Open Access Article
Open Access ArticleElectrolyte coordination environments in wide-temperature aqueous metal batteries: mechanisms and design strategies
Jiashuo
Zhang
ab,
Tao
Liu
ab,
Xusheng
Dong
 ab,
Zhongju
Chen
ab,
Bin
Tang
a,
Fanxing
Bu
ab,
Zhongju
Chen
ab,
Bin
Tang
a,
Fanxing
Bu
 c,
Hongpeng
Li
c,
Hongpeng
Li
 d,
Zhen
Zhou
a,
Dongliang
Chao
d,
Zhen
Zhou
a,
Dongliang
Chao
 *c and
Ruizheng
Zhao
*c and
Ruizheng
Zhao
 *abef
*abef
aInterdisciplinary Research Center for Sustainable Energy Science and Engineering (IRC4SE2), Engineering Research Center of Advanced Functional Material Manufacturing of Ministry of Education, School of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, Henan, China. E-mail: rzzhao@zzu.edu.cn
bZhongyuan Critical Metals Laboratory, Zhengzhou University, Zhengzhou 450001, Henan, China
cLaboratory of Advanced Materials, Aqueous Battery Center, Shanghai Wusong Laboratory of Materials Science, College of Smart Materials and Future Energy, Fudan University, Shanghai 200433, Shanghai, China. E-mail: chaod@fudan.edu.cn
dCollege of Mechanical Engineering, Yangzhou University, Yangzhou 225127, China
eSalt Lake Chemical Engineering Research Complex, Qinghai University, Qinghai 810016, Gansu, China
fNational Key Laboratory of Special Rare Metal Materials, Zhengzhou University, China
First published on 23rd December 2025
Abstract
Aqueous metal batteries (AMBs) are promising for energy storage, which is attributed to their intrinsic safety, low cost, and environmental friendliness. However, they degrade at extreme temperatures, i.e., they undergo crystallization at low temperatures and evaporation or heat-driven side reactions at high temperatures, which have raised performance and safety concerns. Addressing these challenges requires simultaneous thermodynamic and kinetic insights into electrolyte behavior under such conditions. Among various strategies, tuning intermolecular interactions to optimize the electrolyte coordination environment has been proven to be especially effective. However, comprehensive treatments that integrate both low- and high-temperature regulation and provide underlying electrochemical mechanisms remain scarce. This review (i) dissects the failure modes of AMBs under extreme temperature conditions, (ii) discusses advances related to molecular-interaction tuning for wide-temperature performance, and (iii) offers perspectives and design guidelines for future research and development.
1. Introduction
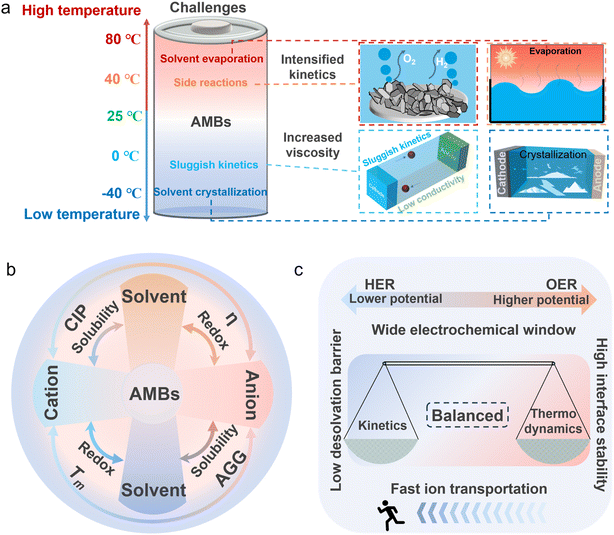
As the demand for electrochemical energy storage has grown, aqueous metal batteries (AMBs) have gained attention for their low cost, intrinsic safety, and eco-friendliness, and high energy density.1–5 AMBs are already used in portable electronics,6–10 yet their performance in extreme environments (e.g., polar and aerospace settings) is constrained by electrolyte phase transitions.11–15 The thermal stability of an aqueous electrolyte is determined by the solvation/coordination structure, which is shaped by intermolecular interactions.16–21 At low temperatures (<−20 °C), elevated desolvation energy can impede ion transport, while strengthened hydrogen bonding (HB) among free water molecules can induce crystallization. At high temperatures (>60 °C), weakened solvation structures may cause solute decomposition and parasitic reactions.22–27 Thus, regulating intermolecular interactions to optimize the coordination environment is essential for electrolytes that can operate reliably over a wide temperature range.Various strategies have been proposed to overcome the dilemma of the wide-temperature adaptability of AMBs, but optimizing the electrolyte remains a core approach (Fig. 1a).28–32 The solvation-structure framework, i.e. the connection between intermolecular interactions and the electrolyte coordination environment, is key for understanding and designing AMBs.33–38 Some researchers have reviewed the solvation process and emphasized its significant impact on electrochemical performance. These effects are intensified at low temperatures due to sluggish ion kinetics, higher viscosity, and crystallization.39,40 To mitigate them, polar additives and highly concentrated electrolytes have been employed to modulate intermolecular interactions and reshape coordination environments.41,42 For example, MgCl2 has been used in aqueous magnesium-based batteries, where Cl− adjusts the coordination environment, lowering the desolvation energy and freezing point.43 However, this approach can raise safety concerns at high temperatures: accelerated transport may trigger electrolyte decomposition, evaporation, and Cl− oxidation, causing pH shifts, corrosion, and parasitic reactions.44 Currently, high-temperature strategies typically employ flame retardants, thermally stable salts, or solid-state electrolytes to suppress evaporation and enhance safety.45–47 Overall, many methods work only within narrow temperature windows and rely heavily on composition tuning, often without mechanistic clarity on how molecular interactions govern thermal stability.48–53 Therefore, designing wide-temperature electrolytes demands systematic regulation of molecular-level interactions and coordination structures to balance low- and high-temperature performance.
 | ||
| Fig. 1 (a) A timeline of key milestones in the engineering of wide-temperature AMBs. (b) The number of publications relating to wide-temperature AMBs. | ||
Recent atomic-level research has advanced our understanding of ion transport in AMBs. These studies have systematically analyzed low-temperature electrolyte regulation through atomic insights and have proposed molecular-design principles for one specific kind of all-weather aqueous metal battery.22,54 However, existing reviews typically address either high- or low-temperature regimes—or focus on a specific battery type—without an integrated wide-temperature perspective (Fig. 1b). This review fills that gap by centering on the regulation of the electrolyte coordination environment via tailored molecular interactions. Unlike traditional perspectives, we designed a “moderate solvation structure” by regulating the intermolecular forces. This strategy is not limited to the regulation of a certain type of AMB, nor is it confined to high or low temperatures. This strategy regulates the delicate balance of solvent–cation–anion interactions, avoiding one-dimensional modifications of intermolecular forces or selective tuning of specific AMB systems. Furthermore, this method is not confined to enhancing battery performance at either high or low temperatures, but is applicable across a wide temperature range. We systematically examine the governing mechanisms, discuss design strategies for stable performance across a broad temperature range, and offer practical recommendations to guide future wide-temperature AMB development.
2. Challenges faced by AMBs at extreme temperatures
Despite the advantages of AMBs in terms of safety, cost, and environmental sustainability, AMBs still face critical challenges (Fig. 2a). Beyond common issues such as dendrite formation and gas evolution, temperature-induced failures, such as electrolyte crystallization, performance degradation caused by extremely sluggish kinetics at low temperatures, thermal runaway and decomposition caused by poor thermodynamics at high temperatures, remain major barriers to achieving wide-temperature operation. Unlike conventional organic-based batteries, aqueous-based batteries contain a large number of free water molecules with high reactivity, whose intrinsic physicochemical properties compromise electrolyte stability under extreme conditions. These challenges are closely linked to the molecular interactions that govern the electrolyte's coordination environment (Fig. 2b). Therefore, this section provides a comprehensive overview of the key failure modes of AMBs at extreme temperatures, elucidating the underlying thermodynamic and kinetic mechanisms and highlighting the significant impact of changes in the intermolecular forces that drive these behaviors (Fig. 2c).552.1 Low-temperature thermodynamic analysis
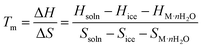
From a thermodynamic perspective, the main challenge facing low-temperature electrolytes is crystallization. The solid–liquid phase transition is governed by changes in the Gibbs free energy (ΔG), described by the following equation:| ΔG = ΔH − TΔS | (1) |
 | (2) |
As the temperature decreases, the thermal motion of water molecules weakens, stable hydrogen bonds take precedence, and liquid water gradually turns into ice. Water transforms from a short-range ordered tetrahedral structure in a liquid state to a long-range ordered hexahedral structure.59–61 Constraints on the degrees of freedom of molecular motion and the formation of long-range ordered structures lead to a significant reduction in structural entropy, raising Tm.62,63 As the enthalpy and entropy of ice and solutes are relatively constant, maintaining the electrolyte in a liquid state at low temperature requires a solution with lower ΔH and higher ΔS.64,65 Introducing strongly polar molecules or co-solvents can reduce ΔH by releasing heat through intermolecular interactions and simultaneously disrupt the HB network and coordination environment, increasing system entropy.66,67 Therefore, creating a multi-component solvation structure that regulates the HB network and coordination environment is an effective strategy for stabilizing aqueous electrolytes at low temperatures.
2.2 Low-temperature kinetic analysis
In addition to thermodynamic limitations, kinetic factors such as desolvation, polarization, and charge transfer also significantly affect the performance of AMBs at low temperatures.68,69 During the desolvation process, higher solvation energy leads to a higher energy barrier, which hinders the dissolution of metal ions. At low temperatures, reduced thermal motion and molecular freedom increase intermolecular interactions, promoting the formation of stable coordination structures and further elevating solvation energy and impeding ion dissolution.70,71 Additionally, the ionic conductivity (σ), which depends on ion concentration (ni), mobility (µi), unit charge (e), and valence (z), is adversely affected. Moreover, strong interactions within large solvation clusters restrict molecular mobility, increasing electrolyte viscosity.72,73 As a result, the associated ionic conductivity (σ) also decreases as a function of temperature. Of these, ion concentration is determined by the salt content, and ion mobility is inversely related to the viscosity (η) and radius (r) of solvated metal ions, following the Stokes–Einstein equation: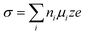 | (3) |
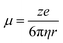 | (4) |
As the temperature decreases, the viscosity of the electrolyte increases, which hinders the ion mobility and reduces electrical conductivity.74 The reduction in kinetics caused by these factors will significantly impact battery performance. Taking one of the essential performance parameters—voltage—as an example, it is also influenced by the solvation structure.75 From the perspective of kinetics, operating at low temperatures induces greater battery polarization, including ohmic, concentration, and electrochemical polarization, all of which contribute to a voltage drop. The working voltage (E) of a battery can be described by the following equation:
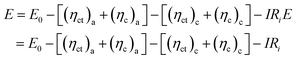 | (5) |
At low temperatures, battery polarization can be categorized into three types. (i) Ohmic polarization arises from increased resistance to ion transport. Lower temperatures lead to higher electrolyte viscosity and reduced conductivity, primarily due to the sluggish movement of large solvated clusters. (ii) Concentration polarization occurs when the ion transport rate lags behind the electrode reaction rate, causing ion accumulation or depletion near the electrode surface. This is exacerbated by the slow diffusion of large solvation clusters and high energy coordination environment structures that hinder ion dissociation and interfacial reactions—especially at high current densities. (iii) Electrochemical polarization results from sluggish interfacial charge transfer. At low temperatures, the increased activation energy for ion desolvation and electrochemical reactions slows down the electron transfer kinetics, requiring higher overpotentials to sustain reactions.76,77 In summary, achieving a stable aqueous electrolyte at low temperatures requires balancing thermodynamic and kinetic factors. This can be realized by regulating intermolecular interactions to construct a robust coordination environment structure with favorable physicochemical properties.
2.3 High-temperature thermodynamic analysis
Unlike at low temperatures, where the main thermodynamic challenge is insufficient energy (e.g., high dissolution barriers and electrolyte crystallization), high-temperature conditions present the opposite problem, viz., excess energy. This leads to thermal decomposition and electrolyte evaporation.78,79 One of the earliest consequences of elevated temperatures is the narrowing of the electrolyte's electrochemical stability window (ESW), which is defined by its oxidation potential (Eox) and reduction potential (Ered). High temperatures alter the equilibrium potential of electrode surface reactions, thereby directly reducing the ESW.80–82 For example, in the case of oxygen evolution, as per:| H2O → O2 + 4H+ + 4e− | (6) |
The equilibrium potential Eox can be described by the Nernst equation:
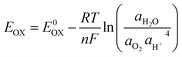 | (7) |
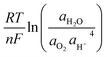 increases, shifting Eox to lower potentials, thereby narrowing the electrochemical stability window and promoting the oxygen evolution reaction.83,84 Thermodynamically, elevated temperatures enhance the thermal energy of electrons, increasing the likelihood of transitions to higher energy levels.85,86 Additionally, this also affects the electrolyte's solvation structure, weakening the dipole–dipole interactions between solvent molecules and reducing solvation stability.87,88 Moreover, the increased thermal agitation raises the probability of electron transitions from the HOMO to the LUMO or higher excited states, effectively narrowing the energy gap. This promotes side reactions at the electrode interface, leading to the growth of a thicker solid electrolyte interphase (SEI). The thickened SEI further depletes electrolyte components and hinders efficient ion transport across the electrode–electrolyte interface.89,90
increases, shifting Eox to lower potentials, thereby narrowing the electrochemical stability window and promoting the oxygen evolution reaction.83,84 Thermodynamically, elevated temperatures enhance the thermal energy of electrons, increasing the likelihood of transitions to higher energy levels.85,86 Additionally, this also affects the electrolyte's solvation structure, weakening the dipole–dipole interactions between solvent molecules and reducing solvation stability.87,88 Moreover, the increased thermal agitation raises the probability of electron transitions from the HOMO to the LUMO or higher excited states, effectively narrowing the energy gap. This promotes side reactions at the electrode interface, leading to the growth of a thicker solid electrolyte interphase (SEI). The thickened SEI further depletes electrolyte components and hinders efficient ion transport across the electrode–electrolyte interface.89,90
2.4 High-temperature kinetic analysis
At low temperatures, the primary challenges are high dissolution energy barriers and sluggish ion transport. In contrast, these limitations are largely alleviated at high temperatures.91–93 According to the Stokes–Einstein equation, self-diffusivity is inversely related to viscosity: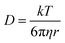 | (8) |
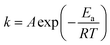 | (9) |
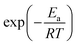 increases, significantly accelerating side-reaction rates.96,97 Most of the side reactions are exothermic, and if the heat generated is not effectively dissipated, it can lead to rapid battery degradation and even trigger thermal runaway, posing serious safety risks.98,99 In summary, while elevated temperatures can enhance battery performance by improving ion transport and reaction kinetics, they can also intensify thermodynamic instability. Achieving stable and safe operation under high-temperature conditions requires careful balancing of thermodynamic and kinetic factors. Therefore, the design of thermally robust coordination environment structures is essential for high-temperature aqueous electrolytes.
increases, significantly accelerating side-reaction rates.96,97 Most of the side reactions are exothermic, and if the heat generated is not effectively dissipated, it can lead to rapid battery degradation and even trigger thermal runaway, posing serious safety risks.98,99 In summary, while elevated temperatures can enhance battery performance by improving ion transport and reaction kinetics, they can also intensify thermodynamic instability. Achieving stable and safe operation under high-temperature conditions requires careful balancing of thermodynamic and kinetic factors. Therefore, the design of thermally robust coordination environment structures is essential for high-temperature aqueous electrolytes.
3. Mechanism and design strategies for wide-temperature AMBs
Tailoring the electrolyte coordination environment is a promising strategy to mitigate the performance limits of AMBs at extreme temperatures. That environment is governed by three interdependent interactions, i.e., solvent-–solvent, solvent–ion, and ion–ion, that together determine the electrolyte's physicochemical properties. Effective regulation, therefore, requires precise, targeted modulation of these interactions. In this section, we examine atomic-scale mechanisms for designing wide-temperature aqueous electrolytes via the controlled tuning of intermolecular interactions.3.1 Regulation of solvent–solvent interactions
Regulating solvent–solvent interactions in AMBs mainly means modulating water–water HBs. Each H2O molecule can form up to four HBs (two donor H atoms and two acceptor lone pairs), producing an extended tetrahedral network (Fig. 3a). At low temperatures, this ordered network promotes crystallization and electrolyte freezing; at high temperatures, increased molecular motion disrupts HBs and accelerates evaporation. Therefore, disrupting or reconfiguring the intrinsic HB network is essential for stabilizing aqueous electrolytes across a wide temperature range.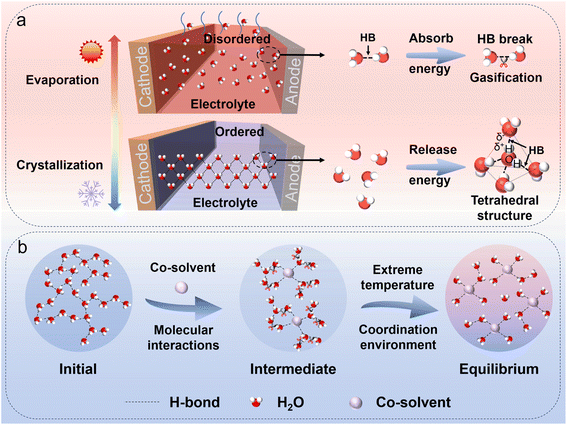 | ||
| Fig. 3 (a) Water-related failure modes of AMB electrolytes at low and high temperatures. (b) A schematic diagram of temperature-driven changes in the electrolyte coordination environment. | ||
A common approach is to introduce co-solvents or additives, which weaken the water–water HBs while strengthening the water-co-solvent interactions (Fig. 3b).100,101 For instance, Naveed et al. used the flame-retardant triethyl phosphate as an additive, which stabilizes aqueous zinc batteries (AZBs) at elevated temperatures. However, this additive does not effectively disrupt the HB network, so it only partially suppresses high-temperature decomposition/combustion and offers limited low-temperature protection.102 Conversely, Hu's team introduced a quaternized galactomannan polysaccharide rich in hydroxyl groups, which effectively binds free water and breaks the HB network, enabling operation down to −30 °C; however, its polymeric nature makes it prone to thermal degradation and it is therefore unsuitable for obtaining high-temperature stability.103
To disrupt the HB network effectively, co-solvents should form strong hydrogen-bonding donor/acceptor (HBD/HBA) interactions with water, preferably stronger than water–water interactions, and multifunctional HB groups further enhance this effect. Typical water-miscible candidates include sulfoxides, sulfones, nitriles, alcohols, amides, phosphate esters and ketones.104–110 An ideal co-solvent, therefore, should combine the following characteristics: (i) good miscibility with water, (ii) abundant HBA/HBD sites, (iii) a high dielectric constant, (iv) chemical and thermal robustness, and (v) low cost. Practical demonstrations support this strategy. A water–eutectic system with ethylene glycol (EG) and SnI4 enabled a Zn//Zn symmetric cell to run for over 6000 h at −30 °C and for 2500 h at 60 °C, with MD simulations confirming strong HB disruption by EG.111 Similarly, EG-based electrolytes enabled >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at −40 °C and 10
000 cycles at −40 °C and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 105 °C in aqueous Na-ion cells.112 These examples show that reconstructing the HB network via appropriately chosen co-solvents is an effective route to wide-temperature aqueous electrolytes, suppressing low-temperature crystallization and high-temperature evaporation.
000 cycles at 105 °C in aqueous Na-ion cells.112 These examples show that reconstructing the HB network via appropriately chosen co-solvents is an effective route to wide-temperature aqueous electrolytes, suppressing low-temperature crystallization and high-temperature evaporation.
3.2 Regulation of solvent–ion interactions
In addition to regulating the solvent–solvent interactions, a primary approach is to regulate the solvent–ion interactions. Solvent molecules form metal-ion solvation shells via electrostatic solvent–ion interactions, establishing the electrolyte's coordination structure (Fig. 4a). The strength and character of these interactions determine the solvation-shell composition and stability, and directly govern ion transport and interfacial desolvation (Fig. 4b). If solvent–ion coupling is too weak, solvation shells are labile, and the HB network persists, degrading low-temperature crystallization resistance and high-temperature stability. Sufficiently strong interactions stabilize the solvation structures and suppress crystallization, but overly strong binding raises desolvation barriers and slows electrochemical kinetics. Therefore, precise tuning of solvent–ion interactions is required to balance thermal robustness and interfacial kinetics. One direct strategy is adjusting the electrolyte concentration.113 Increasing the ion concentration enhances solvent coordination, disrupts HB networks, and stabilizes the solvation shell. For instance, a highly concentrated Al(OTf)3 electrolyte was employed by Wang et al. in aqueous aluminum batteries (AABs), forming a stable “water-in-salt” coordination structure that effectively disrupted HBs.114 However, very high concentrations can increase viscosity and material cost due to the addition of large amount of salt, and may lead to crystallization at low temperatures or decomposition at high temperatures. Thus, the concentration must be carefully optimized, or combined with suitable co-solvents and/or additives. To address this, Zhang et al. formulated a balanced electrolyte using Al(OTf)3, acetonitrile, triethyl phosphate, and water. The resulting AABs achieved stable operation for 450 h at −10 °C and 1500 h at 50 °C, demonstrating excellent wide-temperature performance.89 | ||
| Fig. 4 (a) Ion coordination in electrolytes. (b) Desolvation under different conditions. (c) The principle of eutectic electrolytes. | ||
Another classical approach to strengthen solvent–ion interactions is the use of eutectic electrolytes, which are mixtures of two or more components at specific ratios that exhibit melting points much lower than their individual constituents. Their formation is driven by HBs, Lewis acid–base interactions, and van der Waals forces (Fig. 4c).115 At the molecular level, eutectic electrolytes emerge from the reconstruction of intermolecular interactions among components, which in aqueous systems disrupt the HB network of water, lower the freezing point, and reduce free-water activity. These effects suppress decomposition reactions at both low and high temperatures and stabilize the solvation environment. For example, Zhang et al. developed a hydrated eutectic electrolyte composed of Al(OTf)3, glycerol, sodium β-glycerophosphate pentahydrate (SG), and water for aqueous aluminum-ion batteries. Al//Al symmetric cells exhibited excellent cycling stability, operating for over 500 h at −20 °C and 1000 h at 60 °C.116 This demonstrates that regulating solvent–ion interactions via eutectic systems can “lock” water and construct a stable coordination environment for wide-temperature operation.
3.3 Regulation of cation–anion interactions
Ion transport in electrolytes is primarily governed by solvent–solvent and solvent–ion interactions (Fig. 5a). Cation–anion interactions influence the composition and structure of the SEI, which governs interfacial ion transport; therefore, cation–anion interactions become critical at the electrolyte–electrode interface.117,118 In general, an increase in the content of inorganic components in the SEI film promotes ion diffusion, which is primarily caused by the decomposition of anions in the electrolyte. Strong cation–anion interactions can bring anions closer to the electrode surface, triggering anion decomposition and generating an inorganic-rich SEI layer.119,120 The electrolyte's ion coordination environment determines the priority by which species contribute to SEI formation. A moderate coordination structure favors a dense, homogeneous SEI that suppresses side reactions, enhancing cycling stability and safety. Additionally, a robust SEI mitigates low-temperature embrittlement and cracking, as well as high-temperature decomposition or dissolution. However, interfacial ion transport also depends on desolvation: while strong cation–anion interactions improve SEI formation, they may simultaneously increase solvent–ion binding, raising the desolvation energy barrier. In addition, overly strong interactions between cations and anions can lead to the formation of a thick SEI, which not only leads to the consumption of electrolyte components but also adversely affects ion transport. | ||
| Fig. 5 (a) Deposition of metal ions from different coordination structures. (b) The effects of varying interaction strengths on interfacial processes. | ||
To address this issue, Wang's team introduced bis(2-methoxyethyl)amine (BMEA) as a chelating additive in AABs. The amino group strongly coordinates with metal ions, reshaping the electrolyte's coordination structure and promoting strong cation–anion interactions.121 Characterization results confirmed that BMEA participates in SEI formation, contributing to stable battery operation at room temperature. However, at low temperatures, increased desolvation barriers can reduce conductivity, while at high temperatures, excessive SEI growth may deplete the electrolyte. To overcome these limitations, coordination environments should combine strong ion–ion and weak solvent–ion interactions (Fig. 5b). Weakly solvated electrolytes were studied by Shi et al., showing that with fewer water molecules in the solvation shell, lower desolvation energy was obtained and faster ion transport and uniform metal deposition were enabled.122 Therefore, designing such tailored coordination environments is critical for achieving wide-temperature electrolytes.
Generally speaking, designing a weak solvation structure requires careful consideration of several key factors, as follows: (i) solvent polarity, selecting solvents with low dielectric constants to reduce their solvation strength toward metal ions; (ii) donor ability, choosing solvents with low donor numbers (DNs), ideally lower than that of water, to weaken metal–ion–solvent coordination. Carbonates and nitriles are common weakly interacting solvents that promote the formation of contact ion pairs, facilitating ion desolvation and uniform metal deposition; (iii) hydrogen bonding, choosing solvents with weak HB donor/acceptor abilities to disrupt the water HB network; (iv) aprotic solvents, favoring aprotic solvents such as nitriles (e.g., acetonitrile), esters (e.g., methyl acetate), and ethers (e.g., tetrahydrofuran) that act as HB acceptors and help weaken water–water interactions. Xie et al. employed γ-valerolactone as a weak solvent, simultaneously enabling AZBs to cycle stably for over 120 cycles at 50 °C and 800 cycles at −30 °C.101 This solvent acts as a strong HB acceptor and diluent, breaking HBs between free water molecules and enhancing both thermal and chemical stability.
In fact, designing wide-temperature electrolytes cannot rely on a single interaction type. Solvent–solvent, solvent–ion, and ion–ion interactions are interdependent, and modifying one inevitably affects the others. Therefore, a holistic approach is essential for establishing a stable, optimized coordination environment across a broad temperature range.
3.4 Regulation of multiple interactions
Current strategies for regulating the coordination environment in AMBs almost all focus on tuning individual intermolecular interactions. Traditional single-solvent systems are limited by uniform interactions that struggle to modulate ion coordination effectively. In contrast, introducing multiple solvent species enables multiple adjustable molecular interactions, producing diverse solvation structures and enhanced electrolyte functionality. A multi-component system used by Zhao et al. contains multiple solvents and/or salts that create a disordered coordination environment that improves battery performance by increasing configurational entropy and weakening electrostatic interactions.123 This multi-component approach is epitomized by the concept of high-entropy electrolytes (HEEs, Fig. 6a). Unlike simple solvent mixtures, HEEs require deliberate design to achieve weak solvation, high ionic mobility, and broad electrochemical stability. Key design principles include (i) good miscibility among solvents for homogeneous solvation, (ii) complementary physicochemical properties to offset individual limitations, and (iii) sufficient salt solubility to support efficient ion transport. For instance, Ji et al. utilized a 9 M acetamide-based hybrid solvent in aqueous Zn-metal batteries, achieving over 1000 cycles at −25 °C and 150 cycles at 50 °C.124 Despite challenges such as structural complexity and higher cost, HEEs demonstrate strong potential for finely tuning coordination environments to achieve high-performance AMBs. | ||
| Fig. 6 (a) A schematic diagram of a high-entropy electrolyte. (b) A schematic diagram of a hydrogel electrolyte. | ||
In addition to multi-component systems, maximizing water incorporation into the coordination structure is an effective strategy for wide-temperature electrolytes. Macromolecular polymers, with abundant and regular functional groups, form extensive coordination structures with water. However, their poor solubility limits their liquid electrolyte applications. To solve this problem, Chen's team utilized hydrogel electrolytes to harness the advantages of polymers.125 Hydrogel electrolytes overcome this problem by combining polymer structural advantages with the conductivity of water. Composed of three-dimensional polymer networks, hydrogels retain large amounts of water (Fig. 6b), and functional groups, e.g., hydroxyl, carboxyl, and amino groups, form strong HBs with water, stabilizing the solvation structure and enabling adaptive coordination across a wide temperature range. These hydrogels are temperature-responsive. At high temperatures, polymer chains coil to reduce free volume and water loss, while maintaining ion mobility. At low temperatures, chains re-extend, preserving mechanical flexibility and continuous ion transport. Enhanced HBs at low temperatures improve toughness and suppress embrittlement, ensuring stable electrochemical performance. Optimization can be achieved by introducing functional groups to tune intermolecular interactions (e.g., HBs and van der Waals forces) and by adjusting the crosslinking density to strengthen the polymer network. For example, Liu et al. developed a double-network polyanionic hydrogel based on borax and bacterial cellulose for Zn//I2 batteries, achieving over 2000 h of stable cycling at −50 °C and 600 h at 50 °C at 1 mA cm−2.126 Despite their excellent thermal adaptability, hydrogel electrolytes still face challenges related to mechanical stability under extreme conditions, requiring further material and structural optimization to enhance their durability.
4. Conclusions and perspectives
AMBs are attractive for being low cost, highly secure, and environmentally benign, but their aqueous electrolytes suffer from poor low-temperature kinetics and unstable high-temperature thermodynamics that restrict their wide-temperature operation. This review surveys molecular strategies, e.g. tuning solvent–solvent, solvent–ion, and anion–cation interactions, that can achieve fast low-temperature kinetics and adjust the thermodynamics to expand the usable temperature window. Although these approaches have made significant progress, a universally robust wide-temperature aqueous electrolyte has not yet been realized, leaving ample room for further innovation. We therefore outline several research directions that should be prioritized to advance wide-temperature AMBs (Fig. 7).4.1 Utilize machine learning to screen wide-temperature electrolytes
Compared to traditional methods, machine learning offers faster and more efficient screening of electrolyte materials with varied physical and chemical properties, like dielectric constant and viscosity. It can quickly gather extensive data, facilitating the identification of promising electrolytes. Additionally, machine learning can predict key properties, such as freezing point, conductivity, and viscosity, of prepared electrolytes, guiding more efficient experimental testing.4.2 Explore novel electrolyte formulations for wide-temperature AMBs
In order to overcome the inherent instability of conventional aqueous solution systems at extreme temperatures, it is necessary not merely to adjust the proportions of the components but also to pursue fundamentally new electrolyte classes. Although adjusting component ratios has yielded improvements, traditional aqueous electrolytes still fail at extreme temperatures. Emerging approaches, such as high-entropy and quasi-solid-state aqueous electrolytes, have already shown promise for broader thermal tolerance. However, their stabilizing mechanisms remain poorly understood. Designing novel electrolyte systems and clarifying the underlying physicochemical principles will be essential for realizing truly wide-temperature aqueous electrolytes.4.3 Comprehensively probe mechanisms for wide-temperature AMBs
Although this review systematically examines how various intermolecular forces affect electrolyte properties, most studies consider only one or two interactions in isolation. The coordinated control of multiple intermolecular forces remains underexplored; clarifying their coupled effects requires systematic, multi-variable experiments and multi-scale characterization to extract quantitative parameters. Rigorous, mechanism-first analysis of electrolyte structures and interaction networks is therefore essential for the rational design of aqueous electrolytes that remain stable across a wide temperature range and for advancing wide-temperature AMBs.4.4 Design novel electrode materials for wide-temperature AMBs
At extreme temperatures, common electrode materials suffer accelerated dissolution and capacity loss, while uneven surface electric fields create locally high current densities that favor dendrite nucleation. The development of electrodes with wide-temperature adaptability, therefore, requires high chemical stability, such as utilizing a stable main structure or protective interface, as well as the use of special structures for uniform charge distribution, such as conformal conductive coatings, engineered porosity, or 3D collectors. Combining these strategies reduces parasitic reactions and dendrite formation, significantly enhancing the cycle life, safety and performance of AMBs over a wide temperature range.4.5 Optimize separators, binders, and current collectors for wide-temperature AMBs
Secondary components, such as separators, binders and current collectors, have a significant impact on AMB performance, yet they are not widely studied at extreme temperatures. Separators must retain porosity, wettability, and ionic conductivity without mechanical collapse or blockage; binders require thermal and chemical stability while preserving electrode cohesion; and collectors need corrosion resistance and architectures that homogenize current to avoid hotspots and local failure. The systematic development of temperature-adaptive polymers, functional coatings, and structured collectors, together with component-level testing via cycling over a wide temperature range, is therefore crucial for improving the lifetime, safety, and performance of AMBs.4.6 Scale up the production of wide-temperature AMBs
To meet the growing demand for energy storage, scaling up the production of wide-temperature-range AMBs is urgently needed. Although significant progress has been reported in the research of wide-temperature AMBs, the technical processes for their large-scale, continuous production are currently insufficiently developed. In order to strengthen the market position of wide-temperature AMBs, measures must be taken to reduce production costs, optimize production processes and actively develop and promote advanced research technologies. Awareness should also be raised in both academia and industry regarding the development of wide-temperature AMBs to accelerate the large-scale development and production of wide-temperature AMBs as soon as possible and to promote the transformation and development of more efficient energy systems.Author contributions
R. Z. and D. C. conceived and supervised the research. J. Z. and T. L. originally drafted and wrote the manuscript. X. D., Z. C. and B. T. provided theoretical guidance. F. B., H. L. and Z. Z. checked and revised the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Data availability
All data needed to evaluate the conclusions are present in the paper.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22309165, U24A2060, and 22279023), the National Key R&D Program of China (2024YFE0101100), the Excellent Youth Foundation of Henan Province (242300421126), Zhongyuan Critical Metals Laboratory (GJJSGFYQ202504), the Science and Technology Commission of Shanghai Municipality (25DZ3002901 and 25PY2600100), the Talent Development Funding Project of Shanghai (2021030), the Postdoctoral Science Foundation of China (2023M743170), the Key Research Projects of Higher Education Institutions of Henan Province (24A530010), the Open Project of Salt Lake Chemical Engineering Research Complex, Qinghai University (2025-DXSSKF-06), Graduate Student Independent Innovation Project of Zhengzhou University (20250512), and Projects of Innovation and Entrepreneurship Training Programme for College Students of Zhengzhou University (2025cxcy359).Notes and references
- J. Huang, Z. Guo, Y. Ma, D. Bin, Y. Wang and Y. Xia, Small Methods, 2018, 3, 1800272 CrossRef.
- L. He, C. Lin, P. Xiong, H. Lin, W. Lai, J. Zhang, F. Xiao, L. Xiao, Q. Qian, Q. Chen and L. Zeng, Trans. Tianjin Univ., 2023, 29, 321–346 CrossRef CAS.
- S. Chen, M. Zhang, P. Zou, B. Sun and S. Tao, Energy Environ. Sci., 2022, 15, 1805–1839 RSC.
- Y. Zhang, L. Zhao, Y. Liang, X. Wang and Y. Yao, eScience, 2022, 2, 110–115 CrossRef CAS.
- Z. Jiang, X. Yang, J. Zhang, J. Yang, B. Sun, Z. Sun, J. Xue, J. He, Z. Sun, H. K. Liu and S. X. Dou, Adv. Funct. Mater., 2025, 35, e11754 CrossRef CAS.
- M. Wang, Y. Meng, Y. Xu, D. Shen, P. Tong and W. Chen, ACS Energy Lett., 2024, 9, 1381–1388 CrossRef CAS.
- X. Li, X. Wang, L. Ma and W. Huang, Adv. Energy Mater., 2022, 12, 2202068 CrossRef CAS.
- Y. Duan, C. Li, Z. Ye, H. Li, Y. Yang, D. Sui and Y. Lu, Nanomaterials, 2022, 12, 3954 CrossRef CAS PubMed.
- D. Sui, M. Chang, Z. Peng, C. Li, X. He, Y. Yang, Y. Liu and Y. Lu, Nanomaterials, 2021, 11, 2771 Search PubMed.
- D. Sui, M. Wu, Y. Liu, Y. Yang, H. Zhang, Y. Ma, L. Zhang and Y. Chen, Nanotechnology, 2021, 32, 015403 CrossRef CAS PubMed.
- F. Ai, Z. Wang, N.-C. Lai, Q. Zou, Z. Liang and Y.-C. Lu, Nat. Energy, 2022, 7, 417–426 CrossRef CAS.
- X. Lin, M. Salari, L. M. R. Arava, P. M. Ajayan and M. W. Grinstaff, Chem. Soc. Rev., 2016, 45, 5848–5887 Search PubMed.
- J. Zhang, C. Lin, L. Zeng, H. Lin, L. He, F. Xiao, L. Luo, P. Xiong, X. Yang, Q. Chen and Q. Qian, Small, 2024, 20, 2312116 CrossRef CAS PubMed.
- L. Jiang, Y.-C. Hu, F. Ai, Z. Liang and Y.-C. Lu, Energy Environ. Sci., 2024, 17, 2815–2824 RSC.
- Q. Wang, J. He, B. Sun, Y. Bai, Y. Yan, J. Xue, Z. Sun, X. Wang, J. Wu, J. Wang, R. Zhao, Z. Sun, H. K. Liu and S. X. Dou, ACS Nano, 2025, 19, 28992–29027 Search PubMed.
- X. Jin, G. Lai, X. Xiu, L. Song, X. Li, C. Dai, M. Li, Z. Quan, B. Tang, G. Shao, Z. Zhang, F. Liu, L. Qu and Z. Zhou, Angew. Chem., Int. Ed., 2024, 64, e202418682 CrossRef.
- J.-W. Zhang, J.-L. Sun, D.-N. Zhao, Y.-J. Zhao, X.-Y. Hu, Y.-N. Wang, Y.-J. Yao, N.-S. Zhang, L.-J. Zhang, C.-L. Li, P. Wang, S.-Y. Li and X.-L. Cui, Energy Storage Mater., 2024, 72, 103698 CrossRef.
- B. Sun, F. Huo, C. Zhao, J. He, J. Xue, Z. Sun, J. Wu, X. Wang, J. Wang, R. Zhao and Z. Sun, J. Mater. Chem. A, 2025, 13, 25444–25456 Search PubMed.
- P. Xue, C. Guo, L. Li, H. Li, D. Luo, L. Tan and Z. Chen, Adv. Mater., 2022, 34, 2110047 CrossRef CAS PubMed.
- H. Li, R. Zhao, W. Zhou, L. Wang, W. Li, D. Zhao and D. Chao, JACS Au, 2023, 3, 2107–2116 CrossRef CAS.
- H. Li, C. Guo, T. Zhang, P. Xue, R. Zhao, W. Zhou, W. Li, A. Elzatahry, D. Zhao and D. Chao, Nano Lett., 2022, 22, 4223–4231 CrossRef CAS PubMed.
- C. You, W. Fan, X. Xiong, H. Yang, L. Fu, T. Wang, F. Wang, Z. Zhu, J. He and Y. Wu, Adv. Funct. Mater., 2024, 34, 2403616 CrossRef CAS.
- X. Zhang, Y. Liu, S. Wang, J. Wang, F. Cheng, Y. Tong, L. Wei, Z. Fang and J. Mao, Energy Storage Mater., 2024, 70, 103471 CrossRef.
- Y. Lv, Y. Xiao, L. Ma, C. Zhi and S. Chen, Adv. Mater., 2021, 34, 2106409 CrossRef PubMed.
- X. Gao, J. Yang, Z. Xu, Y. Nuli and J. Wang, Energy Storage Mater., 2023, 54, 382–402 CrossRef.
- C. Cao, S. Tang, X. Wu, H. Huang, S. Liu and H. Li, Adv. Sci., 2025, 12, e11439 CrossRef CAS PubMed.
- H. Li, S. Ding, J. Ding, J. Luo, S. Liu and H. Huang, Energy Storage Mater., 2025, 74, 103907 CrossRef.
- T. Liu, X. Dong, B. Tang, R. Zhao, J. Xu, H. Li, S. Gao, Y. Fang, D. Chao and Z. Zhou, J. Energy Chem., 2024, 98, 311–326 CrossRef CAS.
- M. Wang, Z. Xu, C. He, L. Cai, H. Zheng, Z. Sun, H. K. Liu, H. Ying and S. Dou, ACS Nano, 2025, 19, 9709–9739 CrossRef CAS.
- R. Zhao, A. Elzatahry, D. Chao and D. Zhao, Matter, 2022, 5, 8–10 CrossRef CAS.
- J. Xu, T. Liu, X. Dong, X. Dong, W. Zhou, X. Li, D. Chao, Z. Zhou and R. Zhao, Natl. Sci. Rev., 2025, 12, nwae433 CrossRef CAS.
- Z. Xie, Y. Qu, F. Kong, R. Zhao and X. Wang, Nanomaterials, 2025, 15, 940 CrossRef CAS PubMed.
- Z. Xing, W. Zhao, B. Yu, Y. Wang, L. Zhou, P. Xiong, M. Chen and J. Zhu, Small, 2024, 20, 2405442 CrossRef CAS.
- Y. Shen, B. Liu, X. Liu, J. Liu, J. Ding, C. Zhong and W. Hu, Energy Storage Mater., 2021, 34, 461–474 CrossRef.
- H. Luo, X. Su, Z. Chen, H. Guo, R. Zhao, Q. Gao, M. Lu and T. Liu, Adv. Mater., 2025, 37, 2507978 CrossRef CAS.
- T. Liu, X. Dong, J. Zhang, H. Chen, R. Cao, Z. Sun, W. Zhou, H. Li, D. Chao, Z. Zhou and R. Zhao, Chem. Sci., 2025, 16, 17426–17435 RSC.
- H. Luo, F. Li, M. Wang, S. Sun, M. Zhou, W. Zhang, H. Guo, X. Su, X. Li and L. Ma, Chem. Sci., 2025, 16, 2044–2045 RSC.
- H. Luo, H. Guo, X. Li, S. Li, Y. Li, J. Shi, Q. Gao, H. He, M. Lu, Q. Zhang and D. Chao, Matter, 2025, 8, 102379 CrossRef CAS.
- M. Qin, Z. Zeng, S. Cheng and J. Xie, Interdiscip. Mater., 2023, 2, 308–336 CAS.
- M. Han, T. C. Li, X. Chen and H. Y. Yang, Small, 2023, 20, 2304901 CrossRef.
- Y. Gao, Z. Wang, H. Tu, J. Xue, S. Weng, S. Lu, L. Liu, G. Sun, K. Peng, X. Zhang, D. Li, Y. Liu, J. Xu, H. Li and X. Wu, Adv. Funct. Mater., 2024, 35, 2414652 CrossRef.
- L. Jiang, S. Han, Y.-C. Hu, Y. Yang, Y. Lu, Y.-C. Lu, J. Zhao, L. Chen and Y.-S. Hu, Nat. Energy, 2024, 9, 839–848 CrossRef CAS.
- G. Yang, X. Xu, G. Qu, J. Deng, Y. Zhu, C. Fang, O. Fontaine, P. Hiralal, J. Zheng and H. Zhou, Chem. Eng. J., 2023, 455, 140806 CrossRef CAS.
- X. Ye, X. Xiao, Z. Wu, Y. Zhan, X. Wu and S. Liu, J. Mater. Chem. A, 2024, 12, 23337–23363 RSC.
- Q. Zheng, L. Liu, Z. Hu, Z. Tang, H. Lu, Y. Gao, J. Wang, Y. Song, C. Han and W. Li, Adv. Funct. Mater., 2025, 35, 2504782 CrossRef CAS.
- C. Zhu, D. Wu, C. Wang and J. Ma, Adv. Funct. Mater., 2024, 34, 2406764 CrossRef CAS.
- X. Zheng, Z. Cao, Z. Gu, L. Huang, Z. Sun, T. Zhao, S. Yu, X.-L. Wu, W. Luo and Y. Huang, ACS Energy Lett., 2022, 7, 2032–2042 CrossRef CAS.
- Y. Sui, M. Yu, Y. Xu and X. Ji, J. Electrochem. Soc., 2022, 169, 030537 CrossRef CAS.
- Y. Wang, H. Wei, Z. Li, X. Zhang, Z. Wei, K. Sun and H. Li, Chem. Rec., 2022, 22, e202200132 CrossRef CAS PubMed.
- X. Yang, P. Li, C. Guo, W. Yang, N. Zhou, X. Huang and Y. Yang, J. Power Sources, 2024, 624, 235563 CrossRef CAS.
- F. Yue, Z. Tie, S. Deng, S. Wang, M. Yang and Z. Niu, Angew. Chem., Int. Ed., 2021, 60, 13882–13886 CrossRef CAS.
- M. Li, R. Li, H. Ma, M. Yang, Y. Dai, H. Yu, Y. Hao, Z. Wang, B. Wang, M. Hu and J. Yang, Nano-Micro Lett., 2025, 17, 158 Search PubMed.
- J. Wang, Y. Yang, Y. Wang, S. Dong, L. Cheng, Y. Li, Z. Wang, L. Trabzon and H. Wang, ACS Nano, 2022, 16, 15770–15778 Search PubMed.
- T. Xiong, Y. Guo and X. Wang, Adv. Funct. Mater., 2024, 35, 2421240 Search PubMed.
- R. Zhao, X. Dong, P. Liang, H. Li, T. Zhang, W. Zhou, B. Wang, Z. Yang, X. Wang, L. Wang, Z. Sun, F. Bu, Z. Zhao, W. Li, D. Zhao and D. Chao, Adv. Mater., 2023, 35, 2209288 CrossRef CAS.
- C. Yang, J. Xia, C. Cui, T. P. Pollard, J. Vatamanu, A. Faraone, J. A. Dura, M. Tyagi, A. Kattan, E. Thimsen, J. Xu, W. Song, E. Hu, X. Ji, S. Hou, X. Zhang, M. S. Ding, S. Hwang, D. Su, Y. Ren, X.-Q. Yang, H. Wang, O. Borodin and C. Wang, Nat. Sustain., 2023, 6, 325–335 Search PubMed.
- M. Qiu, P. Sun, K. Han, Z. Pang, J. Du, J. Li, J. Chen, Z. L. Wang and W. Mai, Nat. Commun., 2023, 14, 601 CrossRef CAS PubMed.
- L. Jiang, D. Dong and Y.-C. Lu, Nano Energy, 2022, 1, e9120003 Search PubMed.
- T. Xue, Y. Mu, Z. Zhang, J. Guan, J. Qiu, C. Yang, L. Zang and L. Zeng, Adv. Energy Mater., 2025, 15, 2500674 CrossRef CAS.
- Y. Liu, J. Chen, M. Qiu, P. Sun and W. Mai, Adv. Funct. Mater., 2025, 35, e04127 Search PubMed.
- T. Xue, J. Guan, Y. Mu, M. Han, C. Yang, L. Zang and L. Zeng, Chem. Eng. J., 2025, 514, 162994 Search PubMed.
- Q. Nian, T. Sun, Y. Li, S. Jin, S. Liu, X. Luo, Z. Wang, B. Q. Xiong, Z. Cui, D. Ruan, H. Ji, Z. Tao and X. Ren, Angew. Chem., Int. Ed., 2023, 62, e202217671 Search PubMed.
- M. Qiu, P. Sun, Y. Liang, J. Chen, Z. L. Wang and W. Mai, Nat. Commun., 2024, 15, 10420 Search PubMed.
- C. Yang, X. Liu, Y. Lin, L. Yin, J. Lu and Y. You, Adv. Mater., 2023, 35, 2301817 Search PubMed.
- M. Wang, M. Zheng, J. Lu and Y. You, Joule, 2024, 8, 2467–2482 Search PubMed.
- S. Deng, B. Xu, J. Zhao and H. Fu, Energy Storage Mater., 2024, 70, 103490 CrossRef.
- Q. Dong, H. Ao, Z. Qin, Z. Xu, J. Ye, Y. Qian and Z. Hou, Small, 2022, 18, 2203347 CrossRef CAS PubMed.
- K. Chen, J. Luo and Y. Huang, Chem. Eng. J., 2025, 503, 158260 CrossRef CAS.
- L. Geng, J. Meng, X. Wang, W. Wu, K. Han, M. Huang, C. Han, L. Wu, J. Li, L. Zhou and L. Mai, Chem, 2025, 11, 102302 Search PubMed.
- T. Ma, Y. Ni, Q. Wang, W. Zhang, S. Jin, S. Zheng, X. Yang, Y. Hou, Z. Tao and J. Chen, Angew. Chem., Int. Ed., 2022, 61, e202207927 CrossRef CAS PubMed.
- Z. Wang, T. Zheng, S. Wang, X.-G. Zhang, Y. Gu, S. Tang and Y. Fu, J. Am. Chem. Soc., 2025, 147, 5962–5970 CrossRef CAS PubMed.
- J. Guo, S. Gu, W. Nie, B. Long, S. Ryazantsev, S. Malyshev, J. Li, S. Guo and C. Wu, Adv. Mater., 2025, 37, 2419865 CrossRef CAS.
- A. Cushing, T. Zheng, K. Higa and G. Liu, Polymers, 2021, 13, 4033 CrossRef CAS.
- L. Luo, K. Chen, H. Chen, H. Li, R. Cao, X. Feng, W. Chen, Y. Fang and Y. Cao, Adv. Mater., 2023, 36, 2308881 CrossRef.
- Q. Nian, T. Sun, S. Liu, H. Du, X. Ren and Z. Tao, Chem. Eng. J., 2021, 423, 130253 CrossRef CAS.
- M. Zhao, T. Cheng, T. Li, S. Wang, Y. Yin and X. Li, Energy Environ. Sci., 2025, 18, 378–385 RSC.
- H.-I. Kim, K. M. Lee, W.-Y. Kim, S. H. Kweon, X. Wang, S. Zheng, S.-H. Kim, J. H. Ha, S. J. Kang, Z.-S. Wu, S. K. Kwak and S.-Y. Lee, Energy Environ. Sci., 2024, 17, 1961–1974 RSC.
- J. Wei, P. Zhang, J. Sun, Y. Liu, F. Li, H. Xu, R. Ye, Z. Tie, L. Sun and Z. Jin, Chem. Soc. Rev., 2024, 53, 10335–10369 Search PubMed.
- Y. Feng, L. Zhou, H. Ma, Z. Wu, Q. Zhao, H. Li, K. Zhang and J. Chen, Energy Environ. Sci., 2022, 15, 1711–1759 Search PubMed.
- D. Sui, R. Luo, S. Xie, H. Zhang, T. Ma, H. Sun, T.-T. Jia, J. Sun and X. Li, Chem. Eng. J., 2024, 480, 148007 CrossRef CAS.
- D. Guo, S. Thomas, J. K. El-Demellawi, Z. Shi, Z. Zhao, C. G. Canlas, Y. Lei, J. Yin, Y. Zhang, M. N. Hedhili, M. Arsalan, Y. Zhu, O. M. Bakr, O. F. Mohammed and H. N. Alshareef, Energy Environ. Sci., 2024, 17, 8151–8161 RSC.
- Y. Shang, N. Chen, Y. Li, S. Chen, J. Lai, Y. Huang, W. Qu, F. Wu and R. Chen, Adv. Mater., 2020, 32, 2004017 CrossRef CAS.
- Z. Chen, Y. Wang, Q. Wu, C. Wang, Q. He, T. Hu, X. Han, J. Chen, Y. Zhang, J. Chen, L. Yang, X. Wang, Y. Ma and J. Zhao, Adv. Mater., 2024, 36, 2411004 CrossRef CAS.
- H. Zhang, Y. Zhong, J. Li, Y. Liao, J. Zeng, Y. Shen, L. Yuan, Z. Li and Y. Huang, Adv. Energy Mater., 2022, 13, 2203254 CrossRef.
- Z. Hao, X. Shi, Z. Yang, L. Li and S. L. Chou, Adv. Funct. Mater., 2022, 32, 2208093 CrossRef CAS.
- M. Luo, H. Yu, F. Hu, T. Liu, X. Cheng, R. Zheng, Y. Bai, M. Shui and J. Shu, Chem. Eng. J., 2020, 380, 122557 CrossRef CAS.
- X. Yun, Y. Chen, H. Gao, D. Lu, L. Zuo, P. Gao, G. Zhou, C. Zheng and P. Xiao, Adv. Energy Mater., 2024, 14, 2304341 CrossRef CAS.
- M. Wang, L. Yin, M. Zheng, X. Liu, C. Yang, W. Hu, J. Xie, R. Sun, J. Han, Y. You and J. Lu, Nat. Commun., 2024, 15, 8866 CrossRef CAS PubMed.
- X. Luo, R. Wang, L. Zhang, Z. Liu, H. Li, J. Mao, S. Zhang, J. Hao, T. Zhou and C. Zhang, ACS Nano, 2024, 18, 12981–12993 CrossRef CAS.
- Y. Zhang, S. Shen, K. Xi, P. Li, Z. Kang, J. Zhao, D. Yin, Y. Su, H. Zhao, G. He and S. Ding, Angew. Chem., Int. Ed., 2024, 63, e202407067 CrossRef CAS.
- Y. Fu, X. Cui, Y. Zhang, T. Feng, J. He, X. Zhang, X. Bai and Q. Cheng, J. Chem. Eng. Data, 2018, 63, 1180–1189 CrossRef CAS.
- B. S. Lalia, N. Yoshimoto, M. Egashira and M. Morita, J. Power Sources, 2010, 195, 7426–7431 CrossRef CAS.
- B. Batiot, T. Rogaume, F. Richard, J. Luche, A. Collin, E. Guillaume and J. L. Torero, Appl. Sci., 2021, 11, 4075 CrossRef CAS.
- L. Sun, Z. Song, C. Deng, Q. Wang, F. Mo, H. Hu and G. Liang, Batteries, 2023, 9, 386 Search PubMed.
- J. Landesfeind and H. A. Gasteiger, J. Electrochem. Soc., 2019, 166, A3079–A3097 Search PubMed.
- G. Kucinskis, M. Bozorgchenani, M. Feinauer, M. Kasper, M. Wohlfahrt-Mehrens and T. Waldmann, J. Power Sources, 2022, 549, 232129 CrossRef CAS.
- M. Chen, G. Chen, C. Sun, X. Li, M. Zhang, H. Hua, J. Zhao and Y. Yang, Angew. Chem., Int. Ed., 2025, 64, e202502005 CrossRef CAS PubMed.
- W. Mei, Z. Liu, C. Wang, C. Wu, Y. Liu, P. Liu, X. Xia, X. Xue, X. Han, J. Sun, G. Xiao, H.-y. Tam, J. Albert, Q. Wang and T. Guo, Nat. Commun., 2023, 14, 5251 CrossRef CAS PubMed.
- J. Hou, L. Lu, L. Wang, A. Ohma, D. Ren, X. Feng, Y. Li, Y. Li, I. Ootani, X. Han, W. Ren, X. He, Y. Nitta and M. Ouyang, Nat. Commun., 2020, 11, 5100 CrossRef CAS.
- M. Yang, Z. Yan, J. Xiao, W. Xin, L. Zhang, H. Peng, Y. Geng, J. Li, Y. Wang, L. Liu and Z. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202212666 CrossRef CAS PubMed.
- C. Xie, S. Liu, H. Wu, Q. Zhang, C. Hu, Z. Yang, H. Li, Y. Tang and H. Wang, Sci. Bull., 2023, 68, 1531–1539 CrossRef CAS.
- A. Naveed, H. Yang, J. Yang, Y. Nuli and J. Wang, Angew. Chem., Int. Ed., 2019, 58, 2760–2764 CrossRef CAS.
- B. Hu, T. Chen, Y. Wang, X. Qian, Q. Zhang and J. Fu, Adv. Energy Mater., 2024, 14, 2401470 Search PubMed.
- Q. Guo, R. Luo, Z. Tang, X. Li, X. Feng, Z. Ding, B. Gao, X. Zhang, K. Huo and Y. Zheng, ACS Nano, 2023, 17, 24227–24241 Search PubMed.
- Y. Quan, M. Yang, M. Chen, W. Zhou, X. Han, J. Chen, B. Liu, S. Shi and P. Zhang, Chem. Eng. J., 2023, 458, 141392 CrossRef CAS.
- C. Liu, X. Xie, B. Lu, J. Zhou and S. Liang, ACS Energy Lett., 2021, 6, 1015–1033 CrossRef CAS.
- D. Feng, F. Cao, L. Hou, T. Li, Y. Jiao and P. Wu, Small, 2021, 17, 2103195 CrossRef CAS.
- Y. An, C. Shu, Y. Liu, Y. Xu, L. Kang, X. Zhang, J. Sun, Z. Ma, K. Zhao, Y. Huang, F. Kang, F. Jiang and W. Liu, ACS Nano, 2025, 19, 11146–11163 CrossRef CAS.
- S. Lin, H. Hua, P. Lai and J. Zhao, Adv. Energy Mater., 2021, 11, 2101775 CrossRef CAS.
- Y. Jiang, B. Wujieti, Y. Liu, Q. Zeng, Z. Li, J. Guan, H. Wang, L. Chen, Y. Cao, R. Li, Y. Zhou, H. Zhou, W. Cui and L. Zhang, Adv. Funct. Mater., 2024, 35, 2421160 Search PubMed.
- W. Chen, Y. Wang, F. Wang, Z. Zhang, W. Li, G. Fang and F. Wang, Adv. Mater., 2024, 36, 2411802 Search PubMed.
- S. Huang, Z. Li, P. Li, X. Du, M. Ma, Z. Liang, Y. Su and L. Xiong, J. Mater. Chem. A, 2023, 11, 15532–15539 Search PubMed.
- S. Wang, Z. Xue, F. Chu, Z. Guan, J. Lei and F. Wu, J. Energy Chem., 2023, 79, 201–210 Search PubMed.
- X. Wang, Z. Xi and Q. Zhao, Ind. Chem. Mater., 2025, 3, 7–30 Search PubMed.
- L. Geng, J. Meng, X. Wang, C. Han, K. Han, Z. Xiao, M. Huang, P. Xu, L. Zhang, L. Zhou and L. Mai, Angew. Chem., Int. Ed., 2022, 61, e202206717 Search PubMed.
- X. Zhang, R. Wang, Z. Liu, Q. Ma, H. Li, Y. Liu, J. Hao, S. Zhang, J. Mao and C. Zhang, Adv. Energy Mater., 2024, 14, 2400314 CrossRef CAS.
- X. Zhou, Q. Zhang, Z. Zhu, Y. Cai, H. Li and F. Li, Angew. Chem., Int. Ed., 2022, 61, e202205045 CrossRef CAS.
- Y. Jie, S. Wang, S. Weng, Y. Liu, M. Yang, C. Tang, X. Li, Z. Zhang, Y. Zhang, Y. Chen, F. Huang, Y. Xu, W. Li, Y. Guo, Z. He, X. Ren, Y. Lu, K. Yang, S. Cao, H. Lin, R. Cao, P. Yan, T. Cheng, X. Wang, S. Jiao and D. Xu, Nat. Energy, 2024, 9, 987–998 CrossRef CAS.
- M. Liu, W. Yuan, G. Ma, K. Qiu, X. Nie, Y. Liu, S. Shen and N. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202304444 CrossRef CAS PubMed.
- Z. Chen, B. Wang, Y. Li, F. Bai, Y. Zhou, C. Li and T. Li, ACS Appl. Mater. Interfaces, 2022, 14, 28014–28020 CrossRef CAS.
- D.-Y. Wang, E. Hu, G. Wu, H. Choo, C. Franke, B.-E. Jia, J. Song, A. Sumboja, I. T. Anggraningrum, A. Z. Syahrial, Q. Zhu, M.-F. Ng, T. Li and Q. Yan, Angew. Chem., Int. Ed., 2025, 64, e202508641 Search PubMed.
- X. Shi, J. Xie, J. Wang, S. Xie, Z. Yang and X. Lu, Nat. Commun., 2024, 15, 302 Search PubMed.
- Q. Wang, C. Zhao, J. Wang, Z. Yao, S. Wang, S. G. H. Kumar, S. Ganapathy, S. Eustace, X. Bai, B. Li and M. Wagemaker, Nat. Commun., 2023, 14, 440 CrossRef CAS.
- H. Ji, C. Xie, T. Wu, H. Wang, Z. Cai, Q. Zhang, W. Li, L. Fu, H. Li and H. Wang, Chem. Commun., 2023, 59, 8715–8718 Search PubMed.
- Z. J. Chen, T. Y. Shen, X. Xiao, X. C. He, Y. L. Luo, Z. Jin and C. H. Li, Adv. Mater., 2024, 36, 2413268 CrossRef CAS.
- Y. Liu, F. Li, J. Hao, H. Li, S. Zhang, J. Mao, T. Zhou, R. Wang, L. Zhang and C. Zhang, Adv. Funct. Mater., 2024, 34, 2400517 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |