Open Access Article
Open Access ArticleA Polycondensation–depolymerization strategy enables closed-loop recyclable polyoxalates via ring-opening polymerization of six-membered cyclic oxalates
Yalei Liua,
Zheng Lia,
Dongfang Zhaoa,
Yong Shen *b and
Zhibo Li
*b and
Zhibo Li *ac
*ac
aState Key Laboratory of Advanced Optical Polymer and Manufacturing Technology, College of Polymer Science and Engineering, Qingdao University of Science and Technology, Qingdao, 266042, China. E-mail: zbli@qust.edu.cn
bCollege of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, 266042, China. E-mail: shenyong@qust.edu.cn
cState Key Laboratory of Chemical Engineering and Low-carbon Technology, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, 310058, China. E-mail: zbli@zju.edu.cn
First published on 22nd December 2025
Abstract
Developing a simple and efficient strategy for the scalable production of closed-loop recyclable polymers from abundant and low-cost feedstocks remains highly desirable. Here, we present a facile “polycondensation–depolymerization” approach for the large-scale synthesis of cyclic 1,2-alkylene oxalates, which undergo controlled ring-opening polymerization to yield high-molecular-weight polyoxalates. The influence of alkyl substituents on the polymerization kinetics, thermodynamics, and material properties was systematically investigated. Remarkably, these polyoxalates can be chemically recycled to their pristine monomers with high purity and yield using sodium glycolate as a catalyst. Furthermore, the polyoxalates exhibit excellent marine degradability, providing a promising strategy to tailor the seawater degradation behavior of polyesters through copolymerization.
Introduction
The rapid development of synthetic polymers has enabled their widespread applications in almost every area owing to their lightweight, mechanical robustness, low cost, and durability. However, the prevailing “production-use-disposal” paradigm of the linear plastic economy has led to escalating environmental and resource crises. These challenges have stimulated intensive efforts toward a more sustainable circular plastic economy.1–3 Besides physical recycling, chemical recycling has been a cutting-edge research frontier in both academic and industrial contexts. In particular, polymers with a “monomer–polymer–monomer” closed-loop lifecycle have emerged as a particularly promising solution to address the end-of-life problem of plastics. Such systems allow on-demand depolymerization under mild, energy-efficient conditions to regenerate pristine monomers that can be re-polymerized into polymers of virgin quality.4–6 Significant progress has been achieved in recent decades in designing polymers with intrinsic closed-loop recyclability, including polyesters,7–20 polycarbonates,21–24 polythioesters,25–33 polyacetals,34,35 and polyamides.36–38 Despite these advances, most reported systems rely on tailor-made cyclic monomers that require elaborate, costly, and multistep syntheses, presenting a substantial barrier to broader development. This limitation highlights the urgent need for efficient, conceptually simple strategies to access suitable cyclic monomers from abundant and inexpensive feedstocks.Among these, a particularly attractive approach is the controlled depolymerization of prepolymers, which can directly furnish cyclic monomers in high purity. A classic example is the depolymerization of low molecular weight (MW) polylactide (PLA), typically obtained from lactic acid polycondensation, to yield cyclic lactide in the presence of appropriate catalysts.39–41 Inspired by this paradigm, we envisioned a tandem polycondensation–depolymerization strategy to transform readily available oxalates into cyclic alkyl oxalates with diverse substituents. These monomers can undergo ring-opening polymerization (ROP) to produce polyoxalates with intrinsic closed-loop recyclability.
Polyoxalates represent a particularly promising yet underexplored class of sustainable polymers. Their feedstock, oxalic acid or oxalate, can be obtained on a large scale at low cost via carbohydrate oxidation or electrochemical CO2 reduction.42 In fact, massive oxalic acid/oxalate is already available from industrial processes, warranting various value-added applications. Beyond their accessibility, polyoxalates are especially attractive due to their unique marine degradability, in sharp contrast to widely used biodegradable polymers such as polylactide (PLA) and poly(butylene adipate terephthalate) (PBAT), which degrade extremely slow in seawater.43–46
Traditionally, polyoxalates have been synthesized via step-growth polycondensation of oxalic acid or oxalates with diols. For example, Miller and Garcia reported a series of polyoxalates from dimethyl oxalate and linear diols using p-toluenesulfonic acid catalysis.47 Wei and co-workers demonstrated kilogram-scale synthesis of poly(ethylene oxalate) (PEOx) by carefully tuning the stoichiometry and reaction conditions.46 While effective, the polycondensation route demands stringent stoichiometric control, high temperature, and vacuum conditions to achieve high MWs. In contrast, ROP of cyclic monomers offers superior atom economy, excellent control over MW and dispersity (Đ), and well-defined chain-end fidelity. Although ROP has been extensively exploited to prepare polyesters, polythioesters, and polycarbonates, yet cyclic oxalates remain largely unexplored. Earliest work by Carothers described the spontaneous polymerization of ethylene oxalate, albeit without systematic characterization.48 More recently, Melik-Nubarov and co-workers reported the ROP of cyclic propylene oxalate to yield poly(propylene oxalate) with MW up to 30 kDa.49 Despite these isolated efforts, a systematic exploration of cyclic 1,2-oxalates as ROP monomers as well as the resulting poly(1,2-oxalate)s’ structure–property relationships remains lacking. A key barrier is the facile and versatile synthesis of cyclic oxalates with diverse substituents.
Herein, we demonstrate that a tandem polycondensation–depolymerization strategy enables the efficient synthesis of a family of six-membered cyclic 1,2-oxalates directly from commodity oxalates and diols. Low MW poly(1,2-oxalate)s were first prepared by step-growth polycondensation of dimethyl oxalate with 1,2-alkyl diols, followed by selective depolymerization to afford cyclic oxalates. These monomers then underwent ROP to produce high MW poly(1,2-oxalate)s. The polymerization kinetics, thermodynamics, and thermal properties of the resulting materials were systematically investigated. Importantly, the polyoxalates could be selectively depolymerized back into their cyclic monomers in high yield, thereby completing a fully closed-loop “monomer–polymer–monomer” lifecycle. This work introduces a generalizable strategy to access closed-loop recyclable polymers from commodity oxalates and establishes poly(1,2-oxalate)s as a new platform for sustainable polymer design.
Results and discussion
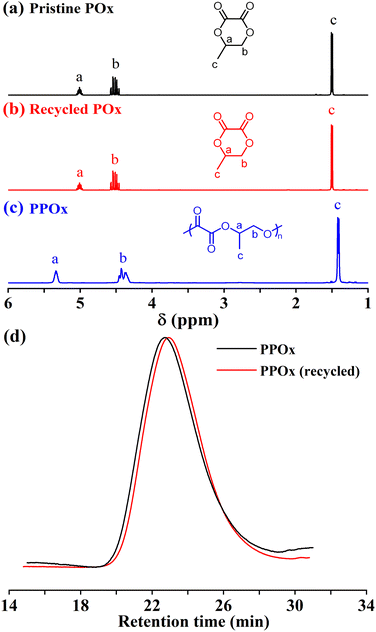
As illustrated in Scheme 1, the step-growth polycondensation of dimethyl oxalate (DMO) and 1,2-propylene diol was conducted using tetrabutyl titanate (TBT) as the catalyst (Fig. S1). Following reported protocol,50 the reaction was firstly performed at 80 °C for 1 hour under a nitrogen atmosphere. Subsequently, the temperature was raised step-wisely to 110 °C for 1 hour, 140 °C for 2 hours, and 150 °C for 1 hour. The system pressure was then gradually reduced to 50 Pa at 150 °C and maintained for 1–2 hours to remove methanol and excess 1,2-propanediol, driving the reaction equilibrium toward polymerization. Poly(propylene oxlate) (PPOx) with a Mn = 4.1 kDa and 96% yield can easily prepared as prepolymer. Then, the obtained PPOx prepolymer was directly used for depolymerization without further purification. The depolymerization and monomer isolation was combined together by stirring the low MW PPOx with catalyst at prescribed temperature under reduced pressure, so the POx monomer was directly collected before being subjected to yield and purity measurements. A series of catalysts was then screened for the depolymerization of PPOx, and the results were summarized in Table S1. When 1 wt% stannous octoate (Sn(Oct)2) was used as the catalyst, the depolymerization conducted at 180 °C (∼50 Pa) produced POx monomer with 82.9% purity and 63.4% yield. In contrast, both ZnCl2 and p-toluenesulfonic acid did not work well as they gave even lower yield and purity (Table S1, runs 2 and 3). When sodium acetate and sodium glycolate were used as the catalyst, the corresponding purity and yield of POx were 83.5%, 87.4% and 86.2%, 90.7%, respectively. We then used strong base KOH as the depolymerization catalyst, and obtained POx monomer with unexpected high purity (93%) and yield (90.2%). We also tried organobase 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as the catalyst and found both purity and yield of POx was lower than that of KOH (Table S1, run 7). Apparently, the KOH exhibited best activity and selectivity for the depolymerization of PPOx towards POx monomer. The depolymerization product analyzed by 1H NMR spectrum confirmed the presence of 1,2-propanediol, oligomers and minor unidentified impurities (Fig. S2). After the first round of workout, the crude product was further purified by rectification to afford high-purity POx suitable for ROP with an overall yield of 80%. The molecular structure was unambiguously verified by NMR and electrospray ionization mass spectroscopy (ESI-MS) techniques (Fig. 1a, S2 and S3). It was worth pointing out that the “polycondensation–depolymerization” strategy can be easily scaled up to produce 500 g POx in one batch in the lab, which demonstrated the feasibility of such strategy to make large quantity monomer and also paved a possible roadmap for large scale production of such cyclic monomers.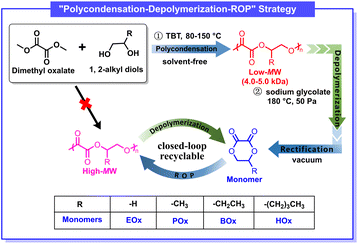 | ||
| Scheme 1 The preparation of high MW poly(1,2-oxalate)s via a “polycondensation–depolymerization” strategy. | ||
Since the (de)polymerization thermodynamics and polymer properties can be tailored by introducing suitable substituents on the six-membered ring, we herein synthesized a series of cyclic 1,2-alkylene oxalates, including ethylene oxalate (EOx), 1,2-propylene oxalate (POx), 1,2-butylene oxalate (BOx) and 1,2-hexylene oxalate (HOx) (Scheme 1). By doing so, the impacts of the alkyl length on the properties of resulting poly(1,2-oxalate)s can be systematically investigated. Several studies have attempted the depolymerization of low MW PEOx but the purity and yield of obtained EOx were not ideal for controlled ROP. On the other hand, the depolymerization of other poly(1,2-oxalate)s was scarcely reported. As such, the preparation of cyclic oxalates via the tandem “polycondensation–depolymerization” strategy was first investigated by screening the catalysts and optimizing the conditions (Table S2). BOx and HOx were successfully prepared via the similar procedure of POx using corresponding diols as the starting reagents (Table S2, runs 1 and 2).
Three cyclic 1,2-alkylene oxalates were obtained as colorless oil. In contrast, EOx is obtained as a white crystal with a high melting temperature of 144 °C. As such, the depolymerization of PEOx was conducted in a sublimation apparatus at a higher temperature of 210 °C. In such case, when the strong base KOH was used as the depolymerization catalyst, no EOx monomer was collected (Table S2, run 3). When the weaker base sodium glycolate was used as the catalyst, the depolymerization of PEOx afforded EOx with satisfactory yield (60.5%) and purity (85.7%) (Table S2, run 4). The crude EOx was further purified by recrystallization in tetrahydrofuran (THF) to produce high-purity monomer suitable for ROP. The chemical structures of purified cyclic oxalates were verified by NMR and ESI-MS (Fig. S5–S10).
The ROP of the obtained POx was then investigated using a series of catalysts in the presence of benzyl alcohol (BnOH) as the initiator at an initial monomer concentration of 4 mol L−1. The ROP of POx was first attempted using an organophosphazene base tBu-P2 as the catalyst at 25 °C with a feeding molar ratio of [POx]/[C]/[BnOH] = 300/1/1, which achieved a high monomer conversion of 97% within 2 min but produced PPOx with a moderate molecular weight distribution of Đ = 1.54 (Table S3,run 1). The weaker base tBu-P1 and DBU also exhibited high catalytic activity but moderate control over the polymerization as supported by the moderate dispersities (Table S3, runs 2 and 3). The typical commercial AlMe3 and TBT showed much lower catalytic activity compared to organobase, achieving low monomer conversion of ∼30% at a much longer time of 24 h (Table S3,runs 4 and 5). The rare-earth metal catalyst La[N(Si(CH3)3)2]3 demonstrated extremely high activity, reaching 96% monomer conversion within 1 min and producing PPOx with a Mn,SEC of 29.6 kDa (Table S3, run 6). However, the SEC curve of obtained PPOx displayed a shoulder peak (Fig. S11). Additionally, ZnEt2 and MeAl[salen] demonstrated moderate catalytic activity and controllability (Table S3, runs 7–9). For example, the ROP of POx catalyzed by MeAl[salen] reached 95% monomer conversion within 2 h and produced PPOx with a Mn of 32.9 kDa and a moderate Đ of 1.59.
Compared with solution polymerization, solvent-free bulk ROP is more environmentally friendly and suitable for industrial production. Therefore, we then investigated the ROP of POx under bulk condition at 130 °C. The organobases, including tBu-P1, tBu-P2, and DBU, still exhibited high catalytic activity, achieving almost quantitative monomer conversion within 2 min. However, significant yellow discoloration of the product was observed, indicating possible side reactions (Table S4, runs 1–3). TBT exhibited much lower catalytic activity and produced PPOx with a much lower Mn compared to the theoretical value (Table S4, run 4). In contrast, ZnEt2, MeAl[salen] and Sn(Oct)2 demonstrated high catalytic activity and good controllability toward the ROP of PPOx (Table S4, runs 5–6 and Table 1, run 1). When Sn(Oct)2 was used as the catalyst, the ROP of POx achieved a monomer conversion of 95% within 20 min. The obtained PPOx had a Mn of 32.3 kDa and a moderate Đ of 1.54 from SEC characterization (Table 1, run 1). Considering its low cost, easy handle and good stability to oxygen and moisture, Sn(Oct)2 was selected as the catalyst for further investigation.
| Run | Monomer | [M]/[C]/[I] | Temp. (°C) | Time (min) | Conv.b (%) | Mn,theoc (kDa) | Mn,SECd (kDa) | Đd |
|---|---|---|---|---|---|---|---|---|
a Conditions: 0.02 mmol Sn(Oct)2 was used as the catalyst and benzyl alcohol (BnOH) was used as the initiator. n.d. = not determined due to the poor solubility.b Determined by 1H NMR spectra.c Theoretical molecular weight was calculated from the feeding molar ratio and monomer conversion as Mn,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) theo = [M]/[I] × conv.(M) × MW(M) + MW(Initiator).d Determined by SEC in THF relative to PS standards. theo = [M]/[I] × conv.(M) × MW(M) + MW(Initiator).d Determined by SEC in THF relative to PS standards. |
||||||||
| 1 | POx | 300/1/1 | 130 | 20 | 95 | 37.1 | 32.3 | 1.54 |
| 2 | POx | 300/1/1 | 100 | 30 | 92 | 36.0 | 32.1 | 1.56 |
| 3 | POx | 300/0.3/1 | 130 | 30 | 94 | 36.7 | 32.2 | 1.57 |
| 4 | BOx | 300/1/1 | 130 | 20 | 94 | 40.4 | 41.0 | 1.57 |
| 5 | HOx | 300/1/1 | 130 | 20 | 94 | 48.6 | 45.2 | 1.58 |
| 6 | EOx | 300/1/1 | 180 | 20 | n.d | n.d | n.d | — |
| 7 | POx | 400/1/1 | 130 | 25 | 95 | 49.3 | 37.2 | 1.60 |
| 8 | BOx | 700/1/1 | 130 | 40 | 93 | 94.1 | 71.1 | 1.59 |
| 9 | HOx | 1000/1/1 | 130 | 60 | 94 | 162.5 | 106.6 | 1.62 |
| 10 | EOx | 1000/1/1 | 180 | 60 | n.d | n.d | n.d | — |
It was found that either decreasing the polymerization temperature to 100 °C or the catalyst loading to [POx]/[Sn(Oct)2]/[BnOH] = 300/0.3/1 did not change the MW and Đ of obtained PPOx, but resulted in a slightly reduced polymerization rate (Table 1, runs 2 and 3). Note that Sn(Oct)2 also exhibited excellent catalytic activity toward the ROP of BOx and HOx, achieving ∼94% monomer conversion within 20 min at 130 °C. For example, ROP of BOx conducted at a feeding molar ratio of [BOx]/[Sn(Oct)2]/[BnOH] = 300/1/1 produced PBOx with a Mn = 41.0 kDa and Đ = 1.57 (Table 1, run 4). The resultant PHOx was characterized by SEC to give a Mn = 45.2 kDa and Đ = 1.58 (Table 1, run 5). The measured Mns agreed well with the theoretical values calculated from the feeding molar ratio and monomer conversion, and the SEC curves were unimodal. These results indicated that ROP of cyclic 1,2-alkylene oxalates catalyzed by Sn(Oct)2 exhibited some controlled chain-growth characteristics (Fig. S12). Note all obtained poly(1,2-alkylene oxalate)s were observed as viscous oil at room temperature, indicating their amorphous characteristic and irregular main chain structures. Among these monomers, an exception is EOx. The ROP of EOx was conducted at 180 °C due to the high melting temperature of PEOx (Tm = 172–182 °C). In contrast to the poly(1,2-alkylene oxalate)s that exhibited good solubility in THF, the obtained PEOx was not soluble in common solvents. As an alternative to the SEC characterization, the intrinsic viscosity (IV) of the obtained PEOx was measured using an Ubbelohde viscometer in hexafluoroisopropanol (HFIP) to give a value of 0.80 dL g−1 (Table 1, run 6).
The obtained poly(1,2-alkylene oxalate)s were carefully examined with NMR and matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS) techniques (Fig. S13–S19). For example, Fig. 1b gives the representative 1H NMR spectrum of PPOx. The characteristic methine (signal b), methylene (signal d), and methyl (signal c) of the repeating units appears at 5.34, 4.43, and 1.42 ppm, respectively. The multiplet observed at 7.38 ppm can be assigned to the phenyl group of BnOH. The molecular weight Mn, NMR calculated from the relative integral ratio of repeating units (signal b) to the chain-end group (signal a) is 13.2 kDa, which is well consistent with the theoretical value and the measured Mn, SEC by SEC (Table S8, run 1). The 13C NMR spectrum shown in Fig. S13 also agrees well with the chemical structure of PPOx. Fig. S17 gives the MALDI-TOF mass spectrum of a PPOx sample (Mn = 7.1 kDa, Đ = 1.57) before subjecting to precipitation into methanol. As expected, a major group of molecular ion peaks with a separation of 130.1 Da can be assigned to the linear PPOx initiated by BnOH. Cyclic PPOx is observed in the low MW region due to the intramolecular transesterification or back-biting (Scheme S2, pathway A). In addition, the third group of molecular ion peaks is tentatively assigned to α, ω-phenylmethoxyl capped PPOx while the final group is assigned to 2-hydroxyl-propanoxyl terminated PPOx, which are attributed to the intermolecular transesterification (Scheme S2, pathway B).51 The MALDI-TOF mass spectra of PBOx and PHOx also exhibit similar four groups of molecular ion peaks due to inter- and intramolecular transesterification (Fig. S18 and S19).
 | ||
| Fig. 1 1H NMR spectra measured in CDCl3 of (a) POx and (b) PPOx obtained at [POx]/[Sn(Oct)2]/[BnOH] = 100/1/1. | ||
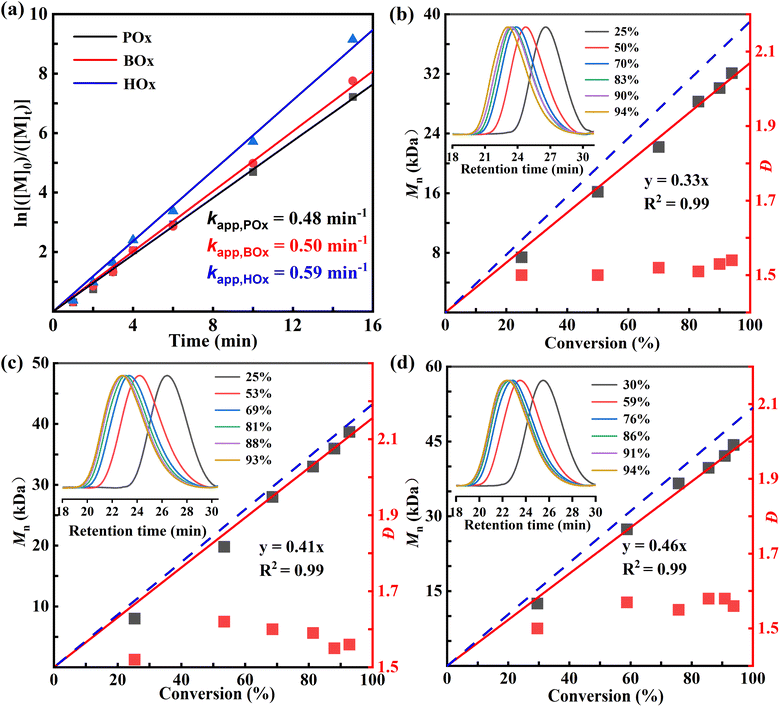
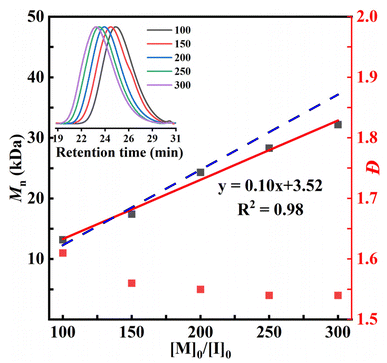
The ROP kinetics of POx, BOx, and HOx were investigated at the molar ratio of [M]0/[Sn(Oct)2]/[BnOH] = 300/1/1 in bulk at 130 °C (Fig. 2). The monomer conversions and the Mns of resultant polyoxalates were monitored by withdrawing aliquots of polymerization mixtures at certain time intervals and measured with 1H NMR and SEC, respectively. The linear correlation of ln([M]0/[M]t) versus time for ROP of POx suggested a first-order kinetic behavior relative to the monomer concentration in the presence of Sn(Oct)2 as the catalyst. The apparent kinetic constant calculated from the slope was kapp = 0.48 min−1. The ROP of POx exhibited some controlled polymerization characteristics as supported by the good linear dependence of measured Mns of PPOx as a function of monomer conversions as well as the unimodal SEC traces despite with moderate dispersities (Fig. 2b). Moreover, a series of PPOx with various MWs can be easily prepared by changing the feeding molar ratio of [M]0/[BnOH]. For example, as the targeted degree of polymerization (DP) increased from 100 to 300, the measured Mns of PPOx linearly increased from 13.2 to 32.2 kDa (Fig. 3). The ROPs of BOx and HOx also exhibited first-order kinetic behaviors, giving a slightly higher kapp value of 0.50 and 0.59 min−1 compared to that of POx, respectively. Similarly, the controlled polymerization of BOx and HOx catalyzed by Sn(Oct)2 was supported by the linear correlation of measured Mns of polyoxalates with the monomer conversion (Fig. 2c and d). The Mns of PBOx and PHOx increased linearly within the range of 500 DP and 700 DP to obtain polyoxalates with Mn up to 60.6 kDa and 94.0 kDa, respectively (Fig. S20 and S21). Note that the minor deviations between Mn,theo and Mn,SEC were attributed to the transesterification and back-biting side reactions.
It is worth pointing out that high MW polyoxalates can be prepared by further increasing the feeding molar ratio of [M]0/[BnOH] (Table 1, runs 7–9). For example, when the ROP of POx was conducted at a feeding molar ratio of [M]0/[Sn(Oct)2]/[BnOH] = 400/1/1, the Mn of PPOx further increased to 37.2 kDa (Table 1, run 7). PHOx with a Mn up to 106.6 kDa can be obtained at [M]0/[Sn(Oct)2]/[BnOH] = 1000/1/1. When the ROP of EOx was conducted at a feeding molar ratio of [M]0/[Sn(Oct)2]/[BnOH] = 1000/1/1, a PEOx sample with a IV = 1.56 was obtained. This value is much higher than that of PEOx prepared by polycondensation of DMO and ethylene glycol (IV = 0.78), highlighting the advantage of ROP strategy in preparing high-molecular-weight polyoxalates.52
We then investigated the polymerization thermodynamics of three monomers (Fig. S22–S24). The ROPs of cyclic 1,2-alkylene oxlates were conducted at different temperatures to measure the equilibrium monomer concentration. The polymerization was considered to reach equilibrium when the monomer conversion measured by 1H NMR remained constant for 30 min. The dependence of the equilibrium monomer concentration ([M]e) on temperature, that is, the Van't Hoff equation ln([M]e) = ΔHpθ/(TR) − ΔSpθ/R was used to calculate the thermodynamic parameters, which are summarized in Table 2. For example, the change of enthalpy (ΔHpθ) and entropy (ΔSpθ) for ROP of POx was calculated to be −14.1 kJ mol−1 and −34.3 J mol−1 K−1, respectively, which corresponded to a Gibbs free energy change of ΔGpθ = −3.87 kJ mol−1 at 25 °C. The ceiling temperature was thus calculated to be 138 °C at an initial monomer concentration of 1 mol L−1. BOx and HOx possessed comparable thermodynamic parameters (ΔHpθ and ΔSpθ) with those of POx, which suggested that the length of the alkyl substituents had a negligible impact on their ROP thermodynamics.
| M | kappa (min−1) | ΔHpθ (kJ mol−1) | ΔSpθ (J mol−1 k−1) | ΔGpθ (kJ mol−1) | Tcb (°C) |
|---|---|---|---|---|---|
| a Conditions: the polymerization was conducted at 130 °C in bulk at [M]0/[Sn(Oct)2]/[BnOH] = 300/1/1.b Calculated at [M]0 = 1.0 mol L−1. | |||||
| POx | 0.48 ± 0.01 | −14.1 ± 0.6 | −34.3 ± 1.4 | −3.87 ± 0.73 | 411 ± 33 |
| BOx | 0.50 ± 0.01 | −12.9 ± 0.4 | −32.0 ± 1.1 | −3.48 ± 0.52 | 403 ± 26 |
| HOx | 0.59 ± 0.01 | −13.5 ± 0.6 | −31.8 ± 1.5 | −4.02 ± 0.75 | 424 ± 38 |
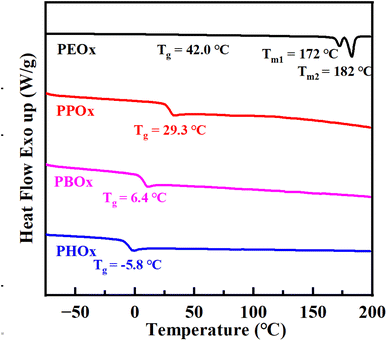
The thermal stability of obtained polyoxalates was then investigated with thermogravimetric analysis (TGA). All samples displayed a one-step decomposition profile (Fig. S25). Compared to PPOx (Td,5% = 193 °C), PBOx and PHOx with longer pendent alkyl groups exhibited improved thermal stability as evidenced by their improved Td,5% (the temperature at 5% weight loss) of 250 and 257 °C, respectively. The thermal behaviors of obtained polyoxalates were further investigated with differential scanning calorimetry (DSC). No endothermal peaks were observed for PPOx, PBOx and PHOx at both the first and second heating scans. The absence of melting transition suggested the amorphous characteristic of poly(1,2-alkylene oxalate)s, which was probably ascribed to the lack of stereoregularity on the polyoxalate backbones. As the pendant alkyl substituent length increased from C1 to C2 to C4, the glass transition temperature (Tg) of PPOx, PBOx, and PHOx slightly decreased from 29.3 to 6.4 to −5.8 °C, respectively (Fig. 4). In contrast, two melting transition peaks at 172 and 182 °C were observed in the second heating scan of DSC curve of PEOx, suggesting that PEOx is a semi-crystalline polyoxalate.
The mechanical properties of obtained PPOx and PEOx were then investigated using uniaxial tensile testing at an extension rate of 50 mm min−1. PBOx and PHOx failed to form films due to their low Tgs and amorphous characteristic, so they were not investigated. Both PPOx and PEOx behaved as brittle plastics and exhibited low enlongation at break. For example, PPOx with a Mn of 37.2 kDa gave a tensile stress of 31.2 ± 1.1 MPa and an elongation at break of 5.4 ± 1.0% at 20 °C. PEOx with a IV = 1.56 exhibited even higher tensile strength of 100.5 MPa, which was 30.5 MPa higher compared to that of PEOx obtained via the polycondensation strategy.52 In sharp contrast to the low degradation rate of PLA and PCL, it is reported that PEOx exhibited rapid degradation in marine environment. We herein compared the hydrolytic degradation behaviors of PEOx and PPOx by immersing their square-shape films in water or artificial seawater. The weigh loss of each sample was monitored by taken out of the specimen from the solution, washed with distilled water and then dried to constant weight. As shown in Fig. 5b, both PEOx and PPOx showed excellent degradability in water and artificial seawater. Compared to PEOx that only achieved 39% degradation in water within 30 days, PPOx demonstrated superior degradability and achieved complete degradation within 5 days. Fig. S27 gives the SEC curves of the remaining polymer during the degradation of PPOx in artificial seawater. As expected, the remaining polymer exhibited a gradually decreased molecular weight but an increased dispersity (Table S15), which was consistent with the random chain scission during hydrolysis. The pH value of the final degradation solution was measured to be 2, suggesting the presence of oxalic acid. Moreover, the 1H NMR spectrum of the solution clearly displays the proton resonances of 1,2-propanediol (Fig. S28). Note that the proton resonances of oxalic acid cannot be observed by 1H NMR spectrum. These results support the good marine degradability of PPOx.
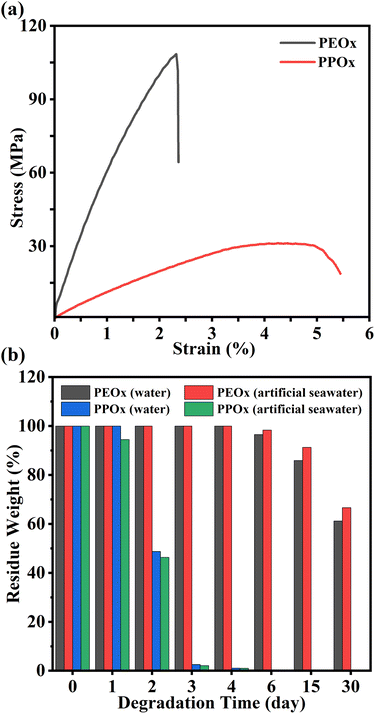 | ||
| Fig. 5 (a) Stress–strain curves of PPOx (Mn = 37.2 kDa, Đ = 1.60) and PEOx (IV = 1.56). (b) Hydrolytic degradation profiles of PEOx and PPOx in water or artificial seawater. | ||
In addition to the low MW polyoxalates, it is supposed that the obtained poly(1,2-alkylene oxalate)s with high MW are also capable of depolymerizing back to pristine monomers given their moderate ceiling temperatures. The depolymerization of PPOx (Mn = 32.2 kDa) that conducted in a distillation apparatus in bulk at 170 °C under reduced pressure (∼50 Pa) in the presence of 0.5 wt% Sn(Oct)2 as the catalyst produced POx with a yield of 70% within 6 h. The 1H NMR spectrum of recycled POx clearly displayed the signal of Sn(Oct)2 (Fig. S29). This result suggested that Sn(Oct)2 was distilled out from the reaction mixture at such a high temperature and vacuum, which accounted for the moderate monomer yield. In contrast, when 0.5 wt% sodium glycolate was used as the catalyst, the depolymerization of PPOx produced clean POx with an almost quantitative yield of 96% within 4 h under similar conditions. The high-purity of recovered POx was evidenced by the 1H NMR spectrum (Fig. 6b). Of note, the recovered POx can be further repolymerized to produce PPOx with almost the same MW and dispersity as the original monomer (Fig. 6d), thus successfully establishing the “monomer–polymer–monomer” closed-loop life cycle. The successful depolymerization of PBOx and PHOx to recover the monomers was also achieved using sodium glycolate as the catalyst to give a high monomer yield of 90% and 91%, respectively (Fig. S30 and S31).
Conclusions
In summary, we have developed a one-pot “polycondensation–depolymerization” strategy to achieve the scalable production of a series of cyclic 1,2-alkylene oxalate with high yield, which underwent ROPs to produce closed-loop recyclable polyoxalates using Sn(Oct)2 as the catalyst. The ROPs of these cyclic 1,2-alkylene oxalates in the presence of Sn(Oct)2 as the catalyst exhibited first-order kinetic behaviors relative to monomer concentration. The cyclic 1,2-alkylene oxalates exhibited slightly higher kinetic constant as the increase of the alkyl substituent length. On the other hand, the pendant alkyl substituent length had a negligible effect on their polymerization thermodynamic parameters. The ROPs of cyclic 1,2-alkylene oxalates catalyzed by Sn(Oct)2 exhibited controlled polymerization characteristics as supported by the linear correlation of measured Mns of obtained polyoxalates as a function of monomer conversion and feeding molar ratio of [M]/[I] despite with moderate dispersities. Compared to the polycondensation strategy, the ROP of cyclic oxalate has a significant advantage in preparing high-molecular-weight polyoxalates. The obtained PPOx, PBOx and PHOx were amorphous polyoxalates with Tg values decreased as their alkyl length increased. In contrast, the high-molecular-weight PEOx sample with an IV = 1.56 was a semi-crystalline polyoxalate and exhibited a high tensile strength of 100.5 MPa but a low enlongation at break of 2.3%. Remarkably, the high MW polyoxalates can be chemically recycled back to recover pristine cyclic oxalates with >90% yield by vacuum distillation at 170 °C in the presence of sodium glycolate as the catalyst. Compared to PEOx, PPOx exhibited superior degradability in both water and artificial sea water. Considering the unique marine degradability of these polyoxalates, it is attractive to further investigate the copolymerization of cyclic oxalates with the commercial lactones or lactides, such as ε-caprolactone and L-lactide, to arbitrarily tune the degradability of obtained copolyesters.Author contributions
Yalei Liu: investigation, formal analysis, visualization, writing – original draft. Zheng Li and Dongfang Zhao: investigation. Yong Shen and Zhibo Li: conceptualization, formal analysis, supervision, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experiment details, 1H and 13C NMR spectra, ESI-MS spectrum, MALDI-TOF mass spectra, SEC curves, DSC and TGA results, degradation results. See DOI: https://doi.org/10.1039/d5sc08361c.Acknowledgements
The authors appreciate the financial support by National Natural Science Foundation of China (No. U24A20558 and 52322304), Taishan Scholar Foundation of Shandong Province (No. tsqn202103078), State Key Laboratory of Chemical Engineering and Low-carbon Technology (No. SKL-ChE-25T01).Notes and references
- Y. Sun, Z. An, Y. Gao, R. Hu, Y. Liu, H. Lu, X.-B. Lu, X. Pang, A. Qin, Y. Shen, Y. Tao, Y.-Z. Wang, J. Wang, G. Wu, G.-P. Wu, T.-Q. Xu, X.-H. Zhang, Y. Zhang, Z. Zhang, J.-B. Zhu, M. Hong and Z. Li, New sustainable polymers with on-demand depolymerization property, Sci. China. Chem., 2024, 67, 2803–2841 Search PubMed.
- F. M. Haque, J. S. A. Ishibashi, C. A. L. Lidston, H. Shao, F. S. Bates, A. B. Chang, G. W. Coates, C. J. Cramer, P. J. Dauenhauer, W. R. Dichtel, C. J. Ellison, E. A. Gormong, L. S. Hamachi, T. R. Hoye, M. Jin, J. A. Kalow, H. J. Kim, G. Kumar, C. J. LaSalle, S. Liffland, B. M. Lipinski, Y. Pang, R. Parveen, X. Peng, Y. Popowski, E. A. Prebihalo, Y. Reddi, T. M. Reineke, D. T. Sheppard, J. L. Swartz, W. B. Tolman, B. Vlaisavljevich, J. Wissinger, S. Xu and M. A. Hillmyer, Defining the Macromolecules of Tomorrow through Synergistic Sustainable Polymer Research, Chem. Rev., 2022, 122, 6322–6373 Search PubMed.
- C. Shi, E. C. Quinn, W. T. Diment and E. Y. X. Chen, Recyclable and (Bio)degradable Polyesters in a Circular Plastics Economy, Chem. Rev., 2024, 124, 4393–4478 Search PubMed.
- C. Shi, L. T. Reilly, V. S. Phani Kumar, M. W. Coile, S. R. Nicholson, L. J. Broadbelt, G. T. Beckham and E. Y. X. Chen, Design principles for intrinsically circular polymers with tunable properties, Chem, 2021, 7, 2896–2912 Search PubMed.
- X. Tang and E. Y. X. Chen, Toward Infinitely Recyclable Plastics Derived from Renewable Cyclic Esters, Chem, 2019, 5, 284–312 Search PubMed.
- Z. Li, Y. Shen and Z. Li, Ring-Opening Polymerization of Lactones to Prepare Closed-Loop Recyclable Polyesters, Macromolecules, 2024, 57, 1919–1940 Search PubMed.
- C. Li, L. Wang, Q. Yan, F. Liu, Y. Shen and Z. Li, Rapid and Controlled Polymerization of Bio-sourced δ-Caprolactone toward Fully Recyclable Polyesters and Thermoplastic Elastomers, Angew. Chem., Int. Ed., 2022, 61, e202201407 Search PubMed.
- J. Li, F. Liu, Y. Liu, Y. Shen and Z. Li, Functionalizable and Chemically Recyclable Thermoplastics from Chemoselective Ring-Opening Polymerization of Bio-renewable Bifunctional α-Methylene-δ-valerolactone, Angew. Chem., Int. Ed., 2022, 61, e202207105 Search PubMed.
- Z. Li, D. Zhao, Y. Shen and Z. Li, Ring-opening Polymerization of Enantiopure Bicyclic Ether-ester Monomers toward Closed-loop Recyclable and Crystalline Stereoregular Polyesters via Chemical Upcycling of Bioplastic, Angew. Chem., Int. Ed., 2023, 62, e202302101 Search PubMed.
- C. Weng, Z. Ding, W. Qiu, B. Wang and X. Tang, Achieving Exceptional Thermal and Hydrolytic Resistance in Chemically Circular Polyesters via In-Chain 1,3-Cyclobutane Rings, Angew. Chem., Int. Ed., 2024, 63, e202401682 Search PubMed.
- Q. Yan, J. Ma, W. Pei, Y. Zhang, R. Zhong, S. Liu, Y. Shen and Z. Li, Chemoselective Ring-Opening Polymerization of α-Methylene-δ-valerolactone Catalyzed by a Simple Organoaluminum Complex to Prepare Closed-Loop Recyclable Functional Polyester, Angew. Chem., Int. Ed., 2025, 64, e202418488 Search PubMed.
- Y. Shen, W. Xiong, Y. Li, Z. Zhao, H. Lu and Z. Li, Chemoselective Polymerization of Fully Biorenewable α-Methylene-γ-Butyrolactone Using Organophosphazene/Urea Binary Catalysts Toward Sustainable Polyesters, CCS Chem., 2021, 3, 620–630 Search PubMed.
- H. Fan, C. Hu, M. Niu, Q. Zhang, B. Li, X. Pang and X. Chen, Modular Access from Acrylate to a Sustainable Polyester Platform with Large-Span Tunability and Chemical Circularity under Mild Conditions, J. Am. Chem. Soc., 2025, 147, 9836–9843 Search PubMed.
- C.-T. Han, K. Ma, Z. Zhang, R. W. Clarke, R. R. Gowda, T.-Q. Xu and E. Y. X. Chen, Circular Polymer Designed by Regulating Entropy: Spiro-Valerolactone-Based Polyesters with High Gas Barriers and Adhesion Strength, J. Am. Chem. Soc., 2025, 147, 4511–4519 Search PubMed.
- H.-Y. Huang, M. Xie, S.-Q. Wang, Y.-T. Huang, Y.-H. Luo, D.-G. Yu, Z. Cai and J.-B. Zhu, Ultratough Thermoplastic Elastomers Based on Chemically Recyclable Cycloalkyl-Substituted Polyhydroxyalkanoates, J. Am. Chem. Soc., 2025, 147, 7788–7798 Search PubMed.
- Y. M. Tu, X. M. Wang, X. Yang, H. Z. Fan, F. L. Gong, Z. Cai and J. B. Zhu, Biobased High-Performance Aromatic-Aliphatic Polyesters with Complete Recyclability, J. Am. Chem. Soc., 2021, 143, 20591–20597 Search PubMed.
- L. Zhou, Z. Zhang, C. Shi, M. Scoti, D. K. Barange, R. R. Gowda and E. Y.-X. Chen, Chemically circular, mechanically tough, and melt-processable polyhydroxyalkanoates, Science, 2023, 380, 64–69 Search PubMed.
- J. B. Zhu, E. M. Watson, J. Tang and E. Y. Chen, A Synthetic Polymer System with Repeatable Chemical Recyclability, Science, 2018, 360, 398–403 Search PubMed.
- L. Wang, Y. Shen and Z. Li, Properties Study of Thermoplastic Elastomers Prepared by Sequential Ring-opening Copolymerization of Bio-based δ-Caprolactone and L-lactide, Acta Polym Sin, 2024, 55, 582–593 Search PubMed.
- D. Zhao, Z. Li, Y. Shen and Z. Li, Synthesis and Ring-opening Polymerization of Benzoxy Substituted Oxa-lactones, Acta Polym Sin, 2023, 54, 1303–1311 Search PubMed.
- W. Zhang, J. Dai, Y.-C. Wu, J.-X. Chen, S.-Y. Shan, Z. Cai and J.-B. Zhu, Highly Reactive Cyclic Carbonates with a Fused Ring toward Functionalizable and Recyclable Polycarbonates, ACS Macro Lett., 2022, 11, 173–178 Search PubMed.
- W.-N. Liu, M. Wang, Z. Ding, Y. Li and B. Wang, Modular Access to Aliphatic Polycarbonates with Tunable Properties and Dual Closed-Loop Recyclability by Polycondensation–Depolymerization–Repolymerization Strategy, Angew. Chem., Int. Ed., 2025, 64, e202505333 Search PubMed.
- C. Shi, W. T. Diment and E. Y.-X. Chen, Closed-Loop Recycling of Mixed Plastics of Polyester and CO2-Based Polycarbonate to a Single Monomer, Angew. Chem., Int. Ed., 2024, 63, e202405083 Search PubMed.
- Y. Yu, B. Gao, Y. Liu and X.-B. Lu, Efficient and Selective Chemical Recycling of CO2-Based Alicyclic Polycarbonates via Catalytic Pyrolysis, Angew. Chem., Int. Ed., 2022, 61, e202204492 Search PubMed.
- K. A. Stellmach, M. K. Paul, M. Xu, Y.-L. Su, L. Fu, A. R. Toland, H. Tran, L. Chen, R. Ramprasad and W. R. Gutekunst, Modulating Polymerization Thermodynamics of Thiolactones Through Substituent and Heteroatom Incorporation, ACS Macro Lett., 2022, 11, 895–901 Search PubMed.
- H.-Z. Fan, X. Yang, J.-H. Chen, Y.-M. Tu, Z. Cai and J.-B. Zhu, Advancing the Development of Recyclable Aromatic Polyesters by Functionalization and Stereocomplexation, Angew. Chem., Int. Ed., 2022, 61, e202117639 Search PubMed.
- W. Pei, Y. Liu, Q. Yan, K. Yuan, S. Li, Y. Shen and Z. Li, Crystallization/Precipitation Driven Non-equilibrium Ring-opening Polymerization of Thiovalerolactone toward Closed-loop Recyclable Polythioester with Excellent Barrier Properties, Angew. Chem., Int. Ed., 2025, 64, e202505104 Search PubMed.
- Y. Wang, M. Li, J. Chen, Y. Tao and X. Wang, O-to-S Substitution Enables Dovetailing Conflicting Cyclizability, Polymerizability, and Recyclability: Dithiolactone vs. Dilactone, Angew. Chem., Int. Ed., 2021, 60, 22547–22553 Search PubMed.
- Y. Zhu, M. Li, Y. Wang, X. Wang and Y. Tao, Performance-Advantaged Stereoregular Recyclable Plastics Enabled by Aluminum-Catalytic Ring-Opening Polymerization of Dithiolactone, Angew. Chem., Int. Ed., 2023, 62, e202302898 Search PubMed.
- W. Xiong, W. Chang, D. Shi, L. Yang, Z. Tian, H. Wang, Z. Zhang, X. Zhou, E.-Q. Chen and H. Lu, Geminal Dimethyl Substitution Enables Controlled Polymerization of Penicillamine-Derived β-Thiolactones and Reversed Depolymerization, Chem, 2020, 6, 1831–1843 Search PubMed.
- Y. Wang, Y. Zhu, W. Lv, X. Wang and Y. Tao, Tough while Recyclable Plastics Enabled by Monothiodilactone Monomers, J. Am. Chem. Soc., 2023, 145, 1877–1885 Search PubMed.
- J. Yuan, W. Xiong, X. Zhou, Y. Zhang, D. Shi, Z. Li and H. Lu, 4-Hydroxyproline-Derived Sustainable Polythioesters: Controlled Ring-Opening Polymerization, Complete Recyclability, and Facile Functionalization, J. Am. Chem. Soc., 2019, 141, 4928–4935 Search PubMed.
- L. Zhou, L. T. Reilly, C. Shi, E. C. Quinn and E. Y. X. Chen, Proton-triggered topological transformation in superbase-mediated selective polymerization enables access to ultrahigh-molar-mass cyclic polymers, Nat. Chem., 2024, 16, 1357–1365 Search PubMed.
- H. G. Hester, B. A. Abel and G. W. Coates, Ultra-High-Molecular-Weight Poly(Dioxolane): Enhancing the Mechanical Performance of a Chemically Recyclable Polymer, J. Am. Chem. Soc., 2023, 145, 8800–8804 Search PubMed.
- B. A. Abel, R. L. Snyder and G. W. Coates, Chemically Recyclable Thermoplastics from Reversible-deactivation Polymerization of Cyclic Acetals, Science, 2021, 373, 783–789 Search PubMed.
- X.-T. Tang, J.-A.-Q. Zhou, Y.-M. Tu, H.-Z. Fan, M.-Y. Wang, Q. Cao, Z. Cai and J.-B. Zhu, Ring-Opening Polymerization Enables Access to High-Performance Aliphatic-Aromatic Polyamides with Chemical Recyclability, Angew. Chem., Int. Ed., 2025, 64, e202505310 Search PubMed.
- R. M. Cywar, N. A. Rorrer, H. B. Mayes, A. K. Maurya, C. J. Tassone, G. T. Beckham and E. Y. X. Chen, Redesigned Hybrid Nylons with Optical Clarity and Chemical Recyclability, J. Am. Chem. Soc., 2022, 144, 5366–5376 Search PubMed.
- J.-J. Tian, X. Liu, L. Ye, Z. Zhang, E. C. Quinn, C. Shi, L. J. Broadbelt, T. J. Marks and E. Y.-X. Chen, Redesigned Nylon 6 Variants with Enhanced Recyclability, Ductility, and Transparency, Angew. Chem., Int. Ed., 2024, 63, e202320214 Search PubMed.
- C. F. Gallin, W.-W. Lee, J. A. Byers and A. Simple, Selective, and General Catalyst for Ring Closing Depolymerization of Polyesters and Polycarbonates for Chemical Recycling, Angew. Chem., Int. Ed., 2023, 62, e202303762 Search PubMed.
- W. Zhao, Z. Guo, J. He and Y. Zhang, Solvent-Free Chemical Recycling of Polyesters and Polycarbonates by Magnesium-based Lewis Acid Catalyst, Angew. Chem., Int. Ed., 2025, 64, e202420688 Search PubMed.
- T. M. McGuire, A. Buchard and C. Williams, Chemical Recycling of Commercial Poly(L-lactic acid) to L-Lactide Using a High-Performance Sn(II)/Alcohol Catalyst System, J. Am. Chem. Soc., 2023, 145, 19840–19848 Search PubMed.
- M. A. Murcia Valderrama, R.-J. van Putten and G.-J. M. Gruter, The potential of oxalic – and glycolic acid based polyesters (review). Towards CO2 as a feedstock (Carbon Capture and Utilization – CCU), Eur. Polym. J., 2019, 119, 445–468 Search PubMed.
- Q. Luan, H. Hu, X. Jiang, C. Lin, X. Zhang, Q. Wang, Y. Dong, J. Wang and J. Zhu, Melt polycondensation of poly (butylene oxalate-co-succinate) with great potential in curbing marine plastic pollution, J. Hazard. Mater., 2023, 457, 131801 Search PubMed.
- Q. Luan, H. Hu, X. Ouyang, X. Jiang, C. Lin, H. Zhu, T. Shi, Y.-L. Zhao, J. Wang and J. Zhu, New modifications of PBAT by a small amount of oxalic acid: Fast crystallization and enhanced degradation in all natural environments, J. Hazard. Mater., 2024, 465, 133475 Search PubMed.
- Z. Tu, L. Wang, Y. Lu, Y. Li, L. Sang, Y. Zhang and Z. Wei, Rapid marine degradable poly(butylene oxalate) by introducing promotion building blocks, J. Hazard. Mater., 2024, 462, 132791 Search PubMed.
- Z. Tu, Y. Lu, L. Sang, Y. Zhang, Y. Li and Z. Wei, Kilogram-Scale Preparation of Poly(ethylene oxalate) toward Marine-Degradable Plastics, Macromolecules, 2023, 56, 3149–3159 Search PubMed.
- J. J. Garcia and S. A. Miller, Polyoxalates from biorenewable diols via Oxalate Metathesis Polymerization, Polym. Chem., 2014, 5, 955–961 Search PubMed.
- W. H. Carothers, J. A. Arvin and G. L. Dorough, Studies on polymerization and ring formation. V. glycol esters of oxalic acid, J. Am. Chem. Soc., 1930, 52, 3292–3300 Search PubMed.
- N. P. Iakimov, E. M. Budynina, A. K. Berkovich, M. V. Serebryakova, V. B. Platonov, E. O. Fomin, A. G. Buyanovskaya, I. V. Mikheev and N. S. Melik-Nubarov, Polymerization of six-membered propylene oxalate, Eur. Polym. J., 2024, 220, 113410 Search PubMed.
- L. Wang, Z. Tu, J. Liang, Y. Wang and Z. Wei, Development of poly(butylene oxalate-co-furanoate) copolymers with enhanced sustainability and hydrolytic degradability, J. Hazard. Mater., 2024, 480, 135997 Search PubMed.
- N. P. Iakimov, E. M. Budynina, E. O. Fomin, A. A. Egorova, A. K. Berkovich, M. V. e. Serebryakova, I. D. Grozdova and N. S. Melik-Nubarov, End-group analysis to characterize the ring-opening polymerization of propylene oxalate, Mendeleev Commun., 2025, 35, 586–588 Search PubMed.
- Z. Tu, Y. Lu, Y. Zhang, Y. Li and Z. Wei, Development of poly(n-alkylene oxalate)s toward a new kind of seawater degradable plastics, Mater. Today Sustainability, 2023, 22, 100378 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |