Open Access Article
Open Access ArticleComment on “Mapping photoisomerization dynamics on a three-state model potential energy surface in bacteriorhodopsin using femtosecond stimulated Raman spectroscopy” by Z. Wang, Y. Chen, J. Jiang, X. Zhao and W. Liu, Chem. Sci., 2025, 16, 3713
I.
Schapiro
abc,
M.
Olivucci
de,
K.
Heyne
f and
S.
Haacke
 *g
*g
aDepartment of Physics, TU Dortmund, 44227 Dortmund, Germany
bResearch Center Chemical Sciences and Sustainability, University Alliance Ruhr, 44801 Bochum, Germany
cInstitute of Chemistry, The Hebrew University of Jerusalem, 9190401, Jerusalem, Israel
dDepartment of Chemistry, Bowling Green State University, Bowling Green, OH 43403, USA
eDipartimento di Biotecnologie, Chimica e Farmacia, Universita di Siena, I-53100 Siena, Italy
fDepartment of Physics, Free University Berlin, D-14195 Berlin, Germany
gUniversity of Strasbourg, CNRS, IPCMS, 67034 Strasbourg, France. E-mail: stefan.haacke@ipcms.unistra.fr
First published on 19th December 2025
Abstract
The article by Wang et al. (Chem. Sci., 2025, 16, 3713) reports an experimental study of the photo-isomerization dynamics of the all-trans protonated Schiff base of retinal (AT-PSBR) in bacteriorhodopsin (bR), based on femtosecond stimulated Raman spectroscopy. In the present comment, we point out a misinterpretation of a new interesting high-frequency vibrational mode, conceptual flaws, like interpreting the data in a C2h symmetry framework, and most importantly the neglect of basic properties of AT-PSBR in bR, which were established over the past 30 years. The comment ends with a few suggestions on how to substantiate the new findings within a correct experimental and theoretical framework.
Introduction
The article by Wang et al.1 reports on a new study of the photo-isomerization dynamics of the all-trans protonated Schiff base of retinal (AT-PSBR) in light-adapted bacteriorhodopsin (bR) by femtosecond stimulated Raman spectroscopy. It complements previous studies performed with the same technique,2,3 which were limited to a spectral range of 700–1700 cm−1. However, in the following we explain in detail four fundamental points of criticism that we invite the authors to consider and comment on in their reply.Main comment
The first aspect is technical. While previous studies were performed under low pump intensities, thus ensuring 1-photon absorption per protein, the new study is performed with excessive intensities leading, as the authors recognize by providing the comparative Fig. S3 and S4, to slower dynamics for the decay of the J state (9.1 instead of 3.5 ps).Unfortunately, the paper does not provide any information about the diameters of the focus spot of these beams, or does not quote the energy density (J cm−2), which is the only value of importance for evaluating the percentage of proteins excited (P). Let us remind here that P = nphσ, is the product of the photon surface density of the pump beam in the focus, nph, and of the absorption cross section at the pump wavelength σ(λx). For protein dynamics studies under relevant physiological conditions, i.e. under low light excitation, P should be <0.3. This central point was unfortunately not documented in the paper.
The second and major aspect concerns the authors’ central new finding: the observation of a high-energy vibronic band at 1820 cm−1, which is then used to assign the electronic character of the I and J intermediates. However, such reported signature depends in a non-linear fashion on the pump energy of the excitation (actinic) pulse Ea. Indeed, an inspection of Raman intensities at 1517 cm−1 and at 1820 cm−1 presented in Fig. S5 and Fig. 2 for 50 nJ and 200 nJ excitation energy, respectively, indicates a different signal dependence of the vibrations. In a linear regime, all signals increase linearly with pump energy/intensity for all delay times. In contrast, the ratios R of the two bands at 1517 cm−1 and 1820 cm−1 at 450 fs are R(50 nJ) = 4.5 and R(200 nJ) = 1.1. The 1820 cm−1 band gains in relative intensity as compared to the one at 1517 cm−1, indicating a super-linear dependence on Ea of the former. This contradicts a linear dependence as a function of the excited state population, as it is assumed in the further analysis. We would like to refer the authors to a recent paper, where the effects of excessive excitation density (multi-photon excitation) on bR's excited state dynamics, including the increase in the lifetime of J, were reported and analysed in detail.4
The super-linear increase in the 1820 cm−1 band as a function of Ea is an observation that should have prompted the authors to investigate such non-linearity in detail. Instead, they have worked in a non-linear response regime thus (i) reporting results that are irrelevant for advancing the understanding of one-photon photo-isomerization in bR and (ii) limiting the significance of the comparison with former well-established studies on ultrafast vibrational dynamics, even if some features like the excited state Raman frequencies are well reproduced.
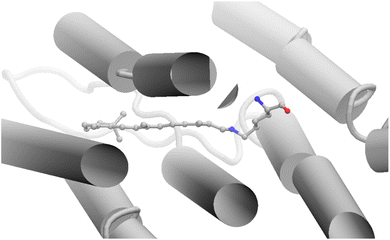
The third and more conceptual aspect concerns the authors' use of the above-mentioned 1820 cm−1 vibronic band for assigning the electronic characters of the I and J intermediates. A similar high energy vibronic signature was reported for polyenes with conjugation length N ≤ 5, and assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching (ref. 18, 20 and 21 in the paper). Based on computational results, and relying on C2h symmetry, the polyene energy up-shift was attributed to vibronic coupling between the 2A−g excited state and the 1A−g ground state (ref. 19, 22 and 23 cited in the paper). However, such finding cannot be directly transferred to the all-trans retinal protonated Schiff base chromophore of (AT-PSBR) in bR. In fact, and in contrast with polyenes, AT-PSBR not only does not have C2h symmetry but features a strongly asymmetric (polarized) π-electron density. This has the consequence that, even when adopting the common practice of retaining the C2h symmetry labels, such polarized electronic structure yields a different state energy ordering that is further biased by the steric and electrostatic interactions with protein environment (Fig. 1: the ground state structure of AT-PSBR in bR is actually distorted out-of-plane). Hence, the authors' assumption that the AT-PSBR has the same electronic structure as polyene hydrocarbons is not correct and this is especially true for the further distorted I and J intermediates with non-zero dihedral angles.
C stretching (ref. 18, 20 and 21 in the paper). Based on computational results, and relying on C2h symmetry, the polyene energy up-shift was attributed to vibronic coupling between the 2A−g excited state and the 1A−g ground state (ref. 19, 22 and 23 cited in the paper). However, such finding cannot be directly transferred to the all-trans retinal protonated Schiff base chromophore of (AT-PSBR) in bR. In fact, and in contrast with polyenes, AT-PSBR not only does not have C2h symmetry but features a strongly asymmetric (polarized) π-electron density. This has the consequence that, even when adopting the common practice of retaining the C2h symmetry labels, such polarized electronic structure yields a different state energy ordering that is further biased by the steric and electrostatic interactions with protein environment (Fig. 1: the ground state structure of AT-PSBR in bR is actually distorted out-of-plane). Hence, the authors' assumption that the AT-PSBR has the same electronic structure as polyene hydrocarbons is not correct and this is especially true for the further distorted I and J intermediates with non-zero dihedral angles.
The confusion generated by the differences in polyene and PSBRs was clarified in a review paper on simulation methods relevant for PSBR in retinal proteins.5 Ground and excited states of PSBR are more appropriately characterised by the degree of diabatic electronic character, namely closed shell covalent (COV) that, in terms of pseudosymmetry, could be interpreted as 1Ag, open-shell diradical (DIR) interpreted as 2Ag or charge transfer (CT) that would be interpreted as a 1Bu. This last state is now reckoned to be the low lying excited state in rhodopsins in general, a conclusion that does not apply to polyenes. The intricate mixing of the electronic characters as a function of the dihedral angle is discussed in detail in the mentioned review and should form the basis or framework for the interpretation of the FSRS data reported by Wang et al. Most relevantly the excited state evolution and decay occurs after propagating on a CT (i.e. 1Bu) state not along a DIR (2Ag) state.
What is also inappropriate, is not having summarized the experimental and computational work done in the past for assigning the nature of the AT-PSBR excited state in bR and, most specifically, of the I state. In fact, when considering a valid pseudo-symmetry denomination, it has been shown to have a strongly mixed 2Ag (diradical)/1Bu (zwitterionic) character,6,7 which is abandoned when the chromophore starts to twist about the reactive double bond and enters a conical intersection formed, as mentioned above, by crossing of a 1Bu excited state and the 1Ag ground state placed half-way along the isomerization coordinate. There is instead no evidence for the crossing between the 1Ag and 2Ag states documented in polyenes, that not only would have a different energy but has a dramatically different geometrical structure not suitable for driving an efficient C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C isomerization reaction even in the gas-phase.5 Notice that a 2Ag/1Ag crossing driving the isomerization was proposed long ago but when suitable electronic structure calculations have become available it was then replaced by a 1Bu/1Ag picture as explained above.
C isomerization reaction even in the gas-phase.5 Notice that a 2Ag/1Ag crossing driving the isomerization was proposed long ago but when suitable electronic structure calculations have become available it was then replaced by a 1Bu/1Ag picture as explained above.
The last aspects concerns the nature of the J intermediate. According to many papers on bR, J is identified as a vibrationally excited ground state population.8–14 Assigning J to a conformer with close to 90° dihedral angle (Fig. 1A in the paper) is not only at odds with this established picture, but is physically incorrect. A state with a 3 or 9 ps lifetime cannot be located at a sloped region of the potential energy surface, since the lifetime in these states is <100 fs due to the presence of a conical intersection. This is also a major misinterpretation made in ref. 10, cited in the paper.
Conclusions
In summary, the observation of the vibronic band at 1820 cm−1 is a clear new experimental finding, but the non-linear increase in its amplitude as a function of the excitation power remains to be explained. In addition, discussing the excited states of the PSBR's in bR in terms of a putative character with C2h symmetry is not only inappropriate but is in contrast with the most recent computational characterization following the diradical, zwitterionic and covalent characters associated with the 2Ag, 1Bu and 1Ag pseudo-symmetries, respectively. We would suggest the authors to perform a detailed study of the amplitudes of the vibronic signatures as a function of excitation power and examine the expected linear relationship. In addition, it might be helpful to perform calculations for the excited state resonance Raman spectra for bR in its I and J intermediates, in order to clarify the origin of the 1820 cm−1 band.Author contributions
All authors contributed equally to this comment.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this article.Acknowledgements
The authors acknowledge funding of joint projects such as the French-German SunHy project (ANR 21-CE50-0040), the ANR project UltrArchae (grant no. ANR-21-CE11-0029), and the Sen§us program for international collaboration funded by the University of Strasbourg.Notes and references
- Z. Wang, Y. Chen, J. Jiang, X. Zhao and W. Liu, Chem. Sci., 2025, 16, 3713 RSC.
- D. W. McCamant, P. Kukura and R. A. Mathies, J. Phys. Chem. B, 2005, 109, 10449–10457 CrossRef CAS PubMed.
- S. Shim, J. Dasgupta and R. A. Mathies, J. Am. Chem. Soc., 2009, 131, 7592–7597 CrossRef CAS PubMed.
- G. Nass Kovacs, J.-P. Colletier, M. L. Grünbein, Y. Yang, T. Stensitzki, A. Batyuk, S. Carbajo, R. B. Doak, D. Ehrenberg, L. Foucar, R. Gasper, A. Gorel, M. Hilpert, M. Kloos, J. E. Koglin, J. Reinstein, C. M. Roome, R. Schlesinger, M. Seaberg, R. L. Shoeman, M. Stricker, S. Boutet, S. Haacke, J. Heberle, K. Heyne, T. Domratcheva, T. R. M. Barends and I. Schlichting, Nat. Commun., 2019, 10, 3177 CrossRef PubMed.
- S. Gozem, H. L. Luk, I. Schapiro and M. Olivucci, Chem. Rev., 2017, 117, 13502–13565 CrossRef CAS PubMed.
- A. Cembran, F. Bernardi, M. Olivucci and M. Garavelli, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 6255–6260 CrossRef CAS PubMed.
- S. Gozem, P. J. M. Johnson, A. Halpin, H. L. Luk, T. Morizumi, V. I. Prokhorenko, O. P. Ernst, M. Olivucci and R. J. D. Miller, J. Phys. Chem. Lett., 2020, 3889–3896 CrossRef CAS PubMed.
- J. Herbst, K. Heyne and R. Diller, Science, 2002, 297, 822–825 CrossRef CAS PubMed.
- G. Haran, K. Wynne, A. Xie, Q. He, M. Chance and R. M. Hochstrasser, Chem. Phys. Lett., 1996, 261, 389–395 CrossRef CAS.
- K. C. Hasson, F. Gai and P. A. Anfinrud, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 15124–15129 CrossRef CAS PubMed.
- F. Gai, Science, 1998, 279, 1886–1891 CrossRef CAS PubMed.
- T. Ye, E. Gershgoren, N. Friedman, M. Ottolenghi, M. Sheves and S. Ruhman, Chem. Phys. Lett., 1999, 314, 429–434 CrossRef CAS.
- S. Ruhman, B. X. Hou, N. Friedman, M. Ottolenghi and M. Sheves, J. Am. Chem. Soc., 2002, 124, 8854–8858 CrossRef CAS PubMed.
- A. Wand, I. Gdor, J. Y. Zhu, M. Sheves and S. Ruhman, Annu. Rev. Phys. Chem., 2013, 64, 437–458 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |