Open Access Article
Open Access ArticleThe structural parameter of spin states of C1-symmetric four-coordinate cobalt complexes: D4
Chenggong
Zheng
 a,
Jiyong
Liu
a,
Jiyong
Liu
 a,
Yongtao
Wang
a,
Yongtao
Wang
 *ac,
Haoran
Li
*ac,
Haoran
Li
 *acd,
Yuwen
Wang
*acd,
Yuwen
Wang
 *b and
Zhan
Lu
*b and
Zhan
Lu
 *ac
*ac
aDepartment of Chemistry, Zhejiang University, Hangzhou 310058, China. E-mail: wyongtao@zju.edu.cn; lihr@zju.edu.cn; luzhan@zju.edu.cn
bHangzhou Institute for Advanced Study, UCAS, Hangzhou 310024, China. E-mail: yuwen.wang@ucas.ac.cn
cCenter of Chemistry for Frontier Technologies, ZJU-NHU United R&D Center, Zhejiang University, Hangzhou 310058, China
dCollege of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310058, China
First published on 3rd November 2025
Abstract
Herein, we reveal the correlation between structure and spin-states for C1-symmetric four-coordinate cobalt complexes. A series of C1-symmetric four-coordinate cobalt complexes with various steric, electronic, anions and chelation modes were synthesized and characterized. X-ray diffraction studies revealed a structural distortion from square-planar to distorted tetrahedral pyramidal geometry. Combining paramagnetic nuclear magnetic resonance (pNMR), magnetic measurement (SQUID), X-ray photoelectron spectroscopy (XPS) and density functional theory (DFT), the electronic structure with a cobalt(II) (SCo = 1/2 to 3/2) ion antiferromagnetically coupled to a radical anion ligand (SLigand = 1/2) was established. A new structural parameter, D4, was introduced as an improved parameter for quantitatively assessing the spin state in four-coordinate complexes.
Introduction
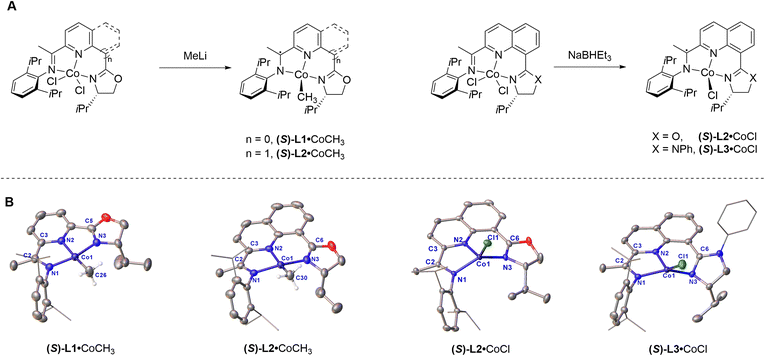
Transition metal catalysis serves as a powerful tool in organic synthesis.1,2 Understanding structure–activity relationships remains key to developing efficient catalytic systems.3,4 Over the past two decades, 3d transition metal catalysts have gained prominence owing to their earth abundance, favorable biocompatibility, and reduced environmental impact.5 Nevertheless, the relationship between structure and catalytic reactivity remain elusive for these systems. This knowledge gap stems from the intrinsic challenges in characterizing 3d metal active species – their propensity for single-electron transfer processes and spin-state interconversions complicates precise determination of both oxidation and spin states during catalysis.6–8Four-coordinate complexes exhibiting high reactivity have been identified as catalytically active species.9–14 It is crucial to understand the relationship between structure and reactivity for four-coordinate complexes. For 3d metals, the fundamental insight lies in elucidating the correlation between structure and spin states. Pioneering studies by Lippard and co-workers established the relationship between the tetrahedral twist angles (Θ) and spin states in D2d-symmetric M(II)–tropocoronand complexes (M = Co, Ni).15 Smith reported the relationship between the Tolman cone angle (θ) and spin states in C3-symmetric complexes with tris(carbene) ligands.16 Peters described that the metal–ligand distances are strongly correlated with the spin state in C3v-symmetric complexes with tris(phosphino)borate [BP3] ligands.17,18 Chirik reported the relationship between distorted planarity and spin states in C2v-symmetric (PDI)Fe19,20 or (PDI)Co21 complexes (PDI = pyridinediimine). Moreover, Chirik and co-workers have also made pioneering contributions to the catalysis of C1-symmetric four-coordinate cobalt complexes.11,22 However, the structure–spin relationship of C1-symmetric four-coordinate complexes has not yet been systematically investigated (Fig. 1A).
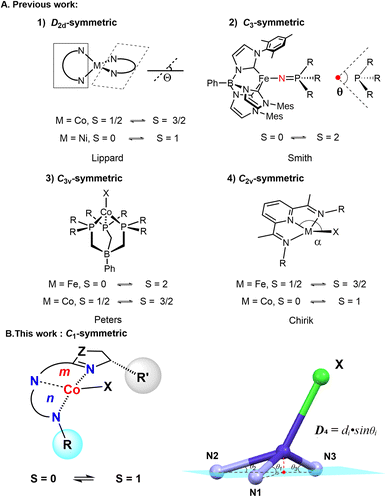 | ||
| Fig. 1 (A) The relationship between structure and spin states in symmetric four-coordinate complexes. (B) The C1-symmetric four-coordinate complexes and structure-spin parameter, D4. | ||
There are several challenges that need to be addressed: (1) four-coordinate complexes with fewer d-electrons are more reactive and less stable, making them more difficult to isolate and characterize. (2) The C1-symmetric skeleton significantly increases the difficulty of obtaining X-ray diffraction structures through recrystallization. (3) The rational design of spin cross-over complexes with a narrow energy window remains an elusive task.23
In our previous work, C1-symmetric four-coordinate cobalt alkyl species were considered as key intermediates in various reactions including hydrogenation,24–27 hydrosilylation,28–30 hydroboration,31–34 and isomerization.35–37 However, the oxidation states, spin states, and coordination modes of these species remain elusive. Herein, we describe a series of chiral four-coordinate cobalt complexes with spin multiplicity, which display singlet to triplet transitions (Fig. 1B). Combining X-ray single-crystal diffraction, paramagnetic nuclear magnetic resonance (pNMR), and magnetic measurement (SQUID), X-ray photoelectron spectroscopy (XPS) and density functional theory (DFT), the electronic structure with a cobalt(II) ion antiferromagnetically coupled to a radical anion ligand was established. The 5,6-chelating mode plays a crucial role in modulating the spin state of the four-coordinate cobalt complexes. Furthermore, a novel structural parameter, D4, was introduced as a metric for the spin states in four-coordinate cobalt complexes.
Results and discussion
Synthesis and characterization of cobalt complexes
In our previous work, the chiral cobalt(II) chloride complex with a 5,5-chelating oxazoline iminopyridine ligand was synthesized and characterized.34 Inspired by the previous work, this work focuses on the synthesis of chiral cobalt methyl complex (S)-L1·CoCH3, 8-oxazoline imine quinoline cobalt methyl complex (S)-L2·CoCH3, 8-oxazoline iminoquinoline cobalt chloride complex (S)-L2·CoCl and 8-imidazoline iminoquinoline cobalt chloride complex (S)-L3·CoCl, following established literature procedures.34,38 The solid-state structures of these complexes were determined using X-ray diffraction, and a representative molecular structure is illustrated in Fig. 2. Notably, although chiral oxazoline iminopyridine cobalt methyl complex (S)-L1·CoCH3 has been synthesized previously,38 to the best of our knowledge, its single-crystal structure has not been reported until now.The representative bond distances and angles of these complexes are summarized in Table 1. The imine bonds C(2)–N(1) are measured to be 1.334(4) Å,1.317(3) Å, 1.325(5) Å and 1.336(3) Å, respectively. Compared with their precursor complexes (S)-L·CoCl2, the imine bonds are elongated (Table S1). Meanwhile, the C(2)–C(3) bonds are contracted to ∼1.434 Å. The variation in bond lengths within the ligands is consistent with a one-electron reduction.39,40 That is to say, the changes in the bond distance indicate that the C1-symmetric ligands play a non-innocent role with a radical anion chelated Co(II) metal center. Notably, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds of the imine are longer than those of oxazoline or imidazoline in four-coordinate complexes, suggesting that the imine modules play a more significant non-innocent role upon one-electron reduction of the chiral ligand.
N double bonds of the imine are longer than those of oxazoline or imidazoline in four-coordinate complexes, suggesting that the imine modules play a more significant non-innocent role upon one-electron reduction of the chiral ligand.
| (S)-L1·CoCla | (S)-L1·CoCH3 | (S)-L2·CoCH3 | (S)-L2·CoClb | (S)-L3·CoCl | |
|---|---|---|---|---|---|
| a From ref. 34. b The bond lengths and angles were averaged over four independent molecules in the unsymmetric unit. c The Coxazoline atom on the oxazoline is labeled as C(5) in (S)-L1·CoCH3 and as C(6) in (S)-L2·CoCH3. | |||||
| C(2)–N(1) | 1.329(2) | 1.334(4) | 1.317(3) | 1.325(5) | 1.336(3) |
| C(2)–C(3) | 1.434(2) | 1.432(4) | 1.432(3) | 1.436(5) | 1.433(4) |
| C(5)/C(6)–N(3)c | 1.300(2) | 1.305(4) | 1.288(3) | 1.301(5) | 1.314(3) |
| Co–N(1) | 1.906(2) | 1.887(2) | 1.898(2) | 1.976(3) | 1.979(2) |
| Co–N(2) | 1.811(2) | 1.838(2) | 1.918(2) | 1.954(3) | 1.957(2) |
| Co–N(3) | 1.934(2) | 1.907(2) | 1.897(2) | 1.984(3) | 1.988(2) |
| Co–X | 2.193(5) | 1.958(3) | 1.970(3) | 2.259(2) | 2.253(1) |
| N(1)–Co–N(3) | 162.6(1) | 162.2(1) | 166.9(1) | 134.8(2) | 146.5(1) |
| N(2)–Co–X | 176.3(1) | 173.2(2) | 164.2(1) | 122.9(1) | 133.6(1) |
| τ 4 | 0.15 | 0.18 | 0.20 | 0.73 | 0.57 |
| D 4 | 0.037 | 0.057 | 0.206 | 0.744 | 0.568 |
In order to precisely quantify the geometric deviation of the cobalt center from the coordination plane, we introduce a new structural parameter, D4, defined as the vertical distance from the metal center to the N(1)–N(2)–N(3) coordination plane (Fig. 3). The parameter can be easily calculated using Olex 2 software or other software programs.41 The cobalt center in (S)-L1·CoCH3 is nearly coplanar with the N(1)–N(2)–N(3) coordination plane, approaching an ideal square planar geometry (D4 = 0.057 Å). This square planar configuration is consistent with the diamagnetic behavior of (S)-L1·CoCH3, as observed by nuclear magnetic resonance, and indicates a strong ligand field.13,21,42 For (S)-L2·CoCH3, the geometry index (τ4 = 0.20) is slightly larger than that of (S)-L1·CoCH3 (τ4 = 0.18),43 while the structural parameter D4 significantly increases to 0.206 Å. Consequently, the molecular geometry of (S)-L2·CoCH3 is best described as a distorted planar structure.
Both (S)-L2·CoCl and (S)-L1·CoCl34 are stabilized by the same coordinating atom but differ in their chelating modes. However, the subtle variation triggers dramatic differences in the coordination modes. Firstly, the X-ray analysis of (S)-L2·CoCl revealed that both cis- and trans- conformations coexist within the unsymmetric unit cell (Fig. S18). Secondly, the N(2)–Co–Cl angle is measured to be 122.9(1)° and the τ4 index is 0.73 in (S)-L2·CoCl. The central metal atom is significantly lifted from the N(1)–N(2)–N(3) coordination plane and the D4 value is measured to be 0.744 Å. Therefore, the molecular geometry of (S)-L2·CoCl is best described as a distorted tetrahedral structure. As for (S)-L3·CoCl, the oxazoline moiety on the ligand was replaced with a more electron-rich imidazoline group. Accordingly, the coordination structures undergo distinct modifications. In contrast to (S)-L2·CoCl, (S)-L3·CoCl presents a single trans-conformation. The N(2)–Co–Cl angle and τ4 index are measured to be 133.6(1)° and 0.57, respectively. The D4 value is measured to be 0.568 Å and is smaller than that of (S)-L2·CoCl. Correspondingly, the molecular geometry of (S)-L3·CoCl is best described as a distorted sawhorse structure.44
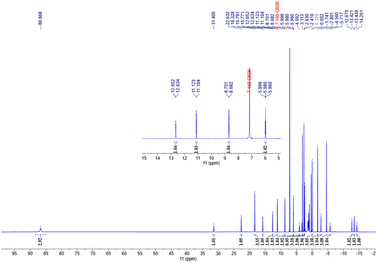
The 1H NMR spectrum of the (S)-L2·CoCH3 complex exhibits an interesting feature, displaying characteristics of both paramagnetic broadening (typical of paramagnetic compounds) and fine coupling splitting (typical of diamagnetic compounds) simultaneously (Fig. 4). The unique phenomenon has not been previously reported in paramagnetic cobalt complexes,9,21,45 providing intriguing insights into the electronic structure of the complex. A solution magnetic moment of 0.77(1) µB was measured in benzene-d6 at room temperature by Evans' method.46 Based on the correlation between the µeff and spin state, the spin population distribution of (S)-L2·CoCH3 can be described as a mixture of 93% low-spin (LS) and 7% high-spin (HS) states.47 This indicates that (S)-L2·CoCH3 exhibits mixed spin multiplicities, with contributions from both singlet and triplet states. The Gibbs energy change (ΔG) between the singlet and triplet states was calculated to be −1.4 kcal mol−1.8,48 The solution magnetic moment of (S)-L2·CoCl was measured to be 2.94(6) µB in benzene-d6 at 291 K, suggesting an absolute high-spin state at this temperature. The 1H NMR spectrum of the (S)-L3·CoCl complex also exhibits both paramagnetic and diamagnetic characteristics (see the SI). The solution magnetic moment of (S)-L3·CoCl was measured to be 2.59(1) µB in benzene-d6 at 288 K, indicating a spin state composition of 0.16 LS + 0.84 HS. Combined analysis of X-ray diffraction and magnetic measurement reveals a clear positive correlation between an increasing population of the high-spin triplet state and a rising value of the structural parameter D4.
The unsaturated magnetic moment of (S)-L2·CoCH3 was further investigated using variable-temperature superconducting quantum interference device (SQUID) magnetometry in the solid state (Fig. 5). At approximately 2 K, the χT value of (S)-L2·CoCH3 approaches zero, indicating a diamagnetic ground state with S = 0. Above 150 K, χT increases with temperature and does not saturate even at 350 K. The SQUID data suggest that the complex undergoes a temperature-induced spin transition from the S = 0 singlet state to the S = 1 triplet state, consistent with spin crossover behavior. The effective magnetic moment reaches 0.98 µB at 290 K, a value essentially consistent with that calculated by Evans' method.
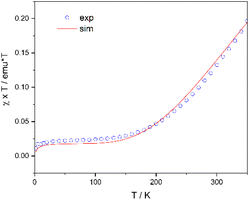 | ||
| Fig. 5 Temperature dependence of magnetic susceptibility under an external magnetic field of 1 T and simulations with the domain model (solid lines) for (S)-L2·CoCH3. | ||
X-ray photoelectron spectroscopy
XPS was performed to reveal the electronic structure and oxidation states of Co sites in the cobalt complexes. (S)-L1·CoCl2, (S)-L2·CoCl2 and (S)-L3·CoCl2 were analyzed by XPS, affording binding energies of 781.0 eV, 780.8 eV and 780.6 eV (Fig. S15–S17). Next, (S)-L1·CoCH3, (S)-L2·CoCH3, (S)-L2·CoCl and (S)-L3·CoCl were carefully analyzed by XPS, affording Co 2p3/2 binding energies of 780.8 eV, 780.4 eV, 780.9 eV and 780.9 eV, respectively (Fig. 6). Their binding energies are slightly higher than that of CoO (780.0 eV),49 supporting the +2 oxidation state of the Co species in L·CoCl2. The binding energies of L·CoCH3 and L·CoCl are similar to those of their precursor, L·CoCl2, before reduction. The slight binding energy change further supports the ligand's redox activity, suggesting that single-electron reduction occurs on the ligand, while the oxidation state of the central metal remains constant. Combining the XRD structure, magnetic measurements, XPS analysis and DFT calculations, (S)-L·CoCH3 and (S)-L·CoCl are best described as radical anions antiferromagnetically coupled to a Co(II) metal center (Fig. 8 and 9).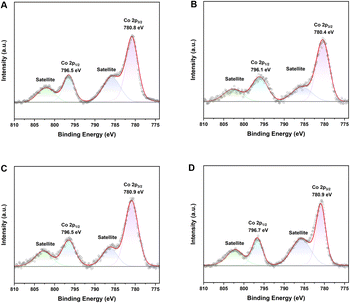 | ||
| Fig. 6 XPS spectrum of Co 2p for cobalt complexes. (a) (S)-L1·CoCH3; (b) (S)-L2·CoCH3; (c) (S)-L2·CoCl; (d) (S)-L3·CoCl. | ||
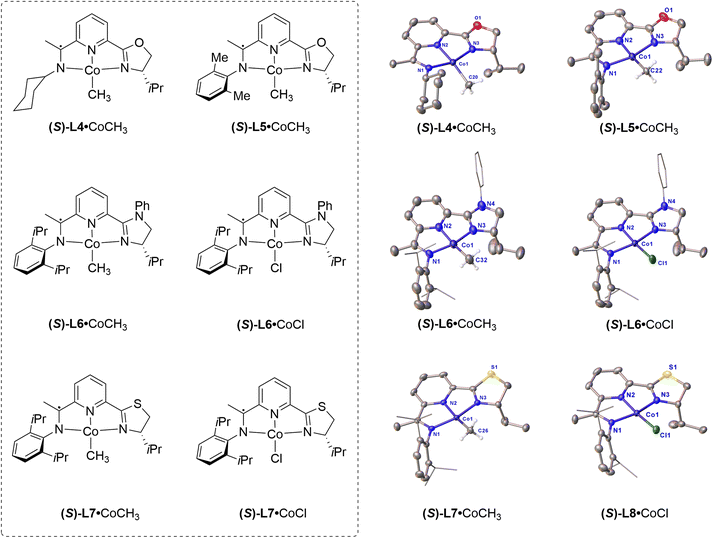
In order to obtain a more systematic understanding of the correlation between structure and spin states, a series of four-coordinated cobalt complexes was further synthesized and characterized (Fig. 7). The effects of steric, electronic, and anions on spin states were systematically investigated. The representative structural parameters are summarized in Table 2.
| Complex | τ 4 | D 4/Å | λ max/nm | Spin states |
|---|---|---|---|---|
| (S)-L1·CoCl | 0.15 | 0.037 | 515 | Low spin |
| (S)-L1·CoCH3 | 0.18 | 0.057 | 558 | Low spin |
| (S)-L2·CoCH3 | 0.20 | 0.206 | 625 | 0.93 LS + 0.07 HS |
| (S)-L2·CoCl | 0.73 | 0.744 | 657 | High spin |
| (S)-L3·CoCl | 0.57 | 0.568 | 650 | 0.16 LS + 0.84 HS |
| (S)-L4·CoCH3 | 0.25 | 0.095 | 545 | Low spin |
| (S)-L5·CoCH3 | 0.18 | 0.011 | 526 | Low spin |
| (S)-L6·CoCH3 | 0.16 | 0.045 | 552 | Low spin |
| (S)-L6·CoCl | 0.16 | 0.045 | 529 | Low spin |
| (S)-L7·CoCH3 | 0.20 | 0.049 | 577 | Low spin |
| (S)-L7·CoCl | 0.17 | 0.082 | 532 | Low spin |
It should be noted that the geometry index τ4 of (S)-L4·CoCH3 (τ4 = 0.25) is larger than that of (S)-L2·CoCH3 (τ4 = 0.20); however, (S)-L4·CoCH3 exhibits diamagnetic behaviour with low-spin states. Correspondingly, the D4 value of (S)-L4·CoCH3 is measured to be 0.095 Å, which is smaller than that of (S)-L2·CoCH3 (D4 = 0.206 Å). Considering that the τ4 index was initially proposed to quantify the deviation from ideal planar geometry for symmetric four-coordinate complexes, like D2d and C2v, the τ4 index may not be suitable for assessing C1-symmetric four-coordinate compounds.43
Comparatively, the parameter D4 is defined geometrically as di![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θi, where di is the M–L bond length (reflecting ligand coordinated strength), and θi is the angle between the M–L bond and the coordination plane, reflecting the dx2−y2 orbital overlap (Fig. 3). In other words, a smaller D4 value is likely associated with a stronger ligand field and a larger splitting energy. Interestingly, in the UV-vis spectra, the maximum absorption wavelength of four-coordinate cobalt complexes exhibits a red shift with an increase in the D4 value.
θi, where di is the M–L bond length (reflecting ligand coordinated strength), and θi is the angle between the M–L bond and the coordination plane, reflecting the dx2−y2 orbital overlap (Fig. 3). In other words, a smaller D4 value is likely associated with a stronger ligand field and a larger splitting energy. Interestingly, in the UV-vis spectra, the maximum absorption wavelength of four-coordinate cobalt complexes exhibits a red shift with an increase in the D4 value.
Computational studies
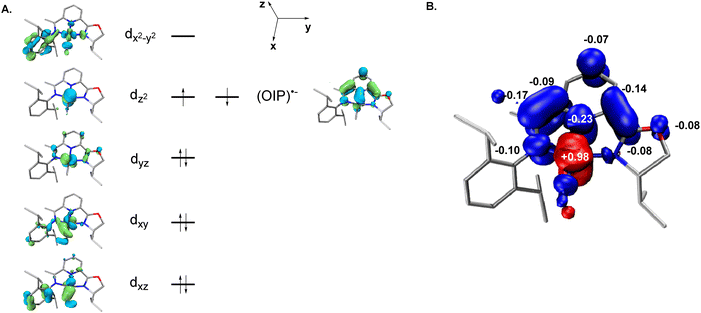
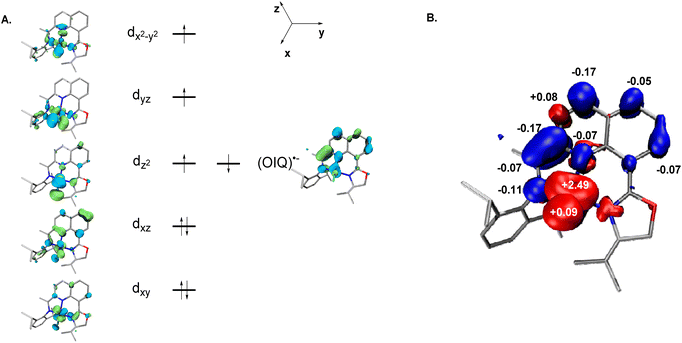
Full-molecule DFT calculations were performed on (S)-L1·CoCH3 and (S)-L2·CoCl using the TPSSh50–52 functional methods and the def2-TZVP basis set.53–56 For (S)-L1·CoCH3, the results reveal that the electronic structure adopts a broken-symmetry (BS) (1, 1) configuration,57 with an electronic energy −1.5 kcal mol−1 lower than that of the BS (3, 1) triplet state. The corresponding molecular orbital diagram and spin–density plot are illustrated in Fig. 8.58–60 The qualitative molecular-orbital diagram is consistent with that expected for a square-planar complex with two doubly occupied cloverleaf d orbitals (dxy and dyz) and dz2 (the z axis being defined as perpendicular to the chelate plane). One unpaired electron is located in the dxz orbital, while the second unpaired electron occupies an oxazoline iminopyridine π* symmetry orbital. An orbital principally of dx2−y2 origin is found among the unoccupied orbitals. The overall singlet ground state (S = 0) is confirmed by the Mulliken spin density plot, which shows antiferromagnetic coupling between a low-spin cobalt(II) ion (SCo = 1/2) and an oxazoline iminopyridine radical anion (SOIP˙− = 1/2).The (S)-L2·CoCl calculation converged to a BS (3, 1) solution, which is lower in energy by 3.7 kcal mol−1 than the BS (1, 1) open-shell singlet state. The corresponding molecular orbital diagram and spin–density plot are shown in Fig. 9. The qualitative molecular orbital diagram is consistent with a distorted tetrahedral complex, in which the cobalt center is significantly displaced from the coordination plane. The electronic structure of (S)-L2·CoCl is corroborated by the Mulliken spin density population analysis, confirming a high-spin cobalt(II) ion (SCo = 3/2) antiferromagnetically coupled to an 8-oxazoline iminoquinoline radical anion (SOIQ˙− = 1/2), resulting in an overall S = 1 triplet state.
Conclusions
In summary, we reported a series of C1-symmetric four-coordination cobalt complexes with various steric effects, electronic effects, anions and chelation modes with spin multiplicities. By modulating the chelation modes and ligand substituents, the spin states of the metal center can be effectively manipulated. The 5,6-chelating mode provides a spin-state manipulation window for four-coordination cobalt complexes. The electronic structure of the radical anion antiferromagnetically coupled Co(II) metal center was established by multiple spectra and DFT calculations. A novel structural parameter for four-coordination complexes, D4, is proposed, which could be better correlated with the spin state of the metal center. It can be anticipated that the D4 index could offer a predictive framework for manipulating spin and understanding the spin states of intermediates and transition states in spin chemistry.Author contributions
Z. Lu conceived the project and supervised the work with Y. W. Wang, Y. T. Wang and H. R. Li. C. G. Zheng conducted the experiments and drafted the original manuscript. J. Y. Liu contributed to the characterization of the single crystals. Y. T. W. performed theoretical calculations. H. R. Li provided critical supervision in electronic structure analysis. Y. W. Wang provided critical guidance on the single-crystal preparation. All authors discussed the results, reviewed, and contributed to the final manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). See DOI: https://doi.org/10.1039/d5sc06683b.CCDC 2325627, 2329552, 2338659–2338660, 2344060, 2344061, 2348647, 2353792, 2355865, 2355871, 2356769, 2362794, 2369004, 2375363, 2375454, 2374877 and 2475931 ((S)-L·CoCl2, (S)-L·CoCH3 and (S)-L·CoCl) contain the supplementary crystallographic data for this paper.61a–q
Acknowledgements
Financial support was provided by the National Key R&D Program of China (2021YFF0701600), the Zhejiang Provincial Natural Science Foundation of China (LDQ24B020001), the National Natural Science Foundation of China (NSFC) (22525109), the Fundamental Research Funds for the Central Universities (226-2022-00224 and 226-2024-00003), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB0610000), and the Research Funds of the Hangzhou Institute for Advanced Study, UCAS (Grant No. 2024HIAS-p003). We thank Wang Chen and Prof. Shengfa Ye from the Dalian Institute of Chemical Physics for SQUID analysis and helpful discussions. We thank Dr Xiang Ren for providing help in synthesizing 8-OIQ ligands. We thank Prof. Qiaohong He for ESI-HRMS analysis. We thank Dr Dier Shi, Shuna Liu, Minghang Li and Jinyi Li for XPS analysis. We thank Dr Hongliang Wang and Peihong Zhou for helpful computational suggestions. We thank Jialian Ding for UV-vis analysis. The authors also thank Lixuan Zheng, Jiale Ying, Zhanchen Ye, Prof. Jianguo Huang and Prof. Longguan Zhu for their helpful discussions.Notes and references
- A. H. Cherney, N. T. Kadunce and S. E. Reisman, Chem. Rev., 2015, 115, 9587–9652 CrossRef CAS PubMed.
- H. Wang, J. Wen and X. Zhang, Chem. Rev., 2021, 121, 7530–7567 CrossRef CAS PubMed.
- J. F. Hartwig, Organotransition Metal Chemistry, University Science Books, Sausalito, CA, 2010 Search PubMed.
- H. R. Crabtree, The Organometallic Chemistry of the Transition Metals, Wiley-Interscience, New York, 6th edn, 2014 Search PubMed.
- J. Guo, Z. Cheng, J. Chen, X. Chen and Z. Lu, Acc. Chem. Res., 2021, 54, 2701–2716 CrossRef CAS PubMed.
- M. Swart and M. Gruden, Acc. Chem. Res., 2016, 49, 2690–2697 CrossRef CAS PubMed.
- K. D. Vogiatzis, M. V. Polynski, J. K. Kirkland, J. Townsend, A. Hashemi, C. Liu and E. A. Pidko, Chem. Rev., 2019, 119, 2453–2523 CrossRef CAS PubMed.
- J. Cirera, M. Via-Nadal and E. Ruiz, Inorg. Chem., 2018, 57, 14097–14105 CrossRef CAS PubMed.
- J. V. Obligacion, S. P. Semproni and P. J. Chirik, J. Am. Chem. Soc., 2014, 136, 4133–4136 CrossRef CAS PubMed.
- J. M. Hoyt, V. A. Schmidt, A. M. Tondreau and P. J. Chirik, Science, 2015, 349, 960–963 CrossRef CAS PubMed.
- M. R. Friedfeld, M. Shevlin, G. W. Margulieux, L. C. Campeau and P. J. Chirik, J. Am. Chem. Soc., 2016, 138, 3314–3324 CrossRef CAS PubMed.
- C. K. Blasius, V. Vasilenko, R. Matveeva, H. Wadepohl and L. H. Gade, Angew. Chem., Int. Ed., 2020, 59, 23010–23014 CrossRef CAS PubMed.
- J. S. Doll, M. L. Heldner, M. Scherr, J. Ballmann and D.-A. Roşca, ACS Catal., 2023, 13, 8770–8782 CrossRef CAS.
- K. E. Berger, R. J. Martinez, J. Zhou and C. Uyeda, J. Am. Chem. Soc., 2023, 145, 9441–9447 CrossRef CAS PubMed.
- B. S. Jaynes, L. H. Doerrer, S. Liu and S. J. Lippard, Inorg. Chem., 1995, 34, 5735–5744 CrossRef CAS.
- H.-J. Lin, D. Siretanu, D. A. Dickie, D. Subedi, J. J. Scepaniak, D. Mitcov, R. Clérac and J. M. Smith, J. Am. Chem. Soc., 2014, 136, 13326–13332 CrossRef CAS PubMed.
- D. M. Jenkins and J. C. Peters, J. Am. Chem. Soc., 2005, 127, 7148–7165 CrossRef CAS PubMed.
- S. E. Creutz and J. C. Peters, Inorg. Chem., 2016, 55, 3894–3906 CrossRef CAS PubMed.
- A. C. Bowman, C. Milsmann, E. Bill, Z. R. Turner, E. Lobkovsky, S. DeBeer, K. Wieghardt and P. J. Chirik, J. Am. Chem. Soc., 2011, 133, 17353–17369 CrossRef CAS PubMed.
- C. B. Kovel, J. M. Darmon, S. C. E. Stieber, G. Pombar, T. P. Pabst, B. Theis, Z. R. Turner, O. Ungor, M. Shatruk, S. DeBeer and P. J. Chirik, J. Am. Chem. Soc., 2023, 145, 5061–5073 CrossRef CAS PubMed.
- A. C. Bowman, C. Milsmann, E. Bill, E. Lobkovsky, T. Weyhermuller, K. Wieghardt and P. J. Chirik, Inorg. Chem., 2010, 49, 6110–6123 CrossRef CAS PubMed.
- S. Monfette, Z. R. Turner, S. P. Semproni and P. J. Chirik, J. Am. Chem. Soc., 2012, 134, 4561–4564 CrossRef CAS PubMed.
- S. Ye and F. Neese, Inorg. Chem., 2010, 49, 772–774 CrossRef CAS PubMed.
- J. Chen, C. Chen, C. Ji and Z. Lu, Org. Lett., 2016, 18, 1594–1597 CrossRef CAS PubMed.
- J. Guo, B. Cheng, X. Shen and Z. Lu, J. Am. Chem. Soc., 2017, 139, 15316–15319 CrossRef CAS PubMed.
- J. Guo, X. Shen and Z. Lu, Angew. Chem., Int. Ed., 2017, 56, 615–618 CrossRef CAS PubMed.
- P. Lu, H. Wang, Y. Mao, X. Hong and Z. Lu, J. Am. Chem. Soc., 2022, 144, 17359–17364 CrossRef CAS PubMed.
- B. Cheng, P. Lu, H. Zhang, X. Cheng and Z. Lu, J. Am. Chem. Soc., 2017, 139, 9439–9442 CrossRef CAS PubMed.
- J. Guo, H. L. Wang, S. P. Xing, X. Hong and Z. Lu, Chem, 2019, 5, 881–895 CAS.
- Z. Cheng, M. Li, X. Y. Zhang, Y. Sun, Q. L. Yu, X. H. Zhang and Z. Lu, Angew. Chem., Int. Ed., 2023, 62, e202215029 CrossRef CAS PubMed.
- J. H. Chen, T. Xi, X. Ren, B. Cheng, J. Guo and Z. Lu, Org. Chem. Front., 2014, 1, 1306–1309 RSC.
- X. Ren and Z. Lu, Org. Lett., 2021, 23, 8370–8374 CrossRef CAS PubMed.
- C. Chen, H. Wang, T. Li, D. Lu, J. Li, X. Zhang, X. Hong and Z. Lu, Angew. Chem., Int. Ed., 2022, 61, e202205619 CrossRef CAS PubMed.
- Y. Bao, C. Zheng, K. Xiong, C. Hu, P. Lu, Y. Wang and Z. Lu, J. Am. Chem. Soc., 2024, 146, 21089–21098 CrossRef CAS.
- J. Zhao, B. Cheng, C. Chen and Z. Lu, Org. Lett., 2020, 22, 837–841 CrossRef CAS PubMed.
- W. Liu, Y. Zheng, Y. Mao, J. Chen, X. Ren, Z. Cheng and Z. Lu, Org. Lett., 2022, 24, 1158–1163 CrossRef CAS PubMed.
- J. Zhao, G. Xu, X. Wang, J. Liu, X. Ren, X. Hong and Z. Lu, Org. Lett., 2022, 24, 4592–4597 CrossRef CAS PubMed.
- L. Zhang, Z. Zuo, X. Wan and Z. Huang, J. Am. Chem. Soc., 2014, 136, 15501–15504 CrossRef CAS PubMed.
- Q. Knijnenburg, D. Hetterscheid, T. M. Kooistra and P. H. M. Budzelaar, Eur. J. Inorg. Chem., 2004, 2004, 1204–1211 CrossRef.
- A. C. Bowman, C. Milsmann, C. C. Atienza, E. Lobkovsky, K. Wieghardt and P. J. Chirik, J. Am. Chem. Soc., 2010, 132, 1676–1684 CrossRef CAS PubMed.
- https://www.bilibili.com/video/BV15y4y1773o/?spm_id_from=333.337.search-card.all.click%26vd_source=0362e9aeb0256418dc579a053f424d21 .
- J. Lee, D. Heo, W. Lee and J. Seo, J. Am. Chem. Soc., 2025, 147, 14997–15005 CrossRef CAS PubMed.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 9, 955–964 RSC.
- M. H. Reineke, M. D. Sampson, A. L. Rheingold and C. P. Kubiak, Inorg. Chem., 2015, 54, 3211–3217 CrossRef CAS.
- C. Chen, T. R. Dugan, W. W. Brennessel, D. J. Weix and P. L. Holland, J. Am. Chem. Soc., 2014, 136, 945–955 CrossRef CAS.
- S. K. Sur, J. Magn. Reson., 1989, 82, 169–173 CAS.
- K. Sun, Y. Huang, Q. Wang, W. Zhao, X. Zheng, J. Jiang and H. L. Jiang, J. Am. Chem. Soc., 2024, 146, 3241–3249 CrossRef CAS PubMed.
- F. Milocco, F. de Vries, I. M. A. Bartels, R. W. A. Havenith, J. Cirera, S. Demeshko, F. Meyer and E. Otten, J. Am. Chem. Soc., 2020, 142, 20170–20181 CrossRef CAS PubMed.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- V. N. Staroverov, G. E. Scuseria, J. M. Tao and J. P. Perdew, J. Chem. Phys., 2003, 119, 12129–12137 CrossRef CAS.
- J. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Phys. Rev. Lett., 2003, 91, 146401 CrossRef PubMed.
- V. N. Staroverov, G. E. Scuseria, J. Tao and J. P. Perdew, J. Chem. Phys., 2004, 121, 11507 CrossRef CAS.
- A. Schäfer, C. Huber and R. Ahlrichs, J. Chem. Phys., 1994, 100, 5829–5835 CrossRef.
- K. Eichkorn, F. Weigend, O. Treutler and R. Ahlrichs, Theor. Chem. Acc., 1997, 97, 119–124 Search PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- F. Neese, J. Phys. Chem. Solids, 2004, 65, 781–785 CrossRef CAS.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- T. Lu, J. Chem. Phys., 2024, 161, 082503 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- (a) CCDC 2325627: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2j207w; (b) CCDC 2329552: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2j62vp; (c) CCDC 2338659: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2jhkm7; (d) CCDC 2338660: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2jhkn8; (e) CCDC 2344060: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2jp5v8; (f) CCDC 2344061: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2jp5w9; (g) CCDC 2348647: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2jtyt4; (h) CCDC 2353792: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2k09sp; (i) CCDC 2355871: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2k0dbb; (j) CCDC 2355865: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2k2gns; (k) CCDC 2356769: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2k3dtx; (l) CCDC 2362794: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2k9p5r; (m) CCDC 2369004: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2kj4hr; (n) CCDC 2374877: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2kq7yh; (o) CCDC 2375363: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2kqrmp; (p) CCDC 2375454: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2kqvkq; (q) CCDC 2475931: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p3drz.
| This journal is © The Royal Society of Chemistry 2026 |