Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High-valent sulfur fluorides as reactivity switches for PFAS-free benzene–azepine skeletal editing
Chavakula
Nagababu
a,
Takuya
Muramatsu
b,
Muhamad Zulfaqar
Bacho
 a,
Shiwei
Wu
b,
Seishu
Ochiai
b,
Jorge
Escorihuela
a,
Shiwei
Wu
b,
Seishu
Ochiai
b,
Jorge
Escorihuela
 cd and
Norio
Shibata
cd and
Norio
Shibata
 *ab
*ab
aDepartment of Nanopharmaceutical Sciences, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555, Japan. E-mail: nozshiba@nitech.ac.jp
bDepartment of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555, Japan
cDepartamento de Química Orgánica, Universitat de València, Avda. Vicente Andrés Estellés s/n, Burjassot 46100, Valencia, Spain
dInstituto de Ciencia Molecular (ICMol), Universitat de València, Calle Catedrático José Beltrán 2, Paterna, Valencia, Spain
First published on 19th November 2025
Abstract
The development of non-PFAS fluorinated heterocycles is a critical challenge in sustainable molecular design. Here we report a reactivity-switching skeletal editing strategy that transforms benzenes into azepines through visible-light activation mediated by high-valent sulfur fluorides. The pentafluorosulfanyl (SF5) and tetrafluorosulfanyl (SF4) groups act as powerful electronic activators that stabilize singlet nitrenes, lowering the barrier for 6π-electrocyclization and enabling efficient benzene-to-azepine interconversion in high yields. Mechanistic and DFT studies reveal that the strong electron-withdrawing capacity of SF5/SF4 is key to promoting this transformation. Importantly, SF5 and SF4 motifs are PFAS-free fluorine architectures that retain the beneficial physicochemical properties of perfluoroalkyl groups while avoiding environmental persistence. This work establishes a sustainable and general strategy for fluorinated skeletal editing, offering a foundation for future design of PFAS-free agrochemical, medicinal, and material scaffolds.
Introduction
Fluorinated substituents are indispensable in the molecular design of pharmaceuticals and agrochemicals, where subtle modifications in lipophilicity, metabolic stability, and target engagement can critically influence whether a lead compound progresses to a viable product.1–3 Among these, the trifluoromethyl (CF3) group has long been a widely adopted motif. However, growing concerns have emerged regarding its environmentally persistent degradation product, trifluoroacetic acid (TFA), which is now regulated as part of the PFAS (per- and polyfluoroalkyl substances) class.4,5 This issue is particularly pressing in sectors with unavoidable environmental release, such as agrochemicals, outdoor coatings, and high-volume polymers, where new fluorinated motifs are urgently needed that retain the performance advantages of CF3 while avoiding its long-term ecological impact. The challenge is especially acute in agrochemistry, where over 40% of fluorinated agrochemicals incorporate CF3.3 These compounds are vital to ensuring global food security in the face of a rapidly growing population. The high-valent sulfur fluorides, such as pentafluorosulfanyl (SF5)6,7 and trans-tetrafluorosulfanyl (trans-SF4)8–10 groups directly address this challenge. The SF5 group is more electronegative than CF3, nearly twice more lipophilic, and adopts a compact “umbrella-like” geometry. Importantly, the S–F bonds in SF5 are significantly weaker than C–F bonds, offering potential for improved environmental degradability (Fig. 1a). Seminal contributions by Sheppard,11,12 and the development of Umemoto's oxidative protocol13 have made aryl-SF5 compounds synthetically accessible, enabling incorporation into structures such as pyridines14,15 and five-membered heterocycles16—key motifs in agrochemicals. However, SF5-containing seven-membered heterocycles remain entirely unexplored.A structurally complementary but similarly underutilized motif is the trans-SF4 group. In contrast to SF5, which functions as a terminal substituent analogous to CF3, the SF4 moiety serves as a linear linker, connecting two carbon units across a C–SF4–C axis measuring just 3.66 Å. This makes trans-SF4 the shortest member of the “linear bioisostere” family, which includes alkynes (4.13 Å), bicyclo[1.1.1]pentane (4.91 Å), cubane (5.73 Å), and para-substituted phenylene (5.86 Å).8–10 When combined with an alkyne, the trans-SF4 group forms the longest fully linear connector known to date—6.23 Å—providing medicinal chemists with an exceptional rod-like linker for fragment-based design and spatial tuning (Fig. 1b).
The azepine ring—a seven-membered nitrogen-containing heterocycle—is a privileged scaffold found in numerous antiviral, anticancer, and neuroactive agents.17,18 One of the most conceptually elegant strategies for its construction is photoinduced dearomative coupling (PDC) of aryl azides, a transformation that effectively repurposes benzene rings into azepine frameworks.19–26 While PDC has been widely investigated, its synthetic utility remains limited by generally moderate yields and poor compatibility with phenolic nucleophiles. In fact, the only reported example involving a phenol—Takeuchi's 1982 study—afforded the desired diaryl ether azepine in a mere 7.7% yield (Fig. 1d).27
The PDC sequence involves four mechanistic steps: photoactivation of the aryl azide to generate a singlet nitrene, intramolecular insertion to form an azirine intermediate, electrocyclic ring-opening to a conjugated ketenimine, and final nucleophilic trapping to yield the azepine product. Since the SF5 group dramatically alters the physiochemical properties of the connected benzene ring,6,7,11,12 we hypothesized that the SF5 group would accelerate nitrene formation, promote efficient azirine ring expansion, and significantly enhance the electrophilicity of the ketenimine intermediate. Together, these effects would break through the long-standing yield barrier and unlock phenol-compatible routes to azepine architectures. We report herein a visible-light-driven (blue LED, 455 nm), base-mediated protocol that transforms SF5-phenyl azides into SF5-azepine diaryl ethers in isolated yields of up to 83%—approximately twice the efficiency achieved with non-SF5 analogues. These azepine products undergo efficient skeletal contraction back to SF5-aryl amines in yields exceeding 90% upon treatment with acid anhydrides, enabling a reversible and programmable interconversion between benzene and azepine frameworks with broad functional group tolerance (Fig. 1e). This concept also extends to trans-SF4 alkynyl azides, which, under 365 nm irradiation, furnish the first azepine derivatives incorporating this uniquely linear, rod-like spacer. Beyond their skeletal reversion, trans-SF4 alkynyl azepines retain the synthetic versatility of alkyne functionality, offering broad opportunities for downstream chemical transformations (Fig. 1f). These findings establish high-valent sulfur fluorides not simply as PFAS-safe sustainable CF3 alternatives, but as reactivity-switching motifs for skeletal editing—unlocking novel fluorinated architectures for next-generation drugs and materials.
Results and discussion
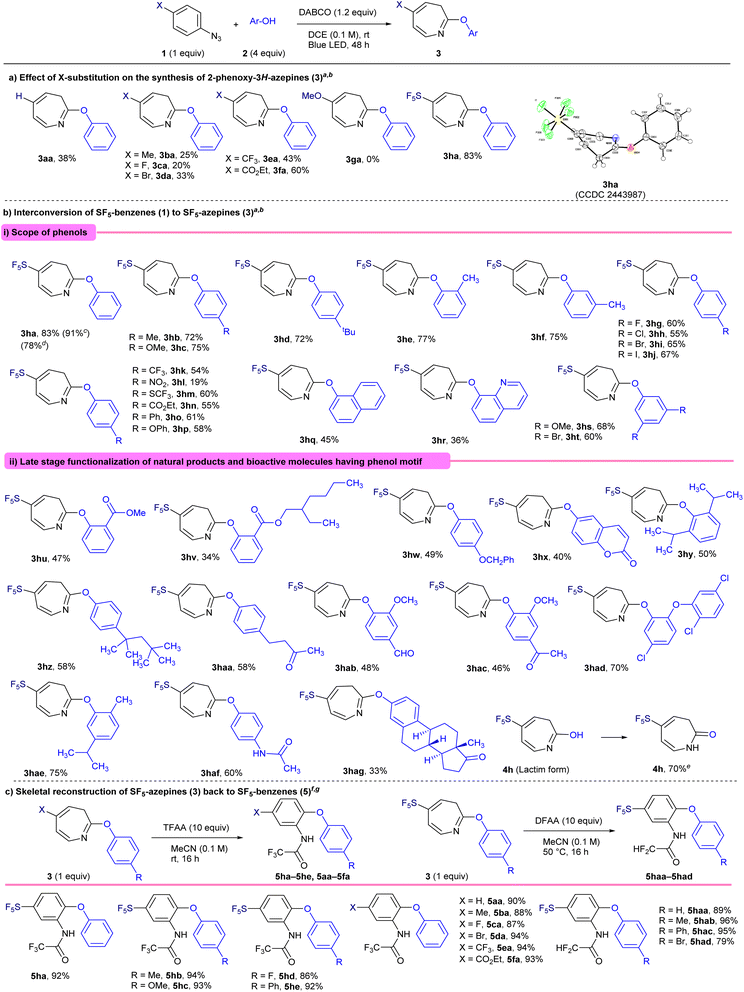
We began our investigation by revisiting the photoinduced dearomative coupling reaction of phenyl azide (1a) with phenol (2a) (Table 1), originally examined by Takeuchi et al. in 1982, which yielded azepine-phenyl-ether (3aa) in a modest yield (7.7%)27 (entry 1). Their protocol was employed in a quartz tube with a 300 W high-pressure mercury lamp emitting aggressive short-wavelength radiation; thus, we aimed to improve this method by utilizing a milder and more environmentally friendly blue LED (40 W, 455 nm) source. Initially, the reaction did not proceed in EtOH under blue LED irradiation for 24 h (entry 2). To enhance the nucleophilicity of phenol, we subsequently added K2CO3 and changed the solvent from EtOH to DCE to avoid the competitive insertion of ethanol. The observed yield of 3aa was 27%. We further screened various bases, including Na2CO3, KOH, Et3N, DBU, and DABCO, and found that DABCO was the optimal choice, yielding desired product 3aa in 38% yield. Changing the irradiation source from a standard blue LED (40 W) to a 365 nm LED reduced the yield to 34%. Control experiments confirmed that LED irradiation is essential for the reaction to proceed. Although the maximum yield of 38% represents a substantial improvement over the original value (7.7%), opportunities remain for further optimization. Given the scarcity of the literature on the synthesis of substituted 2-phenoxy-3H-azepines, we extended our methodology to a diverse range of substituted aryl azides 1 (Scheme 1a). Methyl-substituted azide 1b afforded the corresponding product, 3ba, in 25% yield. The halogen-substituted derivatives fluoro-(1c) and bromo-(1d) were well tolerated, yielding 3ca and 3da in 20% and 33% yields, respectively. Electron-withdrawing substituents such as CF3 (1e) and CO2Et (1f) significantly improved the reaction outcomes, affording products (3ea and 3fa) in 43% and 60% yields, respectively. In contrast, MeO-substituted azide 1g failed to react under the tested conditions, providing no detectable product 3ga. Notably, SF5-substituted phenyl azide (1h) produced the desired SF5-azepine-phenyl-ether 3ha in 83% yield, highlighting the unique enhanced effect of the SF5-group in this synthetic approach. The structure of 3ha was confirmed using X-ray crystallography (CCDC 2443987).| Entry | Irradiation | Solvent | Base | Yieldb (%) |
|---|---|---|---|---|
| a Unless otherwise noted, the reactions were carried out with 1a (0.2 mmol), 2a (0.8 mmol), and DABCO (0.24 mmol) in DCE (0.1 M) irradiated under blue LED light (about 455 nm) at rt for 48 h. b Isolated yields of 3a. | ||||
| 1 | UV | EtOH | — | 7.7 (ref. 27) |
| 2 | Blue LED (40 W) | EtOH | — | 0 |
| 3 | Blue LED (40 W) | DCE | K2CO3 | 27 |
| 4 | Blue LED (40 W) | DCE | Na2CO3 | 12 |
| 5 | Blue LED (40 W) | DCE | KOH | 10 |
| 6 | Blue LED (40 W) | DCE | Et3N | 8 |
| 7 | Blue LED (40 W) | DCE | DBU | 24 |
| 8 | Blue LED (40 W) | DCE | DABCO | 38 |
| 9 | 365 nm LED (30 W) | DCE | DABCO | 34 |
| 10 | — | DCE | DABCO | 0 |
Encouraged by the exceptional performance of the SF5 group rather than the others in phenyl under photo-induced dearomative coupling reactions with phenol 2a, we systematically explored the reaction scope using SF5-phenyl azide 1h with various phenols 2 featuring diverse electronic properties (Scheme 1b-i). Phenol (2a) initially yielded SF5-azepine 3ha in an excellent yield of 83%, which was maintained at 78% upon scaling the reaction to the gram scale. Phenols 2 bearing electron-donating substituents, such as 4-Me (2b), 4-MeO (2c), 4-tert-Bu (2d), 2-Me (2e), and 3-Me (2f), were effectively incorporated, affording the corresponding products (3hb–3hf) in yields ranging from 72% to 77%. Halogen-substituted phenols, including 4-F (2g), 4-Cl (2h), 4-Br (2i), and 4-I (2j), also reacted efficiently under the optimized conditions, producing azepines 3hg–3hj with yields of up to 67%, without any loss of halogen, especially the iodo-group.28 Thus, phenols containing electron-donating substituents demonstrated enhanced efficiency in the PDC reaction. Phenols bearing electron-withdrawing substituents, such as 4-CF3 (2k), 4-NO2 (2l), 4-SCF3 (2m), 4-CO2Et (2n), and 4-phenyl (2o), also reacted effectively, yielding the corresponding azepines (3hk–3ho) in good yields of up to 61%, except for 4-NO2-phenol (2l). Moreover, structurally more complex phenols, including 4-phenoxyphenol (2p), naphthol (2q), and 8-hydroxyquinoline (2r), provided the desired products (3hp, 3hq, and 3hr) in yields of 58%, 45%, and 36%, respectively. Notably, disubstituted phenols, such as 3,5-dibromophenol (2s) and 3,5-dimethoxyphenol (2t), also participated smoothly under the optimized reaction conditions without compromising the reaction efficiency to furnish 3hs and 3ht, respectively.
We further explored our synthetic strategy for the late-stage functionalization of diverse natural products and bioactive molecules featuring phenolic motifs (Scheme 1b-ii). Initially, reactions involving 2-methyl salicylate (2u) and 2-ethylhexyl salicylate (2v) proceeded effectively, delivering the corresponding azepines 3hu and 3hv in yields of 47% and 34%, respectively. Subsequently, substrates such as 4-benzyloxyphenol (2w), 6-hydroxycoumarin (2x), 2,6-diisopropylphenol (2y), 4-(1,1,3,3-tetramethylbutyl) phenol (2z), and 4-(4-hydroxyphenyl)-butan-2-one (2aa) were successfully converted into their corresponding SF5-azepine derivatives (3hw–3haa) in moderate yields (40–58%). Furthermore, more structurally diverse and bioactive phenols such as vanillin (2ab), acetovanillone (2ac), triclosan (2ad), and carvacrol (2ae) participated in the reaction, producing the desired azepines (3hab–3hae) in good yields (up to 75%). Interestingly, pharmaceuticals such as paracetamol (2af) and estrone (2ag) also underwent efficient transformation under the optimized reaction conditions, affording SF5-substituted azepines 3haf and 3hag in yields of 60% and 33%, respectively. Notably, the reaction under aqueous conditions in the absence of phenols furnished SF5-azepinone 4h in 70% yield via its lactim form 4h. Having successfully demonstrated the skeletal transformation of SF5-benzenes into SF5-azepine scaffolds (Scheme 1b), we explored the reverse process, namely the skeletal reconstruction of SF5-azepines back to SF5-benzene derivatives (Scheme 1c). Treatment of 5-(pentafluoro-λ6-sulfaneyl)-2-phenoxy-3H-azepine (3ha) with trifluoroacetic anhydride (TFAA) in acetonitrile efficiently afforded the corresponding SF5-substituted ortho-aminophenol (5ha) in excellent yield (92%). Substituted SF5-azepines bearing Me (3hb), MeO (3hc), and F (3hg) groups were well tolerated under the reaction conditions, furnishing the corresponding aminophenol derivatives 5hb, 5hc, and 5hd, respectively, in excellent yields (up to 94%). Additionally, phenyl-substituted azepine 3ho underwent smooth skeletal editing to deliver ortho-aminophenol 5he in 92% yield. This reverse skeletal transformation was also applicable to non-SF5-substituted azepine-aryl ethers, enabling their conversion into the corresponding diaryl ethers, as proven by non-substituted phenoxy-3H-azepine (3aa), which actively participated in the reaction to deliver the corresponding ortho-aminophenol (5aa) in 90% yield. Likewise, substituted phenoxy-3H-azepines bearing electronically varied substituents, such as Me (3ba), F (3ca), Br (3da), CF3 (3ea), and CO2Et (3fa), smoothly participated in the reaction to furnish the corresponding ortho-aminophenol derivatives 5ba, 5ca, 5da, 5ea, and 5fa in high yields ranging from 87–94%. These results indicated that the opposite skeletal transformation from azepines 3 to benzenes 5 proceeded smoothly, independent of the electronic state of the functional groups, which is different from the skeletal transformation from benzenes to azepines 3. It is noteworthy that this reverse skeletal transformation can also be performed using difluoroacetic anhydride (DFAA) in place of the commonly employed TFAA. Under these conditions, the corresponding COCF2H-substituted diaryl ethers (5haa–5had) were obtained in excellent yields (79–96%). Importantly, the COCF2H group lies outside the OECD PFAS definition, ensuring full PFAS-free compatibility.
Reaction mechanism and electrophilicity analysis
A plausible reaction mechanism for the photoinduced dearomative coupling of phenyl azide 1 with phenol 2 has been proposed based on previous studies (Fig. 2a). Upon photoirradiation, phenyl azide 1 undergoes photolysis to generate a singlet nitrene intermediate (I). This highly reactive species undergoes regioselective insertion at the ortho-position of the aryl ring to form a strained azirine intermediate (II). The azirine subsequently undergoes a thermally allowed 6π-electrocyclic ring-opening reaction to yield a conjugated ketenimine intermediate (III), which acts as a key electrophilic species. Nucleophilic attack by phenol 2 then affords 1H-azepine (VI), which undergoes tautomerization or isomerization to the thermodynamically favored 3H-azepine product 3. To investigate the role of SF5 substitution in this skeletal transformation, we analyzed the full product distribution, including azepine 3, unreacted starting material 1, and the byproduct (Fig. 2b). Notably, SF5-phenyl azide (1h) predominantly yielded azepine 3ha with minimal residual starting material 1h (5%). In contrast, phenyl azides bearing CF3 (1e), H (1a), and MeO (1g) substituents left similar levels of unreacted starting material 1 (1e: 39%; 1a: 42%; 1g: 40%), regardless of their electronic nature. However, a clear divergence was observed in azepine formation: while 1e (X = CF3) and 1a (X = H) provided azepines in ∼43% yield, 1g (X = MeO) failed to produce any azepine (0%), but instead afforded azo-dimer 6 in 48% yield. To rationalize the enhanced reactivity of SF5-substituted phenyl azide 1h, we examined their global electrophilicity index (GEI), calculated as the square of the electronegativity divided by the chemical hardness (Fig. 2c). This ground-state molecular descriptor correlates well with electrophilic reactivity, especially toward soft nucleophiles, making it a valuable predictor of reaction outcomes. The GEI values for para-substituted phenyl azides 1 (X–C6H4–N3) were as follows: 1h (X = SF5), 2.680 eV; 1e (CF3), 1.607 eV; 1a (H), 1.164; and 1g (OMe), 1.044 eV. These results indicate that strongly electron-withdrawing substituents like SF5 substantially enhance the electrophilicity of azide 1 and its nitrene intermediate I, facilitating smooth formation of azirine II and the ketenimine III. This trend closely matches our experimental findings, where the residual starting material followed the order SF5 (5%) <<< CF3 (39%) ≈ H (42%) ≈ MeO (40%). We further computed the GEI values for ketenimine intermediates (III) to assess their susceptibility to phenolic nucleophiles 2. The GEI values were as follows: III-h (SF5), 2.838 eV; III-e (CF3), 1.523 eV; III-a (H), 1.047 eV; and III-g (MeO), 1.004 eV. Again, the electrophilicity of the SF5 group was correlated with the experimental yields of azepine-aryl ethers: SF5 (83%) >>> CF3 (43%) ≈ H (38%), with no product observed for OMe (0%). Density functional theory (DFT) calculations were performed at the SMD-M06-2X/def2-TZVP level of theory for the spin density on the N atom, and it showed a correlation with the experimental yield (Fig. 2d).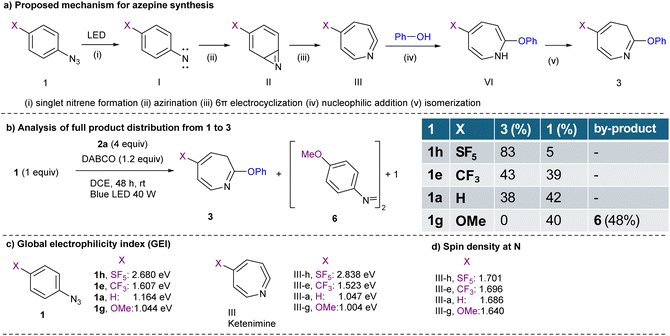 | ||
| Fig. 2 (a) Proposed mechanism. (b) Analysis of full product distribution from 1 to 3. (c) Global electrophilicity index. (d) Spin density on the N atom, DFT, SMD-M06-2X/def2-TZVP. | ||
To get further insights into the reaction mechanism, the Gibbs energy profile for phenyl azide (1a) as the model substrate was investigated at the SMD-M06-2X/def2TZVP level of theory in dichloroethane as solvent (Fig. 3c). The unrestricted open-shell singlet (1A2) was located 9.4 kcal mol−1 higher in the Gibbs energy profile than the phenyl azide (1a), and the corresponding restricted closed-shell singlet (1A1) was found to be 19.0 kcal mol−1 higher in energy than the unrestricted closed-shell singlet 1A1. However, the triplet state (3A2) was found to be 8.6. kcal mol−1 more stable than the phenyl azide (1a). We further studied the possible transformations of aryl azides 1 with different substituents leading to the formation of nitrenes (Fig. 3d). For the p-MeO system (1g), the strong +M effect of MeO stabilizes the empty N π* orbital. Thus, the lone-pair donation both narrows the 1A2–1A1 gap (2.8 kcal mol−1) (cf., 1A2–1A1 gap of SF5, 12.9 kcal mol−1) and increases spin–orbit coupling, accelerating ISC by >102-fold to the triplet surface, becoming the dominant pathway.30–34 The resulting triplet 3A2 nitrene (−12.2 kcal mol−1) from the p-MeO system (1g) dimerizes at the diffusion limit to azo-dimer 6 (48%); azepine 3ga is not detected, a well-established outcome for triplet aryl nitrenes in non-protic solvents. Next, the intramolecular aziridination was investigated from 1A2 and 3A2 (Fig. 3c). DFT calculations demonstrate that both 1A2 and 3A2 intermediates are reactive toward the aziridination, as inferred from the computed Gibbs activation energies for TS1-1A2 and TS1-3A2. The 1A2 intermediate directly gives three-membered azirine IIvia transition structure TS1-1A2 with a Gibbs activation energy of 12.6 kcal mol−1 from intermediate 1A2.
Alternatively, the barrier from 3A2 involving the triplet state was computed to be 11.7 kcal mol−1via transition structure TS1-3A2, showing that the process is also feasible. Calculations reveal that the endergonicity for transformation of three-membered azirine II from phenyl azide (1a) is 1.5 kcal mol−1. After the three-membered ring formation, azirine II is involved in 6π-electrocyclization which is predicted to be fast viaTS2 with a Gibbs activation barrier of 4.3 kcal mol−1, delivering ketenimine intermediate III in a slightly exergonic step (ΔG = −1.0 kcal mol−1). Next, the phenol nucleophilic attack affords 1H-azepine IV in a highly exergonic and irreversible step (ΔG = −42.1 kcal mol−1). Finally, the formed 1H-azepine IV undergoes tautomerization to yield the thermodynamically favored 3H-azepine product 3a. This step is computed to be exergonic by about 11.6 kcal mol−1. In the case of CF3-azepine, the computed activation barriers for aziridination and 6π-electrocyclization were found to be 9.3 and 3.4 kcal mol−1, respectively (Fig. 3e). The relatively high activation barriers for phenyl azide (1a) and CF3-phenyl azide 1e rationalize why the yield is moderate in the case of products 3aa and 3ea. Finally, the computed activation barriers for aziridination and 6π-electrocyclization of SF5-azepine from SF5-phenyl azide 1h were found to be 9.1 and 3.0 kcal mol−1, respectively. In this case, the computed activation barrier for aziridination is more favorable, rationalizing the higher reactivity of SF5-azepines. As inferred from these DFT calculations, it is notable that aziridination, with the highest activation barrier of the entire mechanism, is the rate determining step of the reaction mechanism. The reaction mechanism studied by DFT calculations agrees with the experimental findings as computed barriers are affordable at room temperature. Finally, phenyl azide 1g (OMe case) showed similar barriers for aziridination, however, the computed barrier for 6π-electrocyclization was found to be higher in energy (7.9 kcal mol−1). This data, together with the data in Fig. 3d, indicate that the dimerization reaction would be favored for MeO-substituted phenyl azide 1g.
Extension from SF5 to SF4
We extended our dearomative skeletal editing methodology from SF5-aryl azides (1) to alkynylated trans-SF4-aryl azide (7). Given the electron-deficient and highly polarized nature of the alkynyl moiety in compound 7, which is prone to reactions with nucleophiles, electron-rich species, and carbenes, we initially anticipated potential side reactions involving the alkyne. In particular, the presence of both an aryl azide and an alkyne linked via the SF4 unit raised concerns about competing nitrene insertion pathways. Surprisingly, the photoinduced reaction proceeded with high chemoselectivity at the aryl azide site, leaving the alkyne untouched. This enabled efficient ring expansion to afford the desired alkynyl-trans-SF4-azepine 8a in 68% yield under the same conditions used for the SF5 analogues. The yield further improved to 75% when the reaction was conducted under 365 nm irradiation. The transformation proved broadly applicable across a variety of functionalized trans-SF4 aryl azides. Substituents including electron-donating (Me and OMe), halogen (F and Br), electron-withdrawing (CO2Et), and aryl (Ph) groups were well tolerated, providing the corresponding trans-SF4 azepines 8b–8g in 53–67% yields (Scheme 2, top).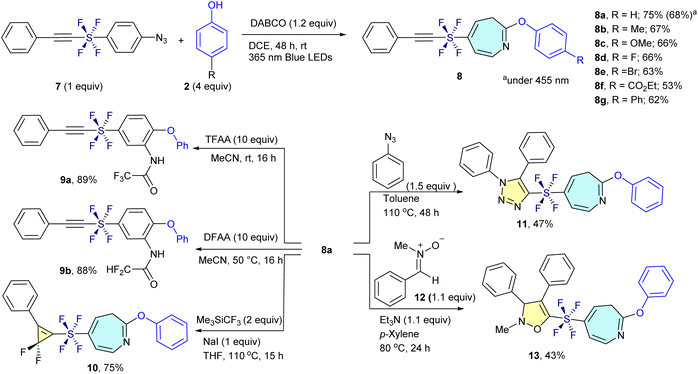 | ||
| Scheme 2 Chemo-selective transformation of alkynylated trans-SF4-aryl azides (7) to azepines (8), and their further chemo-selective derivatization to 9, 10, 11 and 13. | ||
Further derivatization demonstrated the synthetic utility of these scaffolds. The alkynylated SF4-azepine 8a underwent ring re-contraction upon treatment with TFAA and acetonitrile, regenerating the corresponding SF4-benzene derivative 9a bearing amino and ether functionalities in 89% yield. The reaction of 8a with DFAA furnished the PFAS-free ring-reconstructed product 9b in similarly high yield (88%). In addition, selective gem-difluorocyclopropanation of 8a was achieved using Me3SiCF3 and NaI in THF, furnishing the unique SF4-azepine–gem-difluorocyclopropene hybrid 10 in 75% yield. Next, the compound 8a participated in a click reaction with azidobenzene 1a, resulting in the formation of the SF4-azepine–triazole (11) with 47% yield. After that, the compound 8a smoothly involved in a (3 + 2) cycloaddition reaction with nitrone (12) to afford the SF4-azepine–isoxazole (13) with 43% yield (Scheme 2, bottom). These results demonstrate that the trans-SF4 group functions as a stable, linear, and highly versatile linker under photoinduced skeletal editing conditions. The resulting SF4-azepine frameworks represent promising candidates for PFAS-safe agrochemical development, combining structural novelty with tunable electronic properties and synthetic flexibility.
Conclusions
We have developed a bidirectional skeletal-editing platform centered on high-valent sulfur fluorides—namely, pentafluorosulfanyl (SF5) and tetrafluorosulfanyl (SF4)—that enables the reversible interconversion between benzenes and azepine-aryl ethers under mild, visible-light irradiation. These motifs function not merely as PFAS-safe perfluoroalkyl alternatives but as reactivity-switching handles that unlock new chemical transformations. The strongly electron-withdrawing nature of SF5 selectively stabilizes the open-shell singlet nitrene intermediate, accelerating a 6π-electrocyclization/ring-expansion cascade and delivering azepines in yields significantly exceeding those of CF3- or their non-fluorinated counterparts. Computational studies support this unique reactivity enhancement. Importantly, the resulting azepines undergo clean skeletal contraction upon treatment with acid anhydrides, regenerating the original SF5- or SF4-aryl cores while introducing an aryl-ether functionality—marking the first efficient return pathway for any high-valent sulfur fluoride azepine. While demonstrated using phenolic nucleophiles, the underlying mechanism—diradical nitrene capture followed by skeletal reorganization—should be generalizable to other soft nucleophiles such as amines, thiols, and carboxylates. This work thus establishes high-valent sulfur fluorides as programmable scaffolding elements for visible-light skeletal editing and introduces a sustainable route to structurally diverse, fluorinated heterocycles. Because the SF5 group serves as a terminal substituent while the SF4 unit functions as a linear connector, this platform offers a versatile and PFAS-conscious entry point for the design of fluorinated agrochemicals and advanced materials.Author contributions
CN optimized the reaction conditions, surveyed the substrate scope, analyzed the data and discussed the results with NS. TM, MZB, and SW prepared starting materials. SO and JE conducted DFT studies. CN and NS wrote the manuscript. NS supervised the study. All authors contributed to the manuscript and approved the final version of the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Data availability
CCDC 2443987 contains the supplementary crystallographic data for this paper.35The data that support the findings of this study are available within the article and the supplementary information (SI). Supplementary information: materials and methods, experimental procedures, characterization data, and NMR spectra. See DOI: https://doi.org/10.1039/d5sc08177g.
Acknowledgements
This study was supported by the CREST program of the Japan Science and Technology Agency, entitled “Precise Material Science for Degradation and Stability” (grant number: JPMJCR21L1), and by Dr Seiji Motojima (CMC Research Institute, Japan). J. E. thanks Ministerio de Ciencia, Innovación y Universidades for financial support under the program (PID2023-152131NB-I00). The computational resources from the Servei d'Informàtica de la Universitat de València (SIUV) are gratefully acknowledged for providing access to supercomputing resources. Project partially developed within the framework programa propi d'investigació del Vicerrectorat d'investigació de la UV, under the call for visiting researchers (INV25-01-15/382627).References
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC.
- M. Inoue, Y. Sumii and N. Shibata, ACS Omega, 2020, 5, 10633–10640 CrossRef CAS PubMed.
- Y. Ogawa, E. Tokunaga, O. Kobayashi, K. Hirai and N. Shibata, iScience, 2020, 23, 101467 CrossRef CAS PubMed.
- H. P. H. Arp, A. Gredelj, J. Gluge, M. Scheringer and I. T. Cousins, Environ. Sci. Technol., 2024, 58, 19925–19935 CrossRef CAS.
- F. Freeling, M. Scheurer, J. Koschorreck, G. Hoffmann, T. A. Ternes and K. Nodler, Environ. Sci. Technol. Lett., 2022, 9, 400–405 CrossRef CAS.
- P. R. Savoie and J. T. Welch, Chem. Rev., 2015, 115, 1130–1190 CrossRef CAS.
- G. Haufe, Tetrahedron, 2022, 109, 132656 CrossRef CAS.
- Y. Murata, K. Hada, T. Aggarwal, J. Escorihuela and N. Shibata, Angew. Chem., Int. Ed., 2024, 63, e202318086 CrossRef CAS PubMed.
- S. R. Narra, M. Z. Bacho, M. Hattori and N. Shibata, Adv. Sci., 2024, 11, 2306554 CrossRef CAS PubMed.
- T. Aggarwal, K. Hada, Y. Murata, Y. Sumii, K. Tanagawa, K. Niina, S. Mori, J. Escorihuela and N. Shibata, Angew. Chem., Int. Ed., 2023, 62, e202307090 CrossRef CAS PubMed.
- W. A. Sheppard, J. Am. Chem. Soc., 1962, 84, 3064–3072 CrossRef.
- W. A. Sheppard, J. Am. Chem. Soc., 1960, 82, 4751–4752 CrossRef CAS.
- T. Umemoto, L. M. Garrick and N. Saito, Beilstein J. Org. Chem., 2012, 8, 461–471 CrossRef CAS PubMed.
- O. S. Kanishchev and W. R. Dolbier, Angew. Chem., Int. Ed., 2015, 54, 280–284 CrossRef CAS PubMed.
- M. Kosobokov, B. Cui, A. Balia, K. Matsuzaki, E. Tokunaga, N. Saito and N. Shibata, Angew. Chem., Int. Ed., 2016, 55, 10781–10785 CrossRef CAS.
- P. Das, E. Tokunaga and N. Shibata, Tetrahedron Lett., 2017, 58, 4803–4815 CrossRef CAS.
- M. Kaur, S. Garg, D. S. Malhi and H. S. Sohal, Curr. Org. Chem., 2021, 25, 449–506 CAS.
- G. F. Zha, K. P. Rakesh, H. M. Manukumar, C. S. Shantharam and S. Long, Eur. J. Org. Chem., 2019, 162, 465–494 CAS.
- S. C. Patel and N. Z. Burns, J. Am. Chem. Soc., 2022, 144, 17797–17802 CrossRef CAS PubMed.
- L. Song, X. Tian, K. Farshadfar, F. Shiri, F. Rominger, A. Ariafard and A. S. K. Hashmi, Nat. Commun., 2023, 14, 831–839 CrossRef CAS PubMed.
- R. Mykura, R. Sanchez-Bento, E. Matador, V. K. Duong, A. Varela, L. Angelini, R. J. Carbajo, J. Llaveria, A. Ruffoni and D. Leonori, Nat. Chem., 2024, 16, 771–779 CrossRef CAS.
- E. Matador, M. J. Tilby, I. Saridakis, M. Pedron, D. Tomczak, J. Llaveria, I. Atodiresei, P. Merino, A. Ruffoni and D. Leonori, J. Am. Chem. Soc., 2023, 145, 27810–27820 CrossRef CAS.
- G. Li, M. N. Lavagnino, S. Z. Ali, S. Hu and A. T. Radosevich, J. Am. Chem. Soc., 2023, 145, 41–46 CrossRef CAS PubMed.
- B. Li, A. Ruffoni and D. Leonori, Angew. Chem., Int. Ed., 2023, 62, e202310540 CrossRef CAS PubMed.
- R. Sanchez-Bento, B. Roure, J. Llaveria, A. Ruffoni and D. Leonori, Chem, 2023, 9, 3685–3695 CAS.
- T. J. Pearson, R. Shimazumi, J. L. Driscoll, B. D. Dherange, D. Park and M. D. Levin, Science, 2023, 381, 1474–1479 CrossRef CAS PubMed.
- K. Koyama and H. Takeuchi, J. Chem. Soc., Perkin Trans., 1982, 1, 1269–1273 Search PubMed.
- W. Sander, M. Winkler, B. Cakir, D. Grote and H. F. Bettinger, J. Org. Chem., 2007, 72, 715–724 CrossRef CAS.
- S. V. Chapyshev, E. M. Vega and W. Sander, Chem.–Eur. J., 2021, 27, 1258–1269 CrossRef CAS.
- R. Purvis, R. K. Smalley, W. A. Strachan and H. Suschitzky, J. Chem. Soc., Perkin Trans., 1978, 1, 191–195 RSC.
- A. Albini, G. Bettinetti and G. Minoli, J. Am. Chem. Soc., 1999, 121, 3104–3113 CrossRef CAS.
- W. M. Kwok, P. Y. Chan and D. L. Phillips, J. Phys. Chem. A, 2005, 109, 2394–2400 CrossRef CAS PubMed.
- D. Aranda, F. J. Avila, I. Lopez-Tocon, J. F. Arenas, J. C. Otero and J. Soto, Phys. Chem. Chem. Phys., 2018, 20, 7764–7771 RSC.
- J. Soto, J. C. Otero, F. J. Avila and D. Pelaez, Phys. Chem. Chem. Phys., 2019, 21, 2389–2396 RSC.
- CCDC 2424208: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2n1596.
| This journal is © The Royal Society of Chemistry 2026 |