pt0/Pt2+ ratio ensures controlled hydrogenation of C2 intermediates, while H2WO4 selectively cleaves C–C bonds in glucose, directing carbon flux toward ethanol.28 Other studies have demonstrated similar synergy in combined Ni@C–H3PO4 systems, where protonic acids accelerate hydrolysis and retro-aldol steps, and metal sites facilitate hydrogenation.29 The key lies in maintaining an intermediate acidity level that enhances depolymerization and C–C scission while preventing dehydration or carbon loss.
Recent progress in spatially confined hybrid catalysts has further advanced ethanol selectivity. Fu et al.95 developed the Ru-WOx/HZSM-5 and obtained an ethanol yield of 42.3% after 24 h of reaction. The highly dispersed Ru3W17 alloy had a moderate acid site and showed a synergistic catalytic effect with HZSM-5. Hollow Pt@HZSM-5 (ref. 30) and Pd@Al–SiO2189 catalysts integrate acid and metal functions within nanoconfined architectures, promoting tandem hydrolysis–hydrogenation reactions while minimizing diffusion loss of intermediates. The PdZn@S-1 zeolite system exemplifies this principle. The “restricted adjacency” between PdZn alloy sites and nearby Pd–O(H)–Si groups forms bifunctional ensembles of hydrogenation and Lewis-acid centers, achieving 69.2% ethanol selectivity and nearly complete carbon recovery.190
These results collectively reveal that ethanol selectivity is dictated by C–O bond activation preference and the stability of C2 intermediates, both governed by acid–metal synergy and local electronic structure. Rational control over metal–oxide interfaces and confinement thus enables the programmable conversion of cellulose into ethanol with molecular-level precision.
4.2 EG system
Among the various polyols derived from cellulose hydrogenolysis, EG occupies a uniquely central position due to its high carbon efficiency, chemical stability, and broad industrial relevance as an antifreeze, solvent, and polymer precursor. From a mechanistic standpoint, EG formation represents a selectivity-controlled termination point within the C2–C3 scission network, specifically, the selective hydrogenation of glycolaldehyde intermediates derived from glucose RAC. Thus, understanding EG selectivity requires analyzing how catalyst architecture regulates β-C–C bond cleavage, intermediate stabilization, and hydrogenation kinetics within a single catalytic framework.Tungsten-based catalysts uniquely fulfill these requirements through redox-active acid–metal cooperation. Under hydrothermal conditions, WO3 species are partially reduced to hydrogen tungsten bronzes (HxWO3),42 which generate both Brønsted and Lewis acid sites. The variable valence of tungsten enables reversible redox cycling (W6+ ↔ W5+), maintaining continuous activity for C–C scission and hydrogenation.238–240 In Ni–W2C, Ni–WO3/SBA-15, and Ni–W/MOR systems, strong electronic coupling between Ni and W centers enhances hydrogen spillover and moderates EG adsorption, suppressing over-hydrogenolysis. Optimized catalysts achieve EG selectivities exceeding 70% under 230–245 °C and 3–6 MPa H2, with stable performance across multiple cycles.40,71,256
The metal–support interface plays an equally decisive role. Supports such as SBA-15, SiO2, or ZnO254,257 adjust surface acidity and confine intermediates within mesoporous environments, preventing excessive fragmentation. The acidity-reducibility balance of the support defines how long reactive intermediates reside near the metal–oxide interface, directly shaping selectivity. When WO3−x domains remain partially reduced, β-C–C cleavage to glycolaldehyde is favored;252 excessive reduction to W2C or WC drives deeper fragmentation toward methanol or methane.247 Metals such as Ni and Ru maintain the optimal W5+/W6+ ratio, while Cu or Al modifiers introduce weak basicity that further suppresses over-cleavage.261–263
Spatial confinement offers an additional level of selectivity control. Encapsulated nanoreactors, such as core–shell Ru@acid-functionalized oxides, enable hydrolysis near the shell and RAC-hydrogenation near the core, allowing diffusion-mediated regulation of product ratios.127 In temperature-responsive H2WO4 systems, partial dissolution under reaction conditions followed by reprecipitation upon cooling provides a self-regulating supply of active species, ensuring stable W valence distribution.114
Overall, EG formation exemplifies the threefold coordination of catalytic functions: redox-active tungsten oxides provide dynamic acidity, metallic centers control hydrogenation selectivity, and structured supports define the microenvironment for intermediate stabilization. Together, these factors allow EG production to be directed by rational design rather than empirical optimization.
4.3 1,2-PG system
The selective formation of 1,2-PG illustrates how subtle tuning of acid–base and metal functions directs the reaction pathway toward C3 products. While EG arises from β-C–C bond cleavage in glucose, 1,2-PG originates from glucose–fructose isomerization followed by γ-C–C bond scission through RAC of fructose to dihydroxyacetone and glyceraldehyde, which are then hydrogenated into 1,2-PG. Selectivity thus depends on synchronizing these sequential reactions—each requiring distinct catalytic functions.Lewis acid-mediated isomerization initiates this transformation by rearranging glucose to fructose through 1,2-hydride shifts. Catalysts containing W5+, Al3+, or Sn4+ centers (in WOx, Al2O3, Sn-Beta, Sn-MFI) generate coordinatively unsaturated Lewis acid sites that polarize the carbonyl group and enable hydride migration or a ring-opening-ring-closure mechanism of the pyranose form to facilitate glucose–fructose isomerization.298–301 These same centers subsequently promote RAC to form C3 intermediates, while adjacent metallic sites (Ni, Cu, Pd, or Pt) hydrogenate them to 1,2-PG. The acid–metal balance determines product distribution. Excessive acidity accelerates cleavage to C2 species, while insufficient acidity hinders isomerization. Systems such as Pd–WOx/Al2O3,302 Cu–WOx/Al2O3,273 and CuB/Al2O3222 achieve an optimal middle ground, producing 45–56% 1,2-PG from glucose with minimal over-fragmentation.
Basic co-catalysts introduce a complementary control dimension. Moderately basic supports such as MgO,221 ZnO,212 or La2O2CO3223 promote enediol formation via proton abstraction at C2, lowering the barrier for glucose–fructose isomerization. The ionic radius and basicity of these oxides modulate intermediate stability and suppress humin condensation.223 When coupled with metal sites, such as in CuCr–Ca(OH)2 (ref. 86 and 87) or Ni–W–ZnO, Ni–MgO–ZnO212,255 systems, the proximity of base and metal centers ensures rapid turnover between isomerization, RAC, and hydrogenation, producing 1,2-PG yields up to 53% under hydrothermal conditions.
Tin-based systems represent a particularly versatile platform because Sn species provide both Lewis acidity and redox flexibility. Framework-incorporated Sn4+ sites in Sn-Beta or Sn-MFI zeolites act as solid Lewis-acid analogues of enzymes, catalyzing glucose isomerization and RAC with water-tolerant stability.82,89,90 Surface SnOx species further tune adjacent metal electronic states, steering the EG/1,2-PG ratio.84 The Sn/metal ratio and Sn valence state control this bifunctional synergy: low Sn loading favors hydrogenation-dominant PtSn alloys, while higher Sn content enhances Lewis acidity and directs selectivity toward 1,2-PG.83 Redox cycling between Sn2+ and Sn4+ dynamically adjusts oxygen-vacancy concentration, stabilizing intermediates and avoiding over-hydrogenation.85 These tunable Sn-metal ensembles thus offer a “programmable” route to C3 diols, achieving 25–35% 1,2-PG selectivity with compositional control in representative catalysts such as 5% Ru–3% Sn/AC156 and Ni–SnOx/SBA-15.85
In essence, the 1,2-PG system demonstrates how acid–base-metal cooperation and site proximity orchestrate selective cascade catalysis. Lewis acids enable molecular rearrangement, bases stabilize reactive intermediates, and metals initiate hydrogenation precisely at the C3 stage. Through architectural design, reaction networks once governed by competition among pathways become programmable at the molecular scale.
4.4 Polyol system
Polyols such as sorbitol and mannitol represent the high-carbon branch of cellulose hydrogenolysis, where the carbon skeleton of glucose is preserved rather than cleaved. Their selective formation depends on the kinetic synchronization between cellulose depolymerization and glucose hydrogenation.303 Acidic sites catalyze the hydrolysis of β-1,4-glycosidic bonds, while metal sites hydrogenate the resulting glucose into polyols. If these steps are not properly balanced, intermediates undergo side dehydration or condensation, leading to humins or gaseous byproducts. Hence, controlling the relative rates of hydrolysis and hydrogenation is the decisive factor for polyol selectivity.Nickel-based systems remain the most studied non-noble catalysts for polyol formation, combining strong H2 activation304–306 with cost-effectiveness. Ni-rich catalysts benefit from the high H-dissociation capacity of Ni0/Niδ+, but suffer from sintering, leaching, and excessive hydrogenolysis under hydrothermal conditions.307–309 Electronic modulation via P or trace Rh, Ir, Pt to form Ni2P213–215 or Ni–M alloys69 weakens Ni–H bonding and geometrically isolates Ni, raising sorbitol selectivity from ∼40% to >60%. Supports such as CNF,209 MC208 and ZSM-5 (refs. 56 and 218) with low Brønsted acidity and high surface area supply mild hydrolysis sites and anchor Ni through defects or N/O functions, securing high dispersion at minimal acid density. The hydrogenation depth is governed by electron transfer between Ni and the promoter, while surface chemistry of the support controls Ni stability; only their concerted optimization delivers cost-effective, durable Ni catalysts.
Noble metal systems are predominantly based on Ru catalysts. Ru catalysts exhibit 1–2 orders of magnitude higher hydrogenation activity than Ni,309,310 enabling operation under mild conditions that suppress sugar degradation. acid–metal nano-pairing—nanoscale contact of Ru with mineral or heteropoly acids—drives cellulose hydrolysis to glucose and immediate hydrogenation to sorbitol, curbing inhibitor accumulation.116,164 Optimal acid strength and 2–3 nm Ru particles are required to avoid side reactions and over-hydrogenolysis.122,124 Functionalized supports (CNT,136 N-doped carbon,137,138 sulfonated carbon,104–107 niobium phosphate,109 zeolites110,111) stabilize Ru and promote hydrogen spillover, mitigating leaching and oligomer deposition. Systems such as HPA–Ru/C,121,125,126 Ru/CNT,123,134,146–148 Ru–N/C,139 and Ru/NbOPO4 (ref. 109) deliver 70–90% sorbitol yield at >80% selectivity for ≥4 cycles with <3 wt% Ru, decoupling high activity from stability and offsetting noble-metal cost.
Across ethanol, EG, 1,2-PG, and polyol systems, the formation of specific products in cellulose hydrogenolysis is determined by how acid, base, and metal functionalities are balanced and spatially coordinated. Acid strength dictates whether C–O or C–C bonds are cleaved first; the metal phase governs the depth of hydrogenation; and the surrounding framework defines how intermediates migrate and transform. Product selectivity thus emerges from a kinetic and structural hierarchy rather than from any single component. Three factors recur throughout all systems. First, interfacial electronic structure, especially charge transfer at metal–oxide or metal-phosphate boundaries, controls the activation barriers of key elementary steps such as glucose isomerization, RAC, and C–O hydrogenolysis. Second, acid–base balance tunes the ratio of β- to γ-C–C cleavage, guiding carbon flow between C2 (EG and ethanol), C3 (1,2-PG), and C6 (polyol) products. Third, spatial confinement and site proximity enable consecutive reactions to proceed in one domain, preventing undesired condensation and improving carbon efficiency.
Together, these principles define a unified mechanistic logic for product-oriented catalyst design. By integrating acid, metal, and structural functions within a coherent architecture, it becomes possible to steer the cellulose hydrogenolysis network toward predetermined molecular outcomes with both high selectivity and operational stability—a necessary foundation for scalable, sustainable biomass valorization. However, achieving high selectivity under laboratory conditions does not necessarily guarantee process viability. The formation and transformation of these target molecules are strongly influenced by reactor environment, mass and heat transfer, and feedstock heterogeneity, all of which evolve dramatically when moving from gram-scale experiments to continuous industrial operation. To translate these molecular-level insights into scalable technologies, the focus must shift from active-site design to the engineering of entire catalytic processes. Reactor configuration, flow regime, substrate feeding strategy, and pretreatment protocols collectively determine whether catalytic pathways established in fundamental studies can be realized at the process level. Consequently, Section 5 integrates these aspects into a unified engineering framework, linking intrinsic catalytic kinetics with reactor hydrodynamics, feedstock preparation, and industrial-scale implementation.
5 Reaction engineering and process perspectives
The catalytic hydrogenolysis of cellulose to low-carbon alcohols is a quintessential multiscale reaction system, involving molecular transformations at the catalyst surface and collective phenomena in multiphase flow environments. Achieving practical efficiency and selectivity requires uniting molecular-level design with reactor- and process-level optimization. This section thus focuses on the engineering dimensions of biomass hydrogenolysis, highlighting how reaction configuration, feedstock preparation, and process intensification translate catalytic principles into sustainable industrial practice.5.1 Reactor modes and operations
Hydrogenolysis of cellulose proceeds through sequential hydrolysis, RAC, and hydrogenation. The spatial–temporal coupling of these steps is profoundly influenced by reactor configuration and operational parameters.Batch reactors remain indispensable tools for intrinsic kinetic analysis and mechanistic elucidation. They allow precise temperature and pressure control and provide valuable information on activation energies for cellulose hydrolysis (120–180 kJ mol−1),70 glucose RAC (≈145 kJ mol−1),32 and hydrogenation (<50 kJ mol−1).31 Temperatures exceeding 453 K represent a critical threshold for the cleavage of β-1,4-glycosidic bonds24,25,69 and the rate of RAC.71,114 However, the batch reactors' closed nature promotes intermediate accumulation, leading to secondary condensation, humin formation, and a rapid decline in EG selectivity.62,63 Thus, batch operation serves primarily as a diagnostic tool for understanding elementary kinetics rather than for optimizing selectivity.
Semi-continuous reactors bridge kinetic studies and process-level optimization. Continuous substrate feeding at differential rates (typically ∼2 mL min−1 for glucose solutions) maintains a low steady-state concentration of reactive intermediates, reducing the rate of polymerization and enhancing EG selectivity from ∼17% to >50%.33 This “differential substrate input, cumulative product output” principle underpins most kinetic models that describe tandem hydrolysis–hydrogenation systems.
Continuous-flow reactors mark the transition from experimental demonstration to industrial application.311 They ensure steady-state conditions, minimize heat and mass-transfer gradients, and enable real-time control of residence time distribution (RTD). Two major strategies dominate: (i) direct solid feeding, wherein wet or dry cellulose slurries (10–28 wt%) are introduced by screw or piston pumps into pressurized stirred reactors, yielding 40–65% EG at high throughput;51,67 and (ii) two-step saccharification–hydrogenation, where cellulose is pre-hydrolyzed to soluble oligosaccharides before hydrogenation, doubling total diol yield relative to untreated cellulose.68
Emerging reactor concepts, including structured catalytic membranes, microchannel reactors, and slurry-fixed hybrid beds, seek to minimize external mass-transfer resistance while maximizing interfacial contact. These designs also facilitate continuous catalyst regeneration and heat recovery, aligning process intensification with catalyst durability.
5.2 Feedstock and pretreatment
In practical operation, catalytic hydrogenolysis must address the complexity of real lignocellulosic biomass rather than purified cellulose powders. Native biomass contains cellulose fibrils embedded within a matrix of hemicellulose, lignin, extractives, and inorganic minerals.17,312 These non-cellulosic components strongly influence catalyst accessibility, reaction selectivity, and stability, and therefore pretreatment becomes essential for achieving reproducible, high-yield production of low-carbon alcohols.Among all structural components, lignin is the most detrimental to hydrogenolysis performance. Studies using Ni–W2C/AC systems revealed a clear negative correlation between EG yield and lignin content despite nearly complete biomass conversion (Fig. 10).34 Aromatic fragments derived from lignin depolymerization tend to adsorb irreversibly on metallic sites, suppressing hydrogenation and promoting humin formation.244 By contrast, hemicellulose frequently exerts a beneficial effect, as its hydrolysis products include C2–C3 fragments that can be further converted to EG and 1,2-PG, thereby increasing overall carbon efficiency.
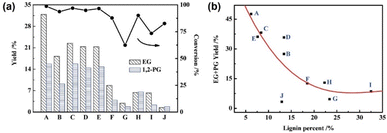 | ||
| Fig. 10 (a) Catalytic performance of corn stalk after various pretreatments. (b) Plot of overall yields of EG and 1,2-PG as functions of lignin percent in corn stalks. (A) Ammonia and H2O2, (B) butanediol, (C) NaOH, (D) H2O2, (E) ammonia, (F) 50% ethanol, (G) hot water, (H) hot limewater, (I) SC-CO2, (J) raw corn stalk (Reaction conditions: 2% Ni–30% W2C/AC, 245 °C, 2.5 h). Reproduced with permission.34 Copyright 2011, American Chemical Society. | ||
Another major challenge arises from inorganic ions, especially Ca2+, Mg2+, and Fe3+, which readily precipitate as inactive tungstates (e.g., CaWO4, Fe2(WO4)3) under hydrogenolysis conditions, irreversibly deactivating W-based catalysts.313 Systematic work on poplar, pine, corn stover, miscanthus, and other feedstocks indicates that maintaining divalent-cation concentrations below ∼4 mmol kg−1 is necessary to preserve catalytic activity and avoid batch-to-batch variation.314
Pretreatment strategies therefore focus on removing lignin and mineral contaminants, opening up the plant cell wall, and exposing cellulose fibrils to catalytic hydrolysis and hydrogenation.34 Alkaline delignification (e.g., ammonia or NaOH) effectively disrupts lignin–carbohydrate complexes, swells cellulose fibers, and enhances hydrolytic accessibility. When combined with mild oxidative steps (e.g., H2O2 treatment), the combined EG and 1,2-PG yield can increase to 48%. Crucially, acid washing following alkaline pretreatment removes residual Ca2+/Fe3+ ions and prevents the formation of inactive tungstate phases, reinstating high catalytic activity and ensuring stable long-term operation.315 These principles hold across a wide spectrum of herbaceous feedstocks, enabling combined diol yields above 50% even at high solid loadings.67,260
Beyond chemical delignification, mechanical activation—including ball milling and steam explosion—partially amorphizes cellulose, lowers crystallinity, and improves wetting and catalyst–substrate contact. Enhanced accessibility yields consistently higher EG production across agricultural residues such as corn cob, wheat straw, and bamboo, and reduces mass-transfer limitations in continuous-flow reactors where solid–liquid contact becomes rate-limiting.257 Mechanical–chemical synergy is thus emerging as a practical approach for improving both selectivity and throughput.
Woody biomass presents a contrasting but equally important scenario. Although more recalcitrant due to its dense fiber architecture, wood typically contains fewer soluble minerals and can be rendered compatible with tungsten-based or noble-metal catalysts through combined mechanical activation and mild acid-assisted delignification. Using Ni–W2C catalysts, birch and pine have been converted into EG and 1,2-PG with total yields of 45–75% (based on sugars), while lignin is simultaneously depolymerized into monophenols with yields around 40–50%.244 Noble-metal catalysts such as Ru/C or Pt/C can also efficiently upgrade pretreated wood, achieving ∼55–62% yields of C4–C6 sugar alcohols after mechanical activation or acid-assisted pretreatment.116,316 These examples highlight how targeted disruption of woody tissue architecture enables its effective incorporation into hydrogenolysis processes.
Looking beyond conventional acid–base routes, organic-solvent fractionation has recently emerged as a low-energy pretreatment option capable of simultaneously deconstructing biomass and stabilizing lignin fragments. Ethanol–water mixtures partially delignify straw and produce clean carbohydrate streams suitable for Ru–W catalysis,317 while formaldehyde-assisted acidic solvents enable near-complete fractionation of cellulose, hemicellulose, and lignin with 76–90% efficiency.318 Importantly, formaldehyde prevents lignin recondensation and generates stable, valorisable aromatic intermediates—offering promising integration with biorefinery operations.
It should be noted that efficient chemical pretreatment methods (such as acid–base treatment and organic solvent fractionation), while effectively removing lignin, may introduce soluble impurities (e.g., residual alkaline agents, inorganic salt ions, or organic solvent molecules). If these impurities are not effectively removed, they will enter the subsequent hydrogenolysis reactor along with the cellulose-rich stream. This can potentially poison the acid/base or metal sites of the catalyst or alter the reaction microenvironment, thereby affecting catalytic activity and product selectivity. Therefore, developing supporting purification steps (such as thorough washing and solvent recovery) or designing pretreatment-catalysis integrated processes compatible with downstream catalytic systems is a crucial link in achieving efficient and stable biomass refining.
Overall, feedstock composition and pretreatment fundamentally determine the kinetics, selectivity, and durability of catalytic hydrogenolysis. Removing lignin, extractives, and mineral ions enhances cellulose accessibility, suppresses catalyst poisoning, and stabilizes active sites—effects that are especially critical under high-solid, continuous-flow conditions. The convergence of mechanical activation, mild chemical delignification, and energy-efficient solvent fractionation defines a new generation of integrated pretreatment strategies capable of converting diverse lignocellulosic biomass into uniform, catalyst-ready intermediates. Such advances will be essential for scaling the continuous production of bio-based low-carbon alcohols.
5.3 Industrial and techno-economic outlook
The industrial realization of bio-based low-carbon alcohols, particularly EG, requires the coordinated optimization of feedstock processing, catalytic system design, reactor operation, and product purification. Moving from laboratory studies to scalable production demands simultaneous advances in feedstock utilization, catalyst durability, process intensification, and energy-efficient separation.A central determinant of commercial viability is feedstock pretreatment, which governs both catalyst accessibility and long-term stability. Green and cost-effective methods such as dilute acid or alkali pretreatment, ammonia fiber expansion, and steam explosion effectively remove lignin and disrupt cellulose crystallinity, improving EG yields while prolonging catalyst lifetime. Sustainable biomass supply chains, optimized within short collection radii, are equally important to reduce logistics costs and carbon footprints. Operationally, moderate hydrogen pressures (3–6 MPa) and temperatures (230–270 °C) strike a balance between conversion and energy consumption; overly harsh conditions accelerate humin formation and catalyst deactivation, while mild conditions supported by bifunctional catalysts sustain continuous, high-selectivity conversion.43
Catalyst stability remains the dominant cost factor in techno-economic analyses. Tungsten-based species such as tungstic acid and WO3 show excellent recyclability through precipitation–calcination or residue distillation after EG recovery. For hydrogenation, skeletal or supported Ni catalysts (e.g., Raney Ni, Ni–P, Ni–Mo alloys) exhibit strong hydrothermal stability and mechanical robustness.319 Nevertheless, lignin-derived aromatics can poison active sites and suppress hydrogenation;244 periodic solvent-assisted regeneration or mild oxidative cleaning is therefore essential. Alloying, phosphidation, or core–shell encapsulation strategies further enhance hydrothermal endurance without sacrificing selectivity.
In large-scale and continuous biomass conversion systems, catalyst deactivation primarily stems from chemical poisoning/blockage (e.g., adsorption of lignin-derived aromatics and humins), leaching of active components (hydrothermal dissolution), and structural sintering. The aforementioned strategies, such as alloying, phosphidation, and core–shell encapsulation, represent proactive designs targeting these failure modes: core–shell structures physically confine and inhibit the sintering and leaching of active components; alloying and phosphidation modulate the surface electronic structure to weaken the strong adsorption of oxygenated intermediates or coke precursors, thereby enhancing anti-coking capability; carrier surface engineering (e.g., hydrophilicity/hydrophobicity tuning) helps reduce the retention of large-molecular-weight by-products. Consequently, the long-term stability of catalysts requires synergistic design encompassing intrinsic material properties, microstructure, and surface/interface characteristics.
Large-scale production depends on process intensification and continuous operation. Plants exceeding 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ton per yearmust employ continuous or semi-continuous reactors to maintain steady operation and consistent product quality.311 Increasing solid content from 5–10 wt% to >20 wt% improves EG concentration and reduces downstream energy use, but introduces mass-transfer limitations due to slurry viscosity. Optimized hydrodynamics have enabled EG yields of 40–65% even at high solids.67 Continuous solid feeding using screw or piston pumps allows direct hydrogenolysis of wet or dry biomass under pressure,51 though mechanical stability remains a challenge. Alternatively, two-stage strategies—acidic saccharification followed by hydrogenation—achieve high efficiency and controllability. Hilgert et al.68 converted ball-milled cellulose to soluble oligosaccharides using 10 wt% H2SO4, which were subsequently hydrogenolyzed to hexitols with greatly improved activity.
000 ton per yearmust employ continuous or semi-continuous reactors to maintain steady operation and consistent product quality.311 Increasing solid content from 5–10 wt% to >20 wt% improves EG concentration and reduces downstream energy use, but introduces mass-transfer limitations due to slurry viscosity. Optimized hydrodynamics have enabled EG yields of 40–65% even at high solids.67 Continuous solid feeding using screw or piston pumps allows direct hydrogenolysis of wet or dry biomass under pressure,51 though mechanical stability remains a challenge. Alternatively, two-stage strategies—acidic saccharification followed by hydrogenation—achieve high efficiency and controllability. Hilgert et al.68 converted ball-milled cellulose to soluble oligosaccharides using 10 wt% H2SO4, which were subsequently hydrogenolyzed to hexitols with greatly improved activity.
Downstream separation is another decisive cost factor. Because ethanol, EG, 1,2-PG, and 1,2-BDO differ in boiling points by only 3–10 K, conventional distillation is energy-intensive. Alternatives such as pervaporation membranes, reactive distillation, or selective catalytic upgrading offer promising low-energy options. Polymer-grade EG (>98%) is required for polyethylene terephthalate (PET) synthesis.320 Minor 1,2-PG or 1,2-BDO impurities (<2 wt%) have a negligible impact on PET properties. Nevertheless, standardized specifications for bio-based alcohols must be established to ensure downstream compatibility and market acceptance.
A landmark demonstration was achieved in October 2024, when the “thousand-ton-scale one-step catalytic conversion of biomass to EG” pilot plant—jointly developed by the Dalian Institute of Chemical Physics (Academician Zhang Tao) and Zhongke Baiyijin New Energy—passed national appraisal.321 Operating continuously for 72 h, the process reached ≈80% EG selectivity and 99.9% purity, satisfying polymer-grade standards. The Ni–W catalytic system and efficient separation sequence confirmed the feasibility of direct biomass-to-EG conversion under mild, energy-saving conditions. The resulting bio-EG has been successfully applied in manufacturing bio-PET, polyethylene furanoate (PEF), and fine-chemical aromatics, offering a tangible pathway toward industrial decarbonization.
Overall, the integration of advanced catalytic systems with reactor engineering, high-solid processing, and low-energy separations has transformed cellulose hydrogenolysis from a mechanistic model into a practicable industrial technology. Continued progress will rely on cross-scale integration—linking rational catalyst design, operando mechanistic insight, feedstock preprocessing, reactor hydrodynamics, and separation intensification. As global chemical production shifts toward renewable carbon and circular-economy paradigms, such convergence will be decisive for achieving economically viable, carbon-neutral manufacture of bio-based low-carbon alcohols and related platform molecules.
6 Challenges and future opportunities
The catalytic hydrogenolysis of cellulose to low-carbon alcohols has advanced from fundamental mechanistic insight to encouraging pilot-scale demonstrations. Yet, the translation of this progress into robust, scalable technology will require deeper convergence between molecular catalysis, materials design, and process engineering.Mechanistic studies have established a coherent picture of the reaction network, identifying cellulose depolymerization and glucose retro-aldol condensation as the kinetically demanding steps. Although substantial progress has been made in correlating tungsten redox chemistry, proton transfer, and hydride delivery, the intrinsic activation barriers of these transformations remain high. A key challenge is thus the development of catalytic environments capable of dynamically stabilizing transient intermediates and lowering reaction energies through coupled proton–hydride pathways. Continued integration of operando spectroscopy, transient kinetic analysis, and microkinetic modelling will be central to revealing active motifs and informing mechanism-driven design.
From a materials perspective, bifunctional systems, particularly tungsten-based acid–metal ensembles and spatially confined nanostructures, have provided a conceptual platform for unifying hydrolysis, isomerization, C–C scission, and hydrogenation. However, hydrothermal stability, structural complexity, and susceptibility to leaching continue to limit long-term performance. Opportunities lie in strategies that impart atomic-level precision—single-atom dispersion, defect engineering, and self-regulating oxide-metal interfaces capable of maintaining activity under dynamic reaction conditions. Hierarchically organized microenvironments that choreograph reaction sequences within confined domains offer another route to simultaneously enhance selectivity and durability.
Notably, single-atom catalysts (SACs) provide a highly promising pathway for achieving catalytic design with atomic-level precision by maximizing the utilization efficiency of metal atoms and constructing unique active microenvironments. For instance, the elaborately designed Ru single-atom catalyst combined with sulfonic acid and nitrogen sites (Ru–SO3H–N) has achieved a significantly enhanced yield in the hydrogenolysis of cellulose to 1,2-PG.322 This is attributed to the optimized electronic structure of Ru sites, which can simultaneously improve key steps such as cellulose hydrolysis and fructose hydrogenolysis. Similarly, integrating high-density ruthenium single atoms (10.1 wt%) into sulfonic acid-functionalized hollow mesoporous carbon enables efficient catalysis of the cascade reaction of cellulose conversion to isosorbide via sorbitol,323 where Ru single-atom sites play a crucial role in the selective hydrogenation of glucose intermediates. Beyond noble metals, durable nickel single-atom catalysts (Ni–N–C) have also demonstrated potential in efficiently catalyzing cellulose conversion reactions under harsh conditions, offering a possibility for reducing catalyst costs.324 These advances highlight the unique advantage of SACs in synergistically catalyzing multiple sequential reactions in cellulose conversion through the precise regulation of the coordination environment of metal centers.
Selectivity control—central to producing EG, 1,2-PG, ethanol, or polyols—illustrates the importance of kinetic steering rather than thermodynamic preference. The next phase of catalyst design will require embedding selectivity determinants into the architecture itself through electronic tuning and spatial programming, reducing reliance on external operational constraints. Machine-learning-assisted discovery of structure-selectivity descriptors is expected to accelerate this transition toward programmable catalytic systems.
Process engineering considerations remain equally decisive. While semi-continuous and continuous-flow reactors have established links between intrinsic kinetics and scalable operation, challenges associated with high-solids processing, mass-transfer limitations, and catalyst fouling persist. Progress will depend on process-intensified reactor concepts that integrate hydrogenolysis, separation, and catalyst regeneration, as well as on catalysts tolerant to feedstock heterogeneity. The co-development of low-energy pretreatment strategies and robust catalytic systems is critical for ensuring broad feedstock compatibility and reducing operational complexity.
Looking forward, several research directions appear particularly promising: (1) unconventional product pathways: exploring novel transformation paradigms, such as the directed synthesis of methanol from cellulose, which requires innovative catalyst design and reaction engineering to control deep C–C bond cleavage. (2) Dynamic multifunctional catalysis: developing catalysts capable of in situ restructuring or valence switching (e.g., W6+/W5+, Sn4+/Sn2+) to adaptively couple hydrolysis, isomerization, and hydrogenation under reaction conditions. (3) Advanced confinement materials: employing novel confinement materials with designable pores and tunable acid–base properties (such as metal organic frameworks, covalent organic frameworks, and modified layered clays) to create precise microenvironments for cascade reactions in cellulose hydrogenolysis. (4) Atomic and interfacial precision: utilizing single-atom anchoring, dual-site ensembles, and well-defined oxide-metal interfaces to regulate elementary reaction steps and suppress over-hydrogenation. (5) Data-driven catalyst discovery: integrating high-throughput experimentation with machine learning and DFT calculations to identify structure–activity descriptors governing selectivity toward C2–C3 alcohols. (6) Integrated reaction-separation modules: combining catalytic hydrogenolysis with membrane separation, reactive distillation, or electrochemical hydrogen management to improve carbon efficiency and reduce energy input. (7) Whole-biomass valorization: extending cellulose-focused studies toward integrated lignocellulosic biorefineries that simultaneously valorize cellulose, hemicellulose, and lignin.
The recent demonstration of thousand-ton-scale biomass-to-EG production underscores the growing maturity of this field and its movement toward industrial relevance. As catalytic innovation increasingly aligns with process design and digital optimization, cellulose hydrogenolysis is poised to contribute meaningfully to a low-carbon chemical economy. Ultimately, the one-step catalytic transformation of renewable carbohydrates into platform alcohols exemplifies a broader paradigm: the integration of molecular precision with systems-level engineering to enable sustainable, carbon-neutral chemical manufacturing.
Author contributions
Y. Cui: data curation, formal analysis, investigation, validation, visualization, writing-original draft. D. Wang: investigation, validation, visualization. H. Ben: project administration, resources. X. Su: investigation, validation, supervision, writing-review & editing. X. Yang: conceptualization, formal analysis, investigation, funding acquisition, project administration, resources, writing-review & editing. Y. Huang: project administration, resources.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 22478209, 22478383), the Natural Science Foundation of Shandong Province (Grant No. ZR2024YQ018), the Youth Innovation Team Project of the Shandong Provincial Department of Education (Grant No. 2023KJ356), the Youth Science and Technology Talent Lifting Project of Shandong Province (Grant No. SDAST2024QTA042).References
- S. Zangoei, N. Salehnia and M. K. Mashhadi, Environ. Sci. Pollut. Res., 2021, 28, 19799–19809 CrossRef CAS PubMed.
- A. M. Ruppert, K. Weinberg and R. Palkovits, Angew. Chem., Int. Ed., 2012, 51, 2564–2601 CrossRef CAS PubMed.
- J. C. Serrano-Ruiz, R. Luque and A. Sepulveda-Escribano, Chem. Soc. Rev., 2011, 40, 5266–5281 Search PubMed.
- P. Yang, H. Kobayashi and A. Fukuoka, Chin. J. Catal., 2011, 32, 716–722 CrossRef CAS.
- S. Wang, A. Cheng, F. Liu, J. Zhang, T. Xia, X. Zeng, W. Fan and Y. Zhang, Ind. Chem. Mater., 2023, 1, 188–206 RSC.
- Biomass Market Size, Share and Growth Trend Report, 2026–2035, https://www.researchnester.com/cn/reports/biomass-market/1355.
- X. Zhang, K. Wilson and A. F. Lee, Chem. Rev., 2016, 116, 12328–12368 CrossRef CAS PubMed.
- Y. C. Feng, S. S. Long, X. Tang, Y. Sun, R. Luque, X. H. Zeng and L. Lin, Chem. Soc. Rev., 2021, 50, 6042–6093 RSC.
- B. M. Upton and A. M. Kasko, Chem. Rev., 2016, 116, 2275–2306 CrossRef CAS PubMed.
- H. C. Ong, K. L. Yu, W.-H. Chen, M. K. Pillejera, X. Bi, K.-Q. Tran, A. Pétrissans and M. Pétrissans, Renew. Sustain. Energy Rev., 2021, 152, 111698 CrossRef CAS.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- M. Kumar, A. O. Oyedun and A. Kumar, Renew. Sustain. Energy Rev., 2018, 81, 1742–1770 CrossRef.
- H. Li, A. Bunrit, N. Li and F. Wang, Chem. Soc. Rev., 2020, 49, 3748–3763 RSC.
- W. Gong, Y. Lin, C. Chen, M. Al-Mamun, H. S. Lu, G. Wang, H. Zhang and H. Zhao, Adv. Mater., 2019, 31, e1808341 CrossRef PubMed.
- C. Li, X. Zhao, A. Wang, G. W. Huber and T. Zhang, Chem. Rev., 2015, 115, 11559–11624 CrossRef CAS PubMed.
- IRENA, Global Energy Transformation: A Roadmap to 2050, International Renewable Energy Agency, 2018, http://refhub.elsevier.com/S2589-5974(19)30149-2/rf0005 Search PubMed.
- M. E. Himmel, S. Ding, D. K. Johnson, W. S. Adney, M. R. Nimlos, J. W. Brady and T. D. Foust, Science, 2007, 315, 804–807 CrossRef CAS PubMed.
- R. M. Wahlström and A. Suurnäkki, Green Chem., 2015, 17, 694–714 RSC.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- N. Wei, J. Quarterman, S. R. Kim, J. H. D. Cate and Y.-S. Jin, Nat. Commun., 2013, 4, 2580 CrossRef PubMed.
- Y. Sun and J. Cheng, Bioresour. Technol., 2002, 83, 1–11 CrossRef CAS PubMed.
- M. Li, W. Zhao, Y. Xu, Y. Zhao, K. Yang, W. Tao and J. Xiao, Ind. Eng. Chem. Res., 2019, 58, 19179–19188 CrossRef CAS.
- Y. Weng, X. Wang and Y. Zhang, Trends Chem., 2022, 4, 374–377 CrossRef CAS.
- C. Luo, S. Wang and H. Liu, Angew. Chem., Int. Ed., 2007, 46, 7636–7639 CrossRef CAS PubMed.
- A. Fukuoka and P. L. Dhepe, Angew. Chem., Int. Ed., 2006, 45, 5161–5163 CrossRef CAS PubMed.
- M. Yang, H. Qi, F. Liu, Y. Ren, X. Pan, L. Zhang, X. Liu, H. Wang, J. Pang, M. Zheng, A. Wang and T. Zhang, Joule, 2019, 3, 1937–1948 CrossRef CAS.
- D. Chu, Z. Luo, Y. Xin, C. Jiang, S. Gao, Z. Wang and C. Zhao, Fuel, 2021, 292, 120311 Search PubMed.
- H. Song, P. Wang, S. Li, W. Deng, Y. Li, Q. Zhang and Y. Wang, Chem. Commun., 2019, 55, 4303–4306 RSC.
- Q. Liu, H. Wang, H. Xin, C. Wang, L. Yan, Y. Wang, Q. Zhang, X. Zhang, Y. Xu, G. W. Huber and L. Ma, ChemSusChem, 2019, 12, 3977–3987 CrossRef CAS PubMed.
- Y. Wu, C. Dong, H. Wang, J. Peng, Y. Li, C. Samart and M. Ding, ACS Sustainable Chem. Eng., 2022, 10, 2802–2810 CrossRef CAS.
- J. Zhang, B. Hou, A. Wang, Z. Li, H. Wang and T. Zhang, AIChE J., 2015, 61, 224–238 CrossRef CAS.
- J. Zhang, B. Hou, A. Wang, Z. Li, H. Wang and T. Zhang, AIChE J., 2014, 60, 3804–3813 CrossRef CAS.
- G. Zhao, M. Zheng, J. Zhang, A. Wang and T. Zhang, Ind. Eng. Chem. Res., 2013, 52, 9566–9572 CrossRef CAS.
- J. Pang, M. Zheng, A. Wang and T. Zhang, Ind. Eng. Chem. Res., 2011, 50, 6601–6608 CrossRef CAS.
- L. Zhang, B. Zhan, Y. He, Y. Deng, H. Ji, S. Peng and L. Yan, Green Chem., 2024, 26, 8794–8807 RSC.
- W. H. Zartman and H. Adkins, J. Am. Chem. Soc., 1933, 55, 4559–4563 CrossRef CAS.
- E. I. Du Pont De Nemours & Co, GB1933035971, 1933.
- M. S. S. R. Tanikella, EP1982303832, 1982.
- U. Saxena, N. Dwivedi and S. R. Vidyarthi, Ind. Eng. Chem. Res., 2005, 44, 1466–1473 CrossRef CAS.
- N. Ji, T. Zhang, M. Zheng, A. Wang, H. Wang, X. Wang and J. G. Chen, Angew. Chem., Int. Ed., 2008, 47, 8510–8513 CrossRef CAS PubMed.
- M. Zheng, A. Wang, J. Pang, N. Li and T. Zhang, Mechanism and Kinetic Analysis of the Hydrogenolysis of Cellulose to Polyols, in Reaction Pathways and Mechanisms in Thermocatalytic Biomass Conversion I: Cellulose Structure, Depolymerization and Conversion by Heterogeneous Catalysts, ed. Schlaf, M., Zhang, Z. C., Springer, Berlin, 2016, pp 227–260 Search PubMed.
- A. Wang and T. Zhang, Acc. Chem. Res., 2013, 46, 1377–1386 CrossRef CAS PubMed.
- M. Zheng, J. Pang, A. Wang and T. Zhang, Chin. J. Catal., 2014, 35, 602–613 CrossRef CAS.
- I. Delidovich, P. J. C. Hausoul, L. Deng, R. Pfützenreuter, M. Rose and R. Palkovits, Chem. Rev., 2016, 116, 1540–1599 CrossRef CAS PubMed.
- Y. Qiao, G. J. Xia, W. Cao, K. H. Zeng, Q. L. Guo, X. F. Yang, A. Q. Wang and Y. G. Wang, J. Catal., 2023, 427, 115114 CrossRef CAS.
- H. S. Kambo and A. Dutta, Renew. Sustain. Energy Rev., 2015, 45, 359–378 CrossRef CAS.
- X. Wang, T. You, W. Zheng, X. Li, S. Chen and F. Xu, Chem. Eng. J., 2024, 483, 148841 CrossRef CAS.
- N. Yan and S. Ding, Trends Chem., 2019, 1, 457–458 CrossRef CAS.
- D. Chu, Y. Xin and C. Zhao, Chin. J. Catal., 2021, 42, 844–854 CrossRef CAS.
- K. Tomishige, M. Yabushita, J. Cao and Y. Nakagawa, Green Chem., 2022, 24, 5652–5690 RSC.
- M. Zheng, J. Pang, R. Sun, A. Wang and T. Zhang, ACS Catal., 2017, 7, 1939–1954 CrossRef CAS.
- C. Montassier, D. Giraud and J. Barbier, Stud. Surf. Sci. Catal., 1988, 41, 165–170 CrossRef CAS.
- K. Wang, M. C. Hawley and T. D. Furney, Ind. Eng. Chem. Res., 1995, 34, 3766–3770 CrossRef CAS.
- J. Zhang, F. Lu, W. Yu, J. Chen, S. Chen, J. Gao and J. Xu, Catal. Today, 2014, 234, 107–112 Search PubMed.
- N. Li and G. W. Huber, J. Catal., 2010, 270, 48–59 CrossRef CAS.
- G. Liang, L. He, H. Cheng, W. Li, X. Li, C. Zhang, Y. Yu and F. Zhao, J. Catal., 2014, 309, 468–476 CrossRef CAS.
- J. Xi, D. Ding, Y. Shao, X. Liu, G. Lu and Y. Wang, ACS Sustainable Chem. Eng., 2014, 2, 2355–2362 CrossRef CAS.
- W. Luo, P. C. A. Bruijnincx and B. M. Weckhuysen, J. Catal., 2014, 320, 33–41 CrossRef CAS.
- B. Zhang, X. Li, Q. Wu, C. Zhang, Y. Yu, M. Lan, X. Wei, Z. Ying, T. Liu, G. Liang and F. Zhao, Green Chem., 2016, 18, 3315–3323 RSC.
- J. Sun and H. Liu, Green Chem., 2011, 13, 135–142 RSC.
- N. Déchamp, A. Gamez, A. Perrard and P. Gallezot, Catal. Today, 1995, 24, 29–34 CrossRef.
- H. Kishida, F. Jin, X. Yan, T. Moriya and H. Enomoto, Carbohydr. Res., 2006, 341, 2619–2623 CrossRef CAS PubMed.
- X. Yan, F. Jin, K. Tohji, A. Kishita and H. Enomoto, AIChE J., 2010, 56, 2727–2733 CrossRef CAS.
- D. K. Mishra, A. A. Dabbawala, J. J. Park, S. H. Jhung and J. S. Hwang, Catal. Today, 2014, 232, 99–107 CrossRef CAS.
- T. Komanoya, H. Kobayashi, K. Hara, W.-J. Chun and A. Fukuoka, ChemCatChem, 2014, 6, 230–236 CrossRef CAS.
- G. Zhao, M. Zheng, R. Sun, Z. Tai, J. Pang, A. Wang, X. Wang and T. Zhang, AIChE J., 2017, 63, 2072–2080 CrossRef CAS.
- J. Pang, M. Zheng, A. Wang, R. Sun, H. Wang, Y. Jiang and T. Zhang, AIChE J., 2014, 60, 2254–2262 CrossRef CAS.
- J. Hilgert, N. Meine, R. Rinaldi and F. Schüth, Energy Environ. Sci., 2013, 6, 92–96 RSC.
- J. Pang, A. Wang, M. Zheng, Y. Zhang, Y. Huang, X. Chen and T. Zhang, Green Chem., 2012, 14, 614–617 RSC.
- E. Crezee, B. W. Hoffer, R. J. Berger, M. Makkee, F. Kapteijn and J. A. Moulijn, Appl. Catal., A, 2003, 251, 1–17 CrossRef CAS.
- M. Y. Zheng, A. Q. Wang, N. Ji, J. F. Pang, X. D. Wang and T. Zhang, ChemSusChem, 2010, 3, 63–66 CrossRef CAS PubMed.
- M. J. Ahmed, Heat Mass Transf., 2012, 48, 343–347 CrossRef CAS.
- L. Shuai and X. Pan, Energy Environ. Sci., 2012, 5, 6889–6894 RSC.
- S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2008, 130, 12787–12793 CrossRef CAS PubMed.
- Z. Yao, J. Zhao, R. J. Bunting, C. Zhao, P. Hu and J. Wang, ACS Catal., 2021, 11, 1202–1221 CrossRef CAS.
- X. Li, Z. Shao, H. Shan and L. Liu, Green Chem., 2025, 27, 5322–5331 RSC.
- X. Hu, C. Lievens and C.-Z. Li, ChemSusChem, 2012, 5, 1427–1434 CrossRef CAS PubMed.
- G. Chen, C. Fu, W. Zhang, W. Gong, J. Ma, X. Ji, L. Qian, X. Feng, C. Hu, R. Long and Y. Xiong, Nat. Commun., 2025, 16, 665 CrossRef CAS PubMed.
- J. Zhang, X. Liu, M. Sun, X. Ma and Y. Han, ACS Catal., 2012, 2, 1698–1702 CrossRef CAS.
- C. Liu, J. M. Carraher, J. L. Swedberg, C. R. Herndon, C. N. Fleitman and J.-P. Tessonnier, ACS Catal., 2014, 4, 4295–4298 CrossRef CAS.
- J. M. Carraher, C. N. Fleitman and J.-P. Tessonnier, ACS Catal., 2015, 5, 3162–3173 CrossRef CAS.
- L. Ren, Q. Guo, P. Kumar, M. Orazov, D. Xu, S. M. Alhassan, K. Andre Mkhoyan, M. E. Davis and M. Tsapatsis, Angew. Chem., Int. Ed., 2015, 127, 10998–11001 CrossRef.
- T. Deng and H. Liu, Green Chem., 2013, 15, 116–124 RSC.
- R. Sun, M. Zheng, J. Pang, X. Liu, J. Wang, X. Pan, A. Wang, X. Wang and T. Zhang, ACS Catal., 2016, 6, 191–201 CrossRef CAS.
- Z. Xiao, J. Mao, C. Jiang, C. Xing, J. Ji and Y. Cheng, J. Renew. Sustain. Energy, 2017, 9, 024703 CrossRef.
- Z. Xiao, S. Jin, M. Pang and C. Liang, Green Chem., 2013, 15, 891–895 RSC.
- Z. Xiao, S. Jin, G. Sha, C. T. Williams and C. Liang, Ind. Eng. Chem. Res., 2014, 53, 8735–8743 CrossRef CAS.
- T. Deng, J. Sun and H. Liu, Sci. China Chem., 2010, 53, 1476–1480 CrossRef CAS.
- M. Moliner, Y. Roman-Leshkov and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6164–6168 CrossRef CAS PubMed.
- R. Bermejo-Deval, R. S. Assary, E. Nikolla, M. Moliner, Y. Roman-Leshkov, S. J. Hwang, A. Palsdottir, D. Silverman, R. F. Lobo, L. A. Curtiss and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9727–9732 CrossRef CAS PubMed.
- Y. Cao, J. Wang, M. Kang and Y. Zhu, RSC Adv., 2015, 5, 90904–90912 RSC.
- X. Yang, Z. Li, M. Guo, T. Zhao, X. Su, W. Jiang, G. Han and H. Ben, Fuel, 2023, 341, 127560 Search PubMed.
- C. Liu, C. Zhang, S. Sun, K. Liu, S. Hao, J. Xu, Y. Zhu and Y. Li, ACS Catal., 2015, 5, 4612–4623 Search PubMed.
- Y. Liu, C. Luo and H. Liu, Angew. Chem., Int. Ed., 2012, 51, 3249–3253 CrossRef CAS PubMed.
- C. Li, G. Xu, C. Wang, L. Ma, Y. Qiao, Y. Zhang and Y. Fu, Green Chem., 2019, 21, 2234–2239 Search PubMed.
- N. Li, Y. Zheng, L. Wei, H. Teng and J. Zhou, Green Chem., 2017, 19, 682–691 Search PubMed.
- J. J. Wiesfeld, P. Peršolja, F. A. Rollier, A. M. Elemans-Mehring and E. J. M. Hensen, Mol. Catal., 2019, 473, 110400 Search PubMed.
- K. Fabičovicová, M. Lucas and P. Claus, Green Chem., 2015, 17, 3075–3083 Search PubMed.
- L. S. Ribeiro, J. Órfão, J. J. de Melo Órfão and M. F. R. Pereira, Cellulose, 2018, 25, 2259–2272 CrossRef CAS.
- L. S. Ribeiro, J. J. Melo Órfão and M. F. R. Pereira, Bioresour. Technol., 2018, 263, 402–409 Search PubMed.
- L. S. Ribeiro, N. Rey-Raap, J. L. Figueiredo, J. J. Melo Órfão and M. F. R. Pereira, Cellulose, 2019, 26, 7337–7353 CrossRef CAS.
- Z. Yang, R. Huang, W. Qi, L. Tong, R. Su and Z. He, Chem. Eng. J., 2015, 280, 90–98 Search PubMed.
- K. Zhang, G. Yang, G. Lyu, Z. Jia, L. A. Lucia and J. Chen, ACS Sustainable Chem. Eng., 2019, 7, 11110–11117 Search PubMed.
- J. W. Han and H. Lee, Catal. Commun., 2012, 19, 115–118 CrossRef CAS.
- M. Qiu, J. Zheng, Y. Yao, L. Liu, X. Zhou, H. Jiao, J. Aarons, K. Zhang, Q. Guan and W. Li, J. Clean. Prod., 2022, 362, 132364 Search PubMed.
- P. A. Lazaridis, S. A. Karakoulia, C. Teodorescu, N. Apostol, D. Macovei, A. Panteli, A. Delimitis, S. M. Comand, V. I. Parvulescud and K. S. Triantafyllidis, Appl. Catal., B, 2017, 214, 1–14 CrossRef CAS.
- M. C. M. Castoldi, L. D. T. Câmara, R. S. Monteiro, A. M. Constantino, L. Camacho, J. W. de M. Carneiro and D. A. G. Aranda, React. Kinet. Catal. Lett., 2007, 91, 341–352 CrossRef.
- H. Kobayashi, Y. Ito, T. Komanoya, Y. Hosaka, P. L. Dhepe, K. Kasai, K. Haraa and A. Fukuoka, Green Chem., 2011, 13, 326–333 Search PubMed.
- J. Xi, Y. Zhang, Q. Xia, X. Liu, J. Ren, G. Lu and Y. Wang, Appl. Catal., A, 2013, 459, 52–58 CrossRef CAS.
- A. Negoi, K. Triantafyllidis, V. I. Parvulescu and S. M. Coman, Catal. Today, 2014, 223, 122–128 CrossRef CAS.
- A. Romero, D. A. Cantero, A. Nieto-Márquez, C. Martínez, E. Alonso and M. J. Cocero, Green Chem., 2016, 18, 4051–4062 RSC.
- O. V. Manaenkov, V. G. Matveeva, E. M. Sulman, A. E. Filatova, O. Y. Makeeva, O. V. Kislitza, A. I. Sidorov, V. Y. Doluda and M. G. Sulman, Top. Catal., 2014, 57, 1476–1482 CrossRef CAS.
- N. V. Gromov, T. B. Medvedeva, O. P. Taran, M. N. Timofeeva, O. Said-Aizpuru, V. N. Panchenko, E. Y. Gerasimov, I. V. Kozhevnikov and V. N. Parmon, Appl. Catal., A, 2020, 595, 117489 CrossRef CAS.
- Z. Tai, J. Zhang, A. Wang, M. Zheng and T. Zhang, Chem. Commun., 2012, 48, 7052–7054 RSC.
- J. Chai, S. Zhu, Y. Cen, J. Guo, J. Wang and W. Fan, RSC Adv., 2017, 7, 8567–8574 Search PubMed.
- R. Palkovits, K. Tajvidi, J. Procelewska, R. Rinaldi and A. Ruppert, Green Chem., 2010, 12, 972–978 Search PubMed.
- K. Shimizu, H. Furukawa, N. Kobayashi, Y. Itaya and A. Satsuma, Green Chem., 2009, 11, 1627–1632 RSC.
- E. N. Dorokhova and I. P. Alimarin, Russ. Chem. Rev., 1979, 48, 502–516 CrossRef.
- S. Yu, X. Cao, S. Liu, L. Li and Q. Wu, RSC Adv., 2018, 8, 24857–24865 RSC.
- A. R. Mankar, A. Modak and K. K. Pant, Fuel Process. Technol., 2021, 218, 106847 CrossRef CAS.
- R. Palkovits, K. Tajvidi, A. M. Ruppert and J. Procelewska, Chem. Commun., 2011, 47, 576–578 Search PubMed.
- G. Liang, C. Wu, L. He, J. Ming, H. Cheng, L. Zhuo and F. Zhao, Green Chem., 2011, 13, 839–842 RSC.
- W. Deng, X. Tan, W. Fang, Q. Zhang and Y. Wang, Catal. Lett., 2009, 133, 167–174 CrossRef CAS.
- J. Geboers, S. Van de Vyver, K. Carpentier, P. Jacobs and B. Sels, Chem. Commun., 2011, 47, 5590–5592 RSC.
- J. Geboers, S. Van de Vyver, K. Carpentier, K. de Blochouse, P. Jacobs and B. Sels, Chem. Commun., 2010, 46, 3577–3579 Search PubMed.
- B. Op de Beeck, J. Geboers, S. Van de Vyver, J. V. Lishout, J. Snelders, W. J. J. Huijgen, C. M. Courtin, P. A. Jacobs and B. F. Sels, ChemSusChem, 2013, 6, 199–208 Search PubMed.
- Y. Yang, D. Ren, C. L. Shang, Z. Ding and X. Luo, Chem. Eng. J., 2023, 452, 139206 Search PubMed.
- S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2008, 130, 12787–12793 Search PubMed.
- J. Pang, A. Wang, M. Zheng and T. Zhang, Chem. Commun., 2010, 46, 6935–6937 Search PubMed.
- T. Komanoya, H. Kobayashi, K. Hara, W. J. Chun and A. Fukuoka, Appl. Catal., A, 2011, 407, 188–194 CrossRef CAS.
- K. Fabičovicová, O. Malter, M. Lucas and P. Claus, Green Chem., 2014, 16, 3580–3588 RSC.
- K. Nakajima, Y. Baba, R. Noma, M. Kitano, J. N. Kondo, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2011, 133, 4224–4227 CrossRef CAS PubMed.
- N. Li, Z. Ji, L. Wei, Y. Zheng, Q. Shen, Q. Ma, M. Tan, M. Zhan and J. Zhou, Bioresour. Technol., 2018, 264, 58–65 CrossRef CAS PubMed.
- L. S. Ribeiro, J. J. Delgado, J. J. de Melo Órfão and M. F. R. Pereira, Catal. Today, 2017, 279, 244–251 Search PubMed.
- L. S. Ribeiro, J. J. M. Órfão and M. F. R. Pereira, Green Chem., 2015, 17, 2973–2980 Search PubMed.
- L. S. Ribeiro, J. J. Delgado, J. J. M. Órfão and M. F. R. Pereira, Appl. Catal., B, 2017, 217, 265–274 Search PubMed.
- X. Xiao, S. H. Lim, W. Chu and Y. Liu, ACS Sustainable Chem. Eng., 2021, 9, 12655–12662 CrossRef CAS.
- S. Carlier, J. Gripekoven, M. Philippo and S. Hermans, Appl. Catal., B, 2021, 282, 119515 Search PubMed.
- J. Yang, L. Liu, H. Zhang, M. Qiu, Q. Guan and W. Li, ACS Sustainable Chem. Eng., 2023, 11, 15663–15673 CrossRef CAS.
- D. Lai, L. Deng, Q. Guo and Y. Fu, Energy Environ. Sci., 2011, 4, 3552–3557 RSC.
- C. Zhang, H. Wang, F. Liu, L. Wang and H. He, Cellulose, 2013, 20, 127–134 CrossRef CAS.
- I. Podolean, A. Negoi, N. Candu, M. Tudorache, V. I. Parvulescu and S. M. Coman, Top. Catal., 2014, 57, 1463–1469 CrossRef CAS.
- O. V. Manaenkov, J. J. Mann, O. V. Kislitza, Y. Losovyj, B. D. Stein, D. G. Morgan, M. Pink, O. L. Lependina, Z. B. Shifrina, V. G. Matveeva, E. M. Sulman and L. M. Bronstein, ACS Appl. Mater. Interfaces, 2016, 8, 21285–21293 CrossRef CAS PubMed.
- M. Lv, Q. Xin, D. Yin, Z. Jia, C. Yu, T. Wang, S. Yu, S. Liu, L. Li and Y. Liu, ACS Sustainable Chem. Eng., 2020, 8, 3617–3625 CrossRef CAS.
- Y. Liu and H. Liu, Catal. Today, 2016, 269, 74–81 CrossRef CAS.
- L. S. Ribeiro, J. J. de Melo Órfão and M. F. R. Pereira, Bioresour. Technol., 2017, 244, 1173–1177 CrossRef CAS PubMed.
- N. Rey-Raap, L. S. Ribeiro, J. J. de Melo Órfão, J. L. Figueiredo and M. F. R. Pereira, Appl. Catal., B, 2019, 256, 117826 CrossRef CAS.
- L. S. Ribeiro, J. J. de Melo Órfão and M. F. R. Pereira, Bioresour. Technol., 2017, 232, 152–158 CrossRef CAS PubMed.
- S. Esposito, B. Silvestri, V. Russo, B. Bonelli, M. Manzoli, F. A. Deorsola, A. Vergara, A. Aronne and M. D. Serio, ACS Catal., 2019, 9, 3426–3436 Search PubMed.
- A. Yepez, A. Pineda, A. Garcia, A. A. Romero and R. Luque, Phys. Chem. Chem. Phys., 2013, 15, 12165–12172 RSC.
- J. Zhang, B. Hou, X. Wang, Z. Li, A. Wang and T. Zhang, J. Energy Chem., 2015, 24, 9–14 CrossRef.
- Y. Liu, Y. Liu, Q. Wu and Y. Zhang, Catal. Commun., 2019, 129, 105731 Search PubMed.
- Y. Liu, Y. Liu and Y. Zhang, Appl. Catal., B, 2019, 242, 100–108 CrossRef CAS.
- J. Ji, Y. Xu, Y. Liu and Y. Zhang, Catal. Commun., 2020, 144, 106074 CrossRef CAS.
- Y. Hirano, K. Sagata and Y. Kita, Appl. Catal., A, 2015, 502, 1–7 CrossRef CAS.
- J. Pang, M. Zheng, X. Li, Y. Jiang, Y. Zhao, A. Wang, J. Wang, X. Wang and T. Zhang, Appl. Catal., B, 2018, 239, 300–308 CrossRef CAS.
- M. Besson, P. Gallezot, A. Perrard and C. Pinel, Catal. Today, 2005, 102–103, 160–165 CrossRef CAS.
- T. Kilpiö, A. Aho, D. Murzin and T. Salmi, Ind. Eng. Chem. Res., 2013, 52, 7690–7703 CrossRef.
- V. N. Sapunov, M. Y. Grigoryev, E. M. Sulman and M. B. Konyaeva, J. Phys. Chem. A, 2013, 117, 4073–4083 CrossRef CAS PubMed.
- A. Aho, S. Roggan, O. A. Simakova, T. Salmi and D. Y. Murzin, Catal. Today, 2015, 241, 195–199 CrossRef CAS.
- B. W. Hoffer, E. Crezee, P. R. M. Mooijman, A. D. van Langeveld, F. Kapteijn and J. A. Moulijn, Catal. Today, 2003, 79, 35–41 CrossRef.
- V. Russo, T. Kilpio, M. Di Serio, R. Tesser, E. Santacesaria, D. Y. Murzin and T. Salmi, Chem. Eng. Res. Des., 2015, 102, 171–185 Search PubMed.
- M. Besson and P. Gallezot, Catal. Today, 2003, 81, 547–559 Search PubMed.
- B. J. Arena, Appl. Catal., A, 1992, 87, 219–229 CrossRef CAS.
- D. K. Mishra, J. M. Lee, J. S. Chang and J. S. Hwang, Catal. Today, 2012, 185, 104–108 CrossRef CAS.
- M. Yadav, D. K. Mishra and J. S. Hwang, Appl. Catal., A, 2012, 425, 110–116 Search PubMed.
- J. Zhang, L. Lin, J. Zhang and J. Shi, Carbohydr. Res., 2011, 346, 1327–1332 CrossRef CAS PubMed.
- A. Romero, A. Nieto-Maŕquez and E. Alonso, Appl. Catal., A, 2017, 529, 49–59 Search PubMed.
- D. K. Mishra, A. A. Dabbawala and J. S. Hwang, J. Mol. Catal. A: Chem., 2013, 376, 63–70 CrossRef CAS.
- X. Yang, X. Li, J. Zhao, J. Liang and J. Zhu, Molecules, 2023, 28, 4830 CrossRef CAS PubMed.
- H.-L. He, Z. Liu, F. Liu, J. Chen, P. Wang, X. Yi, A. Zheng and L. Wang, J. Mater. Chem. A, 2024, 12, 26214–26223 Search PubMed.
- C. Li, G. Xu, X. Zhang and Y. Fu, Chin. J. Chem., 2020, 38, 453–457 CrossRef CAS.
- Y. Jia and H. Liu, Catal. Sci. Technol., 2016, 6, 7042–7052 RSC.
- P. J. C. Hausoul, A. K. Beine, L. Neghadar and R. Palkovits, Catal. Sci. Technol., 2017, 7, 56–63 RSC.
- D. K. Sohounloue, C. Montassier and J. Barbier, React. Kinet. Catal. Lett., 1983, 22, 391–397 CrossRef CAS.
- C. Montassier, J. C. Ménézo, L. C. Hoang, C. Renaud and J. Barbier, J. Mol. Catal., 1991, 70, 99–110 CrossRef CAS.
- L. Zhao, J. Zhou, Z. Sui and X. Zhou, Chem. Eng. Sci., 2010, 65, 30–35 CrossRef CAS.
- J. Zhou, M. Zhang, L. Zhao, P. Li, X. Zhou and W. Yuan, Catal. Today, 2009, 147, S225–S229 CrossRef CAS.
- L. Zhao, J. Zhou, G. Yang, Y. Ji, M. Zhang, H. Chen and X. Zhou, Energy Sources, Part A, 2012, 34, 430–438 CrossRef CAS.
- X. Guo, J. Guan, B. Li, X. Wang, X. Mu and H. Liu, Sci. Rep., 2015, 5, 16451 CrossRef PubMed.
- X. Guo, H. Dong, B. Li, L. Dong, X. Mu and X. Chen, J. Mol. Catal. A: Chem., 2017, 426, 79–87 CrossRef CAS.
- I. M. Leo, M. L. Granados, J. L. G. Fierro and R. Mariscal, Chin. J. Catal., 2014, 35, 614–621 Search PubMed.
- B. Gumina, C. Espro, S. Galvagno, R. Pietropaolo and F. Mauriello, ACS Omega, 2019, 4, 352–357 Search PubMed.
- G. F. Leal, S. F. Moya, D. M. Meira, D. H. Barrett, E. Teixeira-Neto, A. A. S. Curvelo, V. Teixeira da Silva and C. B. Rodella, RSC Adv., 2016, 6, 87756–87766 RSC.
- W. Jia, H. Song, D. Wang, C. Zhou, Y. Zhao, Y. Cui, H. Ben and X. Yang, ACS Sustainable Chem. Eng., 2025, 13, 13274–13283 Search PubMed.
- W. Jia, W. Liu, Y. Cui, H. Song, C. Zhou, W. Jiang, H. Ben, X. Yang and D. Chen, J. Catal., 2025, 442, 115860 CrossRef CAS.
- S. Xu, X. Yan, Q. Bu and H. Xia, Cellulose, 2017, 24, 2403–2413 Search PubMed.
- Q. Xin, L. Jiang, S. Yu, S. Liu, D. Yin, L. Li, C. Xie, Q. Wu, H. Yu, Y. Liu and Y. Liu, J. Phys. Chem. C, 2021, 125, 18170–18179 CrossRef CAS.
- Y. Cui, D. Wang, C. Zhou, X. Zhang, H. Ben and X. Yang, Appl. Catal., B, 2026, 381, 125818 Search PubMed.
- Y. Cui, N. Wang, G. Bi, H. Zhuo, X. Shang, T. Cai, W. Jiang, H. Ben, X. Yang and Y. Huang, Adv. Funct. Mater., 2025, 35, 2421143 CrossRef CAS.
- Q. Xin, S. Yu, L. Jiang, D. Yin, L. Li, C. Xie, Q. Wu, H. Yu, Y. Liu, Y. Liu and S. Liu, J. Phys. Chem. C, 2021, 125, 6632–6642 CrossRef CAS.
- M. Lv, Y. Zhang, Q. Xin, D. Yin, S. Yu, S. Liu, L. Li, C. Xie, Q. Wu, H. Yu and Y. Liu, Chem. Eng. J., 2020, 396, 125274 CrossRef CAS.
- D. Ekeberg, S. Morgenlie and Y. Stenstrøm, Carbohydr. Res., 2005, 340, 373–377 CrossRef CAS PubMed.
- Y. Roman-Leshkov and M. E. Davis, ACS Catal., 2011, 1, 1566–1580 CrossRef CAS.
- X. Chen, G. Clet, K. Thomas and M. Houalla, J. Catal., 2010, 273, 236–244 CrossRef CAS.
- T. Kim, A. Burrows, C. J. Kiely and I. E. Wachs, J. Catal., 2007, 246, 370–381 CrossRef CAS.
- J. Wang, G. Yao, Y. Wang, H. Zhang, Z. Huo and F. Jin, RSC Adv., 2015, 5, 51435–51439 RSC.
- Y. Jia, Q. Sun and H. Liu, Appl. Catal., A, 2020, 603, 117770 CrossRef CAS.
- Y. Jia and H. Liu, Chin. J. Catal., 2015, 36, 1552–1559 CrossRef CAS.
- L. Yang, X. Yan, Q. Wang, Q. Wang and H. Xia, Carbohydr. Res., 2015, 404, 87–92 CrossRef CAS PubMed.
- D. Wang, W. Niu, M. Tan, M. Wu, X. Zheng, Y. Li and N. Tsubaki, ChemSusChem, 2014, 7, 1398–1406 CrossRef CAS PubMed.
- E. Girard, D. Delcroix and A. Cabiac, Catal. Sci. Technol., 2016, 6, 5534–5542 RSC.
- M. Gu, Z. Shen, W. Zhang, M. Xia, J. Jiang, W. Dong, X. Zhou and Y. Zhang, ChemCatChem, 2020, 12, 3447–3452 CrossRef CAS.
- L. Vilcocq, A. Cabiac, C. Especel, S. Lacombe and D. Duprez, Catal. Commun., 2011, 15, 18–22 CrossRef CAS.
- L. Vilcocq, A. Cabiac, C. Especel, S. Lacombe and D. Duprez, Catal. Today, 2012, 189, 117–122 CrossRef CAS.
- L. Vilcocq, A. Cabiac, C. Especel, S. Lacombe and D. Duprez, Catal. Today, 2015, 242, 91–100 Search PubMed.
- L. Vilcocq, R. Koerin, A. Cabiac, C. Especel, S. Lacombe and D. Duprez, Appl. Catal., B, 2014, 148–149, 499–508 Search PubMed.
- H. Kobayashi, Y. Hosaka, K. Hara, B. Feng, Y. Hirosaki and A. Fukuoka, Green Chem., 2014, 16, 637–644 RSC.
- S. Van de Vyver, J. Geboers, M. Dusselier, H. Schepers, T. Vosch, L. Zhang, G. Van Tendeloo, P. A. Jacobs and B. F. Sels, ChemSusChem, 2010, 3, 698–701 Search PubMed.
- S. Van de Vyver, J. Geboers, W. Schutyser, M. Dusselier, P. Eloy, E. Dornez, J. W. Seo, C. M. Courtin, E. M. Gaigneaux, P. A. Jacobs and B. F. Sels, ChemSusChem, 2012, 5, 1549–1558 Search PubMed.
- R. Sun, T. Wang, M. Zheng, W. Deng, J. Pang, A. Wang, X. Wang and T. Zhang, ACS Catal., 2015, 5, 874–883 CrossRef CAS.
- X. Wang, L. Meng, F. Wu, Y. Jiang, L. Wang and X. Mu, Green Chem., 2012, 14, 758–765 RSC.
- L. N. Ding, A. Q. Wang, M. Y. Zheng and T. Zhang, ChemSusChem, 2010, 3, 818–821 CrossRef CAS PubMed.
- Y. K. Lee and S. T. Oyama, J. Catal., 2006, 239, 376–389 CrossRef CAS.
- P. Yang, H. Kobayashi, K. Hara and A. Fukuoka, ChemSusChem, 2012, 5, 920–926 CrossRef CAS.
- C. Li, G. Xu, K. Li, C. Wang, Y. Zhang and Y. Fu, Chem. Commun., 2019, 55, 7663–7666 RSC.
- J. Zhang, S. Wu and Y. Liu, Energy Fuels, 2014, 28, 4242–4246 CrossRef CAS.
- G. Liang, H. Cheng, W. Li, L. He, Y. Yu and F. Zhao, Green Chem., 2012, 14, 2146–2149 RSC.
- G. Liang, L. He, M. Arai and F. Zhao, ChemSusChem, 2014, 7, 1415–1421 CrossRef CAS PubMed.
- A. A. Kirali, S. Sreekantan and B. Marimuthu, New J. Chem., 2020, 44, 15958–15965 RSC.
- Z. Tan, L. Shi, Y. Zan, G. Miao, S. Li, L. Kong, S. Li and Y. Sun, Appl. Catal., A, 2018, 560, 28–36 CrossRef CAS.
- C. Liu, Y. Shang, S. Wang, X. Liu, X. Wang, J. Gui, C. Zhang, Y. Zhu and Y. Li, Mol. Catal., 2020, 485, 110514 CAS.
- P. Yazdani, B. Wang, Y. Du, S. Kawi and A. Borgna, Catal. Sci. Technol., 2017, 7, 4680–4690 RSC.
- L. Ye, X. Duan, H. Lin and Y. Yuan, Catal. Today, 2012, 183, 65–71 CrossRef CAS.
- Z. Zhou, J. Zhang, J. Qin, D. Li and W. Wu, J. Phys. Chem. A, 2018, 92, 456–465 CAS.
- M. Banu, S. Sivasanker, T. M. Sankaranarayanan and P. Venuvanalingam, Catal. Commun., 2011, 12, 673–677 CrossRef CAS.
- M. Banu, P. Venuvanalingam, R. Shanmugam, B. Viswanathan and S. Sivasanker, Top. Catal., 2012, 55, 897–907 CrossRef CAS.
- R. Vijaya Shanthi, T. M. Sankaranarayanan, R. Mahalakshmy and S. Sivasanker, J. Environ. Chem. Eng., 2015, 3, 1752–1757 CrossRef CAS.
- T. Soták, T. Schmidt and M. Hronec, Appl. Catal., A, 2013, 459, 26–33 CrossRef.
- X. Jin, J. Shen, W. Yan, M. Zhao, P. S. Thapa, B. Subramaniam and R. V. Chaudhari, ACS Catal., 2015, 5, 6545–6558 CrossRef CAS.
- R. Vijaya Shanthi, R. Mahalakshmy, K. Thirunavukkarasu and S. Sivasanker, Mol. Catal., 2018, 451, 170–177 CAS.
- X. Chen, X. Wang, S. Yao and X. Mu, Catal. Commun., 2013, 39, 86–89 CrossRef CAS.
- X. Wang, X. Liu, Y. Xu, G. Peng, Q. Cao and X. Mu, Chin. J. Catal., 2015, 36, 1614–1622 CrossRef CAS.
- W. Du, L. Zheng, J. Shi, S. Xia and Z. Hou, Fuel Process. Technol., 2015, 139, 86–90 CrossRef CAS.
- W. Du, L. Zheng, X. Li, J. Fu, X. Lu and Z. Hou, Appl. Clay Sci., 2016, 123, 166–172 CrossRef CAS.
- X. Cao, Q. Zhang, D. Jiang, Q. Liu, L. Ma, T. Wang and D. Li, Chin. J. Chem. Phys., 2015, 28, 338–344 CrossRef CAS.
- C. Cai, H. Wang, H. Xin, C. Zhu, Q. Zhang, X. Zhang, C. Wang, Q. Liu and L. Ma, RSC Adv., 2020, 10, 3993–4001 RSC.
- Y. Qiao, G. J. Xia, W. Cao, K. H. Zeng, Q. L. Guo, X. F. Yang, A. Q. Wang and Y. G. Wang, J. Catal., 2023, 427, 115114 CrossRef CAS.
- Y. Liu, W. Zhang and H. Liu, Chin. J. Catal., 2023, 46, 56–63 CrossRef CAS.
- Z. Li, J. Zhang, B. Hou and A. Wang, AIChE J., 2019, 65, e16585 CrossRef.
- Y. Liu, W. Zhang, C. Hao, S. Wang and H. Liu, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2206399119 CrossRef CAS PubMed.
- N. Ji, M. Zheng, A. Wang, T. Zhang and J. G. Chen, ChemSusChem, 2012, 5, 939–944 Search PubMed.
- L. Zhou, A. Wang, C. Li, M. Zheng and T. Zhang, ChemSusChem, 2012, 5, 932–938 CrossRef CAS PubMed.
- C. Li, M. Zheng, A. Wang and T. Zhang, Energy Environ. Sci., 2012, 5, 6383–6390 RSC.
- N. Ji, T. Zhang, M. Zheng, A. Wang, H. Wang, X. Wang, Y. Shu, A. L. Stottlemyer and J. G. Chen, Catal. Today, 2009, 147, 77–85 CrossRef CAS.
- C. B. Rodella, D. H. Barrett, S. F. Moya, S. J. A. Figueroa, M. T. B. Pimenta, A. A. S. Curvelo and V. Teixeira da Silva, RSC Adv., 2015, 5, 23874–23885 RSC.
- M. Gao, Z. Li, B. Zhao, S. Yu, L. Huang and Q. Wu, Fuel Process. Technol., 2023, 247, 107816 CrossRef CAS.
- Y. Zhang, A. Wang and T. Zhang, Chem. Commun., 2010, 46, 862–864 Search PubMed.
- G. Zhao, M. Zheng, A. Wang and T. Zhang, Chin. J. Catal., 2010, 31, 928–932 CrossRef CAS.
- Z. Tai, J. Zhang, A. Wang, J. Pang, M. Zheng and T. Zhang, ChemSusChem, 2013, 6, 652–658 CrossRef CAS PubMed.
- L. Zhou, J. Pang, A. Wang and T. Zhang, Chin. J. Catal., 2013, 34, 2041–2046 CrossRef CAS.
- Y. Cao, J. Wang, M. Kang and Y. Zhu, J. Mol. Catal. A: Chem., 2014, 381, 46–53 CrossRef CAS.
- Z. Xiao, J. Mao, J. Ji, R. Sha, Y. Fan and C. Xing, J. Fuel Chem. Technol., 2017, 45, 641–650 CrossRef CAS.
- I. G. Baek, S. J. You and E. D. Park, Bioresour. Technol., 2012, 114, 684–690 CrossRef CAS PubMed.
- M. Gu, Z. Shen, L. Yang, W. Dong, L. Kong, W. Zhang, B. Y. Peng and Y. Zhang, Sci. Rep., 2019, 9, 11938 CrossRef PubMed.
- Z. Xiao, R. Sha, J. Ji and J. Mao, J. Fuel Chem. Technol., 2016, 44, 1225–1232 CAS.
- Z. Xiao, Y. Xu, Y. Fan, Q. Zhang, J. Mao and J. Ji, Asia Pac. J. Chem. Eng., 2018, 13, e2153 CrossRef.
- Z. Xiao, Y. Fan, Y. Cheng, Q. Zhang, Q. Ge, R. Sha, J. Ji and J. Mao, Fuel, 2018, 215, 406–416 CrossRef CAS.
- Z. Xiao, Q. Zhang, T. Chen, X. Wang, Y. Fan, Q. Ge, R. Zhai, R. Sun, J. Ji and J. Mao, Fuel, 2018, 230, 332–343 CrossRef CAS.
- M. Li, Y. Ma, X. Ma, Y. Sun and Z. Song, RSC Adv., 2018, 8, 10907–10913 RSC.
- M. S. Hamdy, M. A. Eissa and S. M. A. S. Keshk, Green Chem., 2017, 19, 5144–5151 RSC.
- S. Sreekantan, A. A. Kiralia and B. Marimuthu, New J. Chem., 2021, 45, 19244–19254 RSC.
- H. Liu, L. Qin, X. Wang, C. Du, D. Sun and X. Meng, Catal. Commun., 2016, 77, 47–51 CrossRef CAS.
- N. Li, X. Liu, J. Zhou, Q. Ma, M. Liu and W. Chen, ACS Sustainable Chem. Eng., 2020, 8, 9650–9659 CrossRef CAS.
- J. Yu, J. Liang, X. Chen, L. Wang, X. Wei, Y. Li and Y. Qin, ACS Omega, 2021, 6, 11650–11659 CrossRef CAS PubMed.
- D. Chu and C. Zhao, Catal. Today, 2020, 351, 125–132 CrossRef CAS.
- X. Yang, Z. Li, M. Guo, T. Zhao, X. Su, W. Jiang, G. Han and H. Ben, Fuel, 2023, 341, 127560 CrossRef CAS.
- H. Wang, X. Hu, S. Liu, C. Cai, C. Zhu, H. Xin, Z. Xiu, C. Wang, Q. Liu, Q. Zhang, X. Zhang and L. Ma, Cellulose, 2020, 27, 7591–7605 CrossRef CAS.
- Z. Xiao, X. Wang, Q. Yang, C. Xing, Q. Gea, X. Gai, J. Mao and J. Ji, J. Energy Chem., 2020, 50, 25–36 Search PubMed.
- R. Ooms, M. Dusselier, J. A. Geboers, B. Op de Beeck, R. Verhaeven, E. Gobechiya, J. A. Martens, A. Redl and B. F. Sels, Green Chem., 2014, 16, 695–707 Search PubMed.
- Y. Cao, J. Wang, M. Kang and Y. Zhu, RSC Adv., 2015, 5, 90904–90912 RSC.
- Y. Cao, J. Wang, M. Kang and Y. Zhu, J. Fuel Chem. Technol., 2016, 44, 845–852 CrossRef CAS.
- C. Liu, C. Zhang, S. Hao, S. Sun, K. Liu, J. Xu, Y. Zhu and Y. Li, Catal. Today, 2016, 261, 116–127 CrossRef CAS.
- H. Wang, C. Zhu, D. Li, Q. Liu, J. Tan, C. Wang, C. Cai and L. Ma, Renew. Sustain. Energy Rev., 2019, 103, 227–247 CrossRef CAS.
- S.-M. Wu, X.-Y. Yang and C. Janiak, Angew. Chem., Int. Ed., 2019, 58, 12340–12354 CrossRef CAS.
- H. Tabassum, A. Mahmood, B. Zhu, Z. Liang, R. Zhong, S. Guo and R. Zou, Energy Environ. Sci., 2019, 12, 2924–2956 RSC.
- L. Liu and A. Corma, Nat. Rev. Mater., 2021, 6, 244–263 CrossRef CAS.
- H. Wang, L. Wang and F.-S. Xiao, ACS Cent. Sci., 2020, 6, 1685–1697 CrossRef CAS PubMed.
- X. Liu, Y. Liu, Y. Wu, S. Dong, G. Qi, C. Chen, S. Xi, P. Luo, Y. Dai, Y. Han, Y. Zhou, Y. Guo and J. Wang, J. Hazard. Mater., 2023, 458, 131848 CrossRef CAS PubMed.
- P. He, Q. Yi, H. Geng, Y. Shao, M. Liu, Z. Wu, W. Luo, Y. Liu and V. Valtchev, ACS Catal., 2022, 12, 14717–14726 CrossRef CAS.
- J. Xiao, K. Cheng, X. Xie, M. Wang, S. Xing, Y. Liu, T. Hartman, D. Fu, K. Bossers, M. A. van Huis, A. van Blaaderen, Y. Wang and B. M. Weckhuysen, Nat. Mater., 2022, 21, 572–579 CrossRef CAS.
- J. Li, T. Cai, Y. Feng, X. Liu, N. Wang and Q. Sun, Sci. China Chem., 2024, 67, 2911–2917 CrossRef CAS.
- K. Cheng, L. I. van der Wal, H. Yoshida, J. Oenema, J. Harmel, Z. Zhang, G. Sunley, J. Zečević and K. P. de Jong, Angew. Chem., Int. Ed., 2020, 59, 3592–3600 Search PubMed.
- C. Wang, L. Wang, J. Zhang, H. Wang, J. P. Lewis and F.-S. Xiao, J. Am. Chem. Soc., 2016, 138, 7880–7883 CrossRef CAS PubMed.
- Y. Chai, W. Shang, W. Li, G. Wu, W. Dai, N. Guan and L. Li, Adv. Sci., 2019, 6, 1900299 CrossRef.
- Y. Wang, C. Wang, L. Wang, L. Wang and F.-S. Xiao, Acc. Chem. Res., 2021, 54, 2579–2590 CrossRef CAS PubMed.
- J. Zhang, L. Wang, B. Zhang, H. Zhao, U. Kolb, Y. Zhu, L. Liu, Y. Han, G. Wang, C. Wang, D. S. Su, B. C. Gates and F. S. Xiao, Nat. Catal., 2018, 1, 540–546 CrossRef CAS.
- W. Luo, M. Sankar, A. M. Beale, Q. He, C. J. Kiely, P. C. A. Bruijnincx and B. M. Weckhuysen, Nat. Commun., 2015, 6, 6540 CrossRef CAS PubMed.
- X. Pan, F. Jiao, D. Miao and X. Bao, Chem. Rev., 2021, 121, 6588–6609 CrossRef CAS PubMed.
- K. Cheng, L. C. J. Smulders, L. I. van der Wal, J. Oenema, J. D. Meeldijk, N. L. Visser, G. Sunley, T. Roberts, Z. Xu, E. Doskocil, H. Yoshida, Y. Zheng, J. Zecevic, P. E. de Jongh and K. P. de Jong, Science, 2022, 377, 204–208 CrossRef CAS.
- Q. Zhang, S. Q. Gao and J. H. Yu, Chem. Rev., 2023, 123, 6039–6106 CrossRef CAS PubMed.
- Z. P. Hu, J. Han, Y. Wei and Z. Liu, ACS Catal., 2022, 12, 5060–5076 Search PubMed.
- J. He, Z. Wu, Q. Gu, Y. Liu, S. Chu, S. Chen, Y. Zhang, B. Yang, T. Chen, A. Wang, B. M. Weckhuysen, T. Zhang and W. Luo, Angew. Chem., Int. Ed., 2021, 60, 23713–23721 Search PubMed.
- H. J. Cho, D. Kim, S. Li, D. Su, D. Ma and B. J. Xu, ACS Catal., 2020, 10, 3340–3348 Search PubMed.
- IRENA, Global Energy Transformation: A Roadmap to 2050, International Renewable Energy Agency, 2018, http://refhub.elsevier.com/S2589-5974(19)30149-2/rf0005 Search PubMed.
- G. Xu, A. Wang, J. Pang, X. Zhao, J. Xu, N. Lei, J. Wang, M. Zheng, J. Yin and T. Zhang, ChemSusChem, 2017, 10, 1390–1394 CrossRef CAS PubMed.
- C. Yang, Z. Miao, F. Zhang, L. Li, Y. Liu, A. Wang and T. Zhang, Green Chem., 2018, 20, 2142 RSC.
- N. Olson, N. Deshpande, S. Gunduz, U. S. Ozkan and N. A. Brunelli, Catal. Today, 2019, 323, 69–75 CrossRef CAS.
- L.-J. Liu, Z.-M. Wang, S. Fu, Z.-B. Si, Z. Huang, T.-H. Liu, H.-Q. Yang and C.-W. Hu, Catal. Sci. Technol., 2021, 11, 1537–1543 RSC.
- Y. Román-Leshkov, M. Moliner, J. A. Labinger and M. E. Davis, Angew. Chem., Int. Ed., 2010, 49, 8954–8957 CrossRef.
- V. Choudhary, S. H. Mushrif, C. Ho, A. Anderko, V. Nikolakis, N. S. Marinkovic, A. I. Frenkel, S. I. Sandler and D. G. Vlachos, J. Am. Chem. Soc., 2013, 135, 3997–4006 CrossRef CAS PubMed.
- C. Liu, C. Zhang, S. Sun, K. Liu, S. Hao, J. Xu, Y. Zhu and Y. Li, ACS Catal., 2015, 5, 4612–4623 CrossRef CAS.
- B. Zada, M. Chen, C. Chen, L. Yan, Q. Xu, W. Li, Q. Guo and Y. Fu, Sci. China Chem., 2017, 60, 853–869 CrossRef CAS.
- H. Li, H. Li and J.-F. Deng, Catal. Today, 2002, 74, 53–63 CrossRef CAS.
- H. Li, W. Wang and J.-F. Deng, J. Catal., 2000, 191, 257–260 CrossRef CAS.
- P. Gallezot, P. J. Cerino, B. Blanc, G. Flèche and P. Fuertes, J. Catal., 1994, 146, 93–102 CrossRef CAS.
- B. W. Hoffer, E. Crezee, F. Devred, P. R. M. Mooijman, W. G. Sloof, P. J. Kooyman, A. D. van Langeveld, F. Kapteijn and J. A. Moulijn, Appl. Catal., A, 2003, 253, 437–452 CrossRef CAS.
- J.-P. Mikkola, H. Vainio, T. Salmi, R. Sjöholm, T. Ollonqvist and J. Väyrynen, Appl. Catal., A, 2000, 196, 143–155 CrossRef CAS.
- K. van Gorp, E. Boerman, C. V. Cavenaghi and P. H. Berben, Catal. Today, 1999, 52, 349–361 CrossRef CAS.
- J. Wisnlak and R. Simon, Ind. Eng. Chem. Prod. Res. Dev., 1979, 18, 50–57 CrossRef.
- T. Zhang, A. Q. Wang, M. M. Zheng, C. Li, J. Pang, T. N. Kalnes, J. Q. Chen, and J. A. Kocal, US pat. 08323937, 2012.
- J. D. DeMartini, S. Pattathil, J. S. Miller, H. Li, M. G. Hahn and C. E. Wyman, Energy Environ. Sci., 2013, 6, 898–909 RSC.
- J. Pang, M. Zheng, R. Sun, L. Song, A. Wang, X. Wang and T. Zhang, Bioresour. Technol., 2015, 175, 424–429 CrossRef CAS PubMed.
- T. D. J. te Molder, S. R. A. Kersten, J.-P. Lange and M. P. Ruiz, Ind. Eng. Chem. Res., 2021, 60, 13515–13522 CrossRef CAS.
- J. B. Powell and J. N. Chheda, US pat. US14/574661, 2014.
- A. Yamaguchi, O. Sato, N. Mimura, Y. Hirosaki, H. Kobayashi, A. Fukuoka and M. Shirai, Catal. Commun., 2014, 54, 22–26 CrossRef CAS.
- K. Fabičovicová, M. Lucas and P. Claus, ChemSusChem, 2016, 9, 2804–2815 CrossRef PubMed.
- L. Shuai, M. T. Amiri, Y. M. Questell-Santiago, F. Heŕoguel, Y. Li, H. Kim, R. Meilan, C. Chapple, J. Ralph and J. S. Luterbacher, Science, 2016, 354, 329–333 CrossRef CAS PubMed.
- B. Zong, X. Mu, X. Zhang, X. Meng and M. Qiao, Chin. J. Catal., 2013, 34, 828–837 CrossRef CAS.
- B. Xiao, M. Zheng, J. Pang, Y. Jiang, H. Wang, R. Sun, A. Wang, X. Wang and T. Zhang, Ind. Eng. Chem. Res., 2015, 54, 5862–5869 CrossRef CAS.
- https://www.dicp.ac.cn/xwdt/kyjz/202410/t20241017_7402288.html .
- Y. Yang, D. Ren, Z. Ding, C. Shang, C. Li and S. Lee, Mater. Today Sustain., 2024, 27, 100838 Search PubMed.
- Y. Yang, Z. Ding, D. Ren, C. Shang, C. Li, F. Yang, B. Zhou, S. Hao, K. Sun and S. Lee, Mater. Today Sustain., 2023, 24, 100494 Search PubMed.
- W. Liu, Y. Chen, H. Qi, L. Zhang, W. Yan, X. Liu, X. Yang, S. Miao, W. Wang, C. Liu, A. Wang, J. Li and T. Zhang, Angew. Chem., Int. Ed., 2018, 57, 7071–7075 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |
