Open Access Article
Open Access ArticleLiquid–liquid phase separation of peptides: a molecular foundation for next-generation biomaterials
Liheng
Lu
 and
Huaimin
Wang
and
Huaimin
Wang
 *
*
Department of Chemistry, School of Science, Westlake University, No. 600 Dunyu Road, Hangzhou 310024, Zhejiang Province, China. E-mail: wanghuaimin@westlake.edu.cn
First published on 22nd December 2025
Abstract
Liquid–liquid phase separation (LLPS) is a key mechanism for organizing membraneless cellular compartments, influencing processes like stress response, signaling, and gene regulation. While intrinsically disordered proteins (IDPs) and their regions (IDRs) drive LLPS through weak, multivalent interactions, the complexity of full-length proteins limits systematic design and understanding of phase separation. Peptides, as simpler modular units, offer precise control over sequence composition and provide a scalable platform for studying LLPS. Recent advancements demonstrate that peptide condensates reveal fundamental phase separation rules and enable applications such as artificial cells and drug delivery systems. This perspective highlights recent progress in peptide LLPS, focusing on sequence design, structural characterization, and emerging applications, and discusses challenges and future directions for broader applications.
1 Introduction
Liquid–liquid phase separation (LLPS) has emerged as a central principle for organizing biomolecules in cells,1–4 enabling the formation of dynamic, membraneless condensates such as stress granules, nucleoli, and signaling hubs.5–7 These condensates provide confined environments that concentrate specific molecules, regulate biochemical reactions, and allow rapid responses to environmental cues.8–11 The conceptual origins of LLPS trace back to early solution thermodynamics and phase diagram analyses by Van't Hoff and Gibbs, later expanded by polymer theories such as the Flory–Huggins model.9,12 Since the discovery that C. elegans P granules behave as liquid droplets rather than static particles,5 followed by in vitro reconstitution of protein–RNA condensates, LLPS has been recognized as a general organizing mechanism across diverse cellular contexts.13–20 Its dysregulation is increasingly linked to pathologies including neurodegeneration, cancer, and immune dysfunction, underscoring both its physiological importance and therapeutic relevance.21–23Mechanistic studies of LLPS have traditionally focused on intrinsically disordered proteins (IDPs) and intrinsically disordered regions (IDRs), which contain multivalent motifs that mediate weak but cooperative interactions such as electrostatic interaction, π–π stacking, and cation–π interaction.8,24–26 While these systems have revealed much about the molecular grammar of phase separation, the complexity of full-length proteins poses persistent challenges. Their long sequences, modular domains, and extensive post-translational modifications make it difficult to isolate the contribution of specific residues or to systematically test design rules. Moreover, high valency is often required for proteins to phase-separate at physiological concentrations, which complicates experimental tractability and limits high-throughput exploration.
Peptides are now emerging as simplified and programmable building blocks for LLPS, offering a complementary approach to protein-based studies. As the smallest modular units, peptides retain essential interaction motifs while providing precise control over sequence composition, length, and valency.27–29 Their chemical accessibility enables rapid synthesis, systematic library construction, and facile incorporation of functional groups. In this way, peptides bridge a critical gap: they are biologically relevant enough to mimic features of protein condensates, yet minimal enough to enable mechanistic dissection and rational design.
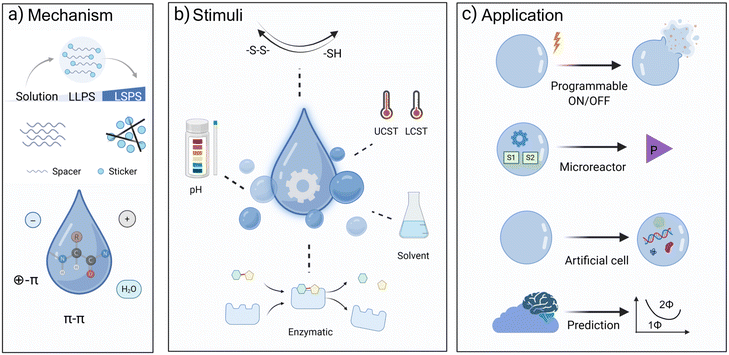
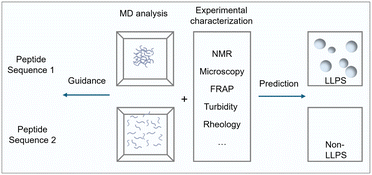
This perspective focuses specifically on peptide materials driven by LLPS. We highlight how sequence determinants and intermolecular forces govern condensate formation, and how external stimuli (e.g., pH, temperature, redox state, and enzymatic activity) regulate condensate dynamics. We then discuss how these principles translate into emerging applications, including programmable delivery systems, microreactors, and artificial organelles. Finally, we outline the challenges that must be addressed, such as predictive sequence design, stability in complex environments, and integration of multifunctional modules, to fully realize peptide LLPS as a versatile platform for chemistry, bioengineering, and medicine (Fig. 1).
2 Mechanistic basis and design of peptide condensates through LLPS
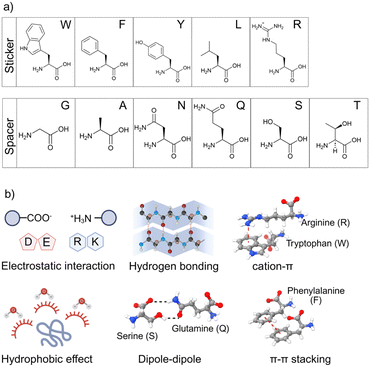
2.1 Sequence composition and the “sticker–spacer” model
Peptide LLPS arises from multivalent interactions between “sticker” residues (typically aromatic or charged amino acids) connected by flexible “spacers” that regulate chain mobility.24,30,31 Compared with long proteins, short peptides have lower valency, and thus require either stronger per-residue interactions or favorable thermodynamic compensation to phase-separate at low concentrations.32–35 Incorporating residues with the capability of providing strong and directional interactions is thus essential for effective LLPS in minimal peptide systems.Aromatic amino acids such as phenylalanine (F), tyrosine (Y), and especially tryptophan (W) serve as potent stickers due to their extended π-electron systems, hydrophobicity,36–38 and in the case of W, additional hydrogen bonding potential from its indole NH group. Artificial enrichment of these residues can significantly enhance LLPS propensity (Fig. 2a). For example, Zhou et al. designed WXXW tetrapeptides (X = amino acid residue) that formed droplets at high concentration, with increased sticker density (e.g., WXWW) reducing the critical concentration for phase separation.39 These studies show that even in minimal sequences, tuning the sticker number and arrangement provides programmable control over condensate behavior.
2.2 Forces for driving LLPS
Beyond sequence composition, condensate stability is governed by the balance and reversibility of underlying noncovalent interactions (Fig. 2b).40,41 Electrostatic interactions are often dominant in charged peptide systems and peptide–nucleic acid coacervates,42 whereas hydrophobic interactions can drive LLPS in neutral peptides by promoting water exclusion and lowering the free energy of phase separation.36 Aromatic–aromatic interactions, π–π stacking and cation–π interactions (e.g., between arginine and aromatics residues) further enhance cohesion, and hydrogen bonding or dipole–dipole interactions from polar residues can modulate internal organization and mobility within condensates.43 Castelletto et al. demonstrated that combining aromatic groups with arginine strongly promotes LLPS in minimal peptides, tuning the formation of droplets and controlling their dynamics.33 Such findings reinforce that condensates are stabilized not by a single force but by a cooperative network of weak interactions.Together, the sticker–spacer framework and the types of driving forces provide a rational design basis for peptide LLPS. By selectively varying residue type, valency, and patterning, one can shift critical concentrations, modulate material states, or introduce responsiveness. Beyond the sticker–spacer framework, peptide LLPS can also emerge from alternative strategies such as solvation–desolvation dynamics, sequence-encoded polarity gradients, or environmentally triggered changes in charge or hydrophobicity, which collectively broaden the molecular strategies available for designing minimal condensate-forming peptides. These principles not only clarify the minimal requirements for condensation but also offer a blueprint for engineering peptide-based assemblies with tailored functions.
3 Physicochemical properties of peptide-based condensates through LLPS
Minimal peptides challenge the traditional view of biomolecular condensates as homogeneous liquid droplets. Instead, their phase-separated assemblies reveal surprising levels of structural complexity, ranging from multiphase organization to solvent–driven transitions and diverse dynamic states.14,44 These properties are not incidental but encode fundamental rules for how short peptide sequences interact with each other and with their environment. We argue that uncovering these rules is central to transforming LLPS of peptides from a descriptive phenomenon into a rationally engineerable platform.3.1 Internal architecture and multiphase organization
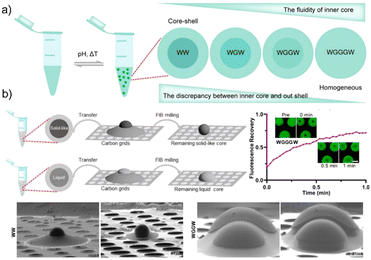
The microenvironment of peptide condensates formed through LLPS has often been overlooked, with droplets typically assumed to be homogeneous in organization. However, recent studies have revealed that condensates can display internal heterogeneity and evolving structural features, motivating closer examination of their microstructural dynamics. For example, Zhou and colleagues45 reported that a library of programmable tetrapeptides can form core–shell condensates even though they are single-component systems (Fig. 3). In their work, they systematically varied sticker density by inserting glycine spacers into di-tryptophan motifs to tune the balance between π–π stacking, hydrophobic contacts, and peptide–water interactions. As shown in Fig. 3a, increasing the number of glycine spacers (e.g., WW → WGGGW) progressively reduces aromatic interaction strength and enhances hydration, providing a design rationale for modulating condensate architecture. Cryo-focused ion beam scanning electron microscopy (cryo-FIB–SEM, Fig. 3b) images revealed that the WW peptide forms droplets with a dense, solid-like tryptophan-rich core enveloped by a more fluid shell, whereas glycine-containing analogues yield fully homogeneous droplets. This structural interpretation is supported by fluorescence-lifetime imaging (FLIM), FRAP fusion dynamics, and Raman/FTIR measurements, which collectively show that weakening peptide–peptide interactions and strengthening peptide–water hydrogen bonding drives the transition from core–shell organization to uniform liquid condensates. These findings highlight how small sequence modifications in minimal peptides can generate diverse internal architectures through controlled modulation of interaction strengths and hydration. Besides, Netzer et al. demonstrated that systematic tuning of aromatic residue type and arrangement could regulate the internal organization of peptide condensates, enabling transitions from homogeneous droplets to layered structures.46 Their work revealed that cation–π interactions act synergistically with hydrophobic forces to drive spatial compartmentalization. Recently, Ye et al.47 showed that sufficient micropolarity contrast between layers favors the emergence of multilayered architecture, with the shell being more polar than the core, and that inversion of micropolarity corresponds to restructuring of layers.These findings indicated that, beyond overall sticker density or spacing, variations in local polarity and interaction strength within the condensate can bias droplets toward internally layered rather than homogeneous organization. In peptide-based systems, even modest gradients in hydrophobicity or polarity may be sufficient to promote such internal segregation. This implies that multiphase organization is not necessarily rare, but can emerge when sequences exceed certain thresholds of heterogeneity. Designing peptides with controlled polarity gradients or differential interaction motifs may therefore provide a route to encode spatially distinct microenvironments within condensates, enabling compartmentalization of functions such as catalysis versus storage.
3.2 Hydration and desolvation processes
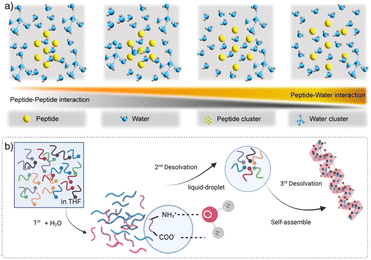
Hydration plays a central role in phase separation, as the balance between peptide–water affinity and peptide–peptide interactions determines whether systems undergo condensation or remain dispersed.48–50 Wang et al. demonstrated that changes in the water structure accompany condensate formation, and that progressive dewetting of hydration shells is closely associated with liquid to solid transitions.45 In this view, strong peptide–peptide networks coupled with weakened hydration promote the formation of solid-like cores, whereas sustained hydration supports more homogeneous, liquid-like droplets (Fig. 4a).More specifically, Yuan et al. proposed a multistep desolvation mechanism in peptide assemblies through LLPS.51 According to their model, condensation proceeds via three stages (Fig. 4b): (1) partial desolvation and solvent exchange, (2) selective desolvation of hydrophobic moieties triggering phase separation into peptide-rich droplets, and (3) further desolvation inside droplets that leads to nucleation and internal ordering. They demonstrated this with model peptides (e.g. TPPGFF in mixed solvents), tracking structural intermediates via cryo-TEM, mass spectrometry, NMR, and MD simulation. This model connects early hydration-governed organization to later droplet-to-fiber or gel transitions, highlighting hydration–desolvation balance as a key axis for tuning condensate properties through solvent engineering.
Solvent is often treated as a passive background in design strategies, but in peptide LLPS it can act as an active co-determinant of behavior. Because minimal peptide sequences are highly sensitive to solvent effects, tuning parameters such as ionic strength, osmolytes, cosolvents, or hydration dynamics can markedly alter phase behavior. In practice, modulating desolvation kinetics (e.g., by adjusting solvent exchange rates or introducing weak cosolutes) offers a route to control droplet stability, morphology, and maturation pathways that is orthogonal to sequence-based design.52,53 In designing peptide-based condensates, stability of the liquid droplet phase requires a balance: interactions must be strong enough to drive LLPS but not so strong as to promote irreversible aggregation or precipitation. This balance can be tuned by modulating sticker density and charge, introducing hydrophilic or steric-protecting chemical modifications (e.g., PEGylation), or using reversible side–chain chemistries to prevent kinetic trapping in solid states. Under appropriate solvent or ionic conditions, these strategies help maintain dynamic, reversible, and fluid condensates.
3.3 Dynamic behavior and mechanistic insights into peptide condensates
The material properties of condensates, from liquids to gels to solids, are central to functional performance.54 In peptide systems, fluorescence recovery after photobleaching (FRAP) commonly reveals slower recovery and reduced mobility in droplets enriched in aromatic or hydrophobic residues, whereas more polar or weakly interacting sequences tend to display faster recovery and more liquid-like dynamics.55 Molecular dynamics (MD) simulations complement these observations by providing atomistic or coarse-grained insight into binding energies, hydration effects, and internal mobility.56 MD studies have shown that stronger peptide–peptide interactions slow diffusion, whereas stronger peptide–water interactions promote mobility, trends that are recapitulated across minimal peptide and coacervate models. However, simulations face practical limitations: atomistic MD is constrained in timescales and system size, limiting access to long-term maturation or multiphase organization, while coarse-grained models extend the spatiotemporal range at the cost of chemical detail and sequence specificity.57,58 Additional challenges include parameterization and benchmarking, as inaccurate force fields can lead to overstabilized interactions or misrepresented solvent dynamics.In functional design, achieving phase separation is only a first step; tuning fluidity is often more critical for task-specific applications. Simulations are valuable for hypothesis generation and for mapping sequence–mobility relationships, but they are most effective when calibrated against experimental measurements such as FRAP, NMR, or rheology. In peptide condensates through LLPS, even small sequence modifications (e.g., replacing an aromatic residue with a less interactive one) can substantially alter mobility, underscoring the value of integrating simulations and experiments. To illustrate this synergy, Fig. 5 provides a schematic overview of how MD analyses and experimental data are combined to assess LLPS propensity and guide sequence design.
4 Stimuli-responsive properties of peptide condensates
Peptide condensates offer a unique opportunity to encode responsiveness into minimal molecular systems. In biology, membraneless organelles assemble, dissolve, or reorganize in response to diverse physicochemical cues associated with stress, development, or metabolism.59,60 Mimicking such dynamic regulation is a defining advantage of peptide LLPS, enabling switch-like control over assembly, internal organization, and function.29,61 Here we highlight how pH, temperature, redox state, enzymatic activity, and metabolites have been used to modulate peptide LLPS, and what design principles emerge from these studies.4.1 pH and temperature
pH and temperature are among the most common and precisely tunable stimuli for modulating peptide LLPS. They regulate phase separation by altering the ionization state, hydrophobicity, and solvent interactions of peptide chains. Changes in pH can directly influence electrostatic interactions and the protonation states of amino acid side chains. For example, in early work by the Ali group, they demonstrated that LLPS of histidine-rich squid beak peptides (HBP-1 and its fragment GY-23) occurred in a narrow pH range of 6.0–7.5 at concentrations above 20–30 µM. High-resolution NMR revealed that as pH gradually increased from 3.3 to 7.0, histidine residues became deprotonated, enabling the formation of transient hydrogen bonds with the phenolic hydroxyl groups of tyrosine, which stabilized the coacervate phase. At pH > 11, deprotonation of tyrosine hydroxyl groups disrupts this hydrogen-bond network, leading to gradual condensate disassembly.62Temperature provides another axis of control. Systems that are homogeneous at elevated temperature and phase-separate upon cooling display upper critical solution temperature (UCST) behavior, whereas systems that are miscible at lower temperature but separate into coexisting phases upon heating exhibit lower critical solution temperature (LCST) behavior (Fig. 6a). Depending on sequence and solvent conditions, peptide-based systems may also show closed-loop or dual UCST/LCST phase diagrams, reflecting the balance between enthalpic interactions and entropy-driven dehydration at different temperatures. Tanaka et al. investigated branched elastin-like peptide (ELP) systems based on an oligo (Glu) backbone with pendant FPGVG pentapeptides.63 Upon heating, the solution became turbid at around 60 °C, corresponding to dehydration and coacervation of the peptides, while further heating to 90 °C restored transparency, indicating rehydration and redissolution. The reversible temperature cycling between 20 °C, 60 °C, and 90 °C was confirmed by turbidity measurements and photographic observation. They found that increasing branching density and concentration reduced the LCST and, at low concentration (0.5 mM), induced an unusual dual LCST/UCST-like transition. These observations provide design strategies for tuning operating windows and for constructing temperature-responsive condensates that can be triggered on demand.
Collectively, these studies show that minimal peptides allow direct control over responsiveness through ionization, hydration, and thermodynamics, offering a route to engineer condensates tailored to physiological environments (e.g., lysosomal pH and fever-range temperature.
4.2 Redox responsiveness
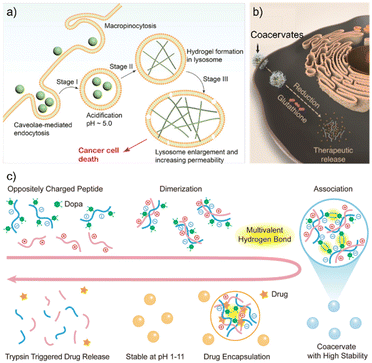
Redox chemistry can alter the covalent connectivity and give rise to reversible structural transitions in condensates. Zhou et al. demonstrated this using the tetrapeptide WCRY, where oxidation induced disulfide crosslinking and transformed homogeneous droplets into core–shell architectures (Fig. 6b).39 Reduction cleaved crosslinks and restored the original morphology, concurrently redistributing encapsulated cargo.Building on the idea of structural switching, Sun et al. introduced an additional level of control by coupling redox sensitivity with pH responsiveness.64 In their disulfide-conjugated HBpep system, the peptides remained as monomers below pH 6.0 but underwent LLPS at neutral pH (∼6.5–7.5) to form positively charged droplets approximately 1 µm in diameter (ζ ≈ +15 mV) (Fig. 6c). In the intracellular reducing environment (1 mM GSH), the droplets disassembled rapidly, releasing up to ∼90% of encapsulated EGFP within 24 h, whereas no release occurred in the absence of GSH. This “two-stage trigger” design substantially improved delivery specificity and efficiency.
Extending beyond disulfide-based designs, Netzer et al. revealed that aromatic residue composition can influence not only the balance between electrostatic and hydrophobic forces but also the coupling of LLPS stability to electron transfer processes in oxidative environments.46 This finding suggests that redox regulation in peptide condensates can be achieved through both covalent bond modulation and subtler adjustments to side–chain chemistry, offering a broader design space for tunable responsiveness.
Redox responsiveness shows how a single chemical motif (cysteine) can encode reversible phase transitions, yet focusing only on disulfides narrows the design space. Expanding to alternative redox-active residues, such as methionine oxidation or catechol-based chemistry, could diversify peptide-based redox control and broaden biomedical utility.
4.3 Enzymatic responsiveness
Compared with physicochemical stimuli, enzyme-triggered LLPS offers higher biological specificity and spatial precision. This responsiveness takes advantage of the unique expression profiles and catalytic activities of enzymes in different cellular contexts, enabling condensates to form only at designated locations.A recent example comes from Li et al., who designed the chimeric peptide DMN-SIPL that remained dispersed in solution. Upon cleavage by hepsin (a membrane-anchored protease overexpressed in prostate cancer cells), the original peptide released a more hydrophobic fragment undergoing LLPS directly on the cell membrane.65 The resulting condensates anchored stably at the reaction site, continuously sequestering and inactivating hepsin. Leveraging LLPS in this way provides unique advantages over conventional assemblies: condensates form in situ to avoid dilution and mislocalization, maintain dynamic molecular exchange for sustained regulation, and achieve high spatial precision through enzyme-guided activation (Fig. 6d). Building on this concept, integrating enzyme responsiveness with redox or pH sensitivity could enable multi-layered logic gating, ensuring that condensates form only under specific combinations of biochemical cues, thereby targeting cell types or disease states with exceptional selectivity.
Enzyme-triggered LLPS highlights how peptides can act as “biochemical logic gates,” assembling condensates only under specific intracellular conditions. The future challenge lies in multiplexing: designing peptides responsive to more than one enzymatic input, or coupling enzymatic cues with pH/redox triggers, to achieve programmable multi-layered logic.
4.4 Metabolite and small-molecule modulation of condensate microenvironments
In addition to external factors such as pH, temperature, redox signals, and enzymes that regulate the phase behavior and material properties of the condensate, it is equally important that the internal microenvironment of the aggregates (including polarity and microviscosity) can also be deliberately designed. By modulating how small molecules/metabolites interact with water and peptide components, one can re-organize hydration layers and molecular mobility without changing sequences, thereby influencing multilayer miscibility, molecular partitioning, and reaction processes as an orthogonal control axis to classical stimuli.Zhang and co-workers quantitatively mapped how various organic solutes reshape condensate microenvironments using fluorescence lifetime imaging microscopy (FLIM), FRAP, and passive microrheology. They found that microenvironmental changes do not correlate with bulk solvent polarity, instead, they track with the relative interaction strengths of solute–water versus solute–protein. Solutes with a single H-bond acceptor tend to decrease micropolarity and increase microviscosity, whereas solutes bearing multiple acceptors or forming multi-water complexes generally increase micropolarity and decrease microviscosity, enhancing molecular mobility. Notably, ATP at physiological concentrations similarly modulates condensate polarity. These microenvironmental shifts alter the miscibility of multilayer condensates and reprogram reaction rates and equilibria via changed product partitioning. Together, these data establish a measurable link from metabolites/cosolutes to hydration organization and functional outputs, offering a practical blueprint for microenvironment engineering in peptide LLPS systems.
Metabolites should be viewed not as perturbations but as designable regulators. By embedding metabolite-binding motifs into peptides, one could create condensates that sense cellular metabolic states or act as synthetic biosensors. This aligns peptide LLPS with systems-level control in synthetic biology.
4.5 Integrative outlook on stimuli-responsive design
The examples above collectively show that peptide condensates are not passive assemblies, but programmable dynamic materials. Each type of stimulus taps into a distinct molecular principle: ionization equilibria, covalent crosslinking, proteolytic processing, or noncovalent competition. Importantly, these mechanisms are not mutually exclusive. In living systems, condensates rarely respond to a single cue, instead, they integrate multiple inputs to produce emergent behaviors such as maturation, dissolution, or functional reorganization. For peptide-based LLPS, this suggests a design map with two axes: (I) triggering mechanism (electrostatic, hydrophobic, covalent, enzymatic, and metabolic). (II) Functional output (assembly/disassembly, multiphase restructuring, fluid-to-solid transition, and selective release). Positioning a peptide system within this map highlights both its immediate utility and potential for modular combination. For instance, a histidine-rich peptide may be extended with cysteine residues to yield dual pH-redox responsiveness, or with protease-cleavable motifs to introduce enzymatic specificity. Similarly, metabolite-binding domains could be appended to confer context-dependent regulation.The next frontier is logic-gated condensates: droplets that form, dissolve, or reprogram only when multiple environmental conditions are satisfied. Achieving this requires layering stimuli in a rational way: for example, engineering peptides that undergo LLPS only under acidic pH and reductive conditions, or droplets that release cargo only when metabolite concentration crosses a threshold. Such “AND/OR” logic recapitulates cellular decision-making and would expand peptide LLPS beyond passive carriers into active, adaptive materials. Ultimately, stimuli-responsive peptide condensates embody the convergence of molecular design and systems-level control. By combining simple sequence motifs with programmable responsiveness, the field moves closer to building minimal synthetic organelles that not only mimic biological dynamics but also achieve functionalities not found in nature.
5 Functionalization strategies and emerging applications of peptide condensates
As peptide-driven LLPS advances from a fundamental concept to a design platform, attention has shifted toward its functional capabilities. Peptide condensates have been engineered for biomolecule delivery, catalysis, protein stabilization, and modulation of cellular processes. Here we highlight representative examples, focusing on the design strategies that enable functionality and point toward future applications.5.1 Intelligent delivery of biomacromolecules
The environmental sensitivity of peptide LLPS allows delivery systems to release cargo only under pre-defined conditions, replacing “blind transport” with precision targeting.66 For example, Wang et al. designed a hexapeptide (LTP) that undergoes a solution-to-condensate transition in the acidic lysosomes of drug-resistant cancer cells. This phase transformation not only disrupted lysosomal homeostasis but also liberated chemotherapeutic agents trapped inside, thereby restoring drug sensitivity.67 In this design, the phase transition functioned both as a delivery “switch” and as an active component of the therapeutic effect, illustrating how condensates can integrate material transformation with biological intervention (Fig. 7a).Another strategy focuses on overcoming biological barriers to achieve direct cytosolic delivery of macromolecules. Sun et al. developed LLPS-forming peptide droplets that can penetrate cell membranes without relying on endocytosis, thereby bypassing degradative trafficking pathways.64 Once inside, these droplets disassembled in response to the reductive intracellular environment, releasing proteins and mRNA while preserving their structural integrity and bioactivity. This work demonstrates how condensates can serve as responsive vehicles for fragile biomacromolecules that are otherwise difficult to deliver effectively (Fig. 7b).
Chen et al. further broadened the adaptability of peptide condensates by addressing oral delivery, a long-standing challenge due to gastric acidity and high-salt conditions.68 They designed Dopa-modified peptide coacervates stabilized by multivalent hydrogen bonding, which maintained integrity in harsh gastric environments but disassembled upon recognition by intestinal enzymes. This enzyme-triggered release strategy enabled precise delivery of therapeutic proteins in the gut, highlighting how rationally engineered condensates can extend LLPS-based delivery systems to routes previously inaccessible to peptide carriers (Fig. 7c).
Collectively, these studies underscore that peptide condensates are not passive “cargo containers” but programmable delivery modules. Their responsiveness is deeply integrated into the release process, actively shaping when, where, and how therapeutic payloads are released. Such integration elevates condensates from carriers to dynamic participants in treatment, positioning peptide LLPS as a versatile platform for next-generation drug delivery. Compared with conventional polymeric nanoparticles and lipid nanoparticles (LNPs), peptide condensates operate through a distinct physical mechanism. Polymeric and lipid carriers are typically stabilized by covalent backbones or lipid bilayers, and cargo release is governed by diffusion through, or disruption of, these relatively fixed nanostructures (for example by polymer degradation, membrane fusion, or endosomal destabilization). In contrast, LLPS-derived peptide droplets are membraneless condensates formed by reversible, multivalent noncovalent interactions. Their assembly, internal viscosity, and cargo partitioning can all be tuned by environmental cues such as pH, redox state, or enzymatic activity, leading to release that is tightly coupled to condensate dissolution or reorganization. This membraneless, dynamically exchangeable nature distinguishes peptide condensates from classical nanoparticles and underpins their potential as responsive carriers for macromolecular therapeutics.
5.2 Peptide condensates as microreactors for bond-forming reactions
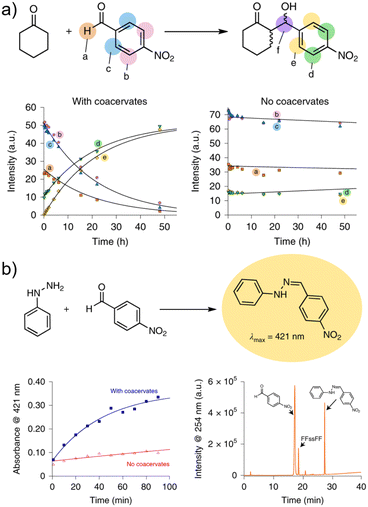
Beyond delivery, peptide condensates provide confined and tuneable microenvironments that mimic cellular crowding and polarity, thereby conferring catalytic advantages. Abbas et al. engineered a disulfide-bridged dipeptide, FFssFF, which undergoes LLPS to form stable condensates in aqueous solution.32 Within these droplets, both aldol (Fig. 8a) and hydrazone (Fig. 8b) condensation reactions were markedly accelerated compared with homogeneous solution.Specifically, aldol formation proceeded with significantly enhanced conversion, while hydrazone synthesis exhibited a ∼13-fold rate increase. This catalytic effect originates from selective substrate partitioning into the condensates and from the polarity-adjusted interior, which stabilizes reactive intermediates and promotes bond formation. Such redox-responsive peptide condensates not only serve as minimal models of primitive catalytic compartments but also represent a versatile strategy to design dynamic and recyclable microreactors for chemical and biochemical transformations.
Peptide condensates represent a step toward programmable catalytic organelles. Unlike conventional nanoreactors, they offer reversibility, adaptability, and responsiveness.69 Future directions include designing condensates that not only enhance specific reactions but also reconfigure dynamically in response to metabolic cues, effectively creating synthetic pathways that behave like adaptive metabolic nodes.
5.3 Preservation and controlled release of biomolecules
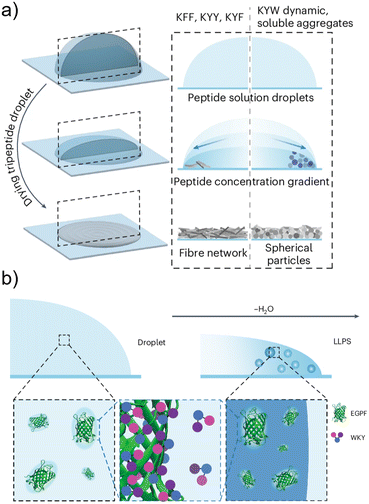
The compartmentalization offered by LLPS can also extend biomolecule lifespan. Recent studies revealed that phase separation can be triggered by localized evaporation, as exemplified by Lys-Tyr-Trp (KYW) tripeptide assemblies in sessile droplets. This evaporation-driven assembly induces condensates that solidify into porous peptide microparticles, providing a robust platform for encapsulation (Fig. 9a). Dave et al. further demonstrated that such condensates can protect enzymes during drying.52 Upon rehydration, the particles rapidly disassemble, releasing fully active biomolecules, with nearly 100% enzymatic activity retained after 15 days of storage. This mechanism echoes natural strategies where organisms employ phase separation to preserve vital components under drought stress and suggests that synthetic peptide condensates could serve as substitutes for cold-chain storage of vaccines or enzyme therapeutics. In addition, evaporation-driven peptide condensates exhibit the capacity to recruit payload molecules through dynamic side–chain interactions, enabling versatile encapsulation and controlled release (Fig. 9b). Such properties highlight their potential as practical platforms for biomolecule preservation and stimulus-responsive delivery in biomedical applications.Peptide LLPS offers a potential alternative to cold-chain storage, with direct implications for vaccines, enzymes, and RNA-based therapeutics.70 The convergence of encapsulation (for preservation) and stimuli-responsive release (for delivery) suggests a continuum where condensates function as both storage reservoirs and delivery vehicles, a duality rarely seen in other material systems.
5.4 Regulation of cell fate and disease intervention
At a higher biological level, the significance of LLPS extends beyond material encapsulation to include information regulation. Kodali et al. comprehensively reviewed how condensates orchestrate transcriptional programs and cell fate in immune systems. In these contexts, condensates integrate environmental and signalling cues to modulate differentiation, activation, and effector functions.71 Although this review focused on protein-driven condensates, the conceptual framework strongly suggests that peptide condensates could be engineered to achieve similar regulatory control. As we mentioned above, Li et al. developed protease-cleavable peptides that undergo LLPS selectively in prostate cancer cells. Upon cleavage, hydrophobic fragments nucleated condensates at the plasma membrane, disrupting signaling and suppressing proliferation. This study demonstrated that peptides can be programmed as context-sensitive therapeutic effectors, with LLPS triggered only in diseased environments,72 which could also be used in the immune system. Pathological proteins such as TDP-43 and FUS undergo LLPS-driven aggregation in neurodegeneration.53,73 Synthetic peptides could intervene by co-condensing with or disrupting these assemblies, offering a new therapeutic strategy for proteinopathies. As application scenarios expand, the research focus is shifting from “whether condensates can form” to “how to ensure they function precisely at the right time and place, and under the right conditions.”We see these strands converging toward a new paradigm of synthetic regulatory biology with peptide LLPS. Peptide condensates could one day act as programmable intracellular logic circuits, forming, dissolving, or remodelling only in response to disease-specific cues. Unlike large proteins, peptides offer tunability, responsiveness, and reduced immunogenicity, making them uniquely suited for therapeutic engineering. The immediate challenge is to bridge the minimalism of peptide motifs with the complexity of cellular networks, while success here could transform peptide LLPS into a modality for immunotherapy, cancer therapy, neurodegeneration, and regenerative medicine.
6 Future perspectives
Peptide condensates through LLPS represent a versatile and programmable class of biomolecular assemblies. They have already shown potential in targeted delivery, catalysis, preservation, and cellular regulation. To transition from proof-of-concept systems to robust technologies, however, several obstacles remain. Below, we outline the major challenges and suggest possible strategies to address them, followed by a vision for the field's broader promise.6.1 Key challenges and possible solutions
Despite these advances, the path from laboratory prototypes to broad deployable dynamic modules still presents significant challenges. Key priorities include establishing predictive sequence–function relationships, developing scalable and cost-effective synthesis methods, achieving robust and reversible control over condensate formation in complex biological environments, and integrating multifunctional modules without compromising stability. Addressing these issues will not only deepen our understanding of liquid–liquid phase separation in biological systems but also unlock the full potential of peptide condensates as programmable tools for biomedicine, synthetic biology, and molecular engineering.
6.2 Outlook
Looking forward, peptide LLPS condensates hold the potential to evolve into a true platform technology that unites chemistry, biology, and materials science. Their minimalism makes them uniquely suited to act as programmable “synthetic organelles” in synthetic biology, where spatial control over enzymatic pathways and signalling can rewire cellular behaviors. At the same time, realizing peptide-based synthetic organelles will require overcoming conceptual challenges such as achieving multi-input control, ensuring orthogonality to native condensates, and establishing reliable links between condensate dynamics and functional outputs.In medicine, they may become highly specific therapeutic effectors, assembling only in diseased environments to regulate signaling or buffer pathological aggregation. In materials science, their tunable responsiveness offers routes to adaptive catalysts, sensors, and preservation systems that outperform conventional polymers. At the same time, as minimal models, peptide condensates will continue to provide fundamental insights into the molecular grammar of phase separation, deepening our understanding of protein condensates and soft matter physics.
Our perspective is that the next decade will be defined by efforts to reconcile the simplicity of peptides with the complexity of cellular systems. By integrating rational design, high-throughput experimentation, and machine learning, peptide LLPS can move from descriptive observation to predictive engineering. Ultimately, this transformation will enable peptide condensates to evolve from biomimetic curiosities into practical and transformative tools for synthetic biology, biomedicine, and materials science.
Author contributions
L. L.: visualization, investigation, writing – original draft, review & editing. H. W.: supervision, resources, funding acquisition, writing – review & editing, project administration.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This project was supported by the National Natural Science Foundation of China (U24A2076).References
- S. F. Banani, H. O. Lee, A. A. Hyman and M. K. Rosen, Biomolecular condensates: organizers of cellular biochemistry, Nat. Rev. Mol. Cell Biol., 2017, 18, 285–298 CrossRef CAS PubMed.
- A. A. Hyman, C. A. Weber and F. Jüelicher, Liquid-Liquid Phase Separation in Biology, Annu. Rev. Cell Dev. Biol., 2014, 30, 39–58 CrossRef CAS.
- Y. Shin and C. P. Brangwynne, Liquid phase condensation in cell physiology and disease, Science, 2017, 357, eaaf4382 CrossRef PubMed.
- B. S. Visser, W. P. Lipinski and E. Spruijt, The role of biomolecular condensates in protein aggregation, Nat. Rev. Chem., 2024, 8, 686–700 CrossRef CAS.
- C. P. Brangwynne, C. R. Eckmann, D. S. Courson, A. Rybarska, C. Hoege, J. Gharakhani, F. Jülicher and A. A. Hyman, Germline P Granules Are Liquid Droplets That Localize by Controlled Dissolution/Condensation, Science, 2009, 324, 1729–1732 CrossRef CAS.
- S. Jain, J. R. Wheeler, R. W. Walters, A. Agrawal, A. Barsic and R. Parker, ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure, Cell, 2016, 164, 487–498 Search PubMed.
- H. X. Zhou, D. Kota, S. B. Qin and R. Prasad, Fundamental Aspects of Phase-Separated Biomolecular Condensates, Chem. Rev., 2024, 124, 8550–8595 Search PubMed.
- A. Abyzov, M. Blackledge and M. Zweckstetter, Conformational Dynamics of Intrinsically Disordered Proteins Regulate Biomolecular Condensate Chemistry, Chem. Rev., 2022, 122, 6719–6748 CrossRef CAS.
- M. L. Huggins, Thermodynamic Properties of Solutions of Long-Chain Compounds, Ann. N. Y. Acad. Sci., 1942, 43, 1–32 Search PubMed.
- Y. Ozawa, H. Anbo, M. Ota and S. Fukuchi, Classification of proteins inducing liquid-liquid phase separation: sequential, structural and functional characterization, J. Biochem., 2022, 173, 255–264 CrossRef.
- S. V. Razin and A. A. Gavrilov, The Role of Liquid-Liquid Phase Separation in the Compartmentalization of Cell Nucleus and Spatial Genome Organization, Biochemistry, 2020, 85, 643–650 CAS.
- P. J. Flory, Thermodynamics of high polymer solutions, J. Chem. Phys., 1942, 10, 51–61 CrossRef CAS.
- M. Kato, T. N. W. Han, S. H. Xie, K. Shi, X. L. Du, L. C. Wu, H. Mirzaei, E. J. Goldsmith, J. Longgood, J. M. Pei, N. V. Grishin, D. E. Frantz, J. W. Schneider, S. Chen, L. Li, M. R. Sawaya, D. Eisenberg, R. Tycko and S. L. McKnight, Cell-free Formation of RNA Granules: Low Complexity Sequence Domains Form Dynamic Fibers within Hydrogels, Cell, 2012, 149, 753–767 Search PubMed.
- P. L. Li, S. Banjade, H. C. Cheng, S. Kim, B. Chen, L. Guo, M. Llaguno, J. V. Hollingsworth, D. S. King, S. F. Banani, P. S. Russo, Q. X. Jiang, B. T. Nixon and M. K. Rosen, Phase transitions in the assembly of multivalent signalling proteins, Nature, 2012, 483, 336–340 CrossRef CAS PubMed.
- W. K. Cho, J. H. Spille, M. Hecht, C. Lee, C. Li, V. Grube and I. I. Cisse, Mediator and RNA polymerase II clusters associate in transcription-dependent condensates, Science, 2018, 361, 412–415 CrossRef CAS PubMed.
- M. J. Du and Z. J. J. Chen, DNA-induced liquid phase condensation of cGAS activates innate immune signaling, Science, 2018, 361, 704–709 CrossRef CAS.
- A. R. Strom, A. V. Emelyanov, M. Mir, D. V. Fyodorov, X. Darzacq and G. H. Karpen, Phase separation drives heterochromatin domain formation, Nature, 2017, 547, 241–245 CrossRef CAS.
- X. L. Su, J. A. Ditlev, E. F. Hui, W. M. Xing, S. Banjade, J. Okrut, D. S. King, J. Taunton, M. K. Rosen and R. D. Vale, Phase separation of signaling molecules promotes T cell receptor signal transduction, Science, 2016, 352, 595–599 CrossRef CAS.
- M. L. Zeng, Y. Shang, Y. Araki, T. F. Guo, R. L. Huganir and M. J. Zhang, Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity, Cell, 2016, 166, 1163–1175 CrossRef CAS.
- G. M. Zhang, Z. Wang, Z. Du and H. Zhang, mTOR Regulates Phase Separation of PGL Granules to Modulate Their Autophagic Degradation, Cell, 2018, 174, 1492–1506 CrossRef CAS PubMed.
- A. Molliex, J. Temirov, J. Lee, M. Coughlin, A. P. Kanagaraj, H. J. Kim, T. Mittag and J. P. Taylor, Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization, Cell, 2015, 163, 123–133 CrossRef CAS.
- B. Wang, L. Zhang, T. Dai, Z. R. Qin, H. S. Lu, L. Zhang and F. F. Zhou, Liquid-liquid phase separation in human health and diseases, Signal Transduction Targeted Ther., 2021, 6, 290 Search PubMed.
- X. Yan, D. Kuster, P. Mohanty, J. Nijssen, K. Pombo-García, J. G. Morato, A. Rizuan, T. M. Franzmann, A. Sergeeva, A. M. Ly, F. L. Liu, P. M. Passos, L. George, S. H. Wang, J. Shenoy, H. L. Danielson, B. Ozguney, A. Honigmann, Y. M. Ayala, N. L. Fawzi, D. W. Dickson, W. Rossoll, J. Mittal, S. Alberti and A. A. Hyman, Intra-condensate demixing of TDP-43 inside stress granules generates pathological aggregates, Cell, 2025, 188, 4123–4140 CrossRef CAS PubMed.
- E. W. Martin, A. S. Holehouse, I. Peran, M. Farag, J. J. Incicco, A. Bremer, C. R. Grace, A. Soranno, R. V. Pappu and T. Mittag, Valence and patterning of aromatic residues determine the phase behavior of prion-like domains, Science, 2020, 367, 694–699 CrossRef CAS.
- S. Rekhi, C. G. Garcia, M. Barai, A. Rizuan, B. S. Schuster, K. L. Kiick and J. Mittal, Expanding the molecular language of protein liquid-liquid phase separation, Nat. Chem., 2024, 16, 1113–1124 CrossRef CAS.
- R. van der Lee, M. Buljan, B. Lang, R. J. Weatheritt, G. W. Daughdrill, A. K. Dunker, M. Fuxreiter, J. Gough, J. Gsponer, D. T. Jones, P. M. Kim, R. W. Kriwacki, C. J. Oldfield, R. V. Pappu, P. Tompa, V. N. Uversky, P. E. Wright and M. M. Babu, Classification of Intrinsically Disordered Regions and Proteins, Chem. Rev., 2014, 114, 6589–6631 CrossRef CAS.
- M. Abbas, W. P. Lipin, J. Wang and E. Spruijt, Peptide-based coacervates as biomimetic protocells, Chem. Soc. Rev., 2021, 50, 3690–3705 RSC.
- R. Kubota, S. Torigoe and I. Hamachi, Temporal Stimulus Patterns Drive Differentiation of a Synthetic Dipeptide-Based Coacervate, J. Am. Chem. Soc., 2022, 144, 15155–15164 CrossRef CAS.
- G. L. Li, C. Q. Yuan and X. H. Yan, Peptide-mediated liquid-liquid phase separation and biomolecular condensates, Soft Matter, 2025, 21, 1781–1812 RSC.
- J. M. Choi and R. V. Pappu, The Stickers and Spacers Framework for Describing Phase Behavior of Multivalent Intrinsically Disordered Proteins, Biophys. J., 2020, 118, 492a CrossRef.
- S. Ranganathan and E. I. Shakhnovich, Dynamic metastable long-living droplets formed by sticker-spacer proteins, Elife, 2020, 9, e56159 Search PubMed.
- M. Abbas, W. P. Lipiński, K. K. Nakashima, W. T. S. Huck and E. Spruijt, A short peptide synthon for liquid–liquid phase separation, Nat. Chem., 2021, 13, 1046–1054 CrossRef CAS.
- V. Castelletto, J. Seitsonen, A. Pollitt and I. W. Hamley, Minimal Peptide Sequences That Undergo Liquid–Liquid Phase Separation via Self-Coacervation or Complex Coacervation with ATP, Biomacromolecules, 2024, 25, 5321–5331 CrossRef CAS PubMed.
- Y. Dai, L. You and A. Chilkoti, Engineering synthetic biomolecular condensates, Nat. Rev. Bioeng., 2023, 1, 466–480 CrossRef CAS.
- J. M. Choi, A. S. Holehouse and R. V. Pappu, Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions, Annu. Rev. Biophys., 2020, 49, 107–133 CrossRef CAS.
- S. L. Perry, L. Leon, K. Q. Hoffmann, M. J. Kade, D. Priftis, K. A. Black, D. Wong, R. A. Klein, C. F. Pierce, K. O. Margossian, J. K. Whitmer, J. Qin, J. J. de Pablo and M. Tirrell, Chirality-selected phase behaviour in ionic polypeptide complexes, Nat. Commun., 2015, 6, 6052 CrossRef CAS.
- F. P. Cakmak, S. Choi, M. O. Meyer, P. C. Bevilacqua and C. D. Keating, Prebiotically-relevant low polyion multivalency can improve functionality of membraneless compartments, Nat. Commun., 2020, 11, 5949 CrossRef CAS.
- B. Gabryelczyk, H. Cai, X. Y. Shi, Y. Sun, P. J. M. Swinkels, S. Salentinig, K. Pervushin and A. Miserez, Hydrogen bond guidance and aromatic stacking drive liquid-liquid phase separation of intrinsically disordered histidine-rich peptides, Nat. Commun., 2019, 10, 5465 Search PubMed.
- L. Zhou, L. Zhu, C. Wang, T. Xu, J. Wang, B. Zhang, X. Zhang and H. Wang, Multiphasic condensates formed with mono-component of tetrapeptides via phase separation, Nat. Commun., 2025, 16, 2706 Search PubMed.
- K. Kaygisiz, D. Sementa, V. Athiyarath, X. Chen and R. V. Ulijn, Context dependence in assembly code for supramolecular peptide materials and systems, Nat. Rev. Mater., 2025, 10, 449–472 CrossRef.
- H. R. Kilgore and R. A. Young, Learning the chemical grammar of biomolecular condensates, Nat. Chem. Biol., 2022, 18, 1298–1306 CrossRef CAS PubMed.
- W. M. Aumiller and C. D. Keating, Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles, Nat. Chem., 2016, 8, 129–137 CrossRef CAS.
- B. Saha, A. Chatterjee, A. Reja and D. Das, Condensates of short peptides and ATP for the temporal regulation of cytochrome activity, Chem. Commun., 2019, 55, 14194–14197 Search PubMed.
- D. L. J. Lafontaine, J. A. Riback, R. Bascetin and C. P. Brangwynne, The nucleolus as a multiphase liquid condensate, Nat. Rev. Mol. Cell Biol., 2021, 22, 165–182 CrossRef CAS.
- L. C. Zhou, L. H. Lu, Y. Zhang, J. Wang, J. J. Cheng and H. M. Wang, Water Dynamics in the Formation of Single-Component-Based Multiphasic Droplets, J. Am. Chem. Soc., 2025, 147, 31290–31299 CrossRef CAS.
- A. Netzer, A. Baruch Leshem, S. Veretnik, I. Edelstein and A. Lampel, Regulation of Peptide Liquid–Liquid Phase Separation by Aromatic Amino Acid Composition, Small, 2024, 20, 2401665 Search PubMed.
- S. Ye, A. P. Latham, Y. Tang, C.-H. Hsiung, J. Chen, F. Luo, Y. Liu, B. Zhang and X. Zhang, Micropolarity governs the structural organization of biomolecular condensates, Nat. Chem. Biol., 2024, 20, 443–451 CrossRef CAS.
- S. S. Ribeiro, N. Samanta, S. Ebbinghaus and J. C. Marco, The synergic effect of water and biomolecules in intracellular phase separation, Nat. Rev. Chem., 2019, 3, 552–561 CrossRef CAS.
- S. Arya, A. K. Sinh, T. Khan, M. Bhattacharya, A. Datta and S. Mukhopadhyay, Water Rearrangements upon Disorder-to-Order Amyloid Transition, J. Phys. Chem. Lett., 2016, 7, 4105–4110 CrossRef CAS.
- D. Thirumalai, G. Reddy and J. E. Straub, Role of Water in Protein Aggregation and Amyloid Polymorphism, Acc. Chem. Res., 2012, 45, 83–92 CrossRef CAS.
- C. Yuan, R. Xing, J. Cui, W. Fan, J. Li and X. Yan, Multistep Desolvation as a Fundamental Principle Governing Peptide Self-Assembly Through Liquid–Liquid Phase Separation, CCS Chem., 2023, 6, 255–265 CrossRef.
- D. R. Dave, S. Kassem, M. Coste, L. Xu, M. Tayarani-Najjaran, D. Podbevsek, P. Colon-De Leon, S. Zhang, L. Ortuno Macias, D. Sementa, M. Pérez-Ferreiro, N. S. Ayati, M. A. Choudhury, K. Veerasammy, S. Doganata, T. Zhong, C. Weng, J. Morales, D. C. Favaro, M. Marianski, S. Y. Ahn, A. C. Obermeyer, T. Wang, T.-D. Li, X. Chen, R. Tu, Y. He and R. V. Ulijn, Adaptive peptide dispersions enable drying-induced biomolecule encapsulation, Nat. Mater., 2025, 24, 1465–1475 Search PubMed.
- H. Gong, Y. Sakaguchi, T. Suzuki, M. Yanagisawa and T. Aida, Near-identical macromolecules spontaneously partition into concentric circles, Nature, 2024, 636, 92–99 CrossRef CAS.
- S. Jiao, D. M. R. Mirabal, A. J. DeStefano, R. A. Segalman, S. Han and M. S. Shell, Sequence Modulates Polypeptoid Hydration Water Structure and Dynamics, Biomacromolecules, 2022, 23, 1745–1756 CrossRef CAS PubMed.
- L. Jawerth, E. Fischer-Friedrich, S. Saha, J. Wang, T. Franzmann, X. J. Zhang, J. Sachweh, M. Ruer, M. Ijavi, S. Saha, J. Mahamid, A. A. Hyman and F. Jülicher, Protein condensates as aging Maxwell fluids, Science, 2020, 370, 1317–1323 CrossRef CAS.
- M. T. Ivanović and R. B. Best, All-atom simulations of biomolecular condensates, Curr. Opin. Struct. Biol., 2025, 93, 103101 CrossRef PubMed.
- L. X. Cao, B. Coventry, I. Goreshnik, B. W. Huang, W. Sheffler, J. S. Park, K. M. Jude, I. Markovic, R. U. Kadam, K. H. G. Verschueren, K. Verstraete, S. T. R. Walsh, N. Bennett, A. Phal, A. Yang, L. Kozodoy, M. DeWitt, L. Picton, L. Miller, E. M. Strauch, N. D. DeBouver, A. Pires, A. K. Bera, S. Halabiya, B. Hammerson, W. Yang, S. Bernard, L. Stewart, I. A. Wilson, H. Ruohola-Baker, J. Schlessinger, S. Lee, S. N. Savvides, K. C. Garcia and D. Baker, Design of protein-binding proteins from the target structure alone, Nature, 2022, 605, 551–560 CrossRef CAS.
- D. E. Shaw, P. Maragakis, K. Lindorff-Larsen, S. Piana, R. O. Dror, M. P. Eastwood, J. A. Bank, J. M. Jumper, J. K. Salmon, Y. B. Shan and W. Wriggers, Atomic-Level Characterization of the Structural Dynamics of Proteins, Science, 2010, 330, 341–346 CrossRef CAS PubMed.
- S. Alberti, A. Gladfelter and T. Mittag, Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates, Cell, 2019, 176, 419–434 CrossRef CAS.
- S. Chagri, D. Y. W. Ng and T. Weil, Designing bioresponsive nanomaterials for intracellular self-assembly, Nat. Rev. Chem., 2022, 6, 320–338 CrossRef.
- Y. Bao, H. Chen, Z. Xu, J. Gao, L. Jiang and J. Xia, Photo-Responsive Phase-Separating Fluorescent Molecules for Intracellular Protein Delivery, Angew. Chem., Int. Ed., 2023, 62, e202307045 CrossRef CAS PubMed.
- B. Gabryelczyk, H. Cai, X. Shi, Y. Sun, P. J. M. Swinkels, S. Salentinig, K. Pervushin and A. Miserez, Hydrogen bond guidance and aromatic stacking drive liquid-liquid phase separation of intrinsically disordered histidine-rich peptides, Nat. Commun., 2019, 10, 5465 CrossRef PubMed.
- N. Tanaka, K. Suyama, K. Tomohara and T. Nose, Exploring LCST- and UCST-like Behavior of Branched Molecules Bearing Repeat Units of Elastin-like Peptides as Side Components, Biomacromolecules, 2024, 25, 7156–7166 CrossRef CAS PubMed.
- Y. Sun, S. Y. Lau, Z. W. Lim, S. C. Chang, F. Ghadessy, A. Partridge and A. Miserez, Phase-separating peptides for direct cytosolic delivery and redox-activated release of macromolecular therapeutics, Nat. Chem., 2022, 14, 274–283 CrossRef CAS.
- Y. Li, T. Xu, Y. Li and H. Wang, Enzyme Induced Solid-Like Condensates Formation of Engineered Peptide in Living Cells for Prostate Cancer Inhibition, Angew. Chem., Int. Ed., 2025, 64, e202504958 CrossRef CAS.
- J. H. Liu, E. Spruijt, A. Miserez and R. Langer, Peptide-based liquid droplets as emerging delivery vehicles, Nat. Rev. Mater., 2023, 8, 139–141 Search PubMed.
- J. Wang, L. Hu, H. Zhang, Y. Fang, T. Wang and H. Wang, Intracellular Condensates of Oligopeptide for Targeting Lysosome and Addressing Multiple Drug Resistance of Cancer, Adv. Mater., 2021, 34, 2104704 CrossRef.
- S. Chen, G. Zou, Q. Guo, X. Qian, H. Li, H. Gao and J. Yu, Extreme pH Tolerance in Peptide Coacervates Mediated by Multivalent Hydrogen Bonds for Enzyme-Triggered Oral Drug Delivery, J. Am. Chem. Soc., 2025, 147, 9704–9715 CrossRef CAS.
- Y. L. M. Ji, Y. Y. Lin and Y. Qiao, Interfacial assembly of biomimetic MOF-based porous membranes on coacervates to build complex protocells and prototissues, Nat. Chem., 2025, 17, 986–996 CrossRef CAS PubMed.
- C. Roden and A. S. Gladfelter, RNA contributions to the form and function of biomolecular condensates, Nat. Rev. Mol. Cell Biol., 2021, 22, 183–195 Search PubMed.
- S. Kodali, C. M. Sands, L. Guo, Y. Huang and B. Di Stefano, Biomolecular condensates in immune cell fate, Nat. Rev. Immunol., 2025, 25, 445–459 CrossRef CAS PubMed.
- T. Ikenoue, M. So, N. Terasaka, W.-E. Huang, Y. Kawata, Y. Miyanoiri, K. Kamagata and H. Suga, De Novo Peptides That Induce the Liquid–Liquid Phase Separation of α-Synuclein, J. Am. Chem. Soc., 2025, 147, 24113–24126 Search PubMed.
- H. R. Li, W. C. Chiang, P. C. Chou, W. J. Wang and J. R. Huang, TAR DNA-binding protein 43 (TDP-43) liquid-liquid phase separation is mediated by just a few aromatic residues, J. Biol. Chem., 2018, 293, 6090–6098 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |