 Open Access Article
Open Access ArticleOne-pot catalytic oxidation for the synthesis of 2-biphenylbenzoxazoles, benzothiazoles and 1-substituted benzimidazoles: a convenient and efficient strategy
Yifan Ouyang a,
Niuniu Zhangabc,
Mingkang Yangac,
Hao Yang
a,
Niuniu Zhangabc,
Mingkang Yangac,
Hao Yang b,
Bei Jiang*ad,
Huilong Xie*a and
Meimei Zhang*b
b,
Bei Jiang*ad,
Huilong Xie*a and
Meimei Zhang*b
aFujian Key Laboratory of Toxicant and Drug Toxicology, School of Medicine, Ningde Normal University, Ningde, Fujian 352100, PR China. E-mail: dalinorthjiang@163.com; T1236@ndnu.edu.cn
bSchool of Basic Medicine, Ningxia Medical University, Yinchuan 750004, PR China. E-mail: zhangmm196@163.com
cCollege of Chemistry, Fuzhou University, Fuzhou 350100, PR China
dYunnan Key Laboratory of Screening and Research on Anti-pathogenic Plant Resources from Western Yunnan, College of Pharmacy, Dali University, Dali, 671000, PR China
First published on 15th January 2026
Abstract
We report a one-pot, catalytic oxidative synthesis of 2-biphenylbenzoxazoles, benzothiazoles and 1-substituted benzimidazoles from 2-aminosubstituted phenols/thiophenols/phenylamines and substituted biphenylcarbaldehydes. Among the advantages of this method are its simple procedure, high efficiency, and broad substrate scope.
Introduction
Biphenyl is an important structural motif widely found in natural compounds, pharmaceuticals, and chemical intermediates.1 Particularly in the field of medicine, biphenyl and its derivatives have demonstrated a broad range of biological activities, such as anti-inflammatory, anticancer, and antibacterial effects, in corresponding drugs.2,3 These drugs often incorporate nitrogen-containing heterocyclic units.4 Therefore, the combination of biphenyl and nitrogen-containing heterocyclic structural motifs is highly valuable for drug development and the study of biological activities.2-Biphenylbenzoxazole, benzothiazole and benzimidazole, as important bioactive molecules, exhibit extensive applications in medicine-related fields (Fig. 1). For instance, compounds featuring the 2-biphenylbenzoxazole, benzothiazole or benzimidazole core can serve as candidates for P2X2/3 antagonists in pain treatment.5 Meanwhile, small molecules containing such core structures also demonstrate significant potential as novel cancer immunotherapy inhibitors targeting the PD-1/PD-L1 interaction.6 Additionally, non-nucleoside inhibitors of non-structural protein 5B (NS5B), which are used for treating hepatitis C virus infection, also incorporate this core scaffold.7
However, most 2-biphenylbenzoxazoles, benzothiazoles and benzimidazoles are synthesized via a two-step procedure.8–11 As shown in Scheme 1a, the first step yields 2-phenylbenzoxazoles, benzothiazoles and benzimidazoles using nanocatalysts,12,13 metal-catalysts,14,15 or ionic-liquid-catalysts,16–18 followed by a classic Suzuki coupling reaction in the second step to obtain the final 2-biphenylbenzoxazole, benzothiazole and benzimidazole products.19,20 This process not only requires the use of structurally complex, expensive, or toxic catalysts but is also not step-economical. Although some 2-phenylbenzoxazole and benzothiazole derivatives can be directly synthesized under the first-step conditions mentioned above, only moderate yields are obtained.13,17 Given the ongoing demand in materials and pharmaceutical fields, developing simple and efficient synthetic methods for 2-biphenylbenzoxazole, benzothiazole and benzimidazole derivatives would provide significant convenience for scientists.
Herein, we report a one-pot oxidative synthesis of 2-biphenylbenzoxazoles, benzothiazoles and 1-substituted benzimidazoles catalyzed by N-heterocyclic carbenes (NHCs) generated in situ from readily accessible bridged bis-triazolium salts (Scheme 1b). This method avoids the use of structurally complex, expensive, or toxic catalysts and eliminates the need for complicated multi-step procedures. Moreover, it provides a series of 2-biphenylbenzoxazole, benzothiazole and 1-substituted benzimidazole derivatives in excellent yields, further enhancing the attractiveness of this approach.
Results and discussion
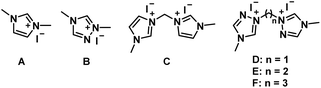
Initially, 4-bromo-2-aminophenol (1a) and 2-methylbiphenylcarbaldehyde (2a) were selected as model substrates to optimize the reaction conditions (Table 1). The reaction was catalyzed by NHCs generated in situ from azolium salts (10 mol%), with reduced catalyst loading leading to lower yield (Table S1, entries 1–2). As summarized in Table 1 (Entries 1–6), the effects of different azolium salts as NHCs precursors were investigated. Bridged azolium salts exhibited superior catalytic performance compared to their non-bridged counterparts, likely due to their enhanced stability at elevated temperatures, which facilitates more efficient NHCs generation. Furthermore, triazolium salts afforded higher yields than imidazolium salts (Table 1, entries 1–4). With increasing chain length of the bridging linker in the azolium salts, the yield gradually decreased (Table 1, entries 5–6), possibly owing to reduced structural stability and increased susceptibility to decomposition under high-temperature conditions. Control experiments demonstrated unequivocally that the NHC precursor is indispensable (Table 1, entries 7). However, the maximum yield obtained under these conditions was only 32%, which was attributed to the insufficient oxidizing capacity of atmospheric oxygen. Therefore, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) was introduced as an oxidant into the reaction system after 1 h to prevent the oxidation of substrate 1a to the corresponding benzoquinone. To our delight, the addition of DDQ significantly improved the yield of product 3a (Table 1, entries 8–11), with the best yield of 78% achieved using 3 equivalents of DDQ. During this period, we investigated the conversion efficiency of alternative oxidants (MnO2, O2 and DMP) and found that the highest yield obtained was 73% (Table S1, entries 3–5), which is lower than the oxidation yield achieved with DDQ (78%). Subsequently, the reaction temperature was screened (Table 1, entries 10, 12–15). The optimal yield of 89% was obtained at 150 °C. A further increase in temperature led to decreased yields, presumably due to decomposition of the azolium salt at higher temperatures. Finally, the influence of reaction time on the yield was examined (Table 1, entries 14, 16–19). The yield initially increased and then decreased with prolonged reaction time, reaching a maximum at 4 h (Table 1, Entry 18).| Entry | NHC precursor | DDQb (equiv.) | T (°C) | Timec (h) | Yieldd (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol, 1 equiv.), 2a (0.36 mmol, 1.2 equiv.), NHC precursor (10 mmol%) and DDQ in 3 ml xylene.b After 1 h of reaction, DDQ was added.c Total reaction time.d Isolated yield.e Without NHC precursor. | |||||
| 1 | A | 0 | 120 | 10 | 10 |
| 2 | B | 0 | 120 | 10 | 12 |
| 3 | C | 0 | 120 | 10 | 25 |
| 4 | D | 0 | 120 | 10 | 32 |
| 5 | E | 0 | 120 | 10 | 30 |
| 6 | F | 0 | 120 | 10 | 26 |
| 7 | — | 0 | 120 | 10 | Tracee |
| 8 | D | 1 | 120 | 10 | 68 |
| 9 | D | 2 | 120 | 10 | 73 |
| 10 | D | 3 | 120 | 10 | 78 |
| 11 | D | 4 | 120 | 10 | 75 |
| 12 | D | 3 | 130 | 10 | 82 |
| 13 | D | 3 | 140 | 10 | 84 |
| 14 | D | 3 | 150 | 10 | 89 |
| 15 | D | 3 | 160 | 10 | 83 |
| 16 | D | 3 | 150 | 8 | 89 |
| 17 | D | 3 | 150 | 6 | 90 |
| 18 | D | 3 | 150 | 4 | 92 |
| 19 | D | 3 | 150 | 2 | 88 |
 |
|||||
Under the optimal reaction conditions (Table 1, entry 18), the substrate scope was further investigated and the results are shown in Table 2. All reactions were completed within 4 h, affording the corresponding 2-biphenylbenzoxazoles, benzothiazoles and 1-substituted benzimidazoles in excellent yields. First, the influence of substituents on the phenol moiety was investigated by reacting various 2-aminosubstituted phenols with 2-methylbiphenylcarbaldehyde (2a). For substituents at the 4-position, both electron-withdrawing groups (–Br, –F, –Cl, –CF3, –NO2) and electron-donating groups (–CH3, –OCH3) afforded the corresponding products (3a–g) in excellent yields with no significant variation. Compared to substituents at the 3-, 5-, and 6-positions, the 5-substituted derivative (3i) exhibited little influence on the yield, while 3- and 6-substituted substrates (3h and 3j) led to diminished yields, likely due to steric hindrance from the ortho-substituents. Disubstituted 2-aminophenols also provided the corresponding products (3k) in excellent yields. Subsequently, reactions between 4-bromo-2-aminophenol (1a) and substituted biphenylcarbaldehydes were examined. Regardless of whether the substituent at R1 is an electron-donating group (3b), an electron-withdrawing group (3m) or a hydrogen atom (3l), excellent yields can be obtained. Substituents at the R2 position, whether electron-donating (3n and 3p) or electron-withdrawing (3o) and regardless of their location (para or meta), showed no significant impact on the yield. Then, 2-aminobenzenethiol derivatives were employed as substrates to synthesize 2-biphenylbenzothiazole derivatives (3q–3s). It was found that the benzothiazole derivatives were obtained in even higher yields compared to their benzoxazole analogues. This outcome may be attributed to the sulfur atom possessing more electrons, thereby providing a stronger driving force for dehydrogenative aromatization. Finally, when 1,2-diaminobenzene was employed as the substrate, only a trace amount of the corresponding fluorescent product was detected by TLC. As reported previously, this is likely due to the tendency of one molecule of o-phenylenediamine to condense with two molecules of the aldehyde.17 Gratifyingly, the use of monoalkylated o-phenylenediamine instead of o-phenylenediamine afforded the desired products (3t–3u) in satisfactory yields.
| a Reaction conditions: 1 (0.3 mmol), 2 (0.36 mmol), D (10 mmol%), DDQ (0.9 mmol), xylene (3 ml), 150 °C, 4 h. |
|---|
 |
To demonstrate the potential synthetic utility of the protocol, a gram-scale reaction was conducted with 4-bromo-2-aminophenol (1a) and 2-methylbiphenylcarbaldehyde (2a). Under the standard reaction conditions, 3a was obtained in 88% yield (1.60 g). Furthermore, the bromo substituent on 3a underwent coupling reactions with various substituted arylboronic acids,21 affording the corresponding products (4 and 5) in high yields (Scheme 2).
Based on the results and related literature, the mechanism of the proposed reaction is as follows (Scheme 3). N-Heterocyclic carbene I is first generated in situ by the bridged bis-triazolium salts (D) at 150 °C, and undergoes a nucleophilic addition to imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, forming the intermediate II. Then the aldehyde proton (red font) is transferred to DDQ, which forms the intermediate III. Driven by the aromatization force and the deprotonation of HX, nucleophilic attack from the anion X− forms the aerobic oxidative product with concomitant regeneration of the NHC catalyst. Simultaneously, DDQ is subsequently converted to 4,5-dichloro-3,6-dihydroxy-phthalonitrile (DDP) by capturing a proton again.
N bond, forming the intermediate II. Then the aldehyde proton (red font) is transferred to DDQ, which forms the intermediate III. Driven by the aromatization force and the deprotonation of HX, nucleophilic attack from the anion X− forms the aerobic oxidative product with concomitant regeneration of the NHC catalyst. Simultaneously, DDQ is subsequently converted to 4,5-dichloro-3,6-dihydroxy-phthalonitrile (DDP) by capturing a proton again.
The synthesized 2-biphenylbenzoxazole, benzothiazole, and benzimidazole derivatives hold significant promise in medicine due to their structural resemblance to several bioactive scaffolds and their inherent photophysical properties. In medicinal chemistry, these compounds are known to exhibit a broad spectrum of biological activities. For instance, derivatives containing such core structures have been reported as potent P2X2/3 receptor antagonists for pain management,5 and they have also shown potential as PD-1/PD-L1 interaction inhibitors for cancer immunotherapy.6 Additionally, such scaffolds are found in non-nucleoside NS5B inhibitors used in the treatment of hepatitis C virus infection.7 The presence of a biphenyl moiety enhances π–π stacking and hydrophobic interactions with protein targets, which is crucial for improving binding affinity and selectivity. The ability to introduce diverse substituents on both the heterocycle and biphenyl rings further allows for fine-tuning of pharmacokinetic properties and bioactivity. Therefore, the efficient and versatile synthetic method presented herein not only facilitates access to these valuable scaffolds but also opens avenues for their further functionalization and application in drug discovery.
Conclusions
In conclusion, we have reported a convenient and efficient synthetic method for 2-biphenylbenzoxazoles, benzothiazoles and 1-substituted benzimidazoles. The reaction proceeds via a one-pot oxidative synthesis catalyzed by an NHC, generated in situ from an easily accessible bridged bis-triazolium salt. This protocol demonstrates excellent substrate tolerance and employs environmentally benign catalysts and oxidants. The feasibility of the method was confirmed through gram-scale synthesis, and the transformability of the products was verified via further derivatization reactions. Meanwhile, our laboratory is currently extending this catalytic system to the preparation of other useful heterocyclic compounds.Experimental
General information
All of the chemical reagents and solvents were obtained from commercial sources and used directly without further purification. 1H NMR and 13C NMR spectra were recorded on a Bruker AV-400 or 600 MHz spectrometer. Chemical shifts (δ) are given in relative to tetramethylsilane (δ 0.00 ppm) in CDCl3. Coupling constants, J, were reported in hertz unit (Hz). High resolution mass spectra (HRMS) were obtained on a Q-STAR Elite ESI-LC-MS/MS Spectrometer. Chemical names were generated using Cambridge Soft. ChemDraw Ultra 16.0.Experimental procedures
NHC precursor D (10 mmol%) was added to a solution of compound 1 (0.3 mmol) and compound 2 (0.36 mmol) in 3 ml of xylene under air at 150 °C. After stirring for 1 h, DDQ (0.9 mmol) was added, and the reaction was continued under reflux for an additional 3 h. After the reaction was completed, the mixture was extracted with ethyl acetate (10 ml × 3). The organic layer was washed with saturated saline and dried over anhydrous sodium sulfate. After removing the solvent in a vacuum, the resulting residue was purified by column chromatography on silica gel to give the desired products 3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (100.2 mg, 92% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.09 (dd, J = 6.8, 2.4 Hz, 1H), 7.95 (t, J = 1.2 Hz, 1H), 7.54–7.31 (m, 9H), 2.61 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.8, 149.4, 144.1, 143.7, 141.5, 136.5, 133.0, 129.5, 129.4, 128.3, 128.1, 127.2, 126.8, 125.7, 123.2, 117.1, 111.8, 19.2. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0335.
1) as a white solid (100.2 mg, 92% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.09 (dd, J = 6.8, 2.4 Hz, 1H), 7.95 (t, J = 1.2 Hz, 1H), 7.54–7.31 (m, 9H), 2.61 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.8, 149.4, 144.1, 143.7, 141.5, 136.5, 133.0, 129.5, 129.4, 128.3, 128.1, 127.2, 126.8, 125.7, 123.2, 117.1, 111.8, 19.2. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0335.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (81.6 mg, 91% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.11 (dd, J = 6.1, 3.3 Hz, 1H), 7.68–7.60 (m, 1H), 7.53–7.37 (m, 8H), 7.22 (dd, J = 8.3, 1.7 Hz, 1H), 2.65 (s, 3H), 2.53 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 163.8, 148.7, 144.0, 142.3, 141.7, 136.2, 134.2, 132.5, 129.4, 129.4, 128.2, 127.6, 127.1, 126.2, 125.6, 120.1, 109.9, 21.6, 19.2. HRMS (ESI) m/z [M + H]+ calcd for C21H18NO+ 300.1383, found 300.1380.
1) as a white solid (81.6 mg, 91% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.11 (dd, J = 6.1, 3.3 Hz, 1H), 7.68–7.60 (m, 1H), 7.53–7.37 (m, 8H), 7.22 (dd, J = 8.3, 1.7 Hz, 1H), 2.65 (s, 3H), 2.53 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 163.8, 148.7, 144.0, 142.3, 141.7, 136.2, 134.2, 132.5, 129.4, 129.4, 128.2, 127.6, 127.1, 126.2, 125.6, 120.1, 109.9, 21.6, 19.2. HRMS (ESI) m/z [M + H]+ calcd for C21H18NO+ 300.1383, found 300.1380.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (86.9 mg, 92% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.11 (dd, J = 5.8, 3.5 Hz, 1H), 7.52–7.33 (m, 9H), 7.01 (dd, J = 8.9, 2.5 Hz, 1H), 3.91 (s, 3H), 2.65 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.5, 157.3, 145.1, 144.0, 142.9, 141.7, 136.2, 132.6, 129.4, 129.4, 128.2, 127.5, 127.1, 125.7, 113.9, 110.7, 102.9, 56.0, 19.2. HRMS (ESI) m/z [M + H]+ calcd for C21H18NO2+ 316.1332, found 316.1330.
1) as a white solid (86.9 mg, 92% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.11 (dd, J = 5.8, 3.5 Hz, 1H), 7.52–7.33 (m, 9H), 7.01 (dd, J = 8.9, 2.5 Hz, 1H), 3.91 (s, 3H), 2.65 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.5, 157.3, 145.1, 144.0, 142.9, 141.7, 136.2, 132.6, 129.4, 129.4, 128.2, 127.5, 127.1, 125.7, 113.9, 110.7, 102.9, 56.0, 19.2. HRMS (ESI) m/z [M + H]+ calcd for C21H18NO2+ 316.1332, found 316.1330.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (85.4 mg, 94% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.13 (dd, J = 7.0, 2.4 Hz, 1H), 7.59–7.36 (m, 9H), 7.14 (m, 1H), 2.66 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 165.5, 160.1 (d, J = 241 Hz), 146.7, 144.1, 142.9 (d, J = 13 Hz), 141.5, 136.4, 132.9, 129.5, 129.4, 128.3, 127.2, 127.1, 125.7, 112.8 (d, J = 26 Hz), 110.8 (d, J = 10 Hz), 106.6 (d, J = 25 Hz), 19.3. HRMS (ESI) m/z [M + H]+ calcd for C20H15FNO+ 304.1132, found 304.1132.
1) as a white solid (85.4 mg, 94% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.13 (dd, J = 7.0, 2.4 Hz, 1H), 7.59–7.36 (m, 9H), 7.14 (m, 1H), 2.66 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 165.5, 160.1 (d, J = 241 Hz), 146.7, 144.1, 142.9 (d, J = 13 Hz), 141.5, 136.4, 132.9, 129.5, 129.4, 128.3, 127.2, 127.1, 125.7, 112.8 (d, J = 26 Hz), 110.8 (d, J = 10 Hz), 106.6 (d, J = 25 Hz), 19.3. HRMS (ESI) m/z [M + H]+ calcd for C20H15FNO+ 304.1132, found 304.1132.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (91.9 mg, 96% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.12 (dd, J = 7.1, 2.3 Hz, 1H), 7.82 (d, J = 2.1 Hz, 1H), 7.57–7.35 (m, 9H), 2.65 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 165.0, 149.0, 144.1, 143.2, 141.5, 136.5, 133.0, 129.9, 129.5, 129.4, 128.3, 127.2, 126.9, 125.7, 125.4, 120.2, 111.3, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C20H15ClNO+ 320.0837, found 320.0838.
1) as a white solid (91.9 mg, 96% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.12 (dd, J = 7.1, 2.3 Hz, 1H), 7.82 (d, J = 2.1 Hz, 1H), 7.57–7.35 (m, 9H), 2.65 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 165.0, 149.0, 144.1, 143.2, 141.5, 136.5, 133.0, 129.9, 129.5, 129.4, 128.3, 127.2, 126.9, 125.7, 125.4, 120.2, 111.3, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C20H15ClNO+ 320.0837, found 320.0838.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (98.5 mg, 93% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.24–8.05 (m, 2H), 7.78–7.64 (m, 2H), 7.53–7.34 (m, 7H), 2.67 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 165.3, 152.1, 144.2, 142.2, 141.4, 136.7, 133.2, 129.5, 129.4, 128.3, 127.3, 127.2 (q, J = 32 Hz), 126.6, 125.8, 124.3 (q, J = 270 Hz), 122.4 (q, J = 4 Hz), 117.9 (q, J = 4 Hz), 111.0, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C21H15F3NO+ 354.1100, found 354.1104.
1) as a white solid (98.5 mg, 93% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.24–8.05 (m, 2H), 7.78–7.64 (m, 2H), 7.53–7.34 (m, 7H), 2.67 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 165.3, 152.1, 144.2, 142.2, 141.4, 136.7, 133.2, 129.5, 129.4, 128.3, 127.3, 127.2 (q, J = 32 Hz), 126.6, 125.8, 124.3 (q, J = 270 Hz), 122.4 (q, J = 4 Hz), 117.9 (q, J = 4 Hz), 111.0, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C21H15F3NO+ 354.1100, found 354.1104.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow solid (93.1 mg, 94% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.73 (d, J = 2.3 Hz, 1H), 8.38 (dd, J = 8.9, 2.3 Hz, 1H), 8.17 (dd, J = 7.5, 1.9 Hz, 1H), 7.73 (d, J = 8.9 Hz, 1H), 7.52–7.36 (m, 7H), 2.68 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 166.5, 153.9, 145.3, 144.4, 142.5, 141.3, 137.1, 133.6, 129.6, 129.3, 128.3, 127.3, 126.0, 125.9, 121.2, 116.6, 110.7, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C20H15N2O3+ 331.1077, found 331.1078.
1) as a yellow solid (93.1 mg, 94% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.73 (d, J = 2.3 Hz, 1H), 8.38 (dd, J = 8.9, 2.3 Hz, 1H), 8.17 (dd, J = 7.5, 1.9 Hz, 1H), 7.73 (d, J = 8.9 Hz, 1H), 7.52–7.36 (m, 7H), 2.68 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 166.5, 153.9, 145.3, 144.4, 142.5, 141.3, 137.1, 133.6, 129.6, 129.3, 128.3, 127.3, 126.0, 125.9, 121.2, 116.6, 110.7, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C20H15N2O3+ 331.1077, found 331.1078.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (95.8 mg, 88% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.13 (dd, J = 7.2, 2.2 Hz, 1H), 7.58 (m, 2H), 7.51–7.34 (m, 7H), 7.28 (t, J = 8.0 Hz, 1H), 2.65 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.3, 150.6, 144.0, 141.5, 141.5, 136.6, 133.0, 129.7, 129.4, 128.3, 127.7, 127.2, 126.9, 125.9, 125.7, 112.9, 109.7, 19.2. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0334.
1) as a white solid (95.8 mg, 88% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.13 (dd, J = 7.2, 2.2 Hz, 1H), 7.58 (m, 2H), 7.51–7.34 (m, 7H), 7.28 (t, J = 8.0 Hz, 1H), 2.65 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.3, 150.6, 144.0, 141.5, 141.5, 136.6, 133.0, 129.7, 129.4, 128.3, 127.7, 127.2, 126.9, 125.9, 125.7, 112.9, 109.7, 19.2. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0334.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (103.5 mg, 95% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.11 (dd, J = 7.0, 2.4 Hz, 1H), 7.81 (d, J = 1.8 Hz, 1H), 7.70 (d, J = 8.4 Hz, 1H), 7.54–7.36 (m, 8H), 2.64 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.2, 150.9, 144.1, 141.5, 141.3, 136.5, 132.9, 129.5, 129.4, 128.3, 127.9, 127.2, 126.8, 125.7, 121.2, 118.0, 114.1, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0334.
1) as a white solid (103.5 mg, 95% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.11 (dd, J = 7.0, 2.4 Hz, 1H), 7.81 (d, J = 1.8 Hz, 1H), 7.70 (d, J = 8.4 Hz, 1H), 7.54–7.36 (m, 8H), 2.64 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.2, 150.9, 144.1, 141.5, 141.3, 136.5, 132.9, 129.5, 129.4, 128.3, 127.9, 127.2, 126.8, 125.7, 121.2, 118.0, 114.1, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0334.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (94.7 mg, 87% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.19 (dd, J = 6.7, 2.6 Hz, 1H), 7.78 (d, J = 7.9 Hz, 1H), 7.55 (d, J = 7.9 Hz, 1H), 7.50–7.37 (m, 7H), 7.28 (d, J = 7.9 Hz, 1H), 2.68 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 163.9, 148.7, 144.1, 142.7, 141.5, 136.6, 133.1, 129.7, 129.4, 128.2, 128.3, 127.2, 126.7, 125.8, 125.6, 119.2, 102.5, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0333.
1) as a white solid (94.7 mg, 87% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.19 (dd, J = 6.7, 2.6 Hz, 1H), 7.78 (d, J = 7.9 Hz, 1H), 7.55 (d, J = 7.9 Hz, 1H), 7.50–7.37 (m, 7H), 7.28 (d, J = 7.9 Hz, 1H), 2.68 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 163.9, 148.7, 144.1, 142.7, 141.5, 136.6, 133.1, 129.7, 129.4, 128.2, 128.3, 127.2, 126.7, 125.8, 125.6, 119.2, 102.5, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0333.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a grey solid (104.8 mg, 96% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.63 (d, J = 2.1 Hz, 1H), 8.38 (d, J = 2.1 Hz, 1H), 8.22 (dd, J = 7.6, 1.8 Hz, 1H), 7.52–7.36 (m, 7H), 2.68 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 166.7, 150.7, 145.4, 144.4, 143.1, 141.1, 137.3, 134.0, 129.8, 129.3, 128.3, 127.4, 126.0, 125.4, 121.4, 116.6, 115.0, 19.4. HRMS (ESI) m/z [M + H]+ calcd for C20H14ClN2O3+ 365.0687, found 365.0688.
1) as a grey solid (104.8 mg, 96% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.63 (d, J = 2.1 Hz, 1H), 8.38 (d, J = 2.1 Hz, 1H), 8.22 (dd, J = 7.6, 1.8 Hz, 1H), 7.52–7.36 (m, 7H), 2.68 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 166.7, 150.7, 145.4, 144.4, 143.1, 141.1, 137.3, 134.0, 129.8, 129.3, 128.3, 127.4, 126.0, 125.4, 121.4, 116.6, 115.0, 19.4. HRMS (ESI) m/z [M + H]+ calcd for C20H14ClN2O3+ 365.0687, found 365.0688.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (94.2 mg, 90% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.50 (t, J = 1.8 Hz, 1H), 8.25–8.21 (m, 1H), 7.95 (d, J = 1.3 Hz, 1H), 7.83–7.79 (m, 1H), 7.74–7.69 (m, 2H), 7.63 (t, J = 7.8 Hz, 1H), 7.54–7.49 (m, 4H), 7.46–7.41 (m, 1H). 13C NMR (101 MHz, Chloroform-d) δ 164.1, 149.8, 143.7, 142.1, 139.9, 130.6, 129.5, 129.0, 128.2, 127.9, 127.2, 127.2, 126.5, 126.4, 123.0, 117.4, 111.9. HRMS (ESI) m/z [M + H]+ calcd for C19H13BrNO+ 350.0175, found 350.0174.
1) as a white solid (94.2 mg, 90% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.50 (t, J = 1.8 Hz, 1H), 8.25–8.21 (m, 1H), 7.95 (d, J = 1.3 Hz, 1H), 7.83–7.79 (m, 1H), 7.74–7.69 (m, 2H), 7.63 (t, J = 7.8 Hz, 1H), 7.54–7.49 (m, 4H), 7.46–7.41 (m, 1H). 13C NMR (101 MHz, Chloroform-d) δ 164.1, 149.8, 143.7, 142.1, 139.9, 130.6, 129.5, 129.0, 128.2, 127.9, 127.2, 127.2, 126.5, 126.4, 123.0, 117.4, 111.9. HRMS (ESI) m/z [M + H]+ calcd for C19H13BrNO+ 350.0175, found 350.0174.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (82.7 mg, 91% yield). 1H NMR (600 MHz, Chloroform-d) δ 8.22 (ddd, J = 8.4, 6.6, 1.8 Hz, 1H), 7.68–7.60 (m, 4H), 7.54–7.48 (m, 3H), 7.47–7.42 (m, 1H), 7.39 (t, J = 7.8 Hz, 1H), 7.22 (dd, J = 8.4, 1.8 Hz, 1H), 2.53 (s, 3H). 13C NMR (151 MHz, Chloroform-d) δ 159.7 (d, J = 5 Hz), 157.7 (d, J = 260 Hz), 148.8, 142.0, 135.0, 134.6, 133.8 (d, J = 5 Hz), 130.8 (d, J = 15 Hz), 129.6, 129.2 (d, J = 5 Hz), 128.5, 128.1, 126.7, 124.5 (d, J = 5 Hz), 120.3, 116.3 (d, J = 12 Hz), 110.1, 21.6. HRMS (ESI) m/z [M + H]+ calcd for C20H15FNO+ 304.1132, found 304.1128.
1) as a white solid (82.7 mg, 91% yield). 1H NMR (600 MHz, Chloroform-d) δ 8.22 (ddd, J = 8.4, 6.6, 1.8 Hz, 1H), 7.68–7.60 (m, 4H), 7.54–7.48 (m, 3H), 7.47–7.42 (m, 1H), 7.39 (t, J = 7.8 Hz, 1H), 7.22 (dd, J = 8.4, 1.8 Hz, 1H), 2.53 (s, 3H). 13C NMR (151 MHz, Chloroform-d) δ 159.7 (d, J = 5 Hz), 157.7 (d, J = 260 Hz), 148.8, 142.0, 135.0, 134.6, 133.8 (d, J = 5 Hz), 130.8 (d, J = 15 Hz), 129.6, 129.2 (d, J = 5 Hz), 128.5, 128.1, 126.7, 124.5 (d, J = 5 Hz), 120.3, 116.3 (d, J = 12 Hz), 110.1, 21.6. HRMS (ESI) m/z [M + H]+ calcd for C20H15FNO+ 304.1132, found 304.1128.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (96.9 mg, 89% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.48 (t, J = 1.8 Hz, 1H), 8.25–8.17 (m, 1H), 7.95–7.94 (m, 1H), 7.81–7.78 (m, 1H), 7.63–7.58 (m, 3H), 7.50–7.49 (m, 2H), 7.32 (d, J = 8.0 Hz, 2H), 2.45 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.2, 149.8, 143.7, 142.0, 137.8, 137.0, 130.4, 129.7, 129.4, 128.2, 127.1, 127.0, 126.3, 126.2, 123.0, 117.4, 111.9, 21.2. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0333.
1) as a white solid (96.9 mg, 89% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.48 (t, J = 1.8 Hz, 1H), 8.25–8.17 (m, 1H), 7.95–7.94 (m, 1H), 7.81–7.78 (m, 1H), 7.63–7.58 (m, 3H), 7.50–7.49 (m, 2H), 7.32 (d, J = 8.0 Hz, 2H), 2.45 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.2, 149.8, 143.7, 142.0, 137.8, 137.0, 130.4, 129.7, 129.4, 128.2, 127.1, 127.0, 126.3, 126.2, 123.0, 117.4, 111.9, 21.2. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0333.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (111.1 mg, 89% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.49 (d, J = 1.8 Hz, 1H), 8.28 (d, J = 7.8 Hz, 1H), 7.94 (s, 1H), 7.83–7.75 (m, 5H), 7.66 (t, J = 7.8 Hz, 1H), 7.51 (s, 2H). 13C NMR (101 MHz, Chloroform-d) δ 163.7, 149.8, 143.6, 143.4, 140.6, 130.7, 130.0 (q, J = 33 Hz), 129.7, 128.4, 127.5, 127.4, 127.3, 126.5, 125.9 (q, J = 4 Hz), 124.2 (q, J = 270 Hz), 123.1, 117.5, 111.9. HRMS (ESI) m/z [M + H]+ calcd for C20H12BrF3NO+ 418.0049, found 418.0050.
1) as a white solid (111.1 mg, 89% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.49 (d, J = 1.8 Hz, 1H), 8.28 (d, J = 7.8 Hz, 1H), 7.94 (s, 1H), 7.83–7.75 (m, 5H), 7.66 (t, J = 7.8 Hz, 1H), 7.51 (s, 2H). 13C NMR (101 MHz, Chloroform-d) δ 163.7, 149.8, 143.6, 143.4, 140.6, 130.7, 130.0 (q, J = 33 Hz), 129.7, 128.4, 127.5, 127.4, 127.3, 126.5, 125.9 (q, J = 4 Hz), 124.2 (q, J = 270 Hz), 123.1, 117.5, 111.9. HRMS (ESI) m/z [M + H]+ calcd for C20H12BrF3NO+ 418.0049, found 418.0050.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (98.0 mg, 90% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.49 (t, J = 1.8 Hz, 1H), 8.24–8.20 (m, 1H), 7.95 (t, J = 1.2 Hz, 1H), 7.82–7.78 (m, 1H), 7.62 (t, J = 7.6 Hz, 1H), 7.56–7.47 (m, 4H), 7.40 (t, J = 7.6 Hz, 1H), 7.29–7.24 (m, 1H), 2.48 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.2, 149.8, 143.7, 142.2, 139.9, 138.6, 130.7, 129.4, 128.9, 128.7, 128.2, 128.0, 127.1, 126.4, 126.4, 124.3, 123.0, 117.4, 111.9, 21.6. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0332.
1) as a white solid (98.0 mg, 90% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.49 (t, J = 1.8 Hz, 1H), 8.24–8.20 (m, 1H), 7.95 (t, J = 1.2 Hz, 1H), 7.82–7.78 (m, 1H), 7.62 (t, J = 7.6 Hz, 1H), 7.56–7.47 (m, 4H), 7.40 (t, J = 7.6 Hz, 1H), 7.29–7.24 (m, 1H), 2.48 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.2, 149.8, 143.7, 142.2, 139.9, 138.6, 130.7, 129.4, 128.9, 128.7, 128.2, 128.0, 127.1, 126.4, 126.4, 124.3, 123.0, 117.4, 111.9, 21.6. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNO+ 364.0332, found 364.0332.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (110.3 mg, 97% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.10 (d, J = 2.0 Hz, 1H), 7.99 (d, J = 8.8 Hz, 1H), 7.69–7.63 (m, 2H), 7.50–7.45 (m, 2H), 7.43–7.36 (m, 5H), 2.46 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 169.0, 152.6, 143.9, 141.6, 137.5, 134.8, 133.7, 132.0, 129.8, 129.7, 129.3, 128.3, 127.2, 125.8, 124.5, 124.0, 118.8, 18.7. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNS+ 380.0103, found 380.0105.
1) as a white solid (110.3 mg, 97% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.10 (d, J = 2.0 Hz, 1H), 7.99 (d, J = 8.8 Hz, 1H), 7.69–7.63 (m, 2H), 7.50–7.45 (m, 2H), 7.43–7.36 (m, 5H), 2.46 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 169.0, 152.6, 143.9, 141.6, 137.5, 134.8, 133.7, 132.0, 129.8, 129.7, 129.3, 128.3, 127.2, 125.8, 124.5, 124.0, 118.8, 18.7. HRMS (ESI) m/z [M + H]+ calcd for C20H15BrNS+ 380.0103, found 380.0105.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (114.4 mg, 98% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.35 (t, J = 1.9 Hz, 1H), 8.10–8.08 (m, 2H), 7.86 (d, J = 8.5 Hz, 1H), 7.84–7.72 (m, 5H), 7.63 (t, J = 7.8 Hz, 1H), 7.41 (dd, J = 8.5, 2.0 Hz, 1H). 13C NMR (101 MHz, Chloroform-d) δ 169.4, 154.9, 143.6, 140.8, 134.0, 133.3, 132.5, 130.0 (q, J = 30 Hz), 129.8, 127.6, 127.4, 126.3, 125.9, 125.9 (q, J = 12 Hz), 125.9, 124.2 (q, J = 270 Hz), 123.1, 122.4. HRMS (ESI) m/z [M + H]+ calcd for C20H12ClF3NS+ 390.0326, found 390.0327.
1) as a white solid (114.4 mg, 98% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.35 (t, J = 1.9 Hz, 1H), 8.10–8.08 (m, 2H), 7.86 (d, J = 8.5 Hz, 1H), 7.84–7.72 (m, 5H), 7.63 (t, J = 7.8 Hz, 1H), 7.41 (dd, J = 8.5, 2.0 Hz, 1H). 13C NMR (101 MHz, Chloroform-d) δ 169.4, 154.9, 143.6, 140.8, 134.0, 133.3, 132.5, 130.0 (q, J = 30 Hz), 129.8, 127.6, 127.4, 126.3, 125.9, 125.9 (q, J = 12 Hz), 125.9, 124.2 (q, J = 270 Hz), 123.1, 122.4. HRMS (ESI) m/z [M + H]+ calcd for C20H12ClF3NS+ 390.0326, found 390.0327.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (97.5 mg, 97% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.13 (d, J = 2.0 Hz, 1H), 7.88 (d, J = 8.5 Hz, 1H), 7.68 (dd, J = 6.3, 2.8 Hz, 1H), 7.50–7.37 (m, 8H), 2.46 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 170.4, 154.5, 143.9, 141.6, 134.8, 134.1, 133.7, 132.2, 132.0, 129.8, 129.3, 128.3, 127.2, 125.8, 125.7, 123.2, 122.2, 18.7. HRMS (ESI) m/z [M + H]+ calcd for C20H15ClNS+ 336.0608, found 336.0608.
1) as a white solid (97.5 mg, 97% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.13 (d, J = 2.0 Hz, 1H), 7.88 (d, J = 8.5 Hz, 1H), 7.68 (dd, J = 6.3, 2.8 Hz, 1H), 7.50–7.37 (m, 8H), 2.46 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 170.4, 154.5, 143.9, 141.6, 134.8, 134.1, 133.7, 132.2, 132.0, 129.8, 129.3, 128.3, 127.2, 125.8, 125.7, 123.2, 122.2, 18.7. HRMS (ESI) m/z [M + H]+ calcd for C20H15ClNS+ 336.0608, found 336.0608.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (79.5 mg, 89% yield). 1H NMR (600 MHz, Chloroform-d) δ 7.87 (dd, J = 7.8, 1.6 Hz, 1H), 7.49–7.41 (m, 3H), 7.45–7.38 (m, 3H), 7.41–7.33 (m, 5H), 3.71 (s, 3H), 2.13 (s, 3H). 13C NMR (151 MHz, Chloroform-d) δ 154.1, 143.2, 143.0, 141.5, 135.6, 135.5, 131.4, 130.8, 129.4, 129.3, 128.2, 127.1, 125.6, 122.7, 122.3, 119.9, 109.5, 30.7, 18.0. HRMS (ESI) m/z [M + H]+ calcd for C21H19N2+ 299.1543, found 299.1545.
1) as a white solid (79.5 mg, 89% yield). 1H NMR (600 MHz, Chloroform-d) δ 7.87 (dd, J = 7.8, 1.6 Hz, 1H), 7.49–7.41 (m, 3H), 7.45–7.38 (m, 3H), 7.41–7.33 (m, 5H), 3.71 (s, 3H), 2.13 (s, 3H). 13C NMR (151 MHz, Chloroform-d) δ 154.1, 143.2, 143.0, 141.5, 135.6, 135.5, 131.4, 130.8, 129.4, 129.3, 128.2, 127.1, 125.6, 122.7, 122.3, 119.9, 109.5, 30.7, 18.0. HRMS (ESI) m/z [M + H]+ calcd for C21H19N2+ 299.1543, found 299.1545.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (91.8 mg, 85% yield). 1H NMR (600 MHz, Chloroform-d) δ 7.93 (d, J = 8.0 Hz, 1H), 7.47–7.42 (m, 3H), 7.42–7.36 (m, 5H), 7.36–7.32 (m, 2H), 7.28–7.22 (m, 4H), 7.21–7.18 (m, 2H), 2.01 (s, 3H). 13C NMR (151 MHz, Chloroform-d) δ 153.3, 142.9, 141.6, 136.4, 135.6, 135.3, 131.1, 130.1, 129.4, 129.2, 128.1, 127.9, 127.0, 126.5, 125.3, 123.3, 122.9, 120.1, 116.2, 115.2, 110.5, 18.5. HRMS (ESI) m/z [M + H]+ calcd for C26H21N2+ 361.1699, found 361.1697.
1) as a white solid (91.8 mg, 85% yield). 1H NMR (600 MHz, Chloroform-d) δ 7.93 (d, J = 8.0 Hz, 1H), 7.47–7.42 (m, 3H), 7.42–7.36 (m, 5H), 7.36–7.32 (m, 2H), 7.28–7.22 (m, 4H), 7.21–7.18 (m, 2H), 2.01 (s, 3H). 13C NMR (151 MHz, Chloroform-d) δ 153.3, 142.9, 141.6, 136.4, 135.6, 135.3, 131.1, 130.1, 129.4, 129.2, 128.1, 127.9, 127.0, 126.5, 125.3, 123.3, 122.9, 120.1, 116.2, 115.2, 110.5, 18.5. HRMS (ESI) m/z [M + H]+ calcd for C26H21N2+ 361.1699, found 361.1697.Synthetic transformations
The compound 3a (1 mmol), substituted arylboronic acids (1.5 mmol), (PPh3)4Pd (0.02 mmol), and Cs2CO3 (2 mmol) in DMF/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 mL) were heated at 80 °C under argon overnight. After the reaction was completed, the mixture was extracted with ethyl acetate (30 ml × 3). The organic layer was washed with saturated saline and dried over anhydrous sodium sulfate. After removing the solvent in a vacuum, the resulting residue was purified by column chromatography on silica gel to give the desired products 4 and 5.
1, 20 mL) were heated at 80 °C under argon overnight. After the reaction was completed, the mixture was extracted with ethyl acetate (30 ml × 3). The organic layer was washed with saturated saline and dried over anhydrous sodium sulfate. After removing the solvent in a vacuum, the resulting residue was purified by column chromatography on silica gel to give the desired products 4 and 5.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (295.6 mg, 78% yield). 1H NMR (400 MHz, Chloroform-d) δ 9.70 (s, 1H), 8.34–8.25 (m, 1H), 8.15 (dd, J = 6.7, 2.7 Hz, 1H), 7.93 (dd, J = 8.6, 1.7 Hz, 1H), 7.70 (dd, J = 8.5, 0.6 Hz, 1H), 7.51–7.36 (m, 8H), 6.92 (d, J = 3.7 Hz, 1H), 2.67 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 177.3, 164.9, 152.1, 151.2, 144.2, 142.9, 141.5, 136.6, 133.0, 129.5, 129.4, 128.3, 127.2, 126.8, 125.9, 125.7, 122.8, 117.2, 111.2, 107.6, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C25H18NO3+ 380.1281, found 380.1285.
1) as a white solid (295.6 mg, 78% yield). 1H NMR (400 MHz, Chloroform-d) δ 9.70 (s, 1H), 8.34–8.25 (m, 1H), 8.15 (dd, J = 6.7, 2.7 Hz, 1H), 7.93 (dd, J = 8.6, 1.7 Hz, 1H), 7.70 (dd, J = 8.5, 0.6 Hz, 1H), 7.51–7.36 (m, 8H), 6.92 (d, J = 3.7 Hz, 1H), 2.67 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 177.3, 164.9, 152.1, 151.2, 144.2, 142.9, 141.5, 136.6, 133.0, 129.5, 129.4, 128.3, 127.2, 126.8, 125.9, 125.7, 122.8, 117.2, 111.2, 107.6, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C25H18NO3+ 380.1281, found 380.1285.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (337.5 mg, 90% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.16 (dd, J = 6.0, 3.2 Hz, 1H), 8.05 (d, J = 1.6 Hz, 1H), 7.73–7.56 (m, 4H), 7.52–7.39 (m, 7H), 7.33 (d, J = 8.0 Hz, 2H), 2.69 (s, 3H), 2.46 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.3, 149.8, 144.0, 142.7, 141.6, 138.3, 137.1, 136.4, 132.7, 129.6, 129.5, 129.4, 128.3, 127.4, 127.3, 127.2, 125.7, 124.6, 124.4, 118.4, 110.5, 21.1, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C27H22NO+ 376.1696, found 376.1698.
1) as a white solid (337.5 mg, 90% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.16 (dd, J = 6.0, 3.2 Hz, 1H), 8.05 (d, J = 1.6 Hz, 1H), 7.73–7.56 (m, 4H), 7.52–7.39 (m, 7H), 7.33 (d, J = 8.0 Hz, 2H), 2.69 (s, 3H), 2.46 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 164.3, 149.8, 144.0, 142.7, 141.6, 138.3, 137.1, 136.4, 132.7, 129.6, 129.5, 129.4, 128.3, 127.4, 127.3, 127.2, 125.7, 124.6, 124.4, 118.4, 110.5, 21.1, 19.3. HRMS (ESI) m/z [M + H]+ calcd for C27H22NO+ 376.1696, found 376.1698.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ra08212a.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 82404405), Fujian Provincial Natural Science Foundation of China (No.2023J05207), Ningxia Natural Science Foundation (No. 2024AAC03291), Major Cultivation Program of Ningde Normal University (No. 2025ZX041), and Scientific Research Fund project of Ningde Normal University (No. 2022Y18). The authors thank the Medical Sci-Tech Research Center of Ningxia Medical University (Medical Science Research Institution of Ningxia Hui Autonomous Region)for valuable help in our experiment.References
- H. A. Ali, M. A. Ismail, A. E. S. Fouda and E. A. Ghaith, RSC Adv., 2023, 13, 18262–18305 RSC.
- M. Migden, A. S. Farberg, R. Dummer, N. Squittieri and C. W. Hanke, J. Drugs Dermatol., 2021, 20, 156–165 CrossRef PubMed.
- A. Rusu, C. Tanase, G. Pascu and N. Todoran, Pharmaceuticals, 2020, 13, 217 CrossRef CAS.
- S. Singh, P. Geetha and R. Ramajayam, Result Chem., 2023, 6, 101135 CrossRef CAS.
- C. Dane, L. Stokes and W. T. Jorgensen, Expert Opin. Ther. Pat., 2022, 7, 769–790 CrossRef.
- P. Sasmal, S. K. Babasahib, B. R. P. Kumar and N. M. Raghavendra, Bioorg. Med. Chem., 2022, 73, 117001 CrossRef CAS.
- Z. Zhou, J. Zhang, E. Zhou, C. Ren, J. Wang and Y. Wang, Eur. J. Med. Chem., 2022, 240, 114595 Search PubMed.
- S. S. Malunavar, P. Prabhala, S. M. Sutar, R. Kapavarapu, M. K. Mittal and R. G. Kalkhambkar, Chem. Data Collect., 2022, 39, 100876 Search PubMed.
- S. Soni, N. Sahiba, S. Teli, P. Teli, L. K. Agarwal and S. Agarwal, RSC Adv., 2023, 34, 24093–24111 RSC.
- V. Dhayalan and M. Hayashi, Synth., 2012, 44, 2209–2216 CrossRef CAS.
- R. Yadav, D. Meena, K. Singh, R. Tyagi, Y. Yadav and R. Sagar, RSC Adv., 2023, 13, 21890–21925 RSC.
- A. Rendon-Patiño, A. Primo, B. Cojocaru, S. Gabriela Ion, D. G. Popescu, V. Parvulescu and H. Garcia, ChemCatChem, 2022, 14, e202200356 CrossRef.
- K. Bahrami and M. Bakhtiarian, ChemistrySelect, 2018, 3, 10875–10880 CrossRef CAS.
- J. Y. Chen, K. M. Li, Y. X. Sun, Y. Xiao, F. S. Guo, Y. B. Huang and Q. Lu, Green Chem., 2024, 26, 4834–4843 RSC.
- M. Bala, P. K. Verma, U. Sharm, N. Kumar and B. Singh, Green Chem., 2013, 15, 1687 RSC.
- T. T. Nguyen, X. T. T. Nguyen, T. L. H. Nguyen and P. H. Tran, ACS Omega, 2019, 4, 368–373 Search PubMed.
- Y. Zhou, W. Liu, Y. Liu, J. Guan, J. Yan, J. J. Yuan, D. J. Tao and Z. Song, Mol. Catal., 2020, 492, 111013 CAS.
- Q. Zhou, S. Liu, M. Ma, H. Z. Cui, X. Hong, S. Huang, J. F. Zhang and X. F. Hou, Synth., 2018, 50, 1315–1322 CrossRef CAS.
- Z. G. Niu, T. Zheng, Y. H. Su, P. J. Wang, X. Y. Li, F. Cui, J. Liang and G. N. Li, New J. Chem., 2015, 39, 6025–6033 Search PubMed.
- N. Gantasala, C. Fournet, M. L. Roch, C. Lalli, S. Pabbaraja and N. Gouault, Org. Biomol. Chem., 2022, 21, 5245–5253 RSC.
- Y. Ouyang, H. Si, C. Zhu, L. Zhong, H. Ma, Z. Li, H. Xiong, T. Liu, Z. Liu, Z. Zhang, Z. Zhang and Q. Cai, J. Med. Chem., 2022, 65, 7833–7842 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |





