 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design strategies and application progress of covalent organic frameworks in photocatalytic oxidation reactions
Tao
Sun†
ab,
Rui
Wang†
c,
Renquan
Guan
d,
Li
Wang
 ab,
Tian
Zhong
ab,
Tian
Zhong
 e,
Chunbo
Liu
e,
Chunbo
Liu
 *ab,
Xueying
Cheng
*ab and
Qianrong
Fang
*ab,
Xueying
Cheng
*ab and
Qianrong
Fang
 *c
*c
aScience and Technology Innovation Center of Jilin Province for Targeted Identification and Photocatalytic Degradation Materials, College of Engineering, Jilin Normal University, Siping, 136000, China. E-mail: chunboliu@jlnu.edu.cn; chengxy@jlnu.edu.cn
bKey Laboratory of Preparation and Application of Environmental Friendly Materials of the Ministry of Education, College of Chemistry, Jilin Normal University, Changchun, 130103, China
cState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry Jilin University, Changchun, 130012, China. E-mail: qrfang@jlu.edu.cn
dState Key Laboratory of Semiconductor Physics and Chip Technologies, Institute of Semiconductors, Chinese Academy of Sciences, Beijing, 100083, China
eSchool of Pharmacy, Faculty of Medicine, Macau University of Science and Technology, Macau
First published on 29th December 2025
Abstract
Photocatalytic generation of reactive oxygen species (ROS) presents a sustainable alternative to traditional oxidation methods, offering precise control over reaction pathways for diverse applications. Covalent organic frameworks (COFs), with their crystalline porous structures and tunable electronic properties, are ideal platforms for maximizing photocatalytic ROS efficiency and selectivity. This review systematically explores the intrinsic connection between COF architecture and ROS activity, framed by a “structure–ROS–substrate” paradigm. We detail how rational design strategies—from foundational band energy engineering and π-conjugation control to functional group integration and pore microenvironment regulation—precisely modulate the material's electronic structure, charge carrier dynamics, and mass transport to govern ROS speciation and concentration. These structural innovations underpin the remarkable performance of COFs in photocatalytic oxidation, including selective organic transformations, sustainable H2O2 production, and efficient degradation of recalcitrant pollutants. The application scope is further extended to biomedical fields, leveraging ROS for antibacterial therapy and photodynamic effects. Finally, we discuss prevailing challenges in quantitative ROS detection, operational stability, and scalable synthesis, while outlining future opportunities in machine-learning-guided design and tandem catalytic systems. This review aims to establish fundamental design principles for the next generation of COF-based photocatalysts, bridging molecular-level engineering to macroscopic oxidative efficacy.
1 Introduction
The development of efficient and sustainable oxidation technologies is a central challenge in modern chemistry.1 Oxidation reactions are central to both chemical manufacturing and environmental management. They are indispensable in synthesizing fine chemicals—including key products such as pharmaceuticals and agrochemicals—as well as in producing polymers. Beyond synthesis, these reactions are fundamental to environmental remediation processes such as wastewater treatment and air purification.2 Conventional oxidation methods, although widely employed in industry, typically rely on stoichiometric oxidants or noble-metal catalysts.3 While these approaches are effective, they suffer from intrinsic limitations, including high energy consumption, low atom economy, poor selectivity, and the generation of toxic by-products.4 These issues not only increase operational costs but also raise severe environmental and sustainability concerns.4 In an era of increasing demand for green and scalable technologies, there is an urgent need to design oxidation platforms that are both efficient and environmentally benign.Photocatalysis offers a compelling solution by harnessing sunlight—a clean, abundant, and renewable energy source—to drive redox processes.5 In particular, photocatalytic oxidation mediated by reactive oxygen species (ROS) such as hydroxyl radicals (·OH), superoxide radicals (·O2−), singlet oxygen (1O2), and hydrogen peroxide (H2O2) has emerged as a versatile and sustainable alternative to conventional oxidation chemistry.6 The selective generation and utilization of ROS under mild conditions not only lowers the energy threshold of transformations but also provides a handle to regulate product selectivity, which is critical for pharmaceutical synthesis and pollutant degradation.7 Beyond its practical advantages, ROS chemistry also represents a model system for exploring fundamental concepts in photochemistry, electron transfer, and interfacial catalysis.8
Covalent organic frameworks (COFs), a rapidly expanding class of crystalline porous polymers, have recently attracted tremendous attention as photocatalytic platforms.9 Distinguished by their ordered π-conjugated skeletons, modular design, and robust structural stability, COFs offer an unprecedented level of tunability in photocatalysis. Unlike traditional inorganic photocatalysts, COFs allow precise integration of donor–acceptor (D–A) architectures, extension of π-conjugation, and incorporation of heteroatoms or metal centres into their frameworks.10 These features enable rational bandgap tuning, efficient charge separation, and targeted engineering of catalytic sites. Importantly, the inherent porosity of COFs facilitates mass transport, while their crystallinity allows detailed mechanistic studies that link structure with function.11 This combination of modularity, crystallinity, and tunability distinguishes COFs as an ideal platform to systematically study and optimize photocatalytic oxidation.12
Over the past decade, COF-based photocatalysis has witnessed remarkable growth, particularly in oxidation chemistry.13 Representative advances include the selective oxidation of sulfides to sulfoxides, a key transformation in drug and fine chemical synthesis; oxidative coupling of amines to imines, important intermediates for pharmaceuticals and agrochemicals; aerobic oxidation of alcohols to aldehydes or ketones; and the activation of inert C–H bonds, which opens new avenues for late-stage functionalization of complex molecules.14 In parallel, COFs have been successfully applied to environmental remediation, where their ability to regulate ROS species enables the degradation of dyes, antibiotics, and emerging pollutants with high selectivity and minimal secondary contamination.15 Another rapidly expanding frontier is the photocatalytic production of H2O2, where COFs have demonstrated significant potential in addressing the kinetic limitations of the water oxidation reaction (WOR) and oxygen reduction reaction (ORR).16 By simultaneously activating ORR and WOR pathways, COFs can achieve efficient overall H2O2 production directly from water and oxygen, offering an attractive alternative to the energy-intensive anthraquinone process.17 Furthermore, COFs are increasingly explored in biomedical contexts, such as photodynamic therapy (PDT) and antibacterial disinfection, where controlled ROS generation under visible or near-infrared light enables targeted therapeutic applications.18
However, the true conceptual power and guiding significance of this “structure–ROS–substrate” paradigm extend beyond cataloguing these individual correlations. It lies in its capacity to serve as a unifying and predictive framework that advances beyond traditional analytical lenses. Conventional frameworks such as band theory or active-site analysis offer invaluable yet compartmentalized insights: the former excels at describing photophysical initiation, while the latter focuses on terminal chemical events at localized sites.19,20 In contrast, the “structure–ROS–substrate” paradigm integrates these aspects into a holistic, dynamic continuum. It uniquely posits that the photocatalytic outcome is an emergent property arising from the continuous interplay among the three vertices of the triad. Therefore, this paradigm does not merely describe structure–property relationships; it provides a causal lens to interpret complex reaction selectivity, reconcile seemingly contradictory literature findings, and, most importantly, guide the rational design of COFs by targeting the optimization of this interplay.
Beyond practical demonstrations, these advances have also deepened fundamental insights into the so-called “structure–ROS–substrate” paradigm. It is now clear that electronic configuration, porosity, interfacial interactions, and functional group distribution within COFs collectively determine ROS speciation and reactivity. For instance, the orientation of linkage bonds can alter dipole moments and influence charge separation efficiency,21 heteroatom doping can accelerate intersystem crossing (ISC) to boost 1O2 generation,22 and pore-size engineering can dictate substrate accessibility and product selectivity.23 Such molecular-level control is rarely achievable in conventional photocatalysts, underscoring the unique role of COFs in bridging fundamental photochemistry with practical oxidation catalysis. Despite significant progress, a clear, mechanistic, and ultimately predictive understanding of the intrinsic correlations between the COF structure and photocatalytic oxidation performance is still developing. This incomplete knowledge base is the root cause of several persistent challenges. First, the synthesis of many high-performance COFs often relies on harsh conditions or specialized monomers, complicating scalable and sustainable manufacturing. Second, the long-term structural and functional stability of these frameworks in the harsh oxidative environments inherent to photocatalysis—particularly in acidic, basic, or biologically relevant media—requires systematic improvement. Finally, the translation of laboratory performance to practical applications necessitates that COFs retain their functionality in complex, real-world matrices containing competing species and under variable operational conditions. Therefore, to advance the field, a dual focus is imperative. A deep, fundamental elucidation of “structure–mechanism–activity” relationships—achieved through advanced in situ characterization, computational modelling, and mechanistic studies—must guide the applied engineering efforts aimed at overcoming challenges in scalability, stability, and performance in complex environments. It is through these concerted and complementary efforts that COF-based photocatalytic oxidation can evolve from a promising laboratory discovery into viable technology for sustainable synthesis, environmental remediation, and biomedical applications.
In this review, we aim to provide a comprehensive overview of recent advances in COF-based photocatalytic oxidation, with a particular emphasis on the structural design principles that govern ROS generation and reactivity. Crucially, we will thread this discussion through the unifying perspective of the “structure–ROS–substrate” paradigm, demonstrating how it offers a coherent framework to organize existing knowledge and inspire future design. We begin by outlining the fundamental mechanisms of ROS generation in COFs, examining how intrinsic structural features such as the degree of π-conjugation set key photophysical parameters like band alignment and exciton binding energy. These parameters fundamentally govern the subsequent efficiency of dynamic processes—including light absorption, exciton dissociation, and charge separation—which collectively dictate the pathways, yields, and selectivity of ROS formation. We then discuss structural engineering strategies—including D–A integration, functional group modification, metal incorporation, and topology/porosity control—that have been developed to enhance photocatalytic efficiency. Building on this foundation, we provide a reaction-oriented analysis of COF applications across four major areas: selective organic transformations, environmental pollutant degradation, H2O2 production, and biomedical disinfection/therapy. Finally, we discuss the remaining challenges and future opportunities for rational COF design, including expanding light harvesting into the near-infrared region, achieving enantioselective oxidation, and developing scalable synthetic protocols. By offering both a mechanistic framework and a synthesis of the latest advances, this review seeks to inspire further innovation in the design of COFs for photocatalytic oxidation. We anticipate that continued progress in this area will not only expand the scope of green oxidation chemistry but also accelerate the translation of COF-based materials into practical applications ranging from chemical manufacturing to environmental protection and healthcare.
2 Mechanistic fundamentals of ROS generation in COFs
2.1 Photocatalytic processes in COFs
Photocatalysis is a process that utilizes light energy to drive chemical reactions. Its core mechanism involves the generation, separation, and migration of photogenerated electron–hole pairs, followed by redox reactions occurring on the catalyst surface.24 COFs exhibit significant advantages in the field of photocatalysis due to their highly ordered pore structures, tunable band structures, excellent charge transport properties, and good stability. Its photocatalytic process mainly encompasses the following key steps:2.2 Pathways of ROS generation
In photocatalytic oxidation reactions, the generation of ROS is central to achieving efficient oxidation reactions. The generation pathways are complex, multi-step processes that depend on the interactions between photogenerated e− and h+ with adsorbed O2 or H2O molecules, leading to the formation of various ROS. The main ROS include ·O2−, ·OH, 1O2, and H2O2. The generation pathways of these ROS are interconnected, forming a complex reaction network. Their types and concentrations are significantly influenced by the band structure of COFs, surface chemical properties, and reaction environment.31,32| O2 + e− → ·O2− | (1) |
The band structure of COFs can be precisely tuned through the linkage chemistry and the energy levels of the building units. For example, COFs constructed with strong electron-donating units (such as pyrene and porphyrin) typically have more negative CB positions, providing a stronger thermodynamic driving force for O2 reduction.34 Additionally, highly extended π-conjugated structures and ordered pores facilitate the rapid migration of photogenerated e−, effectively suppressing electron–hole recombination and enhancing the generation efficiency of ·O2−.35 Surface engineering also plays a crucial role in the generation of ·O2−. Introducing electron-rich functional groups or metal nodes (such as Co and Fe) can act as electron relay stations to facilitate electron transfer and enhance the adsorption and activation of O2, further improving the generation rate and stability of ·O2−.36
| ·O2− + ·O2H + H2O → H2O2 + O2 + OH− | (2) |
| ·O2− + e− + 2H+ → ·H2O2 | (3) |
| 2H2O + 2h+ → H2O2 + 2H+ | (4) |
| H2O + h+ → ·OH + H+ | (5) |
| ·OH + ·OH → H2O2 | (6) |
The unique, programmable architecture of COFs offers exceptional molecular-level control over the selectivity and efficiency of H2O2 production. Photocatalytic H2O2 synthesis predominantly proceeds via two pathways: (1) the two-electron oxygen reduction reaction (2e− ORR), where O2 is reduced by photogenerated electrons, and (2) the water oxidation reaction (WOR), where H2O is oxidized by photogenerated holes. The central challenge lies in selectively promoting the 2e− ORR over the competing four-electron (4e−) pathway to H2O, and in efficiently activating the thermodynamically demanding WOR, which is critical for achieving overall photosynthetic H2O2 generation from H2O and O2 without sacrificial agents.
COFs address these challenges through precise structural and electronic engineering that targets the key reaction intermediates. For the 2e− ORR pathway, the selectivity is governed by the binding strength of the *OOH intermediate. COFs can be designed to favor the 2e− pathway by modulating their electronic structure, for example, through donor–acceptor (D–A) motif engineering, which optimizes the adsorption free energy of *OOH to a value close to the thermodynamic optimum. Furthermore, the strategic incorporation of heteroatoms (e.g., boron and sulfur) or single-metal sites (e.g., Co and Ni) can create defined Lewis acid or redox-active centers that selectively stabilize the *OOH intermediate, thereby suppressing its further reduction to H2O. For the WOR pathway, the oxidation of H2O to H2O2 involves complex multi-step proton-coupled electron transfers. COFs can facilitate this process through functional group engineering; for instance, hydrophilic –OH or –COOH groups can enhance water adsorption and local proton concentration, while specific organic moieties can act as co-catalysts to lower the kinetic barrier for water oxidation. Beyond active site design, the porous and ordered structure of COFs enhances mass transport and enriches reactants (O2 and H2O) near active sites. Concurrently, their extended π-conjugation systems promote efficient light absorption and long-range charge carrier separation, ensuring a sufficient flux of electrons and holes to drive the respective reduction and oxidation reactions. The synergistic interplay of these structural elements enables COFs to achieve superior efficiency and selectivity in photocatalytic H2O2 synthesis compared to many conventional materials.37
| H2O + h+ → ·OH + H+ | (7) |
However, due to its high potential, the direct hole oxidation pathway is usually limited. Under acidic conditions or in the presence of metal sites (such as in Fenton-like reactions), H2O2 can be homolytically reduced by e− to generate ·OH (eqn (8)).
| H2O2 + e− → ·OH + OH− | (8) |
The abundant functional sites on the surface of COFs (such as hydroxyl and carboxyl groups) and metal nodes introduced through post-modification (such as Fe and Cu) can effectively regulate interfacial charge separation and participate in the generation pathway of ·OH.40
3 Structure–activity relationships in ROS regulation
3.1 Linkage chemistry and framework stability
The photocatalytic performance of COFs is fundamentally governed by their molecular structure, with linkage chemistry serving as a key structural determinant.41 The choice of covalent linkages connecting the organic building units not only determines the material's crystallinity and porosity, but also directly impacts the extent of π-conjugation, thermodynamic and kinetic stability, and electronic band structure, ultimately affecting the separation efficiency of photogenerated charge carriers and the generation of ROS.42 Consequently, strategic linkage design forms the foundation of the “structure–ROS–substrate” paradigm for constructing stable, high-performance COF photocatalytic platforms.Common linkage types in COFs include boronic ester, imine, triazine, hydrazone, and sp2 carbon linkages.43 The chemical stability and electronic properties of each linkage type differ significantly, which directly determines their suitability in complex photocatalytic oxidation environments, particularly when involving the generation and consumption of ROS.
Boronic ester-linked COFs are among the first successfully demonstrated crystalline COFs and possess excellent thermal stability. However, their susceptibility to hydrolysis in moist environments is even more pronounced, often leading to the loss of porosity and crystallinity, which restricts their application in photocatalytic reactions.44 Furthermore, imine-linked COFs formed via reversible Schiff base condensation are among the most widely studied types due to their relatively mild synthesis conditions, high crystallinity, and large specific surface area. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond imparts a polarized character that can facilitate intramolecular charge transfer, favouring light absorption. However, their dynamic covalent nature often compromises hydrolytic stability, especially during prolonged operation in aqueous or acidic oxidative environments, limiting their long-term recyclability.45 Other linkages such as triazine and hydrazone present distinct trade-offs in stability, synthesis conditions, and electronic properties.43
N bond imparts a polarized character that can facilitate intramolecular charge transfer, favouring light absorption. However, their dynamic covalent nature often compromises hydrolytic stability, especially during prolonged operation in aqueous or acidic oxidative environments, limiting their long-term recyclability.45 Other linkages such as triazine and hydrazone present distinct trade-offs in stability, synthesis conditions, and electronic properties.43
To address these stability challenges, research has focused on designing irreversible or highly stable linkage types. Among these, fully conjugated sp2 carbon-linked COFs constructed via irreversible reactions such as Knoevenagel condensation represent a significant advancement.46 These fully conjugated frameworks, such as vinylene-linked or sp2c-COFs, demonstrate exceptional chemical stability against hydrolysis, acids, and bases, attributable to the robust carbon–carbon bonds. More importantly, the extended and fully delocalized π-conjugation system significantly enhances charge carrier mobility, reduces exciton binding energy, and improves visible light absorption, thereby leading to superior photocatalytic activity and long-term operational stability.47
Therefore, linkage chemistry is not merely a structural detail but a core design parameter in the “structure–ROS–substrate” paradigm. The development of COFs with highly stable and fully conjugated linkages is crucial for advancing photocatalytic oxidation technologies. Simultaneously, post-synthetic modification strategies that transform hydrolytically labile imine bonds into more stable chemical bonds have become an effective means to enhance the stability of imine-based COFs. Through targeted selection and engineering of the linkage type, the electronic structure, exciton dynamics, and internal chemical environment of COFs can be precisely controlled, laying a solid foundation for subsequent bandgap engineering, D–A design, and pore optimization.
3.2 Band gap and energy level engineering
The photocatalytic performance of COFs is closely related to their electronic structure, particularly the band gap width and the positioning of energy levels (such as HOMO/LUMO or VB/CB), which have a decisive impact on the generation pathways and efficiency of ROS. Through rational structural design, especially by matching the linker chemistry with the electronic properties of the building units, precise control over the band structure of COFs can be achieved, thereby optimizing the separation efficiency of photogenerated carriers and the selective generation of ROS.48Wang et al. systematically studied a series of COFs connected by imine bonds (such as Py–Py, Etta–Py, Py–Td, and Etta–Td), revealing the significant regulatory effect of the electronic properties of the building units on the optical band gap of the materials.49 As shown in Fig. 2, the introduction of electron-withdrawing thiazole (Td) units significantly reduces the band gap of the COFs (Py–Td: 2.18 eV; Etta–Td: 2.12 eV), while purely donor-type building units (such as Py–Py) exhibit a wider band gap (2.39 eV). This narrowing of the band gap is mainly attributed to the enhanced D–A type charge transfer effect, thereby extending the material's absorption range in the visible light region. Furthermore, the regulation of energy level positions is equally crucial as it directly determines the reduction/oxidation capabilities of photogenerated e− and h+, thus governing the pathways of ROS generation. Through Mott–Schottky tests and cyclic voltammetry (CV) measurements, researchers found that all tested COFs exhibited p-type semiconductor characteristics. Among them, the CB positions of Py–Td and Etta–Td are located at −0.34 V and −0.68 V (vs. NHE), respectively, while the VB positions are at 1.84 V and 1.44 V, respectively. These energy level positions directly influence their reduction/oxidation capabilities, thereby determining the pathways of ROS generation (such as ·O2−vs.1O2).
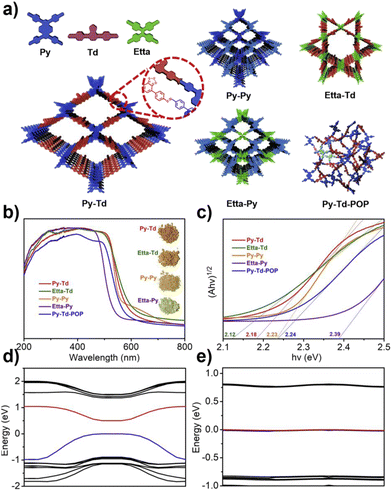 | ||
| Fig. 2 (a) Amine linkage-based connection structures and their corresponding synthesized materials; (b and c) optical properties of the materials; (d) band structure diagrams and (e) symmetry–performance relationship studies of Py–Td and Etta–Td COFs. Reproduced with permission from ref. 49, Copyright 2019, from Elsevier. | ||
Crystallinity also has a significant impact on the band structure. Although the amorphous analogue Py–Td–POP has the same chemical composition as Py–Td, its band gap is wider (2.24 eV) and its visible light absorption capacity is significantly reduced, further confirming the critical role of long-range ordered structures in promoting π-conjugation extension and charge delocalization. Materials with poor crystallinity typically have more defect states, which can act as charge recombination centres, reducing the efficiency of carrier separation and ultimately weakening the ability to generate ROS.49
In summary, band engineering is a core strategy for regulating the photocatalytic ROS generation behaviour of COFs. By reasonably selecting donor and acceptor building units with specific electronic properties, the band gap of the material can be effectively narrowed, the light response range can be broadened, and the energy level positions can be precisely controlled, thereby thermodynamically creating conditions for the generation of specific ROS. Meanwhile, constructing a highly crystalline framework structure is the kinetic foundation for achieving efficient charge separation and migration, which is crucial for ensuring photocatalytic activity.
3.3 Tuning charge delocalization via π-conjugation engineering
The highly ordered and extended π-conjugated framework in COFs is not only the structural basis for efficient light harvesting but also the key to promoting the separation and migration of photogenerated charges. By carefully designing building units to construct strong D–A systems or introducing specific functional groups to enhance intramolecular electronic coupling, the exciton dynamics (such as promoting ISC) and charge delocalization behaviour can be effectively regulated, thereby significantly influencing the pathways and efficiency of ROS generation.π-Conjugated systems have multiple impacts on charge separation efficiency. Firstly, an extended π-conjugated system can broaden the light absorption range of COFs, allowing them to capture more photon energy. For example, pyrene-based COFs achieve a blue shift in the absorption edge through π-conjugation expansion, enhancing their response to visible light and providing more excitation sources for charge separation.50 Secondly, a π-conjugated network helps in the delocalization of e− within and between molecules, forming delocalized π-bonds. This reduces the charge transport barrier and promotes the separation and transport of e− and h+.51 In D–A type COFs, π-conjugated linkers can guide e− to transfer directionally from donor units to acceptor units, further improving charge separation efficiency. Additionally, the ordered stacked structure formed by strong π–π interactions can facilitate charge delocalization, reducing the likelihood of electron–hole recombination.52
Qin and colleagues systematically studied the effects of interlayer π–π interactions on photogenerated charge separation and transport by designing two pyrene-based COF isomers with opposing imine bridge orientations (PY-CN-BIP-Ni and PY-NC-BIP-Ni). Theoretical calculations and experimental results indicate that the asymmetric charge distribution in PY-CN-BIP-Ni, where the donor is attached to imine carbon (Cδ+) and the acceptor to nitrogen (Nδ−), effectively weakens interlayer electronic coupling. This suppression of interlayer exciton formation and energy loss localizes the electron cloud within the layers, promoting efficient charge transfer along the D–A axis to the Ni active centre. In contrast, strong interlayer π-stacking in PY-NC-BIP-Ni leads to electron delocalization across layers, forming exciplexes and hindering intralayer charge separation (Fig. 3a).53 As shown in Fig. 3b and c, Professor Li's team proposed a “linker unit engineering” strategy, inserting a polarized acylhydrazone unit as a π-spacer between the electron donor (benzotrithiophene) and acceptor (triazine). This induces a unique “sequential electron transfer” mechanism under illumination, effectively avoiding the rapid charge recombination common in traditional D–A structures. This significantly enhances charge delocalization, extending the excited state lifetime to 1.35 ns and greatly reducing the exciton binding energy (31.1 meV), thus achieving efficient spatial separation of e− and h+.54
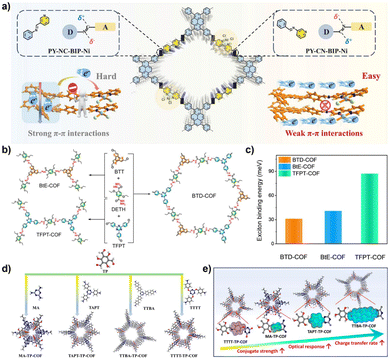 | ||
| Fig. 3 (a) Schematic diagram of interlayer and intralayer charge transfer in imine-based COFs; reproduced with permission from ref. 53, Copyright 2025, from Wiley-VCH. (b) Synthesis diagrams of BTD-COF, BtE-COF, and TFPT-COF, and (c) their corresponding exciton binding energies; reproduced with permission from ref. 54, Copyright 2025, from American Chemical Society. (d) Synthesis diagrams of triazine-based COFs with different degrees of conjugation; (e) relationship between the degree of conjugation and photocatalytic performance of COFs. Reproduced with permission from ref. 55, Copyright 2025, from American Chemical Society. | ||
In regulating ROS generation, π-conjugated systems also play a crucial role. Firstly, their charge delocalization properties can adjust the local charge distribution around active sites. The extended π-conjugation can increase the charge density around active sites, enhancing the adsorption and activation of reactants, thereby indirectly influencing ROS generation. Secondly, the electronic structure of π-conjugated networks can regulate the stability of reaction intermediates, prolong the lifetime of charge-separated states, stabilize key intermediates during reactions, and thus promote the sustained generation of ROS. Professor Wang's team systematically constructed triazine-based COFs with different types (π–π vs. p–π) and degrees of conjugation to deeply reveal the regulatory mechanisms of π-conjugated networks on photocatalytic performance (Fig. 3d and e). The study shows that TTBA–TP-COF, with a strong π–π conjugated network, significantly enhances light response and provides efficient charge transport channels and electron-rich sites, thus exhibiting optimal charge separation efficiency (with the highest carrier mobility and the longest fluorescence lifetime up to 0.95 µs) and interfacial electron transfer capability. These features collectively contribute to its excellent interfacial electron transfer capability and ROS generation efficiency in the photocatalytic ORR.55
3.4 Functional groups and metal integration
Introducing specific functional groups or metal nodes into COFs is a key strategy for molecular-level regulation of their photocatalytic performance, particularly for the precise control of ROS generation pathways and efficiency. Such modifications can effectively tune the local electronic structure and chemical microenvironment of active sites, thereby influencing substrate adsorption behavior, charge transfer pathways, and the stability of key intermediates, ultimately achieving directed promotion of specific ROS generation pathways.Integrating bio-inspired groups with inherent redox activity into the COF is an effective approach to constructing efficient and highly selective photocatalytic systems. Inspired by natural flavin cofactors, Trenker et al. designed and synthesized a COF (FEAx-COF) containing alloxazine structural units (Fig. 4a and b). Alloxazine itself is a known organic photocatalyst, and when integrated into an ordered two-dimensional framework, its photocatalytic functionality is retained and significantly enhanced. This material not only retains the single/double electron transfer capability of alloxazine, but also broadens the visible light absorption range to 650 nm due to the extended conjugation within the framework, outperforming its molecular form. Research indicates that FEAx-COF can simultaneously generate ·O2− and 1O2 during the photocatalytic process, with both species working synergistically in the alcohol oxidation reaction to produce aldehydes and H2O2. This indicates that the presence of the alloxazine functional group directly determines the generation of ROS through a mixed pathway that combines electron transfer and energy transfer, thereby driving efficient and highly selective alcohol oxidation reactions. Moreover, anchoring alloxazine within the COF effectively suppresses the aggregation of its molecular form in solution, allowing FEAx-COF to exhibit excellent and stable catalytic performance across various solvents, as well as good recyclability. Compared to reference COFs that do not contain alloxazine, its activity is significantly higher, confirming that this functional group is a crucial source of catalytic activity.56
 | ||
| Fig. 4 (a) Synthesis of FEAx-COF; (b) photocatalytic oxidation mechanism of MBA by FEAx-COF; reproduced with permission from ref. 56, Copyright 2021, from Author(s). (c) Molecular structure of Mn+@Tp–TAPT; (d) degradation kinetics rate constants of Mn+@Tp–TAPT; (e) photocatalytic degradation mechanism of Mn+@Tp–TAPT; reproduced with permission from ref. 57, Copyright 2023, from Wiley-VCH. (f) Synthesis and photocatalytic mechanism diagram of AlCCOF-1/2; (g) comparison of the photocatalytic H2O2 production performance of AlCCOF-1, AlCCOF-2, and AlOC-17. Reproduced with permission from ref. 58, Copyright 2025, from Wiley-VCH. | ||
Metal integration is another effective strategy to enhance the photocatalytic ROS generation activity of COFs. Introducing metal ions or metal clusters into the COF or pores can create Lewis acid sites, optimizing the adsorption and activation of O2, and facilitating the transfer and separation of photogenerated e−, thereby synergistically enhancing the reactivity and selectivity of ROS. As shown in Fig. 4c–e, Yang and colleagues developed a type of metal-COF single-atom catalyst by accurately anchoring metal single atoms (such as Pd2+, Cu2+, and Fe3+) within imine-linked COFs (Tp–TAPT). This strategy not only successfully introduced abundant Lewis acid sites, significantly enhancing the material's adsorption capacity for Lewis basic gases such as O2, but also positioned the metal sites as efficient centres for electron capture and transfer, greatly improving the separation and migration efficiency of photogenerated e−, while also collaborating with the inherent π-conjugated structure of the COFs to create a fast electron transport channel. Notably, the optimized Pd2+@Tp–TAPT catalyst can direct molecular oxygen along a highly selective route (O2 → ·O2− → H2O2 → ·OH) for conversion, thereby achieving the efficient and directed generation of ·OH.57 Zhang et al. utilized high-connectivity octanuclear aluminum clusters (Al8-8c) as secondary building units and covalently assembled them with linear organic linkers, successfully constructing a three-dimensional metal cluster-based COF (AICCOF-1). This strategy firmly integrates the metal clusters into the COF through covalent bonds, significantly enhancing the structural stability and functional diversity of the material. The core of the aluminum clusters consists of interconnected AlO4N2 octahedra, which exhibit properties similar to those of oxide semiconductors, introducing abundant Lewis acid sites that effectively optimize the adsorption of O2 molecules on the catalyst surface (with an adsorption energy of −23.1 kcal mol−1). This also provides key active sites for photocatalytic reactions, significantly enhancing the generation efficiency and pathway selectivity of ·O2−. In photocatalytic H2O2 synthesis, AICCOF-1 achieved a remarkable yield of up to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794.69 µmol g−1 h−1. Theoretical calculations further revealed that after light excitation, e− migrate directionally from the aluminum clusters to the organic linkers, forming a spatially separated charge distribution that significantly enhances the electron–hole separation efficiency (Fig. 4f and g).58
794.69 µmol g−1 h−1. Theoretical calculations further revealed that after light excitation, e− migrate directionally from the aluminum clusters to the organic linkers, forming a spatially separated charge distribution that significantly enhances the electron–hole separation efficiency (Fig. 4f and g).58
3.5 Pore structure and mass transfer effects
The inherent high specific surface area and pore structure of COFs are crucial foundations for their effectiveness as catalysts.59,60 Nonetheless, conventional COFs are predominantly microporous (pore diameter <2 nm), which can restrict the diffusion of reactants and products during catalytic reactions that involve gas-phase product formation or require rapid mass transfer, hindering the effective utilization of internal active sites.61,62 Constructing COFs with hierarchical pore structures (i.e., coexistence of micropores, mesopores, and macropores) is an effective strategy to address this challenge and achieve breakthroughs in photocatalytic performance. In a hierarchical pore system, micropores primarily provide a high specific surface area and abundant active sites, while mesopores and macropores act as “mass transfer channels,” significantly reducing diffusion resistance and ensuring rapid transport of reactants and products, while also facilitating the penetration and distribution of light within the material.63,64Khalil and colleagues' study offers compelling evidence for this. They successfully synthesized a hierarchical porous COF with a macroporous structure (macro-TpBpy) using the polystyrene (PS) hard template method and systematically compared it with the original COF (TpBpy), which has only a microporous structure (Fig. 5). Structural characterization indicates that macro-TpBpy successfully introduces interconnected macropores of approximately 270 nm while perfectly retaining its crystalline structure and microporous characteristics. This structural advantage directly translates to superior physicochemical properties: its BET surface area (1384 m2 g−1) and total pore volume (0.799 cm3 g−1) are significantly higher than those of the original TpBpy (859 m2 g−1, 0.468 cm3 g−1), demonstrating that the presence of macropores greatly enhances the accessibility of the micropore channels. In the photocatalytic production of H2O2, its yield (2716 µmol g−1 h−1) is significantly higher than that of the original COF with only micropores (2134 µmol g−1 h−1). Mechanistic analysis indicates that the hierarchical pore structure facilitates more efficient charge separation and the generation of ROS (·O2− and ·OH). This performance enhancement arises from the synergistic effects of the hierarchical pores: micropores provide a high surface area and abundant active sites, while macropores serve as efficient transport channels, significantly optimizing the mass transfer process, ensuring the rapid diffusion of reactants and products, enhancing the penetration and utilization of light within the material, promoting the separation of photogenerated charge carriers, and inhibiting the recombination of e− and h+, thus overall enhancing the efficiency of the photocatalytic oxidation reaction.65
 | ||
| Fig. 5 (a) Synthesis of macro-TpBpy using the PS hard template method and its photocatalytic mechanism; (b) FESEM image of macro-TpBpy; (c) EPR CB electron spectra of macro-TpBpy and original TpBpy under dark and visible light irradiation; (d) photocatalytic H2O2 performance of macro- and original TpBpy COFs. Reproduced with permission from ref. 65, Copyright 2024, from Author(s). | ||
In summary, constructing a hierarchical pore structure is a key dimension for optimizing the photocatalytic performance of COFs. This strategy significantly enhances the generation capacity of ROS and the efficiency of photocatalytic oxidation by synergistically improving mass transfer, light absorption, and charge separation behaviors. This indicates that, in addition to regulating the electronic structure of COFs, systematic design of their mesoscopic pore structures is equally crucial, thus laying a solid structural foundation for achieving efficient mass transfer and energy conversion within the “structure–ROS–substrate” paradigm.
Collectively, these design strategies indicate that optimizing photocatalysis in COFs requires integrating initial structural design with the ultimate reaction outcomes. While traditional theoretical frameworks have laid the foundation for subsequent studies, they typically attend only to specific segments of the photocatalytic cascade. Band-gap theory imposes thermodynamic constraints on the initial stage of the reaction, predicting the feasibility, in terms of redox potentials, of charge excitation in the photocatalyst and ROS generation. In contrast, active-site theory concentrates on predictive analyses of substrate adsorption, bond formation and cleavage, and the local chemical environment of the catalyst during the reaction, typically via sophisticated theoretical calculations.19,20 However, photocatalytic oxidation is a dynamic cascade that involves photon absorption, exciton dissociation, charge migration, interfacial ROS generation, and ultimately substrate conversion or product formation.25 A singular reliance on band-gap theory or active-site analysis may overlook critical interactions and kinetic requirements among these steps, leading to an incomplete understanding of the overall photocatalytic process; consequently, even materials with ideal band positions or abundant active sites can exhibit poor activity due to inefficient charge dynamics or ineffective ROS pathways.
The “structure–ROS–substrate” paradigm overcomes this limitation. It integrates the above dimensions into a coherent causal chain: by tuning COF linkage chemistry, energy-level engineering, charge delocalization, functional-group modifications, and the incorporation of metal nodes, it determines the types, yields, and lifetimes of ROS, which in turn dictate the efficiency and selectivity of substrate conversion. This paradigm goes beyond merely predicting thermodynamic feasibility and identifying reaction active sites; it is committed to elucidating the dynamic causal relationships operating throughout the photocatalytic process. It explicitly links molecular-level structural design to the dynamic evolution of ROS and the eventual evolution of substrates, thereby providing a unified analytical perspective for complex photocatalytic oxidation and guiding the rational design of COF materials to achieve specific oxidative transformations.
4 Applications in photocatalytic oxidation
The application of COFs in photocatalytic oxidation is mainly reflected in their wide range of fields from synthetic chemistry to environmental and biomedical engineering.66,67 Guided by the “structure–ROS–substrate” paradigm, precise control over the generation of ROS, including 1O2, ·O2−, and H2O2, can be achieved through strategic designs such as bandgap engineering, D–A unit design, metal integration, and pore environment optimization in COFs. This regulation further determines the efficiency and selectivity of the oxidation process. To provide a coherent narrative and underscore the versatility of COFs, this section is organized by major application domains, each serving as a case study for the governing principles of the “structure–ROS–substrate” paradigm. This section systematically reviews these advances, first discussing the selective oxidation of organic molecules (such as sulfides, amines, and alcohols) for the synthesis of fine chemicals, and then highlighting the sustainable production of H2O2via water oxidation pathways. Subsequently, the field of environmental remediation is discussed, elucidating how COF-driven ROS mineralize persistent pollutants such as dyes, antibiotics, and phenols. Finally, the unique potential of COFs in biomedical applications is explored, with a focus on their roles in ROS-mediated antibacterial activity and PDT. Their programmable structures offer the possibility of achieving synergistic and targeted therapeutic outcomes. In summary, these applications highlight the tremendous potential of COFs as multifunctional platforms in advancing green oxidation reactions.4.1 Selective oxidation of organic molecules
COFs are promising photocatalysts for selective organic oxidation due to their tunable structures, strong visible-light absorption, and efficient charge carrier dynamics.68 This potential is rooted in their designable architectures, which enable precise modulation of the types, yields, and lifetimes of ROS. By band gap engineering, D–A design, π-conjugation control, heavy atom effects, polarization fields, active site construction, and pore optimization, COFs can precisely regulate ROS such as 1O2 and ·O2−.69 This structure–ROS–substrate control enables efficient, mild, and selective oxidation reactions, advancing green synthesis. This section reviews recent progress in COF-catalyzed sulfide oxidation, amine coupling, alcohol-to-carbonyl conversion, C–H activation, and biomass valorization, highlighting links between structural design and catalytic performance.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C connections via Knoevenagel condensation. Its narrow band gap (2.39 eV) significantly enhanced blue light absorption. Additionally, its larger surface area about 680 m2 g−1 provided abundant active sites. Through a unique dual-path mechanism, the TP-COF achieved an 85% conversion rate of phenyl methyl sulfide in 15 minutes under blue light, exhibiting excellent sulfoxide selectivity (99%), with its activity being three times that of the traditional catalyst g-C3N4.70 In 2024, the group further realized precise regulation of COFs' band gap structure and polarization properties through the topological conversion from imines to thiazoles. They designed thiazole-connected isomeric COFs (COF-TZ-1/COF-TZ-2), where COF-TZ-2, due to its larger dipole moment (3.02 debye), significantly improved charge separation efficiency, enabling the high-efficiency selective oxidation of organic sulphides under blue light/O2 conditions, with a 99% oxidation conversion rate of phenyl methyl sulfide within 15 min. EPR confirmed that the ·O2− radical dominated the reaction. By optimizing the conjugated structure, the material's light absorption and reaction kinetics were enhanced.71
C connections via Knoevenagel condensation. Its narrow band gap (2.39 eV) significantly enhanced blue light absorption. Additionally, its larger surface area about 680 m2 g−1 provided abundant active sites. Through a unique dual-path mechanism, the TP-COF achieved an 85% conversion rate of phenyl methyl sulfide in 15 minutes under blue light, exhibiting excellent sulfoxide selectivity (99%), with its activity being three times that of the traditional catalyst g-C3N4.70 In 2024, the group further realized precise regulation of COFs' band gap structure and polarization properties through the topological conversion from imines to thiazoles. They designed thiazole-connected isomeric COFs (COF-TZ-1/COF-TZ-2), where COF-TZ-2, due to its larger dipole moment (3.02 debye), significantly improved charge separation efficiency, enabling the high-efficiency selective oxidation of organic sulphides under blue light/O2 conditions, with a 99% oxidation conversion rate of phenyl methyl sulfide within 15 min. EPR confirmed that the ·O2− radical dominated the reaction. By optimizing the conjugated structure, the material's light absorption and reaction kinetics were enhanced.71
To address the low crystallinity issue of imine-based COFs, the group in 2025 prepared a fully conjugated benzodithiophene COF (BDTT-sp2c-COF). Its fully conjugated structure significantly improved 974 m2 g−1 surface area, charge carrier separation efficiency, and electron transfer ability. The high dipole moment (2.87 D) optimized O2 activation, efficiently catalyzing the sulfoxidation reaction under blue light. Specifically, in 25 min, it achieved a 95% conversion rate and >96% selectivity in oxidizing organic sulfides to sulfoxides, with excellent cycling stability, providing a new paradigm for the application of conjugated COFs in photocatalytic organic synthesis.72
Further enhancement of 1O2 generation efficiency is another effective pathway for improving selective oxidation of sulfides. Introducing heavy atoms into the conjugated framework helps accelerate the ISC process. Benzoselenadiazole, a unit capable of rapid photoinduced charge transfer, is widely used in photocatalysis. In 2024, Dong's group developed a selenium-containing benzoselenadiazole COF (BSe-COF), which utilized the heavy atom effect of selenium to enhance the material's spin–orbit coupling constant (SOC = 2.15 cm−1), promoting the ISC process and thus increasing the 1O2 yield. Under blue light irradiation, a 95% conversion rate for sulfides was achieved. More importantly, its narrow band gap (1.65 eV) extended the light absorption range to the near-infrared region, allowing it to achieve up to 96% sulfoxide yield under natural light, providing a practical solution for green, low-energy photocatalytic oxidation.73 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO) radicals, as highly efficient reactive oxidation mediators, are widely used in organic transformations due to their high selectivity and mild reaction conditions. In 2021, Lang's group overcame the limitations of traditional homogeneous catalysis by constructing a heterogeneous TEMPO and porous porphyrin-based COF (Por-COF) system. Under white light and using O2 as the oxidant, this system efficiently catalyzed the selective oxidation of sulfides, with the electron transfer mechanism mediated by TEMPO improving the reaction efficiency to a >90% conversion rate.74 Additionally, in 2023, the team further explored the synergy between TEMPO and a benzene-selenadiazole COF (TpBSe-COF) achieving a 79% oxidation conversion rate for methyl phenyl sulfide under blue light irradiation, and realizing high selectivity (>94%) for 15 types of sulfides. The study revealed the synergy mechanism between Se atoms enhancing π–π stacking to promote charge separation and TEMPO-mediated hole transfer to facilitate ·O2− generation.75 By incorporating appropriate heteroatoms into COFs to achieve heteroatom substitution, the internal electronic environment of COFs can be altered. In 2024, the team led by Lang designed a benzoxadiazole-based COF (TpBO-COF) using an oxygen atom substitution strategy (Fig. 6). The substitution with oxygen atoms broadened the visible light absorption and enhanced its charge transport properties. This material, in synergy with the TEMPO hole transfer medium, achieved efficient and selective oxidation of sulfides to sulfoxides under blue light irradiation, with a conversion rate of up to 82% (for methyl phenyl sulfide), more than double that of the thiazole-based COF (TpBTD-COF). Further studies demonstrated that the system exhibited good applicability for three methyl phenyl sulfides with different substituents. Mechanistic investigations revealed that in the synergistic catalytic system constructed by TpBO-COF and TEMPO, photogenerated e− reduce O2 to generate ·O2−, while h+ oxidize TEMPO to drive the conversion of sulfides into sulfur radical intermediates, ultimately achieving efficient and highly selective oxidation of sulfides to sulfoxides.76
 | ||
| Fig. 6 (a) Synthesis routes of TpBO-COF and TpBTD-COF; (b) experimental and simulated XRD patterns of TpBO-COF and (c) TpBTD-COF; (d) UV-Vis DRS spectra of TpBO-COF and TpBTD-COF; (e) band structures of TpBO-COF and TpBTD-COF; (f) kinetic curves of the photocatalytic oxidation of methyl phenyl sulfide on TpBO-COF with or without 3% TEMPO; (g) photocatalytic oxidation performance of methyl phenyl sulfides with three different substituents; (h) mechanism of TpBO-COF in conjunction with TEMPO in the oxidation of methyl phenyl sulfide. Reproduced with permission from ref. 76, Copyright 2024, from Elsevier. | ||
Understanding the pore size control of COFs for substrate matching is crucial for improving photocatalytic efficiency. In 2025, Shan's team designed two different pore sizes of two-dimensional porphyrin-based COFs (ETBA–por COF and ETBC–por COF) to study the influence of pore size on photocatalytic organic transformations. The study showed that the small pore-sized ETBA–por COF, with a 2367.9 m2 g−1 high surface area and fast electron transfer capability, achieved a 94% conversion rate and >99% selectivity in sulfide oxidation. The larger pore-sized ETBC–por COF, due to avoiding product blockage in the pores, achieved a 99% conversion rate and 96% yield in amine oxidation coupling. The work also used molecular simulations to confirm that pore size matching with the substrate/product size is key to enhancing photocatalytic efficiency, offering a new paradigm for designing efficient porous photocatalysts.77 The examples above demonstrate that precise control over the pore environment, electronic structure, and heavy-atom effects in COFs is pivotal for optimizing sulfide oxidation. This principle of structure-driven selectivity is equally critical, yet often requires different strategic emphases, for another class of vital organic transformations: the oxidative coupling of amines to imines.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– linkages effectively overcame the degradation issues common in imine-linked COFs under amine-rich conditions.78 Building on this, in 2022, the same group designed an oxime-linked two-dimensional porphyrin COF (Por–DETH-COF). The introduction of oxime linkages significantly enhanced the framework's resistance to amine exchange, while the ordered channels further promoted charge separation. Under red-light irradiation, Por–DETH-COF realized highly selective aerobic oxidation of amines to imines, achieving >90% conversion and >99% selectivity, together with excellent recyclability.79 To further enhance efficiency, integrating donor and acceptor units into COFs has become a widely explored approach to mitigate fast charge recombination. In 2022, the same group developed an innovative “monomer pre-embedding” strategy, successfully incorporating the strongly electron-deficient thiazolo[5,4-d]thiazole (TzTz) unit into a highly crystalline and stable β-ketoenamine-linked framework, affording a robust D–A type COF (TpDTz-COF). This material exhibited markedly enhanced visible-light absorption and efficient charge separation and migration. Under blue-light irradiation, TpDTz-COF catalyzed the selective oxidation of benzylamines and heterocyclic amines to imines with 92% conversion and 99% selectivity, outperforming analogous COFs without TzTz units. The catalyst also showed good recyclability and broad substrate scope. Mechanistic studies confirmed that the reaction followed an electron-transfer pathway, with ·O2− as the key ROS.80 In 2024, Wang et al. developed a pyrene–porphyrin D–A COF (Py–Por-COF), constructing nanoscale heterojunctions through the donor pyrene and acceptor porphyrin units. Band engineering precisely tuned the CB potential (−0.47 V vs. NHE), matching the O2/·O2− redox potential (−0.33 V vs. NHE)—a crucial factor for selectively generating ·O2−. This strategy also optimized the band gap to 2.30 eV. Owing to its broad-spectrum absorption (300–700 nm) and efficient charge separation, Py–Por-COF delivered an outstanding 99% yield in amine oxidative coupling under blue light, with an apparent quantum yield (AQY) of 11.3%. The activity remained above 90% even after four catalytic cycles.81 Wen's group, by varying the number of donor benzene rings in aniline, designed three D–A COFs with Tp as the acceptor and, for the first time, elucidated a polarization–planarity synergistic mechanism (Fig. 7). Tp–Tapb COF, featuring the optimal combination of dipole moment (4.76 D) and planarity (dihedral angles: 5.8°, 36.8°), achieved highly efficient photocatalytic oxidation of amines. DFT calculations confirm that the larger dipole moment of Tp–Tapb COF leads to a stronger internal electric field, thereby facilitating charge separation and transport, while moderate planarity maintains the π-conjugated structure, thus reducing energy loss. This synergistic effect enhances the production efficiency of ROS (1O2/·O2−). Therefore, the Tp–Tapb type COF material achieved a 98% aniline conversion rate within 4.5 h, outperforming similar materials and demonstrating excellent cycle stability. This study established a new paradigm for tuning linear D–A COFs through polarization–planarity synergy.82
C– linkages effectively overcame the degradation issues common in imine-linked COFs under amine-rich conditions.78 Building on this, in 2022, the same group designed an oxime-linked two-dimensional porphyrin COF (Por–DETH-COF). The introduction of oxime linkages significantly enhanced the framework's resistance to amine exchange, while the ordered channels further promoted charge separation. Under red-light irradiation, Por–DETH-COF realized highly selective aerobic oxidation of amines to imines, achieving >90% conversion and >99% selectivity, together with excellent recyclability.79 To further enhance efficiency, integrating donor and acceptor units into COFs has become a widely explored approach to mitigate fast charge recombination. In 2022, the same group developed an innovative “monomer pre-embedding” strategy, successfully incorporating the strongly electron-deficient thiazolo[5,4-d]thiazole (TzTz) unit into a highly crystalline and stable β-ketoenamine-linked framework, affording a robust D–A type COF (TpDTz-COF). This material exhibited markedly enhanced visible-light absorption and efficient charge separation and migration. Under blue-light irradiation, TpDTz-COF catalyzed the selective oxidation of benzylamines and heterocyclic amines to imines with 92% conversion and 99% selectivity, outperforming analogous COFs without TzTz units. The catalyst also showed good recyclability and broad substrate scope. Mechanistic studies confirmed that the reaction followed an electron-transfer pathway, with ·O2− as the key ROS.80 In 2024, Wang et al. developed a pyrene–porphyrin D–A COF (Py–Por-COF), constructing nanoscale heterojunctions through the donor pyrene and acceptor porphyrin units. Band engineering precisely tuned the CB potential (−0.47 V vs. NHE), matching the O2/·O2− redox potential (−0.33 V vs. NHE)—a crucial factor for selectively generating ·O2−. This strategy also optimized the band gap to 2.30 eV. Owing to its broad-spectrum absorption (300–700 nm) and efficient charge separation, Py–Por-COF delivered an outstanding 99% yield in amine oxidative coupling under blue light, with an apparent quantum yield (AQY) of 11.3%. The activity remained above 90% even after four catalytic cycles.81 Wen's group, by varying the number of donor benzene rings in aniline, designed three D–A COFs with Tp as the acceptor and, for the first time, elucidated a polarization–planarity synergistic mechanism (Fig. 7). Tp–Tapb COF, featuring the optimal combination of dipole moment (4.76 D) and planarity (dihedral angles: 5.8°, 36.8°), achieved highly efficient photocatalytic oxidation of amines. DFT calculations confirm that the larger dipole moment of Tp–Tapb COF leads to a stronger internal electric field, thereby facilitating charge separation and transport, while moderate planarity maintains the π-conjugated structure, thus reducing energy loss. This synergistic effect enhances the production efficiency of ROS (1O2/·O2−). Therefore, the Tp–Tapb type COF material achieved a 98% aniline conversion rate within 4.5 h, outperforming similar materials and demonstrating excellent cycle stability. This study established a new paradigm for tuning linear D–A COFs through polarization–planarity synergy.82
 | ||
| Fig. 7 (a) Electrostatic potential (ESP) of Tp–Ta COF, Tp–Tapb COF, and Tp–Abqda COF; (b) HOMO, LUMO, and dihedral angles (isosurface value 0.03) of the three COFs; (c) mechanism diagram of the photocatalytic oxidation of benzylamine by Tp–Tapb COF. Reproduced with permission from ref. 82, Copyright 2025, from American Chemical Society. | ||
Tuning the dimensionality of COFs is also vital for enhancing photocatalytic activity. One-dimensional (1D) COFs, extending along a single direction through covalent bonds combined with π–π stacking and hydrogen bonding, can achieve highly dispersed active sites and significantly improve their utilization. However, due to the anisotropy of organic chains and entropy-driven disordered packing, their synthesis remains challenging. In 2023, Zhao and co-workers developed a 1D COF (JNM-12) based on spirobifluorene-BODIPY units. With a narrow band gap and suitable energy levels, JNM-12 efficiently harvested visible light and activated O2 to generate both ·O2− and 1O2, driving diverse oxidation reactions. Without metal co-catalysts, it achieved 97% conversion in benzylamine oxidative coupling and up to 68% yield in enamine aerobic oxidation under visible light, breaking the bottleneck of 1D COF synthesis and providing new insights for developing efficient metal-free photocatalysts.83
Furthermore, introducing appropriate metal centres can not only accelerate charge separation but also provide abundant active sites, thereby enhancing the selectivity of amine oxidative coupling. In 2024, Duan's team synthesized a bimetallic porphyrin-based COF (COF-Sr2Fe1) by coordinating Sr2+ and Fe2+ into the porphyrin centres of COF-366 via a two-step process. Under visible light, COF-Sr2Fe1 efficiently catalyzed benzylamine oxidative coupling with a yield as high as 97%, far surpassing single-metal and metal-free controls. The synergistic effect of the two metals not only enhanced visible-light absorption but also promoted charge separation. Importantly, DFT calculations revealed for the first time a precise division of labor: Fe sites dominated the dehydrogenation step, while Sr sites facilitated the C–N coupling step. This work provides a rational strategy for designing bimetallic active centres in COFs, and deepens mechanistic understanding of amine oxidative coupling reactions at the molecular level.84 Moving from nitrogen-containing to oxygen-containing compounds, the selective oxidation of alcohols poses distinct challenges, primarily centred on suppressing over-oxidation to carboxylic acids. Here, COF design strategies shift focusses towards precise exciton and polarization engineering to govern the oxidation pathway.
 | ||
| Fig. 8 (a) Synthesis scheme and corresponding electrostatic potential of asymmetrically polarized COFs; (b) band structure diagrams of BA-COF, BP-COF, and BT-COF; (c) H2O2 production performance of different N-doped COFs; (d) aerobic oxidation of various alcohols catalyzed by BT-COF; (e and f) alcohol oxidation conversion and H2O2 production performance and mechanism. Reproduced with permission from ref. 85, Copyright 2025, from Wiley-VCH. | ||
Recently, olefin-linked COFs (OL-COFs) have attracted significant attention due to their outstanding stability and extended π-electron delocalization. Studies have shown that the photocatalytic performance of OL-COFs is closely related to their intrinsic excitonic effects. Reducing excitonic effects facilitates the generation of free e−, thereby enhancing photocatalytic activity. Therefore, developing strategies to regulate excitonic effects in OL-COFs is essential for improving their performance. In 2025, Peng and co-workers proposed an intramolecular exciton regulation strategy by designing an olefin-linked COF (TMO–BDA COF) to achieve precise optimization of the exciton binding energy. Specifically, the introduction of a strong electron-accepting unit, benzoxadiazole (BDA), significantly reinforced intramolecular D–A interactions. Femtosecond transient absorption spectroscopy revealed a strong correlation between charge separation dynamics and photocatalytic activity. This design substantially reduced the exciton binding energy, accelerated charge separation, and enhanced the generation of ·O2− and 1O2. As a result, TMO–BDA COF achieved an 88% yield (aromatic alcohol oxidation–condensation–cyclization synthesis of benzimidazole) in a one-pot cascade reaction under visible light, and the reaction was scalable to the gram level. This study establishes an important new paradigm for tuning photocatalytic performance through exciton engineering.86
 | ||
| Fig. 9 (a) Synthesis of NQ-COFE5-O from NQ-COFE5; (b) substrate scope of the NQ-COFE5-O photocatalytic C–H activation reaction; (c) mechanism of the C–H activation reaction. Reproduced with permission from ref. 87, Copyright 2023, from Wiley-VCH. | ||
 | ||
| Fig. 10 (a) Synthesis of TAD-COF through the CeCl3-catalyzed Mannich reaction and the mechanism of photocatalytic oxidative hydroxylation of arylboronic acids; (b) substrate scope of the TAD-COF photocatalytic oxidative hydroxylation of arylboronic acids; (c) cycle stability of the TAD-COF oxidative hydroxylation reaction of phenylboronic acid; (d) photocatalytic conversion mechanism of arylboronic acids to corresponding phenols. Reproduced with permission from ref. 90, Copyright 2024, from Author(s). | ||
In 2022, Zhou and co-workers applied pore-wall surface engineering to an olefin-linked COF (TBT-COF) by introducing strongly electron-donating methoxy groups (–OMe), yielding MeO–TBT-COF. The strong electron-donating conjugation effect of –OMe groups significantly broadened visible light absorption, narrowed the band gap, and enhanced charge separation efficiency. Its optimized CB potential was thermodynamically favorable for activating O2 to ·O2−. Under air and white LED irradiation, ·O2− nucleophilically attacked the boron centre of arylboronic acids, followed by rearrangement and hydrolysis to generate phenols with 92% yield. Compared with F- or H-modified analogues, the methoxy-functionalized COF exhibited >20% higher catalytic performance, highlighting the fine-tuning ability of pore-wall functionalization for the electronic structure and catalytic activity.91 In 2021, Wen's group designed a fully electron-rich pyrene-based COF (COF-JLU25) that enabled visible-light-driven hydroxylation of arylboronic acids to phenols. Under ambient air and room temperature, with only a low catalyst loading, the system achieved >99% conversion of electron-deficient arylboronic acids within 24 hours, retaining activity over seven cycles without deactivation. The superior performance was attributed to the electron-rich skeleton that promoted ·O2− generation and the stable nanochannels that ensured efficient mass transfer. This study provides a green and practical strategy for synthesizing phenolic intermediates important in pharmaceuticals.92
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 µmol g−1 h−1, significantly enhancing atom economy and overcoming the toxicity and noble-metal dependence of conventional methods. This work establishes a green paradigm for upgrading biomass-derived compounds into pharmaceutical precursors.93 In another example, Zhou and colleagues designed a series of polyimide-based COFs (COF-Nx) with a gradient of nitrogen content. As shown in Fig. 11, by reducing the nitrogen content (COF-N0), they effectively optimized charge carrier dynamics (prolonged lifetime and enhanced separation), thereby promoting efficient 1O2 generation. More importantly, the constructed 1O2-driven photocatalytic system replaced conventional hole-mediated oxidation pathways, suppressing byproduct formation. Under visible-light irradiation, 1O2 selectively triggered the Achmatowicz rearrangement of FFA to yield pyranone (PN) with 99% conversion and 92% selectivity, while simultaneously generating H2O2 at about 4549 µmol g−1 h−1, achieving a solar-to-chemical conversion (SCC) efficiency of 1.73%. In situ DRIFTS, EPR, and DFT calculations confirmed that 1O2 initiated the rearrangement via a [4 + 2] cycloaddition intermediate, reducing the reaction barrier by 1.2 eV compared to the hole oxidation pathway. This avoided traditional oxidative side reactions, and the generated H2O2 was preserved without decomposition. Such a strategy transforms the conventional concept of “sacrificial agent consumption” into “high-value chemical synthesis,” providing a new paradigm for biorefining and sustainable chemical manufacturing.94
080 µmol g−1 h−1, significantly enhancing atom economy and overcoming the toxicity and noble-metal dependence of conventional methods. This work establishes a green paradigm for upgrading biomass-derived compounds into pharmaceutical precursors.93 In another example, Zhou and colleagues designed a series of polyimide-based COFs (COF-Nx) with a gradient of nitrogen content. As shown in Fig. 11, by reducing the nitrogen content (COF-N0), they effectively optimized charge carrier dynamics (prolonged lifetime and enhanced separation), thereby promoting efficient 1O2 generation. More importantly, the constructed 1O2-driven photocatalytic system replaced conventional hole-mediated oxidation pathways, suppressing byproduct formation. Under visible-light irradiation, 1O2 selectively triggered the Achmatowicz rearrangement of FFA to yield pyranone (PN) with 99% conversion and 92% selectivity, while simultaneously generating H2O2 at about 4549 µmol g−1 h−1, achieving a solar-to-chemical conversion (SCC) efficiency of 1.73%. In situ DRIFTS, EPR, and DFT calculations confirmed that 1O2 initiated the rearrangement via a [4 + 2] cycloaddition intermediate, reducing the reaction barrier by 1.2 eV compared to the hole oxidation pathway. This avoided traditional oxidative side reactions, and the generated H2O2 was preserved without decomposition. Such a strategy transforms the conventional concept of “sacrificial agent consumption” into “high-value chemical synthesis,” providing a new paradigm for biorefining and sustainable chemical manufacturing.94
 | ||
| Fig. 11 (a) Synthesis method of nitrogen-doped D–A structured COF-N0–3 and specific functions of different structural fragments; (b) enlarged view of the local unit including pore size and electron flow direction and the AA stacking model of COF-N0 observed along the a-axis; (c) TRPL spectrum of COF-N0–3; (d) photocatalytic H2O2 performance of COF-N0–3; (e) comparison of FFA conversion rate and PN selectivity within 4 h; (f) capture experiment of COF-N0; (g) DFT calculations of the reaction pathways from FFA to PN and FF. Reproduced with permission from ref. 94, Copyright 2025, from Wiley-VCH. | ||
 | ||
| Fig. 12 (a) Structure of Hex–Aza-COF-3; (b) transient absorption spectra of Hex–Aza-COF-3 with and without the addition of substrate 1a and oxidant (NH4)2S2O8 at a delay time of 1.5 ps; (c) kinetic traces of Hex-Aza-COF-3 at 520 nm (black line) and the mixture of Hex–Aza-COF-3 with 1a and (NH4)2S2O8 at 586 nm (red line) at late (main plot) and early (inset) time scales; (d) quenching experiments; (e) cycle stability of Hex–Aza-COF-3; (f) reaction mechanism of the Hex–Aza-COF-3 photocatalytic oxidative [3 + 2] cycloaddition reaction. Reproduced with permission from ref. 98, Copyright 2023, from Author(s). | ||
4.2 H2O2 production
H2O2 is a vital green oxidant and energy carrier, yet its industrial anthraquinone synthesis is energy-intensive and unsustainable. Photocatalytic routes that generate H2O2 from O2 and H2O via the ORR and WOR offer an eco-friendly alternative, but are challenged by unfavorable WOR thermodynamics and H2O2 instability. Band structure tuning is thus critical for selectivity, and COFs, with adjustable electronic structures, high surface areas, and functional pores, emerge as ideal photocatalysts. In contrast to selective organic oxidations that often target a single ROS pathway, efficient H2O2 production frequently requires the synergistic coupling of multiple reaction channels, a task for which the multifunctional and tunable nature of COFs is particularly well-suited. Through rational design, COFs can regulate active sites, charge behavior, and mass transport, advancing ORR and especially WOR pathways for efficient H2O2 production.A central challenge in photocatalytic H2O2 synthesis is activating the kinetically sluggish WOR. Research has shown that by precisely engineering active sites within COFs, this bottleneck can be effectively addressed. The photocatalytic synthesis of H2O2 mainly relies on the ORR, while the slow kinetics of the WOR is the bottleneck for the overall reaction synthesis. Research shows that by precisely regulating the active sites, the efficient WOR process can be activated in COFs. Li and co-workers regulated the number of nitrogen atoms (0–3) in heterocycles of β-ketoenamine COFs to construct biomimetic microenvironments and optimize hole utilization. Among these, the triazine-based N3-COF exhibited the highest hole density, with its nitrogen heterocycles functioning as dedicated WOR active centres. This design enabled a cooperative mechanism between direct 2e−-WOR and indirect 1e−-ORR pathways, ultimately delivering an impressive H2O2 production rate of 4881 µmol g−1 h−1.99 Similarly, Liao and colleagues synthesized three diazine-functionalized COFs (TpDz, TpMd, and TpPz) to systematically investigate the influence of nitrogen atom positioning on photocatalytic H2O2 synthesis. The pyrazine unit in TpDz stabilized endoperoxide intermediates via adjacent nitrogen atoms, which significantly facilitated the direct 2e−-ORR pathway, achieving a high H2O2 yield of 7327 µmol g−1 h−1. Importantly, the VB positions of all three COFs were sufficient to drive the WOR through the 4e− pathway, oxidizing water into O2 while simultaneously supplying protons and e−—without requiring sacrificial agents. TpDz exhibited excellent charge separation efficiency and low transport resistance, further enhancing the synergy between the ORR and WOR.100 Liu's group developed two novel nitrogen-rich COFs (COF-JLU51 and COF-JLU52) by incorporating triazole–triazine (TTT) units. These frameworks efficiently produced H2O2 in pure water and O2 environments via a dual 2e−-ORR and 4e−-WOR mechanism. Specifically, COF-JLU51 achieved a yield of 4260.3 µmol g−1 h−1 in pure water, while COF-JLU52 demonstrated an ultra-high activity of 7624.7 µmol g−1 h−1 and an AQE of 18.2% in a benzyl alcohol/water biphasic system. In situ IR spectroscopy, electrochemical measurements, and DFT calculations confirmed the presence of the 4e−-WOR pathway, in which water molecules are oxidized at nitrogen-rich sites to produce O2 and protons, thereby facilitating the ORR process. The superior performance was attributed to the high surface area, efficient charge separation and transport, favorable band structures, and strong adsorption of O2/H2O at nitrogen-rich sites.101 Ye and co-workers designed a bipyridine-based COF (COF-TfpBpy) that utilized water as the proton source. Upon protonation at the bipyridine sites to form PyH+, two water molecules adsorbed as a (H2O)2 cluster, enabling a direct 2e−-WOR pathway to produce H2O2. This mechanism overcame the traditionally sluggish 2e−-WOR kinetics, achieving a WOR rate of 302 µM h−1 and, together with the 2e−-ORR pathway, nearly 100% atom utilization efficiency. At 333 K, the solar-to-chemical conversion (SCC) efficiency reached 1.08%. In situ IR spectra confirmed the direct 2e−-WOR pathway, while DFT calculations further revealed that the adsorption energy of the (H2O)2 cluster at protonated bipyridine sites was significantly more favorable than single water adsorption, thus reducing the reaction barrier substantially.102 Guo's group proposed a “proton reservoir” strategy by designing a hydroxyl-functionalized COF (TFBP–DHBD COF) to overcome the sluggish kinetics of the WOR in photocatalytic H2O2 synthesis (Fig. 13). Phenolic –OH groups acted as dynamic proton buffers: during the initial illumination stage, they rapidly supplied protons to the ORR to accelerate *OOH intermediate formation, while subsequently capturing delayed protons released from the WOR for cyclic utilization. This bridged the spatiotemporal mismatch between the ORR and WOR. The mechanism was validated by isotope labeling and in situ IR spectroscopy. Consequently, the COF achieved a H2O2 production rate of 1444.0 µmol g−1 h−1 without sacrificial agents—3.3 times higher than its non-hydroxyl analogue.103
 | ||
| Fig. 13 (a) Schematic diagram of the synthesis of TFBP–BD and TFBP–DHBD COFs; (b) band positions of TFBP–BD and TFBP–DHBD COFs. (c) Comparison of photocatalytic H2O2 production performance of TFBP–BD and TFBP–DHBD COFs; (d) DRIFTS spectra of TFBP–DHBD after adsorption of water and O2 saturation; (e) Gibbs free energy diagram for the ORR to H2O2 conversion on the TFBP–DHBD COF. Reproduced with permission from ref. 103, Copyright 2025, from Wiley-VCH. | ||
Yue and colleagues challenged conventional understanding by revealing that in thiophene-based COFs (TD-COF), phenyl rings—rather than heteroatoms—served as efficient WOR active centres when linked by imine bonds to thiophene units. DFT calculations indicated that phenyl rings exhibited lower free energy barriers for *OH adsorption than conventional heteroatom sites. Coupled with a superhydrophilic design, TD-COF achieved a H2O2 production rate of 1080 µmol g−1 h−1 even under an Ar atmosphere. In situ DRIFTS directly detected *OOH and *OH intermediates, confirming the operation of a direct 2e−-WOR pathway.104 Chen and co-workers employed a molecular engineering strategy to design two s-heptazine-based COFs (HEP–TAPT-COF and HEP–TAPB-COF), enabling spatially separated dual active centres for the ORR and WOR within a single framework. Both theoretical calculations and experimental validation revealed that phenyl rings acted as efficient WOR sites, catalyzing the four-electron water oxidation to O2 with a negative Gibbs free energy change (ΔG), indicating a spontaneous process. Isotopic labeling experiments with H218O confirmed that the generated O2 originated exclusively from water oxidation, which was subsequently in situ reduced to H2O2 by adjacent s-heptazine/triazine centres, forming a self-sustained oxygen supply cycle. Benefiting from the dual-ORR-centre design, HEP–TAPT-COF exhibited enhanced charge separation efficiency and accelerated WOR kinetics, achieving a H2O2 production rate of 87.50 µmol h−1 and a SCC efficiency of 0.65%.105 For further mechanistic insights, Pan and colleagues proposed a novel “hydration-triggered” WOR mechanism by synthesizing a series of hydrophilic hydrazone-linked COFs (DETH-COF and BBT-COF). In this mechanism, hydrazone linkages served as active sites where hydration directly initiated O–H bond cleavage, forming N-centred radical intermediates. Subsequently, hole-driven oxidative deprotonation and hydroxyl radical coupling selectively yielded H2O2. Experimental and theoretical studies demonstrated that O2 facilitated the rate-determining step by capturing protons/e−, reducing the energy barrier from 23 to 15.6 kcal mol−1. The intrinsic hydrophilicity was identified as the key factor controlling efficiency. Under visible light in pure water and ambient conditions, the optimized DETH-COF achieved a production rate of 1.0 mmol g−1 h−1, with H218O isotopic labeling confirming that the oxygen in H2O2 originated entirely from water oxidation rather than the ORR.106 Tao and co-workers introduced a “framework-centred radical strategy” by employing carbonyl groups in fluorinated COFs (Kf–F-COF) as photogenerated radical sites. Through a hydrogen atom transfer (HAT) mechanism, both O2 and H2O were simultaneously activated, enabling efficient dual-channel H2O2 synthesis under visible light. Kf–F-COF produced H2O2 directly via the 2e−-WOR pathway without forming hydroxyl radical intermediates, while synergistic ORR further boosted the yield to 6.42 mmol g−1 h−1. Fluorination significantly increased the electron affinity of carbonyl groups, promoting dual radical formation and lowering energy barriers, thereby ensuring excellent stability and activity in both natural sunlight and seawater environments.107
The topology and linkage chemistry of COFs critically influence their electronic structures, hydrophilicity, and charge separation efficiency, thereby dictating WOR performance. Yang and colleagues designed COFs with distinct topologies—cpt (TBD-COF) and hcb (TBC-COF)—to probe these effects. The cpt topology endowed stronger hydrophilicity, enhancing water adsorption and proton transfer. This lowered the energy barrier for the rate-determining step from 4.09 to 2.97 eV, resulting in a H2O2 production rate of 380 µmol g−1 h−1 even under an Ar atmosphere.108 Xu and co-workers further advanced this concept by synthesizing three sp2-carbon-linked 2D COFs with distinct topologies (hcb, sql, and hxl) to systematically investigate topology-dependent photocatalytic performance (Fig. 14). Despite similar band structures and compositions, the hxl-topology QP–HPTP-COF exhibited the best catalytic activity, achieving an SCC efficiency of 1.41%. Combined spectroscopic and computational analyses revealed that the hxl topology significantly reduced exciton binding energy and carrier effective mass, thereby enhancing charge separation and transport. Notably, the WOR occurred via a four-electron pathway at phenyl ring sites, synergistically coupled with a two-electron ORR pathway at cyanoethylene linkage sites, completing the overall H2O2 synthesis.109 Building upon this, Yue and co-workers developed sub-stoichiometric [6 + 6] COFs (HHT-COF) by employing conjugation and linkage-directional strategies. The kgd topology with periodically retained aldehyde groups enhanced hydrophilicity, while mechanistic studies identified phenyl rings—rather than aldehydes—as the true WOR active centres. HHT-COF exhibited the lowest *OH formation barrier, accelerating the WOR. Coupled with the 2e−-ORR pathway, this COF achieved a remarkable H2O2 production rate of 4996 µmol g−1 h−1 in pure water under air.110 Expanding this strategy, two kgd-topology COFs (TPT-COF and TPB-COF) were synthesized. Remarkably, TPB-COF directly oxidized water via a one-step 2e−-WOR pathway to generate H2O2, maintaining 509 µmol g−1 h−1 even under Ar. When combined with efficient 2e−-ORR, the total H2O2 yield reached 7874 µmol g−1 h−1 in pure water and 5696 µmol g−1 h−1 in natural seawater. Both experimental and theoretical analyses demonstrated that the kgd topology effectively facilitated charge separation, reduced energy barriers for *OOH and *OH intermediates, and enhanced water adsorption and activation. Importantly, the in situ generated H2O2 was successfully applied to E. coli disinfection, highlighting its potential for real-world applications.111
 | ||
Fig. 14 (a) Synthesis schemes of QP–TPB-COF, QP–BPT-COF, and QP–HPTP-COF; (b) schematic diagram of the relationship between the topological structures of the three COFs and their effective masses (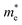 and and 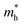 ); (c) band structures of the three COFs; (d) photocatalytic H2O2 production performance (pure water and oxygen-saturated); (e) in situ DRIFT spectra of H2O2 generation by QP–HPTP-COF photocatalysis; (f) mechanism diagram of H2O2 generation by QP–HPTP-COF photocatalysis. Reproduced with permission from ref. 109, Copyright 2024, from Wiley-VCH. ); (c) band structures of the three COFs; (d) photocatalytic H2O2 production performance (pure water and oxygen-saturated); (e) in situ DRIFT spectra of H2O2 generation by QP–HPTP-COF photocatalysis; (f) mechanism diagram of H2O2 generation by QP–HPTP-COF photocatalysis. Reproduced with permission from ref. 109, Copyright 2024, from Wiley-VCH. | ||
Beyond topology engineering, altering the orientation of linkage units can yield compositionally isomeric COFs with distinct optoelectronic properties and catalytic performances. Yang and co-workers synthesized fully conjugated thiophene–pyridine-bridged COFs (TBA-COF/TCA-COF) via a one-pot Pictet–Spengler cyclization reaction. This cyclization precisely positioned WOR active centres at pyridine units while localizing ORR centres at triazine/phenyl sites, thereby enhancing charge separation efficiency and extending the fluorescence lifetime to 2.20 ns. The VB potential was positively shifted to 1.51 V (vs. NHE), thermodynamically driving the 2e−-WOR pathway. Coupled with the 2e−-ORR pathway, TBA-COF achieved a remarkable H2O2 production rate of 8878 µmol g−1 h−1 in real seawater, of which the WOR pathway independently contributed 487 µmol g−1 h−1 under Ar.112 Wang and colleagues synthesized imine-linked isomeric COFs named PB–PT-COF and PT–PB-COF to systematically investigate pathway-dependent differences in photocatalytic H2O2 synthesis. PB–PT-COF, with positively charged TAPB nodes, facilitated *OH intermediate formation, enhancing H2O adsorption and lowering the energy barrier for the WOR, thereby enabling direct water-to-H2O2 conversion. In contrast, PT–PB-COF favoured the generation of ·O2−, producing H2O2 primarily via the ORR pathway. Both experimental and theoretical studies confirmed that isomerization effectively regulates electronic structures and pathway selectivity.113 Li and co-workers proposed a hydroxyl-defect strategy, synthesizing a pyrimidine-based COF (COF-BD) with mixed imine and β-ketoenamine linkages. This architecture lowered the VB maximum (VBM = 1.62 V) and redirected the WOR pathway from 2e− to 4e−, accelerating hole consumption and dramatically enhancing catalyst stability. COF-BD achieved a high H2O2 production rate of 5312 µmol g−1 h−1 in pure water, retaining 70% of its initial activity over 10 h, significantly outperforming β-ketoenamine-linked COF-TP.114
Constructing D–A frameworks is one of the most effective strategies to promote charge separation and has been widely applied to enhance H2O2 production. Jiang and co-workers designed an acetylene-bridged D–A COF (EBBT-COF), where bithienyl (BTT) donor units catalyzed the 2e−-WOR efficiently, as confirmed by in situ DRIFTS and RRDE measurements. Triethynylbenzene (TAEB) served as an electron acceptor, boosting hole transport and acting as an “electron reservoir”. The acetylene linkage enhanced π-conjugation and accelerated charge separation, delivering a WOR activity of 1563 µmol g−1 h−1 and, when coupled with the 2e−-ORR, a total H2O2 yield of 5686 µmol g−1 h−1 with a quantum efficiency of 15.14%.115 Wang and co-workers designed a cyano-functionalized COF (TBTN-COF) by integrating strong electron-accepting TBTN units with hole-rich benzotrithiophene (BTT) donors, enabling efficient sacrificial-agent-free H2O2 synthesis in pure water. Ultrafast intramolecular electron transfer (<500 fs) and long-lived charge-separated states allowed BTT to efficiently drive the WOR to generate O2, which then served as an in situ oxygen source for the ORR. In situ DRIFTS and DFT confirmed that cyano sites stabilized Yeager-type O2 adsorption, suppressing superoxide pathways and directly forming *OOH intermediates, significantly improving H2O2 selectivity. As a result, TBTN-COF achieved an impressive H2O2 production rate of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 013 µmol g−1 h−1.116 Li and co-workers further constructed a triazine-based D–A COF (TPB–TPT-COF) via Schiff-base condensation between triazine acceptors and TPB donors, enabling sacrificial-agent-free H2O2 synthesis from water and oxygen. Under visible light, TPB–TPT-COF delivered a yield of 6740 µmol g−1 h−1, far surpassing the control without triazine units. Mechanistic studies revealed the coexistence of 2e−-ORR and 4e−-WOR pathways. Notably, the 4e−-WOR route was verified using AgNO3 scavenger tests and 18O isotopic labeling, confirming O2 evolution, while in situ IR and DFT identified *O–OH intermediates and active sites near imine linkages.117 Ma and colleagues systematically investigated D–π–A COFs with N-heteroaryl acceptors of varying electron-withdrawing strength (benzene < pyridine < pyrimidine < triazine). Increasing nitrogen content enhanced electron affinity, thereby promoting electron transfer from donor to acceptor across π-bridges and improving charge separation. Among them, Tf–TAPT-COF (triazine acceptor) exhibited the highest H2O2 yield (2700 µmol g−1 h−1) without sacrificial agents. Both experimental and computational analyses indicated that photogenerated h+ localized on donor phenyl aldehyde sites served as the main WOR centres for direct 2e−-water oxidation, while the π-bridge and acceptor jointly facilitated the ORR.118 In another research, Lan and co-workers constructed a redox-active molecular junction COF (TTF–BT-COF) by covalently linking tetrathiafulvalene (TTF, oxidation centre) with benzothiadiazole (Fig. 15). Without sacrificial agents, this framework efficiently generated H2O2via concurrent 2e−-WOR and 2e−-ORR pathways, achieving an extraordinary rate of 276
013 µmol g−1 h−1.116 Li and co-workers further constructed a triazine-based D–A COF (TPB–TPT-COF) via Schiff-base condensation between triazine acceptors and TPB donors, enabling sacrificial-agent-free H2O2 synthesis from water and oxygen. Under visible light, TPB–TPT-COF delivered a yield of 6740 µmol g−1 h−1, far surpassing the control without triazine units. Mechanistic studies revealed the coexistence of 2e−-ORR and 4e−-WOR pathways. Notably, the 4e−-WOR route was verified using AgNO3 scavenger tests and 18O isotopic labeling, confirming O2 evolution, while in situ IR and DFT identified *O–OH intermediates and active sites near imine linkages.117 Ma and colleagues systematically investigated D–π–A COFs with N-heteroaryl acceptors of varying electron-withdrawing strength (benzene < pyridine < pyrimidine < triazine). Increasing nitrogen content enhanced electron affinity, thereby promoting electron transfer from donor to acceptor across π-bridges and improving charge separation. Among them, Tf–TAPT-COF (triazine acceptor) exhibited the highest H2O2 yield (2700 µmol g−1 h−1) without sacrificial agents. Both experimental and computational analyses indicated that photogenerated h+ localized on donor phenyl aldehyde sites served as the main WOR centres for direct 2e−-water oxidation, while the π-bridge and acceptor jointly facilitated the ORR.118 In another research, Lan and co-workers constructed a redox-active molecular junction COF (TTF–BT-COF) by covalently linking tetrathiafulvalene (TTF, oxidation centre) with benzothiadiazole (Fig. 15). Without sacrificial agents, this framework efficiently generated H2O2via concurrent 2e−-WOR and 2e−-ORR pathways, achieving an extraordinary rate of 276![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 µM g−1 h−1. Mechanistically, the WOR proceeded through dehydrogenation of water at TTF sites to form ·OH intermediates, which subsequently coupled to yield H2O2 with an energy barrier of only 3.90 eV. RRDE testing ruled out competing 4e− OER. Isotopic labeling (18O) confirmed near-100% atomic utilization of H2O and O2, while DRIFTS and EPR verified the presence of ·OH and *OOH intermediates.119 Ding and co-workers adopted a one-pot “grafting-to” strategy to incorporate amino-heterocycles such as 2-aminothiazole into COFs, reconstructing local electronic configurations to form intramolecular D–A structures. This design promoted efficient charge separation and optimized band positions, satisfying thermodynamic requirements for the WOR. Notably, COF-Thz synergistically combined 2e−-WOR and 1e−-ORR pathways, achieving an H2O2 yield of 3701 µmol g−1 h−1 and a solar-to-chemical conversion efficiency exceeding that of natural photosynthesis. Moreover, when applied with Fe(II), COF-Thz exhibited nearly 100% bactericidal efficiency and >90% biofilm removal, underscoring its dual potential in photocatalysis and biomedical applications.120
000 µM g−1 h−1. Mechanistically, the WOR proceeded through dehydrogenation of water at TTF sites to form ·OH intermediates, which subsequently coupled to yield H2O2 with an energy barrier of only 3.90 eV. RRDE testing ruled out competing 4e− OER. Isotopic labeling (18O) confirmed near-100% atomic utilization of H2O and O2, while DRIFTS and EPR verified the presence of ·OH and *OOH intermediates.119 Ding and co-workers adopted a one-pot “grafting-to” strategy to incorporate amino-heterocycles such as 2-aminothiazole into COFs, reconstructing local electronic configurations to form intramolecular D–A structures. This design promoted efficient charge separation and optimized band positions, satisfying thermodynamic requirements for the WOR. Notably, COF-Thz synergistically combined 2e−-WOR and 1e−-ORR pathways, achieving an H2O2 yield of 3701 µmol g−1 h−1 and a solar-to-chemical conversion efficiency exceeding that of natural photosynthesis. Moreover, when applied with Fe(II), COF-Thz exhibited nearly 100% bactericidal efficiency and >90% biofilm removal, underscoring its dual potential in photocatalysis and biomedical applications.120
 | ||
| Fig. 15 (a) Synthesis diagram of TTF–BT-COF; (b) SEM and elemental mapping images of TTF–BT-COF; (c) UV-Vis DRS spectra of TTF–BT-COF, TTF–pT-COF, and TPE–BT-COF; (d) comparison of photocatalytic H2O2 production activity of TTF–BT-COF, TTF–pT-COF, and TPE–BT-COF in pure water and O2 atmospheres; (e) DRIFTS spectra of TTF–BT-COF during H2O2 photosynthesis; (f) Gibbs free energy diagrams for photocatalytic H2O2 production via WOR (solid line) and ORR (dashed line) pathways for TTF–BT-COF, TTF–pT-COF, and TPE–BT-COF; (g) adsorption configurations of *OH intermediates on TTF and *OOH intermediates on BT in TTF–BT-COF. Reproduced with permission from ref. 119, Copyright 2022, from Wiley-VCH. | ||
Functional modification of COF skeletons is an effective approach to finely tune their physicochemical properties and catalytic performance. As shown in Fig. 16, Luo and co-workers designed a cyano-functionalized D–A–π–D COF (ECUT-COF-50) via a two-step synthesis strategy, successfully constructing a photocatalyst with a unique electronic structure. Under sacrificial agent-free conditions, ECUT-COF-50 enabled efficient H2O2 production directly from air and water, achieving a yield of 4742 µmol g−1 h−1 with an O2 utilization efficiency of 88%. This advance addressed the key challenge of the WOR pathway: the strong electron-withdrawing effect of cyano groups reconstructed the charge distribution of adjacent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, turning them into efficient WOR active centres that directly oxidized H2O into H2O2 rather than via the conventional O2 intermediate. Both experiments and DFT calculations confirmed that the *HO–OH intermediate at the C
C bonds, turning them into efficient WOR active centres that directly oxidized H2O into H2O2 rather than via the conventional O2 intermediate. Both experiments and DFT calculations confirmed that the *HO–OH intermediate at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C site exhibited a substantially reduced adsorption energy, lowering the energy barrier of the rate-determining step compared to the control COF. Meanwhile, the VB potential satisfied the thermodynamic requirements for the WOR.121 Liu and colleagues employed a multicomponent reaction (MCR) to synthesize six quinoline-linked triazine COFs (R–QN–TA-COFs) with substituent-controlled properties. Among them, the methoxy-modified MeO–QN–TA-COF exhibited outstanding performance, delivering 7384 µmol per g per h H2O2 under sacrificial agent-free conditions in pure water and air. The methoxy groups enhanced the electron density and hydrophobicity of the framework, simultaneously optimizing both the ORR and WOR. Quenching experiments, where EDTA suppressed hole transfer and decreased yields, in situ EPR detection of ·OH intermediates, and DFT analysis showing enhanced HOMO–LUMO overlap confirmed the critical contribution of the WOR. Remarkably, MeO–QN–TA-COF still produced 1353 µmol per g per h H2O2 under a N2 atmosphere, verifying its intrinsic water oxidation capability.122 Shen and co-workers synthesized a hydroxyl-functionalized imine-linked COF (TT-COF-OH). This COF exploited triazine-ring sites to directly oxidize water into H2O2 without free radical intermediates, assisted by a strong internal electric field (IEF) that promoted exciton dissociation and optimized hydrophilic interfaces. TT-COF-OH achieved a high H2O2 yield of 3406 µmol g−1 h−1. In situ DRIFTS confirmed the formation of *OOH intermediates, while fs-TAS revealed that IEF-induced shallow trap states extended carrier lifetimes by sixfold, addressing the fundamental challenge of inefficient exciton dissociation in 2D materials.123
C site exhibited a substantially reduced adsorption energy, lowering the energy barrier of the rate-determining step compared to the control COF. Meanwhile, the VB potential satisfied the thermodynamic requirements for the WOR.121 Liu and colleagues employed a multicomponent reaction (MCR) to synthesize six quinoline-linked triazine COFs (R–QN–TA-COFs) with substituent-controlled properties. Among them, the methoxy-modified MeO–QN–TA-COF exhibited outstanding performance, delivering 7384 µmol per g per h H2O2 under sacrificial agent-free conditions in pure water and air. The methoxy groups enhanced the electron density and hydrophobicity of the framework, simultaneously optimizing both the ORR and WOR. Quenching experiments, where EDTA suppressed hole transfer and decreased yields, in situ EPR detection of ·OH intermediates, and DFT analysis showing enhanced HOMO–LUMO overlap confirmed the critical contribution of the WOR. Remarkably, MeO–QN–TA-COF still produced 1353 µmol per g per h H2O2 under a N2 atmosphere, verifying its intrinsic water oxidation capability.122 Shen and co-workers synthesized a hydroxyl-functionalized imine-linked COF (TT-COF-OH). This COF exploited triazine-ring sites to directly oxidize water into H2O2 without free radical intermediates, assisted by a strong internal electric field (IEF) that promoted exciton dissociation and optimized hydrophilic interfaces. TT-COF-OH achieved a high H2O2 yield of 3406 µmol g−1 h−1. In situ DRIFTS confirmed the formation of *OOH intermediates, while fs-TAS revealed that IEF-induced shallow trap states extended carrier lifetimes by sixfold, addressing the fundamental challenge of inefficient exciton dissociation in 2D materials.123
 | ||
| Fig. 16 (a) Topological views of D–A systems in ECUT-COF-50 and ECUT-COF-51; (b) band positions of ECUT-COF-50 and ECUT-COF-51; (c) H2O2 production performance of ECUT-COF-50 and ECUT-COF-51 under O2 and air conditions; (d and e) Gibbs free energy diagrams for photocatalytic H2O2 production under ORR and WOR conditions for ECUT-COF-50 and ECUT-COF-51; (f) mechanism of photocatalytic H2O2 production from the ORR and WOR using ECUT-COF-50. Reproduced with permission from ref. 121, Copyright 2024, from Wiley-VCH. | ||
Shu and colleagues introduced hydroxyl groups into β-ketoenamine-linked COFs, constructing Tz-THBZ for dual-channel H2O2 production. Tz-THBZ exhibited an optimal VB position and excellent exciton dissociation capability. This design significantly enhanced WOR performance, enabling 940 µmol per g per h H2O2 yield under a N2 atmosphere, thus confirming its direct water oxidation capability. When coupled with efficient ORR, Tz-THBZ achieved a total yield of 4688 µmol g−1 h−1 in pure water. DFT calculations further revealed that the ketonic phenyl ring served as the oxidation centre while the triazine acted as the reduction centre, synergistically promoting dual-channel H2O2 generation.124
Through interfacial engineering, Zhang and co-workers synthesized a series of pyrene-based COFs (PyCOFs) with different functional groups. Among them, hydroxyl-functionalized PyCOF-OH exhibited exceptional hydrophilicity and efficient charge separation. Even under a N2 atmosphere, it produced 115 µmol per g per h H2O2, confirming its ability to generate H2O2via the WOR pathway. Mechanistic studies further revealed a ·OH-mediated two-step reaction mechanism. Coupled with efficient ORR, PyCOF-OH achieved 2961 µmol per g per h H2O2 in 25 mL pure water, while retaining excellent cycling stability.125 Hua and colleagues transformed a cyano-functionalized COF (PTTN-CN) into amidoxime-functionalized PTTN-AO via post-synthetic modification. Although H2O2 was primarily generated via the ORR pathway (yield 6024 µmol g−1 h−1, SCC efficiency 0.61%), the WOR still played a pivotal role, oxidizing water to O2via a 4e− pathway and providing a continuous oxygen supply for the ORR, thus forming a catalytic cycle. In situ DRIFTS and RRDE confirmed WOR involvement and highlighted PTTN-AO's advantages in O2 supply and proton transfer. The amidoxime groups enhanced hydrophilicity, O2 adsorption, and charge separation, while lowering ORR barriers, thereby boosting overall photocatalytic activity.126 Shen's group further developed a thioether-functionalized triazine COF (TDB-COF). Thioether groups broadened visible-light absorption, optimized band structure, and activated a two-step 2e−-WOR pathway rarely achieved in COFs. EPR directly detected ·OH intermediates, while DFT confirmed that thioether sites significantly lowered the Gibbs free energy barrier for ·OH formation, accelerating WOR kinetics. In O2-saturated systems, TDB-COF achieved a H2O2 yield of 723.5 µmol g−1 h−1.127 Tang and colleagues designed partially fluorinated triazine COFs (TP/TAPT-F COFs). Fluorination not only enabled efficient H2O2 generation via the 2e−-ORR but also significantly boosted 4e−-WOR activity. The incorporation of fluorine optimized the electronic structure, improved crystallinity and π–π stacking, and tuned the VB to drive water oxidation to O2 and H+, thereby supplying sustainable reactants for the ORR. Isotope labeling, RRDE, and in situ DRIFTS unambiguously confirmed the presence and contribution of the WOR. TP/TAPT-F COFs exhibited a H2O2 yield of 1655 µmol g−1 h−1 with excellent stability, and were successfully applied in continuous-flow reactors for efficient degradation of organic pollutants.128
4.3 Environmental remediation
RhB molecules feature highly conjugated eosin-type aromatic rings bridged by vinyl linkages, conferring exceptional chemical and photochemical stability and rendering them difficult to degrade rapidly in aqueous environments via conventional approaches.132 Recent COF design strategies have therefore centred on precisely tailoring band structures and energy-level alignments to optimize the generation, separation, and utilization of photogenerated charge carriers.133 For instance, Xiao et al. reported a β-ketoamine-linked, multi-segment D–A backbone that establishes a pronounced built-in electric field at the molecular scale, markedly enhancing spatial charge separation.134 The resulting COF possesses a surface area about 700 m2 g−1, a pore size of 2.3 nm, and visible-light absorption extending to 650 nm. Under simulated solar irradiation, it completely degrades RhB within 3 h, achieving an apparent rate constant of 1.21 h−1. Band structure analysis reveals that its CB is suitably positioned to drive O2 reduction to ·O2−, while its VB can oxidize water to ·OH. Furthermore, the wide-pore architecture facilitates rapid RhB diffusion to active sites, enabling the efficient, synergistic generation and utilization of dual-channel ROS. In another study, Wang et al. constructed a fully conjugated, coplanar sp2-carbon skeleton by condensing three distinct aromatic amine units (TPB, TEB, and TFB) with a triazine aldehyde linker.135 Among these, TEB-COF exhibited the highest activity, achieving complete removal of 10 mg L−1 RhB within 30 min under visible-light irradiation, with an apparent rate constant 2.4 times higher than that of the non-optimized TPB-COF. The photocatalyst retained its performance over five consecutive cycles without significant loss, and the total organic carbon (TOC) removal reached 78%. Active species trapping and ESR analysis revealed that both photogenerated h+ and ·O2− acted synergistically to drive oxidative degradation. In a related study, Jiang and co-workers coupled a β-ketoamine COF with graphitic carbon nitride (g-C3N4) to form a type II heterojunction, establishing a stable channel for electron–hole separation through band alignment.136 This composite displayed a band gap of 2.21 eV with an absorption edge extending to 620 nm, enabling 98% RhB removal within 30 min under visible-light irradiation. Mechanistic analysis identified ·O2− as the primary ROS, with ·OH contributing synergistically to complete mineralization.
In terms of extending π-conjugation and enhancing charge delocalization, Chen et al. developed a COF with extended π-coupling by incorporating large π-conjugated aromatic units into the framework and linking them via β-ketoamine bonds to form a fully conjugated skeleton.137 This design markedly reduced the exciton binding energy, thereby enhancing electron–hole separation efficiency and facilitating the generation of both ·O2− and ·OH under visible-light irradiation. Mechanistic studies revealed that ·O2− predominantly initiated chromophore cleavage during the early decolorization stage, whereas ·OH played a crucial role in aromatic ring scission and deep mineralization; the photocatalyst retained its structural integrity and activity over multiple cycles. In another example, Wang et al. constructed a three-dimensional cross-linked hetero[6]radial diene COF based on radial D–A units.138 The multidirectional electron transport network inherent to this architecture substantially accelerated charge migration and boosted ·O2− yields. Under visible-light illumination, the material enabled rapid RhB removal, with a rate constant significantly exceeding that of analogous two-dimensional COFs. Both radical-trapping and ESR measurements confirmed that ·O2− was the dominant ROS, with ·OH providing supplementary activity during the mineralization phase. In a related approach, Hou et al. enhanced framework polarity, π-conjugation, and structural stability by in situ converting reversible imine linkages into rigid thiazole bonds, thereby facilitating O2 adsorption–activation and exciton dissociation (Fig. 18).139 This thiazole-linked COF achieved 97% RhB removal within 25 min under λ > 420 nm irradiation, with ·O2− and 1O2 identified as the dominant ROS. The photocatalyst retained high activity over five consecutive cycles. The catalyst exhibited excellent MO degradation ability under visible light irradiation, almost completely removing 10 mg per L MO within 60 min, and the reaction rate constant was significantly higher than that of traditional imine COFs. Additionally, Zhang et al. integrated multiple D–A units into a single COF backbone, optimizing intramolecular charge–transfer pathways and increasing the rate of ·O2− generation.140 This structural engineering enhanced the RhB degradation rate constant by 1.7-fold compared with a control COF lacking the heterojunction design, with ·O2− as the primary species responsible for decolorization and mineralization. The framework retained excellent structural stability throughout repeated use.
The incorporation of metal centres and targeted framework functionalization can further enhance the generation and utilization of ROS by modulating local charge-transfer dynamics. For instance, Chen et al. employed an ion thermal synthesis to coordinate triazine nitrogen sites with single-atom Co–N4 active centres, yielding a COF with a specific surface area of 600 m2 g−1, an average pore size of 1.5 nm, a band gap of 2.35 eV, and an absorption edge at 530 nm.141 Under visible-light irradiation, this catalyst achieved 99% RhB degradation within 40 min, approaching complete mineralization. ESR spectroscopy and radical-quenching experiments revealed that ·O2− and ·OH acted synergistically, with ·O2− preferentially cleaving the chromophore and ·OH facilitating deep mineralization of the aromatic backbone. In another example, Khojastegi et al. synthesized a three-dimensional interlocked metal-COF by in situ embedding Cu(I) photosensitizing centres into ordered framework sites, effectively suppressing the flattening and quenching of photosensitizers, extending photocarrier lifetimes, and broadening visible-light absorption.142 This material removed >98% RhB within 30 min under λ > 420 nm irradiation, retained full activity after five cycles, and operated primarily through ·O2− and h+, with ·OH playing a minor role. Building on this concept, Xu et al. integrated photocatalysis with a Fenton-like process in a copper-porphyrin COF, wherein the Cu centre served both as a photosensitizing site to promote electron–hole pair separation and as an active site for H2O2 activation to ·OH.143 This dual-functionality enabled a synergistic ·O2−/·OH pathway, achieving >98% RhB removal and 90% TOC reduction within 50 min under visible light with optimal H2O2 dosage. In addition, the material can efficiently adsorb MB in the dark, and in a photo-Fenton-like system under visible light irradiation and coexistence of H2O2, the removal rate exceeds 99% within 30 minutes, and the TOC removal rate reaches 85%, showing significant mineralization ability. Mechanistic studies confirmed that ·O2− dominated the initial chromophore cleavage, while ·OH and h+ facilitated complete aromatic ring mineralization; PXRD and XPS analysis showed no structural degradation over five cycles. In contrast to metal-containing systems, Li et al. demonstrated a tunable, metal-free COF functionalized with –OH groups, which enhanced pollutant enrichment via electrostatic and hydrogen-bond interactions while promoting electron–hole separation.144 This catalyst achieved 94% RhB removal within 60 min under visible light, with ·O2− and 1O2 as the principal ROS, and exhibited excellent structural and catalytic stability across multiple cycles. In a recent study, Shu et al. synthesized a highly crystalline β-ketoamine COF with continuous π-conjugated channels by solution-phase polycondensation of D–A monomers followed by thermal treatment (Fig. 17).145 The extended conjugation facilitated efficient two-electron reduction of dissolved O2 to H2O2 under visible-light irradiation. Subsequent addition of Fe2+ initiated a Fenton-like process, in which Fe2+ rapidly reacted with in situ–generated H2O2 to produce ·OH in solution. This ·OH, together with photocatalytically generated ·O2−, acted synergistically to accelerate both the decolorization and mineralization of RhB. Notably, this strategy did not require direct photoexcitation at metal centres; instead, it relied on the coupling of metal-free photocatalysis with homogeneous Fenton chemistry. The system exhibited high reaction rates over a broad pH range, underscoring its potential for treating aqueous environments contaminated with diverse organic pollutants.
 | ||
| Fig. 17 (a) Synthetic routes and chemical structures of sulfone COFs; (b) long-term photocatalytic performance of FS–OHOMe-COF under an O2-saturated atmosphere; (c) comparative photocatalytic performance of FS–OHOMe-COF and reference materials in dye degradation; (d) photocatalytic performance of FS–OHOMe-COF over multiple cycles; (e) DFT-calculated interlayer interaction energies of FS–OHOMe-COF and TP-COF. Reproduced with permission from ref. 145, Copyright 2024, from Wiley-VCH. | ||
 | ||
| Fig. 18 (a) Preparation scheme of COF-A, COF-O, and COF-S; (b) photocatalytic performance of COF-S for paracetamol degradation at different initial solution pH; (c) photocatalytic performance of COF-S under different ionic strengths; (d) photocatalytic performance of COF-S with coexisting anions; (e) photocatalytic performance of COF-S in the presence of humic acid; (f) photocatalytic performance of COF-S in different real water samples; (g) photocatalytic degradation of five additional emerging contaminants by COF-S; (h) reusability of COF-A and COF-S for paracetamol degradation under visible-light irradiation; (i) photocatalytic performance of immobilized COF-S in a continuous-flow reactor under visible-light irradiation for 20 h; (j) photocatalytic performance of COF-S in an enlarged 2 L reactor under natural sunlight irradiation for 5 days (insets: digital images of the degradation experiments in different reactors). Reproduced with permission from ref. 139, Copyright 2024, from Springer. | ||
The optimization of pore architecture and mass-transfer properties is equally critical for enhancing RhB degradation efficiency. Zhang et al. fabricated a two-dimensional/two-dimensional BiOBr/TzDa-COF direct Z-scheme heterojunction that maintained high redox potential while markedly improving charge-carrier separation.146 This composite degraded 10 mg per L RhB efficiently across a broad pH range, achieving >95% removal and 80% TOC reduction within 60 min, and displayed a pronounced synergistic effect in RhB/Cr(VI) co-contaminant systems. Xu et al. reported an ordered meso–microporous single-crystal COF, in which precise band-gap engineering optimized both light-harvesting capability and energy-band alignment, enabling exceptionally high RhB degradation rates.147
Under visible-light irradiation, the photocatalyst removed >98% of RhB within 15 min, with an apparent rate constant markedly exceeding that of a non-single-crystalline control. The hierarchical pore network enhanced substrate diffusion and facilitated efficient access to catalytic sites. Reactive species analysis identified ·O2− as the dominant ROS, with ·OH and h+ synergistically driving deep mineralization. In a related study, Qu et al. developed anionic COF aerogels that rapidly concentrated cationic dyes, including RhB, via strong electrostatic interactions.148 Under visible-light photocatalysis, these aerogels achieved 97% RhB removal within 10 min—significantly faster than their non-aerogel counterparts. Mechanistic investigations attributed this performance to the aerogels' large surface area, interconnected pore channels, and enhanced mass-transfer properties, which together facilitated the adsorption–reaction–product diffusion sequence. The photocatalytic activity was primarily governed by ·O2−, with minor contributions from ·OH, and both structural integrity and catalytic performance were retained over multiple cycles. In addition to RhB, this catalyst significantly enhances the adsorption and enrichment of MB and the photocatalytic reaction rate. Under conditions with a wavelength of λ > 420 nm, the material achieves a MB removal rate of 97% within 15 minutes, with ·O2− and h+ as the primary active species. The synergistic adsorption-photocatalytic effect is particularly pronounced in the removal of low-concentration pollutants. Furthermore, Xu et al. combined a β-ketoamine COF with peroxymonosulfate (PMS) in an oxidant-assisted system, generating ·SO4−, ·OH, and ·O2− simultaneously under visible light.149 This system achieved complete RhB degradation within 6 min, representing 12-fold and 8-fold enhancements compared with PMS or the COF alone, respectively. Mechanistically, ·SO4− rapidly cleaved the aromatic framework, while ·OH and ·O2− drove the deep mineralization of intermediates.
MB is a structurally stable, difficult-to-degrade, and potentially toxic cationic dye that causes light attenuation in aquatic environments, ecological imbalance, and health hazards; conventional remediation methods show limited efficacy. COFs have emerged as promising photocatalysts for achieving complete MB mineralization through tailored structural and electronic designs. Zhao et al. constructed a COF featuring an acceptor–donor–acceptor (A–D–A) motif, where a strong electron-acceptor unit was coupled with an electron-rich donor segment to establish a long-range electric dipole within the framework.150 This polarity engineering enhanced the built-in electric field, thereby improving charge separation and transport efficiency. Under visible-light irradiation (10 mg per L MB), this COF exhibited markedly higher degradation rate constants and mineralization efficiencies compared to a non-polarity-engineered control. Radical-trapping and ESR analyses identified ·O2− and h+ as the dominant reactive species, with the dipole field accelerating electron transfer to donor sites for O2 reduction, thus facilitating MB structural breakdown and mineralization. Similarly, Sun et al. employed a one-step polycondensation of olefin- and imine-containing monomers under mild conditions to form a highly crystalline COF with continuous π-conjugation.151 The combined olefin–imine double bonds broadened light absorption and enhanced electron delocalization, reducing electron–hole recombination probability. This photocatalyst achieved >95% MB removal within 30 min under visible light and maintained stable activity over multiple cycles. The photocatalytic degradation of MO by this COF was stable over a wide pH range, with a removal efficiency exceeding 95% at 20 mg per L MO within 50 min and no significant activity decay during five cycles. Mechanistic studies revealed that the conjugated double-bond network enhanced π–π stacking interactions and charge transport, enabling the synergistic generation of ·O2− and ·OH, which accelerated oxidative MB decomposition. Zhang et al. further advanced MB degradation performance by simultaneously introducing electron-withdrawing and electron-donating groups into a triazine-based COF, thereby forming a multi-donor–acceptor heterojunction with a precisely tuned band structure and charge-transfer dynamics.140 The optimized material exhibited high specific surface area, well-ordered mesoporosity, and excellent crystallinity, all of which facilitated active-site exposure and mass transfer. Under visible light, COF-Br@OCH3 demonstrated the highest activity, with significantly increased ·O2− and ·OH yields while lowering the energy barriers for O2 reduction and H2O oxidation, resulting in rapid decolorization and deep MB mineralization.
He et al. designed a 3D COF incorporating a dual A–D–A building block, in which monomer electronic properties were optimized to enhance visible-light absorption and charge-carrier separation efficiency.152 The resulting photocatalyst exhibited excellent redox activity toward multiple organic dyes, including MB and MO. For MO removal, the authors integrated the COF into a photo-Fenton system assisted by H2O2, further improving oxidative capacity. In particular, a functionalized TAPB–DMTA COF was synthesized in which the pore-size distribution and specific surface area were precisely regulated via acetic-acid-assisted synthesis, yielding abundant accessible active sites and facilitating efficient mass transfer. Under visible-light irradiation in the presence of 10 mM H2O2, 15 mg per L MO was removed by 98% within 30 min, with high activity retained even in complex water matrices containing Cl−, SO42−, and humic acid. Mechanistic analysis indicated that ·OH and ·O2− acted synergistically as the dominant ROS, with the optimized pore structure accelerating pollutant–ROS interactions. Building on this strategy, the same group subsequently prepared a highly crystalline COF containing both olefinic and imine linkages via a one-step solvothermal method, thereby achieving simultaneous optimization of π-conjugation and D–A interactions.153 This framework exhibited broadened light absorption, enhanced electron–hole separation efficiency, and superior photocatalytic activity toward both MB and MO. Under visible light, the catalyst achieved >95% MB degradation within 30 min and >93% MO removal within 40 min, with negligible activity loss after multiple cycles. Mechanistic studies confirmed that ·O2− and ·OH were the principal ROS, while the π-conjugated backbone promoted intermolecular electron delocalization and facilitated ROS generation, accelerating oxidative dye decomposition. Gogoi et al. recently developed a metal-free photocatalytic COF via a mechanical pulverization strategy, which eliminates the need for high temperatures or organic solvents, rendering the synthesis process green, facile, and scalable.154 Under visible-light irradiation, the material exhibited outstanding photocatalytic activity toward both RhB and MB, achieving near-complete RhB removal within a short reaction time and high MB degradation efficiency within similarly brief durations. Active-species quenching experiments combined with EPR analysis confirmed that ·OH and ·O2− were the dominant ROS. The synergistic effect of efficient photogenerated charge separation and accelerated mass-transfer kinetics facilitated abundant ROS production, thereby enabling the rapid oxidative decomposition of dye molecules. In a related study, Yang's group reported a functionalized TAPB–DMTA COF synthesized with acetic-acid-mediated pore-size control, yielding higher specific surface area, more optimal pore geometry, and greater active-site accessibility.155 Coupled with a photo-Fenton process, the COF delivered exceptional MO degradation efficiency—98% removal of 15 mg L−1 within 30 min—while retaining performance in the presence of common ionic and organic interferents. The cooperative action of ·OH and ·O2− was again identified as the primary degradation pathway, highlighting the pivotal role of pore engineering in enhancing ROS–pollutant contact kinetics and ensuring high environmental adaptability.
Peng's research group enhanced the mass-transfer efficiency and active-site accessibility of COFs for Fenton-like reactions by tailoring their porosity and specific surface area.156 The resulting high-porosity COFs exhibited markedly accelerated degradation kinetics and superior mineralization efficiency toward malachite green (MG) under photo-Fenton conditions. Mechanistic investigations revealed that the optimized pore architecture not only facilitated the rapid diffusion of pollutant molecules to the catalytic centres, but also promoted the activation of H2O2 to generate ·OH, thereby boosting both reaction rates and resistance to common water-matrix interferences. This work provides a viable structural-engineering strategy for constructing high-performance photocatalytic–Fenton synergistic systems.
Zhao et al. employed band and energy level engineering to tailor an A–D–A motif COF incorporating quinone and triazine dual acceptor units, thereby strengthening the built-in electric field and enhancing charge carrier separation.150 Under visible light irradiation, this material achieved 91% degradation of ciprofloxacin (CIP) within 50 minutes and over 92% removal of erythromycin (ERY), with h+ and ·O2− identified as the dominant reactive species and 1O2 also contributing to the process. Sun et al. designed a highly crystalline COF featuring both ethylene and imine double bonds, where the extended π-conjugation and enhanced framework polarity facilitated >95% removal of amoxicillin (AMX) within 60 minutes. Reactive species analysis confirmed the synergistic roles of ·O2− and h+ in the degradation process.151 A similar band gap tuning approach was reported by Chen et al., who constructed fully π-conjugated vinylene-linked COFs from diacetylene and triazine building blocks.160 The incorporation of diacetylene units markedly extended π-electron delocalization and fine-tuned the band structure, yielding exceptionally high photocatalytic activity and structural stability. This material demonstrated outstanding performance in degrading representative organic pollutants, including phenols and norfloxacin (NOR), achieving >96% NOR removal within 15 minutes, indicative of ultrafast reaction kinetics and broad-spectrum applicability. Shi et al. further developed a COF-TzDa/Ag/AgBr heterojunction, where the surface plasmon resonance effect of photogenerated Ag0 facilitated efficient Z-scheme charge transfer.161 This system removed 97% of tetracycline (TC) within 60 minutes and retained high activity in a TC/Cr(VI) mixed system, with ·O2− and h+ identified as the primary reactive species.
Zhang et al. synthesized a multi-donor–acceptor heterojunction COF that enhanced ·O2− generation via a spatially zoned ICT pathway, leading to a 1.5–2-fold increase in the degradation rate constants of sulfamethoxazole (SMX), ciprofloxacin (CIP), and amoxicillin (AMX) compared with the unmodified framework.140 Tong's group introduced thiazole linkages to increase framework polarity while preserving efficient π–π stacking, thereby facilitating O2 adsorption and activation.139 This COF achieved >88% removal of ofloxacin (OFX) within 40 minutes and nearly 90% removal of chloramphenicol (CAP) within 50 minutes, with ·O2− and 1O2 identified as the primary reactive species. A metal-free tunable COF further improved the degradation efficiency of tetracycline (TC) and SMX through the incorporation of –OH groups, with ·O2− and 1O2 acting as the dominant ROS. Li et al. developed a COF functionalized with carboxyl groups, enabling simultaneous chelation of divalent metal ions and degradation of antibiotics in livestock and poultry wastewater.144 Khojastegi et al. reported a three-dimensional interlocked metal-COF in which Cu(I) photosensitizing centres were anchored at ordered sites within the framework, significantly prolonging the lifetime of photogenerated charge carriers.142 Under visible light, removal efficiencies for TC, oxytetracycline (OTC), norfloxacin (NOR), and SMX all exceeded 90%, with ·O2− and h+ as the dominant active species. To address pharmaceutical contaminants, a β-ketoamine COF with tunable nitrogen positions was designed to modulate the band structure and dipole moment by varying the number and position of heterocyclic nitrogen atoms.162 The ortho-nitrogen variant achieved nearly complete degradation of 10 mg per L acetaminophen (APAP) within 60 minutes under visible light (λ > 420 nm), with a TOC removal rate exceeding 80%. ROS analysis confirmed a synergistic contribution from ·O2− and 1O2.
In the degradation of phenolic pollutants, diverse structural engineering and functionalization strategies have been developed. You et al. constructed a D–A heterojunction within the COF skeleton, which effectively promoted photogenerated charge separation and migration.165 As a result, rapid phenol removal was achieved within 90 minutes under visible light, while simultaneously driving the hydrogen evolution reaction, highlighting the coupling potential of pollutant control with energy conversion. Gao et al. reported an enzyme@COF light-enzyme cascade system prepared by an ionic liquid-mediated dynamic polymerization method (Fig. 19).166 This hybrid platform could simultaneously generate H2O2 and drive enzymatic oxidation under mild aqueous conditions, with a phenol degradation rate 2.63 times higher than that of low-crystalline analogues, underscoring the advantages of light-enzyme synergy in enhancing both degradation efficiency and selectivity. Zadehnazari et al. designed a tetranitrogen-linked COF capable of efficiently producing ·O2− and ·OH under light irradiation,88,167 showing excellent performance in the degradation of phenol and other pollutants and demonstrating the potential of dynamic-bond COFs in complex wastewater treatment. Furthermore, Wang et al. introduced Fe species into a COF skeleton to construct Fe@COF composites.168 These materials could efficiently activate H2O2 under weakly acidic to neutral conditions, markedly improving ·OH generation and exhibiting outstanding degradation activity toward phenol and its derivatives, thus overcoming the conventional Fenton reaction's limitation of requiring strongly acidic media.
 | ||
| Fig. 19 (a) Illustration of phenol degradation by photo-enzyme coupled catalysis using the HRP@TpBpy ensemble; (b) electronic band structures of TpBpy (HAc), TpBpy (IL), HRP@TpBpy (HAc), and HRP@TpBpy (IL); (c) photocatalytic performance of TpBpy (IL) and TpBpy (HAc) for in situ H2O2 production in pure water (λ > 400 nm, 298 K, xenon lamp, light intensity at 400–700 nm: 550 W m−2; 10 mL water, 1 mg catalyst); (d) photocurrent spectra of TpBpy (HAc) and TpBpy (IL); (e) dynamic curves of phenol degradation with different catalysts; (f) apparent degradation rate constants (kd) of phenol over different catalysts; (g) structure of HRP@TpBpy (IL)-based hydrogel beads prepared via double-crosslinked alginate gelatinization; (h) digital image of phenol degradation in real water using HRP@TpBpy (IL)-based hydrogel beads under sunlight; (i) degradation dynamics of phenol over HRP@TpBpy (IL)-based hydrogel beads under sunlight in pure water and WWTP wastewater. Reproduced with permission from ref. 166, Copyright 2024, from Wiley-VCH. | ||
Progress has also been made in the removal of BPA. Sun et al. in situ constructed CdS/COF heterojunctions, which effectively suppressed CdS photocorrosion and enhanced interfacial charge separation.169 Under neutral conditions, the composite achieved 85.68% BPA removal within 3 h, with h+ and ·O2− identified as the dominant reactive species. This strategy ensured both high photostability and degradation efficiency, while avoiding the deactivation issues common to traditional semiconductors, offering important guidance for COF–semiconductor hybrid design. Yang et al. further advanced this concept by covalently integrating the MOF@COF into a Z-scheme heterojunction, combining rapid adsorption with efficient photocatalysis.170 In this system, MOFs provided highly dispersed adsorption sites, whereas the π-conjugated COFpromoted effective electron–hole separation. The composite completely removed 100 ppm of BPA within 10 minutes and exhibited broad-spectrum activity against pollutants such as phenol, tetracycline, and rhodamine B. These findings demonstrate that structural integration to achieve dual functional synergy is a promising route for addressing the challenge of multi-pollutant wastewater. In terms of mechanistic innovation, Deng et al. designed D–A sp2-conjugated COFs with finely tuned band structures, achieving high visible-light activity and continuous in situ H2O2 generation at rates up to 1952 µmol g−1 h−1 without sacrificial agents.171 This cascade pathway of “H2O2 self-generation + ROS-driven degradation” enabled 96% BPA removal within 60 minutes, highlighting a sustainable approach that avoids additional chemical inputs. Building on this principle, Zhou et al. constructed a bipyridine-based polyimide COF platform capable of simultaneously producing H2O2 and driving BPA degradation under visible light.172 The material exhibited outstanding stability and recyclability, retaining high efficiency without structural or functional decay over multiple cycles. This work not only illustrates a green strategy for environmental remediation but also exemplifies the dual role of COFs in clean energy conversion and pollutant degradation driven by renewable light energy.
Although numerous studies have demonstrated the excellent photocatalytic degradation performance of COFs against target pollutants under ideal laboratory conditions,129,140 their performance in complex, multi-phase real environmental systems are the key to assessing their practical application potential. The ubiquitous presence of natural organic matter (NOM), coexisting ions, suspended particles, and pollutant mixtures in real environments primarily affects COF performance through the following mechanisms: (i) pore blockage and active site masking: the physical adsorption of macromolecular NOM or colloidal particles can block micropore channels, hindering mass transfer. (ii) Competitive adsorption and reaction quenching: background species compete with target pollutants for adsorption sites while also acting as scavengers for ROS.149 (iii) Optical shielding effect: the color or turbidity of water attenuates incident light intensity, reducing photon utilization efficiency. (iv) Long-term chemical/structural stability: fluctuating pH, high salinity, or microorganisms in real water bodies may erode the COF, leading to structural collapse and activity loss. Although reports specifically investigating the behaviour of COFs in complex matrices remain relatively limited, preliminary work has begun to focus on this direction, for instance, exploring the performance decay of COFs in degrading antibiotics in simulated seawater or solutions with high background ion concentrations,161 or studying the influence of humic acid on their photogenerated charge behaviour.162 These studies highlight the urgency of evaluating COF performance under near-realistic conditions. Looking forward, advancing the practical environmental application of COFs requires efforts in the following aspects: first, developing COFs with hierarchical pore structures65 or anti-fouling surfaces to alleviate clogging and enhance selective mass transfer. Second, strengthening the specific recognition and adsorption of target pollutants through precise molecular engineering. Third, developing frameworks based on high-stability linkage chemistries to resist environmental erosion. Finally, there is an urgent need to establish standardized performance evaluation protocols that systematically test the long-term activity and stability of COFs in real water bodies containing representative background components or complex simulated matrices, providing a reliable basis for engineering applications.
4.4 Biomedical applications
In recent years, the utilization of light energy for efficient and controllable disease treatment and infection control has emerged as a critical focus in biomedical materials research. Conventional PDT, which relies on photosensitizers to generate ROS, is hindered by several intrinsic limitations, including aggregation-induced quenching of photosensitizer molecules, poor photostability, and low ROS yields in the hypoxic or complex microenvironments of tumors and infections.173 Similarly, photothermal therapy (PTT), which destroys lesions via localized hyperthermia, often suffers from limited selectivity and inconsistent efficacy when used as a monomodal strategy.174COFs provide an elegant solution to these challenges. Their crystalline porous structures offer a stable platform to immobilize and organize photoactive components, while their tunability allows optimization towards specific biological applications. COFs as a new class of crystalline porous materials with ordered π-conjugated skeletons and tunable pore microenvironments, provide a promising platform to overcome these bottlenecks.175 The rigid backbone of COFs can immobilize photosensitizer units, thereby suppressing aggregation-induced quenching. Meanwhile, their extended π-conjugated networks enhance light harvesting and ISC, markedly increasing the yield of 1O2 and other ROS.176 Beyond these inherent photophysical advantages, the chemical versatility of COFs allows for the incorporation of oxygen-storage moieties, the construction of parallel type I/II energy-level architectures, and even the integration of heterojunction or composite systems to couple PDT with PTT for synergistic therapeutic effects.
In the context of PTT, COFs not only act as efficient photothermal conversion scaffolds but also frequently serve as dual-function agents in combined PDT–PTT modalities.177 The synergy between localized hyperthermia and ROS-mediated oxidative damage can promote apoptosis and necrosis, while also stimulating immune responses, thereby enhancing tumor suppression. In antibacterial applications, COF-based photocatalysts show unique advantages under visible or near-infrared irradiation.178 Photosensitizing motifs such as porphyrins or BODIPY embedded in the framework can rapidly produce ROS, which disrupt bacterial membranes and metabolic pathways. When further coupled with photothermal heating or the release of gaseous therapeutic factors, these systems exhibit potent bactericidal activity, even against drug-resistant strains and biofilms. Importantly, this antimicrobial phototherapy is non-invasive, associated with a low risk of resistance development, and benefits from the inherent biocompatibility and biodegradability of COFs, underscoring its translational potential.179 The following subsections detail how COFs are engineered for photodynamic/photothermal therapy and antimicrobial applications, highlighting the translation of photocatalytic ROS generation from chemical concepts to biological efficacy.
Two effective strategies have recently been proposed to overcome the bottleneck posed by the hypoxic tumor microenvironment in solid cancers: (i) oxygen storage/release and “afterglow” PDT and (ii) type I/II parallel reaction pathways with reduced oxygen dependence. Wang et al. post-modified TpDa-COF with a pyridone (Py) side chain to reversibly capture photogenerated 1O2 as an addict, which could then be released on demand under physiological temperature or 808 nm thermal stimulation.184 This design enabled “afterglow” PDT with extended ROS lifetime, achieving an IC50 of 0.54 in HeLa cells and continuous ROS release that led to pronounced tumor regression in mouse models, thereby verifying the advantages of segmented generation and prolonged activity of 1O2. Similarly, Dutta et al. introduced anthracene moieties into COFs as endoperoxide (EPO) oxygen-storage units, combining them with a photothermal component to enable heat-triggered 1O2 release.185 This strategy maintained strong ROS levels under hypoxic conditions and demonstrated clear in vivo tumor inhibition and histological evidence of therapeutic efficacy, highlighting a materials science route toward “oxygen-independent PDT”.
In parallel, type I/II hybrid approaches have also emerged. Zhou et al. designed a PEGylated COF photosensitizer by alternating type I and type II photosensitive units within the framework layers and stacking them interlayer (Fig. 20).186 This architecture enabled the simultaneous generation of 1O2 and O2˙− under both normoxic and hypoxic conditions, effectively inducing immunogenic cell death (ICD). When combined with immune checkpoint blockade (ICB), this system reversed the immunosuppressive tumor microenvironment, ablated large hypoxic in situ tumors, and suppressed distant metastases. Likewise, Wei et al. synthesized Por-DETH-COF, which features dual type I/II channels under 660 nm red light.187 This system not only selectively induced apoptosis in glioma cells but also directionally oxidized thiol groups into disulfides, reflecting the multifunctional and hypoxia-adaptive potential of a single COF platform within complex biological and chemical environments.
 | ||
| Fig. 20 (a) Schematic illustration of the preparation of COF nanophotosensitizers with staggered type I and II photosensitizer motifs; (b) fluorescence microscopy images of 4T1 cells under normoxia or hypoxia after treatment with different groups in the presence of DCFH or DHE upon light irradiation; (c and d) cell viability of 4T1 cells after treatment with TPS-PEG or T-PEG under normoxia and hypoxia at different concentrations upon light irradiation; (e) flow cytometric analysis of CRT expression on the surface of 4T1 tumor cells after treatment with different groups under normoxia or hypoxia; (f) CLSM images and (g) quantitative analysis of CRT expression on 4T1 tumor cells under normoxia or hypoxia; (h) quantitative analysis of HMGB1 release from 4T1 tumor cells using an HMGB1 ELISA kit; (i) quantitative analysis of ATP secretion from 4T1 cells after various treatments using an ATP ELISA kit; (j) quantitative analysis of matured BMDCs after treatment with different groups. Reproduced with permission from ref. 186, Copyright 2024, from American Chemical Society. | ||
Relying on the unique pore environment and designable skeleton of the COF, researchers have been continuously promoting its application in photodynamic and photothermal synergistic therapy in recent years. In terms of coupling monomeric photosensitive units, Zhao et al. covalently integrated porphyrin with BODIPY fragments to construct Tph–BDP-COF.188 By reducing the excited state energy level difference ΔE(S1–T1), ISC was promoted, while non-radiative attenuation was enhanced, so that the material has both strong ROS production capacity and 29.9% photothermal conversion efficiency. This COF achieved 97% killing of Escherichia coli under the synergy of PDT/PTT, which is far better than the 54% achieved by traditional COF-366. EPR and transient absorption experiments further revealed the parallel generation of 1O2 and O2˙− and the improvement of charge separation. In response to this, Sun et al. introduced the NO donor BNN6 into TP-Por covalent organic nanosheets to construct a degradable heterojunction that integrates PDT, PTT and gas therapy. Under 635 nm light irradiation, the system can heat the solution to 55 °C (PTCE ≈ 18.4%) within 6 minutes and exhibit significant antibacterial activity and wound healing ability, while alsoshowing good biodegradability and compatibility.189
Further multimodal expansion is reflected in the combination with other therapeutic methods. Tai et al. constructed Zr-MOF@thin COF shell nanocapsules, which not only enhanced ROS generation through the COF shell, but also induced immunogenic cell death, thereby enhancing radiotherapy sensitivity.190 In a soft tissue sarcoma model, the material significantly delayed tumor progression and reduced the risk of recurrence, demonstrating the high complementarity of phototherapy and radiotherapy. Liang et al. embedded the pillar aromatic unit into the COF skeleton and loaded the thioacetal prodrug to construct a self-amplified antibacterial PDT platform.191 After 20 minutes of illumination, the signal decayed by about 90%, and the in situ release of cinnamaldehyde brought about concentration-dependent bacterial inhibition. Simultaneous imaging and micro-CT confirmed its therapeutic benefit in vivo. In the heterogeneous composite design, Pang 's group used the solvothermal method to synthesize COF-LZU-1 and in situ grew a CuSe shell on its surface to construct a stable core–shell structure, combining the stable 1O2 production capacity of the COF with the strong photothermal performance of CuSe.192 In a mouse model, the material achieved significant tumor inhibition with low toxicity and side effects, showing excellent biosafety. In addition, He et al. reported that AQ4N@THPP/TK-PEG uses a 1O2 cleavable disulfide bond to bind the hypoxia-activated prodrug banoxantrone (AQ4N), designing a cascade pathway from PDT to chemotherapy.193 The drug release rate jumped from 8.9% to 78.8% (4 h) under light irradiation and demonstrated significant tumor inhibition and life extension effects in animal experiments, providing a paradigm for precision treatment in hypoxic environments. The work of Tang et al. further expanded the therapeutic model of the COF. They constructed an iron porphyrin-based COF (FeTPD) through Schiff base condensation and loaded glucose oxidase (GOx) in the pores to form an FeTPD@GOx composite system.194 This design uses the regular pores of the COF to achieve substrate enrichment and interfacial catalysis. GOx can convert glucose in tumors into H2O2, and the Fe3+/Fe2+ cycle in the skeleton further converts H2O2 into O2 and ·OH through a Fenton-like reaction; under ultrasonic excitation, the porphyrin unit can efficiently produce active 1O2. As a result, FeTPD@GOx simultaneously triggers the synergistic effect of SDT (1O2) + CDT (·OH), showing a stronger ROS amplification effect than a single sonosensitizer. In a 4T1 bilateral tumour model, FeTPD@GOx combined with ultrasound achieved proximal tumour clearance within 6 days, inhibited distal tumour growth within 4 days, and reduced tumour weight to only 5–8% of the control group after 15 days, with no toxicity observed in major organs. This result demonstrates that COF pores not only serve as a structurally stable Sono sensing platform but also amplify ROS production through sequential catalysis, opening up new avenues for the application of COF-based materials in SDT/CDT synergistic tumor therapy.
In the design of molecular energy bands, researchers have enhanced the separation of photogenerated carriers and increased ROS production through intermolecular interactions, D–A architectures, and conjugation extension strategies. He et al. constructed a localized electron-regulated micro-environment by introducing C–F⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermolecular interactions into the COF skeleton.196 These non-covalent weak interactions not only modulated the electron cloud distribution within the framework but also promoted charge enrichment at C
O intermolecular interactions into the COF skeleton.196 These non-covalent weak interactions not only modulated the electron cloud distribution within the framework but also promoted charge enrichment at C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O sites, effectively suppressing electron–hole recombination. As a result, the COF generated H2O2 through a dual pathway under visible light, significantly increasing ROS levels. In antibacterial tests, the material almost completely inactivated E. coli within 30 minutes and maintained continuous disinfection activity, highlighting molecular interaction engineering as an effective strategy to enhance photocatalytic antibacterial performance. Building on this principle, Zhu et al. designed a D–A type π-conjugated COF by incorporating benzotrithiophene (BTT) into the framework.197 This modification broadened the light absorption range, improving photon utilization under visible light. More importantly, BTT, as a strong electron donor, enhanced electron–hole separation, thereby boosting ROS production. The material not only decomposed the mustard gas simulant CEES within minutes but also achieved >99% inhibition against both Gram-positive S. aureus and Gram-negative E. coli, demonstrating the dual functionality of detoxification and antibacterial applications. Natural molecule incorporation has also proven effective. Wang et al. employed a quercetin-sensitization strategy, coupling natural polyphenol molecules with the COF skeleton to form a stable D–π–A structure.198 Benefiting from quercetin's strong light-harvesting and energy-level regulation ability, the system exhibited longer excited-state lifetimes and faster electron transfer than the control group. Under illumination, the material rapidly generated H2O2 and effectively inhibited the growth of E. coli and S. aureus, showcasing the unique value of natural molecules in COF photocatalytic antibacterial design.
O sites, effectively suppressing electron–hole recombination. As a result, the COF generated H2O2 through a dual pathway under visible light, significantly increasing ROS levels. In antibacterial tests, the material almost completely inactivated E. coli within 30 minutes and maintained continuous disinfection activity, highlighting molecular interaction engineering as an effective strategy to enhance photocatalytic antibacterial performance. Building on this principle, Zhu et al. designed a D–A type π-conjugated COF by incorporating benzotrithiophene (BTT) into the framework.197 This modification broadened the light absorption range, improving photon utilization under visible light. More importantly, BTT, as a strong electron donor, enhanced electron–hole separation, thereby boosting ROS production. The material not only decomposed the mustard gas simulant CEES within minutes but also achieved >99% inhibition against both Gram-positive S. aureus and Gram-negative E. coli, demonstrating the dual functionality of detoxification and antibacterial applications. Natural molecule incorporation has also proven effective. Wang et al. employed a quercetin-sensitization strategy, coupling natural polyphenol molecules with the COF skeleton to form a stable D–π–A structure.198 Benefiting from quercetin's strong light-harvesting and energy-level regulation ability, the system exhibited longer excited-state lifetimes and faster electron transfer than the control group. Under illumination, the material rapidly generated H2O2 and effectively inhibited the growth of E. coli and S. aureus, showcasing the unique value of natural molecules in COF photocatalytic antibacterial design.
Ding et al. advanced this concept with multi-component electronic configuration reprogramming, introducing multiple electron-acceptor units into the COF backbone to spatially control charge distribution.199 This design minimized local electron–hole recombination, markedly improving H2O2 photosynthesis. Antibacterial experiments confirmed rapid inactivation of multiple bacterial strains, demonstrating excellent bactericidal efficiency. Further extending absorption capability, Zhang et al. developed an acridine-based COF photosensitizer, leveraging acridine's planar rigidity and strong electron affinity to expand light absorption into the visible and near-infrared regions.200 This design significantly improved ROS generation and enabled the COF to efficiently eliminate drug-resistant bacteria such as MRSA, underscoring its potential for clinical translation. A related approach was reported by Wang et al. who introduced polyfluorinated groups into the COF skeleton to act as electron storage units.201 This design allowed temporary electron storage and controlled release, effectively prolonging the carrier lifetime and further enhancing antibacterial performance. In addition to molecular and band-level optimization, amplifying charge utilization efficiency through interfaces and active sites represents another critical design strategy. A representative example is the construction of an S-scheme heterojunction (Fig. 21).202 By coupling COFs with In2S3, a built-in electric field is formed at the interface, which not only preserves strong redox potentials at both ends but also directionally separates e− and h+. This enables a two-step reduction of O2 into H2O2 by photogenerated e−, while h+ oxidize H2O or intermediates, thereby significantly enhancing H2O2 production and achieving rapid sterilization. This highlights the direct value of “interface-driven” photocatalytic antibacterial activity.203 In parallel, ionic COFs achieve a similar acceleration of charge separation without the need for external semiconductors. Symmetrical polarization units within the framework establish a localized built-in electric field, while the positively charged skeleton segments selectively adsorb Gram-positive bacteria. This dual function allows simultaneous imaging and sterilization, achieving >99% clearance efficiency under low-dose, short-term irradiation, demonstrating the potential for precision antimicrobial interventions in complex microbial communities.
 | ||
| Fig. 21 (a) Schematic illustration of the synthesis of TpMA/In2S3; (b) photocatalytic bacterial inactivation efficiency with and without Fe2+ addition; (c) photocatalytic inactivation kinetics of E. coli in the presence of 10% TpMA/In2S3 and Fe2+ under xenon lamp irradiation; (d and e) fluorescence spectra of coumarin solution with (d) and without (e) Fe2+ in 10% TpMA/In2S3 under visible light irradiation; (f) photographic images of coated plates with bacterial solutions sampled at different time intervals. Reproduced with permission from ref. 202, Copyright 2024, from Elsevier. | ||
If heterojunctions and built-in electric fields address the challenge of “separating e− and h+,” then metal centres—particularly single-atom sites—focus on utilizing the separated charges. In a copper–porphyrin-doped polyphenolic aldehyde COF, the porphyrin moiety acts as an efficient photosensitizer, while Cu centres provide quasi-enzyme-like catalytic sites.204 Together, they synergistically amplify the generation of ·O2−, 1O2, and ·OH. Under visible light, this material suppressed the survival of S. aureus and E. coli to extremely low levels and remained effective against drug-resistant strains, underscoring the effectiveness of the dual-channel amplification of photosensitization and metal-site catalysis. Pushing site engineering further, single-atom sites embedded in an sp2-carbon COF were shown to catalyze both exogenous and in situ H2O2 into ·OH under mild conditions, while simultaneously disrupting bacterial iron homeostasis to induce “iron poisoning” and lipid peroxidation.205 This strategy achieved effective bacterial elimination and accelerated wound healing in in vivo infection models, opening a new avenue for integrating metabolic intervention mechanisms into COF antibacterial systems. Similarly, a copper phthalocyanine-based COF incorporated multi-scale active centres consisting of Cu–N4 single-atom sites and Ag nanoparticles.206 The single-atom sites enabled efficient electron capture, while the Ag nanoparticles provided plasmonic and ionic synergy. As a result, this system achieved >99.9% broad-spectrum sterilization and could also be adapted into an electrochemical biosensor platform, enabling the integration of sterilization and monitoring. Finally, COF nanosheets function as light-responsive nanoenzyme enhancers, leveraging their two-dimensional short-range charge transport and large specific surface area.207 By enhancing oxidase-like activity, they simultaneously promote sensing, antibiotic degradation, and antibacterial effects, thereby forming a closed electron cycle spanning “generation–transfer–utilization”.
To advance toward clinical translation, antibacterial materials must not only “kill quickly and steadily” but also demonstrate compatibility with biological environments. This requires degradability, low toxicity, facile application/formability, and reliable performance under realistic conditions such as hypoxia, exudate, and mechanical disturbance. For example, degradable porphyrin-based COFs integrate dynamic bonds or “chain-breaking units” into the skeleton, which are selectively cleaved by ROS or hypoxic stimuli, enabling adaptive release and redistribution of photosensitizer fragments in the infection microenvironment.208 Through the synergistic action of PDT and CDT/Fenton-like pathways, these systems effectively overcome hypoxia-induced limitations. In wound infection models, they markedly reduce bacterial colonies, suppress inflammation, and accelerate tissue repair, achieving a balance between “high efficiency and biosafety”. Alternatively, boron-based COF-1, featuring a simple B–O backbone and mild surface chemistry, offers excellent blood and cellular compatibility while maintaining visible-light-driven 1O2/·O2− generation capacity. It exhibits broad-spectrum antibacterial activity and low toxicity in both in vitro and small-animal experiments, demonstrating that the “simple structure–reliable function” design strategy is more readily applicable in quasi-clinical scenarios.209 Meanwhile, the TCPP–Cu D–A COF hydrogel, which embeds porphyrin photosensitizers and natural curcumin into a three-dimensional network, provides wound sites with adhesion, moisture retention, and mechanical buffering. This hydrogel enables continuous in situ ROS release, leading to near-complete bacterial clearance in infected wounds while simultaneously promoting re-epithelialization. Such integration of “material morphology–photocatalytic effect–tissue repair” exemplifies a holistic design strategy for therapeutic biomaterials.210
The promising performance of COFs in photodynamic therapy and antibacterial applications, primarily observed in simplified in vitro models,180,182 must be critically evaluated against the challenges of the complex biological microenvironment for in vivo translation. Key hurdles include: (i) protein corona formation: surface coating by biomolecules alters colloidal properties, cellular uptake, and biodistribution, potentially triggering immune responses.183 (ii) ROS quenching: endogenous antioxidants, enzymes, and hypoxic conditions in tumors can severely deplete therapeutic ROS, especially 1O2.185,186 (iii) Biodegradation and long-term safety: the metabolic fate, degradation products, and long-term biocompatibility of COFs remain largely unknown, with linkage stability being paramount. (iv) Barrier penetration: effective delivery requires overcoming dense biological barriers, such as biofilms for antibacterial action191 or vascular and tissue barriers for tumor therapy. Emerging studies are beginning to address these complexities, for example, by evaluating COF behavior in serum-containing media182 or biofilm models.191 To advance toward clinical use, future design strategies should focus on: first, intelligent surface engineering to control protein corona formation and enhance targeting. Second, designing microenvironment-responsive or self-oxygenating COFs.186,193 Third, integrating imaging capabilities for theranostic applications. Fourth, establishing robust preclinical evaluation frameworks to systematically assess pharmacokinetics, biodistribution, and long-term toxicity in vivo.
5 Conclusions
Over the past decade, COFs have evolved from an emerging curiosity into a versatile class of crystalline porous materials with far-reaching implications for photocatalytic oxidation chemistry. Distinguished by their long-range ordered yet modular architectures, high surface areas, and tunable electronic structures, COFs have proven to be fertile grounds for harnessing solar energy and mediating the generation of ROS. These reactive intermediates—1O2, ·O2−, ·OH, and other high-valent oxygen species—play decisive roles in driving a wide array of oxidative transformations relevant to environmental remediation, chemical synthesis, and biomedicine.A central theme that emerges from recent research is the capacity of COFs to act as “designer photocatalysts.” Their modular construction from organic linkers and nodes allows precise engineering of frontier orbital energies, charge separation pathways, and the density and distribution of active sites. Through judicious design—such as extending π-conjugation to improve visible-light absorption or incorporating electron-donating and -withdrawing motifs to tune donor–acceptor interactions—researchers can tailor the excited-state properties that govern ROS generation. The introduction of heteroatoms, such as nitrogen or sulfur, or coordination with single-atom metal sites, provides additional levers to control charge localization, spin density distribution, and reaction selectivity. Equally important is the rational manipulation of porosity and topology. COFs offer an unparalleled opportunity to construct ordered porous frameworks that enhance molecular diffusion, facilitate reactant access to catalytic sites, and provide spatial confinement that stabilizes reactive intermediates. Such structural control is central to modulating the relative contributions of type I and type II ROS pathways, thereby enabling a level of selectivity rarely achievable with conventional inorganic semiconductors.
The structural tunability of COFs has been harnessed to realize diverse photocatalytic oxidation processes under mild and environmentally benign conditions. A growing body of studies has demonstrated that COFs can achieve high turnover frequencies and excellent selectivities in the oxidation of organic sulfides to sulfoxides or sulfones, the transformation of amines into imines, and the selective oxidation of alcohols to aldehydes and ketones. These reactions are cornerstones of fine-chemical and pharmaceutical synthesis, highlighting the practical importance of COFs as sustainable alternatives to stoichiometric oxidants or precious-metal catalysts.
Beyond traditional organic synthesis, COF-mediated ROS generation has been explored for environmental purification, particularly in the degradation of persistent organic pollutants such as dyes, pesticides, and antibiotic residues. The ordered pore channels and hydrophilic functionalization of certain frameworks allow efficient mass transfer of water-soluble contaminants to active sites, while the photo-induced ROS enable non-selective degradation of complex pollutants into less harmful by-products. In biomedical research, COFs have shown promise as multifunctional platforms for photodynamic and photothermal therapies. By rationally incorporating photoactive chromophores and catalytic motifs, COFs can generate cytotoxic ROS in situ to eradicate pathogens or induce cancer cell apoptosis, while their tunable pores can encapsulate therapeutic agents for synergistic treatment.
Collectively, the progress summarized above converges on and validates the “structure–ROS–substrate” paradigm as more than a descriptive observation; it is the foundational framework that encapsulates the field's core advancement. The unique contribution of this paradigm lies in its integrative and predictive power. It transcends traditional analytical tools by explicitly linking the initial photophysical event to the final chemical outcome determined by the ROS–substrate encounter within the structural confines. This perspective allows the “structure–ROS–substrate” paradigm to serve a dual function: as an interpretative tool to decode complex selectivity patterns and reconcile disparate catalytic data, and as a forward-looking design blueprint. It shifts the design objective from optimizing isolated properties to purposefully engineering the dynamic interplay within the entire “structure–ROS–substrate” triad to achieve a target photocatalytic function.
Despite this remarkable progress, several challenges remain before COF-based photocatalysts can be deployed widely in industrial or biomedical contexts. A persistent hurdle is the scalable, cost-effective synthesis of highly crystalline and chemically robust frameworks. Many state-of-the-art COFs rely on elaborate organic monomers or demanding reaction conditions, which can limit reproducibility and hinder large-scale implementation. Developing more sustainable synthetic routes—such as low-temperature, aqueous-phase methods or solvent-free mechanochemical strategies—will be vital for broadening accessibility.
Another critical challenge lies in the limited understanding of the mechanistic intricacies that underlie ROS formation and utilization. While techniques such as ESR, operando spectroscopy, and computational modeling have provided invaluable insights into the generation of ·O2−, 1O2, and ·OH, a complete picture of the dynamic interplay between different ROS pathways is still emerging. In particular, the relative contributions of type I versus type II processes, and the role of framework defects or guest–host interactions in directing selectivity, require deeper exploration. Stability is also a recurring concern. Although certain COFs exhibit remarkable tolerance to light irradiation, reactive oxygen environments, and acidic or basic conditions, many others suffer from gradual degradation, loss of crystallinity, or deactivation of catalytic sites. Long-term operational stability, especially under continuous flow or in complex matrices such as industrial effluents and biological fluids, remains a decisive factor for real-world adoption.
More critically, mechanistic understanding of COF-driven photocatalysis is still constrained by the lack of effective in situ ROS detection tools. Current approaches, including radical scavenging, ESR spin trapping, and theoretical simulations, provide valuable but often indirect or static insights. Developing advanced characterization techniques with high spatial and temporal resolution—such as in situ spectroscopies, single-molecule imaging, isotope labeling, or electrochemical probing—will be essential to dynamically monitor ROS generation, evolution, and interplay with substrates. Such breakthroughs would allow a deeper elucidation of the structure–ROS–substrate relationships and guide the precise design of next-generation COFs.
A paramount challenge that curtails the transition from empirical discovery to rational design in COF photocatalysis is the dynamic complexity of the “structure–ROS–substrate” interface, which remains obscured by conventional ex situ characterization. While techniques such as EPR spin trapping and quenching experiments provide essential proof of ROS presence, they offer limited temporal and spatial resolution into the sequence of events: How exactly does charge separation at a donor–acceptor junction dictate the branching ratio between singlet oxygen and superoxide? What is the lifetime and diffusion length of key intermediates within the confined pore before reacting? To decode this black box, the field must pivot towards multimodal in situ and operando diagnostics. For example, time-resolved surface-enhanced Raman spectroscopy could capture vibrational fingerprints of transient surface-bound peroxo or radical species during illumination. Ultrafast transient absorption microscopy would spatially map exciton diffusion and charge separation efficiency across different crystalline domains or defects. In situ liquid-phase transmission electron microscopy could, in principle, visualize the ingress of substrate molecules into pores and their transformation at active sites. Furthermore, coupling isotope-labeled mass spectrometry with photoelectrochemical measurements can quantitatively deconvolute the contributions of water oxidation versus oxygen reduction pathways to H2O2 production. By orchestrating such a suite of techniques, researchers can move beyond correlative “activity–structure” relationships and establish causal mechanistic maps, ultimately enabling the predictive design of COFs with programmed ROS output and substrate selectivity.
Therefore, the path forward must be explicitly guided by the “structure–ROS–substrate” paradigm. Looking ahead, several avenues hold promise for advancing COF-based photocatalytic oxidation. First, synthetic innovation must continue to lower the barriers to access highly crystalline, functionalized frameworks. This includes the development of scalable, green synthetic approaches that leverage renewable feedstocks and avoid harsh reagents. Advances in reticular chemistry, such as topological design, linker engineering, and post-synthetic modification, will be central to constructing COFs with tailored light-absorbing motifs, optimized charge-transport pathways, and controlled porosity.
Second, the rational design of COFs for selective ROS pathways represents a particularly exciting frontier. By controlling the electronic structure through donor–acceptor engineering, heteroatom doping, or the incorporation of catalytic sites such as single-atom metals, researchers can favor the generation of specific ROS. Such control could significantly enhance the energy efficiency and selectivity of photocatalytic oxidation reactions, opening the door to more sustainable processes for chemical synthesis and environmental remediation.
Third, advancing mechanistic understanding remains essential. Integrating transient absorption spectroscopy, in situ or operando spectroscopies, and theoretical modeling will be crucial to unravel the fundamental principles that dictate exciton dissociation, charge transport, and ROS reactivity within COFs. This knowledge will not only guide the rational design of next-generation frameworks but also help establish universal design rules for tailoring photocatalysts at the molecular level.
Fourth, future studies should increasingly consider realistic and complex environments. Many current demonstrations employ model organic dyes or simplified aqueous systems, but practical applications demand robustness under diverse conditions, including the presence of salts, natural organic matter, and fluctuating pH. Translating laboratory findings into industrial water treatment, environmental remediation, or biomedical therapy requires comprehensive evaluation of stability, reusability, and scalability.
Truly advancing COF photocatalysis from a laboratory novelty to a technology with net environmental benefit demands a paradigm shift: performance must be evaluated in tandem with sustainability across the entire life cycle. This necessitates the formal adoption of Green Chemistry principles and rigorous Life Cycle Assessment (LCA) as non-negotiable pillars of future research. First, synthetic elegance must be weighed against environmental cost. The field should celebrate and prioritize COFs derived from biomass-based monomers or synthesized via atom-economical, solvent-free mechanochemistry, even if their initial activity metrics are modest, as their scalable and low-footprint production could offer greater overall sustainability. Second, the circularity of COFs must be proactively engineered. This includes designing frameworks with embedded recyclability, such as COFs that can be depolymerized into pure monomers for re-synthesis, or frameworks with triggered biodegradability via pH- or enzyme-cleavable linkers for safe disposal. Concurrently, pioneering studies must begin to assess the long-term environmental fate of COF nanoparticles, including their ecotoxicity, potential for bioaccumulation, and transformation products in aquatic and soil systems. Funding agencies and journals can accelerate this transition by incentivizing research that reports not only high conversion yields but also Environmental Factor (E-factor) of synthesis and end-of-life impact data. By embedding these holistic metrics into the core R&D ethos, the COF community can ensure its innovations contribute authentically to a circular and green chemical economy, rather than solving one environmental problem while inadvertently creating another.
Fifth, integration with other functional materials provides a fertile path forward. Constructing heterojunctions with semiconductors, coupling COFs with metal–organic frameworks (MOFs), or embedding single-atom catalysts can endow COFs with new functionalities and further enhance ROS yields. Similarly, combining COFs with biomolecules, enzymes, or responsive polymers may enable smart platforms for targeted therapy or controlled release applications, bridging the gap between materials science and biomedicine.
Finally, the field would benefit from embracing computational and data-driven approaches. Machine learning, high-throughput screening, and multiscale modeling could accelerate the discovery of optimal building blocks and linkages, providing predictive insights into how structural variations influence ROS generation and stability. Such approaches, coupled with rigorous life-cycle assessments, will be indispensable for guiding COFs from academic laboratories to real-world deployment.
In summary, COF-based photocatalysts embody a convergence of molecular design precision, structural order, and functional versatility. Their ability to regulate the formation and utilization of ROS under mild, sustainable conditions sets them apart from conventional photocatalysts and positions them as promising candidates for applications ranging from selective organic synthesis to pollutant degradation and biomedical therapy. Most importantly, the research journey has crystallized the “structure–ROS–substrate” paradigm as the central, unifying framework that both explains past successes and illuminates the path ahead. By deepening our mechanistic understanding, innovating synthetic strategies, and fostering interdisciplinary integration, the community is well placed to transform COFs from an emerging concept into a cornerstone of next-generation oxidation technologies. The continuing dialogue between fundamental science and applied engineering will be pivotal in unlocking the full potential of COFs to address pressing challenges in energy, sustainability, and health.
Author contributions
Q. Fang and C. Liu conceived the idea for this review. T. Sun and R. Wang drafted the manuscript, R. Guan and L. Wang conducted data search and verification. X. Cheng and T. Zhong polished the language of the manuscript. Finally, all authors revised the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was supported by the Natural Science Foundation Project of Jilin Province (YDZJ202501ZYTS327), the National Natural Science Foundation of China (22478150 and 22025504), and the “111” project (BP0719036 and B17020).Notes and references
- G. Chen, P. Shao, X. Niu, L.-Z. Wu and T. Zhang, CCS Chem., 2025, 7, 1272–1288 CrossRef CAS.
- Y. Fang, Y. Zheng, T. Fang, Y. Chen, Y. Zhu, Q. Liang, H. Sheng, Z. Li, C. Chen and X. Wang, Sci. China: Chem., 2020, 63, 149–181 Search PubMed.
- C. Liu, F. Chen, B.-H. Zhao, Y. Wu and B. Zhang, Nat. Rev. Chem., 2024, 8, 277–293 CrossRef CAS.
- N. Karousis and D. Tasis, Energy Adv., 2024, 3, 712–740 RSC.
- R. Wang, Z. Zhang, H. Zhou, M. Yu, L. Liao, Y. Wang, S. Wan, H. Lu, W. Xing, V. Valtchev, S. Qiu and Q. Fang, Angew. Chem., Int. Ed., 2024, 63, e202410417 CrossRef CAS PubMed.
- R. Wang, H. Zhou, Q. Fang, Z. Su and S. Qiu, Chem. Commun., 2025, 61, 15346–15361 RSC.
- X. Li, J. Gao, C. Wu, C. Wang, R. Zhang, J. He, Z. J. Xia, N. Joshi, J. M. Karp and R. Kuai, Sci. Adv., 2024, 10, eadl0479 CrossRef CAS PubMed.
- C. Dong, W. Fang, Q. Yi and J. Zhang, Chemosphere, 2022, 308, 136205 CrossRef CAS.
- F. Chen, H. Zheng, Y. Yusran, H. Li, S. Qiu and Q. Fang, Chem. Soc. Rev., 2025, 54, 484–514 RSC; R. Wang, J. Zhao, Q. Fang and S. Qiu, Chem. Synth., 2024, 4, 29 Search PubMed; W. Xia, C. Ji, R. Wang, S. Qiu and Q. Fang, Acta Phys.-Chim. Sin., 2023, 39, 2212057 Search PubMed.
- Y. Yusran, S. Qiu and Q. Fang, EnergyChem, 2025, 7, 100170 CrossRef CAS; L. Liao, R. Wang, Z. Zhang, J. Zhang, S. Huang, W. Xie, Y. Wang, M. Xue, Q. Fang and S. Qiu, Inorg. Chem. Front., 2025, 12, 1881–1889 RSC.
- B. Mishra, A. Alam, A. Chakraborty, B. Kumbhakar, S. Ghosh, P. Pachfule and A. Thomas, Adv. Mater., 2024, 36, 202413118 Search PubMed; J. Song, L. Liao, Z. Zhang, Y. Yusran, R. Wang, J. Fang, Y. Liu, Y. Hou, Y. Wang and Q. Fang, Crystals, 2022, 12, 880 CrossRef CAS; R. Wang, H. Zhou, Q. Pan, Z. Su, S. Qiu, Q. Fang and H. Lu, Chem. Eng. J., 2024, 499, 156033 CrossRef.
- T. Sun, Z. Wang, Y. Wang, Q. Xu, K. Wang and J. Jiang, Angew. Chem., Int. Ed., 2025, 64, 202422814 CrossRef CAS PubMed; R. Wang, Z. Zhang, J. Suo, L. Liao, L. Li, Z. Yu, H. Zhang, V. Valtchev, S. Qiu and Q. Fang, Chem. Eng. J., 2023, 478, 147403 CrossRef.
- L. Xiong and J. Tang, Adv. Energy Mater., 2021, 11, 2003216 CrossRef CAS.
- N.-Y. Huang, Y.-T. Zheng, D. Chen, Z.-Y. Chen, C.-Z. Huang and Q. Xu, Chem. Soc. Rev., 2023, 52, 7949–8004 RSC.
- Y. Chen and D. Jiang, Acc. Chem. Res., 2024, 57, 3182–3193 CrossRef CAS PubMed.
- Z. Yong and T. Ma, Angew. Chem., Int. Ed., 2023, 62, 202308980 CrossRef.
- Y. An, T. Cai, W. Jiang, T. Lei and H. Pang, Green Chem., 2025, 27, 10478–10509 RSC.
- S. Zhu, Z. Zhang, Z. Li, H. Yue and X. Liu, Chem Catal., 2024, 4, 100963 CAS.
- B. Lin and T. W. B. Lo, Coord. Chem. Rev., 2026, 549, 217329 Search PubMed.
- J. Zhang, C. Wang, X. Li, X. Xu, Z. Wang and X. Peng, Coord. Chem. Rev., 2026, 550, 217402 Search PubMed.
- Z. Li, B. Cai, Q. Li, D. Zhang, Y. Liang, Y. Liu, Y. Jiao, A. Thomas and X. Zhao, Angew. Chem., Int. Ed., 2025, 64, 202509444 CrossRef CAS PubMed; H. Ben, G. Yan, H. Liu, C. Ling, Y. Fan and X. Zhang, Adv. Funct. Mater., 2022, 32, 2104519 CrossRef.
- Z. Chen, Z. Shen, X. Zhang, L. Chen, B. Hu, G. Xu, C. Gao and Q. Xu, Appl. Catal., B, 2025, 379, 125731 CrossRef CAS.
- I. E. Khalil, P. Das and A. Thomas, Acc. Chem. Res., 2024, 57, 3138–3150 Search PubMed; J. Ding, X. Guan, J. Lv, X. Chen, Y. Zhang, H. Li, D. Zhang, S. Qiu, H.-L. Jiang and Q. Fang, J. Am. Chem. Soc., 2023, 145, 3248–3254 Search PubMed.
- T. Sun, Y. Deng, G. Wang, H. Liu, L. Chen, R. Guan, C. Liu, G. Che, X. Cheng and Y. Zhu, Appl. Catal., B, 2026, 382, 125907 CrossRef CAS.
- Q. Huang, N. Li, M. Han, J. Liu and Y. Lan, Angew. Chem., Int. Ed., 2025, 64, e202513848 Search PubMed.
- Y. Hou, F. Liu, J. Liang, Z. Li, P. Zhou and M. Tong, Angew. Chem., Int. Ed., 2025, 64, e202505621 CrossRef CAS.
- H. Guo, S. Wang, X. Chen, J. Kou, G. He, Z. Dong and Y. Yan, Nat. Synth., 2025, 4, 1610–1620 Search PubMed.
- J. Qiu, H. Zhai, Y. Zhao, Y. Jin, Z. Li, H. Wang, Z. Li, J. Wang and J. Baek, Angew. Chem., Int. Ed., 2025, 64, e202508078 Search PubMed.
- S. Guo, K. Zhao, L. Liang, Z. Li, B. Han, X. Ou, S. Yao, Z. Lin, Z. Dong, Y. Liu, L. Ye, B. Weng, Y. Cai and Z. Yang, Angew. Chem., Int. Ed., 2025, 64, e202509141 CrossRef CAS.
- X. Wu, M. Sayed, G. Wang, W. Yu and B. Zhu, Adv. Mater., 2025, e11322 Search PubMed.
- Y.-S. Bi, W.-Q. Liu, H. Liu and L.-B. Xing, Chem. Sci., 2025, 16, 16904–16914 RSC.
- D. Yang, Y. Zhang, W. Huang, H. Chen, L. Li, C. Xu, S. Xiang, B. Chen, J. Wang and Z. Zhang, Adv. Funct. Mater., 2025, e18488 CrossRef.
- T. Sun, Y. Deng, G. Wang, L. Yan, T. Sun, R. Guan, S. Zhang and C. Liu, Chem. Eng. J., 2024, 496, 154351 CrossRef CAS.
- J. Lai, Y. Tian, H. Wei, Y. Bai, F. Wu, F. Yu, P. Yu and L. Mao, Anal. Chem., 2025, 97, 3418–3426 Search PubMed.
- S. Alberca, A. M. Nair, P. Escamilla, P. Ferreira, M. Souto and M. Fañanás-Mastral, J. Am. Chem. Soc., 2025, 147, 39582–39589 Search PubMed.
- L. Fang, S. Qiu, H. Xu, T. Ye and L. Li, Adv. Funct. Mater., 2025, 35, 2504676 Search PubMed.
- Y. Wu, Y. Chen, Z. Zhang, X. Zhou, H. Wang, Q. Wu, J. Du and W.-Q. Guo, Trans. Tianjin Univ., 2025, 31, 370–389 CrossRef CAS.
- S. Zhu, X. He, L. Wang, W. Ma, C. Liu, Y. Zhang, X. Hu and J. Jiang, Adv. Funct. Mater., 2025, e19832 CrossRef.
- Y. Zheng, Y. Li, X. Bai, M. Teng, Y. Tang, S. Zhao, Z. Ma, H. Liang, Y. Xie and Q. Wan, Small, 2025, 21, 2410816 CrossRef CAS.
- X. Bai, X. Liu and R. Zong, Appl. Catal., B, 2025, 366, 125062 CrossRef CAS.
- X. Yang, Q. Xu, W. Wei and G. Zeng, Angew. Chem., Int. Ed., 2025, 64, e202504355 CrossRef CAS PubMed.
- C. He, S. Tao, R. Liu, Y. Zhi and D. Jiang, Angew. Chem., Int. Ed., 2024, 63, e202403472 Search PubMed.
- P. Costa, A. Vega-Peñaloza, L. Cognigni and M. Bonchio, ACS Sustainable Chem. Eng., 2021, 9, 15694–15721 CrossRef CAS.
- D. Asgari, J. Grüneberg, Y. Luo, H. Küçükkeçeci, S. Ghosh, V. Chevelkov, S. Fischer-Lang, J. Roeser, A. Lange, B. Dunn, M. Gradzielski and A. Thomas, Nat. Commun., 2024, 15, 7031 CrossRef CAS.
- X. Li, C. Zhang, S. Cai, X. Lei, V. Altoe, F. Hong, J. J. Urban, J. Ciston, E. M. Chan and Y. Liu, Nat. Commun., 2018, 9, 2998 Search PubMed.
- P. Zhang, Z. Wang, S. Wang, J. Wang, J. Liu, T. Wang, Y. Chen, P. Cheng and Z. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202213247 Search PubMed.
- J. Wei, R. Li, P. Zhang, H. Jin, Z. Zhang, Y. Li and Y. Chen, Nat. Commun., 2023, 14, 2805 Search PubMed.
- S. Yang, D. Si, L. Zou, M. Shi, Y. Huang and R. Cao, J. Am. Chem. Soc., 2025, 147, 30287–30295 Search PubMed.
- S. Wang, Q. Sun, W. Chen, Y. Tang, B. Aguila, Y. Pan, A. Zheng, Z. Yang, L. Wojtas, S. Ma and F.-S. Xiao, Matter, 2020, 2, 416–427 CrossRef CAS.
- J. Li, S. Zhu, L. Yang, X. Xiao, H. Yue and X. Liu, Macromol. Rapid Commun., 2025, e00470 CrossRef CAS.
- M. Deng, J. Chakraborty, G. Wang, K. S. Rawat, L. Bourda, J. Sun, I. Nath, Y. Ji, P. Geiregat, V. Van Speybroeck, X. Feng and P. Van Der Voort, J. Am. Chem. Soc., 2025, 147, 10219–10230 CrossRef CAS PubMed.
- C. Li, A. Yu, W. Zhao, G. Long, Q. Zhang, S. Mei and C. Yao, Angew. Chem., Int. Ed., 2024, 63, e202409421 CrossRef CAS PubMed.
- L. Qin, D. Sun, D. Ma, Z. Wang, Y. Liu, Q. Li, F. Song, K. Wu, L. Gan, T. Zhou and J. Zhang, Adv. Mater., 2025, 37, 2504205 CrossRef CAS PubMed.
- H. Pan, Q. Niu, M. Yu, L. Li and Y. Yu, ACS Energy Lett., 2025, 10, 4580–4588 CrossRef CAS.
- Z. Sun, L. Li, S. Fan, Y. Xu, Z. Gao and C. Wang, ACS Appl. Mater. Interfaces, 2025, 17, 25828–25838 CrossRef CAS.
- S. Trenker, L. Grunenberg, T. Banerjee, G. Savasci, L. M. Poller, K. I. M. Muggli, F. Haase, C. Ochsenfeld and B. V. Lotsch, Chem. Sci., 2021, 12, 15143–15150 RSC.
- L. Yang, Z. Chen, Q. Cao, H. Liao, J. Gao, L. Zhang, W. Wei, H. Li and J. Lu, Adv. Mater., 2024, 36, 2306758 CrossRef CAS.
- M. Zhang, P. Huang, R. Li, Y. Huang, M. Qin, Y. Peng, S. Wang, Y. Yan, M. Lu and Y. Lan, Angew. Chem., Int. Ed., 2025, 64, e202507624 CrossRef CAS PubMed.
- S. Manzoor, M. A. Younis, Y. Yao, Q. Tariq, B. Zhang, B. Tian, L. Yan and C. Qiu, Coord. Chem. Rev., 2025, 541, 216840 CrossRef CAS.
- R. Shen, J. Xing, Q. Yue, S. Wang, Y. Li, P. Zhang and X. Li, Sci. China Mater., 2025, 68, 3925–3954 CrossRef.
- L. Sun, W. Yan, W. Wang, L. Wang, S. Osella, G. Liang, W. A. Goddard, R. Zbořil, Y. Zhou, J. Yang and Q. Liu, Angew. Chem., Int. Ed., 2025, 64, e202511080 Search PubMed.
- M. Guo, X. Guan, Q. Meng, M. Gao, Q. Li and H. Jiang, Angew. Chem., Int. Ed., 2024, 63, e202410097 CrossRef CAS.
- L. Huang, W. Li, F. Wei, S. Ke, H. Chen, C. Jing, J. Cheng and S. Liu, Chem, 2024, 10, 3100–3113 CAS.
- H. Xu, W. Wang, X. Zhao, C. Liu, P. Tian, Q. Qi, S. Xu, Y. Fu and X. Zhao, Adv. Funct. Mater., 2025, e15586 CrossRef.
- I. E. Khalil, P. Das, H. Küçükkeçeci, V. Dippold, J. Rabeah, W. Tahir, J. Roeser, J. Schmidt and A. Thomas, Chem. Mater., 2024, 36, 8330–8337 CrossRef CAS.
- A. López-Magano, S. Daliran, A. R. Oveisi, R. Mas-Ballesté, A. Dhakshinamoorthy, J. Alemán, H. Garcia and R. Luque, Adv. Mater., 2023, 35, 2209475 CrossRef.
- T. Luo, L. Gilmanova and S. Kaskel, Coord. Chem. Rev., 2023, 490, 215210 CrossRef CAS.
- H. Chen, H. S. Jena, X. Feng, K. Leus and P. Van Der Voort, Angew. Chem., Int. Ed., 2022, 61, e202204938 Search PubMed.
- G.-B. Wang, K.-H. Xie, H.-P. Xu, Y.-J. Wang, F. Zhao, Y. Geng and Y.-B. Dong, Coord. Chem. Rev., 2022, 472, 214774 Search PubMed.
- H. Hao, F. Zhang, X. Dong and X. Lang, Appl. Catal., B, 2021, 299, 120691 CrossRef CAS.
- Y. Wang, F. Zhang, F. Huang, X. Dong, B. Zeng, X.-K. Gu and X. Lang, Appl. Catal., B, 2024, 354, 124103 CrossRef CAS.
- K. Zhang, F. Zhang, Y. Wang, K. Xiong, S. Zhang and X. Lang, ChemSusChem, 2025, 18, e202500552 CrossRef CAS PubMed.
- F. Yang, X. Li, H. Qu, J. Kan, Y. Guo and Y. Dong, Chin. J. Chem., 2024, 42, 1960–1966 CrossRef CAS.
- K. Feng, H. Hao, F. Huang, X. Lang and C. Wang, Mater. Chem. Front., 2021, 5, 2255–2260 RSC.
- H. Zhao, F. Zhang, X. Dong and X. Lang, J. Mater. Chem. A, 2023, 11, 18115–18125 RSC.
- H. Zhao, Y. Wang, F. Zhang, X. Dong and X. Lang, Appl. Catal., B, 2024, 350, 123899 CrossRef CAS.
- H. Shan, Z. Zhang, Y. Jiang, D. Cai, P. Qin and J. Baeyens, Sep. Purif. Technol., 2025, 363, 132102 CrossRef CAS.
- J. Shi, R. Chen, H. Hao, C. Wang and X. Lang, Angew. Chem., Int. Ed., 2020, 59, 9088–9093 CrossRef CAS PubMed.
- S. Wu, Y.-F. Zhang, H. Ding, X. Li and X. Lang, J. Colloid Interface Sci., 2022, 610, 446–454 CrossRef CAS PubMed.
- F. Zhang, X. Li, X. Dong, H. Hao and X. Lang, Chin. J. Catal., 2022, 43, 2395–2404 CrossRef CAS.
- L. Wang, J. Chakraborty, M. Deng, J. Sun and P. Van Der Voort, ChemCatChem, 2024, 16, e202400200 CrossRef CAS.
- Z. Chen, J. Guo, F.-H. Song, S.-D. Wang, S. A. C. Carabineiro, S.-X. Ouyang and L.-L. Wen, ACS Catal., 2025, 15, 8284–8296 CrossRef.
- J. Zhao, M. Xie, X. Chen, J. Jin, W. Zhao, J. Luo, G. Ning, J. Liu and D. Li, Chem.–Asian J., 2023, 18, e202300328 Search PubMed.
- S. Shi, W. Liu, Y. Li, S. Lu, H. Zhu, M. Du, X. Chen and F. Duan, J. Colloid Interface Sci., 2024, 655, 611–621 CrossRef CAS PubMed.
- K. Xiong, X. Jia, Y. Kong, J. Yang, J. Guo, S. Li, M. Adeli, Y. Wang, X. Luo, X. Han and C. Cheng, Adv. Funct. Mater., 2025, 2510257 CrossRef CAS.
- J. Zhang, W. Zhou, J. Zhao, L. Xu, X. Jiang, Z. Li, Y. Peng and G. Li, Small, 2025, 21, 2408324 Search PubMed.
- H. Pang, G. Liu, D. Huang, Y. Zhu, X. Zhao, W. Wang and Y. Xiang, Angew. Chem., Int. Ed., 2023, 62, e202313520 Search PubMed.
- A. Zadehnazari, A. Khosropour, A. A. Altaf, A. S. Rosen and A. Abbaspourrad, Adv. Mater., 2024, 36, 2311042 CrossRef CAS PubMed.
- B. Ma, X. Yang, J. Yuan, X. Yang, D. Han, K. Zhao, C. Lin, L. Wang, G. Liu and L. Mi, Appl. Catal., A, 2023, 666, 119403 CrossRef CAS.
- J. Wang, T. Sun, J. Zhang, Z. Chen, J. Du, J. Kan and Y. Dong, Chem. Sci., 2024, 15, 18634–18639 Search PubMed.
- K. Cai, W. Wang, J. Zhang, L. Chen, L. Wang, X. Zhu, Z. Yu, Z. Wu and H. Zhou, J. Mater. Chem. A, 2022, 10, 7165–7172 Search PubMed.
- G. Xiao, W. Li, T. Chen, W. Hu, H. Yang, Y. A. Liu and K. Wen, Eur. J. Org Chem., 2021, 2021, 3986–3991 CrossRef CAS.
- L. Li, L. Wang, G. Jia, L. Xu, J. Chen, Z. Hu and J. C. Yu, ACS Nano, 2024, 18, 33142–33151 CrossRef CAS.
- Q. Xue, H. Li, P. Jin, X. Zhou and F. Wang, Angew. Chem., Int. Ed., 2025, 64, e202423368 CrossRef CAS.
- Z. Li, S. Han, C. Li, P. Shao, H. Xia, H. Li, X. Chen, X. Feng and X. Liu, J. Mater. Chem. A, 2020, 8, 8706–8715 RSC.
- K.-K. Niu, T.-X. Luan, J. Cui, H. Liu, L.-B. Xing and P.-Z. Li, ACS Catal., 2024, 14, 2631–2641 CrossRef CAS.
- H. Chen, W. Liu, C. Liu, J. Sun, L. Bourda, R. Morent, N. De Geyter, R. Van Deun, K. Van Hecke, K. Leus and P. Van Der Voort, Appl. Catal., B, 2022, 319, 121920 CrossRef CAS.
- P. T. Parvatkar, S. Kandambeth, A. C. Shaikh, I. Nadinov, J. Yin, V. S. Kale, G. Healing, A.-H. Emwas, O. Shekhah, H. N. Alshareef, O. F. Mohammed and M. Eddaoudi, J. Am. Chem. Soc., 2023, 145, 5074–5082 CrossRef CAS PubMed.
- Y. Zhang, Y. Wu, H. Ma, Y. Gao, X. Fan, Y. Zhao, F. Kang, Z. Li, Y. Liu and Q. Zhang, Small, 2025, 21, 2500674 CrossRef CAS.
- Q. Liao, Q. Sun, H. Xu, Y. Wang, Y. Xu, Z. Li, J. Hu, D. Wang, H. Li and K. Xi, Angew. Chem., Int. Ed., 2023, 62, e202310556 CrossRef CAS.
- Z. Zhang, Q. Zhang, Y. Hou, J. Li, S. Zhu, H. Xia, H. Yue and X. Liu, Angew. Chem., Int. Ed., 2024, 63, e202411546 CrossRef PubMed.
- M. Kou, Y. Wang, Y. Xu, L. Ye, Y. Huang, B. Jia, H. Li, J. Ren, Y. Deng, J. Chen, Y. Zhou, K. Lei, L. Wang, W. Liu, H. Huang and T. Ma, Angew. Chem., Int. Ed., 2022, 61, e202200413 CrossRef CAS.
- C. Sun, Y. Han, H. Guo, R. Zhao, Y. Liu, Z. Lin, Z. Xiao, Z. Sun, M. Luo and S. Guo, Adv. Mater., 2025, 37, 2502990 CrossRef CAS.
- J. Yue, L. Song, Y. Fan, Z. Pan, P. Yang, Y. Ma, Q. Xu and B. Tang, Angew. Chem., Int. Ed., 2023, 62, e202309624 CrossRef CAS.
- D. Chen, W. Chen, Y. Wu, L. Wang, X. Wu, H. Xu and L. Chen, Angew. Chem., Int. Ed., 2023, 62, e202217479 CrossRef CAS.
- G. Pan, X. Hou, Z. Liu, C. Yang, J. Long, G. Huang, J. Bi, Y. Yu and L. Li, ACS Catal., 2022, 12, 14911–14917 CrossRef CAS.
- W. Tao, Y. Wang, L. Cong, C. Zhang, Y. Gao, H. Zheng, W. Shi, D. Zhong and T. Lu, Sci. China: Chem., 2025, 68 DOI:10.1007/s11426-025-2840-0.
- J. Yue, J. Luo, Z. Pan, R. Zhang, P. Yang, Q. Xu and B. Tang, Angew. Chem., Int. Ed., 2024, 63, e202405763 CrossRef CAS PubMed.
- J. Cheng, Y. Wu, W. Zhang, L. Wang, X. Wu and H. Xu, Adv. Mater., 2025, 37, 2410247 CrossRef CAS.
- Z. Pan, Y. Guo, J. Luo and J. Yue, Adv. Funct. Mater., 2025, e12475 Search PubMed.
- J.-Y. Yue, L.-P. Song, Z.-X. Pan, M. Cheng, X. Wang, Q. Xu and P. Yang, Chem. Eng. J., 2025, 504, 158983 CrossRef CAS.
- J. Yue, Z. Pan, R. Zhang, Q. Xu, P. Yang and B. Tang, Adv. Funct. Mater., 2025, 35, 2421514 CrossRef CAS.
- W. Wang, R. Zhang, H. Chu, Z. Zhan, Q. Huang, Z. Li, X. Wang, F. Bai and W. Zhou, Small, 2025, 21, 2406527 CrossRef CAS PubMed.
- Y. Li, J. Zhang, X. Zhang, W. Li and A. Kong, Inorg. Chem. Front., 2025, 12, 6571–6581 RSC.
- B. Li, J. Chen, K. Wang, D. Qi, T. Wang and J. Jiang, Adv. Energy Mater., 2025, 15, 2404497 CrossRef CAS.
- E. Zhou, F. Wang, X. Zhang, Y. Hui and Y. Wang, Angew. Chem., Int. Ed., 2024, 63, e202400999 CrossRef CAS PubMed.
- L. Li, X. Yao, W. Ou, J. Chai, R. Ma, C. Ran, A. Ma, X. Shi, P. Wei, H. Dong, H. Zhou, W. Yang, H.-C. Hu, J.-F. Wu, H. Peng and G. Ma, Green Chem., 2025, 27, 9144–9152 RSC.
- H. Ma, Y. Zhang, Y. Wu, Q. Gu, Z. Li and Q. Zhang, Chem. Sci., 2025, 16, 16668–16677 RSC.
- J. Chang, Q. Li, J. Shi, M. Zhang, L. Zhang, S. Li, Y. Chen, S. Li and Y. Lan, Angew. Chem., Int. Ed., 2023, 62, e202218868 CrossRef CAS PubMed.
- Z. Ding, J. Yang, Z. Wu, M. Adeli, X. Luo, X. Wang, X. Xie, X. Xu, C. Cheng and C. Zhao, Chem. Mater., 2025, 37, 1972–1982 CrossRef CAS.
- L. Guo, L. Gong, Y. Yang, Z. Huang, X. Liu and F. Luo, Angew. Chem., Int. Ed., 2025, 64, e202414658 CrossRef CAS PubMed.
- R. Liu, M. Zhang, F. Zhang, B. Zeng, X. Li, Z. Guo and X. Lang, Small, 2025, 21, 2411625 CrossRef CAS.
- Y. Yao, C. Zhu, R. Liu, Q. Fang, S. Song, B. Chen and Y. Shen, Small, 2024, 20, 2404885 Search PubMed.
- C. Shu, P. Xie, X. Yang, X. Yang, H. Gao, B. Tan and X. Wang, J. Mater. Chem. A, 2024, 12, 25927–25933 Search PubMed.
- M. Zhang, R. Liu, F. Zhang, H. Zhao, X. Li, X. Lang and Z. Guo, J. Colloid Interface Sci., 2025, 678, 1170–1180 Search PubMed.
- Z. Yu, F. Yu, M. Xu, S. Feng, J. Qiu and J. Hua, Adv. Sci., 2025, 12, 2415194 CrossRef CAS.
- Z. Zhou, M. Sun, Y. Zhu, P. Li, Y. Zhang, M. Wang and Y. Shen, Appl. Catal., B, 2023, 334, 122862 Search PubMed.
- L. Jin, M. Deng, J. Gao, L. Wang, Q. Zhou, X. Tang, Q. Li, H. Du, D. Hao and Q. Wang, Chem. Eng. J., 2025, 515, 163722 CrossRef CAS.
- X. Du, H. Nie, Y. Qu, H. Jia, Y. Liu and B. Yin, J. Environ. Chem. Eng., 2025, 13, 115660 Search PubMed.
- J. Song, C. Yu, Y. Liu, J. Ren, J. Liu, Z. Wang, L. Zhu, J. Fu, B. Tang, S. Qiu, Y. Wang and Q. Fang, Mater. Chem. Front., 2023, 7, 1431–1436 Search PubMed; L. Yang, Y. Wang and J. Yuan, Chem. Eng. J., 2022, 446, 137095 Search PubMed.
- Z. Hu, Y. Wang, L. Wang, Q. Wang, Q. Zhang, F. Cui and G. Jiang, Chem. Eng. J., 2024, 479, 147534 CrossRef CAS.
- T. L. Yusuf, B. O. Orimolade, D. Masekela, B. Mambab and N. Mabuba, RSC Adv., 2022, 12, 26176–26191 RSC; R. Wang, Z. Zhang, H. Zhou, M. Yu, L. Liao, Y. Wang, S. Wan, H. Lu, W. Xing, V. Valtchev, S. Qiu and Q. Fang, Angew. Chem., Int. Ed., 2024, 63, e202410417 Search PubMed.
- F. Chen, H. Zheng, Y. Yusran, H. Li, S. Qiu and Q. Fang, Chem. Soc. Rev., 2025, 54, 484–514 Search PubMed; J. Liu, C. Tuo, W. Xiao, M. Qi, Y. Yusran, Z. Wang, H. Li, C. Guo, J. Song, S. Qiu, Y. Xu and Q. Fang, Angew. Chem., Int. Ed., 2025, 64, e202416240 Search PubMed.
- Y. Xiao, A. A. K. Mohammed, S.-W. Kuo and A. F. M. EL-Mahdy, Sep. Purif. Technol., 2025, 356, 129950 CrossRef CAS.
- L. Wang, J. Chakraborty, K. S. Rawat, M. Deng, J. Sun, Y. Wang, V. Van Speybroeck and P. Van Der Voort, Sep. Purif. Technol., 2025, 359, 130368 CrossRef CAS.
- Y. Hou, C. Cui, E. Zhang, J. Wang, Y. Li, Y. Zhang, Y. Zhang, Q. Wang and J. Jiang, Dalton Trans., 2019, 48, 14989 RSC.
- Y. Chen, Y. Guo, T. Wang, S. Ji, H. Shao, M. Lin, S. Seki, N. Yan and D. Jiang, Nat. Commun., 2025, 16, 6495 CrossRef CAS PubMed.
- J. Wang, A. E. Hassan, A. M. Elewa and A. F. M. EL-Mahdy, J. Mater. Chem. A, 2024, 12, 14005 RSC.
- Y. Hou, P. Zhou, F. Liu, K. Tong, Y. Lu, Z. Li, J. Liang and M. Tong, Nat. Commun., 2024, 15, 7350 CrossRef CAS.
- L. Zhang, H. Zhang, D. Zhu, Z. Fu, S. Dong and C. Lyu, Chin. J. Catal., 2024, 66, 181–194 Search PubMed.
- J. Chen, G. Li, N. Lu, H. Lin, S. Zhou and F. Liu, Mater. Today Chem., 2022, 24, 100832 Search PubMed.
- A. Khojastegi, A. Khosropour, F. Auras and A. Abbaspourrad, Small, 2025, 21, 2408151 CrossRef.
- Z. Xu, Q. Duan and X. Cui, Chem. Eng. J., 2025, 511, 162241 CrossRef CAS.
- R. Li, L. Liu and H. Wu, ACS Appl. Mater. Interfaces, 2025, 17, 26671–26678 CrossRef CAS PubMed.
- C. Shu, X. Yang, L. Liu, X. Hu, R. Sun, X. Yang, A. I. Cooper, B. Tan and X. Wang, Angew. Chem., Int. Ed., 2024, 63, e202403926 CrossRef CAS.
- Y. Zhang, Z. Chen, Z. Shi, T. Lu, D. Chen, Q. Wang and Z. Zhan, Sep. Purif. Technol., 2021, 275, 119216 CrossRef CAS.
- Y. Xu, M. Song, Y. Ren, X. Pang, J. Cheng, L. Chen and G. Lu, ACS Appl. Mater. Interfaces, 2025, 17, 8136–8146 CrossRef CAS PubMed.
- L. Qu, Y. Zhang, Y. Shang, B. Gu, X. Hong, S. Zhao, Y. Gu, G. Yang and X. Dong, Adv. Funct. Mater., 2025, 35, e10861 CrossRef CAS.
- N. Xu, K. Liu, Q. Liu, Q. Wang, A. Zhu and L. Fan, Sci. Rep., 2024, 14, 8183 CrossRef CAS.
- H. Zhao, L. Liu, B. Lu, M. Wu, H. Lu, J. Kang, J. Su, X. Jiang, Y. Wang, H. Miao, H. Zhu, Y. Dong and Y. Zhu, Appl. Catal., B, 2025, 377, 125498 CrossRef CAS.
- Q. Sun, S. Jiang, H. Niu, Y. Li, X. Wu, Y. Shi and Y. Cai, J. Mater. Chem. A, 2024, 12, 5254 RSC.
- S. He, Q. Rong, H. Niu and Y. Cai, Chem. Commun., 2017, 53, 9636 RSC.
- S. He, B. Yin, H. Niu and Y. Cai, Appl. Catal., B, 2018, 239, 147–153 CrossRef CAS.
- R. Gogoi, S. K. Jena, A. Singh, K. Sharma, K. Khanna, S. Chowdhury, R. Kumar and P. F. Siril, J. Environ. Chem. Eng., 2024, 12, 112006 CrossRef CAS.
- Y. Zhang, S. Zhang, Y. Lin, S. Wu, X. Li and C. Yang, Environ. Res., 2025, 272, 121152 CrossRef CAS.
- G. Si, L. Zhang, J. Gao, J. Yang, A. Miyazawa and Y. Peng, Environ. Res., 2024, 262, 119912 CrossRef CAS.
- D. Li and W. Shi, Chin. J. Catal., 2016, 37, 792–799 CrossRef CAS.
- X. Bai, W. Chen, B. Wang, T. Sun, B. Wu and Y. Wang, Int. J. Mol. Sci., 2022, 23, 8130 CrossRef CAS PubMed.
- R. Wang, J. Zhao, Q. Fang and S. Qiu, Chem. Synth., 2024, 4, 29 Search PubMed; H. Zhou, K. Li, Q. Pan, Z. Su and R. Wang, Nanomaterials, 2024, 14, 1907 CrossRef CAS.
- X. Chen, W. Cui, R. Liang, C. Zhang, R. Xu, W. Jiang and J. Qiu, ACS Appl. Bio Mater., 2021, 4, 6502–6511 CrossRef CAS PubMed.
- Z. Shi, Z. Chen, Y. Zhang, X. Wang, T. Lu, Q. Wang, Z. Zhan and P. Zhang, Sep. Purif. Technol., 2022, 288, 120717 CrossRef CAS.
- F. Liu, Z. Ma, Y. Deng, M. Wang, P. Zhou, W. Liu, S. Guo, M. Tong and D. Ma, Environ. Sci. Technol., 2021, 55, 5371–5381 Search PubMed.
- M. Motamedi, L. Yerushalmi, F. Haghighat and Z. Chen, Chemosphere, 2022, 296, 133688 CrossRef CAS PubMed.
- J. Sun, Q. Mu, H. Kimura, V. Murugadoss, M. He, W. Du and C. Hou, Adv. Compos. Hybrid Mater., 2022, 5, 627–640 Search PubMed.
- Y. You, X. Shi, L. Huang, J. Zhao, W. Ji, L. Li, D. Bu and S. Huang, Mater. Horiz., 2025, 12, 2965 RSC.
- R. Gao, X. Kou, L. Tong, Z.-W. Li, Y. Shen, R. He, L. Guo, H. Wang, X. Ma, S. Huang, G. Chen and G. Ouyang, Angew. Chem., Int. Ed., 2024, 63, e202319876 CrossRef CAS.
- R. Liu, H. Hao, K. Zhang, X. Li, Z. Guo and X. Lang, Appl. Catal., B, 2026, 382, 125975 CrossRef CAS.
- H. Wang, L. Song, M. Xu, F. Zhang, Z. Liu, G. Zheng and Q. Han, Angew. Chem., Int. Ed., 2025, e202517545 CAS.
- C. Sun, L. Karuppasamy, L. Gurusamy, H.-J. Yang, C.-H. Liu, J. Dong and J. J. Wu, Sep. Purif. Technol., 2021, 271, 118873 CrossRef CAS.
- L. Yang, Y. Wang, J. Yuan, G. Wang, Q. Cao, H. Fei, M. Li, J. Shao, H. Li and J. Lu, Chem. Eng. J., 2022, 446, 137095 CrossRef.
- M. Deng, L. Wang, Z. Wen, J. Chakraborty, J. Sun, G. Wang and P. Van Der Voort, Green Chem., 2024, 26, 3239–3248 RSC.
- H. Zhou, K. Li, Q. Pan, Z. Su and R. Wang, Nanomaterials, 2024, 14, 1907 CrossRef CAS PubMed.
- Q. Guan, L. Zhou, W. Li, Y. Li and Y. Dong, Chem.–Eur. J., 2020, 26, 5583–5591 CrossRef CAS PubMed.
- J. Feng, W. Ren, F. Kong and Y. Dong, Inorg. Chem. Front., 2021, 8, 848–858 RSC.
- J. Zhao, C. Zhang, Z. Qiu, Z. Zhang, X. Lin, S. Huang, J. Zhang, J. Wu, L. Liao and R. Wang, Sensors, 2025, 25, 2304 CrossRef CAS.
- G. Wang, S. Li, C. Yan, F. Zhu, Q. Lin, K. Xie, Y. Geng and Y. Dong, J. Mater. Chem. A, 2020, 8, 6957–6965 Search PubMed.
- M. Lismont, L. Dreesen and S. Wuttke, Adv. Funct. Mater., 2017, 27, 1606314 CrossRef.
- G. Gao, Y. Wang, Y. Jiang, S. Luo, M. Li, Y. Cao, Y. Ma and B. Tang, Inorg. Chem. Front., 2024, 11, 1186–1197 RSC.
- P. Tong, J. Yang, Y. Zhou, Y. Tang, M. Tang, Y. Zang, Y. Pan, L. Dong, Y. Tan, K.-T. Nam, X. Hu, H. Huang, J. Li, H. Wang, T. D. James, J. Yoong and X. He, Coord. Chem. Rev., 2025, 526, 216381 CrossRef CAS.
- L. Zhang, S. Wang, Y. Zhou, C. Wang, X.-Z. Zhang and H. Deng, Angew. Chem., Int. Ed., 2019, 58, 14213–14218 CrossRef CAS.
- T. Luan, L. Du, J. Wang, K. Li, Q. Zhang, P. Li and Y. Zhao, ACS Nano, 2022, 16, 21565–21575 Search PubMed.
- S. Chen, T. Sun, M. Zheng and Z. Xie, Adv. Funct. Mater., 2020, 30, 2004680 Search PubMed.
- P. Gao, M. Wang, Y. Chen, W. Pan, P. Zhou, X. Wan, N. Li and B. Tang, Chem. Sci., 2020, 11, 6882–6888 RSC.
- J. Wang, L. Bai, T. Huang, Y. Wang, Z. Cheng, Q. Liu, X. Su, L. Zhao and F. Lu, J. Colloid Interface Sci., 2024, 673, 679–689 CrossRef CAS PubMed.
- D. Dutta, J. Wang, X. Li, Q. Zhou and Z. Ge, Small, 2022, 18, 2202369 CrossRef CAS.
- Q. Zhou, G. Huang, J. Si, Y. Wu, S. Jin, Y. Ji and Z. Ge, ACS Nano, 2024, 18, 35671–35683 CrossRef CAS PubMed.
- H. Wei, X. Li, F. Huang, S. Wu, H. Ding, Q. Chen, M. Li and X. Lang, Chin. Chem. Lett., 2023, 34, 108564 CrossRef CAS.
- S. Zhao, X. Guo, X. Pan, Y. Huang and R. Cao, Chem. Eng. J., 2023, 457, 141017 CrossRef CAS.
- B. Sun, Z. Ye, M. Zhang, Q. Song, X. Chu, S. Gao, Q. Zhang, C. Jiang, N. Zhou, C. Yao and J. Shen, ACS Appl. Mater. Interfaces, 2021, 13, 42396–42410 CrossRef CAS.
- Y. Tai, Z. Chen, T. Luo, B. Luo, C. Deng, Z. Lu, S. Wen and J. Wang, Adv. Healthcare Mater., 2024, 13, 2303911 CrossRef CAS.
- S. Liang, M. Li, M. Qi, H. Hui, H. Zhang, J. Zhou, L. Wang and Y. Yang, Nano Lett., 2024, 24, 13708–13717 CrossRef CAS PubMed.
- C. Hu, Z. Zhang, S. Liu, X. Liu and M. Pang, ACS Appl. Mater. Interfaces, 2019, 11, 23072–23082 Search PubMed.
- H. He, L. Du, H. Xue, J. Wu and X. Shuai, Acta Biomater., 2022, 149, 297–306 CrossRef CAS.
- Y. Tang, L. Ge, L. Jiang and X. Jiang, Nano Today, 2024, 55, 102166 CrossRef CAS.
- F. Al-dolaimy, S. K. Saraswat, B. A. Hussein, U. A. Hussein, S. M. Saeed, A. T. Kareem, A. S. Abdulwahid, T. L. Mizal, K. Muzammil, A. H. Alawadi, A. Alsalamy, F. Hussin and M. H. Kzar, Micron, 2024, 179, 103595 CrossRef CAS.
- Y. He, G. Huang, X. Guo, S. Chen, Q. Chen, W. Yang, W. Peng, X. Xu, J. Ye, J. Bi and P. K. Wong, Water Res., 2025, 285, 124112 CrossRef CAS.
- Y. Zhu, L. Qin, M. Yang, Z. Shi, H. Chen, N. Wen, Y. Wang, J. Long, S. Han, M. Zhu and H. Xi, Small, 2025, 21, 2412118 CrossRef CAS.
- F. Wang, H. Zhang, S. Ma, D. Kong, P. Hao and L. Zhu, J. Colloid Interface Sci., 2025, 693, 137593 CrossRef CAS PubMed.
- Z. Ding, J. Yang, Z. Wu, M. Adeli, X. Luo, X. Wang, X. Xie, X. Xu, C. Cheng and C. Zhao, Chem. Mater., 2025, 37, 1972–1982 CrossRef CAS.
- C. Zhang, J. Guo, X. Zou, S. Guo, Y. Guo, R. Shi and F. Yan, Adv. Healthcare Mater., 2021, 10, 2100775 CrossRef CAS PubMed.
- B. Ma, X. Lin, T. Zhu, X. Zheng and J. Zhu, Colloids Surf., B, 2024, 242, 114101 CrossRef CAS PubMed.
- H. Chen, S. Gao, G. Huang, Q. Chen, Y. Gao and J. Bi, Appl. Catal., B, 2024, 343, 123545 CrossRef CAS.
- J. Li, H. Jin, T. Qin, F. Liu, S. Wu and L. Feng, ACS Nano, 2024, 18, 4539–4550 Search PubMed.
- Z. Zhou, C. Liu, C. Cai, Y. Wei, K. Li, X. Yu, Y. Liu and N. Wang, J. Environ. Chem. Eng., 2024, 12, 113533 Search PubMed.
- B. Sun, X. Wang, Z. Ye, J. Zhang, X. Chen, N. Zhou, M. Zhang, C. Yao, F. Wu and J. Shen, Adv. Sci., 2023, 10, 2207507 Search PubMed.
- J. Liu, C. Guo, Z. Liu, F. Cheng, S. Zhang and Z. Zhang, Chem. Eng. J., 2024, 494, 153139 CrossRef CAS.
- A. Santhana Krishna Kumar, W.-B. Tseng, E. Arputharaj, P.-J. Huang, W. Tseng and T. Bajda, ACS Sustainable Chem. Eng., 2023, 11, 6956–6969 CrossRef.
- Y. Liu, Y. Li, L. Jiao, Y. Kang, B. Du, W. Cai, H. Cui and R. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 65907–65917 CrossRef CAS.
- Y. Zhang, X. Xu, Q. Liao, Q. Wang, Q. Han, P. Chen and K. Xi, J. Mater. Chem. B, 2022, 10, 3285–3294 RSC.
- P. Li, B. Li, C. Wang, X. Zhao, Y. Zheng, S. Wu, J. Shen, Y. Zhang and X. Liu, Composites, Part B, 2023, 252, 110506 CrossRef CAS.
Footnote |
| † Tao Sun and Rui Wang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |

