Open Access Article
Open Access ArticleRecent advances and design strategies of cathode materials for aqueous aluminum-ion batteries
Jiayou
Feng
,
Shuimei
Chen
,
Yuzheng
Wu
,
Xiaodan
Huang
 * and
Chengzhong
Yu
* and
Chengzhong
Yu
 *
*
Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, QLD 4072, Australia. E-mail: x.huang@uq.edu.au; c.yu@uq.edu.au
First published on 30th December 2025
Abstract
The growing global energy demand stimulates the urgent need for safe, sustainable, and cost-effective energy storage technologies. Aqueous aluminum-ion batteries (AAIBs) have emerged as promising candidates owing to their abundant resources, intrinsic safety, environmental compatibility, and high capacity. AAIB research has seen rapid development, focusing on high-capacity cathodes compatible with Al anodes. The high charge density of trivalent Al3+ induces strong electrostatic interactions with the cathode, impeding ion transport, causing structural distortion, and accelerating capacity decay, while side reactions in aqueous electrolytes further limit reversibility and stability. Addressing these challenges requires a mechanistic understanding of how the cathode composition and structure regulate Al3+–cathode interactions and redox behavior. This review analyzes the fundamental Al3+ storage mechanisms in AAIBs, emphasizing the influence of cathode chemistry, structural characteristics, and electrolyte environment on ion transport, Al3+–cathode interactions and electrochemical performance. Furthermore, representative cathodes, including manganese-based oxides, vanadium-based compounds, Prussian blue analogues and organic cathode materials, are systematically summarized in terms of structure, performance, and optimization strategies. Finally, the review outlines current challenges and prospective research directions for advancing next-generation high-performance AAIB cathodes. This review is expected to provide valuable insights for guiding cathode material design and inspire future strategies to enhance the capacity, stability, and rate performance of AAIBs.
1. Introduction
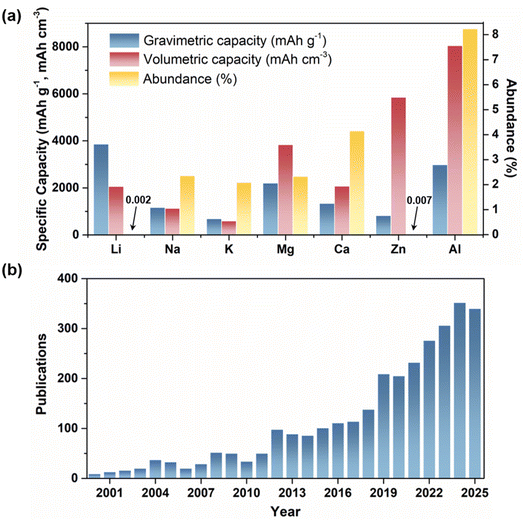
The fast-growing global energy consumption has intensified the demand for sustainable and efficient energy storage, driving the advancement of next-generation electrochemical storage technologies. In recent decades, lithium-ion batteries (LIBs) have dominated portable electronics, electric vehicles, and grid-scale applications, owing to their high energy density and long cycle life.1–3 However, their further advancement is hindered by intrinsic limitations: scarce lithium resources increase cost and supply risks, while flammable and toxic organic carbonate electrolytes pose serious safety hazards such as leakage, combustion, and even explosion.4,5 Moreover, repeated lithium plating and stripping also induce dendrite formation, which can trigger internal short circuits and thermal runaway.6–8 These issues highlight the urgent need for the development of next-generation electrochemical storage systems that offer enhanced safety, environmental friendliness, and cost-effectiveness.In response to these challenges, researchers have turned their attention to post-lithium-ion batteries based on abundant elements such as sodium (Na), potassium (K), zinc (Zn), magnesium (Mg), calcium (Ca), and aluminum (Al).9–16 Among these, aluminum has attracted particular attention as the most abundant metal in the Earth's crust (∼8.2 wt%) and for its low cost (Fig. 1a).17 Furthermore, aluminum can reversibly transfer three electrons per atom, yielding a high theoretical gravimetric capacity of ∼2980 mAh g−1 and a volumetric capacity of ∼8046 mAh cm−3, which is approximately four times that of lithium metal.18 These attributes position aluminum as a promising candidate for the development of high-capacity and cost-effective energy storage systems. Aluminum-ion batteries (AIBs) are typically categorized into two types based on the electrolyte: organic and aqueous systems.19,20 Organic AIBs generally utilize ionic liquids as electrolytes, whereas aqueous AIBs (AAIBs) employ aqueous solutions of aluminum salts. Compared to organic systems, aqueous electrolytes offer several advantages, including non-toxicity, low cost, and non-flammability, thereby ensuring enhanced safety and stability.21,22 Additionally, their low viscosity and high dielectric constant contribute to high ionic conductivity, improving electrochemical kinetics.18 Consequently, AAIBs have attracted widespread attention as a safe, low-cost, and high-performance energy storage technology, with a rapidly increasing number of publications in recent decades (Fig. 1b).
Nevertheless, AAIBs still face several scientific and technical challenges. Although trivalent Al3+ offers a high theoretical capacity, its small ionic radius and high charge density (364C mm−3) lead to strong electrostatic interactions within the cathode matrix.18,23–25 These interactions hinder ion migration and charge transfer, limit the reversible insertion/extraction of Al3+, and induce structural distortion and collapse during cycling, resulting in rapid capacity decay. Additionally, side reactions in aqueous electrolytes, such as hydrogen evolution, anode corrosion, and the formation of passivating oxide layers, further deteriorate coulombic efficiency and cycling stability.26,27 These issues highlight that one of the key directions for advancing AAIBs lies in designing cathode materials capable of regulating Al3+–host interactions to achieve reversible ion storage with minimal structural deformation. A mechanistic understanding of how the cathode structure and chemical characteristics govern Al3+ accessibility, migration kinetics, and redox reversibility is therefore essential for guiding the rational development of high-capacity, high-rate and high-stability cathode systems. To date, most published reviews on AAIBs have primarily provided general overviews of recent progress and challenges in cathodes, anodes, and electrolytes.21,28–30 However, comprehensive reviews dedicated to AAIBs cathode materials remain scarce, and systematic comparisons of their structural characteristics, energy storage mechanisms, and electrochemical behaviors are still lacking.
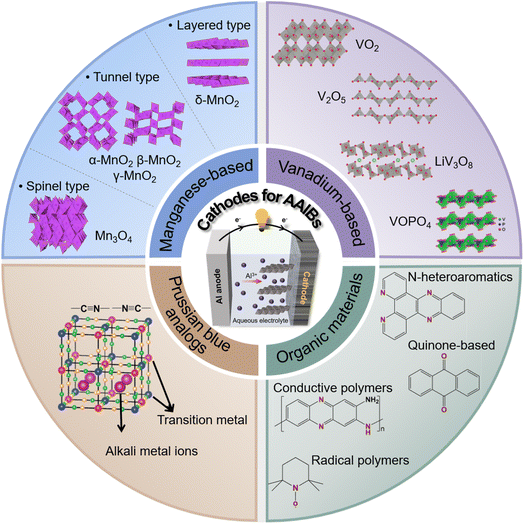
Therefore, this review presents a systematic analysis of cathode materials for AAIBs. We first outline the fundamental energy storage mechanisms, with particular emphasis on how the chemical nature and structural characteristics of the cathode, along with the electrolyte environment, govern Al3+ transport behavior and host–guest interactions, thereby determining the electrochemical performance in terms of capacity, reversibility, rate capability, and cycling stability. Subsequently, we categorize representative cathodes into four categories: manganese-based oxides, vanadium-based compounds, Prussian blue analogues, and organic materials (Fig. 2), and discuss their structural features, corresponding electrochemical behaviors and optimization strategies. By comparing their distinct Al3+–host interactions mechanisms and structure–performance relationships, this review aims to provide comprehensive scientific guidance for the rational design and future development of high-performance cathode materials for AAIBs. Finally, the challenges and promising research directions for each cathode category are summarized to inspire further progress in this emerging field.
2. Energy storage mechanisms of AAIBs
AAIBs are typically composed of a cathode, an anode, electrolyte and a separator. The cathode materials are primarily transition-metal oxides or organic compounds, while aluminum metal or aluminum alloys serve as the anode. Glass fiber is commonly used as the separator, and the electrolyte usually consists of aqueous solutions containing aluminum salts such as Al(OTf)3 or Al2(SO4)3.During the charge–discharge process, reversible redox reactions occur at both electrodes. The Al3+ ions in solvated form (denoted as Al3+ for simplicity) serve as charge carriers that migrate through the electrolyte, while electrons are transferred in the external circuit between the anode and cathode. Specifically, during discharge, the aluminum anode undergoes oxidation, releasing three electrons to form Al3+ (eqn (1)). The Al3+ ions migrate toward the cathode through the electrolyte, whereas the released electrons travel through the external circuit to reach the cathode. The active cathode material in the oxidation form (Cathode[ox]) gains electrons and undergoes transformation into the reduced form (Cathode[red]), accompanied by host–guest interaction between the incoming Al3+ species and the reduced cathode material (eqn (2)). In the subsequent charging process, this reaction reverses: Al3+ ions are extracted from the cathode, migrate back to the anode, and are reduced to metallic aluminum, thereby completing a reversible charge–discharge cycle.
Anode:
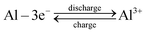 | (1) |
Cathode:
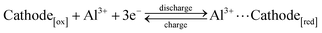 | (2) |
Overall:
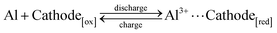 | (3) |
2.1 Factors affecting the electrochemical mechanism
The energy storage mechanism of AAIBs is related to the reaction shown in eqn (3), i.e., the host–guest interactions between Al3+ and the cathode active species, the reversibility of reaction 3 in the charge–discharge process, and the possible occurrence of side reactions. These factors are ultimately dictated by the structural and chemical characteristics of the cathode material and the electrolyte environment, including the anion salt type, concentration and pH, which in turn determine the key electrochemical performance metrics of AAIBs.Firstly, the chemical nature of the cathode material is a key factor governing the energy storage mechanism of AAIBs. The type of electroactive center not only determines the redox reversibility but also influences the type and strength of interaction with Al3+. For inorganic transition-metal oxide cathodes, specific redox couples control both electron-transfer reversibility and reaction kinetics, depending on the electronic structure of the metal centers. Transition metals with partially filled 3d orbitals enable reversible redox reactions, such as Mn (Mn2+/Mn3+/Mn4+) and V (V3+/V4+/V5+) oxides. Their tunable valence ranges allow multiple electron transfers with minimal structural distortion, making them the most widely studied inorganic cathode materials.31–33 Nevertheless, strong coulombic interactions between Al3+ and oxygen in the metal oxide can induce lattice stress or even structural collapse during repeated Al3+ extraction/insertion, thereby limiting the rate capability and cycling stability. For organic cathodes, the redox reversibility primarily depends on the intrinsic reversibility of the functional groups, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–OH. Functional groups within conjugated π-systems or capable of stabilizing negative charges or radical intermediates tend to exhibit higher reversibility.34,35 During discharge, Al3+ coordinates with these negatively charged sites to maintain charge neutrality, so the stability of Al3+-functional group interaction also dictates the redox reversibility. Compared with inorganic cathodes, organic cathodes exhibit weaker Al3+ coordination, leading to less structural distortion during ion transport and consequently superior rate capability and cycling stability.
O/C–OH. Functional groups within conjugated π-systems or capable of stabilizing negative charges or radical intermediates tend to exhibit higher reversibility.34,35 During discharge, Al3+ coordinates with these negatively charged sites to maintain charge neutrality, so the stability of Al3+-functional group interaction also dictates the redox reversibility. Compared with inorganic cathodes, organic cathodes exhibit weaker Al3+ coordination, leading to less structural distortion during ion transport and consequently superior rate capability and cycling stability.
Secondly, the structural characteristics of the cathode material also affect the energy storage mechanism of AAIBs. Parameters such as interlayer spacing, cavity size, and the exposure of active sites directly determine Al3+ accessibility and diffusion kinetics. Cathodes featuring larger interlayer spacing or more open frameworks offer wider migration channels, thereby facilitating faster Al3+ transport and enhancing redox reactions during charge and discharge. For inorganic transition-metal oxide cathodes, layered structures generally provide larger diffusion pathways for Al3+ compared with tunnel-type frameworks, resulting in improved ion transport kinetics. However, the relatively weak structural rigidity of layered materials makes them prone to lattice strain or even collapse upon repeated Al3+ insertion and extraction. Therefore, achieving a balance between sufficient diffusion space and structural stability remains a critical challenge. For organic cathodes, the highly exposed active centers facilitate ion accessibility, while the flexible molecular backbones can effectively accommodate volume changes, thereby mitigating structural stress during cycling. As a result, despite relatively lower specific capacity, organic cathodes generally exhibit superior structural stability and rate performance compared with their rigid inorganic counterparts.
In addition, the electrolyte environment, including anion salt type, concentration and pH, also significantly affects the charge–discharge behavior and energy storage mechanisms of AAIBs. These factors determine the solvation structure of Al3+ and the likelihood of side reactions. Typically, in highly concentrated electrolytes, the proportion of free water is reduced. This suppresses water activity, mitigates the hydrogen evolution reaction (HER), and facilitates the reversible deposition of Al on the anode.36 The type of anion (e.g., Cl−, OTf−, and SO42−) also regulates the coordination environment of Al3+. For instance, CF3SO3− (OTf−) can reduce the number of water molecules surrounding the cation, thereby promoting ion transport and charge transfer, making it a commonly used salt in aqueous batteries.37 Moreover, the electrolyte pH also influences the insertion behavior of cations. Adjusting the pH allows selective regulation of Al3+ and H+ insertion during the electrochemical process, with H+ insertion typically occurring under relatively low pH conditions.38 Sometimes, the introduction of co-solvents such as acetonitrile (AN) and triethyl phosphate (TEP) into a hydrated eutectic Al3+ electrolyte can reduce water activity, suppress side reactions like the HER and corrosion, and widen the electrochemical stability window, thereby enhancing the stability and reversibility of AAIBs.39
2.2 Impact on electrochemical performance
The aforementioned chemical and structural characteristics of the cathode, together with the electrolyte environment, influence the redox behavior and host–guest interactions with Al3+, ultimately affecting the overall electrochemical performance of AAIBs, including capacity performances and the cycling stability.The capacity performances, including rate capacities, are fundamentally dictated by the redox activity and ion transport dynamics within the cathodes. The theoretical specific capacity is primarily determined by the number of electrons involved in redox reactions, governed by the valence changes of metal centers in inorganic cathodes and by the number of redox-active functional groups in organic cathodes. The achievable capacity depends on the structural accessibility of Al3+ ions and the efficiency of redox reactions. Inorganic cathode materials with larger interlayer spacing or more open frameworks can better accommodate Al3+, providing wider diffusion channels and sufficient storage sites, thereby enabling higher capacity. Generally, layered structures are more favorable for Al3+ accommodation than tunnel-type frameworks, leading to superior capacity performance. For organic cathodes, flexible backbones or porous structures can facilitate Al3+ diffusion, while densely packed molecules often exhibit lower practical capacities due to limited ion accessibility. The rate capability is largely determined by the diffusion kinetics of Al3+ within the cathode and the efficiency of charge transport. Slow ion diffusion limits charge transfer per unit time, resulting in significant capacity decay at high current densities. For inorganic cathodes, constructing materials with high specific surface area and abundant defects can accelerate ion transport and enhance reaction kinetics. Additionally, hybridization with conductive carbon improves electronic conductivity, further supporting high-rate performance. For organic cathodes, highly exposed active sites facilitate rapid Al3+ access and diffusion, promoting excellent rate capability.
For cycling stability, both the intrinsic redox reversibility of the cathode and the strength of Al3+–host interactions play decisive roles. In inorganic metal oxide cathodes, the high charge density of Al3+ leads to strong electrostatic interactions with the lattice, which hinders ion diffusion and induces lattice strain, phase transitions, or even partial dissolution, ultimately reducing reaction reversibility and leading to structural collapse and capacity fading. To mitigate this, introducing stabilizing species, such as metal cations or organic molecules, has been shown to weaken these electrostatic interactions and tune the host–guest coupling strength to a moderate level. This strategy effectively buffers volumetric changes, suppresses structural degradation and maintains framework integrity, thereby enhancing both redox reversibility and cycling stability. In contrast, the interactions between Al3+ and organic cathodes are generally mild, making the redox reversibility primarily depend on the intrinsic reversibility of the functional groups. Functional groups within conjugated π-systems or capable of stabilizing negative charges or radical intermediates tend to exhibit good reversibility. Moreover, the flexible molecular backbones of organic cathodes can effectively accommodate volumetric changes during Al3+ interaction, helping to preserve framework integrity and enhance cycling stability. Additionally, dissolution of cathode materials leads to active material loss and structural degradation, thereby accelerating capacity fading and shortening cycling life.
Collectively, the intrinsic redox behavior of the cathode active centers and the host–guest interactions between Al3+ and the cathode material jointly determine the key electrochemical parameters of AAIBs, including specific capacity, rate capability, reversibility, and cycling stability. Therefore, a rational selection of cathode materials and modulation of Al3+–host interactions are of great significance for the design of next-generation high-performance cathode materials for AAIBs.
3. Cathode materials for AAIBs
3.1 Manganese-based cathode materials
Manganese oxides have attracted intensive attention as cathode materials for energy storage, primarily due to their abundant natural reserves, low cost and nontoxicity.31,40 The multiple oxidation states of manganese (Mn2+/Mn3+/Mn4+/Mn7+) support multi-electron transfer reactions, thereby enabling a high theoretical specific capacity of 616 mAh g−1 (based on the Mn4+/Mn2+ redox couple). In addition, this redox flexibility leads to the formation of a variety of manganese oxides with distinct structures (Fig. 3). MnO2 and Mn2O3 are generally composed of edge- or corner-sharing [MnO6] octahedra, while MnO adopts a face-centered cubic structure, and Mn3O4 is considered a mixture of Mn2O3 and MnO.41,42 Notably, MnO2 exhibits diverse phases, including tunnel-type (e.g., α-MnO2, β-MnO2, R-MnO2, T-MnO2, and γ-MnO2), layered-type (e.g., δ-MnO2, birnessite) and three-dimensional spinel-type (e.g., ε-MnO2). This structural diversity endows Mn-based oxides with tunability in Al3+ storage behavior, making them highly attractive cathode materials for AAIBs, with ongoing efforts focused on elucidating their intercalation mechanisms and enhancing electrochemical performance through structural engineering.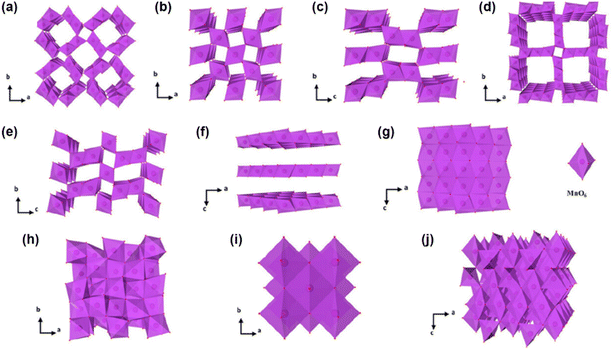 | ||
| Fig. 3 Crystal structures of (a) α-MnO2, (b) β-MnO2, (c) R-MnO2, (d) T-MnO2, (e) γ-MnO2, (f) δ-MnO2, (g) ε-MnO2, (h) Mn2O3, (i) MnO, and (j) Mn3O4. Reproduced with permission.41 Copyright 2024, American Chemical Society. | ||
The electrochemical stability and ion transport properties of manganese oxides are strongly dependent on their crystal structure.43 Among tunnel-type MnO2, β-MnO2 possesses the narrowest channels (1 × 1, 2.3 × 2.3 Å2) and is considered the most thermodynamically stable.41 However, its narrow tunnels severely limit ion diffusion, resulting in poor electrochemical performance. To date, no studies have reported its application as a cathode in AAIBs. In contrast, α-MnO2 features larger 2 × 2 (4.6 × 4.6 Å2) tunnels, which facilitate ion transport and accommodate larger ionic species, making it the most widely investigated tunnel-type MnO2 for AAIB cathodes. γ-MnO2, with mixed 1 × 1 (2.3 × 2.3 Å2) and 1 × 2 (2.3 × 4.6 Å2) tunnels, exhibits intermediate structural openness, and some studies have focused on defect engineering to improve its ion diffusion capabilities. Layered-type δ-MnO2 offers a larger interlayer spacing (∼0.7 nm), significantly exceeding the tunnel sizes of other MnO2. This enhanced ion-accessible volume and facilitates ion intercalation, and it has thus attracted considerable interest as a high-capacity cathode material.44 In the following sections, we will systematically discuss the electrochemical behavior of different manganese oxides in AAIBs.
Given the intrinsic limitations of pristine α-MnO2, such as sluggish Al3+ transport within the narrow tunnels and structural instability arising from repeated Al3+–cathode interactions, subsequent studies have focused on structural optimization strategies, such as introducing defects and expanding tunnel dimensions, to improve ionic accessibility and electrochemical performance. In 2024, Li et al. developed α-MnO2 with oxygen vacancies (denoted as Od-MnO2) by calcination under an argon atmosphere and employed it as a cathode material for AAIBs.46 The introduction of oxygen vacancies facilitated the opening of the microstructure, enhancing electrolyte accessibility and accelerating electrochemical reaction kinetics. As a result, the Od-MnO2 cathode exhibited improved rate performance and cycling stability. In 5 M Al(OTf)3 electrolyte, it delivered a discharge plateau at ∼1.5 V and a high specific capacity of 480 mAh g−1 at a current density of 100 mA g−1. Compared to pristine α-MnO2, Od-MnO2 retained 100 mAh g−1 after 180 cycles at 500 mA g−1, whereas the capacity of α-MnO2 rapidly decayed within 40 cycles (Fig. 4a). This enhancement was attributed to the presence of oxygen vacancies, which provided structural flexibility to accommodate volume changes during cycling, thereby improving stability. Therefore, oxygen vacancies can enhance the electrochemical performance of α-MnO2 by improving ion/electron transport and increasing structural flexibility, making it an effective strategy for MnO2 optimization in AAIBs.
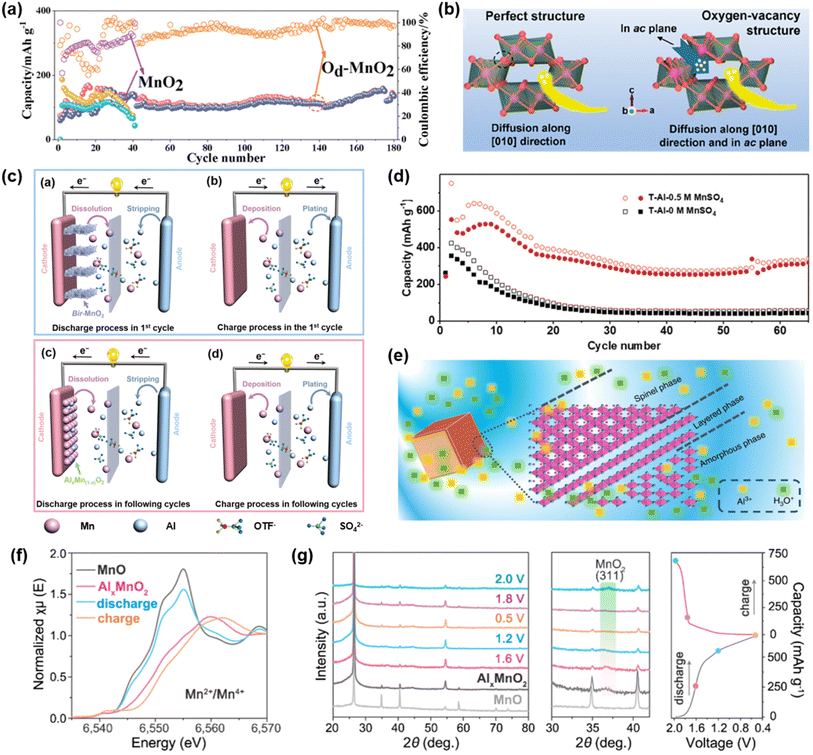 | ||
| Fig. 4 (a) Cycling performance of Od-MnO2 and MnO2 at 500 mA g−1. Reproduced with permission.46 Copyright 2024, Wiley-VCH. (b) Diffusion of H+ into the perfect structure of MnO2 and the structure of MnO2 with an oxygen vacancy. Reproduced with permission.47 Copyright 2023, American Chemical Society. (c) The schematic diagram of aluminum–manganese electrochemistry in the first discharge process, the first charge process, and the discharge process and charge process in the following cycles. (d) Cycling performance of T-Al/δ-MnO2 and T-Al/0.5Mn/δ-MnO2 batteries. Reproduced with permission.48 Copyright 2019, Wiley-VCH. (e) The schematic profile of the structure of AlxMnO2·nH2O. Reproduced with permission.52 Copyright 2019, Springer Nature. (f) Mn K-edge XANES spectra of AlxMnO2 cathodes after full discharge and charge. (g) XRD patterns of AlxMnO2 cathodes at selected states during the first cycle and typical charge/discharge curves at 100 mA g−1. Reproduced with permission.53 Copyright 2020, American Chemical Society. | ||
γ-MnO2 features a mixed tunnel structure composed of 1 × 1 and 1 × 2 channels, which are narrower than the tunnels of α-MnO2 and thus less favorable for Al3+ intercalation. To overcome this limitation, γ-MnO2 enriched with oxygen vacancies (denoted as Ov-MnO2) was synthesized via annealing under an argon atmosphere.47 The introduction of oxygen vacancies can open the MnO6 octahedral framework, generating additional ion diffusion pathways and extending the proton diffusion path along the a–c plane, thereby enhancing the overall capacity (Fig. 4b). In 5 M Al(OTf)3 electrolyte, Ov-MnO2 delivered a high discharge capacity of 481.9 mAh g−1 at 200 mA g−1 and maintained 128.6 mAh g−1 after 200 cycles at 0.4 A g−1. To the best of our knowledge, β-MnO2, which possesses only 1 × 1 tunnel structures, has not yet been explored as a cathode material for AAIBs, likely due to its severely limited channel size that restricts Al3+ intercalation.
Overall, the ion storage capacity of tunnel-type MnO2 is strongly influenced by tunnel size. Narrow tunnels, particularly in β- and γ-MnO2, limit the intercalation of bulky multivalent ions such as Al3+, while α-MnO2 shows appreciable Al3+ storage capacity. To address the inherent diffusion constraints in these tunnel-structured MnO2, introducing oxygen vacancies has emerged as an effective strategy to expand ion diffusion pathways and enhance the electrochemical performance of these materials.
In 2019, Yu et al. employed δ-MnO2 as the cathode material for AAIBs.48 During the initial discharge, δ-MnO2 underwent a conversion reaction, in which Mn4+ was reduced and dissolved in the electrolyte as Mn2+. Upon subsequent charging, the dissolved Mn2+ was oxidized and combined with Al3+ to form amorphous AlxMn1−xO2, which then served as the active material for the following charge/discharge cycles (Fig. 4c). The resulting TAl/2 M Al(OTf)3/δ-MnO2 coin cell delivered a capacity of approximately 350 mAh g−1 at 100 mA g−1. Moreover, increasing the Mn2+ concentration in the electrolyte was shown to facilitate the formation of AlxMn1−xO2 and mitigate further Mn dissolution. By introducing 0.5 M MnSO4, the modified cell exhibited a significantly enhanced capacity of 554 mAh g−1 with a discharge plateau at around 1.35 V and retained 320 mAh g−1 after 65 cycles, demonstrating improved cycling stability (Fig. 4d). These results highlight the critical role of Mn2+ in stabilizing the cathode structure and enhancing the electrochemical performance of δ-MnO2-based AAIBs. In a separate study, δ-MnO2 paired with a Pt-sputtered Al anode in a 5 M Al(OTf)3 electrolyte also delivered a high capacity of 452 mAh g−1 at 50 mA g−1 without any Mn2+ supplementation.49
Although layered δ-MnO2 possesses a large interlayer spacing favorable for Al3+ transport, its poor structural stability leads to rapid capacity decay during cycling. In the initial charge/discharge process, δ-MnO2 undergoes an irreversible electrochemically induced structural rearrangement with Al3+ to form a relatively more stable layered amorphous AlxMnO2 phase, which subsequently serves as the active material during cycling and mitigates the excessively strong Al3+–MnO2 interaction in subsequent cycles.48,50 Inspired by this electrochemical conversion, direct pre-intercalation of Al3+ has been employed to construct relatively stable layered manganese oxides. For instance, Ran et al. synthesized layered AlxMnO2·nH2O cathodes via a modified hydrothermal method, which, when coupled with an Al–Cu alloy anode in a 2 M Al(OTf)3 electrolyte, delivered an initial discharge capacity of approximately 400 mAh g−1 at 500 mA g−1, with 83% capacity retention after 400 cycles.51
Similar conversion behavior to layered AlxMnO2 during the first charge–discharge process has also been observed in other Mn oxide cathodes. In 2019, Lu et al. reported that Mn3O4 could be electrochemically converted into a layered AlxMnO2·nH2O structure, resulting in a significant enhancement in capacity.52 During the initial charging process, a portion of Mn3+ in octahedral sites was oxidized to Mn4+, while Mn2+ in tetrahedral sites and the remaining Mn3+ in octahedral sites dissolved into the electrolyte, triggering the formation of a layered phase. The dissolved Mn species subsequently reassembled along the edges of the layered domains in the Al(OTf)3 electrolyte, forming amorphous regions (Fig. 4e). As a result, the obtained AlxMnO2·nH2O exhibits a mixed-phase structure and delivers a high discharge capacity of 467 mAh g−1 at a current density of 30 mA g−1, with a distinct discharge plateau at around 1.2 V and a retained capacity of 272 mAh g−1 after 60 cycles. The superior capacity of AlxMnO2·nH2O arises from its layered structure, which enables easier Al3+ diffusion, and the presence of crystal water, which reduces electrostatic interactions between the Al3+ and the host anions. Similarly, in 2020, Yan et al. employed MnO as a precursor, which was electrochemically converted into a layered AlxMnO2 phase during the initial charge.53 The resulting Zn–Al/2 M Al(OTf)3/AlxMnO2 coin cell exhibited a high discharge plateau (∼1.6 V), a stable reversible capacity (460 mAh g−1 after 80 cycles at 100 mA g−1), and excellent rate performance (100 mAh g−1 at 3 A g−1). Ex situ XANES and XRD analyses confirmed the reversible Mn4+/Mn2+ redox behavior and structural evolution during interaction between Al3+ and the host lattice, providing solid evidence for a reversible Al3+ storage mechanism in MnO-derived AlxMnO2 (Fig. 4f and g).
In summary, current studies indicate that manganese oxide materials with layered structures offer advantages over tunnel-type structures by providing more space for Al3+ storage. However, these layered phases are often structurally unstable and undergo a transformation into more stable layered AlxMnO2 phases during the initial charge/discharge process, facilitated by the pre-incorporation of Al3+ within the host lattice. Despite these advancements, no systematic comparisons have been conducted to determine which type of manganese oxide converts more effectively into AlxMnO2 and delivers better electrochemical performance. The extent of this transformation, including the degree of interaction between Al3+ and the cathode material and the ratio between the newly formed AlxMnO2 phase and the residual parent phase, remains unclear. In addition, the atomic ratio of Al to Mn in the AlxMnO2 phase, which could reveal the quantitative level of Al3+ participation in the structure, has yet to be clearly established.
Jiang et al. reported a layered manganese oxide cathode pre-intercalated with benzoquinone-coordinated Al3+ (BQ-AlxMnO2) (Fig. 5a).55 In this design, organic benzoquinone (BQ) molecules coordinate with Al3+ to form BQ-Al3+ complexes, which are subsequently introduced between MnO2 layers. This coordination lowers the effective charge of Al3+, weakening its electrostatic interaction with the host lattice and facilitating reversible Al3+–host intercalation. Simultaneously, the co-intercalation of BQ-Al3+ complexes expands the interlayer spacing of MnO2, further improving ion transport and structural stability. As a result, the BQ-AlxMnO2 cathode, paired with a Zn50Al50 alloy anode and a mixed electrolyte of 2 M Al(OTf)3 and 0.2 M Mn(OTf)2, exhibited outstanding electrochemical performance. The assembled coin cell delivered a discharge capacity of 300 mAh g−1 at a current density of 1 A g−1 and maintained ∼99% of its capacity after over 800 cycles, demonstrating both high capacity and cycling stability. In addition to organic molecule intercalation, cation pre-intercalation has also proven effective in stabilizing layered MnO2 frameworks and enhancing electrochemical kinetics. For instance, Cu2+ pre-intercalated δ-MnO2 synthesized via a simple hydrothermal method exhibited a high discharge capacity of 363.4 mAh g−1 at 400 mA g−1 and retained 164.5 mAh g−1 of its capacity after 300 cycles.56 The incorporated Cu2+ increased the electronegativity of lattice oxygen, moderated the strong electrostatic interaction with Al3+ and optimized H+ adsorption energy, thereby enhancing proton participation and maintaining the layered structure.
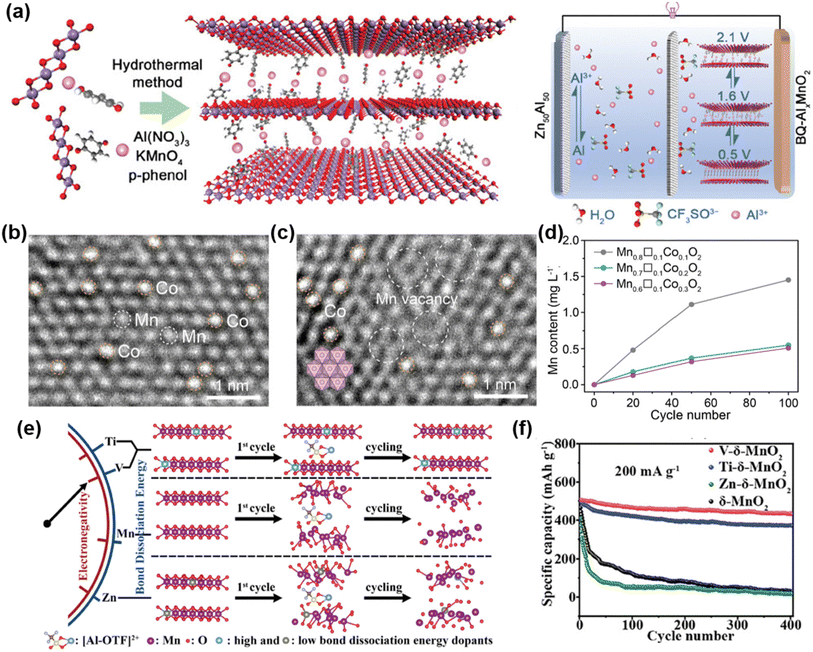 | ||
| Fig. 5 (a) Schematic illustration of preparation of layered BQ-AlxMnO2 by a hydrothermal method, during which highly coordinated BQ-Al3+ pre-intercalated in MnO2 layers, and the scheme of an aqueous AIB full cell that is composed of a Zn50Al50 alloy anode and titanium foil-supported BQ-AlxMnO2 cathode, with 2 M Al(OTf)3 solution as aqueous electrolyte. Reproduced with permission.55 Copyright 2024, Wiley-VCH. Atomic-resolution HAADF-STEM images of (b) Mn0.8Co0.2O2 and (c) acid-coagulated Mn0.7□0.1Co0.2O2. Inset: the hexagonal arrangement of Mn atoms in ideal MnO2 sheets. (d) ICP-AES measurement of dissolved manganese in 2 M Al(OTF)3 electrolyte after different numbers of cycles. Reproduced with permission.59 Copyright 2023, Wiley-VCH. (e) Schematic illustration of the impact of heteroatom doping on the electrochemical stability and performance of δ-MnO2 cathodes during AAIB cycling. (f) Electrochemical cycling performances of pristine and doped δ-MnO2 cathodes at 200 mA g−1. Reproduced with permission.60 Copyright 2024, Wiley-VCH. | ||
Metal cation substitution, especially with aliovalent ions, can simultaneously tailor the host structure and introduce charge carriers (holes or electrons) to maintain charge neutrality, thereby enhancing the structural stability of the cathode material.57,58 Building on this concept, Geng et al. developed a defective cobalt-substituted layered manganese oxide (δ-MnO2) as a cathode material for AAIBs (Fig. 5b and c).59 The synergistic effect between the incorporated Co atoms and Mn vacancies improved the electrical conductivity, facilitated Al3+ diffusion, and effectively suppressed Mn dissolution during cycling (Fig. 5d). As a result, the assembled battery exhibited a high specific capacity of 585 mAh g−1 at a current density of 100 mA g−1, with 78% capacity retention after 300 cycles. However, the Co-doped δ-MnO2 exhibited relatively poor rate capacity, delivering only 185 mAh g−1 at a high current density of 500 mA g−1. To improve this, our group introduced metal heteroatoms with higher metal–oxygen bond dissociation energies, such as V and Ti, to reinforce the δ-MnO2 framework while expanding the interplanar spacing (Fig. 5e and f).60 The enlarged interlayer spacing reduces diffusion resistance and accelerates Al3+ transport and adsorption, resulting in enhanced rate performance. Meanwhile, the stronger metal–oxygen bonds improve structural stability and suppress Mn dissolution. As a result, the V-doped δ-MnO2 cathode achieved a high reversible capacity of 518 mAh g−1 at 200 mA g−1 with 86% capacity retention over 400 cycles. Moreover, its rate performance was significantly improved, delivering 468, 339, and 285 mAh g−1 at 0.5, 1, and 2 A g−1, respectively.
Another strategy to enhance the structural stability and electrochemical performance of MnO2 cathodes is the composite approach. By integrating MnO2 with other functional materials such as conductive carbon, the resulting hybrid structures can enhance structural stability, suppress manganese dissolution, stabilize oxygen vacancies, or provide additional active sites for ion storage.32,61 For instance, a hybrid α-MnO2 cathode with Al3+ pre-insertion and g-C3N4 was developed, where Al3+ expands the tunnel structures and reduces strain during intercalation, while g-C3N4 occupies oxygen vacancies and forms Mn–N bonds with the MnO6 lattice.62 This dual modification not only improves structural robustness but also significantly enhances electrochemical performance, delivering an excellent capacity of 329.25 mAh g−1 at 0.5 A g−1 and a rate capability of 137.67 mAh g−1 at 1.5 A g−1, highlighting the effectiveness of composite engineering in stabilizing MnO2 cathodes for AAIBs.
Overall, tunnel-type MnO2 cathodes suffer from limited capacity due to their narrow channels, whereas layered δ-MnO2 offers greater interlayer spacing, making it a more promising host for Al3+ storage. However, the layered structure is inherently unstable and typically undergoes an in situ transformation into an AlxMnO2 phase during the initial charge/discharge cycle. In addition to δ-MnO2, other manganese oxides such as MnO and spinel-type Mn oxides can also be electrochemically converted into AlxMnO2 during initial cycling. Nevertheless, the stability of the resulting AlxMnO2 remains limited. Therefore, recent research has focused on interlayer engineering strategies, such as organic molecule intercalation, metal-ion pre-intercalation, and heteroatom doping, to stabilize or enlarge the interlayer spacing and suppress structural degradation. These methods aim to preserve the advantageous layered framework while enhancing both the cycling stability and rate performance of Mn-based cathodes. Future research could further refine and integrate these strategies to develop high-performance and durable manganese-based cathode materials for AAIBs.
3.2 Vanadium-based cathode materials
Metallic vanadium, with its wide range of oxidation states from +2 to +5, enables multi-electron transfer and thus delivers high capacities, making vanadium-based compounds an important class of cathode materials. Vanadium-based compounds include vanadium oxides, vanadates, and vanadium phosphates. Among them, vanadium oxides are the most widely studied. Owing to the variable oxidation states of vanadium, vanadium oxides exhibit diverse crystal structures, primarily including orthorhombic layered V2O5, bilayer V2O5·nH2O and V3O7·H2O, and monoclinic tunnel-structured VO2, V6O13, and V2O3. Vanadates, generally denoted as MxVnOm (where M represents metal cations or ammonium ions), are derived from the pre-intercalation of metal ions into vanadium oxides. Such pre-intercalation is beneficial for enhancing the structural stability of vanadium oxides. Vanadium phosphates are constructed from [PO4] tetrahedra and [VO6] octahedra, forming a three-dimensional open framework. They typically exhibit a robust structural framework and remarkable thermal stability and good structural integrity during ion insertion/extraction. However, the separation of [VO6] octahedra by phosphate groups leads to intrinsically low electronic conductivity. The variations in crystal structures and chemical compositions among these vanadium-based compounds lead to differences in electrochemical performance, which provides both opportunities and challenges for their application as cathodes in AAIBs.Subsequently, in 2020, Zhao et al. proposed that in AAIBs, protons can reversibly interact with V2O5, whereas direct Al3+ participation is difficult.38 By tuning electrolyte composition and pH, they regulated H+ intercalation selectivity (Fig. 6a). In 2 M Al(OTf)3 acidic electrolyte, V2O5 delivered a discharge capacity of ∼200 mAh g−1 at 20 mA g−1, with a discharge plateau at around 0.9 V and 120 mAh g−1 remaining after 50 cycles (Fig. 6b). DFT calculations revealed that proton insertion induced a structural transformation from orthorhombic layered V2O5 to a triclinic phase, accompanied by reductions in both interlayer spacing and volume. The insertion of one proton could deliver ∼147 mAh g−1 with good structural stability, whereas insertion of two protons increased the capacity to 296 mAh g−1 but caused significant volume contraction and severe structural degradation (Fig. 6c). These studies clarified the ion storage behavior in V2O5, though the dominant mechanism remains under debate.
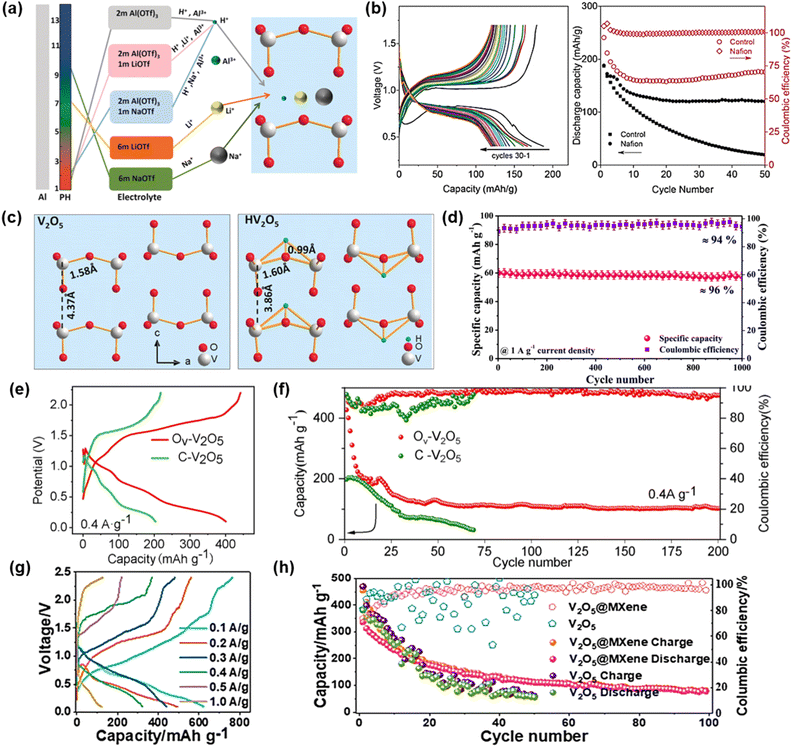 | ||
| Fig. 6 (a) Different cation intercalating behaviors of V2O5 through regulating the electrolytes. (b) Galvanostatic discharge/charge profiles of aqueous Al/V2O5 batteries using a Nafion modified separator and corresponding cycling performance and coulombic efficiency. (c) Structure of pristine V2O5 and V2O5 after proton insertion. Reproduced with permission.38 Copyright 2020, Wiley-VCH. (d) Cycling stability performance of the synthesized V2O5 material. Reproduced with permission.64 Copyright 2023, American Chemical Society. (e) The initial GCD curves for Ov-V2O5 and C-V2O5 at 0.4 A g−1 and (f) cycling performance at 0.4 A g−1. Reproduced with permission.65 Copyright 2025, Elsevier. (g) Rate performance of V2O5@MXene and (h) comparison of the cycle performance between V2O5@MXene and V2O5 at 0.4 A g−1. Reproduced with permission.66 Copyright 2024, American Chemical Society. | ||
To enhance the electrochemical performance of V2O5, in 2023, De et al. synthesized interconnected nanosheet-structured V2O5via a hydrothermal method using NH4VO3 followed by high-temperature calcination.64 This two-dimensional nanosheet architecture increased the electrode–electrolyte contact area, facilitating rapid ion diffusion. In a three-electrode setup with 0.5 M AlCl3 electrolyte, the electrode delivered an initial discharge capacity of ∼140 mAh g−1 at 0.5 A g−1 and maintained 96% of its capacity after 1000 cycles at 1 A g−1 (Fig. 6d). The excellent capacity retention and rate performance were attributed to the high specific surface area provided by the interconnected nanosheet structure, which promoted ion accumulation at the electrode–electrolyte interface, enhancing the electric double-layer contribution to the total capacity.
Introducing oxygen vacancies is another effective strategy. Wang et al. introduced oxygen vacancies (Ov) into V2O5via oxalic acid treatment.65 The resulting Ov-V2O5 exhibited a high initial capacity of 400 mAh g−1 at 0.4 A g−1 in 5 M Al(OTf)3 electrolyte, nearly double that of pristine V2O5 (∼200 mAh g−1) (Fig. 6e). Theoretical calculations indicated that Ov-V2O5 had lower adsorption energies for both H+ and Al3+ compared with pristine V2O5, implying reduced diffusion barriers and accelerated ion transport. Although oxygen vacancies improved cycling stability (Fig. 6f), the capacity of Ov-V2O5 still declined to 103 mAh g−1 after 200 cycles, indicating rapid fading.
Besides defect engineering, compositing with conductive materials has also been investigated to improve the electrochemical performance of V2O5. Yang et al. fabricated a V2O5@MXene composite cathode by depositing rod-like V2O5 onto monolayer MXene.66 In 5 M Al(OTf)3 electrolyte, the composite exhibited a wide voltage window (0.1–2.4 V) and an exceptionally high initial capacity of 626 mAh g−1 at 100 mA g−1 (Fig. 6g). Compared with pristine V2O5, the composite demonstrated approximately twice the cycle life, improved coulombic efficiency, and retained 200 mAh g−1 after 100 cycles at 400 mA g−1 (Fig. 6h). The enhanced performance was attributed to the excellent electrical conductivity and pseudocapacitive characteristics of MXene, which improved electron transport, ion diffusion, and electrode stability.
In summary, layered V2O5 enables reversible interactions with Al3+ or H+ in AAIBs but suffers from limited capacity and structural instability. Although defect engineering and conductive composite design have improved capacity and cycle life, capacity fading remains significant. Therefore, stabilizing the crystal structure and enhancing the reversible ion storage are critical directions for the future development of V2O5-based cathodes.
Compared with layered V2O5, tunnel-type vanadium oxides generally exhibit higher lattice stability during repeated ion–host interaction, which is beneficial for achieving long cycle life.67,68 Among them, the most representative is the metastable monoclinic bronze-type vanadium dioxide (VO2(B)), whose crystal structure consists of alternating [VO6] octahedra and tetrahedra, forming characteristic one-dimensional diffusion tunnels. The tunnel size (4.984 Å × 3.281 Å) is significantly larger than the ionic radius of Al3+ (0.53 Å), thus providing sufficient space for guest ion transport and storage.
Recent studies have further optimized the VO2 structure to enhance its electrochemical performance in AAIBs. In 2020, Cai et al. synthesized porous VO2(B) nanobelts via a hydrothermal method and employed them as cathode materials for AAIBs.69 The robust VO2(B) framework effectively resisted lattice shear during cycling, while the porous nanobelt morphology shortened ion diffusion pathways and increased electrode–electrolyte contact area. As a result, the electrode delivered a specific capacity of 234 mAh g−1 at 150 mA g−1 and retained 77.2% of its capacity after 1000 cycles at 1 A g−1 in a three-electrode system with 5 M Al(OTf)3 electrolyte (Fig. 7a and b). In another study, Wang et al. reported that the unique interconnected tunnel structure of monoclinic VO2 enabled rapid Al3+ diffusion while effectively mitigating volume changes, achieving 235 mAh g−1 at 200 mA g−1 and retaining 49.3% of its capacity when the current density increased to 2 A g−1 (Fig. 7c).70
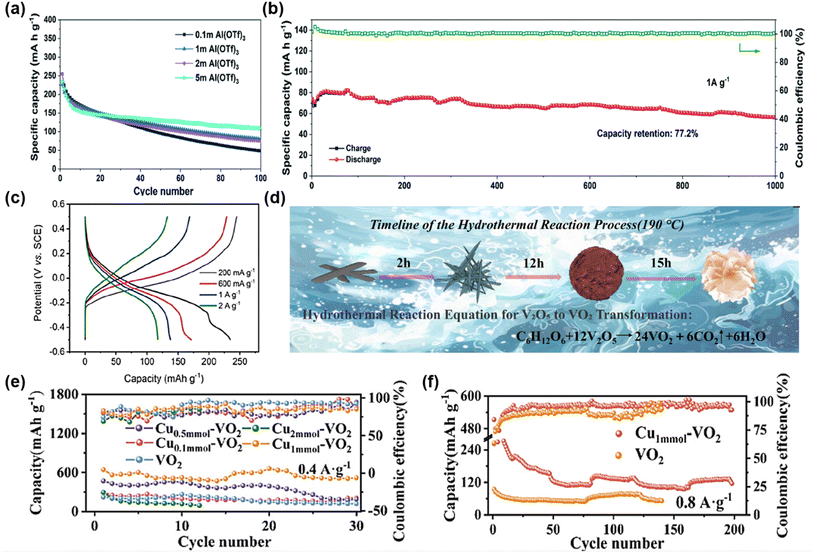 | ||
| Fig. 7 (a) Cycling performances of the VO2-B electrodes in the Al(OTF)3 electrolytes with different concentrations at 150 mA g−1. (b) Long-term cycling performance of the VO2-B electrodes at 1 A g−1 in the 5 M Al(OTF)3 electrolyte. Reproduced with permission.69 Copyright 2020, Royal Society of Chemistry. (c) GCD curve of VO2 at different current densities. Reproduced with permission.70 Copyright 2021, Elsevier. (d) Schematic diagram of preparation and morphology evolution of Cu1mmol-VO2. (e) Cycling performance at 0.4 A g−1. (f) Cycling performance of VO2 and Cu1mmol-VO2 at 0.8 A g−1. Reproduced with permission.71 Copyright 2025, Wiley-VCH. | ||
More recently, in 2025, Wang et al. achieved both a compositional transformation from V2O5 to VO2 and a crystallographic transition from the orthorhombic to the monoclinic phase via glucose-assisted hydrothermal reduction, while simultaneously introducing Cu2+ doping (Fig. 7d).71 The introduced Cu 3d orbitals enhanced the hybridization between V 3d and O 2p orbitals and strengthened electronic coupling, optimizing the band structure and improving both ion and electron transport. At an optimal Cu content of 1 mmol, the resulting VO2 cathode, paired with 5 M Al(OTf)3 electrolyte and an ionic-liquid-treated Al anode, delivered an initial discharge capacity of 642 mAh g−1 at 0.4 A g−1 and maintained 116 mAh g−1 after 200 cycles at 0.8 A g−1 (Fig. 7e and f), outperforming pristine VO2 in both capacity and cycling stability.
In summary, VO2(B) demonstrates considerable potential for AAIB applications owing to its spacious tunnel structure. However, unlike layered vanadium oxides, where interlayer spacing can be tuned to enhance ion diffusion kinetics, the tunnel dimensions of VO2 are relatively fixed, restricting further improvement in ion diffusion kinetics. Therefore, effective strategies to accelerate ion migration within the tunnels are urgently needed. Current strategies, including nanostructure design to shorten diffusion paths and doping to optimize the electronic structure, have enhanced performance. Nevertheless, there remains substantial room for improvement in their long-term cycling stability and high-rate capability.
In 2022, Soundharrajan et al. reported the application of LiV3O8 cathodes in AAIBs.72 The presence of Li+ in its layered structure can mitigate the strong coulombic attraction between Al3+ and the host lattice, thereby facilitating Al3+ diffusion kinetics (Fig. 8a). In 2 M Al(OTf)3 electrolyte, LiV3O8 delivered a high capacity of 289 mAh g−1 at 0.29C and maintained 147 mAh g−1 after 500 cycles at 0.59C, corresponding to a capacity retention of 77.3% (Fig. 8b and c).
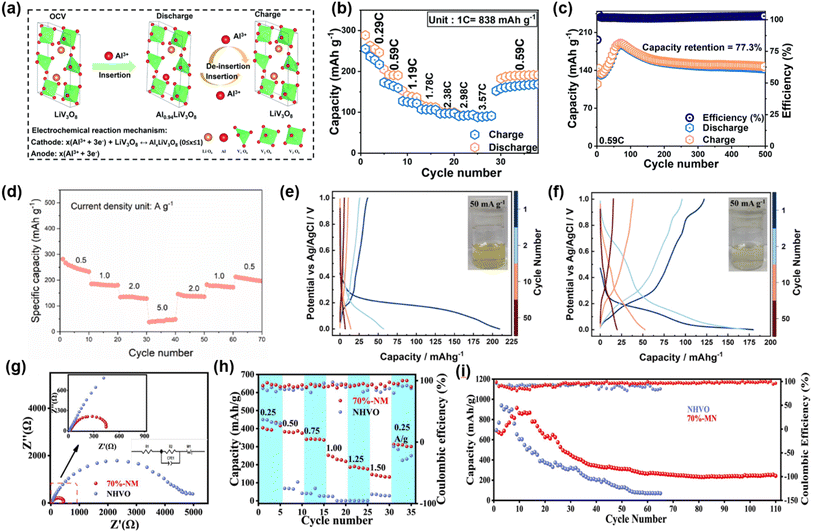 | ||
| Fig. 8 (a) Schematic representation of the Al3+ storage properties of the LiV3O8 cathode during the charge and discharge course. (b) Rate performances of LiV3O8 at different current densities and (c) cycle life curve at 0.59C. Reproduced with permission.72 Copyright 2022, Royal Society of Chemistry. (d) Rate tests of layered NH4V4O10 nanosheets at sets of current densities from 0.5 to 5 A g−1. Reproduced with permission.73 Copyright 2023, Elsevier. (e and f) GCD (current density = 50 mA g−1) profile of NH4V4O10 electrodes cycled in 1 M AlCl3 (e) and 1 M AlCl3 + 0.5 M NH4Cl (f) electrolytes, respectively. Reproduced with permission.74 Copyright 2024, American Chemical Society. (g) Multiplicity curve of 70%-NM ((NH4)2V10O25·8H2O/Ti3C2Tx composites). (h) Rate performance of 70%-NM and NHVO ((NH4)2V10O25·8H2O). (i) Cycling performance graphs for 70%-NM and NHVO. Reproduced with permission.75 Copyright 2024, Elsevier. | ||
In 2023, Liu et al. for the first time applied NH4V4O10 as a cathode for AAIBs.73 The large and lightweight NH4+ act as pillars in the crystal structure, expanding the interlayer spacing and promoting fast ion transport. As a result, the layered NH4V4O10 achieved 257.78 mAh g−1 at 0.5 A g−1 in 2 M Al(OTf)3 and retained 87.80 mAh g−1 even at a high current density of 5 A g−1 (Fig. 8d). Subsequent work further examined electrolyte optimization for the NH4V4O10 cathode material. Radhakantha et al. demonstrated that adding 0.5 M NH4Cl to 1 M AlCl3 electrolyte effectively suppressed vanadium dissolution.74 The first-cycle capacity decay decreased from 84% to 32%, and the electrolyte color changed from deep yellow to light yellow (Fig. 8e and f), indicating that the additive suppressed V dissolution and improved structural stability.
In addition, Wu et al. prepared (NH4)2V10O25·8H2O/Ti3C2Tx composites via van der Waals self-assembly.75 The incorporation of MXene reduced the charge-transfer resistance to one-tenth that of the pristine material and significantly enhanced both rate capability and cycling stability (Fig. 8g and h). At 1.0 A g−1, the electrode maintained 268.7 mAh g−1 after 110 cycles (Fig. 8i). This result indicates that MXene not only accelerates ion transport but also acts as a mechanical support to stabilize structure during cycling, highlighting the advantages of composite design.
In 2018, Nacimiento et al. first applied NASICON-type Na3V2(PO4)3 as a cathode for rechargeable aluminum batteries, delivering a reversible capacity of 60–100 mAh g−1 at current densities ranging from 10 to 1000 mA g−1 in a three-electrode configuration with AlCl3-based electrolyte (Fig. 9a).78 In 2020, Wang et al. employed VOPO4 as the cathode and incorporated a mechanically robust gelatin–polyacrylamide hydrogel electrolyte to construct a flexible AAIB, achieving a capacity of 88 mAh g−1 at 0.8 A g−1 and maintaining 22 mAh g−1 even at a high rate of 6 A g−1 (Fig. 9b).79 Remarkably, this device retained 86.2% of its initial capacity after 2800 cycles at 1 A g−1 (Fig. 9c), demonstrating excellent cycling stability and mechanical flexibility. Subsequently, Pang et al. utilized layered VOPO4·2H2O nanosheets as a cathode material for AAIBs, achieving reversible trivalent Al3+ storage in 2.5 M Al(OTf)3 electrolyte.80 At a current density of 20 mA g−1, the material delivered an initial discharge capacity of 125.4 mAh g−1 with an operating voltage of ∼0.9 V, retaining 60% of its capacity after 40 cycles (Fig. 9d and e), highlighting its cycling stability and application potential. More recently, Liu et al. demonstrated that phenylamine intercalation can effectively expand the interlayer spacing of VOPO4, enabling high-capacity and long-life Al3+ storage in aqueous batteries.81
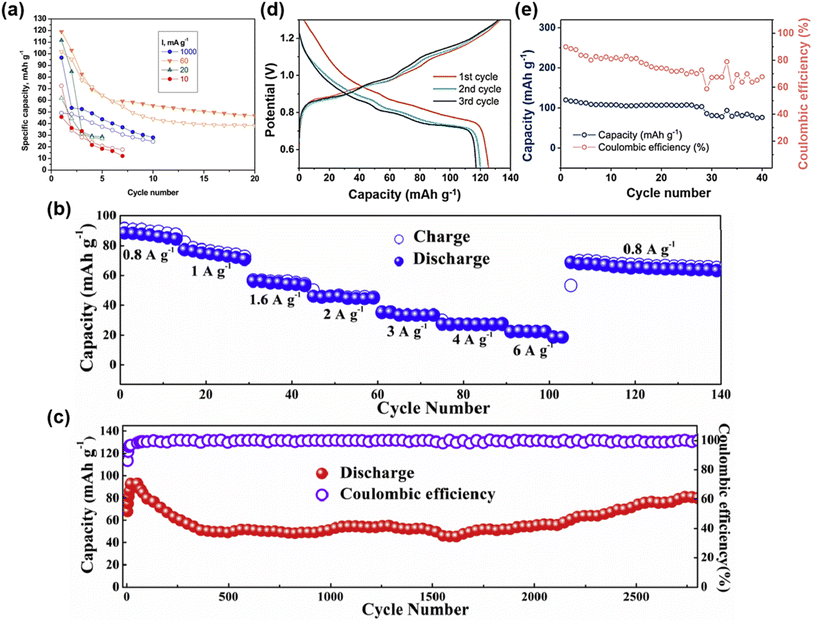 | ||
| Fig. 9 (a) Capacity–cycle number at several rates of Na3V2(PO4)3 (charge: open symbols; discharge: closed symbols). Reproduced with permission.78 Copyright 2018, Elsevier. (b) Rate capability at various rates and (c) long-term cycling performance and the corresponding coulombic efficiency at 1 A g−1 of the rechargeable solid-state aqueous AIB. Reproduced with permission.79 Copyright 2020, Elsevier. (d) First three charge/discharge curves and (e) cycling performance of VOPO4·2H2O/Al metal batteries at a current density of 20 mA g−1. Reproduced with permission.80 Copyright 2021, Elsevier. | ||
Overall, the capacities and operating voltages of vanadium-based cathodes in AAIBs are generally lower than those of their manganese-based counterparts, primarily due to their intrinsic structural and electronic limitations, including relatively low electrical conductivity and suboptimal ion transport pathways. Future research should focus on improving the capacity, stability, and conductivity through strategies such as nanostructure engineering, conductive network construction, and doping modifications, while combining in situ characterization with theoretical calculations to gain deeper insights into their energy storage mechanisms.
3.3 Prussian blue analogs
Prussian blue analogs (PBAs) were first discovered in the 18th century and widely employed as dyes. It was not until 2011 that Cui et al. first utilized KNi-PB as a cathode material for potassium-ion batteries, followed by their report on the application of KCu-PB in lithium-ion batteries.82,83 These pioneering studies not only broadened the application scope of PBAs in rechargeable ion batteries but also revealed their great potential as cathode materials.PBAs are generally represented by the formula AxM[M′(CN)6]1−y·□y·nH2O (0 < x < 2), where A denotes alkali metal ions (commonly K, Na, or Li), M corresponds to N-coordinated transition metal ions, M′ refers to C-coordinated transition metal ions (most commonly Fe), and □ represents vacancies arising from the loss of M′(CN)6 units and the occupation by coordination water.84,85 Prussian blue and its analogues are often abbreviated as AM-PB. In AM-PB, the Fe2+/Fe3+ redox couple at the M′ site generally serves as the dominant redox pair. When the M-site metal is also electrochemically active (e.g., Fe, Co, and Mn), an additional M2+/M3+ redox couple is introduced, enabling two redox-active centers and thus a higher theoretical capacity. In contrast, when M is electrochemically inactive (e.g., Ni, Cu, and Zn), only the Fe2+/Fe3+ redox couple contributes to charge storage, resulting in a lower theoretical capacity.86
The ideal PBA structure adopts a face-centered cubic framework with a lattice parameter of ∼10.2 Å (Fig. 10a). In this structure, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif)
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups are located along the cube edges, where M and M′ ions form octahedral coordination with N and C atoms of cyanide ligands respectively, constructing a three-dimensional open framework. The interstitial sites between octahedra create diffusion pathways for ion transport, thereby allowing the reversible transport and storage of cations. The crystal structure of PBAs can transform among cubic, monoclinic, rhombohedral, trigonal, and tetragonal phases (Fig. 10) depending on the A+ content and the amount of crystalline water.87,88 Such structural evolutions significantly influence their electrochemical stability. Moreover, PBAs commonly contain [Fe(CN)6] vacancies, which are often occupied by coordinated water molecules (Fig. 10f). These vacancies not only reduce the number of active sites but may also disrupt the continuity of the Fe–CN–M framework, leading to structural distortion or collapse and, consequently, inferior cycling performance. Owing to PBA open three-dimensional frameworks, facile synthesis, abundant resources, and tunable operating voltage, PBAs have attracted widespread attention as promising electrode materials for energy storage applications.
N groups are located along the cube edges, where M and M′ ions form octahedral coordination with N and C atoms of cyanide ligands respectively, constructing a three-dimensional open framework. The interstitial sites between octahedra create diffusion pathways for ion transport, thereby allowing the reversible transport and storage of cations. The crystal structure of PBAs can transform among cubic, monoclinic, rhombohedral, trigonal, and tetragonal phases (Fig. 10) depending on the A+ content and the amount of crystalline water.87,88 Such structural evolutions significantly influence their electrochemical stability. Moreover, PBAs commonly contain [Fe(CN)6] vacancies, which are often occupied by coordinated water molecules (Fig. 10f). These vacancies not only reduce the number of active sites but may also disrupt the continuity of the Fe–CN–M framework, leading to structural distortion or collapse and, consequently, inferior cycling performance. Owing to PBA open three-dimensional frameworks, facile synthesis, abundant resources, and tunable operating voltage, PBAs have attracted widespread attention as promising electrode materials for energy storage applications.
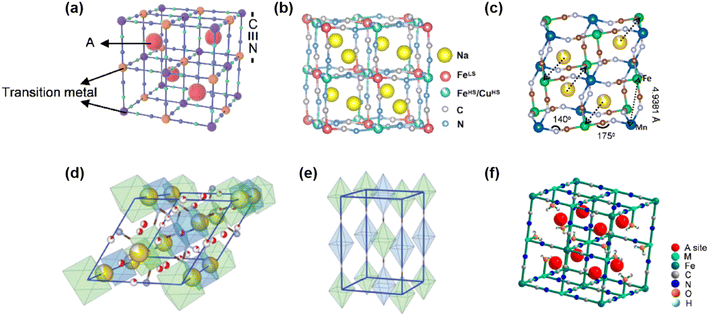 | ||
| Fig. 10 Schematic crystal structures of PBAs: (a) cubic phase, (b) monoclinic phase, (c) rhombohedral phase, (d) trigonal phase, (e) tetragonal phase, and (f) PBA framework with [Fe(CN)6] vacancies. Reproduced with permission.86 Copyright 2025, Springer Nature. | ||
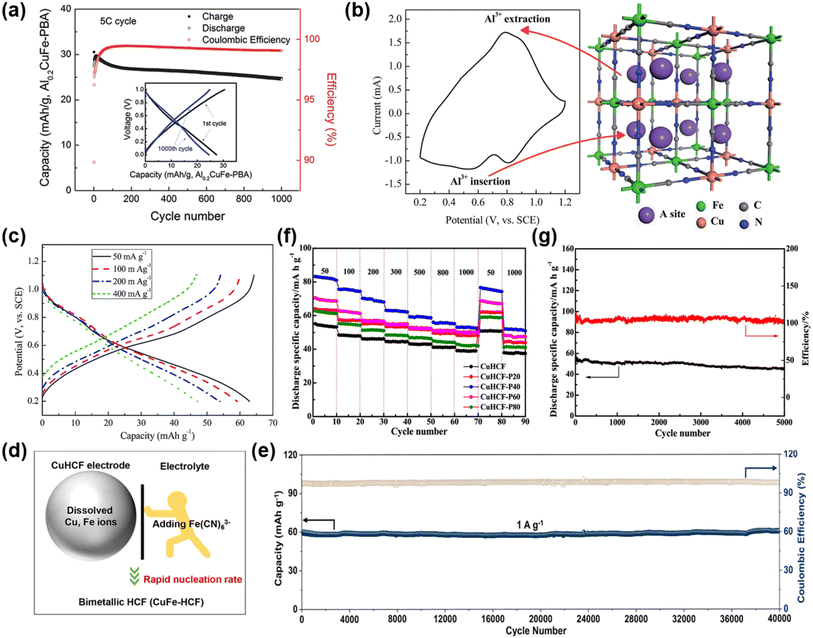 | ||
| Fig. 11 (a) Cycling performance and coulombic efficiency of an AC/Al0.2CuFe-PBA cell at a 5C rate. The inset figure shows the 1st and 1000th charge and discharge curves. Reproduced with permission.89 Copyright 2015, Wiley-VCH. (b) Typical CV curve of the CuHCF electrode in aqueous Al2(SO4)3 and the schematic positions of Al3+ in the CuHCF framework and (c) charge/discharge curves at different current densities of the CuHCF electrode in 0.5 M Al2(SO4)3 aqueous solution. Reproduced with permission.90 Copyright 2015, Royal Society of Chemistry. (d) Schematic diagram of inhibiting metal ion dissolution and (e) long-term stability performance. Reproduced with permission.91 Copyright 2023, American Chemical Society. (f) The rate performance of the as-prepared samples at a current density of 50–1000 mA g−1 and (g) cycle performance at a current density of 1000 mA g−1. Reproduced with permission.93 Copyright 2023, American Chemical Society. | ||
To address the issues of low capacity, short cycle life, and dissolution of CuHCF in aqueous electrolytes, Zhao et al. proposed an electrolyte-engineering strategy by introducing [Fe(CN)6]3− into the electrolyte to drive the re-nucleation of dissolved transition metal ions, thereby preventing framework degradation (Fig. 11d).91 In the optimized electrolyte, no Cu2+ were detected, confirming the effectiveness of this approach. Remarkably, CuHCF delivered ultra-stable electrochemical performance, achieving 99.8% capacity retention after 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 in 1 M (NH4)2SO4 electrolyte with 5 mmol K3Fe(CN)6 added (Fig. 11e). This approach effectively suppressed transition-metal dissolution and enabled ultralong cycling stability of CuHCF. Radhakantha et al. demonstrated that higher electrolyte concentrations can enhance the structural stability of the CuHCF framework, exhibiting near-zero lattice strain during Al3+ insertion.92
000 cycles at 1 A g−1 in 1 M (NH4)2SO4 electrolyte with 5 mmol K3Fe(CN)6 added (Fig. 11e). This approach effectively suppressed transition-metal dissolution and enabled ultralong cycling stability of CuHCF. Radhakantha et al. demonstrated that higher electrolyte concentrations can enhance the structural stability of the CuHCF framework, exhibiting near-zero lattice strain during Al3+ insertion.92
In addition, Zheng et al. investigated the pre-intercalation of Al3+ into CuHCF at different temperatures and found that the sample treated at 40 °C (CuHCF-P40) exhibited the highest capacity and the best cycling performance.93 CuHCF-P40 delivered a discharge capacity of 83.1 mAh g−1 at 50 mA g−1, ∼1.6 times higher than that of pristine CuHCF (Fig. 11f). Even after 5000 cycles at 1000 mA g−1, a capacity of 54.5 mAh g−1 was retained, corresponding to 80.3% capacity retention (Fig. 11g). This result suggested that moderate Al3+ pre-intercalation expands ion transport channels and mitigates structural distortion during cycling, enhancing both capacity and stability, whereas excessive or insufficient Al3+ respectively blocks diffusion pathways or fails to stabilize the framework effectively. Building upon this, Sayeed et al. fabricated flexible full cells pairing Al3+ pre-intercalated CuHCF cathodes with MoO3 anodes in a PVA-based 3 M AlCl3 gel electrolyte.94 The device delivered 48 mAh g−1 at a high current density of 3 A g−1, with negligible capacity fading over 150 cycles. This work not only further validated the effectiveness of Al3+ pre-intercalation but also demonstrated its potential for stable and efficient energy storage in flexible and wearable electronic devices.
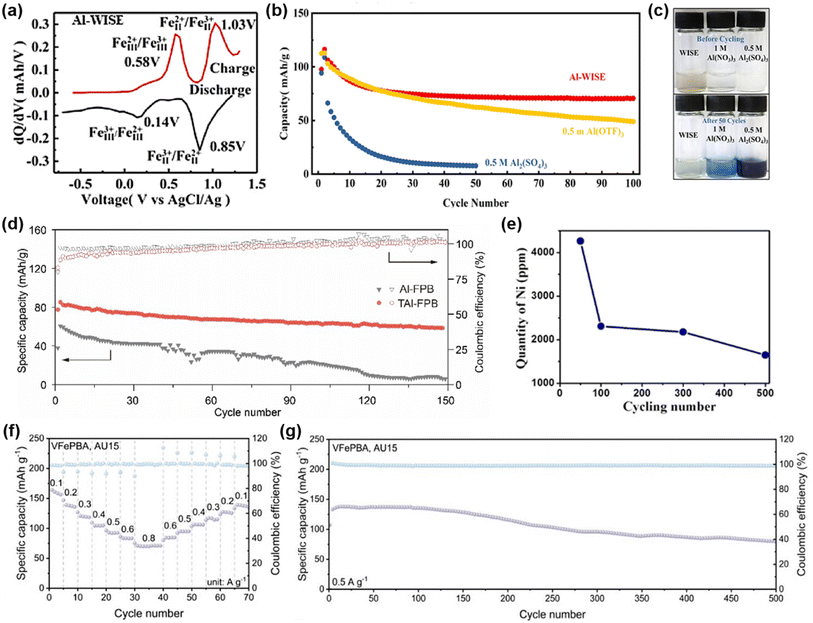 | ||
| Fig. 12 (a) The differential profiles of the 50th cycle in Al-WISE. (b) Cycling life and coulombic efficiency of the FF-PBA cathode in Al-WISE, 0.5 M Al2(SO4)3, and 0.5 M Al(OTF)3 electrolytes. (c) The cathode dissolution experiment. The upper photos are obtained before cycling and the bottom photos are obtained after 50 cycles. Reproduced with permission.95 Copyright 2019, American Chemical Society. (d) Cycling performance and corresponding coulombic efficiency of aqueous Al-FPB batteries using Al and TAl with 2 M Al(OTF)3 at 100 mA g−1. Reproduced with permission.96 Copyright 2021, Elsevier. (e) Ni dissolution concentration from the KNHCF electrode after the 50th, 100th, 300th, 500th cycles measured by ICP-OES. Reproduced with permission.97 Copyright 2020, Wiley-VCH. (f) Capacitive contribution ratios of VFePBA at various scan rates. (g) Long-term cycling performance of VFePBA. Reproduced with permission.98 Copyright 2025, Wiley-VCH. | ||
Beyond Fe–Fe PBAs, other bimetallic redox-site PBAs (e.g., V–Fe, Mn–Fe, and Co–Fe) have been investigated to enhance the capacity and cycling stability. Gao et al. reported potassium nickel hexacyanoferrate (KNHCF) as a cathode paired with an Al foil anode in 5 M Al(CF3SO3)3 electrolyte, revealing for the first time the charge-compensation mechanism of PBAs in multi-metal redox systems.97 The coin cell achieved an initial discharge capacity of 46.5 mAh g−1 at 20 mA g−1. During cycling, Ni gradually dissolved while Fe redox activity increased, compensating for the capacity loss and sustaining overall performance (Fig. 12e). Furthermore, owing to the multiple valence states of vanadium, vanadium-based PBAs (V-PBAs) can undergo multi-electron redox processes, thereby providing more active sites for charge storage. Feng et al. synthesized V-PBAs via an acid-assisted hydrothermal method, and the synergistic effect between V and Fe redox centers was found to significantly enhance capacity.98 In a full cell with a Zn anode and a deep eutectic electrolyte (denoted as AU15, composed of Al2(SO4)3, urea, and H2O), V-PBAs delivered a high capacity of 161.37 mAh g−1 at 0.1 A g−1 and maintained ∼100 mAh g−1 after 500 cycles at 0.5 A g−1 (Fig. 12f and g), demonstrating excellent capacity retention and cycling stability.
3.3.3.1 Defect engineering. Introducing defects or vacancies into the PBA framework can broaden the diffusion channels for Al3+ transport and improve ionic kinetics, thereby enhancing the electrochemical performance. In 2021, Wang et al. designed a defective manganese hexacyanoferrate (MnFe-PBA) cathode containing [Fe(CN)6] vacancies, which effectively expanded the Al3+ transport pathways and weakened the coulombic interactions between Al3+ and the host framework (Fig. 13a and b).99 Benefiting from this structural feature and the presence of dual electrochemically active sites (Mn3+/Mn2+ and Fe3+/Fe2+), the defective MnFe-PBA delivered an initial discharge capacity of 106.3 mAh g−1 at 0.2 A g−1 and maintained higher capacity at 1.0 A g−1. In contrast, the vacancy-free MnFe-PBA-0 exhibited much lower capacity, highlighting the critical role of vacancies in improving both capacity and rate performance (Fig. 13c).
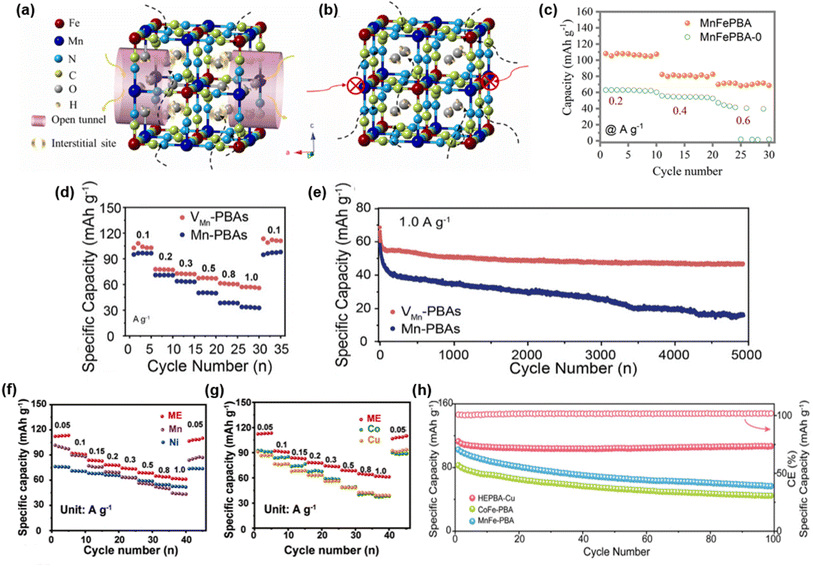 | ||
| Fig. 13 (a) Corresponding crystal structure of a MnFe-PBA unit cell. The yellow and pink hollow cylinders are used to mark interstitial sites for intercalated ions and enlarged open tunnels for the diffusion of carriers, respectively. (b) Corresponding crystal structure of a non-defective MnFe-PBA unit cell, and the possible pathways for Al ion ingress. (c) The effects of vacancy on the rate performance. MnFePBA is with a vacancy and MnFePBA-0 is a non-defective material. Reproduced with permission.99 Copyright 2021, Elsevier. (d) Rate performance and (e) long-term cycling stability of the PBAs//AC cell at 1 A g−1. Reproduced with permission.100 Copyright 2025, Wiley-VCH. (f and g) Rate performance at current densities from 0.05 to 1.0 A g−1 for ME-PBAs and the different single-metal PBAs. Reproduced with permission.101 Copyright 2024, Wiley-VCH. (h) Cycling performance of HEPBA-Cu and bimetallic PBA at 1.0 A g−1. Reproduced with permission.102 Copyright 2024, Wiley-VCH. | ||
Similarly, Shang et al. utilized the strong chelating effect of Na2EDTA to introduce a controlled number of Mn cation vacancies (VMn) into the Mn-PBA framework, obtaining VMn-PBAs.100 Compared with conventional Mn-PBAs, VMn-PBAs delivered an initial discharge capacity of 108 mAh g−1 at 0.1 A g−1 and consistently outperformed at higher current densities (0.1–1.0 A g−1). At 1.0 A g−1, VMn-PBAs retained 67.4% of their capacity after 5000 cycles, in sharp contrast to 26.6% retention for Mn-PBAs (Fig. 13d and e). This improvement was attributed to the introduction of vacancies, which effectively suppressed structural deformation and alleviated the unfavorable Jahn–Teller distortion in Mn–N octahedra. As a result, vacancy engineering enhanced both discharge capacity and cycling stability.
3.3.3.2 Entropy regulation. Increasing system entropy (ΔS) can reduce the Gibbs free energy (ΔG), enhancing structural stability and improving redox activity, thereby leading to better electrochemical performance. Liu et al. adopted an entropy-production strategy to design structurally reinforced medium-entropy PBA (ME-PBA) frameworks containing multiple redox-active metals, with the composition Na1.69Mn0.34Co0.33Ni0.13Cu0.20[Fe(CN)6]0.93·2.51H2O.101 Compared with single-phase PBAs, ME-PBAs exhibited higher discharge capacities across 0.05–1.0 A g−1 and ultra-long cycling stability, retaining 66.9% capacity after 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1.0 A g−1 (Fig. 13f and g). In a full cell with a MoO anode, ME-PBAs delivered 60.9 mAh g−1 at 0.5 A g−1 and maintained 89.7% of their capacity after 600 cycles. This superior performance was attributed to the synergistic contribution of multiple redox centers: Mn and Fe boost capacity, Co and Cu increase operating voltage, and Ni improves long-term stability, collectively enabling higher capacities and faster kinetics.
000 cycles at 1.0 A g−1 (Fig. 13f and g). In a full cell with a MoO anode, ME-PBAs delivered 60.9 mAh g−1 at 0.5 A g−1 and maintained 89.7% of their capacity after 600 cycles. This superior performance was attributed to the synergistic contribution of multiple redox centers: Mn and Fe boost capacity, Co and Cu increase operating voltage, and Ni improves long-term stability, collectively enabling higher capacities and faster kinetics.
Du et al. further employed high-entropy PBAs (HE-PBAs) as cathodes for AAIBs.102 By introducing multiple transition metals, the intrinsic d-band (εd) of individual metals was broadened and the electronic degeneracy reduced, thereby improving electron-transfer efficiency and accelerating local charge compensation. Benefiting from the long-range disorder and strong lattice strain fields inherent in high-entropy structures, HE-PBAs accommodated lattice breathing effects while minimizing volume fluctuations during cycling. As a result, HE-PBAs outperformed bimetallic PBAs in both capacity and cycling stability, achieving a remarkable 91.2% capacity retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a high current density of 5.0 A g−1 (Fig. 13h).
000 cycles at a high current density of 5.0 A g−1 (Fig. 13h).
3.3.3.3 Nanostructure engineering. PBAs with disordered dispersed morphologies often suffer from long ion-transport pathways and insufficient utilization of electrochemically active sites, which limit their overall electrochemical performance. Nanostructure engineering provides an effective means of regulating ion diffusion and structural stability, thereby improving capacity, rate capability, and cycling stability.
In 2020, Ru et al. synthesized potassium cobalt hexacyanoferrate (K2CoFe(CN)6) nanocube assemblies via a facile hydrothermal method followed by low-temperature calcination, forming a structure reminiscent of the traditional Chinese Burr puzzle framework (Fig. 14a).103 In 1 M Al(NO3)3 electrolyte, the material retained 76% of its initial capacity after 1600 cycles at 0.1 A g−1. Zheng et al. prepared one-dimensional Co-PBA (1D-CoPBA) via in situ reduction with hydrazine hydrate (Fig. 14b).104 The 1D architecture significantly improved electrical conductivity and hydrophilicity, thereby enhancing electrolyte accessibility. As a result, 1D-CoPBA delivered 100.1 mAh g−1 at 50 mA g−1 and retained 87% of its capacity after 2000 cycles at 1000 mA g−1, outperforming conventional Co-PBA. Chang et al. employed tannic acid (TA) etching and cation exchange to synthesize Mg-substituted double-walled Mn-PBA nanocubes (MgMn-PBA DWNCs) (Fig. 14c).105 Mg substitution and Mn vacancies provided abundant active sites, buffered volume changes, and improved ion diffusion. Consequently, MgMn-PBA DWNCs delivered a high capacity of 148 mAh g−1 with 99.5% retention after 2000 cycles at 1 A g−1 and maintained 74 mAh g−1 even at 4 A g−1. Furthermore, a full cell assembled with an α-MoO3 anode exhibited 78.6 mAh g−1 after 2000 cycles at 1 A g−1, with an average coulombic efficiency of 98.5%, demonstrating outstanding Al3+ storage capability.
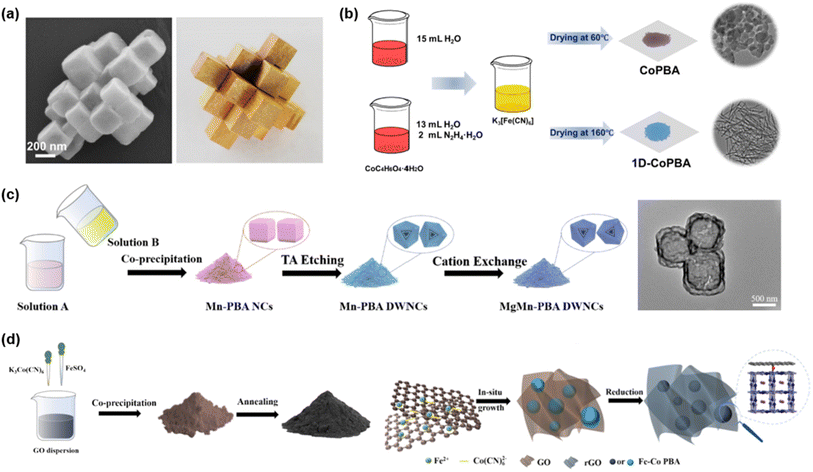 | ||
| Fig. 14 (a) SEM images of the K2CoFe(CN)6 nanocube and structure of burr puzzles. Reproduced with permission.103 Copyright 2020, Elsevier. (b) Schematic illustration of the synthesis and structural characteristics of the CoPBA and 1D-CoPBA materials. Reproduced with permission.104 Copyright 2024, American Chemical Society. (c) Schematic representation of the synthetic process of Mn-PBA NCs, Mn-PBA DWNCs and MgMn-PBA DWNCs, respectively. Reproduced with permission.105 Copyright 2024, Elsevier. (d) Schematic representation of the synthetic process of Fe–Co PBA/rGO. Reproduced with permission.106 Copyright 2024, Elsevier. | ||
The intrinsic low conductivity of PBAs remains a bottleneck, prompting the development of nanocomposites that combine complementary materials to improve electron transport and overall performance. Zhao et al. fabricated Fe–Co PBA/rGO composites via an in situ synthesis method (Fig. 14d).106 Owing to the interfacial chemical coupling between the Fe–Co PBA and rGO, the composite exhibited enhanced electronic structure and accelerated charge transfer. Compared with the pristine Fe–Co PBA, physically mixed Fe–Co PBA/rGO, and rGO, the Fe–Co PBA/rGO composite cathode demonstrated markedly improved rate capability and long-term cycling stability, achieving 112.5 mAh g−1 at 0.1 A g−1 and maintaining 66.7 mAh g−1 after 1500 cycles at 1 A g−1.
In summary, PBAs with their three-dimensional open framework and tunable redox potential hold great promise as cathode materials for AAIBs. However, their relatively limited capacity and structural instability arising from lattice distortion during cycling remain key bottlenecks. To address these issues, defect engineering enhances ion diffusion, entropy engineering introduces multiple redox-active centers and reinforces structural robustness, and nanostructure engineering shortens diffusion pathways and buffers volume change. These strategies have collectively improved capacity, rate capability and cycling stability. Looking forward, achieving both high capacity and long lifespan while maintaining framework integrity will be crucial for advancing PBAs in AAIBs. The synergistic optimization of defect regulation, entropy modulation, and nanostructure design, coupled with in-depth mechanistic understanding via in situ characterization and theoretical modeling, is expected to drive PBAs toward next-generation high-performance cathodes.
3.4 Organic cathode materials
In recent years, organic cathode materials have attracted extensive attention due to their abundance, environmental friendliness, structural tunability, and facile synthesis. Compared with inorganic metal oxides, organic materials are composed of common elements (C, H, O, and N) and possess flexible molecular backbones that can be rationally tailored to optimize electrochemical performance.107,108 Furthermore, their intrinsic structural flexibility helps mitigate volume strain during charge/discharge processes, thereby enhancing cycling stability. In addition, the relatively weak coordination or ion–dipole interactions within organic frameworks facilitate the rapid migration of Al3+, avoiding strong electrostatic interactions typically observed in inorganic cathode materials with high Al3+ charge density.109–111In terms of energy storage mechanisms, the electrochemical behavior of organic cathodes mainly relies on reversible redox-active groups. Through electron transfer involving carbonyl groups, nitrogen heterocycles, conjugated π frameworks, or radical sites, organic cathodes enable reversible storage and release of Al3+. Based on the type of redox-active center, organic cathode materials can be classified into quinone-based compounds, N-heteroaromatic compounds, conductive polymers, and radical polymers.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) redox centers within the molecular backbone, where each C
O) redox centers within the molecular backbone, where each C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group participates in one-electron transfer, enabling high theoretical capacities. In AAIBs, these carbonyl groups can coordinate with Al3+ to store and release charge.
O group participates in one-electron transfer, enabling high theoretical capacities. In AAIBs, these carbonyl groups can coordinate with Al3+ to store and release charge.
In 2021, He et al. reported tetrachloro-1,4-benzoquinone (TCQ) as a cathode material for AAIBs.112 TCQ contains two carbonyl groups that serve as redox-active sites for Al3+ storage, undergoing a reversible transformation between carbonyl and hydroxyl groups. During discharge, TCQ is reduced to tetrachlorohydroquinone (TCHQ) by accepting two electrons, and the coordinated Al3+ substitutes the hydrogen atoms of the hydroxyl groups, while in the charging process Al3+ is released and TCHQ is oxidized back to TCQ (Fig. 15a). The Al//TCQ full cell with 1 M Al(OTf)3 electrolyte delivered a specific capacity of 147.7 mAh g−1 at 0.2 A g−1 and retained 70.7% capacity after 200 cycles at 2 A g−1 (Fig. 15b and c), demonstrating good cycling stability. This study provided a solid proof-of-concept for quinone derivatives as stable organic cathodes in AAIBs.
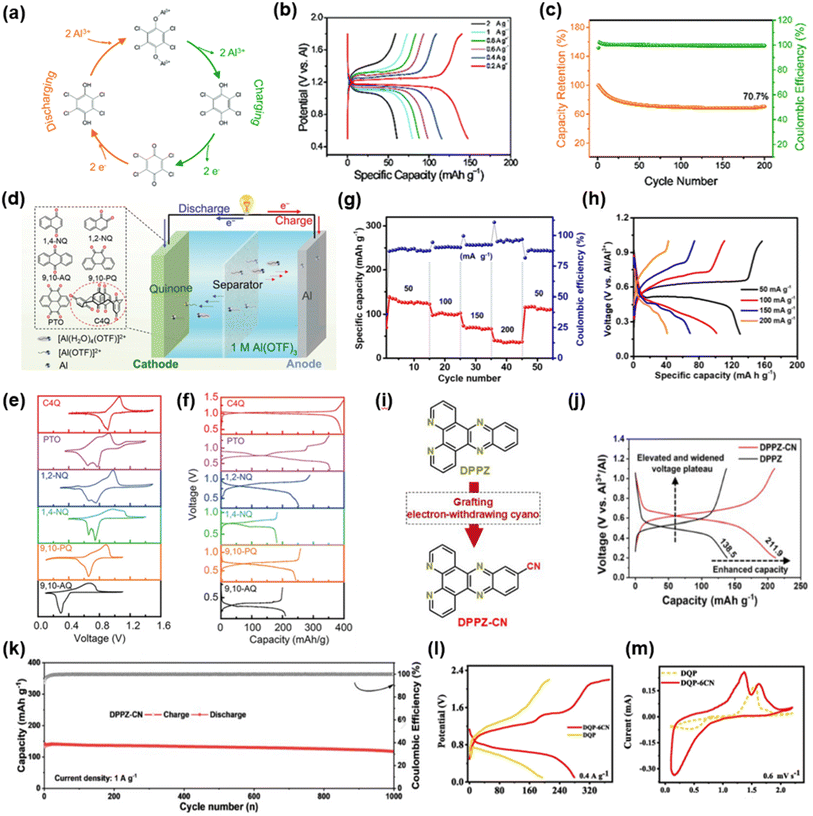 | ||
| Fig. 15 (a) Schematic diagram of the structure transformation of TCQ in the electrochemical process. (b) GCD curves at various current densities and (c) cycling performance of the Al//TCQ battery. Reproduced with permission.112 Copyright 2021, Royal Society of Chemistry. (d) Schematic illustration of fabricated aqueous Al-quinone batteries. (e) CV curves at 0.1 mV s−1 and (f) GCD curves at a current density of 40 mA g−1 of different quinone compounds. Reproduced with permission.113 Copyright 2021, Wiley-VCH. (g) Rate performance of PZ at various current densities from 50 to 200 mA g−1 and (h) corresponding discharge/charge curves. Reproduced with permission.114 Copyright 2021, Wiley-VCH. (i) Typical molecular structure. (j) Charge/discharge profiles at 0.1 A g−1 for Al//DPPZ-CN and Al//DPPZ batteries with 1 M Al(ClO4)3 aqueous electrolyte. (k) Long-term cycling performance of the Al//DPPZ-CN battery at 1 A g−1. Reproduced with permission.116 Copyright 2025, Wiley-VCH. (l) GCD curves of DQP-6CN and DQP and (m) CV curves of DQP-6CN. Reproduced with permission.117 Copyright 2025, Elsevier. | ||
Li et al. further systematically evaluated six representative quinone compounds, namely 1,4-naphthoquinone (1,4-NQ), 1,2-naphthoquinone (1,2-NQ), anthraquinone (9,10-AQ), phenanthrenequinone (9,10-PQ), pyrene-4,5,9,10-tetraone (PTO), and macrocyclic calix[4]quinone (C4Q), in coin-cell configurations (Fig. 15d).113 Among them, C4Q exhibited superior performance due to its large cavity and eight adjacent carbonyl groups, each serving as a redox-active site. As a result, C4Q delivered a higher discharge voltage of almost up to 1 V and a specific capacity of 400 mAh g−1 at 40 mA g−1 (Fig. 15e and f), along with excellent rate capability (300 mAh g−1 at 800 mA g−1), good cycling stability (81% capacity retention after 50 cycles), and outstanding low-temperature performance (224 mAh g−1 at −20 °C). The pouch-type Al-C4Q cell achieved an energy density of 93 Wh kg−1, highlighting the potential of quinone-based materials for practical AAIB applications.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N imine groups, represent a versatile class of organic cathodes for AAIBs. The C
N imine groups, represent a versatile class of organic cathodes for AAIBs. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups act as redox-active centers that reversibly convert to –C–N– during discharge while interacting with Al3+. Their extended π-conjugated frameworks and flexible molecular structures provide abundant active sites, offering high theoretical capacities, analogous to carbonyl-based organic cathodes.
N groups act as redox-active centers that reversibly convert to –C–N– during discharge while interacting with Al3+. Their extended π-conjugated frameworks and flexible molecular structures provide abundant active sites, offering high theoretical capacities, analogous to carbonyl-based organic cathodes.
In 2021, Chen et al. demonstrated that phenazine (PZ) utilizes its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups as redox-active centers, undergoing reversible –C
N groups as redox-active centers, undergoing reversible –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–/–C–N– transformations during Al3+ insertion/extraction in 5 M Al(OTf)3 electrolyte.114 The PZ cathode delivered a high specific capacity of 132 mAh g−1 at 50 mA g−1 and retained 76.5% of its capacity after 300 cycles (Fig. 15g and h). The excellent cycling stability was attributed to minimal structural changes, extensive π-conjugation, and intrinsic molecular flexibility of PZ, which maintain structural integrity during cycling.
N–/–C–N– transformations during Al3+ insertion/extraction in 5 M Al(OTf)3 electrolyte.114 The PZ cathode delivered a high specific capacity of 132 mAh g−1 at 50 mA g−1 and retained 76.5% of its capacity after 300 cycles (Fig. 15g and h). The excellent cycling stability was attributed to minimal structural changes, extensive π-conjugation, and intrinsic molecular flexibility of PZ, which maintain structural integrity during cycling.
However, small-molecule N-heteroaromatic electrodes often suffer from limited electronic conductivity and partial solubility in aqueous electrolytes, compromising long-term performance.115 To address these limitations, Li et al. designed dipyrido[3,2-a:2′,3′-c]phenazine-11-carbonitrile (DPPZ-CN), a multifunctional small-molecule cathode incorporating pyridine, pyrazine, and cyano groups (Fig. 15i).116 The highly conjugated N-heteroaromatic backbone enhances π–π stacking and hydrogen bonding, while the electron-withdrawing cyano group modulates electronic delocalization, improving conductivity and increasing working voltage. DPPZ-CN exhibited 211.9 mAh g−1 at 100 mA g−1 with a voltage plateau of 0.62 V and retained 117.7 mAh g−1 after 1000 cycles at 1 A g−1, corresponding to a capacity retention of 82.4% (Fig. 15j and k). Building on this strategy, Lu et al. synthesized diquinoxalino[2,3-a:2′,3′-c]phenazine-2,3,8,9,14,15-hexacarbonitrile (DQP-6CN) functionalized with six cyano groups.117 The introduction of multiple cyano groups not only increased the number of redox-active sites but also strengthened π-conjugation and reduced local electron density, thus enhancing capacity, electronic conductivity and structural stability. In 5 M Al(OTf)3 electrolyte, DQP-6CN delivered a high reversible capacity of 279 mAh g−1 at 400 mA g−1, surpassing that of unsubstituted DQP (193.5 mAh g−1) (Fig. 15l). Cyclic voltammetry (CV) revealed multiple distinct redox peaks for DQP-6CN, confirming the simultaneous participation of imine and cyano groups in the redox processes (Fig. 15m). Despite these advances, N-heteroaromatic cathodes generally operate at discharge voltages below 0.8 V, lower than that for quinone-based materials, which remains a limitation for their broader applications in AAIBs.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups into a planar π-conjugated framework enables multiple redox-active sites and enhanced intermolecular interactions. The carbonyl and imine groups both provide reversible electron-transfer centers, increasing theoretical capacity, while the extended π-conjugation improves electronic conductivity, suppresses dissolution, and enhances structural stability. Consequently, π-conjugated carbonyl-imine hybrid compounds have emerged as a promising design strategy for high-performance organic cathodes in AAIBs.
N groups into a planar π-conjugated framework enables multiple redox-active sites and enhanced intermolecular interactions. The carbonyl and imine groups both provide reversible electron-transfer centers, increasing theoretical capacity, while the extended π-conjugation improves electronic conductivity, suppresses dissolution, and enhances structural stability. Consequently, π-conjugated carbonyl-imine hybrid compounds have emerged as a promising design strategy for high-performance organic cathodes in AAIBs.
In 2024, Su et al. designed a fully conjugated heterocyclic molecule, tribenzoquinoxaline-5,10-dione (3BQ), derived from 9,10-anthraquinone (AQ), featuring both carbonyl and imine groups for AAIBs (Fig. 16a).118 The multiple redox centers and expanded π-conjugated framework endowed 3BQ with a high theoretical capacity of 515 mAh g−1. When composited with conductive carbon nanotubes (CNTs) featuring highly delocalized π-bonds, the resulting 3BQ-CNT hybrid exhibited enhanced π–π interactions, improved electronic delocalization, and stabilized molecular packing, facilitating efficient Al3+ transport. Consequently, 3BQ-CNTs delivered 445.84 mAh g−1 at 0.05 A g−1 and maintained 125.7 mAh g−1 after over 4000 cycles at 1.0 A g−1, with a per-cycle decay rate of 0.011% (Fig. 16b and c).
 | ||
| Fig. 16 (a) Schematic illustration of molecular design from AQ to TAQC and 3BQ via expanding the π-conjugation strategy. (b) Typical galvanostatic charge/discharge profiles of the 3BQ-CNTs//Al and 3BQ//Al batteries at 0.05 A g−1. (c) Long-cycle performance of 3BQ-CNTs electrodes at 1.0 A g−1. Reproduced with permission.118 Copyright 2024, Wiley-VCH. (d) Synthesis pathway of DDQP. (e) LUMO and HOMO of PQ, TABQ and DDQP and the energy level gap. (f) First charge/discharge curves of PQ, TABQ and DDQP at 400 mA g−1. Reproduced with permission.119 Copyright 2024, Elsevier. (g) Mechanistic description of the reversible redox reaction of BQPT and (h) GCD curves of DND, PTO, and BQPT electrodes at 0.4 A g−1. Reproduced with permission.120 Copyright 2024, American Chemical Society. | ||
Building on this hybrid design concept, Lu et al. synthesized DDQP, a π-conjugated planar carbonyl and imine conjugated backbone, via dehydration condensation of phenanthrenequinone (PQ) and 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione (TABQ) (Fig. 16d).119 Theoretical calculations revealed that DDQP exhibits higher electronic density of states near the Fermi level, a lower lowest unoccupied molecular orbital (LUMO) energy, and a reduced LUMO–HOMO gap than its precursors, indicating stronger electron affinity and improved charge transport ability (Fig. 16e). Electrochemical measurements revealed that DDQP delivered 239.54 mAh g−1 at 400 mA g−1, nearly twice that of PQ and TABQ (Fig. 16f), along with improved rate capability and cycling stability. Similarly, Hu et al. synthesized BQPT (benzo[i]benzo[6,7]quinoxalino[2,3,9,10]phenanthrol[4,5-abc]phenazine-5,10,16,21-tetraone), a multi-redox hybrid containing four carbonyl and four imine groups, via a dehydration condensation strategy.120 Both C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups participate in redox processes during discharge, collectively accepting eight electrons and coordinating with eight Al(OTF)2+ cations (Fig. 16g). This multi-electron mechanism enabled a high capacity of 321.71 mAh g−1 at 0.4 A g−1 (Fig. 16h). Therefore, rational functional group integration and conjugated backbone design offer a feasible and effective route to enhance the electrochemical performance of organic cathodes.
N groups participate in redox processes during discharge, collectively accepting eight electrons and coordinating with eight Al(OTF)2+ cations (Fig. 16g). This multi-electron mechanism enabled a high capacity of 321.71 mAh g−1 at 0.4 A g−1 (Fig. 16h). Therefore, rational functional group integration and conjugated backbone design offer a feasible and effective route to enhance the electrochemical performance of organic cathodes.
Overall, these studies demonstrate that incorporating both carbonyl and imine groups within an extended π-conjugated framework is an effective strategy to enhance the electrochemical performance of AAIB cathodes. Such hybrid molecular design overcomes the limitations of single-type redox centers, providing a promising pathway for the development of high-performance organic cathodes for AAIBs. Nevertheless, both small and macro-molecular organic cathodes are prone to dissolution, which can be mitigated by incorporating them into conductive polymers, as discussed in the following section.
Building on this foundation, in 2022, Sariyer et al. synthesized poly(o-phenylenediamine) (PoPD) via electropolymerization. Using 0.25 M AlCl3 as the electrolyte in a three-electrode system, PoPD delivered an average discharge potential of −0.20 V and a capacity of 156 mAh g−1 at 5C (Fig. 17a).123 Even after 1000 cycles, 110 mAh g−1 was retained (Fig. 17b), demonstrating excellent cycling stability. Building upon this, Meng et al. functionalized PANI with sulfonic acid groups to obtain SPANI, which enhanced both conductivity and ion–polymer interactions.124 In an aqueous deep eutectic electrolyte composed of Al(ClO4)3·9H2O and succinonitrile (SN), SPANI exhibited a capacity of 185 mAh g−1 at 0.1 A g−1, outperforming pristine PANI (143 mAh g−1) (Fig. 17c), and retained 165 mAh g−1 with 89% capacity retention after 300 cycles. XPS analysis revealed the redox mechanism: during charging, the reduced –NH– groups in SPANI were oxidized to –NH+– and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH+–, which coordinated with ClO4–, while during discharging, the oxidized groups were reduced back and coordinated with Al3+ (Fig. 17d). This study highlighted that incorporating the –SO3H group simultaneously improved conductivity, ion interaction and electrochemical reversibility, offering valuable guidelines for the molecular design of conductive polymer cathodes.
NH+–, which coordinated with ClO4–, while during discharging, the oxidized groups were reduced back and coordinated with Al3+ (Fig. 17d). This study highlighted that incorporating the –SO3H group simultaneously improved conductivity, ion interaction and electrochemical reversibility, offering valuable guidelines for the molecular design of conductive polymer cathodes.
 | ||
| Fig. 17 (a) C-Rate performance in 0.25 M AlCl3. (b) Long-term cycling performance at 5C in 0.25 M AlCl3. Reproduced with permission.123 Copyright 2022, American Chemical Society. (c) The discharge/charge curves at different current densities and (d) schematic diagram of the charge/discharge mechanism of the SPANI/ASHEE/Al battery. Reproduced with permission.124 Copyright 2022, Wiley-VCH. (e) Illustration of the electropolymerization process of PDAP. (g) The UV-vis spectra of DAP-soaked, PDAP-soaked electrolyte, and fresh electrolyte (1 M Al(ClO4)3). (h) HOMO–LUMO energy gaps for the optimized models with different molecular chain lengths (monomer, dimer, trimer, and tetramer). Reproduced with permission.125 Copyright 2024, Wiley-VCH. (f) Schematic illustration of the solvent-dependent redox behavior of TEMPO in acetonitrile and aqueous electrolytes. (i) Typical charge/discharge voltage profiles of the PTMA cathodes cycled at 0.5 to 8C. (j) Long cycling performance and coulombic efficiency of PTMA-based AAIBs at 4C. Reproduced with permission.129 Copyright 2023, American Chemical Society. | ||
Extending these advances, Wang et al. developed carbon-supported poly(2,3-diaminophenazine) (PDAP) cathodes via electropolymerization (Fig. 17e).125 The PDAP framework, containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and –NH– groups, provided abundant redox-active sites. In 1 M Al(ClO4)3 electrolyte, the Al//PDAP cell delivered a high capacity of 338 mAh g−1 at 0.2 A g−1 and maintained 233 mAh g−1 after 100 cycles. Even at 2 A g−1, 86 mAh g−1 was retained after 1000 cycles (65% retention). PDAP also exhibited excellent temperature tolerance, delivering 155 mAh g−1 at −20 °C and up to 348 mAh g−1 at 45 °C. These outstanding properties originated from the strong π–π stacking and N–H⋯N hydrogen bonding among PDAP chains, which suppressed dissolution, as evidenced by UV-vis spectroscopy of electrolyte after soaking (Fig. 17g). Theoretical calculations further confirmed that increasing the chain length from monomer to tetramer narrowed the bandgap (from 1.525 to 1.058 eV) and increased the density of states near the Fermi level, indicating enhanced electron delocalization and faster charge transfer (Fig. 17h). This work not only provided a promising strategy for designing high-performance organic cathodes for AAIBs, but also demonstrated their stable operation over a wide temperature range.
N and –NH– groups, provided abundant redox-active sites. In 1 M Al(ClO4)3 electrolyte, the Al//PDAP cell delivered a high capacity of 338 mAh g−1 at 0.2 A g−1 and maintained 233 mAh g−1 after 100 cycles. Even at 2 A g−1, 86 mAh g−1 was retained after 1000 cycles (65% retention). PDAP also exhibited excellent temperature tolerance, delivering 155 mAh g−1 at −20 °C and up to 348 mAh g−1 at 45 °C. These outstanding properties originated from the strong π–π stacking and N–H⋯N hydrogen bonding among PDAP chains, which suppressed dissolution, as evidenced by UV-vis spectroscopy of electrolyte after soaking (Fig. 17g). Theoretical calculations further confirmed that increasing the chain length from monomer to tetramer narrowed the bandgap (from 1.525 to 1.058 eV) and increased the density of states near the Fermi level, indicating enhanced electron delocalization and faster charge transfer (Fig. 17h). This work not only provided a promising strategy for designing high-performance organic cathodes for AAIBs, but also demonstrated their stable operation over a wide temperature range.
Overall, these studies illustrate the versatility of conductive polymers as AAIB cathodes. Progressing from PoPD to functionalized PANI and PDAP, rational molecular design through functional group incorporation and extension of the conjugated backbone has proven effective in improving capacity, conductivity, and cycling stability. These findings highlight conductive polymers as a tunable platform for designing robust, high-performance organic cathodes for AAIBs.
In 2023, Jiang et al. systematically investigated the electrochemical behavior of TEMPO radicals in both organic and aqueous media.129 They found that TEMPO undergoes irreversible disproportionation in acetonitrile due to the formation of aminoxyl anions (TEMPO−), which coordinate with excess Al(OTf)3, rendering the redox process irreversible. In contrast, in aqueous electrolytes, TEMPO− in [Al(OTf)3·TEMPO]− can be substituted by OH− or water molecules, enabling a fully reversible TEMPO+/TEMPO− redox cycle and preserving the radical activity (Fig. 17f). Building on this understanding, the authors prepared the first TEMPO radical polymer cathode, poly(TEMPO methacrylate) (PTMA). In 1 M Al(OTf)3 electrolyte, the PTMA cathode delivered a discharge capacity of approximately 120 mAh g−1 at 4C and retained 77% after 800 cycles, corresponding to a low per-cycle decay rate of 0.028% (Fig. 17i and j). This work highlights the critical role of aqueous environments in stabilizing TEMPO radicals and demonstrates the potential of non-conjugated radical polymers as high-performance cathodes for AAIBs. Overall, the electrochemical performance of various cathode materials discussed in this review is summarized in Table 1.
| Cathode material | Anode material | Electrolyte | Discharge voltage (V) | Current density (mA g−1) | Specific capacity (mAh g−1) | Cycle number | Ref. |
|---|---|---|---|---|---|---|---|
| α-MnO2 | Al | 2 M Al(OTf)3 | 1.3 | 100 | 380 | 40 | 45 |
| α-MnO2 | Al | 5 M Al(OTf)3 | 1.5 | 100 | 480 | 180 | 46 |
| γ-MnO2 | Al | 5 M Al(OTf)3 | 1.5 | 200 | 482 | 200 | 47 |
| δ-MnO2 | Al | 2 M Al(OTf)3 + 0.5 M MnSO4 | 1.35 | 100 | 554 | 65 | 48 |
| δ-MnO2 | Pt-sputtered Al | 5 M Al(OTf)3 | 1.1 | 50 | 452 | 50 | 49 |
| AlxMnO2·nH2O | Al–Cu | 2 M Al(OTf)3 | 1.5 | 500 | 400 | 400 | 51 |
| AlxMnO2·nH2O | Al | 5 M Al(OTf)3 | 1.2 | 30 | 467 | 60 | 52 |
| AlxMnO2 | Zn–Al | 2 M Al(OTf)3 | 1.6 | 100 | 460 | 80 | 53 |
| BQ-AlxMnO2 | Zn50Al50 alloy | 2 M Al(OTf)3 + 0.2 M Mn(OTf)2 | 1.6 | 1000 | 300 | 800 | 55 |
| Cu2+-pre-intercalated δ-MnO2 | Al | 5 M Al(OTf)3 | 1.5 | 400 | 363 | 300 | 56 |
| Co-doped δ-MnO2 | Al | 2 M Al(OTf)3 | 1.4 | 100 | 585 | 300 | 59 |
| V-doped δ-MnO2 | Al–Cu | 2 M Al(OTf)3 | 1.14 | 200 | 518 | 400 | 60 |
| V2O5 | Al | 2 M Al(OTf)3 | 0.9 | 20 | 200 | 50 | 38 |
| V2O5 | Al | 5 M Al(OTf)3 | — | 400 | 400 | 200 | 65 |
| V2O5@MXene | Al | 5 M Al(OTf)3 | — | 100 | 626 | 100 | 66 |
| VO2 | Al | 5 M Al(OTf)3 | — | 150 | 234 | 1000 | 69 |
| Cu-doped VO2 | Al | 5 M Al(OTf)3 | — | 400 | 642 | 200 | 71 |
| LiV3O8 | Activated carbon | 2 M Al(OTf)3 | — | 0.29C | 289 | 500 | 72 |
| VOPO4·2H2O | Al | 2.5 M Al(OTf)3 | 0.9 | 20 | 125 | 40 | 80 |
| CuHCF | Graphite | 0.5 M Al2(SO4)3 | — | 50 | 63 | 1000 | 90 |
| CuHCF | — | 1 M AlCl3 | — | 1000 | 54.5 | 5000 | 93 |
| CuHCF | MoO3 | 3 M AlCl3 | 0.6 | 3000 | 48 | 150 | 94 |
| FeFe-PBA | Deep eutectic-modified Al | 2 M Al(OTf)3 | — | 100 | 85 | 150 | 96 |
| KNHCF | Al | 5 M Al(OTf)3 | 0.7 | 20 | 46.5 | 500 | 97 |
| V-PBA | Zn | Deep eutectic | 1.2 | 100 | 161 | 500 | 98 |
| MnFe-PBA | Al | 1 M Al(OTf)3 | 0.9 | 200 | 106 | 50 | 99 |
| Medium-entropy PBA | MoO | 3 M Al(OTf)3 | — | 500 | 60.9 | 600 | 101 |
| MgMn-PBA | MoO3 | 1 M Al(NO3)3 | — | 1000 | 148 | 2000 | 105 |
| Fe–Co PBA/rGO | MoO3 | 1 M AlCl3 | — | 100 | 112.5 | 1500 | 106 |
| TCQ | Zn–Al | 1 M Al(OTf)3 | — | 200 | 148 | 200 | 112 |
| C4Q | Al | 1 M Al(OTf)3 | 1.0 | 40 | 400 | 50 | 113 |
| PZ | Al | 5 M Al(OTf)3 | 0.6 | 50 | 132 | 300 | 114 |
| DPPZ-CN | Al | 1 M Al(ClO4)3 | 0.6 | 100 | 212 | 1000 | 116 |
| DQP-6CN | Al | 5 M Al(OTf)3 | 0.8 | 400 | 279 | 200 | 117 |
| 3BQ-CNT | Al | 1 M Al(OTf)3 | — | 50 | 446 | 4000 | 118 |
| SPANI | Al | Al(ClO4)3·9H2O + succinonitrile | 0.5 | 100 | 185 | 300 | 124 |
| PDAP | Al | 1 M Al(ClO4)3 | — | 200 | 338 | 100 | 125 |
| poly(TEMPO methacrylate) | Al | 1 M Al(OTf)3 | 1.1 | 4C | 120 | 800 | 129 |
4. Summary and prospects
AAIBs as an emerging electrical energy storage technology, combine high safety, environmental friendliness and low cost, demonstrating promising potential for green energy applications. Despite notable progress in electrode material design and electrolyte optimization in recent years, the development of AAIBs remains at an early stage, and their commercial deployment is still a considerable distance away. Key limiting factors include the sluggish migration kinetics of multivalent Al3+ and insufficient structural reversibility of cathode materials during repeated charge/discharge cycles, which lead to capacity fading and restricted cycle life. These issues significantly constrain AAIBs from achieving high capacity, high power density, and long-term stability.Regarding cathode materials, different types exhibit distinct structural features and ion/electron transport properties, which determine their electrochemical performance and associated challenges. This review systematically summarizes recent advances in AAIBs cathode materials, focusing on four representative systems: manganese-based oxides, vanadium-based compounds, PBAs, and organic materials. Specifically:
(1) Manganese oxides are among the most widely studied AAIB cathodes due to their abundance, low cost, diverse crystal structures and multiple electron-transfer capability. Layered δ-MnO2, with large interlayer spacing and two-dimensional diffusion channels, facilitates Al3+ diffusion and generally exhibits high specific capacity, whereas tunnel-type MnO2 (α, β, and γ) with narrower channels limits Al3+ diffusion and storage, resulting in lower capacity and sluggish kinetics. Although layered structures offer higher capacity, they often suffer from structural instability during cycling, undergoing in situ phase transformations to thermodynamically more stable AlxMnO2 phases. While this process can partially relieve lattice strain, it often causes capacity decay. Unmodified MnO2 cathodes can deliver initial capacities of 500–600 mAh g−1, but structural collapse and Mn dissolution hinder stable cycling beyond 200 cycles. Strategies such as molecular or metal-ion pre-intercalation or doping have been developed to expand interlayer spacing, mitigate lattice strain, enhance structural flexibility, and prolong cycling stability. However, while pre-intercalation or doping strategies can effectively enlarge the interlayer spacing, they may slightly perturb the crystal structure, which could influence long-term structural stability during repeated cycling. In addition, the strong interaction between Al3+ ions and the metal–oxygen framework may further affect such structural changes. Future strategies should aim to balance high initial capacity with durable structural integrity.
(2) Vanadium-based cathodes mainly include layered V2O5, tunnel-type VO2, vanadates, and vanadium phosphates. Layered V2O5 provides large interlayer spacing conducive to Al3+ storage but suffers from lattice contraction and structural degradation upon cycling. Tunnel-type VO2 maintains structural integrity but limits ion diffusion due to fixed tunnel size. Vanadates utilize pre-inserted cations as “pillars” to expand interlayer spacing, enhancing structural stability, whereas vanadium phosphates feature robust 3D frameworks but limited electronic conductivity due to [PO4] units. Overall, V-based materials often exhibit lower capacity than Mn-based oxides. Similar to Mn-based oxides, although strategies such as defect engineering, metal-ion doping, nanoscale structuring, and conductive composite design can improve the electrochemical performance of V-based cathodes, achieving high capacity, fast kinetics, and long-term stability simultaneously remains challenging.
(3) PBAs offer tunable frameworks, long cycle life, and multiple redox-active centers, showing great promise in AAIBs. Depending on the number of active centers, PBAs are classified as single or dual redox-active center systems. Single-center PBAs are limited by Fe2+/Fe3+ charge transfer, with capacities typically in tens of mAh g−1, while dual-center PBAs leverage multi-metal synergy to achieve capacities above 100 mAh g−1. Their remarkable advantage lies in cycling stability, with thousands to tens of thousands of cycles achievable, far exceeding those of Mn- and V-based cathodes. Current strategies to enhance performance include defect engineering to improve ion diffusion, entropy regulation to increase structural robustness via multi-metal centers, and nanostructure design to shorten ion transport paths and buffer volume changes. Nevertheless, the relatively low intrinsic capacity of PBAs, together with the limited utilization of redox-active sites, remains a key bottleneck for their application in high-energy AAIBs. Future work should focus on further optimizing framework design to achieve high capacity, high rate, and long lifespan concurrently.
(4) Organic cathodes, composed mainly of C, H, O, and N, possess tunable molecular backbones and flexible structures, alleviating volume strain during charge/discharge. Compared with the strong electrostatic interactions in inorganic cathodes, weak coordination or ion–dipole interactions within the organic framework facilitate Al3+ ion migration. Energy storage relies on reversible redox-active functional groups, such as carbonyls, imines, or TEMPO radicals. Based on the type of redox-active site, organic cathodes are categorized into quinone-based compounds, N-heteroaromatics, π-conjugated carbonyl-imine hybrids, conductive polymers, and radical polymers. Overall, organic materials typically exhibit better cycling stability than their inorganic counterparts, with capacities higher than those of PBAs but lower than those of Mn oxides. Performance can be improved via functional group integration, backbone conjugation optimization, and conductive composite formation. Nevertheless, challenges such as active material dissolution, limited energy density, and balancing molecular stability with high redox activity still hinder practical applications, calling for rational molecular and composite design to achieve high capacity, fast kinetics, and long-term cycling stability.
To further advance the development of high-performance AAIBs, future research should focus on the following directions:
(1) Design and optimization of cathode materials. The structural and chemical characteristics of the cathode critically govern Al3+ accessibility, migration kinetics, and the reversibility of Al3+–cathode interactions. For inorganic cathodes, strategies such as defect engineering, molecular pre-intercalation, heteroatom doping, and conductive network construction should be further explored to expand interlayer spacing, stabilize frameworks, and create flexible coordination environments. Importantly, future efforts should emphasize establishing clear structure–chemistry–electrochemistry relationships, enabling predictive and rational cathode design. These approaches are expected to mitigate lattice strain during Al3+–cathode interactions and enhance charge transfer, enabling higher capacity, improved rate capability, and long-term cycling stability. For organic cathodes, future research should focus on the rational design of multifunctional groups and the modulation of molecular backbone flexibility to facilitate more Al3+ interactions with active sites. Combined with optimized π-conjugation and conductive composite formation, these strategies can improve ion/electron transport, suppress dissolution and achieve synergistic enhancements in electrochemical performance. Overall, a multiscale, material-tailored design strategy offers a promising pathway toward high-performance and durable AAIB cathodes.
(2) Mechanism insight through advanced characterization and theoretical modeling. In situ techniques (XRD, XAS, Raman spectroscopy, etc.) combined with first-principles calculations and molecular dynamics simulations can provide atomic-level understanding of ion migration pathways, redox mechanisms, and interfacial processes. Particular attention should be placed on distinguishing Al3+-dominated storage from proton-assisted or co-insertion mechanisms, which remains a key challenge in aqueous systems. Correlating structural evolution induced by Al3+–cathode interactions with electrochemical performance will offer theoretical guidance for rational cathode design.
(3) Sustainable materials and scalable device engineering. Research should integrate material sustainability with device-level optimization, bridging laboratory studies and practical applications. Development of earth-abundant, environmentally friendly, and low-cost materials, including bio-derived organic and inorganic–organic hybrid frameworks, will enhance AAIB sustainability. Simultaneously, full-cell design optimization and systematic evaluation of long-term stability, safety, and durability under realistic conditions, including extreme cold or high-temperature environments, are essential. Coordinated design of sustainable materials and scalable devices will provide a solid foundation for the practical deployment of AAIBs in green energy storage.
Author contributions
J. Feng collected papers related to the topic and wrote the manuscript. C. Yu and X. Huang conceived the overall idea of the perspective. The manuscript was revised by all the authors.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
The authors acknowledge the support from the Australian Research Council, the Queensland node of the NCRIS-enabled Australian National Fabrication Facility (ANFF), and the Centre for Microscopy and Microanalysis, The University of Queensland.References
- J. Li, J. Fleetwood, W. B. Hawley and W. Kays, From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing, Chem. Rev., 2022, 122, 903–956 CrossRef CAS PubMed.
- A. Manthiram, A reflection on lithium-ion battery cathode chemistry, Nat. Commun., 2020, 11, 1550 CrossRef CAS PubMed.
- J. Xu, X. Cai, S. Cai, Y. Shao, C. Hu, S. Lu and S. Ding, High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications, Energy Environ. Mater., 2023, 6, e12450 CrossRef CAS.
- E. J. M. Dugamin, A. Richard, M. Cathelineau, M. C. Boiron, F. Despinois and A. Brisset, Groundwater in sedimentary basins as potential lithium resource: a global prospective study, Sci. Rep., 2021, 11, 21091 Search PubMed.
- L. Han, L. Wang, Z. Chen, Y. Kan, Y. Hu, H. Zhang and X. He, Incombustible Polymer Electrolyte Boosting Safety of Solid-State Lithium Batteries: A Review, Adv. Funct. Mater., 2023, 33, 2300892 Search PubMed.
- Y. Chen, Y. Kang, Y. Zhao, L. Wang, J. Liu, Y. Li, Z. Liang, X. He, X. Li, N. Tavajohi and B. Li, A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards, J. Energy Chem., 2021, 59, 83–99 CrossRef CAS.
- X. Gao, Y.-N. Zhou, D. Han, J. Zhou, D. Zhou, W. Tang and J. B. Goodenough, Thermodynamic Understanding of Li-Dendrite Formation, Joule, 2020, 4, 1864–1879 Search PubMed.
- X. Cui, J. Wang, S. Sun, X. Chen, Y. Wang, D. Han, J. Wang, X. Yao and W. Yan, Safety Hazards of Lithium Metal Batteries: From the Perspective of Lithium Dendrites and Thermal Runaway, Energy Fuels, 2025, 39, 7665–7690 CrossRef CAS.
- P. K. Nayak, L. Yang, W. Brehm and P. Adelhelm, From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises, Angew. Chem., Int. Ed., 2018, 57, 102–120 CrossRef CAS.
- T. L. Kulova, V. N. Fateev, E. A. Seregina and A. S. Grigoriev, A Brief Review of Post-Lithium-Ion Batteries, Int. J. Electrochem. Sci., 2020, 15, 7242–7259 CrossRef.
- W. Du, E. H. Ang, Y. Yang, Y. Zhang, M. Ye and C. C. Li, Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries, Energy Environ. Sci., 2020, 13, 3330–3360 RSC.
- D. Wang, Z. Zhang, Y. Hao, H. Jia, X. Shen, B. Qu, G. Huang, X. Zhou, J. Wang, C. Xu and F. Pan, Challenges and Progress in Rechargeable Magnesium-Ion Batteries: Materials, Interfaces, and Devices, Adv. Funct. Mater., 2024, 34, 2410406 CrossRef CAS.
- T. Dutta and J. Mary Gladis, Recent developments on electrode materials and electrolytes for aluminium-ion batteries, J. Energy Storage, 2024, 86, 111287 CrossRef.
- M. Ye, Y. Guan, R. Xu, P. Wang, Y. Zhang, J. Yu, D. Li, L. Li, Q. Zhao, Z. Wang, J. Liang and Y. Wu, Aqueous potassium-ion battery cathodes: Current status and prospects, J. Energy Chem., 2025, 106, 650–670 CrossRef CAS.
- H. Zhang, L. Qiao and M. Armand, Organic Electrolyte Design for Rechargeable Batteries: From Lithium to Magnesium, Angew. Chem., Int. Ed., 2022, 61, e202214054 Search PubMed.
- H. Aziam, B. Larhrib, C. Hakim, N. Sabi, H. Ben Youcef and I. Saadoune, Solid-state electrolytes for beyond lithium-ion batteries: A review, Renew. Sustain. Energy Rev., 2022, 167, 112694 CrossRef CAS.
- A. A. Yaroshevsky, Abundances of chemical elements in the Earth's crust, Geochem. Int., 2006, 44, 48–55 CrossRef.
- X. Zheng, C. Han, C.-S. Lee, W. Yao, C. Zhi and Y. Tang, Materials challenges for aluminum ion based aqueous energy storage devices: Progress and prospects, Prog. Mater. Sci., 2024, 143, 101253 CrossRef CAS.
- Y. Zhang, S. Liu, Y. Ji, J. Ma and H. Yu, Emerging Nonaqueous Aluminum-Ion Batteries: Challenges, Status, and Perspectives, Adv. Mater., 2018, 30, 1706310 CrossRef.
- J. Tu, W. L. Song, H. P. Lei, Z. J. Yu, L. L. Chen, M. Y. Wang and S. Q. Jiao, Nonaqueous Rechargeable Aluminum Batteries: Progresses, Challenges, and Perspectives, Chem. Rev., 2021, 121, 4903–4961 Search PubMed.
- B.-E. Jia, A. Q. Thang, C. Yan, C. Liu, C. Lv, Q. Zhu, J. Xu, J. Chen, H. Pan and Q. Yan, Rechargeable Aqueous Aluminum-Ion Battery: Progress and Outlook, Small, 2022, 18, 2107773 CrossRef CAS.
- D. Chao, W. Zhou, F. Xie, C. Ye, H. Li, M. Jaroniec and S.-Z. Qiao, Roadmap for advanced aqueous batteries: From design of materials to applications, Sci. Adv., 2020, 6, eaba4098 Search PubMed.
- E. Levi, M. D. Levi, O. Chasid and D. Aurbach, A review on the problems of the solid state ions diffusion in cathodes for rechargeable Mg batteries, J. Electroceram., 2009, 22, 13–19 CrossRef CAS.
- F. Wu, H. Yang, Y. Bai and C. Wu, Paving the Path toward Reliable Cathode Materials for Aluminum-Ion Batteries, Adv. Mater., 2019, 31, 1806510 Search PubMed.
- D. Ma, J. Li, H. Li, D. Yuan, Z. Ji, M. Manawan, C. P. de León Albarran, C. Wu and J. H. Pan, Progress of Advanced Cathode Materials of Rechargeable Aluminum-Ion Batteries, Energy Mater. Adv., 2024, 5, 0088 CrossRef CAS.
- K. Bhimani, A. Anjan, V. Mahajani, R. M. Manoj and N. Koratkar, Tuning the Aluminum–Water Interface in Aqueous Aluminum Metal Batteries, ACS Appl. Energy Mater., 2025, 8, 6194–6202 CrossRef CAS.
- X. Geng, X. Hou, X. He and H. J. Fan, Challenges and Strategies on Interphasial Regulation for Aqueous Rechargeable Batteries, Adv. Energy Mater., 2024, 14, 2304094 CrossRef CAS.
- S. Chen, N. A. Gadelhak, Y. Wu, J. Feng, C. Yu, X. Huang and A. K. Nanjundan, Aqueous Aluminium-Ion Batteries: Cathode Material Design, Anode Engineering and Electrolyte Innovation, Small, 2025, e07888 Search PubMed.
- R. Mori, Aqueous rechargeable aluminum battery - a mini review, Energy Adv., 2025, 4, 1321–1336 RSC.
- H. Sun, B. Chen, M. Tan, Y. Li, X. Lang and Q. Jiang, Rational design and construction of aqueous multivalent metal ion battery systems, J. Mater. Chem. A, 2025, 13, 17159–17196 Search PubMed.
- V. Mathew, B. Sambandam, S. Kim, S. Kim, S. Park, S. Lee, M. H. Alfaruqi, V. Soundharrajan, S. Islam, D. Y. Putro, J.-Y. Hwang, Y.-K. Sun and J. Kim, Manganese and Vanadium Oxide Cathodes for Aqueous Rechargeable Zinc-Ion Batteries: A Focused View on Performance, Mechanism, and Developments, ACS Energy Lett., 2020, 5, 2376–2400 CrossRef CAS.
- Y. Xu, G. Zhang, J. Liu, J. Zhang, X. Wang, X. Pu, J. Wang, C. Yan, Y. Cao, H. Yang, W. Li and X. Li, Recent Advances on Challenges and Strategies of Manganese Dioxide Cathodes for Aqueous Zinc-Ion Batteries, Energy Environ. Mater., 2023, 6, e12575 Search PubMed.
- R. Liu, Z. Liang, Y. Xiang, W. Zhao, H. Liu, Y. Chen and K. An, Recognition of V/V/V Multielectron Reactions in Na3V(PO4)2: A Potential High Energy Density Cathode for Sodium-Ion Batteries, Molecules, 2020, 25, 1000 CrossRef CAS.
- P. M. Hari Prasad, G. Malavika, A. Pillai, S. Sadan and Z. S. Pillai, Emerging organic electrode materials for sustainable batteries, NPG Asia Mater., 2024, 16, 37 Search PubMed.
- Z. Song and H. Zhou, Towards sustainable and versatile energy storage devices: an overview of organic electrode materials, Energy Environ. Sci., 2013, 6, 2280–2301 RSC.
- X. Wang, Z. Xi and Q. Zhao, Progress on aqueous rechargeable aluminium metal batteries, Ind. Chem. Mater., 2024, 3, 7–30 Search PubMed.
- H. Liu, Q. Zhou, Q. Xia, Y. Lei, X. Long Huang, M. Tebyetekerwa and X. Song Zhao, Interface challenges and optimization strategies for aqueous zinc-ion batteries, J. Energy Chem., 2023, 77, 642–659 Search PubMed.
- Q. Zhao, L. Liu, J. Yin, J. Zheng, D. Zhang, J. Chen and L. A. Archer, Proton Intercalation/De-Intercalation Dynamics in Vanadium Oxides for Aqueous Aluminum Electrochemical Cells, Angew. Chem., Int. Ed., 2020, 59, 3048 CrossRef CAS PubMed.
- X. Luo, R. Wang, L. Zhang, Z. Liu, H. Li, J. Mao, S. Zhang, J. Hao, T. Zhou and C. Zhang, Air-Stable and Low-Cost High-Voltage Hydrated Eutectic Electrolyte for High-Performance Aqueous Aluminum-Ion Rechargeable Battery with Wide-Temperature Range, ACS Nano, 2024, 18, 12981–12993 Search PubMed.
- Y. Guo, Y. Zhang and H. Lu, Manganese-based materials as cathode for rechargeable aqueous zinc-ion batteries, Battery Energy, 2022, 1, 20210014 CrossRef CAS.
- B. Zhang, P. Dong, S. Yuan, Y. Zhang, Y. Zhang and Y. Wang, Manganese-Based Oxide Cathode Materials for Aqueous Zinc-Ion Batteries: Materials, Mechanism, Challenges, and Strategies, Chem Bio Eng., 2024, 1, 113–132 Search PubMed.
- Y. Yuan, C. Liu, B. W. Byles, W. Yao, B. Song, M. Cheng, Z. Huang, K. Amine, E. Pomerantseva, R. Shahbazian-Yassar and J. Lu, Ordering Heterogeneity of [MnO6] Octahedra in Tunnel-Structured MnO2 and Its Influence on Ion Storage, Joule, 2019, 3, 471–484 CrossRef CAS.
- O. Ghodbane, J.-L. Pascal, B. Fraisse and F. Favier, Structural in Situ Study of the Thermal Behavior of Manganese Dioxide Materials: Toward Selected Electrode Materials for Supercapacitors, ACS Appl. Mater. Interfaces, 2010, 2, 3493–3505 CrossRef CAS PubMed.
- D. Wang, L. Wang, G. Liang, H. Li, Z. Liu, Z. Tang, J. Liang and C. Zhi, A Superior δ-MnO2 Cathode and a Self-Healing Zn-δ-MnO2 Battery, ACS Nano, 2019, 13, 10643–10652 CrossRef CAS PubMed.
- Q. Zhao, M. J. Zachman, W. I. Al Sadat, J. Zheng, L. F. Kourkoutis and L. Archer, Solid electrolyte interphases for high-energy aqueous aluminum electrochemical cells, Sci. Adv., 2018, 4, eaau8131 CrossRef CAS.
- X. Yang, Q. Sun, L. Chai, S. Chen, W. Zhang, H. Y. Yang and Z. Li, α-MnO2 Cathode with Oxygen Vacancies Accelerated Affinity Electrolyte for Dual-Ion Co-Encapsulated Aqueous Aluminum Ion Batteries, Small, 2024, 20, 2400335 CrossRef CAS.
- H. Gu, X. Yang, S. Chen, W. Zhang, H. Y. Yang and Z. Li, Oxygen Vacancies Boosted Proton Intercalation Kinetics for Aqueous Aluminum–Manganese Batteries, Nano Lett., 2023, 23, 11842–11849 CrossRef CAS.
- S. He, J. Wang, X. Zhang, J. Chen, Z. Wang, T. Yang, Z. Liu, Y. Liang, B. Wang, S. Liu, L. Zhang, J. Huang, J. Huang, L. A. O'Dell and H. Yu, A High-Energy Aqueous Aluminum-Manganese Battery, Adv. Funct. Mater., 2019, 29, 1905228 CrossRef CAS.
- G. Li, Y. Zhao, B. Guo, J. Zhang, J. Jia, A. Wang and C. Liu, Architecting a High Specific Energy Aqueous Aluminum–Manganese Battery, Battery Energy, 2025, e70016 CrossRef CAS.
- C. Yan, C. Lv, B.-E. Jia, L. Zhong, X. Cao, X. Guo, H. Liu, W. Xu, D. Liu, L. Yang, J. Liu, H. H. Hng, W. Chen, L. Song, S. Li, Z. Liu, Q. Yan and G. Yu, Reversible Al Metal Anodes Enabled by Amorphization for Aqueous Aluminum Batteries, J. Am. Chem. Soc., 2022, 144, 11444–11455 CrossRef CAS.
- Q. Ran, H. Shi, H. Meng, S.-P. Zeng, W.-B. Wan, W. Zhang, Z. Wen, X.-Y. Lang and Q. Jiang, Aluminum-copper alloy anode materials for high-energy aqueous aluminum batteries, Nat. Commun., 2022, 13, 576 CrossRef CAS PubMed.
- C. Wu, S. Gu, Q. Zhang, Y. Bai, M. Li, Y. Yuan, H. Wang, X. Liu, Y. Yuan, N. Zhu, F. Wu, H. Li, L. Gu and J. Lu, Electrochemically activated spinel manganese oxide for rechargeable aqueous aluminum battery, Nat. Commun., 2019, 10, 73 CrossRef CAS.
- C. Yan, C. Lv, L. Wang, W. Cui, L. Zhang, K. N. Dinh, H. Tan, C. Wu, T. Wu, Y. Ren, J. Chen, Z. Liu, M. Srinivasan, X. Rui, Q. Yan and G. Yu, Architecting a Stable High-Energy Aqueous Al-Ion Battery, J. Am. Chem. Soc., 2020, 142, 15295–15304 CrossRef CAS.
- Y. Liu, G. He, H. Jiang, I. P. Parkin, P. R. Shearing and D. J. L. Brett, Cathode Design for Aqueous Rechargeable Multivalent Ion Batteries: Challenges and Opportunities, Adv. Funct. Mater., 2021, 31, 2010445 CrossRef CAS.
- H. Meng, Q. Ran, M.-H. Zhu, Q.-Z. Zhao, G.-F. Han, T.-H. Wang, Z. Wen, X.-Y. Lang and Q. Jiang, Benzoquinone-Lubricated Intercalation in Manganese Oxide for High-Capacity and High-Rate Aqueous Aluminum-Ion Battery, Small, 2024, 20, 2310722 CrossRef CAS PubMed.
- H. Gu, M. Chen, Z. Wang, W. Zhang and Z. Li, Enhancing H+ intercalation kinetics and stability in Cu2+ pre-intercalated δ-MnO2 for aqueous aluminum batteries, J. Energy Chem., 2025, 102, 126–133 CrossRef CAS.
- A. Zhang, Y. Liang, H. Zhang, Z. Geng and J. Zeng, Doping regulation in transition metal compounds for electrocatalysis, Chem. Soc. Rev., 2021, 50, 9817–9844 RSC.
- P. Gao, Z. Chen, Y. Gong, R. Zhang, H. Liu, P. Tang, X. Chen, S. Passerini and J. Liu, The Role of Cation Vacancies in Electrode Materials for Enhanced Electrochemical Energy Storage: Synthesis, Advanced Characterization, and Fundamentals, Adv. Energy Mater., 2020, 10, 1903780 CrossRef CAS.
- J. Yang, W. Gong and F. Geng, Defect Modulation in Cobalt Manganese Oxide Sheets for Stable and High-Energy Aqueous Aluminum-Ion Batteries, Adv. Funct. Mater., 2023, 33, 2301202 CrossRef CAS.
- S. Chen, Y. Kong, C. Tang, N. A. Gadelhak, A. K. Nanjundan, A. Du, C. Yu and X. Huang, Doping Regulation Stabilizing δ-MnO2 Cathode for High-Performance Aqueous Aluminium-ion Batteries, Small, 2024, 20, 2312229 Search PubMed.
- B. Baatiyah, Y. S. Wudil and M. A. Gondal, Recent advances and challenges in MnO2-based composite cathodes for high-performance flexible zinc-ion batteries: A critical review, J. Energy Storage, 2025, 125, 117000 CrossRef.
- Y. Hu, H. Li, H. Gu, W. Zhang and Z. Li, Al3+ pre-intercalation and g-C3N4 coating synergistically modulate gibbs free energy for robust and compatible MnO2 cathodes in aqueous aluminum batteries, Chem. Eng. J., 2025, 507, 160532 CrossRef CAS.
- J. R. González, F. Nacimiento, M. Cabello, R. Alcántara, P. Lavela and J. L. Tirado, Reversible intercalation of aluminium into vanadium pentoxide xerogel for aqueous rechargeable batteries, RSC Adv., 2016, 6, 62157–62164 Search PubMed.
- P. De, J. Halder, S. Priya, A. K. Srivastava and A. Chandra, Two-Dimensional V2O5 Nanosheets as an Advanced Cathode Material for Realizing Low-Cost Aqueous Aluminum-Ion Batteries, ACS Appl. Energy Mater., 2023, 6, 753–762 CrossRef CAS.
- Z. Wang, H. Gu, T. Wu, W. Zhang and Z. Li, Enhanced dynamics of Al3+/H+ ions in aqueous aluminum ion batteries: Construction of metastable structures in vanadium pentoxide upon oxygen vacancies, J. Energy Chem., 2025, 101, 562–569 Search PubMed.
- X. Yang, H. Gu, L. Chai, S. Chen, W. Zhang, H. Y. Yang and Z. Li, Construction of V2O5@MXene Cathodes toward a High Specific Capacity Aqueous Aluminum-Ion Battery, Nano Lett., 2024, 24, 8542–8549 CrossRef CAS PubMed.
- S. Lee, X.-G. Sun, A. A. Lubimtsev, X. Gao, P. Ganesh, T. Z. Ward, G. Eres, M. F. Chisholm, S. Dai and H. N. Lee, Persistent Electrochemical Performance in Epitaxial VO2(B), Nano Lett., 2017, 17, 2229–2233 CrossRef CAS.
- Q. He, T. Hu, Q. Wu, C. Wang, X. Han, Z. Chen, Y. Zhu, J. Chen, Y. Zhang, L. Shi, X. Wang, Y. Ma and J. Zhao, Tunnel-Oriented VO2 (B) Cathode for High-Rate Aqueous Zinc-Ion Batteries, Adv. Mater., 2024, 36, 2400888 CrossRef CAS.
- Y. Cai, S. Kumar, R. Chua, V. Verma, D. Yuan, Z. Kou, H. Ren, H. Arora and M. Srinivasan, Bronze-type vanadium dioxide holey nanobelts as high performing cathode material for aqueous aluminium-ion batteries, J. Mater. Chem. A, 2020, 8, 12716–12722 RSC.
- Y. Wang, X. Shi, J. Wang, X. Liu and X. Lu, Nanobelt-like vanadium dioxide with three-dimensional interconnected tunnel structure enables ultrafast Al-ion storage, Mater. Today Energy, 2021, 19, 100578 Search PubMed.
- Z. Wang, H. Gu and Z. Li, Orbital-Modulated Cu-Doped VO2 Nanoflowers via Glucose-Assisted Synthesis: Structural Optimization and Electronic Coupling Engineering for High-Capacity Aqueous Aluminum Ion Batteries, Small, 2025, 21, 2503861 Search PubMed.
- V. Soundharrajan, S. Nithiananth, J. Lee, J. H. Kim, J.-Y. Hwang and J. Kim, LiV3O8 as an intercalation-type cathode for aqueous aluminum-ion batteries, J. Mater. Chem. A, 2022, 10, 18162–18169 RSC.
- R. Liu, J. Liu, Y. Li, C. Zhang and H. Li, Aqueous aluminum-ion batteries based on layered NH4V4O10 nanosheets as cathode, Solid State Sci., 2023, 145, 107315 CrossRef CAS.
- V. P. H. Radhakantha, S. Pradhan and A. J. Bhattacharyya, Exploring Aluminum-Ion (Al3+) Insertion in Ammonium Vanadium Bronze (NH4V4O10) for Aqueous Aluminum-Ion Rechargeable Batteries, J. Phys. Chem. C, 2024, 128, 20025–20034 CrossRef CAS.
- T. Wu, Y. Wang, Z. Wang, W. Zhang and Z. Li, Achieving (NH4)2V10O25·8H2O reversible stable phase transition, fast energy storage, and dynamic characteristics with MXene for aqueous aluminum batteries, Chem. Eng. J., 2024, 499, 155926 CrossRef CAS.
- X. Xu, F. Xiong, J. Meng, X. Wang, C. Niu, Q. An and L. Mai, Vanadium-Based Nanomaterials: A Promising Family for Emerging Metal-Ion Batteries, Adv. Funct. Mater., 2020, 30, 1904398 CrossRef CAS.
- A. Kraytsberg and Y. Ein-Eli, Higher, Stronger, Better … A Review of 5 Volt Cathode Materials for Advanced Lithium-Ion Batteries, Adv. Energy Mater., 2012, 2, 922–939 CrossRef CAS.
- F. Nacimiento, M. Cabello, R. Alcántara, P. Lavela and J. L. Tirado, NASICON-type Na3V2(PO4)3 as a new positive electrode material for rechargeable aluminium battery, Electrochim. Acta, 2018, 260, 798–804 Search PubMed.
- P. Wang, Z. Chen, H. Wang, Z. Ji, Y. Feng, J. Wang, J. Liu, M. Hu, J. Fei, W. Gan and Y. Huang, A high-performance flexible aqueous Al ion rechargeable battery with long cycle life, Energy Storage Mater., 2020, 25, 426–435 CrossRef.
- Q. Pang, S. Yang, X. Yu, W. He, S. Zhang, Y. Tian, M. Xing, Y. Fu and X. Luo, Realizing reversible storage of trivalent aluminum ions using VOPO4·2H2O nanosheets as cathode material in aqueous aluminum metal batteries, J. Alloys Compd., 2021, 885, 161008 CrossRef CAS.
- Y. Liu, J.-L. Yang, H.-H. Liu, J.-M. Cao, Y. Liu and X.-L. Wu, Effective reversible calcium/aluminum ion intercalation into VOPO4 enabled by organic molecular assistance, J. Mater. Chem. A, 2025, 13, 17404–17410 RSC.
- C. D. Wessells, S. V. Peddada, R. A. Huggins and Y. Cui, Nickel Hexacyanoferrate Nanoparticle Electrodes For Aqueous Sodium and Potassium Ion Batteries, Nano Lett., 2011, 11, 5421–5425 CrossRef CAS PubMed.
- C. D. Wessells, R. A. Huggins and Y. Cui, Copper hexacyanoferrate battery electrodes with long cycle life and high power, Nat. Commun., 2011, 2, 550 CrossRef PubMed.
- J. Peng, W. Zhang, Q. Liu, J. Wang, S. Chou, H. Liu and S. Dou, Prussian Blue Analogues for Sodium-Ion Batteries: Past, Present, and Future, Adv. Mater., 2022, 34, 2108384 CrossRef CAS.
- Q. Liu, Z. Hu, M. Chen, C. Zou, H. Jin, S. Wang, S.-L. Chou, Y. Liu and S.-X. Dou, The Cathode Choice for Commercialization of Sodium-Ion Batteries: Layered Transition Metal Oxides versus Prussian Blue Analogs, Adv. Funct. Mater., 2020, 30, 1909530 CrossRef CAS.
- X.-Y. Fu, L.-L. Zhang, C.-C. Wang, H.-B. Sun and X.-L. Yang, Recent progress of Prussian blue analogues as cathode materials for metal ion secondary batteries, Rare Met., 2025, 44, 34–59 CrossRef CAS.
- J. Song, L. Wang, Y. Lu, J. Liu, B. Guo, P. Xiao, J.-J. Lee, X.-Q. Yang, G. Henkelman and J. B. Goodenough, Removal of Interstitial H2O in Hexacyanometallates for a Superior Cathode of a Sodium-Ion Battery, J. Am. Chem. Soc., 2015, 137, 2658–2664 CrossRef CAS PubMed.
- W. Wang, Y. Gang, J. Peng, Z. Hu, Z. Yan, W. Lai, Y. Zhu, D. Appadoo, M. Ye, Y. Cao, Q.-F. Gu, H.-K. Liu, S.-X. Dou and S.-L. Chou, Effect of Eliminating Water in Prussian Blue Cathode for Sodium-Ion Batteries, Adv. Funct. Mater., 2022, 32, 2111727 CrossRef CAS.
- Z. Li, K. Xiang, W. Xing, W. C. Carter and Y.-M. Chiang, Reversible Aluminum-Ion Intercalation in Prussian Blue Analogs and Demonstration of a High-Power Aluminum-Ion Asymmetric Capacitor, Adv. Energy Mater., 2015, 5, 1401410 CrossRef.
- S. Liu, G. L. Pan, G. R. Li and X. P. Gao, Copper hexacyanoferrate nanoparticles as cathode material for aqueous Al-ion batteries, J. Mater. Chem. A, 2015, 3, 959–962 RSC.
- Z. Zhao, W. Zhang, M. Liu, S. J. Yoo, N. Yue, F. Liu, X. Zhou, K. Song, J.-G. Kim, Z. Chen, X.-Y. Lang, Q. Jiang, C. Zhi and W. Zheng, Ultrafast Nucleation Reverses Dissolution of Transition Metal Ions for Robust Aqueous Batteries, Nano Lett., 2023, 23, 5307–5316 CrossRef CAS PubMed.
- V. P. H. Radhakantha, J. Tiongson, U. S. Manjunatha, L. O'Dell and A. J. Bhattacharyya, The critical impact of electrolyte concentration on Al3+ redox and stability of CuHCF in aqueous aluminum-ion batteries, J. Mater. Chem. A, 2026 Search PubMed.
- J. Zheng, D. Li, Z. Feng, Y. Wang and T. Sun, Preintercalated Copper Hexacyanoferrate as a Long-Time Cycle Cathode Material for Aqueous Aluminum-Ion Batteries, ACS Sustain. Chem. Eng., 2023, 11, 6280–6291 Search PubMed.
- K. Sayeed, A. Sadhanala, C. V. Yelamaggad and K. Pandey, Inceptive high-performance flexible aqueous aluminum-ion batteries derived from pre-intercalated copper hexacyanoferrate cathode, J. Energy Storage, 2025, 132, 117881 Search PubMed.
- A. Zhou, L. Jiang, J. Yue, Y. Tong, Q. Zhang, Z. Lin, B. Liu, C. Wu, L. Suo, Y. S. Hu, H. Li and L. Chen, Water-in-Salt Electrolyte Promotes High-Capacity FeFe(CN)(6) Cathode for Aqueous Al-Ion Battery, ACS Appl. Mater. Interfaces, 2019, 11, 41356–41362 CrossRef CAS PubMed.
- R. Bai, J. Yang, G. Li, J. Luo and W. Tang, Rechargeable aqueous aluminum-FeFe(CN)6 battery with artificial interphase through deep eutectic solution, Energy Storage Mater., 2021, 41, 41–50 CrossRef.
- Y. Gao, H. Yang, X. Wang, Y. Bai, N. Zhu, S. Guo, L. Suo, H. Li, H. Xu and C. Wu, The Compensation Effect Mechanism of Fe–Ni Mixed Prussian Blue Analogues in Aqueous Rechargeable Aluminum-Ion Batteries, ChemSusChem, 2020, 13, 732–740 CrossRef CAS PubMed.
- W. Feng, B. Li, G. Yuan, Y. Li, Y. Zhang, M. Du, Y. Su, Y. Tang, H. Yue, Y. Li, M. Shakouri, H.-C. Chen, W. Li, Z. Liu and H. Pang, High Performance Aluminum Ion Batteries Enabled by the Coordination Between Vanadium-Based PBAs Cathode and Aqueous Eutectic Electrolyte, Adv. Sci., 2025, e11274 CrossRef CAS PubMed.
- D. Wang, H. Lv, T. Hussain, Q. Yang, G. Liang, Y. Zhao, L. Ma, Q. Li, H. Li, B. Dong, T. Kaewmaraya and C. Zhi, A manganese hexacyanoferrate framework with enlarged ion tunnels and two-species redox reaction for aqueous Al-ion batteries, Nano Energy, 2021, 84, 105945 CrossRef CAS.
- W. Shang, Y. Liu, Y.-N. Liu, J.-L. Yang, H.-H. Liu, H. Yu, J.-M. Cao and X.-L. Wu, Lattice Distortion Confinement Induced by Cationic Vacancy for Ambient Temperature Durable Aluminum-Ion Batteries, Adv. Funct. Mater., 2025, 35, 2422805 CrossRef CAS.
- Y.-N. Liu, J.-L. Yang, Z.-Y. Gu, X.-Y. Zhang, Y. Liu, M.-Y. Su, X.-L. Zhang, I. V. Zatovsky, K. Li, J.-M. Cao and X.-L. Wu, Entropy-Regulated Cathode with Low Strain and Constraint Phase-Change Toward Ultralong-Life Aqueous Al-Ion Batteries, Angew. Chem., Int. Ed., 2024, 63, e202316925 CrossRef CAS PubMed.
- K. Du, Y. Liu, Y. Zhao, H. Li, H. Liu, C. Sun, M. Han, T. Ma and Y. Hu, High-Entropy Prussian Blue Analogues Enable Lattice Respiration for Ultrastable Aqueous Aluminum-Ion Batteries, Adv. Mater., 2024, 36, 2404172 CrossRef CAS.
- Y. Ru, S. Zheng, H. Xue and H. Pang, Potassium cobalt hexacyanoferrate nanocubic assemblies for high-performance aqueous aluminum ion batteries, Chem. Eng. J., 2020, 382, 122853 CrossRef CAS.
- J. Zheng, J. Liu, M. Ma, R. Zhang, S. Zhang, Y. Wang and T. Sun, Prussian Blue Analogue-Based Nanowires as Cathode Materials for Aqueous Aluminum-Ion Storage, ACS Appl. Nano Mater., 2024, 7, 28659–28668 CrossRef CAS.
- W. Chang, J. Peng, W. Mao, Q. Wang, Y. Zhu and N. Peng, Mg-Substituted dual-walled Prussian blue analogous achieving enhanced active sites and stability for aqueous Al-ion batteries, Chem. Eng. J., 2024, 500, 157204 CrossRef CAS.
- A. Zhao, J. Peng, W. Mao, Q. Wang, Y. Zhu and N. Peng, Fe-Co PBA/rGO interface strategy enabling fast Al3+ intercalation for stable aqueous Al-ion batteries, Chem. Eng. J., 2024, 493, 152790 Search PubMed.
- Y. Lu, Q. Zhang, L. Li, Z. Niu and J. Chen, Design Strategies toward Enhancing the Performance of Organic Electrode Materials in Metal-Ion Batteries, Chem, 2018, 4, 2786–2813 CAS.
- Y. Qi, H. Zhao and Y. Lei, Organic molecular design for high-power density sodium-ion batteries, Chem. Commun., 2025, 61, 2375–2386 RSC.
- W. Walker, S. Grugeon, O. Mentre, S. Laruelle, J.-M. Tarascon and F. Wudl, Ethoxycarbonyl-Based Organic Electrode for Li-Batteries, J. Am. Chem. Soc., 2010, 132, 6517–6523 CrossRef CAS PubMed.
- Y. Liang, Y. Jing, S. Gheytani, K.-Y. Lee, P. Liu, A. Facchetti and Y. Yao, Universal quinone electrodes for long cycle life aqueous rechargeable batteries, Nat. Mater., 2017, 16, 841–848 CrossRef CAS.
- X. Peng, Y. Xie, A. Baktash, J. Tang, T. Lin, X. Huang, Y. Hu, Z. Jia, D. J. Searles, Y. Yamauchi, L. Wang and B. Luo, Heterocyclic Conjugated Polymer Nanoarchitectonics with Synergistic Redox-Active Sites for High-Performance Aluminium Organic Batteries, Angew. Chem., Int. Ed., 2022, 61, e202203646 Search PubMed.
- J. He, X. Shi, C. Wang, H. Zhang, X. Liu, Z. Yang and X. Lu, A quinone electrode with reversible phase conversion for long-life rechargeable aqueous aluminum–metal batteries, Chem. Commun., 2021, 57, 6931–6934 RSC.
- Y. Li, L. Liu, Y. Lu, R. Shi, Y. Ma, Z. Yan, K. Zhang and J. Chen, High-Energy-Density Quinone-Based Electrodes with [Al(OTF)]2+ Storage Mechanism for Rechargeable Aqueous Aluminum Batteries, Adv. Funct. Mater., 2021, 31, 2102063 CrossRef CAS.
- J. Chen, Q. Zhu, L. Jiang, R. Liu, Y. Yang, M. Tang, J. Wang, H. Wang and L. Guo, Rechargeable Aqueous Aluminum Organic Batteries, Angew. Chem., Int. Ed., 2021, 60, 5794–5799 Search PubMed.
- J. Cui, Z. Guo, J. Yi, X. Liu, K. Wu, P. Liang, Q. Li, Y. Liu, Y. Wang, Y. Xia and J. Zhang, Organic Cathode Materials for Rechargeable Zinc Batteries: Mechanisms, Challenges, and Perspectives, ChemSusChem, 2020, 13, 2160–2185 Search PubMed.
- H. Li, M. Cao, R. Wang, P. Xiong, Y. Liu, L. Zhang, L. Zhang, L. Zhang, D. Chao and C. Zhang, Design Strategy for Small-Molecule Organic Cathodes: Regulated Active Groups Enable High Capacity and Voltage in Aqueous and Seawater Aluminum Ion Batteries, Angew. Chem., Int. Ed., 2025, 64, e202508057 CrossRef CAS.
- Y. Lu, Q. Ge, C. Hu, H. Chen, W. Zhang and Z. Li, An π-conjugated organic cathode with multiple cyano-substituted for stable aqueous aluminum batteries, J. Colloid Interface Sci., 2025, 682, 281–287 Search PubMed.
- J. Su, M. Zhang, H. Tian, M. Han, Z. Sun, K. Du, F. Cui, J. Li, W. Huang and Y. Hu, Synergistic π-Conjugation Organic Cathode for Ultra-Stable Aqueous Aluminum Batteries, Small, 2024, 20, 2312086 CrossRef CAS.
- Y. Lu, C. Hu, Y. Hu, W. Zhang and Z. Li, Carbonyl and imine conjugated frameworks for aqueous Organo-Aluminum batteries with high specific capacity and low dissolution, J. Colloid Interface Sci., 2024, 665, 181–187 Search PubMed.
- C. Hu, Y. Lu, H. Gu, W. Zhang and Z. Li, Multiple Redox Site π-Conjugated Materials for Aqueous Aluminum–Organic Battery Cathodes, ACS Energy Lett., 2024, 9, 4353–4360 Search PubMed.
- F. Wan, L. Zhang, X. Wang, S. Bi, Z. Niu and J. Chen, An Aqueous Rechargeable Zinc-Organic Battery with Hybrid Mechanism, Adv. Funct. Mater., 2018, 28, 1804975 CrossRef.
- X. Li, X. Xie, R. Lv, B. Na, B. Wang and Y. He, Nanostructured Polypyrrole Composite Aerogels for a Rechargeable Flexible Aqueous Zn-Ion Battery with High Rate Capabilities, Energy Technol., 2019, 7, 1801092 Search PubMed.
- S. Sariyer, A. Ghosh, S. N. Dambasan, E. M. Halim, M. El Rhazi, H. Perrot, O. Sel and R. Demir-Cakan, Aqueous Multivalent Charge Storage Mechanism in Aromatic Diamine-Based Organic Electrodes, ACS Appl. Mater. Interfaces, 2022, 14, 8508–8520 CrossRef CAS.
- P. Meng, J. Huang, Z. Yang, F. Wang, T. Lv, J. Zhang, C. Fu and W. Xiao, A Low-Cost and Air-Stable Rechargeable Aluminum-Ion Battery, Adv. Mater., 2022, 34, 2106511 CrossRef CAS.
- W. Wang, S. Zhang, L. Zhang, R. Wang, Q. Ma, H. Li, J. Hao, T. Zhou, J. Mao and C. Zhang, Electropolymerized Bipolar Poly(2,3-diaminophenazine) Cathode for High-Performance Aqueous Al-Ion Batteries with An Extended Temperature Range of −20 to 45 °C, Adv. Mater., 2024, 36, 2400642 Search PubMed.
- Y. Xiong, Y. Li, X. Cui, S. Li, X. Peng, Y. Ju, T. Zhou, R. Feng, Y. Zhang, Z. Wang, Q. Wang and L. Dong, Stable Radical Polymers as New Electroactive Materials: Synthesis, Properties, and Emerging Applications, Adv. Funct. Mater., 2025, 35, 2419661 Search PubMed.
- D. R. Nevers, F. R. Brushett and D. R. Wheeler, Engineering radical polymer electrodes for electrochemical energy storage, J. Power Sources, 2017, 352, 226–244 Search PubMed.
- K. Sato, R. Ichinoi, R. Mizukami, T. Serikawa, Y. Sasaki, J. Lutkenhaus, H. Nishide and K. Oyaizu, Diffusion-Cooperative Model for Charge Transport by Redox-Active Nonconjugated Polymers, J. Am. Chem. Soc., 2018, 140, 1049–1056 CrossRef CAS.
- S. Jiang, Y. Xie, Y. Xie, L.-J. Yu, X. Yan, F.-G. Zhao, C. J. Mudugamuwa, M. L. Coote, Z. Jia and K. Zhang, Lewis Acid-Induced Reversible Disproportionation of TEMPO Enables Aqueous Aluminum Radical Batteries, J. Am. Chem. Soc., 2023, 145, 14519–14528 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |