Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controllable copper-catalysed photo-induced carbonylative cyclization to access dihydroquinolinones and oxindoles
Yan-Hua Zhaoa,
Le-Cheng Wangab and
Xiao-Feng Wu *ab
*ab
aLeibniz-Institut für Katalyse e.V., Albert-Einstein-Straße 29a, Rostock 18059, Germany. E-mail: Xiao-Feng.Wu@catalysis.de
bDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China. E-mail: xwu2020@dicp.ac.cn
First published on 8th January 2026
Abstract
Oxindoles and dihydroquinolinones are pivotal heterocyclic scaffolds in medicinal and synthetic chemistry. Herein, we describe a controllable visible-light-induced, copper-catalyzed carbonylative cyclization of arylthianthrenium salts with alkenes, enabling the efficient synthesis of structurally diverse oxindoles and dihydroquinolinones. Notably, this transformation proceeds under mild conditions without the need for expensive photocatalysts, and regioselective acyl radical addition is achieved simply by tuning the substitution pattern of the alkene, which enables the switchable synthesis of carbonylated five- and six-membered heterocycles. Mechanistic studies indicate that blue-light irradiation promotes the copper-mediated reduction of arylthianthrenium salts, generating aryl radicals that subsequently capture CO to afford acyl radicals and initiate a tandem cyclization sequence. This method exhibits broad functional-group tolerance and offers a versatile platform for the late-stage functionalization of bioactive molecules.
Introduction
3,3-Disubstituted oxindoles and dihydroquinolinones are important nitrogen-containing heterocycles that are widely found in natural products and bioactive molecules,1–8 and their efficient synthesis continues to attract significant attention.9–15 Over the past decades, numerous transition-metal-catalyzed and metal-free oxidative strategies have been developed for assembling these frameworks.16–20 Among them, the palladium-catalyzed domino-Heck reaction is typically one of the most efficient methods for constructing heterocycles. This approach employs (pseudo)halides, chloroform, aldehydes, and other substrates, coupling them with alkenes to form oxygen-containing indoles and dihydroquinolones through ring-closure reactions.21–24 In recent years, significant progress has been made in radical-based cyclizations (Scheme 1a). Wallentin, Banerjee, and others developed visible-light-driven strategies for generating acyl radicals that subsequently undergo transformations with N-aryl acrylamides.25,26 In 2023, the Maruoka group reported an iron-catalyzed method in which alkyl silyl peroxides serve as precursors to acyl radicals, enabling cascade reactions with N-aryl acrylamides.27 Shortly thereafter, Liu and co-workers reported a visible-light-mediated radical cascade between N-aryl acrylamides or N-methylacrylamide benzamides and carbon dioxide.28 However, these approaches still suffer from limitations such as limited substrate scope. Therefore, the development of a direct, efficient, sustainable, and controllable strategy for the construction of carbonyl-containing heterocyclic frameworks remains highly desirable.Carbonylation chemistry offers one of the most direct and versatile platforms for accessing carbonyl-containing molecules.29–35 Since Heck's pioneering report of palladium-catalyzed carbonylation in 1974, carbon monoxide (CO) has become an essential C1 synthon for transforming simple and readily available substrates into diverse carbonylated derivatives.36–42 Despite its broad synthetic utility, achieving chemoselective and regioselective carbonylation remains challenging, as conventional protocols often rely on noble-metal catalysts, strong Brønsted acids, and high temperatures, thereby limiting functional-group tolerance and substrate scope.43–49 N-arylacrylamides, as structurally privileged activated alkenes, offer an attractive platform for achieving regioselective carbonylation via substitution-controlled reactivity, enabling streamlined access to carbonylated oxindoles and dihydroquinolinones.50–56 Meanwhile, arylthianthrenium salts have attracted considerable interest due to their high reactivity and ready accessibility, which allows them to be readily prepared from simple arenes via C(sp2)–H thianthrenation.57–64 In recent years, Ritter and co-workers have systematically investigated thianthrenium salts, revealing their broad utility in diverse C–H functionalization reactions.65–70 However, only a few examples of carbonylation reactions using thianthrenium salts have been reported, and there are no related reports on the selective controllable carbonylation associated with them.
Building on our previous investigations into the photochemical behavior of thianthrenium salts,68,71–75 we demonstrated that these reagents undergo efficient visible-light-induced homolytic C(sp2)-S bond cleavage to generate aryl radicals. Leveraging this reactivity, we developed a controllable single electron carbonylative cyclization manifold. Under visible-light-promoted copper catalysis, the in situ formed aryl radicals readily capture CO to produce acyl radicals, which undergo selective addition with N-aryl acrylamides to deliver oxindoles and dihydroquinolinones. N-aryl acrylamides are readily accessible from a variety of α,β-unsaturated carboxylic acid derivatives, and their tunable substitution patterns provide an ideal platform for controlling reaction selectivity. Remarkably, tuning the alkene substitution pattern alone dictates the regioselectivity of acyl-radical addition, granting switchable access to carbonylated five- and six-membered heterocycles without altering reaction conditions. This platform thus provides a general, mild, and highly controllable approach to carbonylation, addressing key limitations in current radical carbonylation chemistry (Scheme 1b).
Results and discussion
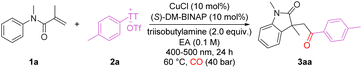
We began our investigation using N-arylacrylamide 1a and arylthianthrenium salt 2a as the model substrates to evaluate the feasibility of the carbonylative cyclization (Table 1). Under blue-light irradiation at 60 °C in EtOAc, with CuCl as the catalyst, (S)-DM-BINAP as the ligand, and triisobutylamine as the base under 40 bar CO for 24 h, the desired oxindole 3aa was obtained in 80% yield (78% isolated, entry 1). Control experiments confirmed that the reaction did not proceed in the absence of the copper catalyst, ligand, base, or light (Table 1, entries 3–5). Screening of copper salts revealed that Cu(acac)2 could also promote the reaction, even with a slightly lower yield, while other copper catalysts gave comparable results (Table 1, entries 6–8). Bidentate phosphine ligands facilitated product formation but did not improve the yield, and the reaction outcomes for (R)-DM-BINAP and (S)-DM-BINAP were similar (Table 1, entries 9 and 12). In contrast, nitrogen-based or monodentate phosphine ligands failed to produce detectable amounts of 3aa (Table 1, entries 10 and 11). The choice of base significantly affected the reaction outcome, with organic bases outperforming inorganic bases (Table 1, entries 13 and 14). Solvent screening indicated that EtOAc was the optimal, while acetonitrile or toluene led to decreased yields (Table 1, entries 15 and 16). Reducing the amount of 2aa or increasing the reaction concentration also lowered the yield (Table 1, entries 17 and 18). The reaction proceeded at 40 °C to give 3aa in 73% yield (Table 1, entry 19), and lowering the CO pressure similarly decreased the reaction efficiency (Table 1, entry 20). For the reaction time, the conversion cannot be completed after 15 hours and no better yield can be produced if we extend the reaction time to 36 hours.| Entry | Variation from the standard conditions | Yield (%)b |
|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.15 mmol), CO (40 bar), catalyst (10 mol%), ligand (10 mol%), base (0.2 mmol), solvent (1.0 mL), blue-light irradiation at 60 °C, 24 h.b Yield was determined by GC using nHexadecane as the internal standard.c Isolated yield.d 70% yield on 10 mmol scale. | ||
| 1 | None | 80 (78)c,d |
| 2 | No catalyst | n.d. |
| 3 | No ligand | n.d. |
| 4 | No base | Trace |
| 5 | No light irradiation | n.d. |
| 6 | CuBr instead of CuCl | 36 |
| 7 | Cu(CH3CN)4BF4 instead of CuCl | 56 |
| 8 | Cu(acac)2 instead of CuCl | 31 |
| 9 | BINAP instead of (S)-DM-BINAP | 69 |
| 10 | PCy3 instead of (S)-DM-BINAP | n.d. |
| 11 | 1,10-Phen instead of (S)-DM-BINAP | n.d. |
| 12 | (R)-DM-BINAP instead of (S)-DM-BINAP | 79 |
| 13 | DIPEA instead of triisobutylamine | 74 |
| 14 | K3PO4 instead of triisobutylamine | 16 |
| 15 | CH3CN as solvent | 25 |
| 16 | Toluene as solvent | 56 |
| 17 | 1.0 equiv. 2a | 50 |
| 18 | 0.2 mol L−1 EA | 75 |
| 19 | 40 °C instead of 60 °C | 73 |
| 20 | 20 bar CO | 69 |
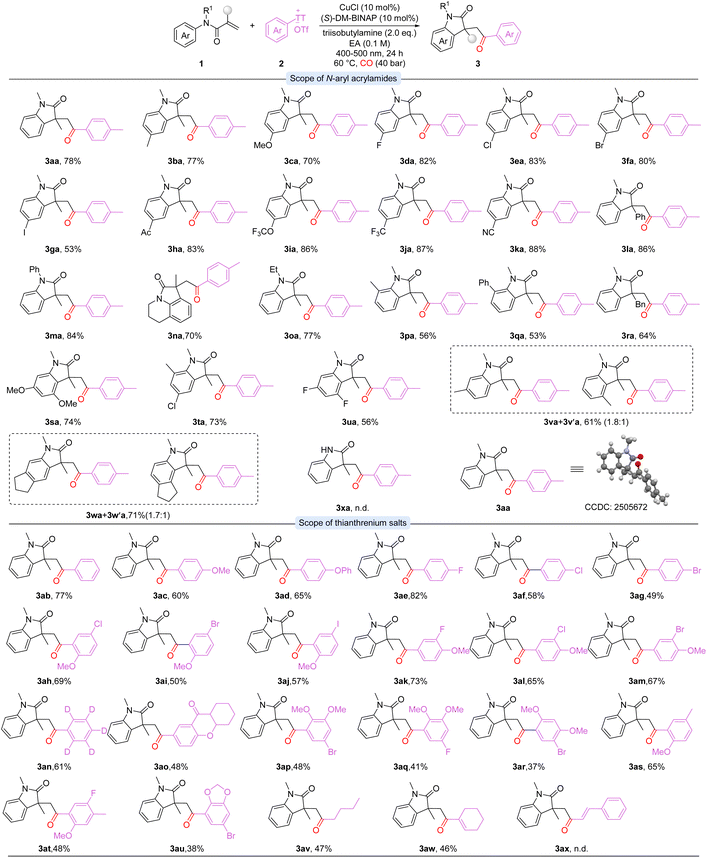
With the optimal conditions established, the substrate scope of N-aryl acrylamides was investigated at the first stage (Scheme 2). Unsubstituted and electron-donating substrates, such as methyl- and methoxy-substituted N-aryl acrylamides, were well tolerated, affording the desired products in good to high yields (3aa-3ca). Halogen-substituted substrates (F, Cl, Br, I) were also compatible, providing the corresponding products in moderate to excellent yields (3da-3ga). Notably, substrates bearing electron-withdrawing groups, including acyl, trifluoromethoxy, trifluoromethyl, and cyano, proceeded smoothly to give oxindoles in excellent yields (3ha-3ka). Alkenes bearing other substituents delivered products 3la and 3ra in 86% and 64% yields, respectively. N-protected acrylamides with phenyl or ethyl groups were also compatible, yielding 3ma and 3oa in 84% and 77% yields. In contrast, unprotected N-aryl acrylamides failed to afford the desired product (3xa). The ester analogue was also tested, but no lactone product could be detected. Tetrahydroquinoline derivatives were tolerated, providing the target product 3na in 70% yield. Ortho-Substituted substrates furnished the products 3pa and 3qa in moderate yields, while meta-substituted substrates led to regioisomeric mixtures (3va/3v′a and 3wa/3w′a). Furthermore, multi-substituted substrates were also well tolerated, afforded the corresponding products in excellent yields (3sa-3ua).
The substrate scope of thianthrenium salts was further investigated (Scheme 2). Unsubstituted and electron-rich aryl thianthrenium salts gave the desired products in good yields (3ab-3ad, 3as). Aryl thianthrenium salts bearing C(sp2)-X bonds (X = F, Cl, Br) were also compatible, providing the corresponding products in moderate to excellent yields (3ae-3ag). Similarly, di- and tri-substituted aryl thianthrenium salts (3ah-3am and 3ap-3ar, 3at-3au) performed well under the reaction conditions, delivering the target products in good yields in general. These results highlight the high chemoselectivity of the catalytic system and its potential for subsequent structural modifications. Complex scaffolds, including flavone-derived salts, also participated smoothly, delivering the corresponding product in moderate yield (3ao). Deuterated substrates afforded the desired oxindole 3an in 61% yield. Notably, thianthrenium salts bearing alkyl and alkenyl substituents reacted smoothly under the standard conditions, delivering the desired products (3av-3aw) in moderate yields. However, the styrenyl-substituted thianthrenium salt showed no reactivity under the standard reaction conditions (3ax). These results indicate that the catalytic system features broad substrate scope and high chemoselectivity. Additionally, instead of thianthrenium salt, iodobenzene was checked as well but lead to no desired product formed.
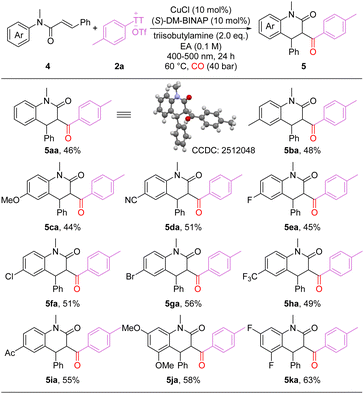
Under the standard reaction conditions, employing alkenes with different substitution patterns, six-membered dihydroquinolinones were selectively obtained rather than five-membered oxindoles (Scheme 3). As shown in the scheme, a variety of carbonylated six-membered heterocycles bearing diverse functional groups were synthesized in moderate to good yields (5aa-5ka). Unsubstituted and electron-rich substrates afforded the corresponding products in moderate yields (5aa-5ca). Internal alkenes bearing halogens (F, Cl, Br) or electron-withdrawing groups (cyano, acyl, trifluoromethyl) were compatible with the reaction, delivering the desired products in moderate yields (5da-5ia). Multi-substituted substrates also performed well, providing the target products in good yields (5ja-5ka). It's worth to mention that non-carbonylation product was the main by-product during this process and response for the moderate yields.
The atom economy of the reaction was also evaluated, and thianthrene was recovered in yields of up to 96% (Scheme 4a). To gain some insight into the reaction mechanism, several mechanistic experiments were conducted. Under the standard conditions, the addition of 2.0 equiv. of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) completely suppressed the formation of the desired product. Similarly, the addition of 2.0 equiv. of 1,1-diphenylethylene (1,1-DPE) led to no detectable target product, while radical adducts 6 and 7 were observed (Scheme 4b). Furthermore, light-on/off experiments revealed that the reaction proceeds only under visible-light irradiation, and no conversion occurs in the dark (Scheme 4c). Collectively, these results indicate that the transformation does not follow a radical chain process, but rather proceeds via an aryl radical intermediate.
Based on the above results and literature precedents, a plausible mechanism is proposed (Scheme 4d).76–80 Under blue-light irradiation, the CuIL complex is excited to CuIL*, which reacts with the aryl thianthrenium salt to generate an aryl radical and copper(II) complex CuIIL. The aryl radical captures CO to form an acyl radical, which adds to the alkene of N-methyl-N-phenylacrylamide 1a, affording intermediate A. Intramolecular cyclization on the aryl ring then gives intermediate B, which undergoes single-electron transfer with CuIIL and subsequent deprotonation to yield oxindole 3. Similarly, when the acyl radical reacts with N-methyl-N-phenylcinnamamide, addition to the alkene forms intermediate C, followed by intramolecular cyclization to intermediate D. Subsequent single-electron transfer with CuIIL and deprotonation furnishes the six-membered dihydroquinolinone 5. This mechanism highlights the key roles of aryl radicals, CO trapping, and Cu-mediated single-electron transfer in enabling selective carbonylative cyclization.
Conclusions
In summary, we have developed a visible-light-induced, copper-catalyzed controllable carbonylative cyclization that enables selective synthesis of carbonylated five- and six-membered heterocycles by simply tuning the substitution pattern of the starting alkenes. The method employs inexpensive CO as the carbonyl source, proceeds under mild conditions, and allows efficient one-pot access to 3,3-disubstituted oxindoles and dihydroquinolinones. It features broad substrate scope, generally good yields, excellent functional-group tolerance, and potential for downstream derivatization, providing a practical, economical, and sustainable approach to nitrogen-containing carbonylated heterocycles.Author contributions
Y. H. Z. and L. C. W. designed and carried out the reactions and analyzed the data. X.-F. W. designed and supervised the project. X.-F. W. and Y. H. Z. wrote and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: general comments, general procedure, analytic data, and NMR spectra. See DOI: https://doi.org/10.1039/d5sc09434h.Acknowledgements
We thank the Chinese Scholarship Council for financial support. We also thank the analytical department of Leibniz-Institute for Catalysis for their excellent analytical service.Notes and references
- J. F. Austin, S.-G. Kim, C. J. Sinz, W.-J. Xiao and D. W. C. MacMillan, Enantioselective organocatalytic construction of pyrroloindolines by a cascade addition–cyclization strategy: Synthesis of (–)-flustramine B, Proc. Natl. Acad. Sci. U.S.A., 2004, 101, 5482–5487 CrossRef CAS.
- J. J. Badillo, N. V. Hanhan and A. K. Franz, Enantioselective synthesis of substituted oxindoles and spirooxindoles with applications in drug discovery, Curr. Opin. Drug Discov. Dev., 2010, 13, 758–776 CAS.
- N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Strategies for the enantioselective synthesis of spirooxindoles, Org. Biomol. Chem., 2012, 10, 5165–5181 RSC.
- C. Zheng and S.-L. You, Catalytic asymmetric dearomatization (CADA) reaction-enabled total synthesis of indole-based natural products, Nat. Prod. Rep., 2019, 36, 1589–1605 RSC.
- X.-H. Yang, R.-J. Song, Y.-X. Xie and J.-H. Li, Iron Catalyzed Oxidative Coupling, Addition, and Functionalization, ChemCatChem, 2016, 8, 2429–2445 CrossRef CAS.
- M. Chen, X. Wang, P. Yang, X. Kou, Z.-H. Ren and Z.-H. Guan, Palladium-Catalyzed Enantioselective Heck Carbonylation with a Monodentate Phosphoramidite Ligand: Asymmetric Synthesis of (+)-Physostigmine, (+)-Physovenine, and (+)-Folicanthine, Angew. Chem., Int. Ed., 2020, 59, 12199–12205 CrossRef CAS.
- T. Ma, Y. Chen, Y. Li, Y. Ping and W. Kong, Nickel-Catalyzed Enantioselective Reductive Aryl Fluoroalkenylation of Alkenes, ACS Catal., 2019, 9, 9127–9133 CrossRef CAS.
- A. D. Marchese, E. M. Larin, B. Mirabi and M. Lautens, Metal-Catalyzed Approaches toward the Oxindole Core, Acc. Chem. Res., 2020, 53, 1605–1619 CrossRef CAS PubMed.
- K. Fang, W. Huang, C. Shan, J. Qu and Y. Chen, Synthesis of 3,3-Dialkyl-Substituted Isoindolinones Enabled by Nickel-Catalyzed Reductive Dicarbofunctionalization of Enamides, Org. Lett., 2021, 23, 5523–5527 CrossRef CAS.
- Q. Pan, Y. Ping, Y. Wang, Y. Guo and W. Kong, Ni-Catalyzed Ligand-Controlled Regiodivergent Reductive Dicarbofunctionalization of Alkenes, J. Am. Chem. Soc., 2021, 143, 10282–10291 Search PubMed.
- N. Qin, X. Lu, Y. Liu, Y. Qiao, W. Qu, F. Feng and H. Sun, Recent research progress of Uncaria spp. based on alkaloids: phytochemistry, pharmacology and structural chemistry, Eur. J. Med. Chem., 2021, 210, 112960 CrossRef CAS.
- K. Wang, Z. Ding, Z. Zhou and W. Kong, Ni-Catalyzed Enantioselective Reductive Diarylation of Activated Alkenes by Domino Cyclization/Cross-Coupling, J. Am. Chem. Soc., 2018, 140, 12364–12368 Search PubMed.
- J.-X. Yan, H. Li, X.-W. Liu, J.-L. Shi, X. Wang and Z.-J. Shi, Palladium-Catalyzed C(sp3)-H Activation: A Facile Method for the Synthesis of 3,4-Dihydroquinolinone Derivatives, Angew. Chem., Int. Ed., 2014, 53, 4945–4949 CrossRef CAS PubMed.
- L. Zhang, Z. Qureshi, L. Sonaglia and M. Lautens, Sequential Rhodium/Palladium Catalysis: Enantioselective Formation of Dihydroquinolinones in the Presence of Achiral and Chiral Ligands, Angew. Chem., Int. Ed., 2014, 53, 13850–13853 CrossRef CAS.
- X. Zheng, Y. Xu, G. Huang and W. Yuan, Catalyst-controlled 5-exo and 6-endo cyclization: regiodivergent access to indolinones and dihydroquinolinones, Org. Chem. Front., 2025, 12, 5018–5026 RSC.
- P. Biswas, S. Mandal and J. Guin, Aerobic Acylarylation of α,β-Unsaturated Amides with Aldehydes, Org. Lett., 2020, 22, 4294–4299 CrossRef CAS.
- P. Biswas, S. Paul and J. Guin, Aerobic Radical-Cascade Alkylation/Cyclization of α,β-Unsaturated Amides: an Efficient Approach to Quaternary Oxindoles, Angew. Chem., Int. Ed., 2016, 55, 7756–7760 CAS.
- X.-W. Chen, J.-P. Yue, K. Wang, Y.-Y. Gui, Y.-N. Niu, J. Liu, C.-K. Ran, W. Kong, W.-J. Zhou and D.-G. Yu, Nickel-Catalyzed Asymmetric Reductive Carbo-Carboxylation of Alkenes with CO2, Angew. Chem., Int. Ed., 2021, 60, 14068–14075 CrossRef CAS PubMed.
- M. He, Y. Yang, H. Zhang, Z. Guan, Z.-B. Dong, H. Yi and A. Lei, General radical difluoromethylation using difluoroacetic anhydride via photoredox catalysis, Sci. China Chem., 2024, 67, 2637–2646 Search PubMed.
- M.-Z. Zhang, L. Liu, Q. Gou, Q. Wang, Y. Li, W.-T. Li, F. Luo, M. Yuan, T. Chen and W.-M. He, Synthesis of hydroxyl-containing oxindoles and 3,4-dihydroquinolin-2-ones through oxone-mediated cascade arylhydroxylation of activated alkenes, Green Chem., 2020, 22, 8369–8374 Search PubMed.
- H. Hu, F. Teng, J. Liu, W. Hu, S. Luo and Q. Zhu, Enantioselective Synthesis of 2-Oxindole Spirofused Lactones and Lactams by Heck/Carbonylative Cylization Sequences: Method Development and Applications, Angew. Chem., Int. Ed., 2019, 58, 9225–9229 Search PubMed.
- Y. Kobayashi, H. Kamisaki, R. Yanada and Y. Takemoto, Palladium-Catalyzed Intramolecular Cyanoamidation of Alkynyl and Alkenyl Cyanoformamides, Org. Lett., 2006, 8, 2711–2713 CrossRef CAS PubMed.
- X. Liu, B. Li and Z. Gu, Palladium-Catalyzed Heck-type Domino Cyclization and Carboxylation to Synthesize Carboxylic Acids by Utilizing Chloroform as the Carbon Monoxide Source, J. Org. Chem., 2015, 80, 7547–7554 Search PubMed.
- D. Wen, Z. Shen, X. Qi and X.-F. Wu, Palladium-Catalyzed Cascade Carbonylative Synthesis of Functionalized Isoquinoline-1,3-diones and Oxindoles Using Dimethyl Carbonate as both Solvent and Reactant, Eur. J. Org Chem., 2022, 2022, e202200971 Search PubMed.
- G. Bergonzini, C. Cassani and C.-J. Wallentin, Acyl Radicals from Aromatic Carboxylic Acids by Means of Visible-Light Photoredox Catalysis, Angew. Chem., Int. Ed., 2015, 54, 14066–14069 CrossRef CAS.
- C. S. Nishad, P. Suman, H. Saha and B. Banerjee, Visible-Light-Induced Metal- and Photocatalyst-Free Radical Cascade Cyclization of Cinnamamides for Synthesis of Functionalized Dihydroquinolinones, J. Org. Chem., 2023, 88, 11010–11022 CrossRef CAS PubMed.
- J. Liu, S. Liu, Z. Wang, T. Kato, Y. Liu and K. Maruoka, Synthetic utility of functionalized alkylsilyl peroxides for Fe-catalyzed and visible-light-promoted radical transformation, Chem. Sci., 2024, 15, 4757–4762 RSC.
- T. Zhang, Z. Chen, F. Xue, X. Ren, B. Wang, W. Jin, Y. Xia, S. Wu, A. Iqbal, Y. Zhang and C. Liu, Visible-Light-Mediated Cascade Arylcarboxylation/Cyclization of N-Aryl/Benzoyl Acrylamide with CO2, Org. Lett., 2025, 27, 8423–8428 CrossRef CAS.
- Z.-P. Bao, L.-C. Wang and X.-F. Wu, Carbonylation: Unlocking Opportunities for Bioactive Molecule and Pharmaceutical Development, ACS Catal., 2025, 15, 19580–19606 CrossRef CAS.
- M. Beller and X.-F. Wu, Transition Metal Catalyzed Carbonylation Reactions, 2013, pp. 115–145 Search PubMed.
- B. Cornils, W. A. Herrmann and M. Rasch, Otto Roelen, Pioneer in Industrial Homogeneous Catalysis, Angew. Chem., Int. Ed., 1994, 33, 2144–2163 CrossRef.
- T. Kawamoto, T. Fukuyama, B. Picard and I. Ryu, New directions in radical carbonylation chemistry: combination with electron catalysis, photocatalysis and ring-opening, Chem. Commun., 2022, 58, 7608–7617 Search PubMed.
- Q. Tian, X. Yin, R. Sun, X. F. Wu and Y. Li, The lower the better: Efficient carbonylative reactions under atmospheric pressure of carbon monoxide, J. Coord. Chem., 2023, 475, 214900 CrossRef CAS.
- L.-C. Wang, H. Yang, Z.-W. Liu, R.-G. Miao, M. Hou and X.-F. Wu, Recent Advances in Single-Electron-Transfer-Mediated Carbonylation, Chem. Rev., 2026 DOI:10.1021/acs.chemrev.5c00664.
- F.-P. Wu, Y. Yang, D. P. Fuentes and X.-F. Wu, Copper-catalyzed carbonylative catenation of olefins: Direct synthesis of γ-boryl esters, Chem, 2022, 8, 1982–1992 CAS.
- J. Falbe, H. Bahrmann and B. Cornils, New Syntheses with Carbon Monoxide, Springer, 1980 Search PubMed.
- S. D. Friis, A. T. Lindhardt and T. Skrydstrup, The Development and Application of Two-Chamber Reactors and Carbon Monoxide Precursors for Safe Carbonylation Reactions, Acc. Chem. Res., 2016, 49, 594–605 CrossRef CAS.
- B. Gabriele, Carbon Monoxide in Organic Synthesis: Carbonylation Chemistry, John Wiley & Sons, 2021 Search PubMed.
- A. Haynes, P. M. Maitlis, G. E. Morris, G. J. Sunley, H. Adams, P. W. Badger, C. M. Bowers, D. B. Cook, P. I. P. Elliott, T. Ghaffar, H. Green, T. R. Griffin, M. Payne, J. M. Pearson, M. J. Taylor, P. W. Vickers and R. J. Watt, Promotion of Iridium-Catalyzed Methanol Carbonylation: Mechanistic Studies of the Cativa Process, J. Am. Chem. Soc., 2004, 126, 2847–2861 Search PubMed.
- A. Schoenberg and R. Heck, Palladium-catalyzed formylation of aryl, heterocyclic, and vinylic halides, J. Am. Chem. Soc., 1974, 96, 7761–7764 CrossRef.
- Q. Tian, X. Yin, R. Sun and Y. Li, The lower the better: Efficient carbonylative reactions under atmospheric pressure of carbon monoxide, J. Coord. Chem., 2023, 475, 214900 CrossRef CAS.
- X.-F. Wu, B. Han, K. Ding and Z. Liu, The Chemical Transformations of C1 Compounds, John Wiley & Sons, 2022 Search PubMed.
- Z. Li, P. Ma, Y. Wang and J. Wang, Substrate-Controlled Divergent Synthesis of Multisubstituted Cyclopentenones and 1,4-Diketones, Org. Lett., 2025, 27, 8946–8951 Search PubMed.
- B.-X. Liu, S.-C. Li, H.-W. Rao, Q.-Q. Yang, W. Du and Y.-C. Chen, Palladium-Catalyzed Substrate-Controlled Regiodivergent Hydroamination of gem-Difluoroallenes, Org. Lett., 2025, 27, 8288–8292 CrossRef CAS PubMed.
- W. Liu, W. Ren, J. Li, Y. Shi, W. Chang and Y. Shi, A Ligand-Directed Catalytic Regioselective Hydrocarboxylation of Aryl Olefins with Pd and Formic Acid, Org. Lett., 2017, 19, 1748–1751 CrossRef CAS PubMed.
- J.-B. Peng and X.-F. Wu, Ligand- and Solvent-Controlled Regio- and Chemodivergent Carbonylative Reactions, Angew. Chem., Int. Ed., 2018, 57, 1152–1160 CrossRef CAS.
- X. Wang, J. Zhang, X. Zhao, Y. Wang, J. Sima, Z. Wang, X. Jia, S. Song and F. Yu, Substrate-controlled regioselective dibenzylation of enaminones, Org. Chem. Front., 2025, 12, 6209–6214 RSC.
- Y. Zhang, S. Torker, M. Sigrist, N. Bregović and P. Dydio, Binuclear Pd(I)–Pd(I) Catalysis Assisted by Iodide Ligands for Selective Hydroformylation of Alkenes and Alkynes, J. Am. Chem. Soc., 2020, 142, 18251–18265 CrossRef CAS.
- F. Zhao, H.-J. Ai and X.-F. Wu, Copper-Catalyzed Substrate-Controlled Carbonylative Synthesis of α-Keto Amides and Amides from Alkyl Halides, Angew. Chem., Int. Ed., 2022, 61, e202200062 CrossRef CAS.
- R. K. Dhungana, S. KC, P. Basnet and R. Giri, Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins, Chem. Rec., 2018, 18, 1314–1340 Search PubMed.
- Z.-L. Li, G.-C. Fang, Q.-S. Gu and X.-Y. Liu, Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes, Chem. Soc. Rev., 2020, 49, 32–48 Search PubMed.
- J. Lin, R.-J. Song, M. Hu and J.-H. Li, Recent Advances in the Intermolecular Oxidative Difunctionalization of Alkenes, Chem. Rec., 2019, 19, 440–451 CrossRef CAS.
- J. Xie, P. Xu, H. Li, Q. Xue, H. Jin, Y. Cheng and C. Zhu, A room temperature decarboxylation/C–H functionalization cascade by visible-light photoredox catalysis, Chem. Commun., 2013, 49, 5672–5674 Search PubMed.
- L. Zheng, H. Huang, C. Yang and W. Xia, UV Light-Mediated Difunctionalization of Alkenes through Aroyl Radical Addition/1,4-/1,2-Aryl Shift Cascade Reactions, Org. Lett., 2015, 17, 1034–1037 Search PubMed.
- M.-B. Zhou, R.-J. Song, X.-H. Ouyang, Y. Liu, W.-T. Wei, G.-B. Deng and J.-H. Li, Metal-free oxidative tandem coupling of activated alkenes with carbonyl C(sp2)–H bonds and aryl C(sp2)–H bonds using TBHP, Chem. Sci., 2013, 4, 2690–2694 RSC.
- M.-B. Zhou, C.-Y. Wang, R.-J. Song, Y. Liu, W.-T. Wei and J.-H. Li, Oxidative 1,2-difunctionalization of activated alkenes with benzylic C(sp3)–H bonds and aryl C(sp2)–H bonds, Chem. Commun., 2013, 49, 10817–10819 Search PubMed.
- M. J. Cabrera-Afonso, A. Granados and G. A. Molander, Sustainable Thioetherification via Electron Donor–Acceptor Photoactivation Using Thianthrenium Salts, Angew. Chem., Int. Ed., 2022, 61, e202202706 Search PubMed.
- L.-C. Campeau, Thianthrenium ions pair-up for Suzuki–Miyaura reactions, Nat. Synth., 2024, 3, 1448–1450 CrossRef CAS.
- S. Happy, M. Saleem and D. Yadagiri, Pd-Catalyzed Late-Stage Installation of Nitrile and Carbamoyl Groups via a Cross-Coupling Approach of Thianthrenium Salts with Isonitriles, ACS Catal., 2025, 15, 13401–13411 Search PubMed.
- F. Juliá, Q. Shao, M. Duan, M. B. Plutschack, F. Berger, J. Mateos, C. Lu, X.-S. Xue, K. N. Houk and T. Ritter, High Site Selectivity in Electrophilic Aromatic Substitutions: Mechanism of C–H Thianthrenation, J. Am. Chem. Soc., 2021, 143, 16041–16054 CrossRef.
- H. Meng, M.-S. Liu and W. Shu, Organothianthrenium salts: synthesis and utilization, Chem. Sci., 2022, 13, 13690–13707 RSC.
- S. Tang, X. Zhao, L. Yang, B. Li and B. Wang, Copper-Catalyzed Carboxylation of Aryl Thianthrenium Salts with CO2, Angew. Chem., Int. Ed., 2022, 61, e202212975 CrossRef CAS.
- K. Targos, A. R. Gogoi, R.-G. Ángel, M. J. Kim, O. Gutierrez and Z. K. Wickens, Mechanism of Z-Selective Allylic Functionalization via Thianthrenium Salts, J. Am. Chem. Soc., 2024, 146, 13689–13696 Search PubMed.
- Y. Yuan, C. Bai, Y. Tao, Y.-N. Zhang, X. Bao, D. Ji, L. Wang, X. Qi and C. Huo, Electrochemical ring-opening oxidation of aryl thianthrenium salts, Green Chem., 2025, 27, 9777–9786 RSC.
- Z. Bai, B. Lansbergen and T. Ritter, Bicyclopentylation of Alcohols with Thianthrenium Reagents, J. Am. Chem. Soc., 2023, 145, 25954–25961 Search PubMed.
- Z. Bai and T. Ritter, Applications of thianthrene chemistry in organic synthesis, Nat. Synth., 2025, 4, 1187–1199 Search PubMed.
- M. Mrozowicz, S. Chatterjee, M. Aliki Mermigki, D. A. Pantazis and T. Ritter, Meta-Dimethylation of Arenes via Catellani Reaction from Aryl Thianthrenium Salts, Angew. Chem., Int. Ed., 2025, 64, e202419472 Search PubMed.
- S. Pandit and T. Ritter, Copper-Photoredox-Catalyzed C(sp3)–C(sp3) Reductive Cross-Coupling of Alkyl Bromides with BCP-Thianthrenium Reagents, Angew. Chem., Int. Ed., 2025, 64, e202506785 Search PubMed.
- X. Sun and T. Ritter, Decarboxylative Polyfluoroarylation of Alkylcarboxylic Acids, Angew. Chem., Int. Ed., 2021, 60, 10557–10562 CrossRef CAS PubMed.
- J. Zhang, M. Jiao, Z. Lu, H. Lu, M. Wang and Z. Shi, Hydrodeuteroalkylation of Unactivated Olefins Using Thianthrenium Salts, Angew. Chem., Int. Ed., 2024, 63, e202409862 Search PubMed.
- M. Liu, Y. Qian, Y. Wu and F. Zhang, Multicomponent synthesis of di-aryl dithiocarbamates via electron donor–acceptor photoactivation with thianthrenium salts, Green Chem., 2023, 25, 3852–3856 RSC.
- C. M. Panos, A. D. Matthews and Z. K. Wickens, Homologative 1,3-Functionalization of Alkenes via an Iodomethyl Thianthrenium Reagent, Angew. Chem., Int. Ed., 2025, 64, e202519207 Search PubMed.
- R. I. Patel, B. Saxena and A. Sharma, Photoactivation of Thianthrenium Salts: An Electron-Donor–Acceptor (EDA)-Complex Approach, J. Org. Chem., 2025, 90, 6617–6643 CrossRef CAS.
- K. Sun, C. Ge, X. Chen, B. Yu, L. Qu and B. Yu, Energy-transfer-enabled photocatalytic transformations of aryl thianthrenium salts, Nat. Commun., 2024, 15, 9693 CrossRef CAS PubMed.
- Y. Zhang, J. Mao, Z. Wang, L. Tang and Z. Fan, Visible light-promoted defluorinative alkylation/arylation of α-trifluoromethyl alkenes with thianthrenium salts, Green Chem., 2024, 26, 9371–9377 Search PubMed.
- K. Cheng, E. W. Webb, G. D. Bowden, J. S. Wright, X. Shao, M. S. Sanford and P. J. H. Scott, Photo- and Cu-Mediated 11C Cyanation of (Hetero)Aryl Thianthrenium Salts, Org. Lett., 2024, 26, 3419–3423 CrossRef CAS PubMed.
- X. Fan, T. Lei, B. Chen, C.-H. Tung and L.-Z. Wu, Photocatalytic C–C Bond Activation of Oxime Ester for Acyl Radical Generation and Application, Org. Lett., 2019, 21, 4153–4158 Search PubMed.
- Y.-Y. Jiang, Z. Chen, Y.-X. Jiang, W.-J. Zhu, J.-C. Xu, J.-H. Ye, W. Zhang and D.-G. Yu, Visible-Light-Driven Thiolate-Catalyzed Carbo-Carboxylation of Alkenes with CO2: Facile Synthesis of Oxindole-3-acetic Acid Derivatives, Chin. J. Chem., 2025, 43, 2341–2346 Search PubMed.
- N. Kvasovs and V. Gevorgyan, Contemporary methods for generation of aryl radicals, Chem. Soc. Rev., 2021, 50, 2244–2259 Search PubMed.
- W.-P. Mai, G.-C. Sun, J.-T. Wang, G. Song, P. Mao, L.-R. Yang, J.-W. Yuan, Y.-M. Xiao and L.-B. Qu, Silver-Catalyzed Radical Tandem Cyclization: An Approach to Direct Synthesis of 3-Acyl-4-arylquinolin-2(1H)-ones, J. Org. Chem., 2014, 79, 8094–8102 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |