Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemoselectivity in the cationic Phospha-Wittig reaction: accessing phosphorus heterocycles, phosphaalkenes, and their annulated [4 + 2] dimers
Philipp Royla a,
Kai Schwedtmann
a,
Kai Schwedtmann a,
Rosa M. Gomila
a,
Rosa M. Gomila b,
Antonio Frontera
b,
Antonio Frontera b and
Jan J. Weigand
b and
Jan J. Weigand *a
*a
aFaculty of Chemistry and Food Chemistry, Technische Universität Dresden, 01062 Dresden, Germany. E-mail: jan.weigand@tu-dresden
bDepartment of Chemistry, Universitat de Illes Balears, 07122 Palma de Mallorca, Spain
First published on 26th December 2025
Abstract
Triflate salts of phosphito-phosphanides [LCP–P(OR)3]+ (1[OTf], R = alkyl, LC = N-heterocyclic carbene) were obtained via nucleophilic fragmentation of the tetraphosphetane [(LC)4P4][OTf]4 (3[OTf]4) with organophosphites P(OR)3. The salts 1[OTf] act as versatile reagents in the cationic Phospha-Wittig reaction, converting aldehydes into imidazoliumyl-substituted phosphaalkenes 2[OTf] and, via a competing pathway, into diphosphiranes 4[OTf]2. The product distribution is governed by the aldehyde substituent, enabling selective access to isolable derivatives of both compound classes. The resulting phosphaalkenes 2[OTf] serve as precursors to diverse phosphorus heterocycles, undergoing expected [2 + 2] dimerisation to 1,3-diphosphetanes syn/anti-(2)2[OTf]2 and trapping reactions with 1,3-dienes to yield the tetrahydrophosphinine 8[OTf] and bicyclic derivative 9[OTf]. Most notably, an unprecedented annulative [4 + 2] dimerisation pathway for cationic C-aryl phosphaalkenes is uncovered that furnishes benzannulated tetrahydro-1,2-diphosphinines 7[OTf]2. Computational studies reveal that the operative mechanism of this transformation involves a Phospha-Diels–Alder step followed by an acid–base-catalytic proton transfer, which is calculated to be energetically more accessible than the classical [2 + 2] dimerisation.
Introduction
Phosphaalkenes are defined by the presence of a localized phosphorus–carbon double bond (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). Despite the electronegativity difference between the two elements, the 2p-3p π-bond in these molecules is nearly apolar,1 imparting phosphaalkenes with reactivity patterns more closely resembling those of alkenes than imines.2,3 A small HOMO–LUMO gap in phosphaalkenes confers a unique electronic structure that makes them attractive as scaffolds for transition metal-mediated catalysis and as versatile building blocks in polymer synthesis and organophosphorus chemistry.2,4–6
C). Despite the electronegativity difference between the two elements, the 2p-3p π-bond in these molecules is nearly apolar,1 imparting phosphaalkenes with reactivity patterns more closely resembling those of alkenes than imines.2,3 A small HOMO–LUMO gap in phosphaalkenes confers a unique electronic structure that makes them attractive as scaffolds for transition metal-mediated catalysis and as versatile building blocks in polymer synthesis and organophosphorus chemistry.2,4–6
In the absence of sufficient electronic or steric stabilisation, the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond engages in dimerisation reactions (Fig. 1). The most commonly observed pathway is a [2 + 2] cycloaddition, occurring either in a head-to-head or head-to-tail fashion to furnish 1,2- or 1,3-diphosphetanes A or B, respectively.7–11 Alternative dimerisation modes are reported for conjugated 1-phosphabutadienes, which can behave as both dienes and dienophiles in [4 + 2] Phospha-Diels–Alder reactions. Depending on the phosphorus substituent, distinct regioisomeric outcomes are observed. Sterically small substituents favour P–P bond formation to give diphosphacyclohexenes C (R1 = Me, Cy, tBu, Ph),12,13 whereas the bulky Mes* (Mes* = 2,4,6-tBu3C6H2) group promotes formation of phosphaalkene-tethered phosphacyclohexenes D.14
C double bond engages in dimerisation reactions (Fig. 1). The most commonly observed pathway is a [2 + 2] cycloaddition, occurring either in a head-to-head or head-to-tail fashion to furnish 1,2- or 1,3-diphosphetanes A or B, respectively.7–11 Alternative dimerisation modes are reported for conjugated 1-phosphabutadienes, which can behave as both dienes and dienophiles in [4 + 2] Phospha-Diels–Alder reactions. Depending on the phosphorus substituent, distinct regioisomeric outcomes are observed. Sterically small substituents favour P–P bond formation to give diphosphacyclohexenes C (R1 = Me, Cy, tBu, Ph),12,13 whereas the bulky Mes* (Mes* = 2,4,6-tBu3C6H2) group promotes formation of phosphaalkene-tethered phosphacyclohexenes D.14
Synthetic access to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C motif is achieved by approaches that mirror classical organic transformations used to construct C
C motif is achieved by approaches that mirror classical organic transformations used to construct C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds.9 A historically important method is the Phospha-Wittig reaction, which involves the transfer of a phosphinidene fragment from phosphanylidene-phosphoranes, R1P–PR3 or R1P[M]–PR3 (E, M = metal fragment), or -phosphonates, R1P–P(O)(OR)2 or R1P[M]–P(O)(OR)2 (F, M = metal fragment) to a carbonyl compound (Fig. 1).15–18
C double bonds.9 A historically important method is the Phospha-Wittig reaction, which involves the transfer of a phosphinidene fragment from phosphanylidene-phosphoranes, R1P–PR3 or R1P[M]–PR3 (E, M = metal fragment), or -phosphonates, R1P–P(O)(OR)2 or R1P[M]–P(O)(OR)2 (F, M = metal fragment) to a carbonyl compound (Fig. 1).15–18
We are investigating the chemistry of cationic P-imidazoliumyl-substituted phosphaalkenes, accessible via an adapted route from the reaction of phosphonio-phosphanides [LCP–PR3]+ (R = aryl, alkyl, LC = N-heterocyclic carbene) with thiocarbonyls.19 The imidazoliumyl group stabilises the low-coordinated phosphorus environment and simultaneously acts as a leaving group, permitting post-synthetic modification.20,21 This modularity could provide a general route to tailor phosphaalkenes for specific electronic or steric environments from a common precursor, avoiding optimization of multi-step procedures. However, extending this approach to prepare C–H-functionalised phosphaalkenes 2+ from aldehydes via a cationic Phospha-Wittig reaction was challenging.
The Horner–Wadsworth–Emmons (HWE) reaction offers a compelling alternative to the classical Wittig reaction for the construction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds.22 This well-established method employs phosphonate-stabilised carbanions, which are more nucleophilic than their ylide counterparts. Indeed, the heavier metal-coordinated phosphorus analogues F (Fig. 1) readily react with ketones, while the reactivity of E is limited to aldehydes. Inspired by this logic, we targeted phosphito-phosphanides 1+ and hypothesized that they could react with aldehydes to give access to imidazoliumyl-substituted phosphaalkenes 2+.
C double bonds.22 This well-established method employs phosphonate-stabilised carbanions, which are more nucleophilic than their ylide counterparts. Indeed, the heavier metal-coordinated phosphorus analogues F (Fig. 1) readily react with ketones, while the reactivity of E is limited to aldehydes. Inspired by this logic, we targeted phosphito-phosphanides 1+ and hypothesized that they could react with aldehydes to give access to imidazoliumyl-substituted phosphaalkenes 2+.
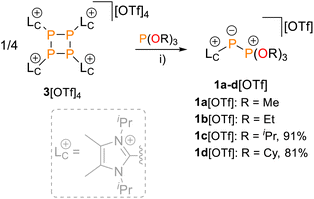
We now report the synthesis of isolable triflate salts of 1+ via the nucleophilic fragmentation of tetraphosphetane [(LC)4P4][OTf]4 (3[OTf]4) by organophosphites P(OR)3 (R = alkyl). Derivatives of 1+ enable the cationic Phospha-Wittig reaction with a range of aldehydes for the first time. We systematically assess the chemoselectivity of this transformation leading to selective access to isolable phosphaalkenes and diphosphiranes. In addition, we uncover a hitherto unknown [4 + 2] dimerisation pathway of C-aryl substituted phosphaalkenes that furnishes benzannulated tetrahydro-1,2-diphosphinines. Computational studies reveal an underlying Phospha-Diels–Alder mechanism succeeded by a proton shift mediated by acid-base catalysis. Crucially, our findings highlight that cationically charged P-imidazoliumyl-substituted phosphaalkenes unlock new reactivity profiles that are inaccessible to their neutral counterparts.
Results and discussion
Synthesis of phosphito-phosphanides
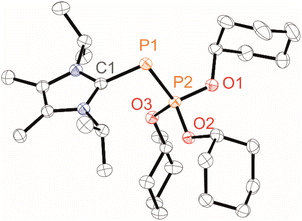
Treating 3[OTf]4 with a slight excess (4.2 equiv.) of organophosphites P(OR)3 (R = Me, Et, iPr, Cy) in CH3CN or CD2Cl2 gave pale-yellow solutions after 16 h at room temperature (Scheme 1). In all cases, 31P NMR spectroscopic investigations of aliquots removed from the reaction mixture revealed the complete conversion of 3[OTf]4. The products displayed the expected AX spin systems with characteristically shielded resonances of the A parts and were assigned to 1a–d[OTf] [δ(31PA) = −199.4–(–182.4) ppm, δ(31PX) = 78.3–90.2 ppm, 1J(PP) = −608–(–601) Hz; see the SI for details, Section S2.1].19,23Whereas work-up of 1a,b[OTf] led to their decomposition to unidentified products and starting material (see the SI, Fig. S1), analytically pure 1c,d[OTf] were obtained in isolated yields of 91% and 81%, respectively. Their synthesis is conveniently adaptable to a multigram scale (∼3.5 g). Structural confirmation of 1d[OTf] was achieved through single-crystal X-ray diffraction analysis after recrystallisation from a saturated C6H5F/Et2O solution at room temperature (Fig. 2). The observed P–P bond length [2.0832(5) Å] is markedly shorter than those reported for cationic phosphonio-phosphanides [LCP–PR3]+ [R = alkyl, 2.1162(4)–2.1446(4) Å]19 and falls at the lower end of the range for structurally characterised phosphanylidene-phosphoranes (2.06–2.15 Å),23 approaching values characteristic of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P double bonds (cf. P
P double bonds (cf. P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P 2.04 Å, P–P 2.22 Å).24,25
P 2.04 Å, P–P 2.22 Å).24,25
Phosphito-phosphanides as Phospha-Wittig reagents
The reactivity of 1c,d[OTf] was tested in reactions with an excess (2–10 equiv.) of a series of aldehydes R1CHO in CH3CN solution (Scheme 2). 31P NMR spectroscopic analysis of aliquots removed from the reaction mixtures after 4 h to 7 d (see Table 1) showed successful Phospha-Wittig conversion to the phosphoric acid esters, OP(OiPr)3 [δ(31P) = −2.8 ppm]26 or OP(OCy)3 [δ(31P) = −2.5 ppm], and phosphaalkenes E-2a–i+, indicated by diagnostic, deshielded resonances [δ(31P) = 173.8–200.5 ppm, 2J(PH) = 14–23 Hz, see the SI, Fig. S13]. Minor, slightly upfield-shifted [δ(31P) = 154.0–176.6 ppm] resonances were assigned to the corresponding Z-isomers due to the characteristically large 2J(PH) coupling constant [2J(PH) = 43–44 Hz].27 The reactions are highly stereoselective with typical E/Z ratios > 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The 31P NMR resonances of phosphaalkenes 2a–i+ are shifted to lower frequencies compared to the reported values of neutral C–H substituted phosphaalkenes [δ(31P) = avg. 250 ppm].8,16,18,28,29 Similar trends are known for inversely polarized phosphaalkenes.30
3. The 31P NMR resonances of phosphaalkenes 2a–i+ are shifted to lower frequencies compared to the reported values of neutral C–H substituted phosphaalkenes [δ(31P) = avg. 250 ppm].8,16,18,28,29 Similar trends are known for inversely polarized phosphaalkenes.30
| R1 = | Reagent | Timea | Equiv. | 2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42+ ratio 42+ ratio |
δ(31P):E-2+, Z-2+ in ppm | 2J(PH):E-2+, Z-2+ in Hz | |
|---|---|---|---|---|---|---|---|
| a Time required for full conversion of 1c,d[OTf] at room temperature, if not specified differently.b 80 °C. | |||||||
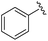 |
2a+ | 1c[OTf] | 7d | 5.0 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
178.2, 154.0 | 19, 44 |
| 1d[OTf] | 7d | 5.0 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
||||
| 1c[OTf] | 4hb | 5.0 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57 |
||||
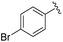 |
2b+ | 1c[OTf] | 5d | 5.0 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
182.5, 157.8 | 21, 43 |
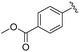 |
2c+ | 1c[OTf] | 4d | 5.0 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
191.5, 167.0 | 22, 44 |
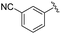 |
2d+ | 1c[OTf] | 4d | 5.0 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
192.0, 168.4 | 22, 43 |
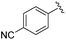 |
2e+ | 1c[OTf] | 16h | 5.0 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
197.5, 172.3 | 20, 43 |
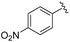 |
2f+ | 1c[OTf] | 16h | 5.0 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
200.5, 175.5 | 22, 43 |
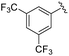 |
2g+ | 1c[OTf] | 16h | 5.0 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
200.3, 176.6 | 23, 43 |
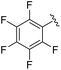 |
2h+ | 1c[OTf] | 4h | 2.0 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
215.5, – | 20, – |
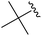 |
2i+ | 1c[OTf] | 4d | 10.0 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
173.8, – | 14, – |
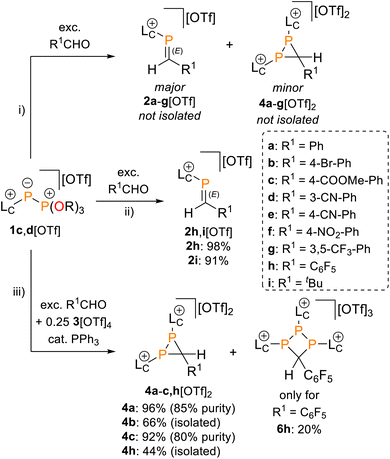
In addition to phosphaalkenes, the 31P{1H} NMR spectra of most reaction mixtures (for 2a–g+) showed sets of shielded resonances of AX spin systems [e.g., R1 = Ph (a): δ(31PA) = −174.1 ppm, δ(31PX) = −138.9 ppm, 1J(PP) = 128 Hz, 2J(PXH) = 31 Hz] in line with the formation of diphosphiranes 4a–g2+ (Scheme 2, for the integral ratio see Table 1).31 Diphosphirane formation was rationalised by cyclopropanation of the in situ–formed phosphaalkenes by phosphinidene [LC–P]+, transferred from 1c,d[OTf], in a ligand displacement reaction.19,32,33 Supporting evidence includes 31P NMR resonances consistent with the transient formation of free P(OiPr)3 [δ(31P) = 140.7 ppm] over the course of the conversion (see the SI, Fig. S14). In the presence of excess aldehyde, released P(OiPr)3 is consumed in an Abramov reaction34 to produce diisopropyl (isopropoxy(aryl)methyl)phosphonates (iPrO)2OP–C(Ar)OiPr [δ(31P) = ca. 25.0 ppm; cf. (MeO)2OP–C(Ph)OMe: δ(31P) = 21.9 ppm].35 Conversion rates of the Phospha-Wittig reaction were accelerated by elevated temperatures, increasing diphosphirane formation, albeit at the expense of lower chemoselectivity, and the formation of several other unidentified products (see the SI, Section S2.6). Notably, while diphosphiranes are typically not observed in the Phospha-Wittig reaction using phosphanylidene-phosphoranes, Gates et al. reported diphosphirane formation in the Phospha–Peterson reaction.8
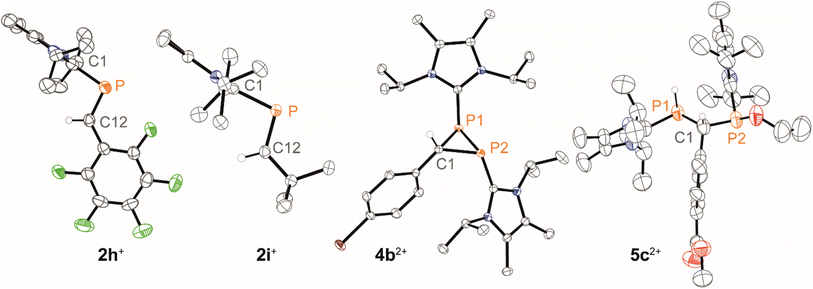
In general, reactions of 1c[OTf] with aldehydes bearing electron-withdrawing groups or tBuCHO favoured phosphaalkene formation (see Table 1), while reactions with 4-methoxybenzaldehyde or mesitylaldehyde did not result in satisfactory phosphaalkene or diphosphirane production (see the SI, Section S2.7). Similar solubilities of the triflate salts of phosphaalkenes 2a–g+ and diphosphiranes 4a–g2+ prevented their separation. However, the phosphaalkenes 2h,i+ [R1 = C6F5 (2h+), tBu (2i+)] were obtained chemoselectively leading to the isolation of their triflate salts in excellent yields of 98% and 91%, respectively. The E-configuration of the cations was verified by single-crystal X-ray diffraction analysis (Fig. 3). In the solid state the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond lengths {2h[OTf]: P1–C12 1.692(4) Å, 2i[OTf]: P1–C12 1.671(2) Å} are similar to other structurally characterised, C–H substituted phosphaalkenes (1.61–1.71 Å),36 for instance E-Mes*P
C double bond lengths {2h[OTf]: P1–C12 1.692(4) Å, 2i[OTf]: P1–C12 1.671(2) Å} are similar to other structurally characterised, C–H substituted phosphaalkenes (1.61–1.71 Å),36 for instance E-Mes*P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)Ph [P
C(H)Ph [P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C 1.660(6) Å],29 and close to the calculated values for the parent HP
C 1.660(6) Å],29 and close to the calculated values for the parent HP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 [P
CH2 [P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C 1.652 Å (at the 6-31G* level of theory)].37 We also note that the P
C 1.652 Å (at the 6-31G* level of theory)].37 We also note that the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in 2h[OTf] is co-planar with the C6F5 substituent, indicating a delocalization of the π-electron density.9
C bond in 2h[OTf] is co-planar with the C6F5 substituent, indicating a delocalization of the π-electron density.9
The direct and chemoselective formation of the diphosphiranes 4a–c2+ was achieved independently by reacting 1c[OTf] with the corresponding aldehydes in the presence of 0.25 equivalents of 3[OTf]4 and catalytic PPh3, as a source of [LC–P]+ (Scheme 2). After work-up, the triflate salts 4a,c[OTf]2 were obtained as crude solids (purity 80–85%, determined by 31P NMR spectroscopy), enabling the unambiguous assignment of all atoms with multinuclear NMR analysis. Efforts to purify these solids by washing with various solvent mixtures, recrystallisation, or separation over a silica plug under inert conditions did not improve purity and in some cases resulted in decomposition to unidentified products. In contrast, diphosphirane 4b[OTf]2 precipitated as an analytically pure solid from a saturated C6H5F solution of the crude product and was isolated in 66% yield.
X-ray diffraction analysis of suitable crystals of 4b[OTf]2 verified the molecular structure and the anti-disposition of the two LC-substituents (Fig. 3). The central three-membered ring features acute bond angles [e.g. P1–C1–P2 73.10(5)°] and C–P bond distances [P1–C1 1.8668(13) Å, P2–C1 1.8729(13) Å], consistent with other structurally identified diphosphiranes.19,31,33,38 The P1–P2 bond length [P1–P2 2.2270(5) Å] is at the longer end of comparable structures.
Diphosphiranes are known to engage in ring opening reactions via P–P bond cleavage upon photo- or thermal treatment or in reactions with electro- and nucleophiles.39 Similarly, crude compound 4c[OTf]2 activates the O–H bond in EtOH leading to the isolation of 5c[OTf]2 in 50% yield, verified by analysis of the molecular structure of 5c[OTf]2 by single crystal X-ray diffraction analysis (Fig. 3). The product was isolated as a mixture of two diastereomers and variable temperature NMR experiments showed no interconversion between them in a range of 240–340 K (see the SI, Fig. S32).
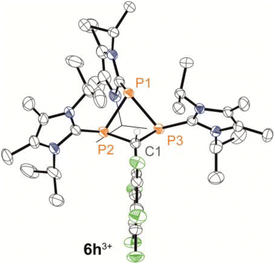
We further conducted experiments of the in situ-generated or isolated phosphaalkenes 2h,i[OTf] with 0.25 equivalents of 3[OTf]4 and catalytic PPh3 to access the diphosphiranes 2h,i[OTf]2. No signs of conversion were observed for 2i+ (R1 = tBu) after over a week of stirring at room temperature or treatment at elevated temperatures (up to 70 °C). In contrast, the reaction of phosphaalkene 2h+ (R1 = C6F5) afforded diphosphirane 4h2+ as the major product. Minor amounts of another previously unobserved product showed sets of resonances in agreement with a AX2 spin system in the 31P{19F} NMR spectrum (see the SI, Sections S2.14 and S2.15), which led to the tentative assignment of the product as triphosphetane 6h[OTf]3 (Scheme 2). A crude solid isolated from the mixture contained both products in a 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 integral ratio. Separation of the products afforded the triflate salts of diphosphirane 4h[OTf]2 and triphosphetane 6h[OTf]3 as analytically pure solids in 44% and 20% yield, respectively, with only minimal contamination (<5%) of the other product according to multinuclear NMR analysis. Attempts to bias the product distribution to selectively form 6h[OTf]3 by performing the reaction with 0.5 equivalents of 3[OTf]4 did not lead to a meaningful difference in the observed chemoselectivity (see the SI, Section S2.3). Single crystal X-ray diffraction analysis of both products unambiguously confirmed their molecular structures (for 4h[OTf]2 see the SI, Fig. S49; for 6h[OTf]3 see Fig. 4). The formation of 6h[OTf]3 underpins the previously observed capability of 3[OTf]4 to simultaneously act as a source of [LC–P]+ and [(LC–P)2]2+.21,40
24 integral ratio. Separation of the products afforded the triflate salts of diphosphirane 4h[OTf]2 and triphosphetane 6h[OTf]3 as analytically pure solids in 44% and 20% yield, respectively, with only minimal contamination (<5%) of the other product according to multinuclear NMR analysis. Attempts to bias the product distribution to selectively form 6h[OTf]3 by performing the reaction with 0.5 equivalents of 3[OTf]4 did not lead to a meaningful difference in the observed chemoselectivity (see the SI, Section S2.3). Single crystal X-ray diffraction analysis of both products unambiguously confirmed their molecular structures (for 4h[OTf]2 see the SI, Fig. S49; for 6h[OTf]3 see Fig. 4). The formation of 6h[OTf]3 underpins the previously observed capability of 3[OTf]4 to simultaneously act as a source of [LC–P]+ and [(LC–P)2]2+.21,40
Dimerisation of imidazoliumyl-phosphaalkenes
While phosphaalkene 2h[OTf] can be isolated as a monomer from solutions of CH3CN, colorless precipitates are obtained in solvents of lower polarity, i.e. THF or C6H5F, after 16 hours at room temperature.The 31P NMR spectrum of this solid in CH3CN shows two broadened resonances with low frequencies at δ(31P) = 19.0 ppm {anti-(2h)2[OTf]2} and δ(31P) = −10.9 ppm {syn-(2h)2[OTf]2} in a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio, indicating formation of dimers of 2h+ and loss of P
50 ratio, indicating formation of dimers of 2h+ and loss of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond character (Scheme 3). Recrystallisation of the solid afforded two sets of colorless crystals suitable for X-ray diffraction analysis, confirming the formation of a syn- and anti-diastereomer of 1,3-diphosphetane syn/anti-(2h)2[OTf]2, respectively (Fig. 5). The syn/anti nomenclature refers to the relative position of the transannular imidazoliumyl substituents at the P2C2 core. Selected structural parameters are given in Fig. 5. Most importantly, the central P2C2 core in anti-(2h)2[OTf]2 adopts a planar geometry [Σ°(P2C2) = 360°, C1–P1–C1 86.30(8)°, P1–C1–P2 93.70(8)°], while the P2C2 core in syn-(2h)2[OTf]2 arranges in a butterfly motif with significantly reduced bond angles [C1–P1–C2 avg. 82.07°, P1–C1–P2 avg. 85.95°].
C double bond character (Scheme 3). Recrystallisation of the solid afforded two sets of colorless crystals suitable for X-ray diffraction analysis, confirming the formation of a syn- and anti-diastereomer of 1,3-diphosphetane syn/anti-(2h)2[OTf]2, respectively (Fig. 5). The syn/anti nomenclature refers to the relative position of the transannular imidazoliumyl substituents at the P2C2 core. Selected structural parameters are given in Fig. 5. Most importantly, the central P2C2 core in anti-(2h)2[OTf]2 adopts a planar geometry [Σ°(P2C2) = 360°, C1–P1–C1 86.30(8)°, P1–C1–P2 93.70(8)°], while the P2C2 core in syn-(2h)2[OTf]2 arranges in a butterfly motif with significantly reduced bond angles [C1–P1–C2 avg. 82.07°, P1–C1–P2 avg. 85.95°].
In line with metric parameters found in other structurally characterised 1,3-diphosphetanes, both diastereomers feature long P–C bond lengths [anti-(2h)2[OTf]2: P1–C1 1.8783(19) Å, P1–C2 1.9027(19) Å; syn-(2h)2[OTf]2: P1–C1 avg. 1.891 Å, P1–C2 avg. 1.894 Å, cf. P–C 1.855 Å41] reflecting strained ring systems.31,33,38 Importantly, this dimerisation is driven by the polarity of the solvent, reflecting another dimension of control for the dimerisation of cationic phosphaalkenes, next to steric and electronic stabilisation of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. Although representatives of 1,3-diphosphetanes have been structurally verified in both configurations,10,11,19,42 to the best of our knowledge (2h)2[OTf]2 is the first 1,3-diphosphetane for which both diastereomers are characterised by single crystal X-ray diffraction. Isolated syn-(2h)2[OTf]2 was obtained upon fractional recrystallisation and only mixed fractions were obtained for anti-(2h)2[OTf]2. No interconversions between the diastereomers or to the monomer were observed in a temperature range from 240–340 K using multinuclear NMR spectroscopy (see the SI, Fig. S61).
C double bond. Although representatives of 1,3-diphosphetanes have been structurally verified in both configurations,10,11,19,42 to the best of our knowledge (2h)2[OTf]2 is the first 1,3-diphosphetane for which both diastereomers are characterised by single crystal X-ray diffraction. Isolated syn-(2h)2[OTf]2 was obtained upon fractional recrystallisation and only mixed fractions were obtained for anti-(2h)2[OTf]2. No interconversions between the diastereomers or to the monomer were observed in a temperature range from 240–340 K using multinuclear NMR spectroscopy (see the SI, Fig. S61).
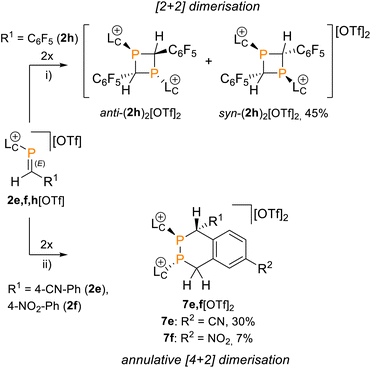
Attempts to isolate the phosphaalkenes 2e,f+ from their reaction mixtures led to the serendipitous formation of annulated tetrahydro-1,2-diphosphinines 7e,f[OTf]2 (Scheme 3), which were obtained as crude solids in up to 85% (7e[OTf]2, 80–90% purity) and 58% yield (7f[OTf]2, 95% purity), respectively. The main impurities of the crude compounds were identified as the respective 1,3-diphosphetanes syn/anti-(2e,f)2[OTf]2 and the formation of syn-(2e)2[OTf]2 could be verified crystallographically (see the SI, Fig. S65). Analytically pure samples of 7e,f[OTf]2 were obtained by recrystallisation of the crude products, allowing the confirmation of their molecular structures by single crystal X-ray diffraction analysis (Fig. 5 and S84 in the SI). In the solid state the central six-membered ring of 7e2+ adopts a distorted boat conformation with anti-oriented imidazoliumyl substituents. Bond lengths and angles are as expected. Notably, the P–P bond distance [2.2073(6) Å] is consistent with a P–P single bond (Σrcov. = 2.22 Å),24 the only other structurally characterised annulated tetrahydro-1,2-diphosphinine [P–P 2.2072(7) Å],43 and monocyclic 1,2,3,6-tetrahydrodiphosphinines.44,45 The diastereoselective formation of the stereogenic centers at P1, P2 and C1 in 7e,f[OTf]2 is supported by single sets of 31P NMR resonances that were iteratively fitted to AB spin systems [7e2+ (CD2Cl2, 300 K): exp., δ(31PAB): centered at −51.1 ppm; iteratively fitted, δ(31PA) = −52.2 ppm, δ(31PB) = −50.4 ppm, 1J(PP) = −190 Hz; 7f2+ (CD3CN, 300 K): exp., δ(31PAB): centered at −53.2 ppm; δ(31PA) = −55.8 ppm, δ(31PB) = −52.2 ppm, 1J(PP) = −193 Hz]. Additionally, the diastereotopic H4 protons are anisochronous at 300 K [7e2+: δ(1H4ax) = 4.52 ppm, δ(1H4eq) = 3.70 ppm, 2J(HH) = 15.1 Hz, 7f2+: δ(1H4ax) = 4.16 ppm, δ(1H4eq) = 3.84 ppm, 2J(HH) = 15.6 Hz], consistent with the absence of detectable conformational changes in solution (for details see the SI, Section S2.17 and S2.18). The diastereoselective formation of the six-membered ring in 7e,f2+ suggests a [4 + 2] dimerisation via a Phospha-Diels–Alder type reaction to be the operative pathway. Although auto-Phospha-Diels–Alder processes have been reported for 1-phosphabutadienes12–14 and 2H-phospholes46 they are elusive to C-aryl substituted phosphaalkenes as interactions of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds with arenes are exceedingly rare.47 To rationalize this unusual reactivity we performed quantum chemical calculations (at the RI-BP86-D4/def2-TZVP level of theory) on the formation of 7e2+ from two molecules of 2e+, using a truncated imidazoliumyl substituent for computational efficiency (Fig. 6).
C double bonds with arenes are exceedingly rare.47 To rationalize this unusual reactivity we performed quantum chemical calculations (at the RI-BP86-D4/def2-TZVP level of theory) on the formation of 7e2+ from two molecules of 2e+, using a truncated imidazoliumyl substituent for computational efficiency (Fig. 6).
The reaction initiates with a concerted annulative [4 + 2] dimerisation of two molecules of 2e+, proceeding through transition state TS1 with a low activation barrier of 12.1 kcal mol−1. The resulting adduct INT1 is nearly isoenergetic with the reactants (ΔG° = +0.8 kcal mol−1), consistent with a reversible first step. Frontier orbital analysis (see the SI, Fig. S96) supports a normal-electron-demand Phospha-Diels–Alder process, where the HOMO is delocalized over the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (diene) and the LUMO localizes on the P
C bonds (diene) and the LUMO localizes on the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C unit (dienophile).48,49
C unit (dienophile).48,49
From INT1, two mechanistic scenarios were evaluated. An intramolecular 1,3 H shift via transition state TS2′ is kinetically inaccessible, with a barrier of 38.9 kcal mol−1. In contrast, an acid-base pathway mediated by the triflate counterion proved highly favourable. The acidic aryl C–H bond in INT1, destabilised by the electron-withdrawing cyano substituents and overall dicationic framework, is readily deprotonated by [OTf]− (or another weak base in the reaction mixture), giving transition state TS2 (ΔG°‡ = 8.2 kcal mol−1). Subsequent reprotonation at the benzylic position occurs barrierless, directly yielding 7e2+, which is strongly stabilised at −30.5 kcal mol−1 relative to the starting materials. For comparison, the competing head-to-tail [2 + 2] dimerisation was calculated to be less favourable, with transition state TS3 at 31.8 kcal mol−1 for the formation of the corresponding 1,3-diphosphetane syn-(2e)22+. Although the product is thermodynamically stable (ΔG° = −10.6 kcal mol−1), the high kinetic barriers render its formation pathway less viable. Notably, the calculations predict the formation of 1,2-diphosphetane to be more feasible than the 1,3-isomer (see the SI, Fig. S97). This discrepancy, along with the generally high absolute values of some calculated barriers (>30 kcal mol−1), is attributed to steric and electronic effects not fully captured by the truncated imidazoliumyl substituent used in the computational model. The preference for the annulative pathway is consistent with dominant formation of 7e,f[OTf]2 under the mild workup conditions used for 2e,f[OTf].
Trapping reactions with 1,3-dienes
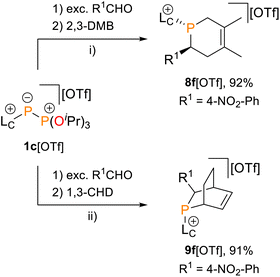
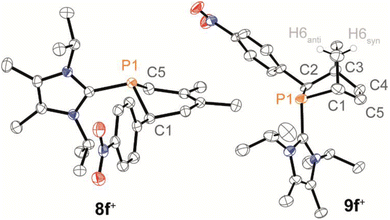
The Phospha-Diels–Alder reaction is an important tool to demonstrate the existence of thermodynamically unstable phosphaalkenes.5,6,17,49,50In this context, phosphaalkene 2f+ was trapped in reactions with dienes, namely 2,3-dimethylbuta-1,3-diene (2,3-DMB) and 1,3-cyclohexadiene (1,3-CHD), affording the anti-1,2,5,6-tetrahydrophosphinine 8f+ and endo-phosphabicyclo[2.2.2]oct-5-ene 9f+, respectively (Scheme 4). Both products were isolated as their triflate salts in excellent yields (92% and 91%), and their molecular structures were confirmed by single-crystal X-ray diffraction analysis (Fig. 7). The solid-state structures show the expected anti arrangement of the LC- and 4-NO2-phenyl substituent in line with the dominant E-configuration of 2f+. Bond lengths and angles fall within the expected ranges [e.g., 8f+: P1–C1 1.854(2) Å, 9f+: P1–C2 1.869(2) Å, cf. P–C 1.855 Å],41 and compare well with structurally related 1,2,3,6-tetrahydrodiphosphinines and diphosphabicyclo[2.2.1]hept-5-enes.45 Compound 9f+ was identified as the endo-diastereomer, and multinuclear NMR spectroscopic investigation supports a diastereoselective formation of both products (see the SI for details, Section S2.20). For instance, the 31P NMR spectra of both heterocycles display only a single resonance at 300 K [8f+: δ(31P) = −47.6 ppm; 9f+: δ(31P) = −29.9 ppm].
Conclusions
To conclude, we present a scalable synthesis of (imidazoliumyl)phosphito-phosphanides 1c,d[OTf] via nucleophilic fragmentation of tetraphosphetane 3[OTf]4. These reagents facilitate the cationic Phospha-Wittig reaction with a broad range of aldehydes for the first time, providing previously inaccessible P-imidazoliumyl-substituted and C–H functionalised phosphaalkenes 2[OTf]. These phosphaalkenes exhibit rich and chemoselective cycloaddition reactivity. They undergo: (I) [2 + 1] cyclopropenation reaction with cationic phosphinidene [LC–P]+ transfer reagents to afford the diphosphiranes 4[OTf] as well as rare triphosphetane 6h[OTf]3; (II) solvent-polarity-controlled [2 + 2] head-to-tail dimerisation to yield 1,3-diphosphetanes (2)2[OTf]2; (III) Phospha-Diels–Alder reactions with 1,3-dienes to tetrahydrophosphinine 8f[OTf] and phosphabicyclo[2.2.2]oct-5-ene 9f[OTf]; and (IV) a hitherto unknown annulative [4 + 2] dimerisation pathway of 2e,f[OTf], furnishing benzannulated tetrahydro-1,2-diphosphinines 7e,f[OTf]2. Mechanistic studies reveal that this transformation proceeds through an auto-Phospha-Diels–Alder step followed by a counter anion-guided proton-transfer and re-aromatisation sequence.Collectively, these findings significantly expand the reactivity landscape of C-aryl-substituted phosphaalkenes and demonstrate that the cationic charge in P-imidazoliumyl phosphaalkenes unlocks new synthetic pathways inaccessible to their neutral analogues, a concept that could provide a blueprint for other transformations in main group chemistry. The new cationic heterocycles presented here are promising platforms for post-functionalisation, and further studies in this direction are underway and will be reported separately.
Author contributions
P. R., K. S., and J. J. W. conceptualized the study; P. R. conducted the experiments and optimized the syntheses, isolations, and purifications; R. G. and A. F. were responsible for mechanistic studies; P. R. and J. J. W. were responsible for X-ray data collection and refinement; K. S. and J. J. W. conceived, oversaw, and directed the project; P. R. prepared the initial draft of the paper; A. F. and J. J. W. secured funding. All authors contributed to data analysis, manuscript review and editing, and discussion.Conflicts of interest
There are no conflicts to declare.Data availability
Additional data can be obtained from the corresponding author upon reasonable request.CCDC 2501246–2501257, 2517773 and 2517774 contain the supplementary crystallographic data for this paper.51a–n
Supplementary information (SI): the data supporting the findings of this study, including CIF files, NMR spectra, and computational details. See DOI: https://doi.org/10.1039/d5sc08693k.
Acknowledgements
This work was supported by the German Science Foundation (DFG; WE 4621/6–1 and WE 4621/6-2). P. R. thanks the Fonds der Chemischen Industrie (Kekulé scholarship). A. F. and R. G. M. thank MICIU/AEI from Spain for financial support, project PID2023-148453NB-I00, FEDER funds. We also thank Technische Universität Dresden (TUD) for financial support.Notes and references
- W. W. Schoeller, J. Chem. Soc., Chem. Commun., 1985, 334 10.1039/C39850000334.
- F. Mathey, Angew Chem. Int. Ed. Engl., 2003, 42, 1578 CrossRef CAS PubMed.
- P. Le Floch, Coord. Chem. Rev., 2006, 250, 627 CrossRef CAS.
- D. P. Gates, Expanding the Analogy Between P=C and C=C Bonds to Polymer Science, Springer Berlin Heidelberg, 2005, 250(250), 107 CAS.
- K. B. Dillon, F. Mathey and J. F. Nixon, Phosphorus. The carbon analogy: from organophosphorus to phospha-organic chemistry, Wiley, Chichester, New York, 1998 Search PubMed.
- R. Appel, ed Multiple Bonds and Low Coordination in Phosphorus Chemistry, Thieme, Stuttgart, 1990 Search PubMed.
- (a) N. H. T. Huy, L. Ricard and F. Mathey, Organometallics, 1988, 7, 1791 CrossRef; (b) A. I. Arkhypchuk, M.-P. Santoni and S. Ott, Organometallics, 2012, 31, 1118 CrossRef CAS; (c) A. I. Arkhypchuk, N. D'Imperio, J. A. L. Wells and S. Ott, Chem. Sci., 2022, 13, 12239 RSC; (d) N. D'Imperio, A. I. Arkhypchuk, J. Mai and S. Ott, Eur. J. Inorg. Chem., 2019, 2019, 1562 CrossRef; (e) G. Becker, M. Rssler and W. Uhl, Z. Anorg. Allg. Chem., 1981, 473, 7 CrossRef CAS; (f) G. Becker, W. Uhl and H.-J. Wessely, Z. Anorg. Allg. Chem., 1981, 479, 41 CrossRef CAS; (g) H. Grützmacher and H. Pritzkow, Angew Chem. Int. Ed. Engl., 1992, 31, 99 CrossRef.
- S. Wang, K. Samedov, S. C. Serin and D. P. Gates, Eur. J. Inorg. Chem., 2016, 2016, 4144 CrossRef CAS.
- M. Yam, J. H. Chong, C.-W. Tsang, B. O. Patrick, A. E. Lam and D. P. Gates, Inorg. Chem., 2006, 45, 5225 CrossRef CAS PubMed.
- G. Becker and O. Mundt, Z. Anorg. Allg. Chem., 1980, 462, 130 CrossRef CAS.
- G. Becker, W. Massa, O. Mundt and R. Schmidt, Z. Anorg. Allg. Chem., 1982, 485, 23 CrossRef CAS.
- R. Appel, F. Knoch and H. Kunze, Chem. Ber., 1984, 117, 3151 CrossRef CAS.
- G. Martin and E. Ocando-Mavarez, Heteroat. Chem., 1991, 2, 651 CrossRef CAS.
- K. Ohtsuki, H. T. G. Walsgrove, Y. Hayashi, S. Kawauchi, B. O. Patrick, D. P. Gates and S. Ito, Chem. Commun., 2020, 56, 774 RSC.
- (a) S. Shah and J. Protasiewicz, Coord. Chem. Rev., 2000, 210, 181 CrossRef CAS; (b) S. Shah, M. C. Simpson, R. C. Smith and J. D. Protasiewicz, J. Am. Chem. Soc., 2001, 123, 6925 CrossRef CAS; (c) S. Shah, T. Concolino, A. L. Rheingold and J. D. Protasiewicz, Inorg. Chem., 2000, 39, 3860 CrossRef CAS; (d) P. Le Floch and F. Mathey, Synlett, 1990, 1990, 171 CrossRef; (e) A. Marinetti, S. Bauer, L. Ricard and F. Mathey, Organometallics, 1990, 9, 793 Search PubMed; (f) P. Le Floch, A. Marinetti, L. Ricard and F. Mathey, J. Am. Chem. Soc., 1990, 112, 2407 CrossRef CAS; (g) T. L. Breen and D. W. Stephan, J. Am. Chem. Soc., 1995, 117, 11914 CrossRef CAS; (h) A. I. Arkhypchuk, Y. V. Svyaschenko, A. Orthaber and S. Ott, Angew Chem. Int. Ed. Engl., 2013, 52, 6484 Search PubMed; (i) K. Esfandiarfard, A. I. Arkhypchuk, A. Orthaber and S. Ott, Dalton Trans., 2016, 45, 2201 Search PubMed.
- S. Shah and J. D. Protasiewicz, Chem. Commun., 1998, 1585 Search PubMed.
- A. Marinetti and F. Mathey, Angew Chem. Int. Ed. Engl., 1988, 27, 1382 CrossRef.
- C. C. Cummins, R. R. Schrock and W. M. Davis, Angew Chem. Int. Ed. Engl., 1993, 32, 756 Search PubMed.
- P. Royla, K. Schwedtmann, Z. Han, J. Fidelius, D. P. Gates, R. M. Gomila, A. Frontera and J. J. Weigand, J. Am. Chem. Soc., 2023, 145, 10364 Search PubMed.
- (a) J. Fidelius, K. Schwedtmann, S. Schellhammer, J. Haberstroh, S. Schulz, R. Huang, M. C. Klotzsche, A. Bauzá, A. Frontera, S. Reineke and J. J. Weigand, Chem, 2024, 10, 644 Search PubMed; (b) K. Schwedtmann, G. Zanoni and J. J. Weigand, Chem.–Asian J., 2018, 13, 1388 CrossRef CAS.
- P. Royla, K. Schwedtmann, R. M. Gomila, A. Frontera and J. J. Weigand, Angew Chem. Int. Ed. Engl., 2025, 64, e202419502 Search PubMed.
- W. S. Wadsworth and W. D. Emmons, J. Am. Chem. Soc., 1961, 83, 1733 Search PubMed.
- J. D. Protasiewicz and C. Hering-Junghans, in Encyclopedia of inorganic and bioinorganic chemistry, ed R. A. Scott, Wiley, Chichester, 2012, vol. 13, pp. 1–27 Search PubMed.
- P. Pyykkö and M. Atsumi, Chem.–Eur. J., 2009, 15, 186 CrossRef.
- P. Pyykkö and M. Atsumi, Chem.–Eur. J., 2009, 15, 12770 Search PubMed.
- Y. Chen, Y.-F. Zhao, Y.-W. Yin and X.-Q. Yang, Phosphorus, Sulfur, and Silicon Relat. Elem., 1991, 61, 31 CrossRef CAS.
- O. Kühl, Phosphorus-31 NMR Spectroscopy, Springer Berlin Heidelberg, Berlin, Heidelberg, 2009 Search PubMed.
- (a) J. I. Bates, B. O. Patrick and D. P. Gates, New J. Chem., 2010, 34, 1660 Search PubMed; (b) M. Brym, C. Jones, M. Waugh, E. Hey-Hawkins and F. Majoumo, New J. Chem., 2003, 27, 1614 Search PubMed; (c) J. D. Masuda, K. C. Jantunen, O. V. Ozerov, K. J. T. Noonan, D. P. Gates, B. L. Scott and J. L. Kiplinger, J. Am. Chem. Soc., 2008, 130, 2408 Search PubMed; (d) M. Yoshifuji, K. Toyota and N. Inamoto, Tetrahedron Lett., 1985, 26, 1727 CrossRef CAS.
- M. Yoshifuji, K. Toyota, I. Matsuda, T. Niitsu, N. Inamoto, K. Hirotsu and T. Higuchi, Tetrahedron, 1988, 44, 1363 CrossRef CAS.
- L. Weber, Eur. J. Inorg. Chem., 2000, 2000, 2425 CrossRef.
- G. Etemad-Moghadam and M. Koenig, in Phosphorus-Carbon Heterocyclic Chemistry, Elsevier, 2001, pp. 57–86 Search PubMed.
- (a) R. Streubel, N. H. T. Huy and F. Mathey, Synthesis, 1993, 1993, 763 CrossRef; (b) G. Etemad-Moghadam, J. Bellan, C. Tachon and M. Koenig, Tetrahedron, 1987, 43, 1793 Search PubMed; (c) K. Lammertsma and M. J. M. Vlaar, Eur. J. Inorg. Chem., 2002, 2002, 1127 CrossRef.
- R. Streubel, M. Nieger and E. Niecke, Chem. Ber., 1993, 126, 645 CrossRef CAS.
- R. Engel, in Organic Reactions, ed S. E. Denmark, Wiley, 2004, pp. 175–248 Search PubMed.
- I. J. Colton, D. Yin, P. Grochulski and R. J. Kazlauskas, Adv. Synth. Catal., 2011, 353, 2529 CrossRef CAS.
- M. Regitz, ed. Multiple Bonds and Low Coordination in Phosphorus Chemistry, Thieme, Stuttgart, 1990 Search PubMed.
- M. Yoshifuji, K. Toyota, N. Inamoto, K. Hirotsu, T. Higuchi and S. Nagase, Phosphorus Sulfur Silicon Relat. Elem., 1985, 25, 237 CrossRef CAS.
- M.-L. Y. Riu, A. K. Eckhardt and C. C. Cummins, J. Am. Chem. Soc., 2022, 144, 7578 CrossRef CAS PubMed.
- (a) M. Gouygou, C. Tachon, G. Etemad-Moghadam and M. Koenig, Tetrahedron Lett., 1989, 30, 7411 CrossRef CAS; (b) M. Gouygou, C. Tachon, M. Koenig, A. Dubourg, J. P. Declercq, J. Jaud and G. Etemad-Moghadam, J. Org. Chem., 1990, 55, 5750 CrossRef CAS; (c) R. El-Ouatib, C. Garot, G. Etemad-Moghadam and M. Koenig, J. Org. Chem., 1992, 436, 169 CrossRef CAS; (d) M. J. Herve, G. Etemad-Moghadam, M. Gouygou, D. Gonbeau, M. Koenig and G. Pfister-Guillouzo, Inorg. Chem., 1994, 33, 596 CrossRef CAS; (e) M. Gouygou, M. Veith, C. Couret, J. Escudié, V. Huch and M. Koenig, J. Org. Chem., 1996, 514, 37 CrossRef CAS.
- (a) K. Trabitsch, S. Hauer, K. Schwedtmann, P. Royla, J. J. Weigand and R. Wolf, Inorg. Chem. Front., 2025, 12, 2013 Search PubMed; (b) K. Schwedtmann, J. Haberstroh, S. Roediger, A. Bauzá, A. Frontera, F. Hennersdorf and J. J. Weigand, Chem. Sci., 2019, 10, 6868 RSC.
- W. M. Haynes, D. R. Lide and T. J. Bruno, CRC Handbook of Chemistry and Physics, CRC Press, 2016 Search PubMed.
- G. Becker and O. Mundt, Z. Anorg. Allg. Chem., 1978, 443, 53 CrossRef CAS.
- A. N. Chernega, M. Y. Antipin, Y. T. Struchkov, V. P. Kukhar', I. V. Shevchenko, O. I. Kolodyazhnyi and I. E. Boldeskul, J. Struct. Chem., 1984, 25, 280 CrossRef.
- J. Grobe, A. Armbrecht, D. Le Van, B. Krebs, J. Kuchinke, M. Läge and E.-U. Würthwein, Z. Anorg. Allg. Chem., 2001, 627, 1241 CrossRef CAS.
- A. Beil, R. J. Gilliard and H. Grützmacher, Dalton Trans., 2016, 45, 2044 RSC.
- (a) T. Möller, M. B. Sárosi and E. Hey-Hawkins, Chem.–Eur. J., 2012, 18, 16604 CrossRef; (b) P. Wonneberger, N. König, F. B. Kraft, M. B. Sárosi and E. Hey-Hawkins, Angew Chem. Int. Ed. Engl., 2019, 58, 3208 CrossRef CAS PubMed; (c) F. Mathey, Acc. Chem. Res., 2004, 37, 954 CrossRef CAS.
- L. L. Liu, J. Zhou, L. L. Cao, Y. Kim and D. W. Stephan, J. Am. Chem. Soc., 2019, 141, 8083 CrossRef CAS.
- M. Abbari, P. Cosquer, F. Tonnard, Y. Y. C. Y. L. Ko and R. Carrié, Tetrahedron, 1991, 47, 71 CrossRef CAS.
- H. T. Teunissen, J. Hollebeek, P. J. Nieuwenhuizen, B. L. M. van Baar, F. J. J. de Kanter and F. Bickelhaupt, J. Org. Chem., 1995, 60, 7439 CrossRef CAS.
- (a) R. de Vaumas, A. Marinetti, L. Ricard and F. Mathey, J. Am. Chem. Soc., 1992, 114, 261 CrossRef CAS; (b) H. Grützmacher and H. Pritzkow, Angew Chem. Int. Ed. Engl., 1989, 28, 740 Search PubMed; (c) L. Chen, S. Wang, P. Werz, Z. Han and D. P. Gates, Heteroat. Chem., 2018, 29, e21474 Search PubMed; (d) T. van der Knaap and F. Bickelhaupt, Tetrahedron, 1983, 39, 3189 Search PubMed; (e) P. Le Floch, D. Carmichael and F. Mathey, Organometallics, 1991, 10, 2432 CrossRef CAS.
- (a) CCDC 2501246: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrcs; (b) CCDC 2501247: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrdt; (c) CCDC 2501248: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrfv; (d) CCDC 2501249: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrgw; (e) CCDC 2501250: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrhx; (f) CCDC 2501251: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrjy; (g) CCDC 2501252: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrkz; (h) CCDC 2501253: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrl0; (i) CCDC 2501254: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrm1; (j) CCDC 2501255: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrn2; (k) CCDC 2501256: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrp3; (l) CCDC 2501257: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pyrq4; (m) CCDC 2517773: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2qhyhp; (n) CCDC 2517774: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2qhyjq.
| This journal is © The Royal Society of Chemistry 2026 |