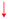Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unusual nitrene reactivity: imine formation in the photochemical reaction of a borylnitrene with ethene
Virinder
Bhagat
 and
Holger F.
Bettinger
and
Holger F.
Bettinger
 *
*
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany. E-mail: holger.bettinger@uni-tuebingen.de
First published on 24th December 2025
Abstract
The cycloaddition of nitrenes with olefins is an important method for the synthesis of aziridines. We report here that the reaction of catecholato borylnitrene CatBN with ethene upon long wavelength photoirradiation (λ > 550 nm) gives not only the expected aziridine, but also the imine CatBN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3 under cryogenic matrix isolation conditions in solid neon at 4 K. Computational analysis reveals that the photochemically generated singlet CatBN (1A1 electronic state) can react with ethene to form the aziridine and a singlet 1,3-diradical intermediate. The latter arises from multi-state reactivity involving the nearly degenerate 1A1 and 1A2 nitrene singlet states. The singlet 1,3-diradical has a very low barrier for 1,2-H migration to give the imine. The observation of an imine in the reaction with an alkene reveals chemodivergence by highlighting a previously unobserved reactivity pathway in nitrene chemistry.
CHCH3 under cryogenic matrix isolation conditions in solid neon at 4 K. Computational analysis reveals that the photochemically generated singlet CatBN (1A1 electronic state) can react with ethene to form the aziridine and a singlet 1,3-diradical intermediate. The latter arises from multi-state reactivity involving the nearly degenerate 1A1 and 1A2 nitrene singlet states. The singlet 1,3-diradical has a very low barrier for 1,2-H migration to give the imine. The observation of an imine in the reaction with an alkene reveals chemodivergence by highlighting a previously unobserved reactivity pathway in nitrene chemistry.
Introduction
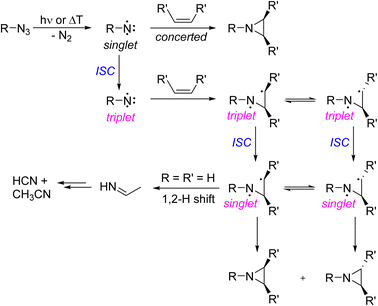
The reaction of nitrenoids and free nitrenes with carbon–carbon double bonds is an important method for producing aziridines in organic synthesis.1–10 The hallmark of singlet nitrenes R–N, electron deficient nitrogen species with an electron sextet, is their stereospecific cycloaddition reaction with olefins to yield aziridines.2 The cycloaddition of singlet nitrenes with alkenes is a concerted reaction that preserves the stereochemistry of the alkene substituents (Scheme 1). In contrast, triplet nitrenes react in a stepwise fashion involving a triplet 1,3-diradical intermediate. As the rate of intersystem crossing to the lower energy singlet 1,3-diradical can be competitive with the rate of rotation about the C–C bond of the diradical, triplet nitrenes show only low or even no stereoselectivity in aziridine formation.2In the gas phase reaction of imidogen NH and ethene, the primary products, aziridine and triplet 1,3-diradical, are formed in a vibrationally hot state and fragment to the ultimate stable products CH3CN and HCN (Scheme 1, bottom).11–14 Vibrationally excited aziridine is assumed to be in dynamic equilibrium with the singlet 1,3-diradical that can readily rearrange to an imine by 1,2-hydrogen shift.13,15 The imine is assumed to be an intermediate of gas phase product formation, but has been rarely observed in the reaction of nitrenes with olefins.8
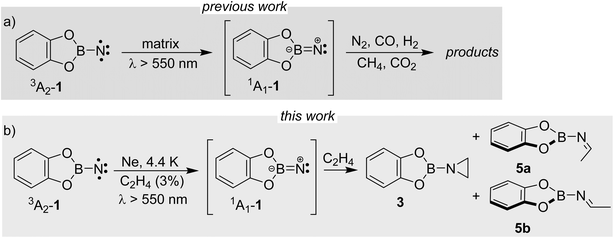
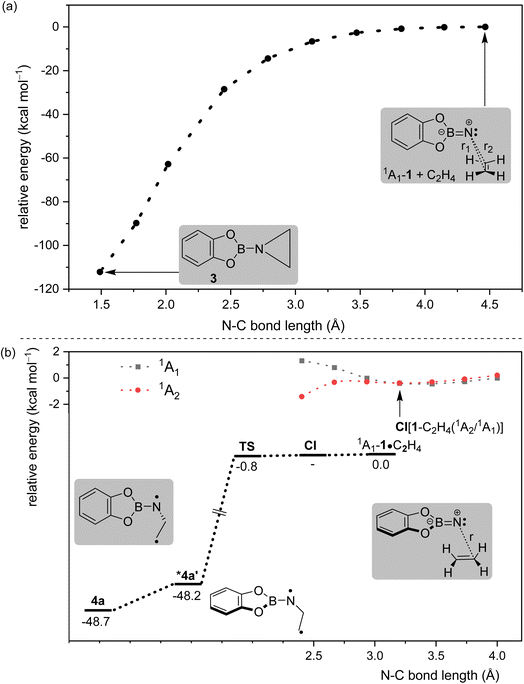
We here report detection of an imine in the reaction of a singlet borylnitrene with ethene. Borylnitrenes are a rather unusual class of nitrenes. The catecholato borylnitrene CatBN 1, a triplet ground state species (3A2 electronic state) that can be observed using matrix isolation methods,16 reacts upon very mild annealing with dioxygen O2 to form nitritoborane CatBONO revealing that 3A2-1 is much more reactive than typical organic nitrenes.16,17 Upon long wavelength irradiation (>550 nm), matrix isolated 3A2-1 forms 1A1-1 as supported by computational analysis of the excited state potential energy surfaces.18 The highly electrophilic vinylidene-like 1A1-1 readily activates unreactive small closed-shell molecules by addition (N2, CO, and CO2) or insertion (CH4 and H2) into single bonds (Scheme 2a).16,19–23 The triplet state of 1 does not react with these unreactive species. In solution or the gas phase, borylnitrenes of the 1,3,2-dioxaborolane type insert into unactivated CH bonds allowing for amination of unusual substrates, such as cycloalkanes, methane, and tetramethylsilane presumably via the transient singlet nitrene.19,21,24
By combining matrix isolation IR spectroscopy and computational methods, we provide evidence that the reaction of CatBN with ethene yields, along with the expected aziridine, the isomeric imine, demonstrating chemodivergence25 in this simple system (Scheme 2b). The imine likely results from the singlet 1,3-diradical, which in turn arises from the multi-state reactivity of the borylnitrene.
Results and discussion
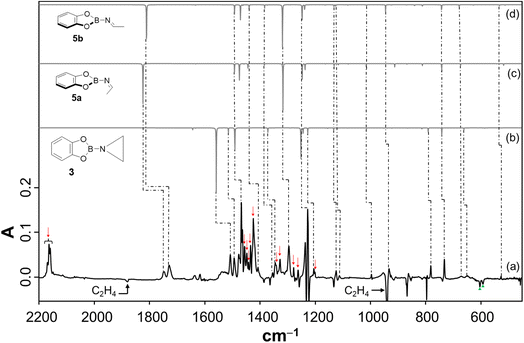
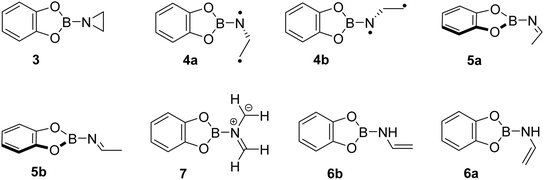
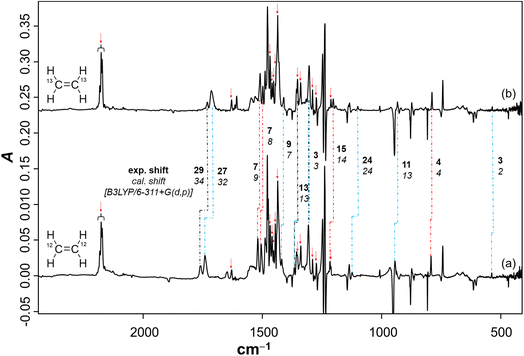
To study the reaction of borylnitrene 1 with ethene C2H4, the borylazide CatBN32 was deposited with a large excess of neon gas doped with 3% C2H4 onto a cold CsI window maintained at 4 K. Thereafter, the deposited matrix was irradiated with light of wavelength λ = 254 nm, which resulted in the formation of borylnitrene 1 in its triplet ground state 3A2 (Fig. S1a). The infrared (IR) signals assigned to 1 showed a shift compared to the reported IR data,16 which is probably due to the complexation26 to the dopant C2H4. In fact, we have experimental evidence supporting the observation of a complex between 1 and ethene (Fig. S2). Especially, the B–N/B–O wagging mode of the complex between 1 and ethene (3A2-1·C2H4) show a prominent red shift of 17 cm−1 compared to bare 3A2-1, which matches exactly with the corresponding computed shift. In addition to the signals due to 3A2-1, weak signals at 1760, 1742, 1520, 1506, and 1307 cm−1 were also observed. Subsequently, the matrix was irradiated with λ > 550 nm, as performed in our previous studies,16,19,20,23 to populate the 1A1 state of 1 that undergoes intermolecular reaction with C2H4.18 The resulting difference spectrum illustrated in Fig. 1a shows the formation of azide 2 that we observed previously under these conditions,16,19–23 the bleaching of IR bands related to C2H4, and the appearance of many additional IR bands at 1760, 1742, 1520, 1506, 1480, 1475, 1462, 1456, 1419, 1366, 1357, 1307, 1240, 1217, 1137, 1124, 1008, 944, 794, 744, 684, 660, and 539 cm−1. The number of new IR bands indicates the formation of more than one species from the photoinduced reaction between 1A1-1 and ethene. There are a number of potential reaction products of this reaction, including aziridine 3, diastereoisomeric imines 5a and 5b, conformational isomeric enamines 6a and 6b, and azomethine ylide 7 (Chart 1).27–29Based on the computed spectra of these species (Fig. S3), the formation of isomeric enamines 6a and 6b, as well as azomethine ylide 7, can safely be excluded. On the other hand, we surmised the formation of aziridine 3 and imine isomers, based on the IR bands positioned between 1700 and 1800 cm−1. To further support the formation of these products, a comparison was made between the experimental difference spectrum resulting from λ > 550 nm irradiation and the computed IR spectra of 3, 5a, and 5b, as shown in Fig. 1. The IR bands, such as 1520 and 1217 cm−1, are composed of three-membered ring breathing modes and the one at 1506 cm−1 is composed of 3-membered ring CH2 scissor motion, which are the signature IR bands of three-membered ring compounds.30 Additionally, the band at 794 cm−1 is predominantly described by the characteristic C–C stretch of the terminal three-membered ring. In the 1800–1700 cm−1 region, strong IR bands signify the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, while bands at 1307 cm−1 and 1366 cm−1 are due to the B–N
C stretching, while bands at 1307 cm−1 and 1366 cm−1 are due to the B–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H scissor mode and symmetric bend of the terminal CH3 groups, respectively, of 5a and 5b. These IR features are indicative of compounds containing the imine functional group.31
C–H scissor mode and symmetric bend of the terminal CH3 groups, respectively, of 5a and 5b. These IR features are indicative of compounds containing the imine functional group.31
To further support the spectral assignments, the reaction between 1 and the 13C2H4 isotopologue of ethene was studied. The difference spectra resulting from λ > 550 nm irradiation (Fig. 2) show that the characteristic ring breathing modes are red shifted by 7 and 15 cm−1, respectively. The CH2 scissor mode and the characteristic C–C stretching mode of the terminal three-membered ring are red shifted by 7 cm−1 and 4 cm−1, respectively. The experimental isotopic shifts match well with the corresponding computed isotopic shifts (Fig. 2). Similarly, the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretches in 5a and 5b show isotopic red shifts of 29 and 27 cm−1, which match well with the corresponding computed shifts of 34 and 32 cm−1, respectively. Also, the common IR modes in the isomeric imines, CH3 symmetric bend and B–N
C stretches in 5a and 5b show isotopic red shifts of 29 and 27 cm−1, which match well with the corresponding computed shifts of 34 and 32 cm−1, respectively. Also, the common IR modes in the isomeric imines, CH3 symmetric bend and B–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H scissor, show isotopic red shifts of 13 and 3 cm−1, respectively, which have a perfect match with the computed shifts. Moreover, the assignment of the products was further corroborated by performing the reaction of 3A2-1 with C2D4 (Fig. S4). In addition, the species from the previous step was tested under photoirradiation with λ = 254 nm, showing that aziridine 3 remains largely intact while 5b photoisomerizes to 5a (Fig. S10).
C–H scissor, show isotopic red shifts of 13 and 3 cm−1, respectively, which have a perfect match with the computed shifts. Moreover, the assignment of the products was further corroborated by performing the reaction of 3A2-1 with C2D4 (Fig. S4). In addition, the species from the previous step was tested under photoirradiation with λ = 254 nm, showing that aziridine 3 remains largely intact while 5b photoisomerizes to 5a (Fig. S10).
The unexpected formation of isomeric imines necessitated further computational investigation to understand the reaction mechanism. Previously, we have obtained computational evidence that the irradiation of 1 in its triplet ground state with λ > 550 nm populates the 1A1 state.18 Therefore, we consider the 1A1 state as the starting point of our computational investigations that comprise two components: (1) the formation of primary products resulting from the reaction between 1A1-1 and ethene, and (2) the subsequent formation of secondary products. First, we studied the formation of the primary product aziridine 3via a relaxed potential energy surface (PES) scan. The scan parameters r1 and r2, as shown in Fig. 3a, were constrained to the same value at each step of the scan to ensure quasi-Cs symmetry throughout the process. The PES has no barrier for the formation of aziridine and therefore supports the experimental observation of 3.
In addition, the reaction was studied with a different reaction coordinate, as shown in Fig. 3b, as the second part of the primary reaction. In this reaction, the planes containing 1 and ethene lie parallel to each other at the start of the reaction (pre-reactive complex). The pre-reactive complex is a good starting point considering the available experimental evidence for such a complex, as discussed above (Fig. S2). Considering the possibility of the multireference character of the wavefunctions of different species along this reaction coordinate, complete active space second-order perturbation (CASPT2) theory was chosen. The calculated stationary points show the barrierless formation of 1,3-diradical 4a (Fig. 3b). A partial PES scan was performed with r as the scan parameter (Fig. 3b) to show the transfer of population from initially populated 1A1 to the 1A2 state of 1 along such a reaction coordinate through a conical intersection (CI).
The observation of imine products in the experimental studies could be explained by the second part of our computational studies. Herein, we have computed a PES starting from aziridine 3 and 1,3-diradical 4a, as shown in Fig. 4 (highlighted in grey). Conversion of 3 into isomeric imines 5a and 5b can proceed via isomeric 1,3-diradicals 4a and 4b, respectively. Since these steps involve high energy barriers of around 57–59 kcal mol−1, these paths are not feasible for the formation of isomeric imines under the cryogenic conditions employed in our experiments if energy transfer to the surrounding matrix is efficient. Furthermore, IR measurements of the 13C-labeled reaction (Fig. S11) at various time intervals reveal the formation of 5a/5b without accumulation of 3, suggesting that the imines form independently rather than as secondary photoproducts. On the other hand, the formation of 5avia a 1,2-H shift to the terminal carbon atom of 4a has a barrier of merely 1.7 kcal mol−1, which can be surpassed in cryogenic matrices at 4 K. In addition, the conversion of 4a into 4b has a negligible barrier, which in turn has an accessible energy barrier of 1.8 kcal mol−1 for its conversion into 5b. Furthermore, starting from 4a and 4b, we explored the possibility of forming enamine products 6a and 6b through a 1,2-H shift to the nitrogen atom, as shown in Fig. S5. High barriers for the formation of isomeric enamines from 4a and 4b, compared to the isomeric imines, support their experimental absence.
 | ||
| Fig. 4 Potential energy surface initiating from aziridine 3 and syn-1,3-diradical 4a calculated at the B2PLYPD3/def2-TZVPP level of theory. | ||
Conclusions
The combined matrix isolation and computational studies regarding the photochemical reactivity of 1 with ethene demonstrate the formation of aziridine 3 and isomeric imines 5a and 5b, as shown in Scheme 3. Notably, the formation of isomeric imines is rare for such a reaction. Computational studies support the formation of singlet 1,3-diradical 4a, which could not be observed experimentally, as an alternate primary product alongside the expected aziridine 3. Finally, the diradical 4a has accessible energy barriers for its conversion into imines 5a and 5b. The observations are remarkable as the formation of the 1,3-diradical intermediate in cycloaddition reactions of nitrenes is the hallmark of triplet state reactivity, while here the singlet state is involved. The low barrier for collapse of the diradical to the imines 5 and aziridine 3 suggests that these processes are very fast. We are currently investigating the dual mode reactions of borylnitrenes with olefins in solution to shed more light onto this unusual reactivity.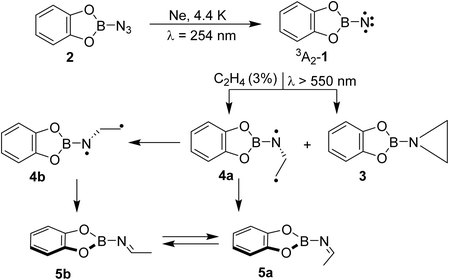 | ||
| Scheme 3 Formation of aziridine 3 and isomeric imines 5a and 5b resulting from the reaction of borylnitrene 1 and ethene. | ||
Author contributions
V. B. conducted all calculations, experiments involving matrix isolation, and data analysis. V. B. and H. F. B. jointly wrote the first draft of the manuscript. H. F. B. conceived the research idea, revised the manuscript, and secured funding.Conflicts of interest
The authors declare no competing interests.Data availability
The data that support the findings of this study are available in the supplementary information (SI) of this article. Supplementary information: matrix isolation spectra, peak tables, computational details, and Cartesian coordinates. See DOI: https://doi.org/10.1039/d5sc07994b.Acknowledgements
The authors thank the German Research Foundation (DFG) for support of this work through project 460087109. The computations were performed on the BwForCluster JUSTUS2 cluster. The author acknowledges support by the state of Baden-Württemberg through bwHPC and the DFG through grant no. INST 40/575-1 FUGG (JUSTUS 2 cluster) for computation facilities.References
-
K. Lee, K. Seo, M. Dehghany, Y. Hu, A. Trinh and J. M. Schomaker, in Heterocycles from Carbenes and Nitrenes : Methods, Reactions and Synthetic Applications, ed. M. P. Doyle and X. Xu, Springer International Publishing, Cham, 2023, pp. 313–377 Search PubMed
.
- W. Lwowski, Angew Chem. Int. Ed. Engl., 1967, 6, 897–906 Search PubMed
.
- N. Baris, M. Dračínský, L. J. Tóth, B. Klepetářová and P. Beier, Org. Chem. Front., 2025, 12, 5610–5615 RSC
.
- N. Baris, M. Dračínský, J. Tarábek, J. Filgas, P. Slavíček, L. Ludvíková, S. Boháčová, T. Slanina, B. Klepetářová and P. Beier, Angew. Chem., Int. Ed., 2024, 63, e202315162 CrossRef CAS
.
- Y.-A. Xu, S.-H. Xiang, J.-T. Che, Y.-B. Wang and B. Tan, Chin. J. Chem., 2024, 42, 2656–2667 CrossRef CAS
.
- A. R. Meyer, M. V. Popescu, A. Sau, N. H. Damrauer, R. S. Paton and T. P. Yoon, ACS Catal., 2024, 14, 12310–12317 CrossRef CAS
.
-
M. S. Platz, in Reactive Intermediate Chemistry, 2003, pp. 501–559 Search PubMed
.
- S. O. Scholz, E. P. Farney, S. Kim, D. M. Bates and T. P. Yoon, Angew. Chem., Int. Ed., 2016, 55, 2239–2242 CrossRef CAS PubMed
.
- M. Holzinger, J. Abraham, P. Whelan, R. Graupner, L. Ley, F. Hennrich, M. Kappes and A. Hirsch, J. Am. Chem. Soc., 2003, 125, 8566–8580 CrossRef CAS
.
- M. Holzinger, O. Vostrowsky, A. Hirsch, F. Hennrich, M. Kappes, R. Weiss and F. Jellen, Angew. Chem., Int. Ed., 2001, 40, 4002–4005 CrossRef CAS
.
- D. W. Cornell, R. S. Berry and W. Lwowski, J. Am. Chem. Soc., 1966, 88, 544–550 Search PubMed
.
- S. P. Devi, T. Salam and R. H. Duncan Lyngdoh, J. Chem. Sci., 2016, 128, 681–693 CrossRef CAS
.
- B. Du, W. Zhang, L. Mu and C. Feng, J. Mol. Struct.: THEOCHEM, 2007, 816, 21–29 CrossRef CAS
.
- M. Röhrig and H. G. Wagner, Ber. Bunsenges. Phys. Chem., 1994, 98, 864–868 Search PubMed
.
- T. Fueno, K. Yamaguchi and O. Kondo, Bull. Chem. Soc. Jpn., 2006, 63, 901–912 CrossRef
.
- H. F. Bettinger and H. Bornemann, J. Am. Chem. Soc., 2006, 128, 11128–11134 CrossRef CAS
.
- V. Bhagat, J. Schumann and H. F. Bettinger, Chem.–Eur. J., 2020, 26, 12654–12663 CrossRef CAS
.
- V. Bhagat and H. F. Bettinger, J. Phys. Chem. A, 2022, 126, 7660–7666 CrossRef CAS PubMed
.
- H. F. Bettinger, M. Filthaus, H. Bornemann and I. M. Oppel, Angew. Chem., Int. Ed., 2008, 47, 4744–4747 CrossRef CAS
.
- H. F. Bettinger, M. Filthaus and P. Neuhaus, Chem. Commun., 2009, 2186–2188 RSC
.
- H. F. Bettinger and M. Filthaus, Org. Biomol. Chem., 2010, 8, 5477–5482 RSC
.
- H. F. Bettinger and H. Bornemann, Z. Anorg. Allg. Chem., 2011, 637, 2169–2174 Search PubMed
.
- V. Bhagat, J. Schumann and H. F. Bettinger, Angew. Chem., Int. Ed., 2021, 60, 23112–23116 CrossRef CAS
.
- M. Filthaus, L. Schwertmann, P. Neuhaus, R. W. Seidel, I. M. Oppel and H. F. Bettinger, Organometallics, 2012, 31, 3894–3903 CrossRef CAS
.
- I. P. Beletskaya, C. Nájera and M. Yus, Chem. Soc. Rev., 2020, 49, 7101–7166 Search PubMed
.
- Y. Du, X. Li, J. Jiang, W. Fan, D. Mu, Q. Li, R. Ma, Q. Yu and X. Zeng, J. Phys. Chem. Lett., 2025, 16, 7127–7133 Search PubMed
.
- A. V. Kuzmin and B. A. Shainyan, J. Phys. Org. Chem., 2014, 27, 794–802 CrossRef CAS
.
- T. Fueno, V. Bonacic-Koutecky and J. Koutecky, J. Am. Chem. Soc., 1983, 105, 5547–5557 CrossRef CAS
.
- E. Jacox and D. E. Milligan, J. Am. Chem. Soc., 1963, 85, 278–282 CrossRef
.
-
G. Herzberg, Infra-Red and Raman Spectra of Polyatomic Molecules, D. Van Nostrand Co., Inc., New York, 1945 Search PubMed
.
- A. Melli, M. Melosso, N. Tasinato, G. Bosi, L. Spada, J. Bloino, M. Mendolicchio, L. Dore, V. Barone and C. Puzzarini, Astrophys. J., 2018, 855, 123 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2026 |