Open Access Article
Open Access ArticleL-region-selective annulative π-extension through dearomative activation of polycyclic aromatic hydrocarbons
Kanami Nakataa,
Wataru Matsuoka a,
Hideto Ito
a,
Hideto Ito *a and
Kenichiro Itami
*a and
Kenichiro Itami *bcd
*bcd
aDepartment of Chemistry, Graduate School of Science, Nagoya University, Nagoya 464-8602, Japan. E-mail: ito.hideto.p4@f.mail.nagoya-u.ac.jp
bMolecule Creation Laboratory, Pioneering Research Institute (PRI), RIKEN, Wako, Saitama 351-0198, Japan. E-mail: kenichiro.itami@riken.jp
cExpanded Chemical Space Research Team, Center for Sustainable Resource Science (CSRS), RIKEN, Wako, Saitama 351-0198, Japan
dInstitute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Nagoya 464-8602, Japan
First published on 29th January 2026
Abstract
Annulative π-extension (APEX) reaction is a useful aromatic ring-fusion method for the synthesis of large polycyclic aromatic hydrocarbons (PAHs) from unfunctionalized small PAHs. While APEX reactions in the K-, M-, and bay-regions of PAHs have been developed, L-region selective APEX is yet to be achieved. Herein, we report a stepwise L-region selective APEX of unfunctionalized PAHs by dearomative activation with N-methyltriazoline dione, followed by Pd-catalyzed annulation with aryl Grignard reagents. Various difficult-to-synthesize core-expanded PAHs can be synthesized by L-APEX from unfunctionalized naphthalene, phenanthrene, chrysene, and [4]helicene.
Introduction
Annulative π-extension (APEX)1 reaction, employed to extend a new fused aromatic ring(s) to unfunctionalized aromatics, has attracted attention in recent years as a powerful method for the precise synthesis of polycyclic aromatic compounds and nanographenes (Fig. 1a). The advantageous characteristics of APEX, such as unnecessary prefunctionalized aromatics and direct C–H bond transformations, enable late-stage modification, successive elongation, and diversity-oriented synthesis of nanographenes. In recent years, various research groups, including our group, have developed APEX reactions.2–7 In particular, with regard to the APEX reaction of polycyclic aromatic hydrocarbons (PAHs), the Diels–Alder reactions with alkenes, alkynes, and arynes for the bay-region (concave armchair edge) of perylene derivatives are well-known.2 We have also developed the K-region selective APEX reaction of PAHs using a Pd catalyst,3 and the M-region APEX selective APEX using N-methyl-1,2,4-triazoline-3,5-dione (MTAD) and Fe-catalyzed diarylation.4 In addition, a limited example of fissure-region (zig-zag edge surrounded by two neighboring peri-carbon atoms in naphthalene) selective APEX reactions was recently achieved by a Li(0)-mediated mechanochemical Birch reductive arylation/cyclodehydrogenation sequence.5 However, no L-region (C1–C2 positions of naphthalene)-selective APEX (L-APEX) reaction of PAHs has been developed to date. The development of this missing-piece L-APEX can facilitate a more comprehensive synthesis of a wider variety of nanographenes and will lead to a further step forward in nanographene synthetic chemistry.8Taking L-APEX of naphthalene (1a) to benzo[g]chrysene as an example, the conventional stepwise π-extension seems to be applicable by C1-position selective bromination of 1a, Suzuki–Miyaura coupling with (1,1′-biphenyl)-2-ylboronic acid, and cyclodehydrogenation, so-called Scholl reaction, of 1-([1,1-biphenyl]-2-yl)naphthalene (A) with triflic acid (TfOH) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (Fig. 1b). However, a synthetically fatal problem occurs in the final Scholl reaction step. The Scholl reaction of A with DDQ/TfOH is initiated by protonation, and the obtained arenium cation B predominantly undergoes a 1,2-aryl shift to afford the thermodynamically more stable cation species C Aromatic electrophilic substitution does not give benzo[g]chrysene (L-APEX product), but its isomer M-APEX product, benzo[f]tetraphene, is the major product. This counterintuitive aromatic rearrangement often occurs in the synthesis of nanographenes when Scholl reactions are applied to sterically congested aromatics.9,10 Therefore, the development of L-APEX reactions is highly important in terms of not only filling in missing pieces in APEX chemistry but also providing a complementary method in synthetic chemistry for various nanographenes.
Results and discussion
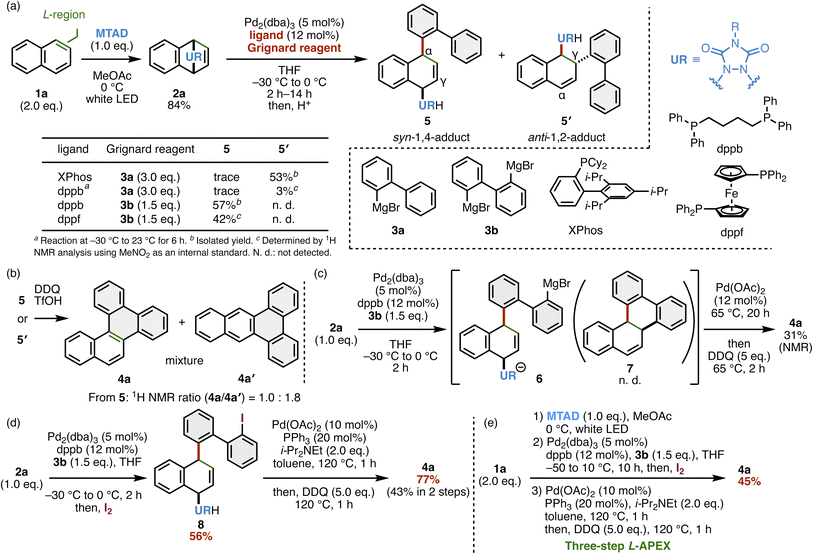
To achieve the unprecedented L-APEX reaction, we considered that Sarlah's work on dearomative functionalization of arenes with MTAD would be valid.11 Indeed, in 2021, he and our group jointly demonstrated the M-APEX reaction of PAHs through (i) regioselective dearomatization of PAHs 1 with MTAD, (ii) Fe-catalyzed diarylation of MTAD-adducts 2 with bis-Grignard reagents 3, and (iii) aromatization by elimination of a bridged urea moiety, affording M-APEX products 4′ (Fig. 1c).4 On one hand, MTAD-adducts 2 possess olefinic moieties, which are suitable for Fe-catalyzed arylation,12 and on the other hand, they possess allylic urea moieties, which are active for various allylic substitution reactions as actively demonstrated by Sarlah and other groups.11,13 As a part of our continuous investigation into APEX chemistry, herein, we report the L-region selective APEX reaction of PAHs by dearomative activation with MTAD, followed by Pd-catalyzed stepwise allylic substitution and aromatization (Fig. 1d). The use of di-Grignard reagents and Pd catalysts enabled intermolecular and intramolecular allylic substitution, affording sterically congested L-region extended products 4.Our initial investigation toward L-APEX is intermolecular α-allylic substitution of naphthalene-MTAD adduct 2a, which was easily obtained by the photoinduced cycloaddition of 1a with MTAD (Fig. 2a). In the previous Sarlah's study, α-allylic substitution of 2a with phenyl Grignard reagent (PhMgBr) was achieved by catalytic amounts of Pd2(dba)3 (dba: dibenzylideneacetone) and DPEphos, affording the syn-1,4-adduct.11a Inspired by this result, we applied a similar catalytic system using Pd2(dba)3, phosphine ligands and biphenyl Grignard reagent 3a. Unlike our expectation to obtain syn-1,4-adduct 5 as a major product, anti-1,2-adduct 5′ was preferentially obtained via γ-allylic substitution even using various phosphine ligands such as XPhos and 1,4-bis(diphenylphosphino)butane (dppb) (see SI for the results using other phosphine ligands). The reaction with 3a in the presence of Pd(dba)2/XPhos gave 5′ in 53% yield, whose structure was elucidated by X-ray diffraction (XRD) analysis of its N-methylated derivative 5′-Me (Fig. S1). Then, the bis-Grignard reagent 3b was used to anticipate changes in the reaction profile and simultaneous cyclization. Interestingly, the use of dppb and 1,1′-bis(diphenylphosphino)ferrocene (dppf) afforded the desired 1,4-adduct 5 in 57% and 42% yields, respectively, through α-allylic substitution. In these reactions, the simultaneous cyclization to afford 7 (Fig. 2c) did not occur. The relative configuration and regioselectivity of 5 were also confirmed by XRD analysis (see Fig. S3). Next, we attempted simple oxidative/acidic cyclization of 5 or 5′ in the presence of DDQ and TfOH14 or FeCl3 (ref. 15) (Fig. 2b). However, both reactions gave a mixture of L-APEX product 4a and M-APEX product 4a′, which implies a 1,2-aryl shift9,10 and undesired cyclization occur in those cationic intermediates. Other examinations of the one-pot annulation of 2a were performed by (i) Pd(0)-catalyzed α-allylic substitution with 3b, (ii) Pd(II)-catalyzed intramolecular γ-allylic substitution of 6 by the remaining aryl magnesium bromide moiety, and (iii) oxidation of the in situ generated precursor 7, yielding 4a in 31% NMR yield (Fig. 2c). While this one-pot procedure is attractive in terms of step economy, the reaction mixture contained various side products; thus, the isolation of 4a was expected to be difficult. To improve the yield of final product 4a, we further examined the reaction conditions and found a stepwise L-APEX via the preparation and isolation of iodoarene 8 by trapping aryl Grignard species 6 with iodine, (ii) Pd(OAc)2/PPh3-catalyzed intramolecular γ-allylic substitution of 8 in a SN2′ fashion, and (iii) oxidation by DDQ (Fig. 2d). This protocol enabled the isolation of 8 and 4a in 56% and 77% yields, respectively, and 43% of the overall yield from 2a was satisfactory. Finally, a one-pot three-step L-APEX sequence from 1a to 4a was demonstrated to afford 4a in 45% isolated yield (Fig. 2e), which is a reasonable result considering the result shown in Fig. 2d.
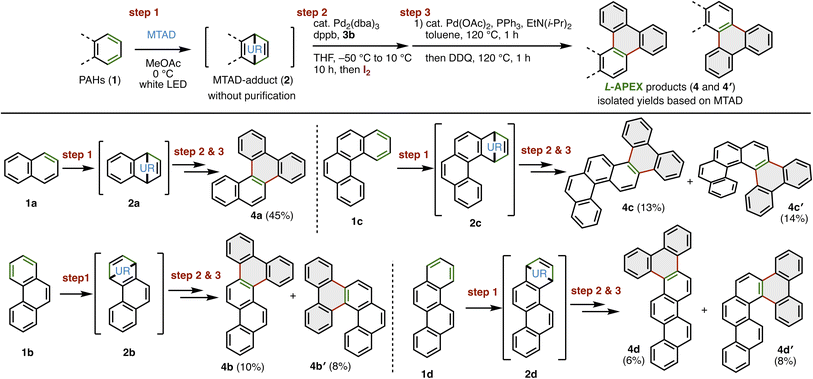
Using the optimized conditions for the three-step L-APEX from naphthalene, we examined the scope of PAHs in this reaction (Fig. 3). In the first step (step 1), the cycloaddition of phenanthrene (1b), benzo[c]phenanthrene (1c), and chrysene (1d) with MTAD gave cycloadducts 2b, 2c, and 2d selectively. Using crude MTAD-adducts without purification, α-allylic arylation/iodination (step 2) and intramolecular γ-allylic arylation/oxidation were successively demonstrated, affording 4a in 45%, benzo[f]picene (4b)/dibenzo[c,g]chrysene (4b′) in 10%/8%, dibenzo[a,j]picene (4c)/benzo[g]naphtho[2,1-c]chrysene (4c′) in 13%/14% and dibenzo[a,c]picene (4d)/benzo[g]naphtho[1,2-c]chrysene (4d′) in 6%/8% isolated yields. In the allylic arylation of 2b, 2c and 2d, nearly no regioselectivity was observed, although they had two sterically different positions. Interestingly, sterically congested helicenes 4b′, 4c′ and 4d′ were also obtained.
Motivated by the presence of K- and L-regions in the L-APEX products 4a and 4b′, we demonstrated further π-extension by APEX reactions. Previously, we achieved M-APEX of 4a to afford 9,4a and K, bay-APEX of 4a to afford 10 (ref. 3e) (Fig. 4a). The newly developed L-APEX reaction was applied to 4a to afford tribenzo[a,c,j]picene (11) in 5% yield. In this reaction, 4a, which can reversibly form the corresponding MTAD-4a adduct, as well as uncyclized intermediates structurally related to 8 and its dehalogenated analogue, were observed during purification, leading to a decreased yield of 11. As another demonstration, pentabenzo[a,c,fg,ij,rst]pentaphene (13) was obtained in overall 10% yield by Pd-catalyzed K-APEX of 4b′ with 2,2′-diiodo-1,1′-biphenyl (12). As often encountered in a previous study,3e these oxidative reaction conditions induced simultaneous cyclodehydrogenation in the fjord-region, giving fully graphitized nanographene 13.
 | ||
| Fig. 4 (a) K, L, M, and bay-APEX of 4a. (b) Further π-extension of 4b′ using Pd-catalyzed K-APEX with diiodobiphenyl 12 and simultaneous cyclodehydrogenation in the fjord-region. | ||
Conclusions
In conclusion, we have developed an untapped L-APEX reaction of unfunctionalized PAHs by dearomative activation with MTAD, followed by Pd-catalyzed one-step or two-step annulation with aryl Grignard reagents. MTAD was selectively attached to the terminal benzo-fusion moieties of PAHs, and the generated allyl urea moieties were arylated by Pd-catalyzed α- and γ-allylic substitutions with bis-Grignard reagents. While the regioselectivity between different L-regions and the total yields was low, this represents the first example of an L-region APEX reaction, and difficult-to-synthesize PAH molecules can be obtained by this method.Author contributions
K. N. mainly synthesized, analyzed, and characterized all the compounds and performed the theoretical calculations. W. M. developed the preliminary experimental results related to this study. H. I. and K. I. designed and directed the study and supervised the experiments. The manuscript was written by H. I., and all authors finalized the manuscript through proofreading. All the authors approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
CCDC 2506075–2506078 (5′, 8, 11 and 5) contain the supplementary crystallographic data for this paper.16a–dSupplementary information (SI): experimental and characterization data, including crystallo-graphic data, and NMR spectra. See DOI: https://doi.org/10.1039/d5sc09309k.
Acknowledgements
This study was supported by the JST-ERATO program (JPMJER1302 to K. I.), JSPS KAKENHI (JP19H05463 and JP25H00429 to K. I., JP21H01931, JP25K01764 and JP25H01408 to H. I.), Tatematsu Foundation (22B025 to H. I.), and Kondo Memorial Foundation (2022-03 to H. I.). We thank Dr Keigo E. Yamada, Dr Hiroki Shudo, Mr Daiki Imoto, Dr Kou P. Kawahara, and Mr Takato Mori (Nagoya University) for their assistance with X-ray diffraction analysis, and Mr Kumpei Hasebe for assistance with MS analysis. The DART-MS measurements were conducted using resources from the Chemical Instrumentation Facility (CIF) of the Research Center for Materials Science (RCMS) at Nagoya University. The computations were performed at the Research Center for Computational Science, Okazaki, Japan (Project No. 23-IMS-C061, 24-IMS-C059, and 25-IMS-C061).Notes and references
- Reviews on APEX reactions: (a) H. Ito, K. Ozaki and K. Itami, Angew. Chem., Int. Ed., 2017, 56, 11144–11148 CrossRef CAS PubMed; (b) H. Ito, Y. Segawa, K. Murakami and K. Itami, J. Am. Chem. Soc., 2019, 141, 3–10 CrossRef CAS PubMed; (c) H. Ito, K. P. Kawahara and K. Itami, Synthesis, 2024, 2024(56), 1335–1354 CrossRef.
- Bay-region selective APEX reaction of PAHs: (a) E. Clar, Ber. Dtsch. Chem. Ges., 1932, 65, 846–853 CrossRef; (b) E. Clar and M. Zander, J. Chem. Soc., 1957, 4616–4619 RSC; (c) E. Clar, Nature, 1948, 161, 238–239 Search PubMed; (d) E. H. Fort, P. M. Donovan and L. T. Scott, J. Am. Chem. Soc., 2009, 131, 16006–16007 CrossRef CAS PubMed; (e) E. H. Fort and L. T. Scott, Angew. Chem., Int. Ed., 2010, 49, 6626–6628 CrossRef CAS PubMed; (f) E. H. Fort and L. T. Scott, J. Mater. Chem., 2011, 21, 1373–1381 RSC; (g) E. H. Fort, M. S. Jeffreys and L. T. Scott, Chem. Commun., 2012, 48, 8102–8104 Search PubMed; (h) E. H. Fort and L. T. Scott, Tetrahedron Lett., 2011, 52, 2051–2054 Search PubMed; (i) H. Hopff and H. R. Schweizer, Helv. Chim. Acta, 1959, 42, 2315–2333 CrossRef CAS; (j) J. Li, C. Jiao, K.-W. Huang and J. Wu, Chem.–Eur. J., 2011, 17, 14672–14680 CrossRef CAS PubMed; (k) A. Konishi, Y. Hirao, K. Matsumoto, H. Kurata and T. Kubo, Chem. Lett., 2013, 42, 592–594 CrossRef CAS; (l) B. Schuler, S. Collazos, L. Gross, G. Meyer, D. Pérez, E. Guitián and D. Peña, Angew. Chem., Int. Ed., 2014, 53, 9004–9006 Search PubMed; (m) T. Nakamuro, K. Kumazawa, H. Ito and K. Itami, Synlett, 2019, 30, 423–428 Search PubMed; (n) N. Zink-Lorre, A. Doncel-Giménez, E. Font-Sanchis, J. Calbo, Á. Sastre-Santos, E. Ortí and F. Fernández-Lázaro, Org. Chem. Front., 2019, 6, 2860–2871 Search PubMed.
- K-region selective APEX reaction of PAHs: (a) K. Ozaki, K. Kawasumi, M. Shibata, H. Ito and K. Itami, Nat. Commun., 2015, 6, 6251 Search PubMed; (b) Y. Yano, H. Ito, Y. Segawa and K. Itami, Synlett, 2016, 27, 2081–2084 Search PubMed; (c) Y. Morinaka, H. Ito, K. J. Fujimoto, T. Yanai, Y. Ono, T. Tanaka and K. Itami, Angew. Chem., Int. Ed., 2024, 63, e202409619 Search PubMed; (d) M. Shibata, H. Ito and K. Itami, J. Am. Chem. Soc., 2018, 140, 2196–2200 CrossRef CAS PubMed; (e) W. Matsuoka, H. Ito and K. Itami, Angew. Chem., Int. Ed., 2017, 56, 12224–12228 Search PubMed; (f) M. Kubo, K. Noguchi and K. Nakano, ChemPlusChem, 2121, 86, 171–175 CrossRef PubMed; (g) A. Swain, K. Radacki, H. Braunschweig and P. Ravat, Chem. Sci., 2024, 15, 11737–11747 Search PubMed; (h) K. Song, Y. Zhang, S. Wu, S. Yang, S. Wei, S. Chen, H. Ito, K. Itami and Y. Li, Org. Chem. Front., 2025, 12, 2225–2231 RSC; (i) Y. Nakashige, H. Nakatsuji, K. Matsushima, K. Murakami, H. Ito and K. Itami, Precis. Chem., 2025, 3, 535–540 Search PubMed.
- M-region selective APEX reaction of PAHs: (a) W. Matsuoka, H. Ito, D. Sarlah and K. Itami, Nat. Commun., 2021, 12, 3940 CrossRef CAS PubMed; (b) W. Matsuoka, K. P. Kawahara, H. Ito, D. Sarlah and K. Itami, J. Am. Chem. Soc., 2023, 145, 658–664 CrossRef CAS PubMed; (c) V. M. Pham and N. Cramer, Angew. Chem., Int. Ed., 2014, 53, 3484–3487 Search PubMed.
- Fissure-region selective APEX reaction of PAHs: (a) Y. Toyama, A. Yagi, K. Itami and H. Ito, Nat. Commun., 2025, 16, 5044 Search PubMed; (b) M. Murata, M. Togo, D. Mishima, A. Harada and M. Muraoka, Org. Lett., 2020, 22, 4160–4163 Search PubMed.
- Other APEX reactions from our group: (a) H. Kitano, W. Matsuoka, H. Ito and K. Itami, Chem. Sci., 2018, 9, 7556–7560 Search PubMed; (b) K. P. Kawahara, W. Matsuoka, H. Ito and K. Itami, Angew. Chem., Int. Ed., 2020, 59, 6383–6388 Search PubMed; (c) K. P. Kawahara, H. Ito and K. Itami, Chem. Commun., 2023, 59, 1157–1160 Search PubMed; (d) K. P. Kawahara, H. Ito and K. Itami, Org. Chem. Front., 2023, 10, 1880–1889 Search PubMed.
- Other related transition metal-catalyzed APEX reactions of simple aromatics: (a) T. Sakakibara, Y. Tanaka and S. Yamaki, Chem. Lett., 1986, 15, 797–800 CrossRef; (b) M. Yamashita, H. Horiguchi, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 7481–7488 CrossRef CAS PubMed; (c) S. Mochida, N. Umeda, K. Hirano, T. Satoh and M. Miura, Chem. Lett., 2010, 39, 744–746 CrossRef CAS; (d) N. Umeda, H. Tsurugi, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2008, 47, 4019–4022 CrossRef CAS PubMed; (e) Y.-T. Wu, K.-H. Huang, C.-C. Shin and T.-C. Wu, Chem.–Eur. J., 2008, 14, 6697–6703 CrossRef CAS PubMed; (f) J. Wu, X. Cui, X. Mi, Y. Li and Y. Wu, Chem. Commun., 2010, 46, 6771–6773 RSC; (g) Z. Shi, C. Tang and N. Jiao, Adv. Synth. Catal., 2012, 354, 2695–2700 Search PubMed; (h) E. Tomita, K. Yamada, Y. Shibata, K. Tanaka, M. Kojima, T. Yoshino and S. Matsunaga, Angew. Chem., Int. Ed., 2020, 59, 10474–10478 CrossRef CAS PubMed; (i) Y. Wu, W. Zhang, Q. Peng, C.-K. Ran, B.-Q. Wang, P. Hu, K.-Q. Zhao, C. Feng and S.-K. Xiang, Org. Lett., 2018, 20, 2278–2281 Search PubMed; (j) Q. Peng, W. Zhang, K. Zhao, Y. Du, C. Feng, B.-Q. Wang, D.-M. Fang, X.-Z. Chen, H.-L. Ni and S.-K. Xiang, Adv. Synth. Catal., 2020, 362, 206–212 CrossRef CAS; (k) K. Zhao, Y. Du, Q. Peng, W.-H. Yu, B.-Q. Wang, C. Feng and S.-K. Xiang, J. Org. Chem., 2021, 86, 2986–2997 CrossRef CAS PubMed; (l) H.-C. Hsiao, P. Annamalai, J. Jayakumar, S.-Y. Sun and S.-C. Chuang, Adv. Synth. Catal., 2021, 363, 1695–1701 CrossRef CAS.
- Reviews on the synthesis of nanographenes: (a) X. Feng, W. Pisula and K. Müllen, Pure Appl. Chem., 2009, 81, 2203–2224 Search PubMed; (b) M. Grzybowski, B. Sadowski, H. Butenschön and D. T. Gryko, Angew. Chem., Int. Ed., 2020, 59, 2998–3027 Search PubMed; (c) A. Narita, Z. Chen, Q. Chen and K. Müllen, Chem. Sci., 2019, 10, 964–975 Search PubMed; (d) J. Wu, W. Pisula and K. Müllen, Chem. Rev., 2007, 107, 718–747 Search PubMed; (e) M. D. Watson, A. Fechtenkötter and K. Müllen, Chem. Rev., 2001, 101, 1267–1300 CrossRef CAS PubMed; (f) Z. Sun, Q. Ye, C. Chi and J. Wu, Chem. Soc. Rev., 2012, 41, 7857–7889 RSC; (g) C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2012, 112, 2208–2267 Search PubMed; (h) A. Facchetti, Chem. Mater., 2011, 23, 733–758 CrossRef CAS; (i) R. Rieger and K. Müllen, J. Phys. Org. Chem., 2010, 23, 315–325 CrossRef CAS; (j) Y. Segawa, H. Ito and K. Itami, Nat. Rev. Mater., 2016, 1, 15002 CrossRef CAS.
- S. L. Skraba-Joiner, E. C. Mclaughlin, A. Ajaz, R. Thamatam and R. P. Johnson, J. Org. Chem., 2015, 80, 9578–9583 CrossRef CAS PubMed.
- Review on rearrangements in the Scholl reaction: N. Ponugoti and V. Parthasarathy, Chem.–Eur. J., 2022, 28, e202103530 CrossRef CAS PubMed.
- (a) C. Tang, M. Okumura, Y. Zhu, A. R. Hooper, Y. Zhou, Y.-H. Lee and D. Sarlah, Angew. Chem., Int. Ed., 2019, 58, 10245–10249 CrossRef CAS PubMed; (b) C. Tang, M. Okumura, H. Deng and D. Sarlah, Angew. Chem., Int. Ed., 2019, 58, 15762–15766 Search PubMed; (c) W. C. Wertjes, M. Okumura and D. Sarlah, J. Am. Chem. Soc., 2019, 141, 163–167 CrossRef CAS PubMed; (d) M. Okumura, A. S. Shved and D. Sarlah, J. Am. Chem. Soc., 2017, 139, 17787–17790 Search PubMed.
- (a) A. Matsumoto, L. Illies and E. Nakamura, J. Am. Chem. Soc., 2011, 133, 6557–6561 Search PubMed; (b) Y. Sun, H. Tang, K. Chen, L. Hu, J. Yao, S. Shaik and H. Chen, J. Am. Chem. Soc., 2016, 138, 3715–3724 Search PubMed.
- Representative reviews and recent examples on palladium-catalyzed allylic substitutions: (a) B. M. Trost and D. L. Van Vranken, Chem. Rev., 1996, 96, 395–422 CrossRef CAS PubMed; (b) O. Pàmies, J. Margalef, S. Cañellas, J. James, E. Judge, P. J. Guiry, C. Moberg, J.-E. Bäckvall, A. Pfaltz, M. A. Pericàs and M. Diéguez, Chem. Rev., 2021, 121, 4373–4505 Search PubMed; (c) K. Ikeda, R. Kojima, K. Kawai, T. Murakami, T. Kikuchi, M. Kojima, T. Yoshino and S. Matsunaga, J. Am. Chem. Soc., 2023, 145, 9326–9333 CrossRef CAS PubMed.
- L. Zhai, R. Shukla and R. Rathore, Org. Lett., 2009, 11, 3474–3477 CrossRef CAS PubMed.
- (a) R. Dorel, C. Manzano, M. Grisolia, W. Soe, C. Joachim and A. M. Echavarren, Chem. Commun., 2015, 51, 6932–6935 RSC; (b) L. Zhai, R. Shukla, S. H. Wadumethrige and R. Rathore, J. Org. Chem., 2010, 75, 4748–4756 Search PubMed.
- (a) CCDC 2506075: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2q3s4s; (b) CCDC 2506076: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2q3s5t; (c) CCDC 2506077: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2q3s6v; (d) CCDC 2506078: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2q3s7w.
| This journal is © The Royal Society of Chemistry 2026 |