 Open Access Article
Open Access ArticleEffects of graphene oxide incorporation via non-covalent interfacial interactions on the properties of small intestinal submucosa
Mianshu Hu a,
Xiuyun Chuan*a,
Yiting Wang*b,
Wenyue Chengc,
Yun Yan
a,
Xiuyun Chuan*a,
Yiting Wang*b,
Wenyue Chengc,
Yun Yan d,
Wenbin Zhang
d,
Wenbin Zhang d,
Kun Zhangb,
Jinsong Hanb and
Jian Zhangc
d,
Kun Zhangb,
Jinsong Hanb and
Jian Zhangc
aKey Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sceiences, Peking University, Beijing 100871, China. E-mail: xychuan@pku.edu.cn; bysywangyiting@126.com
bPeking University Third Hospital, Beijing 100191, China
cNaval Medical University, Shanghai Changzheng Hospital, Shanghai 200003, China
dCollege of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
First published on 19th January 2026
Abstract
To investigate the modifying effect of graphene oxide (GO) on small intestinal submucosa (SIS), GO-SIS biocomposite films were fabricated via a non-covalent coating strategy. Structural analyses confirmed the non-covalent interactions between GO and SIS collagen fibers and the preservation of SIS's native fibrous structure. The GO-SIS biocomposite film showed significantly improved hydrophilicity (contact angle: 71.3 ± 1.0°, p < 0.001; water absorption: 159.00 ± 5.60%, p < 0.01) compared to the SIS film. It also exhibited superior mechanical properties under both dry and wet conditions, with significantly higher tensile strength (dry: 24.46 ± 0.99 MPa; wet: 10.16 ± 0.37 MPa) and elongation at break (dry: 11.41 ± 0.55%; wet: 21.26 ± 0.65%) than the SIS film (p < 0.001 for all comparisons). After in vitro degradation, the GO-SIS biocomposite film showed better morphological stability compared to the SIS film. At 4, 8, 16, 24, 48, and 72 hours, the in vitro degradation of the GO-SIS biocomposite film was significantly slower than that of the SIS film. Furthermore, at 4, 8, 16, and 24 hours, the tensile strength and elongation at break of the degraded GO-SIS biocomposite film were significantly higher than those of the degraded SIS film. Biocompatibility assessment indicated no impact on L929 fibroblast viability or proliferation, along with favorable hemocompatibility. Collectively, the non-covalent incorporation of GO effectively enhances the hydrophilicity and mechanical performance of the GO-SIS biocomposite film and slows down its in vitro degradation, offering a promising strategy for the design of advanced tissue repair materials.
1. Introduction
Small intestinal submucosa (SIS) is an excellent natural extracellular matrix (ECM) material with a porous three-dimensional (3D) structure, processed via decellularization.1–3 SIS is primarily composed of type I and III collagen fibers, with small amounts of type IV and V collagen, and preserves various bioactive factors.3–5 Owing to its collagen fiber structure, excellent biocompatibility, low immunogenicity, and resorbability, SIS has attracted widespread interest in the field of tissue repair.1,6,7 However, its clinical application is limited by rapid degradation and poor long-term mechanical support.6,8 Therefore, modifications of SIS are desired to address these shortcomings.4,8–11 Cao et al.8 combined chitosan (CS)/elastin (ES) electrospun nanofibers with SIS, improving its biodegradability and antimicrobial activity. Tang et al.9 designed a composite patch by in situ solidification of viscous polyvinyl alcohol (PVA) solution on the SIS surface, which provided dynamic mechanical support.Graphene oxide (GO) has attracted considerable attention in the biomedical field. As an important derivative of graphene, GO retains the large planar structure of graphene, while carboxyl (–COOH) groups are introduced at the edges, and hydroxyl (–OH) and epoxy (C–O–C) groups are incorporated on the basal plane.12,13 Due to its excellent properties, such as mechanical performance,14,15 electrochemical properties,16–18 optical performance,19,20 amphiphilicity,21 antimicrobial and antiviral activities,22,23 biocompatibility,24,25 and biodegradability,26,27 many researchers have considered applying GO and its polymers to tissue engineering. Li et al.28 developed a polydopamine-mediated graphene oxide (PGO)-hydroxyapatite (PHA)-alginate/gelatin (AG) composite scaffold, which exhibited excellent electrical conductivity and immunomodulatory ability, facilitating the repair and regeneration of periodontal bone tissue. Heidari et al.29 fabricated poly(ε-caprolactone) (PCL)/gelatin/GO electrospun scaffolds, where the incorporation of GO resulted in outstanding antibacterial properties. Wan et al.30 produced PCL-GO nanofiber membranes via electrospinning, reporting that the addition of GO nanosheets significantly enhanced the mechanical properties and bioactivity of the PCL membrane. In another study, Cifuentes et al.31 prepared a SIS-reduced graphene oxide (rGO) scaffold by mixing dissolved SIS powder with a GO dispersion, followed by freeze-drying and reduction. The results confirmed that the presence of GO or rGO did not compromise the scaffold's excellent biocompatibility, highlighting their potential for tissue engineering applications.
Based on the above, we aim to combine the natural structural advantages of intact SIS with the enhanced functionalities of GO. Therefore, we can avoid using methods such as electrospinning or freeze-drying to mimic the collagen fiber structure of SIS. Moreover, compared to covalent modifications, non-covalent approaches generally avoid harsh chemical reactions, simplify the preparation process, and prevent the introduction of potentially harmful chemical groups.32 It has been reported that non-covalent interfacial interactions (such as electrostatic interactions and hydrogen bonding) exist between GO and collagen.12,33–35 These strong non-covalent interactions not only enable GO to guide the self-assembly of collagen molecules but also enhance the mechanical properties and biocompatibility of GO/collagen composites.34,35
Therefore, we propose introducing GO into the SIS matrix via a coating method based on non-covalent interactions. This approach aims to develop a composite with enhanced hydrophilicity, mechanical properties and slower in vitro degradation, while leveraging and preserving the inherent advantages of SIS.
In this study, GO dispersions were coated onto single-layer SIS films, and GO-SIS biocomposite films were subsequently prepared via vacuum drying. The effects of GO incorporation and the non-covalent interfacial interactions between GO and SIS on the hydrophilicity, mechanical properties, in vitro degradation behavior, and biocompatibility of the composite films were investigated.
2. Experimental
2.1. Materials
Graphene oxide (GO) dispersion (SE3522, solid content 1.0%, D50 ≤ 4 µm) was purchased from Sixth Element (Changzhou) Materials Technology Co., Ltd (Changzhou, China). According to the supplier's datasheet, the GO was synthesized via the Hummers' method,36 and exhibits a high oxidation degree. A single-layer small intestinal submucosa (SIS) film (5 cm × 15 cm) was used as the base material, supplied by Zhuo Ruan Medical Technology Co., Ltd (Suzhou, China) as a decellularized, sterilized, ready-to-use dry film. Phosphate buffer saline (PBS, 0.01 M, pH 7.4) was purchased from Beijing Labgic Technology Co., Ltd (Beijing, China). Collagenase I (C0130) was purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd (Shanghai, China). Hydroxyproline (Hyp) content assay kit (BC0250) was purchased from Beijing Solarbio Science & Technology Co., Ltd (Beijing, China). L929 fibroblasts (iCell-m026) and the recommended culture medium (iCell-m026-001b) were purchased from iCell Bioscience Inc (Shanghai, China). The cell counting kit-8 (CCK-8, HY-K0301) was purchased from MedChemExpress, USA. The live/dead double staining kit (Calcein-AM/PI, BB-4126-2) was obtained from Shanghai BestBio Science & Technology Co., Ltd (Shanghai, China). Fetal bovine serum (FBS, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 099-141) and trypsin-EDTA (25
099-141) and trypsin-EDTA (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200-056) were acquired from Gibco, USA.
200-056) were acquired from Gibco, USA.
2.2. Preparation of GO-SIS biocomposite films
GO-SIS biocomposite films were prepared by using an automatic coating machine (MRX-TM300, Shenzhen Mingruixiang Automation Equipment Co., Ltd, China). The process is schematically illustrated in Fig. 1. Prior to coating, the as-received GO dispersion was diluted with deionized water to achieve a working concentration of 0.5 mg mL−1 and ultrasonicated for 30 minutes. The resulting stable, homogeneous dispersion showed no visible sedimentation for at least 24 h. First, a single-layer SIS film was removed from its sterile packaging and laid flat directly on a glass plate. Then, the 0.5 mg mL−1 GO dispersion was dropped onto the SIS film and spread using a scraper with a gap of 150 µm at a speed of 10 mm s−1. After coating, the GO-SIS biocomposite films were dried in a vacuum drying oven (DZF-6020, Sobo Instrument Equipment Co., Ltd, China) at 25 °C for 8 h. The resulting GO loading on the composite films was approximately 0.05 mg cm−2 (SI, Table S1). Finally, the dried films were cut into desired shapes and sterilized with ethylene oxide for subsequent experiments. | ||
| Fig. 1 Schematic of the fabrication process and non-covalent interfacial interactions in the GO-SIS biocomposite film. | ||
2.3. Structural characterization of GO, SIS and GO-SIS biocomposite films
The thickness of the SIS and GO-SIS biocomposite films was measured using a digital micrometer (DL321025B, Deli, China, ±2 µm error). Measurements were taken at 20 random points on each of five independent films (5 cm × 15 cm), yielding 100 data points from which the average and standard deviation were calculated.
2.4. Performance characterization of SIS and GO-SIS biocomposite films
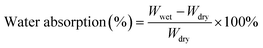 | (1) |
In vitro degradation experiment: the experimental procedure was conducted with reference to established methods for collagen-based biomaterials.8,37 SIS and GO-SIS biocomposite films were cut into 1 cm × 7 cm rectangular strips. The initial dry weight of each film (W1) was measured. The films were then immersed in a 0.05 mg mL−1 type I collagenase solution (prepared in 0.01 M PBS, pH 7.4) at 37 °C with shaking at 200 rpm to simulate physiological collagen degradation. Predetermined time points (4, 8, 16, 24, 48, 72, 96, and 120 h) were selected to cover the complete degradation process. At each time point, the degradation solution was collected and the films were removed, dried, and weighed (W2). For the longer incubation periods (48, 72, 96, and 120 h), the degradation solution was collected daily when the enzyme solution was refreshed. Photographs were taken at each time point. All experiments were performed in triplicate for each time point. The dried films after degradation were retained for subsequent ESEM observation and mechanical properties testing.
The weight loss rates of the films were calculated using the following equation:
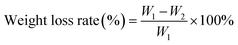 | (2) |
Hyp content determination: the Hyp content in the degradation solutions at each time point was determined according to the instructions of the Hydroxyproline assay kit. The absorbance for hydroxyproline determination (AbsHyp) was measured at 560 nm using a multifunctional microplate reader (EnSpire, PerkinElmer, USA). A standard curve was plotted using gradient concentrations of Hyp standard solution (SI Table S2 and Fig. S1). The Hyp content in the degradation solution at each time point was calculated using the following equation:
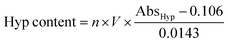 | (3) |
Live/Dead staining assay: L929 fibroblasts (iCell-m026) were seeded in confocal dishes at a density of 5 × 104 cells per dish and cultured overnight under standard conditions (37 °C, 5% CO2). The original culture medium was then aspirated. Cells in the control group were replenished with fresh culture medium (iCell-m026-001b), while those in the experimental groups were treated with the corresponding material extract solutions. All groups were then subjected to further incubation. A staining solution was prepared according to the instructions of the live/dead double staining kit (Calcein-AM/PI). After 1, 3, and 7 days of culture, the medium was removed, and the cells were washed twice with PBS. Subsequently, 200 µL of the staining solution was added to each dish, and the cells were incubated in the dark at 4 °C for 15–20 minutes. The stained cells were observed using a confocal microscope (TCS SP8, Leica, Germany), where live and dead cells were stained green and red, respectively.
Cell proliferation assay: L929 fibroblasts (iCell-m026) were seeded in 96-well plates at a density of 1 × 104 cells per well and cultured overnight under standard conditions (37 °C, 5% CO2). The original medium was then aspirated. Cells in the control group were replenished with 200 µL of fresh culture medium, while cells in the experimental groups were treated with 200 µL of the corresponding material extract solutions. All experimental conditions were performed in triplicate. After 1, 3, and 7 days of incubation, the medium was removed, and the wells were washed three times with PBS. Following this, 100 µL of medium containing 10% CCK-8 reagent was added to each well, and the plates were incubated for 1-2 hours at 37 °C. The absorbance at 450 nm was measured using a microplate reader (Epoch2, BioTek, USA) to assess cell proliferation.
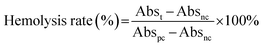 | (4) |
2.5. Statistical analysis
All statistical analyses were performed using Origin 2021 software (OriginLab Corporation, USA). Data were expressed as mean ± standard deviation (SD). Normality (Shapiro–Wilk test) and homogeneity of variances (Levene's test) were verified prior to parametric testing. For data meeting these assumptions, two-group comparisons used the independent samples t-test, and multi-group comparisons used one-way analysis of variance (ANOVA) with Tukey's post-hoc test. Otherwise, the non-parametric Mann–Whitney test (two groups) or Kruskal–Wallis test with Dunn's post-hoc test (multi-group) was applied. Statistical significance was denoted as *p < 0.05, **p < 0.01, and ***p < 0.001.3. Results and discussion
3.1. Structural characterization of GO, SIS and GO-SIS biocomposite films
 | ||
| Fig. 2 Characterization of GO, SIS, and GO-SIS biocomposite films. (a) XRD patterns, (b) FTIR-ATR spectra, and (c) Raman spectra. | ||
The collagen fibers of the SIS film showed a diffraction peak at 2θ = 8.0°, reflecting a lateral spacing of 1.10 nm between the collagen molecules.40,41 In the GO-SIS biocomposite film, the diffraction peak of collagen fibers was observed, suggesting that coating SIS with the 0.5 mg mL−1 GO dispersion did not disrupt the collagen molecular structures. However, no distinct GO diffraction peak was detected in the GO-SIS biocomposite film, which could be attributed to the low concentration and good dispersion of GO.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C–O stretching vibrations, respectively.42–44 These absorption peaks indicated that GO contained functional groups such as hydroxyl (–OH), carboxyl (–COOH), and epoxy (C–O–C) groups.
C, and C–O stretching vibrations, respectively.42–44 These absorption peaks indicated that GO contained functional groups such as hydroxyl (–OH), carboxyl (–COOH), and epoxy (C–O–C) groups.In the FTIR-ATR spectrum of the SIS film, four typical bands of collagen fibers were observed, namely amide A (3297 cm−1), amide I (1632 cm−1), amide II (1543 cm−1), and amide III (1236 cm−1). The amide A band is associated with N–H stretching vibrations.31,34 The amide I band is associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration.31 The amide II band consists of N–H bending vibration and C–N stretching vibration.45 The amide III band is primarily composed of C–N stretching vibration and N–H bending vibration, which is strongly influenced by the constituent amino acids.45,46 Importantly, the amide I, II, and III bands are closely associated with the triple-helical structure of collagen molecules.47 Therefore, these results confirm the presence of amide bonds in SIS collagen fibers. Furthermore, collagen is known to contain hydroxyl (–OH) groups, primarily derived from hydroxyproline and hydroxylysine.48
O stretching vibration.31 The amide II band consists of N–H bending vibration and C–N stretching vibration.45 The amide III band is primarily composed of C–N stretching vibration and N–H bending vibration, which is strongly influenced by the constituent amino acids.45,46 Importantly, the amide I, II, and III bands are closely associated with the triple-helical structure of collagen molecules.47 Therefore, these results confirm the presence of amide bonds in SIS collagen fibers. Furthermore, collagen is known to contain hydroxyl (–OH) groups, primarily derived from hydroxyproline and hydroxylysine.48
The FTIR-ATR spectrum of the GO-SIS biocomposite film was similar to that of the SIS film, indicating that the incorporation of GO did not change the collagen molecular structures. Compared to the SIS film spectrum, the amide A band in the GO-SIS biocomposite film exhibited a slight shift toward lower wavenumber (from 3297 cm−1 to 3295 cm−1). This shift may be attributed to non-covalent interactions between the oxygen-containing functional groups of GO and the functional groups of SIS collagen fibers.33,34
In the Raman spectrum of GO, the D band at 1349 cm−1 and the G band at 1584 cm−1 were observed, with an ID/IG ratio of 0.97 (Table 1). Additionally, the D + G band appeared at 2911 cm−1, confirming the defective and oxidized structure of the GO.31,50,52
The Raman spectrum of the SIS film showed characteristic features at 800–1000 cm−1 (proline and hydroxyproline region), 1237 cm−1 (Amide III band), 1669 cm−1 (Amide I band), and 2800–3100 cm−1 (lipids region), consistent with reported Raman spectra of collagen structure.40,48
For the GO-SIS biocomposite film, the Raman spectrum exhibited both the D and G bands corresponding to GO and the lipids region of SIS collagen fibers, confirming the successful incorporation of GO into the SIS matrix. Compared to the Raman spectrum of GO, the G band in the GO-SIS biocomposite film shifted to a higher wavenumber of 1591 cm−1, and the intensity ratio of the D to G bands (ID/IG) increased to 1.07 (Table 1). These changes were attributed to the non-covalent interfacial interactions between the oxygen-containing functional groups of GO and the functional groups of the SIS collagen fibers,35 which further disordered the local structure of GO nanosheets and thus enhanced the intensity of the D band.
In contrast, the GO-SIS biocomposite film exhibited an overall darker coloration (Fig. 4b), which is attributed to the relatively uniform addition of dark GO nanosheets to the SIS matrix. The average thickness of the GO-SIS biocomposite films was measured to be 16 ± 3 µm (SI Table S4). ESEM images of the GO-SIS biocomposite films (Fig. 4d and f) also displayed a fibrous network structure, confirming that the GO incorporation via the coating process did not disrupt the native collagen fiber structure of SIS. Furthermore, the collagen fibers appeared more densely interconnected compared to those in pure SIS, resulting from the coating of GO nanosheets and the non-covalent interfacial interactions between GO and SIS collagen fibers.
3.2. Performance characterization of SIS and GO-SIS biocomposite films
| Sample | Contact angle (°) | Water absorption (%) |
|---|---|---|
| SIS | 79.9 ± 2.4 | 140.62 ± 7.27 |
| GO-SIS | 71.3 ± 1.0 | 159.00 ± 5.60 |
These results indicate that the non-covalent incorporation of GO significantly enhanced the hydrophilicity of the GO-SIS biocomposite film. This improvement is attributed to the ability of the abundant oxygen-containing functional groups (such as –OH and –COOH) on the GO nanosheets to form hydrogen bonds with water,29,54 thereby enabling the GO-SIS biocomposite film to combine with more water molecules.
| Sample | Tensile strength (MPa) | Elongation at break (%) | ||
|---|---|---|---|---|
| Dry | Wet | Dry | Wet | |
| SIS | 19.97 ± 1.06 | 7.28 ± 0.24 | 9.28 ± 0.66 | 18.53 ± 0.67 |
| GO-SIS | 24.46 ± 0.99 | 10.16 ± 0.37 | 11.41 ± 0.55 | 21.26 ± 0.65 |
These results indicate that the non-covalent incorporation of GO significantly enhanced the mechanical properties of the GO-SIS biocomposite film. This enhancement can be attributed to the relatively uniform incorporation of GO and the resulting non-covalent interactions (e.g., electrostatic interactions and hydrogen bonding) between the abundant oxygen-containing functional groups on GO nanosheets and the functional groups on SIS collagen fibers,12,33–35 as schematically shown in Fig. 1. These non-covalent interactions establish a robust interface between the GO nanosheets and the collagen fibers. When the GO-SIS biocomposite film is subjected to load, this interface effectively transfers and distributes stress from the SIS matrix to the high-strength GO nanosheets, thereby markedly improving the mechanical properties of the composite.34,54 Furthermore, this efficient stress distribution helps delay premature failure caused by local stress concentration, which contributes positively to maintaining the ductility of the composite material.33,34
It is well recognized that the GO content and its dispersion state within the matrix are pivotal parameters governing the ultimate mechanical performance balance of GO-based composites.12,13,55–57 Therefore, future work will involve fabricating and testing a series of GO-SIS biocomposite films through systematic variation of these parameters, aiming to define the optimal processing window.
 | ||
| Fig. 7 Representative digital photographs of SIS and GO-SIS biocomposite films after different periods of in vitro degradation. Scale bars: 1 cm. | ||
To further investigate the morphological changes of the SIS and GO-SIS biocomposite films after in vitro degradation, ESEM analysis was performed. As shown in Fig. 8, the collagen fiber networks in both films were progressively degraded over time. After 24 hours of degradation, the interwoven fibrous network in the SIS film had become indistinct, while the GO-SIS biocomposite film retained clearly discernible fibrous networks. By 48 hours, the surface of the SIS film had become slightly flattened, whereas the interwoven fibrous network in the GO-SIS biocomposite film had become less distinct. As the enzymatic degradation proceeded, the fibrous network of the GO-SIS biocomposite film appeared partially disintegrated and shortened by 72 hours, leading to a relatively flattened surface by 96 hours.
 | ||
| Fig. 8 Representative ESEM images showing the morphology of SIS and GO-SIS biocomposite films at key time points (24 h, 48 h, 72 h, 96 h) after in vitro degradation. Scale bars: 50 µm. | ||
We also conducted a quantitative analysis of the in vitro degradation of the SIS and GO-SIS biocomposite films (Fig. 9). As shown in Fig. 9a, the weight loss rates of both SIS and GO-SIS biocomposite films increased over time during the degradation process. At 4, 8, 16, 24, 48, and 72 hours, the weight loss rate of the GO-SIS biocomposite film was significantly lower than that of the SIS film. Moreover, the SIS film was completely degraded after 120 hours.
Hydroxyproline (Hyp), a characteristic amino acid of collagen, is commonly used to assess the collagen content in collagen-based materials.58 Therefore, we measured the Hyp content in the degradation solution to quantitatively evaluate the extent of collagen fiber degradation in the SIS and GO-SIS biocomposite films. As shown in Fig. 9b, during the in vitro degradation process, the Hyp content in the degradation solution of both SIS and GO-SIS biocomposite films showed an increasing trend over time. At 4, 8, 16, 24, 48, and 72 hours, the Hyp content in the degradation solution of the GO-SIS biocomposite film was significantly lower than that of the SIS film.
By integrating the morphological changes (Fig. 7 and 8) and quantitative analysis results (Fig. 9) of both SIS and GO-SIS biocomposite films, it can be concluded that the GO-SIS biocomposite film not only showed better morphological stability but also degraded distinctly slower in vitro compared to the SIS film. This indicates that the non-covalent incorporation of GO effectively delayed the degradation of the GO-SIS biocomposite film. This effect can be attributed to the GO nanosheets, which coat the collagen fibers of SIS through non-covalent interfacial interactions such as electrostatic interactions and hydrogen bonding (Fig. 1). This coating forms a barrier between collagenase and the collagen fibers, potentially obscuring or altering the enzyme cleavage sites on the collagen.59 Consequently, this inhibits the enzyme–substrate interaction and ultimately retards the enzymatic degradation process.
To further evaluate the biodegradation performance, the mechanical properties of the dry SIS and GO-SIS biocomposite films after degradation were measured. The representative stress–strain curves of the degraded dry SIS and GO-SIS biocomposite films are shown in Fig. S2 (SI). Their tensile strength and elongation at break are shown in Fig. 10a and b, respectively. As the degradation time increased, the tensile strength of both the dry SIS and GO-SIS biocomposite films gradually decreased. At 4, 8, 16, and 24 hours, the tensile strength of the dry GO-SIS biocomposite film was significantly higher than that of the dry SIS film. Similarly, the elongation at break of both the dry SIS and GO-SIS biocomposite films also decreased progressively with longer degradation time. At the same time points (4, 8, 16, and 24 hours), the elongation at break of the dry GO-SIS biocomposite film was significantly higher than that of the dry SIS film. After 48 hours of degradation, the pure SIS films could no longer undergo valid mechanical testing due to extensive degradation, whereas the GO-SIS biocomposite films retained measurable mechanical properties.
These results demonstrate that the GO-SIS biocomposite film exhibits better mechanical properties after in vitro degradation compared to the pure SIS film. The observed enhancement is attributed to the incorporation of high-strength GO nanosheets and the non-covalent interfacial interactions between GO and the SIS collagen fibers. These factors collectively facilitate load transfer and stress distribution, thereby reinforcing the mechanical properties, while concurrently forming a protective barrier around the collagen fibers to retard the enzymatic degradation process. Consequently, the better mechanical performance of the GO-SIS biocomposite film after degradation establishes a foundation for its future evaluation in dynamic physiological environments.
In summary, the GO-SIS biocomposite film demonstrated favorable in vitro biocompatibility comparable to that of pure SIS. These in vitro assessments lay an essential safety foundation for the further development of the GO-SIS biocomposite film. To comprehensively evaluate its potential for tissue repair, future animal studies are warranted. These will elucidate the material's performance in a complex physiological environment, focusing on long-term safety, the dynamic evolution of biomechanical properties, and effects on inflammation and ECM remodeling.
4. Conclusion
In this study, a novel GO-SIS biocomposite film was successfully developed. The introduction of GO via non-covalent interactions (e.g., electrostatic interactions and hydrogen bonding) demonstrated favorable modification effects on SIS. Compared to the pure SIS film, the GO-SIS biocomposite film exhibited enhanced hydrophilicity, improved mechanical properties under both dry and wet conditions, and slower in vitro degradation. After in vitro degradation, the GO-SIS biocomposite film exhibited better morphological stability and mechanical properties than the SIS film. Furthermore, the GO-SIS biocomposite film had no impact on the viability or proliferation of L929 fibroblasts. Therefore, the GO-based non-covalent coating strategy proposed here offers a promising approach for developing high-performance tissue repair patches while preserving the inherent advantages of SIS. Future work will involve systematically varying the GO content and dispersion state, conducting comprehensive characterization and in vivo studies, to identify the optimal and rational processing parameters for enhanced clinical translation potential.Author contributions
Mianshu Hu: writing – original draft, data curation, formal analysis, investigation, methodology, visualization. Xiuyun Chuan and Yiting Wang: writing – review & editing, conceptualization, funding acquisition, supervision. Wenyue Cheng and Kun Zhang: project administration, methodology. Yun Yan and Wenbin Zhang: resources. Jinsong Han and Jian Zhang: resources, conceptualization.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting the findings of this study are available within the article and its supplementary information (SI) file. Supplementary information is available. See DOI: https://doi.org/10.1039/d5ra09329e.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (52074015 and 51774016), the Peking University Health Science Center Discipline Development Program—Clinical Medicine + X Youth Project (PKU2022LCXQ033), and the Peking University Third Hospital Fund for Interdisciplinary Research (BYSYJC2025055).References
- H. Capella-Monsonís, R. J. Crum, G. S. Hussey and S. F. Badylak, Adv. Drug Deliv. Rev., 2024, 211, 13 Search PubMed.
- S. Hinderer, S. L. Layland and K. Schenke-Layland, Adv. Drug Deliv. Rev., 2016, 97, 260–269 CrossRef CAS PubMed.
- S. F. Badylak, D. O. Freytes and T. W. Gilbert, Acta Biomater., 2015, 23, S17–S26 Search PubMed.
- P. Zhao, X. Li, Q. Fang, F. L. Wang, Q. Ao, X. H. Wang, X. H. Tian, H. Tong, S. L. Bai and J. Fan, Regen. Biomater., 2020, 7, 339–348 Search PubMed.
- L. Shi and V. Ronfard, Int. J. Burns Trauma, 2013, 3, 173–179 Search PubMed.
- G. X. Cao, Y. Huang, K. Li, Y. B. Fan, H. Q. Xie and X. M. Li, J. Mat. Chem. B, 2019, 7, 5038–5055 RSC.
- L. Wang, W. P. Wang, J. G. Liao, F. Wang, J. Z. Jiang, C. Cao and S. R. Li, Mater. Sci. Eng. C, 2018, 85, 162–169 Search PubMed.
- G. X. Cao, C. Y. Wang, Y. B. Fan and X. M. Li, Mater. Sci. Eng. C, 2020, 109, 14 CrossRef.
- F. X. Tang, D. T. Miao, R. K. Huang, B. N. Zheng, Y. Yu, P. W. Ma, B. Y. Peng, Y. Li, H. Wang and D. C. Wu, Adv. Mater., 2024, 36, 10 Search PubMed.
- Y. T. Wang, K. Zhang, J. F. Yang, Y. Yao, Y. Q. Guan, W. Y. Cheng, J. Zhang and J. S. Han, Int. Urogynecol. J., 2023, 34, 1501–1511 Search PubMed.
- K. Kim, J. Kim, S. W. Kwak, J. Y. Lee and H. Y. Kim, Fiber. Polym., 2022, 23, 2557–2564 CrossRef CAS.
- S. Tiwari, R. Patil, S. K. Dubey and P. Bahadur, Adv. Colloid Interface Sci., 2020, 281, 14 Search PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 Search PubMed.
- J. W. Suk, R. D. Piner, J. An and R. S. Ruoff, ACS Nano, 2010, 4, 6557–6564 CrossRef CAS.
- S. Ganguly, P. Das, P. P. Maity, S. Mondal, S. Ghosh, S. Dhara and N. C. Das, J. Phys. Chem. B, 2018, 122, 7201–7218 Search PubMed.
- A. T. Smith, A. M. LaChance, S. Zeng, B. Liu and L. Sun, Nano Mater. Sci., 2019, 1, 31–47 Search PubMed.
- I. Jung, D. A. Field, N. J. Clark, Y. Zhu, D. Yang, R. D. Piner, S. Stankovich, D. A. Dikin, H. Geisler, C. A. Ventrice, Jr. and R. S. Ruoff, J. Phys. Chem. C, 2009, 113, 18480–18486 Search PubMed.
- M. M. Mwaurah, J. Mathiyarasu and A. M. Vinu Mohan, Carbohydr. Polym., 2025, 350, 123060 Search PubMed.
- V. Georgakilas, J. N. Tiwari, K. C. Kemp, J. A. Perman, A. B. Bourlinos, K. S. Kim and R. Zboril, Chem. Rev., 2016, 116, 5464–5519 CrossRef CAS PubMed.
- C. Zhu, D. Du and Y. Lin, 2D Mater., 2015, 2, 032004 CrossRef.
- J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. X. Huang, J. Am. Chem. Soc., 2010, 132, 8180–8186 CrossRef CAS.
- H. J. Zhang, T. Yan, S. Xu, S. N. Feng, D. D. Huang, M. Fujita and X. D. Gao, Mater. Sci. Eng. C, 2017, 73, 144–151 CrossRef PubMed.
- H. N. Lim, N. M. Huang and C. H. Loo, J. Non-Cryst. Solids, 2012, 358, 525–530 CrossRef CAS.
- J. K. Wychowaniec, J. Litowczenko and K. Tadyszak, J. Mech. Behav. Biomed. Mater., 2020, 103, 13 Search PubMed.
- L. Pang, C. Q. Dai, L. Bi, Z. S. Guo and J. J. Fan, Nanoscale Res. Lett., 2017, 12, 9 Search PubMed.
- K. L. Zhao, Y. Hao, M. Zhu and G. S. Cheng, Acta Chim. Sin., 2018, 76, 168–176 CrossRef CAS.
- R. Kurapati, J. Russier, M. A. Squillaci, E. Treossi, C. Ménard-Moyon, A. E. Del Rio-Castillo, E. Vazquez, P. Samorì, V. Palermo and A. Bianco, Small, 2015, 11, 3985–3994 Search PubMed.
- Y. Li, L. Yang, Y. Hou, Z. Zhang, M. Chen, M. Wang, J. Liu, J. Wang, Z. Zhao, C. Xie and X. Lu, Bioact. Mater., 2022, 18, 213–227 Search PubMed.
- M. Heidari, S. H. Bahrami, M. Ranjbar-Mohammadi and P. B. Milan, Mater. Sci. Eng. C, 2019, 103 Search PubMed.
- C. Y. Wan and B. Q. Chen, Biomed. Mater., 2011, 6, 8 Search PubMed.
- J. Cifuentes, C. Muñoz-Camargo and J. C. Cruz, Nanomaterials, 2022, 12, 15 CrossRef.
- H. Alimadadi, A. Yahyazadeh, A. Soltani, B. Sharifzadeh and N. O. Mahmoodi, Res. Chem. Intermed., 2025, 51, 4647–4664 CrossRef CAS.
- C. F. Yue, C. K. Ding, X. Du and B. W. Cheng, Compos. Interfaces, 2022, 29, 413–429 CrossRef CAS.
- C. F. Yue, C. K. Ding, X. Du, Y. J. Wang, J. L. Su and B. W. Cheng, Int. J. Biol. Macromol., 2021, 193, 173–182 Search PubMed.
- A. F. Girao, G. Gonçalves, K. S. Bhangra, J. B. Phillips, J. Knowles, G. Irurueta, M. K. Singh, I. Bdkin, A. Completo and P. Marques, RSC Adv., 2016, 6, 49039–49051 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- F. Tang, D. Miao, R. Huang, B. Zheng, Y. Yu, P. Ma, B. Peng, Y. Li, H. Wang and D. Wu, Adv. Mater., 2024, 36, 2307845 Search PubMed.
- H. H. Huang, K. K. H. De Silva, G. R. A. Kumara and M. Yoshimura, Sci. Rep., 2018, 8, 9 CrossRef.
- D. Liu, Q. B. Bian, Y. Li, Y. R. Wang, A. M. Xiang and H. F. Tian, Compos. Sci. Technol., 2016, 129, 146–152 Search PubMed.
- S. Y. Bak, S. W. Lee, C. H. Choi and H. W. Kim, Materials, 2018, 11, 16 Search PubMed.
- E. C. Green, Y. Zhang, H. Li and M. L. Minus, J. Mech. Behav. Biomed. Mater., 2017, 65, 552–564 Search PubMed.
- M. Yadav, K. Y. Rhee and S. J. Park, Carbohydr. Polym., 2014, 110, 18–25 Search PubMed.
- X. Yan, J. Chen, J. Yang, Q. Xue and P. Miele, ACS Appl. Mater. Interfaces, 2010, 2, 2521–2529 CrossRef CAS.
- S. Stankovich, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Carbon, 2006, 44, 3342–3347 Search PubMed.
- T. Riaz, R. Zeeshan, F. Zarif, K. Ilyas, N. Muhammad, S. Z. Safi, A. Rahim, S. A. A. Rizvi and I. U. Rehman, Appl. Spectrosc. Rev., 2018, 53, 703–746 Search PubMed.
- L. L. Fernandes, C. X. Resende, D. S. Tavares, G. A. Soares, L. O. Castro and J. M. Granjeiro, Polímeros, 2011, 21, 1–6 Search PubMed.
- P. G. Kumar, T. Nidheesh and P. V. Suresh, Food Res. Int., 2015, 76, 804–812 CrossRef.
- M. G. Martinez, A. J. Bullock, S. MacNeil and I. U. Rehman, Appl. Spectrosc. Rev., 2019, 54, 509–542 Search PubMed.
- D. G. Henry, I. Jarvis, G. Gillmore and M. Stephenson, Earth-Sci. Rev., 2019, 198, 102936 CrossRef CAS.
- F. H. Lyn, T. C. Peng, M. Z. Ruzniza and Z. A. N. Hanani, Food Packag. Shelf Life, 2019, 21, 9 Search PubMed.
- R. Saito, M. Hofmann, G. Dresselhaus, A. Jorio and M. S. Dresselhaus, Adv. Phys., 2011, 60, 413–550 Search PubMed.
- K. Krishnamoorthy, M. Veerapandian, K. Yun and S. J. Kim, Carbon, 2013, 53, 38–49 Search PubMed.
- H. Bai, C. Li and G. Shi, Adv. Mater., 2011, 23, 1089–1115 Search PubMed.
- L. Zhang, Z. P. Wang, C. Xu, Y. Li, J. P. Gao, W. Wang and Y. Liu, J. Mater. Chem., 2011, 21, 10399–10406 RSC.
- M. Hussain, S. M. Khan, M. Shafiq, M. Al-Dossari, U. F. Alqsair, S. U. Khan and M. I. Khan, Energy, 2024, 308, 132917 CrossRef CAS.
- X. Sun, C. Huang, L. Wang, L. Liang, Y. Cheng, W. Fei and Y. Li, Adv. Mater., 2021, 33, 2001105 CrossRef CAS.
- C. Liao, Y. Li and S. C. Tjong, Int. J. Mol. Sci., 2018, 19, 3564 CrossRef PubMed.
- R. R. Lareu, D. I. Zeugolis, M. Abu-Rub, A. Pandit and M. Raghunath, Acta Biomater., 2010, 6, 3146–3151 Search PubMed.
- H. C. Liang, Y. Chang, C. K. Hsu, M. H. Lee and H. W. Sung, Biomaterials, 2004, 25, 3541–3552 Search PubMed.
- P. Das, S. Ganguly, P. K. Marvi, M. Sherazee, S. R. Ahmed, X. Tang, S. Srinivasan and A. R. Rajabzadeh, Adv. Funct. Mater., 2024, 34, 2314520 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |







