Open Access Article
Open Access ArticleDDQ-promoted synthesis of 2-benzoylbenzofurans and benzofuro[2,3-c]pyridines using 2′-hydroxy ethyl cinnamate and phenacyl bromides
Surbhi Mahender Saini ,
Harshada Rambaboo Singh
,
Harshada Rambaboo Singh ,
Raunak Katiyar
,
Raunak Katiyar and
Sandeep Chandrashekharappa
and
Sandeep Chandrashekharappa *
*
Department of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research, Raebareli (NIPER-R), Lucknow, UP-226002, India. E-mail: c.sandeep@niperraebareli.edu.in; c.sandeep@niperrbl.ac.in; Fax: +91-522-2975587; Tel: +91-522-2499703
First published on 14th January 2026
Abstract
A cascade oxidation, cyanation/cyclization facilitated by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), has been developed as an efficient synthetic route for producing a range of 2-benzoylbenzofurans and benzofuro[2,3-c]pyridines from 2′-hydroxyethyl cinnamate and phenacyl bromides. Mechanistic studies indicate that the CN radical, generated through the homolytic cleavage of the C–C bond of DDQ, served as a key intermediate in the reaction. DDQ-mediated single-electron transfer initiates the cascade process of radical addition, and 1–2 addition of DDQ promotes cyclization. A wide range of bicyclic and tricyclic ring systems of benzofuran and benzofuropyridine are obtained in this methodology with moderate to good yields. This transformation features a broad substrate scope, operates under organometallic catalyst-free conditions, utilises readily accessible starting materials, and demonstrates potential for scale-up. Additionally, it allows for the simultaneous formation of two new C–C and C–N bonds in a single operation.
Introduction
Fused multi-heterocyclic scaffolds are important structural frameworks in organic chemistry and are ubiquitous in pharmacologically active synthetic and natural products. Among these scaffolds, benzofuran and benzofuropyridine have attracted the attention of synthetic and medicinal chemists, as they display potential as promising medicinal candidates.1–6 Due to their significant pharmacological and diagnostic potential, various methods for synthesizing benzofuran and benzofuropyridine analogues have been developed in the past two decades (Scheme 1). In 2014, Hua Zheng and colleagues synthesized analogues of 3-methyl-1-phenylbenzofuro[2,3-c]pyridine using a multicomponent one-pot method with 2-bromoacetophenone, a 2′-hydroxyphenyl-functionalized α,β-unsaturated ketone, and ammonium acetate.6 In 2014, Guodong Yin reported a one-pot method for the preparation of 1,3-diphenylbenzofuro[2,3-c]pyridine, using starting materials 2′-hydroxychalcones, α-bromoketones, and ammonium acetate.7 (Scheme 1a) In 2020, Jiuxi Chen described a palladium-catalyzed direct C–H addition and cyclization of 2-(cyanomethoxy)chalcones with thiophenes, resulting in analogues of 3-aryl-1-(thiophe-2-yl)benzofuro[2,3-c]pyridines.8 (Scheme 1b) In 2022, G. Clarkson and S. Roesner introduced a multi-metal catalyzed one-pot process for synthesizing benzofuropyridines and dibenzofurans using fluoropyridines and 2-bromophenyl acetates9 (Scheme 1c).In 2023, Nirmal K. Rana demonstrated a stereoselective synthesis of trans-2,3-dihydrobenzofurans with reusable Merrifield resin-anchored pyridinium ylide and ortho-hydroxy chalcones. Additionally, the synthesized trans-2,3-dihydrobenzofuran was subjected to cyclization and aromatization with ammonium acetate to obtain 1,3-diphenylbenzofuro[2,3-c]pyridine.10 In 2022, Jiuxi Chen published a study detailing the synthesis of the 1,3-diarylbenzofuro[2,3-c]pyridine framework through a Pd(II)-catalyzed reaction between 2-(cyanomethoxy)chalcones and aryl boronic acids.11 In 2017, Hai-Lei Cui reported a metal-free one-pot synthesis of benzofurans from ynones and quinones via an aza-Michael/Michael/annulation sequence.12 (Scheme 1b) In 2025, Nirmal K. Rana developed a chiral thiourea-catalyzed asymmetric synthesis of trans-2,3-dihydrobenzofurans through cascade Michael addition and oxa-substitution, using an in situ pyridinium ylide and inorganic base.13 The existing methods for preparing benzofuropyridines involve multiple reaction steps and specific conditions. The previous techniques in literature employed harsh conditions and utilized expensive starting materials, reagents, and organometallic catalysts. Most studies focus on synthesizing benzofuro[3,2-b]pyridines, with limited research on benzofuro[2,3-c]pyridine scaffolds, which restricts their functionalization, industrial production, and applications. A straightforward method for preparing benzofuro[2,3-c]pyridine compounds using readily accessible starting materials, non-metallic reagents, and catalysts remains necessary. In this article, we report a novel protocol for constructing 2-benzoylbenzofurans and benzofuro[2,3-c]pyridines through cascade reactions involving 2′-hydroxyethyl cinnamate, phenacyl bromides, and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (Scheme 1d). The main contribution of this work is the identification of DDQ as a cyanation agent for the synthesis of novel ethyl 1-phenylbenzofuro[2,3-c]pyridine-4-carboxylate compounds from 2-benzoylbenzofurans.
Results and discussion
Recently, our research group has reported the synthesis of various fused multi-heterocyclic scaffolds.14–19 Owing to our research interest in this area, we envisioned the synthesis of 2,3-disubstituted benzofuran from 2′-hydroxyethyl cinnamate and phenacyl bromide. Initially, to identify the mild and efficient reaction condition, we selected 2′-hydroxyethyl cinnamate (1a), phenacylbromide (2a), and 1.2 equiv. of K2CO3 as a base. The reaction was conducted in acetonitrile solvent at room temperature (Table 1). After four hours of continuous stirring, a significant formation of O-alkylated intermediate I (see mechanism) with unreacted starting materials was detected. To improve the desired product formation, 2.2 equiv. of K2CO3 with acetonitrile was used for six hours (Table 1, entry 5), and to our delight, 62% yield of ethyl 2-(2-benzoyl-2,3-dihydrobenzofuran-3-yl)acetate (3a) was obtained. To further improve the yield of 3a, various solvents, including EtOH, acetone, DMF, CHCl3, and DCM, were screened for a reaction duration of 6 hours (Table 1, entries 6–10). We observed that the solvents have a considerable effect on the yield outcome of 3a. Among the tested solvents, DMF yielded the cleanest reaction, resulting in a 92% yield of 3a. Additionally, a range of inorganic and organic bases was evaluated to optimize the yield of 3a. Replacing K2CO3 with weaker inorganic bases such as NaHCO3, KHCO3, and Na2CO3 resulted in incompatibility under the examined conditions (Table 1, entries 1–3). However, use of 1.2 equiv. of base Na2CO3 and K2CO3 in the solvent acetonitrile led to incomplete conversion to intermediate I. In contrast, using a strong base, KOH, led to a 78% yield of 3a in DMF solvent (Table 1, entry 11). Organic bases such as piperidine, triethylamine (TEA), and diisopropylethylamine (DIPEA) were screened in DMF for 6 hours (Table 1, entries 12–14). Piperidine did not facilitate the transformation; however, TEA and DIPEA were compatible, albeit producing lower yields of 3a. It is important to emphasize that yields of 3a are suboptimal when less than two equivalents of base are employed in the reaction.| Entry | Base (equiv.) | Solvent | Time (h) | 3a-Yield (%) |
|---|---|---|---|---|
a General reaction conditions: 2′-hydroxy ethyl cinnamate 1a (1.04 mmol), phenacyl bromide 2a (1.04 mmol), base (equivalents) in solvents (2–3 mL) at room temperature for the mentioned time (hours).b The dr 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was determined by 1H NMR analysis of the crude reaction mixture. 1 was determined by 1H NMR analysis of the crude reaction mixture. |
||||
| 1 | NaHCO3 (1.2) | CH3CN | 4 | — |
| 2 | KHCO3 (1.2) | CH3CN | 4 | — |
| 3 | Na2CO3 (1.2) | CH3CN | 4 | — |
| 4 | K2CO3 (1.2) | CH3CN | 4 | — |
| 5 | K2CO3 (2.2) | CH3CN | 6 | 62 |
| 6 | K2CO3 (2.2) | EtOH | 6 | 52 |
| 7 | K2CO3 (2.2) | Acetone | 6 | 48 |
| 8 | K2CO3(2.2) | DMF | 6 | 92b |
| 9 | K2CO3 (2.2) | CHCl3 | 6 | 60 |
| 10 | K2CO3 (2.2) | DCM | 6 | 54 |
| 11 | KOH (2.2) | DMF | 6 | 78 |
| 12 | TEA (2.2) | DMF | 6 | 24 |
| 13 | Piperidine (2.2) | DMF | 6 | — |
| 14 | DIPEA (2.2) | DMF | 6 | 86 |
All the derivatives of 3 were synthesized with the optimized reaction conditions; 1 and 2 (1 equiv.), 2.2 equiv. of K2CO3, and DMF at room temperature for 6 hours (General procedure B, SI). The dr for all entries was determined by 1H NMR analysis of the crude reaction mixture (Fig. S141). Para-halogen substituted phenacyl bromides such as –F (2b), –Cl (2c), –Br (2d) reacted smoothly with 1a and corresponding products 3b, 3c, and 3d were obtained in good yield and excellent diastereoselectivities (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
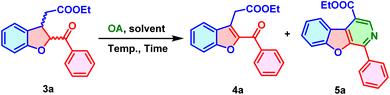
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Scheme 2). The relative configurations of all the derivatives of 3 were assigned by analogy according to the literature.10 In the cases of the 4′-cyano and 3′-nitro derivatives, DIPEA was found to be the most suitable base among the tested organic and inorganic bases (K2CO3, Cs2CO3, TEA, piperidine, and DIPEA). Through a clean and efficient reaction, it enabled complete conversion to the respective derivatives 3h and 3j. Furthermore, the oxidation of compound 3a was envisioned to produce ethyl 2-(2-benzoylbenzofuran-3-yl)acetate (4a) using an oxidizing agent (OA), which is readily accessible, environmentally friendly, and easy to handle. First, Inorganic OA were selected to examine the reaction conditions (Table 2, entries 1–4). The reaction consisted of 3a with two equivalents of potassium permanganate (KMnO4) in ethanol solvent, under reflux conditions for a duration of 2 to 6 hours (Table 2, entries 1 and 2). However, the reaction showed no signs of progress. The investigation was expanded to assess alternative inorganic oxidizing agents, specifically manganese dioxide (MnO2) and chromium dioxide (CrO2), in the solvents acetonitrile and tetrahydrofuran (THF), respectively, for 6 hours under reflux conditions (Table 2, entries 3 and 4). Unfortunately, no progress was observed in the reaction towards the formation of 4a. Later, the focus was shifted to organic OA. An attempt was made to dehydrogenate 3a using the benzoquinone-based organic dehydrogenative agent DDQ in the solvent 1,4-dioxane under reflux conditions for 24 hours (Table 2, entry 5). Among the inorganic and organic OAs tested, DDQ was the only effective reagent for this transformation, leading to the successful formation of 4a with a yield of 72% (Table 2).
1) (Scheme 2). The relative configurations of all the derivatives of 3 were assigned by analogy according to the literature.10 In the cases of the 4′-cyano and 3′-nitro derivatives, DIPEA was found to be the most suitable base among the tested organic and inorganic bases (K2CO3, Cs2CO3, TEA, piperidine, and DIPEA). Through a clean and efficient reaction, it enabled complete conversion to the respective derivatives 3h and 3j. Furthermore, the oxidation of compound 3a was envisioned to produce ethyl 2-(2-benzoylbenzofuran-3-yl)acetate (4a) using an oxidizing agent (OA), which is readily accessible, environmentally friendly, and easy to handle. First, Inorganic OA were selected to examine the reaction conditions (Table 2, entries 1–4). The reaction consisted of 3a with two equivalents of potassium permanganate (KMnO4) in ethanol solvent, under reflux conditions for a duration of 2 to 6 hours (Table 2, entries 1 and 2). However, the reaction showed no signs of progress. The investigation was expanded to assess alternative inorganic oxidizing agents, specifically manganese dioxide (MnO2) and chromium dioxide (CrO2), in the solvents acetonitrile and tetrahydrofuran (THF), respectively, for 6 hours under reflux conditions (Table 2, entries 3 and 4). Unfortunately, no progress was observed in the reaction towards the formation of 4a. Later, the focus was shifted to organic OA. An attempt was made to dehydrogenate 3a using the benzoquinone-based organic dehydrogenative agent DDQ in the solvent 1,4-dioxane under reflux conditions for 24 hours (Table 2, entry 5). Among the inorganic and organic OAs tested, DDQ was the only effective reagent for this transformation, leading to the successful formation of 4a with a yield of 72% (Table 2).
| Entry | OA (equiv.) | Solvent | Temp. | Time (h) | Yieldb (%) | 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a 5a |
|
|---|---|---|---|---|---|---|---|
| 4a | 5a | ||||||
| a Reaction conditions: ethyl 2-(2-benzoyl-2,3-dihydrobenzofuran-3-yl) acetate (0.65 mmol) 3a, oxidizing agents (equiv.) in solvents (4–5 mL) at the mentioned temp of the used solvent for the mentioned time (hours).b Isolated yield of 4a and 5a via chromatography. | |||||||
| 1 | KMnO4 (2) | EtOH | Reflux | 2 | — | — | — |
| 2 | KMnO4 (2) | EtOH | Reflux | 6 | — | — | — |
| 3 | MnO2 (2) | CH3CN | Reflux | 6 | — | — | — |
| 4 | CrO2 (2) | THF | Reflux | 6 | — | — | — |
| 5 | DDQ (2) | 1,4-Dioxane | Reflux | 24 | 72 | 00 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0 0 |
| 6 | DDQ (2) | DMF | 120 °C | 24 | 60 | 00 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 7 | DDQ (2.5) | DMF | 150 °C | 24 | 60 | 22 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
To develop a one-pot methodology for preparing 4a, a reaction of 1a with 2a in presence of K2CO3 was attempted in solvent DMF at room temperature for 6 hours until complete conversion to 3a was observed followed by the addition of 2 equiv. of DDQ in the same pot and allowed the reaction to stir at 120 °C for the next 24 hours (Table 2, entry 6). Fortunately, the 60% yield of 4a was achieved. To enhance the yield of 4a, the number of DDQ equivalents increased to 2.5, and the temperature was raised to 150 °C for 24 hours (General procedure D, SI). During this reaction, the formation of product 4a was observed along with one byproduct (later confirmed as 5a) in approximately a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. To elucidate the complete structure of the byproduct, 1D and 2D NMR spectroscopy were utilized for the derivative 5r (Fig. 1).
1 ratio. To elucidate the complete structure of the byproduct, 1D and 2D NMR spectroscopy were utilized for the derivative 5r (Fig. 1).
 | ||
| Fig. 1 Structure of 5r with respective 1H and 13CNMR signal assignments with 1H–13C correlation via 2D-NMR analysis. | ||
Upon complete 2D-NMR analysis, we confirmed the formation of ethyl 1-phenylbenzofuro[2,3-c]pyridine-4-carboxylate (5r), where DDQ possibly donated a CN unit to ethyl 2-(2-benzoylbenzofuran-3-yl)acetate (4r), and subsequently, cyclization led to the formation of a benzofuran-fused pyridine ring (Fig. S140).
To our knowledge, the formation of a pyridine ring using DDQ is unprecedented in the literature. This unique transformation motivated us to examine further the scope of the reaction for the generality towards the synthesis of novel benzofurans and benzofuropyridines (Scheme 3). Utilizing established conditions, we systematically evaluated the response with a range of functionalized phenacyl bromides and cinnamic esters using a one-pot methodology (General Procedure D-SI).
As shown in Scheme 3, we first explored the scope with respect to the phenyl group substitutions on the meta and para positions of benzofurans (4a–4k) and benzofuropyridines (5a–5k). With electron-withdrawing substituents (EWGs) such as e.g. F, Cl, Br, CN present at the para positions of the phenyl ring, the target compounds (4b–d, 4h) were obtained with good to moderate yield (45–70%). Similarly, electron-donating groups (EDGs) such as CH3, Ph, and OCH3 on the para position of the phenyl ring were also found to be compatible under standard conditions, affording the corresponding benzofurans (4e–g) with good yields (55–62%). The meta-substituted benzofurans 4i and 4j bearing EDG (–OCH3) and EWG (–NO2) also displayed good yield (67%) under the standard procedure, whereas meta and para-dihalogen substrate was also compatible in the established condition with a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of yield of benzofuran (4k-49%) and corresponding benzofuropyridine (5k-33%). Substrate containing both EWGs, such as halogens –F (5b), Br (5d), and EDGs -methyl (5e), methoxy (5f), and phenyl (5g) on the para position of the phenyl ring, readily underwent cyanation and subsequent pyridine ring formation except the substrate with a cyano group (5h). Moreover, the meta-substituted substrate was found to be weakly reactive towards the pyridine ring transformation, resulting in a negligible yield of 5i and 5j. Furthermore, 5′-bromo substituted 2′-hydroxyethyl cinnamate was evaluated with different phenacyl bromides bearing meta- and para-substituted phenyl groups, and as anticipated, para-substituted substrates with both EWGs and EDGs exhibited good compatibility towards both the transformations (4l–s, and 5l–r), but the meta-substituted substrates were found to be completely incompatible. The compatibility of the reaction with halogen-containing substrates also enables potential for subsequent metal-mediated late-stage modifications of the scaffold.
3 ratio of yield of benzofuran (4k-49%) and corresponding benzofuropyridine (5k-33%). Substrate containing both EWGs, such as halogens –F (5b), Br (5d), and EDGs -methyl (5e), methoxy (5f), and phenyl (5g) on the para position of the phenyl ring, readily underwent cyanation and subsequent pyridine ring formation except the substrate with a cyano group (5h). Moreover, the meta-substituted substrate was found to be weakly reactive towards the pyridine ring transformation, resulting in a negligible yield of 5i and 5j. Furthermore, 5′-bromo substituted 2′-hydroxyethyl cinnamate was evaluated with different phenacyl bromides bearing meta- and para-substituted phenyl groups, and as anticipated, para-substituted substrates with both EWGs and EDGs exhibited good compatibility towards both the transformations (4l–s, and 5l–r), but the meta-substituted substrates were found to be completely incompatible. The compatibility of the reaction with halogen-containing substrates also enables potential for subsequent metal-mediated late-stage modifications of the scaffold.
Limitations
Furthermore, the scope of the methodology was tested with aliphatic bromoacetyl derivatives, such as 2-bromoethylacetate (6a), ethyl 3-bromopyruvate (6b), and bromoacetonitrile (6c) (Scheme 4). With the established reaction conditions, experiments were conducted with an equimolar amount of 2′-hydroxyethyl cinnamate (1a) and aliphatic 2-bromoacetyl derivatives (6a/6b/6c) and 2.2 equivalents of base K2CO3 in solvent DMF for 6 hours at room temperature. Unfortunately, the uncyclized O-alkylated intermediate 7a was observed mainly in the case of 6a, and 7c was observed mainly in the case of 6c. In the experiment containing 1a and 6b, the reaction did not proceed, and unreacted 1a was observed with no other product formation. Considering the possible energy requirement, the same reaction mixtures were allowed to stir at 100 °C for 12 hours. Still, no substantial progress in reaction was observed from the O-alkylated intermediate (7a/7c) to the anticipated dihydrobenzofuran products (8a/8c). In the reaction of 1a and 6a, 7a was converted to 8a after heating at 100 °C for 12 hours in a traceable amount. Hence, the methodology is currently limited to 2-bromoacetophenones, with the optimised reaction conditions.Control experiments
A mechanistic study was conducted to gain a deeper understanding of the chemistry involved in this transformation, and several control experiments were performed using established reaction conditions. Three reactions of compound 3f were carried out using solvents: DMF (scheme 5a), 1,4-dioxane (scheme 5b), and under neat conditions (scheme 5c) in parallel. This approach was taken to verify that DDQ is the actual donor of the cyanide (CN) unit, rather than DMF. The formation of products 5f alongside 4f in all three reactions indicates that the CN unit required for pyridine ring formation comes exclusively from DDQ. At the same time, DMF merely acts as a solvent without participating in the transformation. Next, reactions of 3f were conducted in parallel with four, five, and six equivalents of DDQ in DMF at reflux for 24 hours. The ratio of the products 4f and 5f was determined by analytical HPLC, which involved calculating the percentage of the area under the curve (AUC) for both products in the HPLC chromatogram. As the amount of DDQ increased, the production of 5f was amplified, while the amount of 4f decreased in the reaction mixtures (Fig. 2). Additionally, other quinone-based oxidizing agents, such as chloranil (scheme 5d) and 1,4-benzoquinone (scheme 5e), were evaluated. Since chloranil lacks the cyano (CN) group present in DDQ, this structural difference was clearly reflected in the results. Specifically, chloranil led to a 57% formation of compound 4f, with no production of compound 5f.The reaction of 3f with 1,4-benzoquinone failed to form 4f and 5f. It is well known that DDQ oxidizes its substrate via a radical mechanism. In this context, an experiment was conducted under standard reaction conditions with the addition of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), a free radical scavenger (Scheme 5f). The results clearly demonstrate a halt in 4f–5f production, highlighting the likely involvement of a free radical pathway. One possibility is that the formation of compound 4f with DDQ was interrupted by the presence of TEMPO, which prevented its conversion into 5f. To investigate this, a reaction involving isolated 4f and DDQ was conducted under standard conditions (Scheme 5g), and the anticipated formation of 5f was observed.
A different synthetic method was then used to prepare 5f from 3f, ensuring the formation of the 5f and eliminating any potential ambiguity related to its structure (Scheme 6). In this approach, 3f was treated with N,N-dimethylformamide dimethyl acetal (DMFDMA) (5 equiv.) as a single carbon synthon and ammonium acetate (3 equiv.) as a Nitrogen atom source. To our delight, formation of 5f was observed and characterized by NMR and HRMS and compared with the 5f synthesized with the present methodology (Fig. S142).
 | ||
| Scheme 6 Synthesis of ethyl 1-([1,1′-biphenyl]-4-yl)benzofuro[2,3-c]pyridine-4-carboxylate (5f) using 3f, DMFDMA and ammonium acetate. | ||
Given the significant discoveries made, the reaction conditions for synthesizing 5f from 3f were refined to enhance efficiency and effectiveness (Table 3). Various solvents, including dioxane, THF, DMSO, CH3CN, and EtOH, were screened with 2.5 equivalents of DDQ at a constant temperature of 150 °C and a reaction time of 24 hours (Table 3, entries 2–6). THF, CH3CN, and EtOH did not facilitate synthesis, whereas dioxane and DMSO favored 4f over 5f formation. We investigated the effect of varying equivalents of DDQ on the formation of 5f (Table 3, entries 7–10). An increase in DDQ equivalents from 2.5 to 4, 5, and 6 resulted in a gradual improvement in the yield of 5f. However, when the DDQ equivalent was increased to 7, a decline in the yield of 5f was observed, possibly because of multiple side reactions. Consequently, we determined that the optimal conditions for synthesizing 5f and its derivatives are using 6 equivalents of DDQ in DMF solvent at 150 °C for 24 hours. Under optimized reaction conditions, one-pot gram-scale synthesis was conducted to demonstrate the feasibility of this methodology. The reaction proceeded effectively, yielding the desired products 4g (77%) and 5d (68%) in comparable yields and within the same timeframe as observed on the sub-millimolar scale (Scheme SI-5 and 6).
| Entry | DDQ equiv. | Solvent | Temp | Yieldb (%) | 4f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5f 5f |
|
|---|---|---|---|---|---|---|
| 4f | 5f | |||||
| a Reaction conditions: 3f (0.5 mmol), DDQ (equiv.) in solvent (5 mL) at 150 °C for 24 hours.b Isolated yield of 4f and 5f via chromatography. | ||||||
| 1 | 2.5 | DMF | 150 °C | 41 | 49 | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 2.5 | 1,4-Dioxane | 150 °C | 60 | 08 | 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 2.5 | THF | 150 °C | — | — | — |
| 4 | 2.5 | DMSO | 150 °C | 17 | 00 | — |
| 5 | 2.5 | CH3CN | 150 °C | — | — | — |
| 6 | 2.5 | EtOH | 150 °C | — | — | — |
| 7 | 4 | DMF | 150 °C | 38 | 55 | 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 5 | DMF | 150 °C | 22 | 64 | 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 6 | DMF | 150 °C | 00 | 72 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1 |
| 10 | 7 | DMF | 150 °C | 00 | 60 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Based on the above experimental results, we suggest a reaction mechanism for synthesizing 2-benzoyl benzofurans and benzofuro[2,3-c]pyridine-4-carboxylate analogues from 1, 2, and DDQ.
Plausible mechanism
The transformations demonstrated in the present work regarding the synthesis of bi- and tricyclic fused ring systems from 2′-hydroxyethylcinnamate (1) and phenacyl bromide (2) follow the sequence of O-alkylation of 1, forming the intermediate I, and Michael addition to generate 2,3-dihydrobenzofuran compound (3) in the presence of a base. Further DDQ-mediated dehydrogenation of 3 resulted in benzofuran (4) and an unusual CN-transfer/1,2-addition and cyclization to form compound 5 (Fig. 3). | ||
| Fig. 3 Plausible mechanism of DDQ-mediated synthesis of bi- and tricyclic ring system-benzofuran and benzofuro[2,3-c]pyridine-based compounds from 2-hydroxy ethyl cinnamate and phenacyl bromide. | ||
To understand the mechanism behind the CN-unit donation/addition and cyclization promoted by DDQ, an additional experiment for intermediate trapping via ESI-HRMS20 reaction monitoring was conducted. A DDQ-mediated single electron transfer (SET) and 1,2-addition based mechanistic pathway is proposed as per the observations and interpretations of ESI-HRMS results (Fig. 3). An ESI-HRMS spectrum of the unprocessed reaction mixture of 3f with DDQ was recorded at regular intervals of 2 hours till 24 hours and analyzed simultaneously to trap the intermediates formed in situ. The HRMS spectra displayed the peak at m/z 387.1547 (cald.(M + H)+ 387.1596) for the unreacted starting material 3f and at m/z 266.1227 (cald.(M + H)+ 226.9415) for unreacted DDQ in the reaction. The other two intense peaks at m/z 385.1471 (cald.(M + H)+ 385.1440) for the 4f and at m/z 394.1443 (cald.(M + H)+ 394.1443) for the 5f were observed. A peak corresponding to a potential mono-nitrile intermediate IV was observed at m/z 410.1432 (calculated (M + H)+: 410.1392). The DDQ-promoted formation of benzofuro[2,3-c]pyridine (5) involves, SET-based nitrile transfer followed by 1,2-addition to DDQ to activate the imine intermediate V, which undergoes cyclization to form a new pyran ring (VI). Further, the Nu− attack of the N atom opens the pyran ring and initiates the construction of the pyridine ring (VII). Sequential dehydration of VII, followed by proton transfer and elimination of DDQH2, yielded the final product, a 1-phenylbenzofuro[2,3-c]pyridine-4-carboxylate-based compound 5 (Fig. 3).
Conclusion
In summary, an efficient process has been developed for preparing a novel series of 2-benzoylbenzofurans and benzofuro[2,3-c]pyridines, using substituted cinnamic esters, phenacyl bromides and DDQ. The synthesis of 2-benzoyl benzofurans from 2′-hydroxyethyl cinnamate involves O-alkylation, Michael addition and an oxidation reaction. At the same time, the construction of 1-phenylbenzofuro[2,3-c]pyridine-4-carboxylate molecules involves DDQ-mediated single-electron transfer-based nitrile (CN) transfer to the benzylic CH2, followed by 1,2 addition of DDQ. Notably, this newly developed protocol utilizes readily accessible starting materials and straightforward reaction conditions, eliminating the need for metal-containing reagents or catalysts. This method for synthesizing fused bicyclic and tricyclic compounds holds excellent potential for developing green and efficient processes for the industrial production of pharmacologically and medicinally essential candidates.Author contributions
S. M. S: conceptualization, methodology, synthesis, characterization, data curation, formal analysis, investigation, writing – original draft and editing, H. R. S.: synthesis, characterization. R. K.: synthesis, characterization. S.C.: conceptualization, methodology, characterization, investigation, writing – review and editing, supervision.Conflicts of interest
The authors declare no competing financial interest.Data availability
The data underlying this study are available in the published article and its supplementary information (SI). Supplementary information: material and methods, experimental details, characterisation data, copies of 1H, 13C{1H}, 19F{1H} NMR, HRMS spectra of all compounds, mechanistic study details, and additional figures as mentioned in the text. See DOI: https://doi.org/10.1039/d5ra09530a.Acknowledgements
The authors thank Dr Shubhini A Saraf, Director, NIPER Raebareli, and Dr USN Murty, former Director, NIPER Raebareli, for supporting and encouraging this research work. The authors thank the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, and the Government of India for providing a fellowship. NIPER-R/Communication/367.References
- B. Voigt, L. Meijer, O. Lozach, C. Schächtele, F. Totzke and A. Hilgeroth, Novel CDK inhibition profiles of structurally varied 1-aza-9-oxafluorenes, Bioorg. Med. Chem. Lett., 2005, 15(3), 823–825 CrossRef CAS PubMed.
- R. Menegatti, G. M. Silva, G. Zapata-Sudo, J. M. Raimundo, R. T. Sudo, E. J. Barreiro and C. A. Fraga, Design, synthesis, and pharmacological evaluation of new neuroactive pyrazolo [3, 4-b] pyrrolo [3, 4-d] pyridine derivatives with in vivo hypnotic and analgesic profile, Bioorg. Med. Chem., 2006, 14(3), 632–640 CrossRef CAS PubMed.
- M. Anzini, A. Cappelli, S. Vomero, G. Giorgi, T. Langer, M. Hamon, N. Merahi, B. M. Emerit, A. Cagnotto and M. Skorupska, Novel, potent, and selective 5-HT3 receptor antagonists based on the arylpiperazine skeleton: synthesis, structure, biological activity, and comparative molecular field analysis studies, J. Med. Chem., 1995, 38(14), 2692–2704 CrossRef CAS.
- B. Xia, W. Ma, B. Zheng, X. Zhang and B. Fan, Quantitative structure–activity relationship studies of a series of non-benzodiazepine structural ligands binding to benzodiazepine receptor, Eur. J. Med. Chem., 2008, 43(7), 1489–1498 Search PubMed.
- D. G. Wishka, S. C. Reitz, D. W. Piotrowski and V. E. Groppi Jr, Quinuclidines-substituted-multi-cyclic-heteroaryls for the Treatment of Disease, Google Patents, 2002 Search PubMed.
- J. Hu, Z. Deng, X. Zhang, F. Zhang and H. Zheng, Synthesis of benzofuro [2, 3-c] pyridines via a one-pot three-component reaction, Org. Biomol. Chem., 2014, 12(27), 4885–4889 Search PubMed.
- Y. Rao, Z. Li and G. Yin, Clean and efficient assembly of functionalized benzofuro [2, 3-c] pyridines via metal-free one-pot domino reactions, Green Chem., 2014, 16(4), 2213–2218 Search PubMed.
- W. Xiong, Z. Chen, Y. Shao, R. Li, K. Hu and J. Chen, The synthesis of fluorescent benzofuro [2, 3-c] pyridines via palladium-catalyzed heteroaromatic C–H addition and sequential tandem cyclization, Org. Chem. Front., 2020, 7(5), 756–762 RSC.
- G. J. Clarkson and S. Roesner, Synthesis of benzofuropyridines and dibenzofurans by a metalation/negishi cross-coupling/SNAr reaction sequence, J. Org. Chem., 2022, 88(1), 684–689 Search PubMed.
- A. Kumari, A. Jain, K. Shukla, R. Patra and N. K. Rana, A reusable polymer anchored pyridine mediated formal [4+ 1] annulation reaction for the diastereoselective synthesis of 2, 3-dihydrobenzofurans, Org. Biomol. Chem., 2023, 21(27), 5542–5546 RSC.
- W. Xiong, K. Hu, Y. Lei, Q. Zhen, Z. Zhao, Y. Shao, R. Li, Y. Zhang and J. Chen, Palladium-catalyzed cascade reactions of 2-(cyanomethoxy) chalcones with arylboronic acids: selective synthesis of emissive benzofuro [2, 3-c] pyridines, Org. Lett., 2019, 22(4), 1239–1243 Search PubMed.
- H.-L. Cui, H.-Q. Deng and J.-J. Lei, Metal-free one-pot synthesis of benzofurans with ynones and quinones through aza-Michael/Michael/annulation sequence, Tetrahedron, 2017, 73(52), 7282–7290 CrossRef CAS.
- A. Kumari, A. Jain and N. K. Rana, Chiral Thiourea Catalyzed Enantioselective Cascade Synthesis of 2,3-Dihydrobenzofurans, Eur. J. Org Chem., 2025, 28(12), 1–6 CrossRef.
- K. Lakshmikanth, R. Katiyar, S. T. Dorai, P. Tiwari, T. K. Sagar and S. Chandrashekharappa, Efficient One-Pot Multicomponent Approach to 3-Phenylpyrrolo [1, 2-a] Pyrazine and Novel 3-Phenylpyrazino [1, 2-a] Indole in Aqueous Medium, ChemistrySelect, 2024, 9(13), 1–8 Search PubMed.
- S. T. Dorai, K. Lakshmikanth, P. Tiwari, S. M. Saini and S. Chandrashekharappa, One-pot construction of novel trifluoromethyl dihydro-imidazo [1, 2-a] pyridine: A greener approach, Tetrahedron, 2023, 148, 133691 CrossRef CAS.
- G. Sumanth, S. M. Saini, K. Lakshmikanth, G. S. Mangubhai, K. Shivaprasad and S. Chandrashekharappa, Microwave-assisted improved regioselective synthesis of 3-benzoyl indolizine derivatives, J. Mol. Struct., 2023, 1286, 135561 CrossRef CAS.
- K. Lakshmikanth, S. M. Saini, S. T. Dorai and S. Chandrashekharappa, Tandem-Michael-cyclization cascade to make pyridines: Use of electron-deficient acetylenes for the synthesis of indolizines in aqueous media, Tetrahedron, 2023, 142, 133516 CrossRef CAS.
- P. Mundhe, N. Bhanwala, S. M. Saini, G. Sumanth, K. Shivaprasad, S. U. Shende, K. Reddy and S. Chandrashekharappa, Domino synthesis of novel 3-alkenyl benzofuran derivatives-base mediated condensation cascade reaction, Tetrahedron, 2023, 132, 133265 CrossRef CAS.
- G. Sumanth, K. Lakshmikanth, S. M. Saini, P. Mundhe, K. Shivaprasad and S. Chandrashekharappa, Phenyl pyrrolo [1, 2-a] quinolines-finding of a key by-product during quinolinium salt preparation, J. Mol. Struct., 2023, 1273, 134350 CrossRef CAS.
- I. V. Nechaev and G. V. Cherkaev, 3-Thioxo-3H-indolizinium-1-olates and Related Pseudo-Cross-Conjugated Heterocyclic Mesomeric Betaines: Synthesis and Spectral Features, J. Org. Chem., 2024, 89(16), 11215–11232 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |