Open Access Article
Open Access ArticleCharged two-dimensional nanochannels with high ion density enabling ultrafast monovalent and multivalent ion conductivity
Lingjie
Zhang
ab,
Yunjia
Ling
 ab,
Jianglin
Yan
a,
Zhenlei
Wang
ab,
Yanhui
Miao
a,
Haoyu
Bai
a,
Tingting
Zhang
a,
Shaoxian
Song
a,
Mildred
Quintana
b and
Yunliang
Zhao
ab,
Jianglin
Yan
a,
Zhenlei
Wang
ab,
Yanhui
Miao
a,
Haoyu
Bai
a,
Tingting
Zhang
a,
Shaoxian
Song
a,
Mildred
Quintana
b and
Yunliang
Zhao
 *ac
*ac
aSchool of Resources and Environmental Engineering, Wuhan University of Technology, Wenzhi Street 34, Wuhan, Hubei 430070, China. E-mail: zyl286@whut.edu.cn
bFacultad de Ciencias, Universidad Autonoma de San Luis Potosi, Av. Parque Chapultepec 1570, San Luis Potosi 78210, Mexico
cWuhan Clayene Technology Co., Ltd, Tangxunhu North Road 36, Wuhan, Hubei 430223, China
First published on 20th November 2025
Abstract
Ion conductors with fast ion transport and reliable stability are highly desired for energy storage and conversion devices. While solid-state ion conductors with high safety and energy density are promising materials for a new generation of electrochemical devices, it remains challenging to achieve high ion conductivity, especially for multivalent ions due to the stronger steric effect and electrostatic interactions. Here, we report the well-ordered charged nanochannels with high ion density, typically fabricated by stacking montmorillonite (MMT) nanosheets, to serve as versatile solid-state ion conductors. Characterization studies and molecular dynamics simulations reveal that the “adaptive” nanochannel height of MMT membranes, combined with Coulomb interaction-induced concerted ion movement and surface-charge-governed ion transport arising from the high-packing-density cations inside the negatively charged nanochannels, jointly suppress the steric effect and strong interactions for various cations. As a result, our MMT nanochannels achieve considerably high conductivity for both monovalent (K+, Na+, and Li+) and multivalent ions (Mg2+ and Al3+), ∼80 to 210 mS cm−1 at 80 °C, higher than that of the corresponding bulk solutions and state-of-the-art ion conductors. This work provides fresh perspectives on fast ion transport in nanoconfined environments, and presents a promising route for developing next-generation ionic devices.
Introduction
The decarbonization and utilization of sustainable energy are driving the development of electrochemical energy storage devices, such as ion batteries, fuel cells, supercapacitors and nanofluidics.1–4 Ion conductors are the core components that directly determine the performance and lifetime of these devices and are typically expected to rapidly transport charge carrier ions with reliable safety and stability.5–7 The intrinsic thermal stability and high energy density of solid-state ion conductors render them promising candidates to overcome the limitations of conventional liquid electrolytes.8–10 Despite notable progress, crucial concerns remain regarding advanced solid-state ion conductors: (i) limited ion conductivity and electrochemical stability, typically 10−2 to 1 mS cm−1 at room temperature;11 (ii) poor compatibility with multivalent ions, although multivalent-ion batteries offer higher theoretical energy densities, lower costs, and sustainable resource availability.12–14Two-dimensional (2D) layered materials with large active surface areas and ordered ion nanochannels offer ideal platforms to construct highways for fast ion transport.15–21 So far, many 2D lamellar ion conductors have been developed to exhibit high conductivity for monovalent ions (e.g., K+, Na+, and Li+), involving charge-free graphene-based angstrom-scale slits,22 reduced graphene oxide-based atomic-scale ion transistors,23 layered metal compounds,24 and 2D MoS2 membranes.25 Nevertheless, the transport behavior of cations in 2D nanochannels is curbed by steric effects caused by ion hydration and electrostatic attraction interaction with the channel walls.13,26–28 As these effects are strongly dependent on cation valence, the conductivity for multivalent ions in such lamellar ion conductors is typically at least an order of magnitude lower than that for monovalent ions.22,29
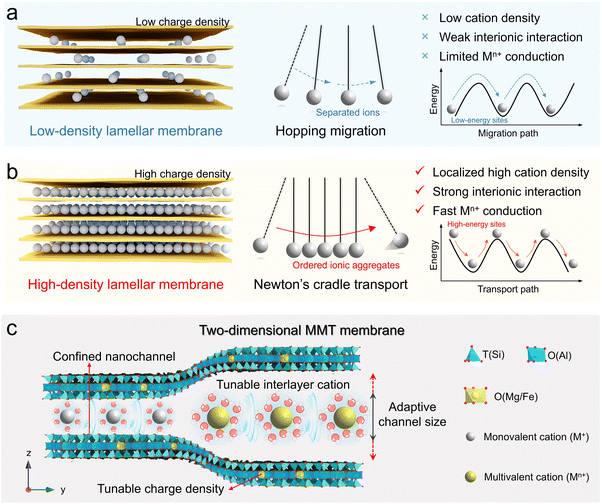
Given the sandwich microstructures of 2D ion conductors, enhancing channel wall–ion and ion–ion interactions presents two potential pathways toward efficient and versatile ion transport. Nanofluidic studies demonstrate that when electrolytes confined in charged nanochannels with dimensions comparable to the Debye length, ion transport markedly accelerates.30,31 Accordingly, constructing 2D ion conductors from negatively charged nanosheets appears favorable for facilitating multivalent ion transport. Nevertheless, this introduces low-energy sites with strong electrostatic attraction near negative charges locations to impede ion migration, especially for multivalent cations (Scheme 1a).12,32 Modulating the interionic interaction between adjacent mobile ions offers another promising solution. In widely studied lamellar ion conductors (e.g., graphene oxide and MXene membranes), however, the nanochannels inherently contain few or no cations, which causes cations to preferentially occupy low-energy sites, encountering high energy barriers to hop between adjacent positions, thus resulting in slow or even suppressed ion transport, especially for multivalent ions (Scheme 1a).33 We conjecture that constructing high-density cation transport networks within the nanoconfined channels can induce localized packing effects, forcing some cations to gather around the high-energy sites (Scheme 1b). During transport, the downhill migration of these cations can partially compensate for the energy barriers encountered by the following uphill-climbing cations, enabling rapid transport through Coulomb knock-on mechanisms reminiscent of a Newton's cradle.34,35
Herein, we demonstrate that leveraging naturally negatively charged montmorillonites (MMTs) as building blocks to assemble into ion-conductive membranes with ordered angstrom-scale channels enables the fast transport of diverse cations. Unlike other 2D nanosheets (e.g., graphene oxide, MXenes, and MoS2), MMT nanosheets consist of one octahedral alumina sheet sandwiched between two tetrahedral silica sheets. Owing to geochemical isomorphic substitution of Al3+ in octahedra by Mg2+ and Fe2+, MMT nanosheets exhibit intrinsic negative charge, balanced by exchangeable interlayer cations (Scheme 1c).36 This natural feature enables tunable charge density, abundant and exchangeable interlayer cations, adaptive channel size and even controllable ion density, highlighting its potential in fabricating intrinsically versatile ion conductors. Consequently, the MMT nanochannel with high charge density and thereby ion density shows universally high ion conductivity for both monovalent (K+, Na+, and Li+) and multivalent ions (Mg2+ and Al3+), ranging from ∼80 to 210 mS cm−1 at 80 °C, substantially outperforming corresponding bulk solutions and advanced solid ionic conductors. Moreover, the developed MMT nanochannels combine long-term durability, exceptional thermal stability, and scalability, offering great promises in diverse ionic devices.
Results and discussion
Preparation of the 2D MMT nanoconfined ion channels
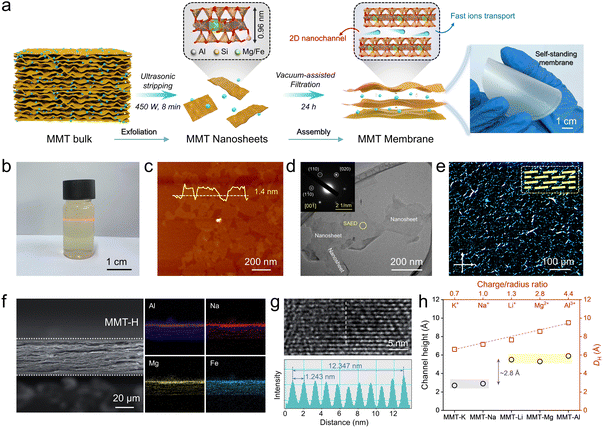
The MMT nanosheets as the building blocks of the nanochannels were prepared by mechanical exfoliation of bulk MMT minerals (Fig. 1a). The resultant dispersions show a pronounced Tyndall effect, indicating the excellent dispersibility of as-obtained nanosheets (Fig. 1b). The increased isomorphic substitutions of Mg and Fe in high-charge-density MMT nanosheets (MMT-H) causes enhanced negative surface charge compared to low-charge-density MMT nanosheets (MMT-L), along with a visible color shift from white to light yellow (Fig. S1 and S2). Atomic force microscopy (AFM) measurements present that MMT nanosheets are predominantly monolayers with a typical thickness of ∼1.4 nm and an average lateral size of ∼200 nm (Fig. 1c, S3 and S4). Transmission electron microscopy (TEM) images and the corresponding selected area electron diffraction (SAED) patterns affirm atomically thin nanosheets with monoclinic crystallinity (Fig. 1d and S5). Polarized optical microscopy (POM) images of MMT nanosheet dispersions exhibit bright birefringence stripes, demonstrating the well self-ordering of nanosheets in dispersions, which favors the construction of uniform and ordered 2D channels (Fig. 1e and S6).37Free-standing MMT membranes were readily fabricated by vacuum-assisted assembly of nanosheets due to the self-ordering nature of nanosheets and inherent flexibility of clays (Fig. 1a). Scanning electron microscopy (SEM) images show the uniform and defect-free surface of the assembled membranes (Fig. S7). The cross-sectional images reveal the aligned laminated structures of membranes with uniform distribution of charge-balanced cations and substitutional cations (Fig. 1f and S8). The results of the energy dispersive X-ray spectroscopy (EDS) prove the markedly higher Mg/Fe substitution degree and thereby interlayer cation amount in the MMT-H membrane (Fig. S9 and Table S1). Notably, MMT membranes illustrate well-defined nanochannels (Fig. 1g and S10). According to small-angle X-ray diffraction (SAXRD) patterns, the interlayer spacing (d) of MMT membranes is ∼1.25 nm (Fig. S11), in accordance with the high-resolution TEM (HRTEM) results, indicating the interplanar channels with a height of 0.29 nm after subtracting the single aluminosilicate layer of 0.96 nm.38 Such nanochannels facilitate the formation of ion transport networks to accelerate the ion conduction.39 It is noteworthy that the increase in charge density of MMT has not extended the channel dimension, which is attributed to the fact that the interlayer cations also increase, resulting in stronger sheet–ion–sheet attraction interactions.40
The nanoconfined channels with other cations can be obtained for versatile ion conductors via ion exchange, that is, immersion of MMT membranes into saturated solutions of the desired cation (including K+, Li+, Mg2+, and Al3+) for 24 h to replace Na+ (Fig. S12). Notably, the height of MMT channels does not scale linearly with the hydrated diameter of intercalated cations (DH), but falls into two distinct values: ∼2.8 Å or ∼5.6 Å, corresponding to one or two layers of H2O molecules (Fig. 1h). This suggests that spacing is governed by the formation of mono- or bilayer hydration shells around intercalated cations. Such behavior correlates with the empirical charge-to-radius ratio (e/r): when e/r ≤ 1 (low-charge, large ions), monolayer H2O forms; when e/r > 1 (high-charge, small ions), bilayer H2O is stabilized, which helps screen electrostatic attraction with channel walls.14 Such “adaptive” channel height feature minimizes both steric and electrostatic effects, promoting multivalent ion transport. Moreover, the thickness of membranes can be controlled by varying the concentration and volume of the dispersion employed for vacuum-assisted filtration (Fig. S13).
Constructing high-density ion transport networks in MMT nanochannels
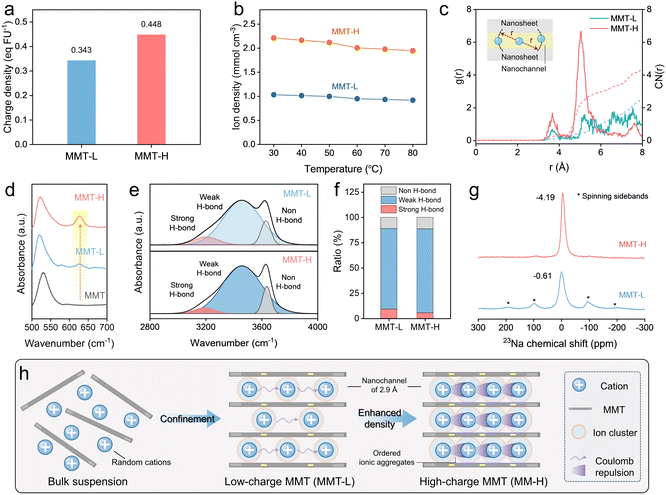
Utilizing the typical alkylammonium method (for details, see Text S2),41 the charge densities of MMT-L and MMT-H membranes were measured to be 0.343 and 0.448 eq FU−1 (Fig. 2a), which is consistent with the results of surface potential (Fig. S2), suggesting that the larger the MMT charge density, the more negative the surface charge. The electrostatic potential (ESP) distribution further affirms the charge characteristic of MMT units (Fig. S14), which enables the formation of the surface-charged regions for enhancing ion conductivity.25 To trace the ion transport networks in nanochannels varying with the charge density of MMT, we first determined the ion exchange capacity of MMT-L and MMT-H membranes, which is 0.72 and 0.94 mmol g−1, respectively (Fig. S15). Furthermore, the ion densities in nanochannels were calculated after stabilization at 98% relative humidity (RH) (for details, see Text S4). As shown in Fig. 2b, the MMT-H membrane with high charge density presents a significantly higher ion density of ∼2 mmol cm−3 than the MMT-L membrane. Such high ion density in atomically scale channels facilitates the formation of aligned and high-density packed ionic aggregates, which creates strong Coulomb knock-on interactions among adjacent ions to enable partial occupation of high-energy sites for concerted and fast transport of multiple ions.24,34 To quantitatively evaluate the ionic aggregation behavior and interionic interaction, we calculated the radial distribution function (RDF) and the corresponding coordination number (CN) of Na+ in the MMT nanochannels using molecular dynamics (MD) simulations. As shown in Fig. 2c, both MMT-L and MMT-H membranes exhibit first Na–Na characteristic peak at r = 3.67 Å, while the density probability (g(Na–Na)) of MMT-H is about three times higher than MMT-L, and similarly appears in the second Na–Na characteristic peak at r = 5.05 Å, which demonstrates the reinforced aggregation and interaction of Na+ in MMT-H nanochannels.42 This is also proved by the results of CN (represents the average number of adjacent Na atoms).Fourier transform infrared (FTIR) spectra were used to provide experimental proof of the high-density ion transport networks within MMT nanochannels. As for the MMT nanosheet dispersion, the Si–O–Al bending vibration band appears at 529 cm−1,43 which is red-shifted to 523 cm−1 when the nanochannels are formed, unveiling the sheet-ion-sheet attraction interactions within MMT nanochannels (Fig. 2d). Notably, an additional peak is observed at 627 cm−1 in MMT-L and is more pronounced in MMT-H membranes, which can ascribe to the ordered and high-density Na+ aggregate networks within the nanochannels.17,44 Moreover, considering the high-density cations confined in the nanochannels containing 1–2-layer water, we hypothesize that the coordination of water molecules with Na+ disrupts the hydrogen-bond (H-bond) interactions between water molecules.45 As shown in Fig. 2e, the O–H stretching vibration of water molecules (3000–3800 cm−1) exhibits a typical wide peak convolved into three components corresponding to water molecules with strong (∼3200 cm−1), weak (∼3400 cm−1) and non H-bonds (∼3600 cm−1), respectively.46 Furthermore, the ratios are determined according to the area of fitted peaks to quantify the H-bond states. With the charge density increasing, the strong H-bond reduces while the weak and non H-bonds increase, reconfirming the high-density cation networks in MMT-H nanochannels (Fig. 2f). The results are supported by Raman spectra as well (Fig. S16). 23Na magic angle spinning nuclear magnetic resonance (MAS-NMR) was also used as a sensitive indicator to investigate ion transport behavior in MMT nanochannels. The 23Na MAS-NMR spectrum of MMT-L membrane shows a strong resonance at −0.61 ppm with multiple spinning sidebands (Fig. 2g). Such a dominant and sharp 23Na signal is attributed to the active Na+ with high mobility in nanochannels.47,48 As for MMT-H membrane , the 23Na signal shifts to the upfield, suggesting the increased electron density and enhanced interionic Coulomb interaction of Na+.49,50 In addition, the intensified signal but reduced full width at half-maximum (FWHM) in the 23Na MAS-NMR spectrum of MMT-H with almost disappeared spinning sidebands, also confirms the enhanced Na+ diffusion.50 Also, the MMT-H membrane presents a larger specific surface area pore size and peak pore size than that of MMT-L (Fig. S17 and Table S2), suggesting that the high charge density might effectively tune the stacking of the MMT layers to provide more ion transport pathways, facilitating ion mobility.51 Overall, the confinement of random ions within charged nanochannels accelerates ion conduction via offering ordered transport networks, and high-density ionic aggregate networks can be constructed by increasing charge density of building blocks, which induces strong Coulomb interaction between adjacent ions to enhance ion mobility (Fig. 2h).
Ion transport behaviors in MMT nanochannels
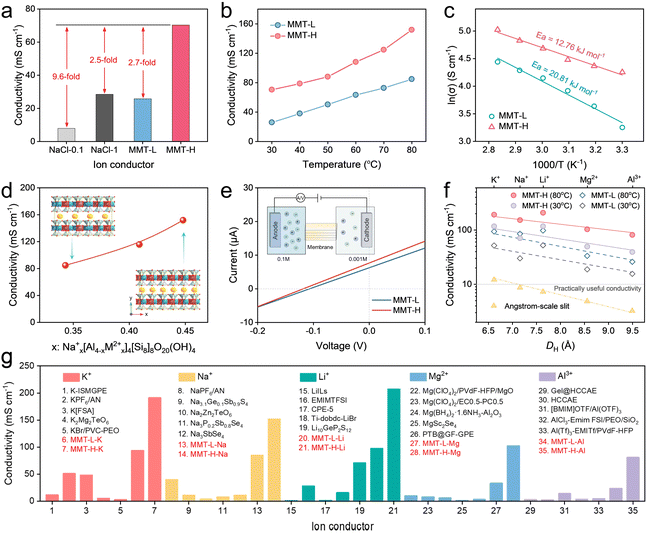
To investigate the effect of nanoconfined channels on ion transport, Na+ conductivities in the MMT membranes and bulk solutions with 0.1 or 1.0 M NaCl were first determined. Generally, liquid ion conductors present high conductivity, but obviously depend on temperatures and concentrations. As shown in Fig. 3a, aqueous NaCl exhibits an ion conductivity of 7.95 mS cm−1 at 0.1 M, and the value increases to 28.5 mS cm−1 at a higher concentration of up to 1.0 M. In contrast, the MMT-L membrane shows a conductivity comparable to that of high-concentration NaCl, indicating that charged nanochannels facilitate ion conductivity. To speak of, the conductivity of the MMT-H membrane is as high as 70.35 mS cm−1, providing a solid proof on the positive influence of high-density ion transport networks.We then measured the temperature-dependent Na+ conductivity of MMT membranes at 98% RH with two-electrode alternating current impedance. The Nyquist impedance plots present semicircles and spikes in the high and low frequency regions, respectively, demonstrating the purely ion-conducting properties of MMT membranes (Fig. S18).10 Across the entire tested temperature range, the Na+ conductivity of MMT-H is much higher than MMT-L (Fig. 3b). Notably, the MMT-H membrane exhibits superior conductivity of 151.87 mS cm−1 at a temperature of 80 °C. We further calculated the activation energy (Ea) for Na+ transport based on the Arrhenius plots (Fig. 3c). As expected, the MMT-H shows a lower Ea of 12.76 kJ mol−1 than MMT-L (20.81 kJ mol−1), suggesting the improved cation kinetic conduction within high-density ion transport nanochannels. This is also affirmed by the conductivity in different humidities (Fig. S19). Moreover, to validate the impact of the charge density of nanochannels, a medium-charge-density membrane (MMT-M) was prepared using nanosheets with the medium Mg/Fe substitution amount, and conductivities were measured (Fig. S20–S22). Accordingly, along with the increased substitution degree and thereby charge density, the conductivity of MMT membranes rapidly grows (Fig. 3d). These results illustrate that adjusting the isomorphic substitution can tune the charge density of MMTs, which modulates the ion density and interionic interaction within assembled nanochannels for fast ion transport.
We further measured the ionic transference numbers (tion) of MMT membranes via the concentration-driven electrodialysis diffusion (as illustrated in the inset of Fig. 3e). The open-cell voltages of both MMT-L and MMT-H were negative, certifying the negatively charged nanochannels (Fig. 3e).52 Based on the Nernst equation, the calculated tion of the MMT-L and MMT-H membranes are 0.764 and 0.829, respectively, suggesting that MMT membranes are Na+ conductors (Fig. S23).53 Furthermore, the effect of ion species on the ionic conduction was explored to evaluate the universality of MMT membranes as ion conductors. As shown in Fig. 3f, all the MMT membranes present considerable high conductivities for monovalent (K+, Na+, and Li+) and multivalent (Mg2+ and Al3+) ions, which are higher than the practically useful value (10 mS cm−1). Note that the nanochannel height of MMT membranes is much smaller than the DH of intercalated cations, thus these cations have to sherd or compress hydration shells as they enter and reside in the nanochannels (Fig. 1h). While monovalent ions with the small DH show the higher ion conductivities within MMT membranes, the conductivity only presents a slight reduction with the increasing DH and cation charges due to the “adaptive” channel height feature of MMT membranes. In particular, MMT-H membranes still have excellent conductivities over 42 and 80 mS cm−1 at 30 and 80 °C for Mg2+ and Al3+, respectively. By contrast, common ion conductors, even uncharged angstrom-scale slits, have conductivities of multivalent ions more than an order of magnitude greater than those of monovalent ions (as illustrated by the yellow line in Fig. 3f).22,29 Apparently, the charged nanochannels and inner high-density ions also play conspicuous roles in conquering the steric effect and strong electrostatic interactions with channel walls.
Additionally, a comparative analysis of the existing ion conductors, involving liquid, gel, solid and 2D ion conductors, highlights the significant advances and universality of charged high-density ion nanochannels in MMT membranes for ion transport. As affirmed in Fig. 3g and Tables S3–S7, the cation conductivities of our MMT membranes exceed those of most state-of-the-art ion conductors under identical conditions.
Ion transport mechanisms of MMT nanochannels
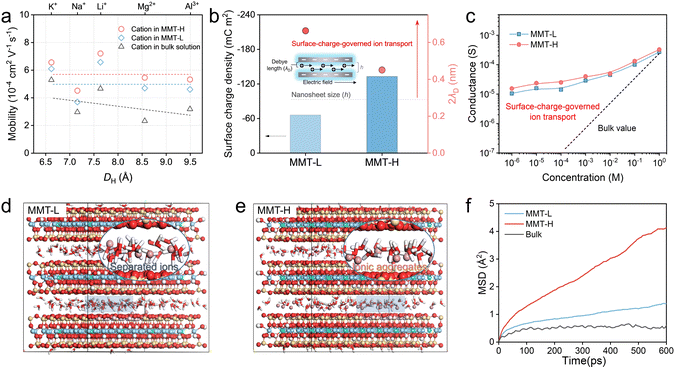
To elucidate the ion transport processes and the mechanisms in MMT nanochannels, we first estimated the effective mobility of cations in the bulk solutions and nanochannels of MMT membranes based on the Nernst–Einstein relationship (eqn (1)) to explore the comprehensive influence from tortuosity and connectivity of transport channel, and spatial proximity of neighboring ionic groups.12,54| σ = F(c+µ+ + c−µ−) | (1) |
To explore the effect of the channel charge, we estimated the thickness of the electrical double layer (Debye length, λD) of MMT walls based on the Debye–Hückel equation (for details, see Text S5). As displayed in Fig. 4b, the calculated λD of MMT-L and MMT-H walls are 0.33 and 0.22 nm, implying that the electrical double layer overlaps in channels with heights of 0.66 and 0.44 nm, respectively, which are higher than the MMT nanochannel size. Accordingly, both MMT nanochannels feature unipolar ion transport governed by surface charge, where conductivity relies on surface charge density (σc) of channel walls.55 Furthermore, we calculated σc by using the equation:56
 | (2) |
Given the synergistic effect of the ordered nanochannels, Coulomb interaction-induced multi-ion concerted migration, and the charge-governed transport, the MMT-H membranes perform universal and ultrafast cation conductivity. This is verified by the mean square displacement (MSD) in MD simulations. Fig. 4d and e and S26 show equilibrated configuration snapshots of MMT-L, MMT-H, and bulk solution, which offer insights into the coordination environment and Na+ diffusion dynamics. The MSD of Na+ was calculated to track kinetic conduction characteristics (Fig. 4f). For MMT-H, the Na+ diffusion coefficient is 61.40 × 10−4 Å2 ps−1, which is 3.84 and 15.31 times that of MMT-L and bulk solution, respectively (Fig. S27), consistent with the experimental results. The results of activation energy barriers for various cations are also in agreement (Fig. S28).
Practicality evaluation of MMT nanochannels
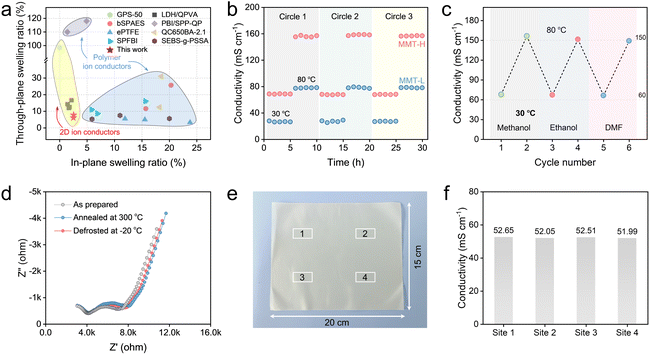
The stability is a common challenge for ion conductors in practical applications. Thus, we comprehensively evaluated the stability of MMT membranes. The hydration of ions is crucial for their rapid transport in channels, while it tends to cause the dimensional swelling, which affects ion conductivity. Fig. S29 and S30 illustrate that the membranes exhibit no noticeable structural change and performance degradation after immersion in NaCl solutions for 48 h, demonstrating their hydro-stability. In addition, MMT membranes show anisotropic dimensional swelling with the in-plane swelling <2.55% and through-plane swelling <8.89%, demonstrating the horizontal orientation of the nanochannels (Fig. S31). Compared to the most polymer and 2D ion conductors, MMT membranes showed excellent dimensional stability, which might result from the aligned interlayer space and sheet–ion–sheet attraction interactions between adjacent MMT nanosheets (Fig. 5a). We further assessed the cycling stability of MMT membranes. Under continuous cooling–heating cycles from 30 to 80 °C (Fig. 5b) and drying–wetting cycles from 30% to 98% RH (Fig. S32), MMT membranes show the unaffected conductivity under each condition, suggesting the long-term durability of MMT membranes resulting from the intrinsic structural stability caused by the close stacking of lamellae.Moving forward from the intrinsic ion conduction and inorganic nature,57 the MMT-H membrane also exhibits high stability in organic solvents, where a negligible change in the ion conductivity occurs for the MMT-H membrane after soaking in various solvents (Fig. 5c). As well, the temperature tolerance of the MMT-H membrane was investigated by determining the ion conductivity before and after annealing at 300 °C and defrosting at −20 °C for 12 h at different temperatures (Fig. 5d and S33). The almost overlapping Nyquist plots of impedance data display the excellent thermal and frost durability of MMT membranes, which is difficult to achieve with polymer electrolytes, organic framework-based and even graphene-based ion conductors.
Beyond conductivity, scalability is also critical for practical applications. To evaluate the scale-up potential of MMT nanochannels, a large-area membrane of 20 × 15 cm2 was prepared by the blade-coating approach. Fig. 5e displays good consistency of the MMT-H membrane during scale-up. Ion conductivity tests at four random selected sites reveal nearly identical values, only slightly lower than that of small membranes prepared by vacuum-assisted filtration. This indicates that large-scale preparation of MMT membranes can be achieved by designing and optimizing the membrane fabrication process.
Overall, the excellent thermal, solvent, and hydration stability, along with scalability, endows the MMT membrane with enhanced safety, processability and interfacial chemical compatibility for electrochemical device fabrication and optimization, while preventing mechanical and chemical failures, thereby being capable of broadening the electrochemical stability window.57
Conclusions
In summary, we develop a versatile solid-state ion conductor by stacking charged MMT nanosheets. By tuning the charge density and intercalated cation, the aligned high-density ion transport networks are constructed in nanochannels of MMT membranes, enabling unexpectedly considerable high ion conductivities for monovalent and multivalent cations, which are hardly realized in conventional ion conductors and even in free-charge nanoslits. It is uncovered that the “adaptive” channel height feature and synergetic contribution of Coulomb interaction-induced concerted ion movement between high-density cations and surface-charge-governed ion transport in charged nanochannels suppress the steric effect and strong electrostatic interactions with channel walls for different cations, thereby facilitating ion conduction. The developed nanochannels also exhibit outstanding conductive, thermal stability, and scalability for practically electrochemical applications. This demonstration not only provides insights into fast ion transport in nanoconfined systems, but also opens a promising avenue for designing and developing advanced ion conductors for batteries, supercapacitors, and nanofluidic devices.Author contributions
Lingjie Zhang: Conceptualization, investigation, methodology, data curation, formal analysis, visualization, software, writing–original draft, writing-review & editing. Yunjia Ling: Data curation, methodology. Jianglin Yan: Data curation, formal analysis, investigation. Zhenlei Wang: Investigation, software. Yanhui Miao: Data curation, methodology, software. Haoyu Bai: Conceptualization, validation, writing-review & editing. Tingting Zhang: Project administration, resources. Shaoxian Song: Supervision, resources. Mildred Quintana: Project administration, resources, supervision, validation, writing-review & editing. Yunliang Zhao: Conceptualization, supervision, funding acquisition, resources, project administration, validation, writing-review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
Data supporting the findings of this work are presented in the paper and its supplementary information (SI). Supplementary information: additional experimental details and data, including detailed preparation procedures, determination methods, membrane characterization and performance. See DOI: https://doi.org/10.1039/d5sc08076b.All data included in this work are available from the corresponding authors upon request.
Acknowledgements
The financial support for this work from the National Natural Science Foundation of China (52574323 and 52374275) and Natural Science Foundation of Hubei Province of China (2023AFA084) was gratefully acknowledged. L. Z. would like to thank CONAHCYT for granting him the scholarship no. 847199 during his PhD study. Y. L. would like to thank CONAHCYT for granting him the scholarship no. 4030488 during his PhD study.References
- H. Zhang, W. Xu, W. Song, K. Peng, L. Sun, C. Yang, X. Zhang, H. Zhang, B. Ye, X. Liang, Z. Yang, L. Wu, X. Ge and T. Xu, Nat. Sustain., 2024, 7, 910–919 CrossRef.
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS.
- D. Larcher and J. M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS.
- L. Zhang, J. Yan, S. Kang, Z. Wang, Y. Miao, R. Gao, T. Zhang, H. J. Ojeda-Galván, M. Quintana, H. Bai, S. Song and Y. Zhao, ACS Nano, 2025, 19, 33620–33631 CrossRef CAS.
- W. Song, K. Peng, W. Xu, X. Liu, H. Zhang, X. Liang, B. Ye, H. Zhang, Z. Yang, L. Wu, X. Ge and T. Xu, Nat. Commun., 2023, 14, 2732 CrossRef CAS PubMed.
- A. Manthiram, X. Yu and S. Wang, Nat. Rev. Mater., 2017, 2, 16103 CrossRef CAS.
- T. Zhu, Y. Kong, B. Lyu, L. Cao, B. Shi, X. Wang, X. Pang, C. Fan, C. Yang, H. Wu and Z. Jiang, Nat. Commun., 2023, 14, 5926 CrossRef CAS.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba and R. Kanno, Nat. Energy, 2016, 1, 16030 CrossRef CAS.
- H. Lian, R. Momen, Y. Xiao, B. Song, X. Hu, F. Zhu, H. Liu, L. Xu, W. Deng, H. Hou, G. Zou and X. Ji, Adv. Funct. Mater., 2023, 33, 2306060 CrossRef CAS.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, Nat. Mater., 2011, 10, 682–686 CrossRef CAS.
- C. Sun, J. Liu, Y. Gong, D. P. Wilkinson and J. Zhang, Nano Energy, 2017, 33, 363–386 CrossRef CAS.
- X. Yu and W. Ren, Nat. Commun., 2023, 14, 3998 CrossRef CAS PubMed.
- Z. W. B. Iton and K. A. See, Chem. Mater., 2022, 34, 881–898 CrossRef CAS.
- Z. W. B. Iton, Z. Irving-Singh, S.-J. Hwang, A. Bhattacharya, S. Shaker, T. Das, R. J. Clément, W. A. Goddard, III and K. A. See, J. Am. Chem. Soc., 2024, 146, 24398–24414 CrossRef CAS PubMed.
- M. K. Sarango-Ramírez, M. Donoshita, Y. Yoshida, D.-W. Lim and H. Kitagawa, Angew. Chem., Int. Ed., 2023, 62, e202301284 CrossRef PubMed.
- C. Yan, C. Lv, Y. Zhu, G. Chen, J. Sun and G. Yu, Adv. Mater., 2017, 29, 1703909 CrossRef.
- X. Qian, L. Chen, L. Yin, Z. Liu, S. Pei, F. Li, G. Hou, S. Chen, L. Song, K. H. Thebo, H.-M. Cheng and W. Ren, Science, 2020, 370, 596–600 CrossRef CAS PubMed.
- S. Acosta, H. J. Ojeda-Galván and M. Quintana, Phys. Chem. Chem. Phys., 2023, 25, 24264–24277 RSC.
- L. Zhang, J. Yan, Z. Wang, T. Zhang, S. Song, M. Quintana and Y. Zhao, Adv. Mater., 2025, e12998 CrossRef.
- J. Wang, X. Zhang, Z. Yu, Y. Gao, Q. Lu, C. Ma, K. Liu, Q. Yuan and Y. Yang, Natl. Sci. Rev., 2025, 12, nwae482 CrossRef CAS.
- Y. Zhang, D. Chen, W. He, J. Tan, Y. Yang and Q. Yuan, Adv. Mater. Technol., 2023, 8, 2202014 CrossRef CAS.
- A. Esfandiar, B. Radha, F. C. Wang, Q. Yang, S. Hu, S. Garaj, R. R. Nair, A. K. Geim and K. Gopinadhan, Science, 2017, 358, 511–513 CrossRef CAS.
- Y. Xue, Y. Xia, S. Yang, Y. Alsaid, K. Y. Fong, Y. Wang and X. Zhang, Science, 2021, 372, 501–503 CrossRef CAS PubMed.
- J. Peng, Y. Liu, Y. Pan, J. Wu, Y. Su, Y. Guo, X. Wu, C. Wu and Y. Xie, J. Am. Chem. Soc., 2020, 142, 18645–18651 CrossRef CAS PubMed.
- J. Park, S. Bhoyate, Y.-H. Kim, Y.-M. Kim, Y. H. Lee, P. Conlin, K. Cho and W. Choi, ACS Nano, 2021, 15, 12267–12275 CrossRef CAS.
- J. Abraham, K. S. Vasu, C. D. Williams, K. Gopinadhan, Y. Su, C. T. Cherian, J. Dix, E. Prestat, S. J. Haigh, I. V. Grigorieva, P. Carbone, A. K. Geim and R. R. Nair, Nat. Nanotechnol., 2017, 12, 546–550 CrossRef CAS PubMed.
- Y. Yang, X. Yang, L. Liang, Y. Gao, H. Cheng, X. Li, M. Zou, R. Ma, Q. Yuan and X. Duan, Science, 2019, 364, 1057–1062 CrossRef CAS.
- Z. Dai, P. Jin, S. Yuan, J. Yang, K. V. Agrawal and H. Wang, Nat. Commun., 2025, 16, 4626 CrossRef CAS.
- Q. Zhao, S. Stalin, C.-Z. Zhao and L. A. Archer, Nat. Rev. Mater., 2020, 5, 229–252 CrossRef CAS.
- J. Shen, G. Liu, Y. Han and W. Jin, Nat. Rev. Mater., 2021, 6, 294–312 CrossRef CAS.
- T. Zhang, H. Bai, Y. Zhao, B. Ren, T. Wen, L. Chen, S. Song and S. Komarneni, ACS Nano, 2022, 16, 4930–4939 CrossRef CAS PubMed.
- X. He, Y. Zhu and Y. Mo, Nat. Commun., 2017, 8, 15893 CrossRef CAS PubMed.
- D. Lei, Y. Wang, Q. Zhang, S. Wang, L. Jiang and Z. Zhang, Nat. Commun., 2025, 16, 754 CrossRef CAS.
- D. A. Köpfer, C. Song, T. Gruene, G. M. Sheldrick, U. Zachariae and B. L. de Groot, Science, 2014, 346, 352–355 CrossRef PubMed.
- B. J. Hinds, Science, 2021, 372, 459–460 CrossRef CAS.
- X. Jiang, L. Zhang, Y. Miao, L. Chen, J. Liu, T. Zhang, S. Cheng, Y. Song and Y. Zhao, Water Res., 2025, 276, 123291 CrossRef CAS.
- S. Chang, J. Liu, J. Chen, Y. Zhang, Z. Wang, Z. Huang and B. Liu, Small, 2025, 21, 2504191 CrossRef CAS.
- W. Wang, Y. Zhao, L. Chen, L. Zhang, T. Wen, Z. Wang and T. Zhang, Chem. Eng. J., 2024, 500, 156801 CrossRef CAS.
- J. Hu, X. Tang, Q. Dai, Z. Liu, H. Zhang, A. Zheng, Z. Yuan and X. Li, Nat. Commun., 2021, 12, 3409 CrossRef CAS PubMed.
- L. Zhang, Z. Wang, T. Zhang, X. Jiang, L. Chen, H. J. Ojeda-Galván, M. Quintana, S. Song, S. Komarneni and Y. Zhao, ACS Mater. Lett., 2025, 7, 2835–2843 CrossRef.
- L. Zhong, S. Hu, X. Yang, M. Yang, T. Zhang, L. Chen, Y. Zhao and S. Song, Colloids Surf., A, 2021, 617, 126364 CrossRef CAS.
- W. Yu, Z. Ge, K. Zhang, X. Liang, X. Ge, H. Wang, M. Li, X. Shen, Y. Xu, L. Wu and T. Xu, Ind. Eng. Chem. Res., 2022, 61, 4329–4338 CrossRef CAS.
- J. Hao, C. Bi, S. Li, S. Zhao, S. Yang, Y. Li and T. E, Int. J. Biol. Macromol., 2025, 284, 138160 CrossRef CAS PubMed.
- H. Wang, P. Wen, Y. Liu, S. Han, Z. Zhang, Y. Xu, M. Chen and X. Lin, J. Am. Chem. Soc., 2025, 147, 14554–14563 CrossRef CAS.
- Q. Zhang, Y. Ma, Y. Lu, L. Li, F. Wan, K. Zhang and J. Chen, Nat. Commun., 2020, 11, 4463 CrossRef CAS PubMed.
- Y. Okazaki, T. Taniuchi, G. Mogami, N. Matubayasi and M. Suzuki, J. Phys. Chem. A, 2014, 118, 2922–2930 CrossRef CAS.
- M. Botta, S. Merk, R. J. Spranger, A. Senyshyn, V. Baran, V. Dyadkin, L. van Wüllen and T. F. Fässler, Angew. Chem., Int. Ed., 2025, 64, e202419381 CrossRef CAS PubMed.
- Z. Zhang, L. Liang, J. Feng, G. Hou and W. Ren, Nat. Commun., 2024, 15, 2706 CrossRef CAS.
- X. Zhou, Y. Huang, B. Wen, Z. Yang, Z. Hao, L. Li, S.-L. Chou and F. Li, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2316914121 CrossRef CAS.
- Z. Lu, H. Yang, Y. Guo, H. Lin, P. Shan, S. Wu, P. He, Y. Yang, Q.-H. Yang and H. Zhou, Nat. Commun., 2024, 15, 3497 CrossRef CAS PubMed.
- M. Ding, H. Xu, W. Chen, Q. Kong, T. Lin, H. Tao, K. Zhang, Q. Liu, K. Zhang and Z. Xie, J. Mater. Chem. A, 2020, 8, 22666–22673 RSC.
- X. Yang, C. Li, P. Liu, Y. Zhai, I. Hussain, X. Sui, L. Gao and L. Jiang, Chem. Mater., 2023, 35, 7266–7272 CrossRef CAS.
- R. Guo, Y. Zhou, W. Wang, Y. Zhai, X. Liu, W. He, W. Ou, R. Ding, H.-L. Zhang, M. Wu, Z. Jiang and K.-G. Zhou, Sci. Adv., 2025, 11, eadr5374 CrossRef CAS PubMed.
- Z. Li, W. Wang, Y. Chen, C. Xiong, G. He, Y. Cao, H. Wu, M. D. Guiver and Z. Jiang, J. Mater. Chem. A, 2016, 4, 2340–2348 RSC.
- A. R. Koltonow and J. Huang, Science, 2016, 351, 1395–1396 CrossRef CAS PubMed.
- J. Tang, Y. Wang, H. Yang, Q. Zhang, C. Wang, L. Li, Z. Zheng, Y. Jin, H. Wang, Y. Gu and T. Zuo, Nat. Commun., 2024, 15, 3649 CrossRef CAS PubMed.
- L. Cao, H. Wu, X. He, H. Geng, R. Zhang, M. Qiu, P. Yang, B. Shi, N. A. Khan and Z. Jiang, J. Mater. Chem. A, 2019, 7, 25657–25664 RSC.
| This journal is © The Royal Society of Chemistry 2026 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: