 Open Access Article
Open Access ArticleElectrochemically grown Cu,Ni-BTC film for the electrochemical detection of caffeic acid in fruits
Nguyen Tien Dat a,
Tran Vinh Hoanga,
Phan Thi Quyenb,
Dau Thi Ngoc Ngac and
Vu Thi Thu
a,
Tran Vinh Hoanga,
Phan Thi Quyenb,
Dau Thi Ngoc Ngac and
Vu Thi Thu *c
*c
aHanoi University of Science and Technology (HUST), 1 Dai Co Viet, Bach Mai, Hanoi, Vietnam
bHanoi University of Industry (HaUI), 298 Cau Dien, Tay Tuu, Hanoi, Vietnam
cUniversity of Science and Technology of Hanoi (USTH), Vietnam Academy of Science and Technology (VAST), 18 Hoang Quoc Viet, Nghia Do, Hanoi, Vietnam. E-mail: thuvu.edu86@gmail.com; vu-thi.thu@usth.edu.vn
First published on 19th January 2026
Abstract
Caffeic acid (CA) is an important antioxidant often found in many kinds of fruits. In this study, we developed an electrochemical sensor based on an electrochemically grown bimetallic metal–organic framework film (Cu,Ni-BTC) for the in situ determination of CA in fruits. The Cu,Ni-BTC film was grown directly onto a glassy carbon electrode (GCE) via a cathodic electrodeposition method under optimal conditions. The results showed that a uniform film made of Cu,Ni-BTC metal–organic frameworks with a Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni molar ratio of 3.67 was grown on the electrode surface. It was found that the charge transfer kinetics were much improved on the electrode modified with the Cu,Ni-BTC material. Consequently, the caffeic acid sensor based on the Cu,Ni-BTC film showed a detection limit and sensitivity as low as 0.7 µM (1.5 times less than that of the unmodified electrode) and 0.924 µA µM−1 cm−2 (13.2 times higher than that of the unmodified electrode), respectively. The developed sensor also exhibited high reproducibility (RSD = 2.61%), good stability (RSD = 6.47%) after 7 days, and good selectivity towards CA. Real sample tests using apple juice showed good recovery (92.2–98.4%). Further studies will be conducted in the near future to better understand the growth mechanism of the MOF films, control their morphology and utilize them in other electronic applications.
Ni molar ratio of 3.67 was grown on the electrode surface. It was found that the charge transfer kinetics were much improved on the electrode modified with the Cu,Ni-BTC material. Consequently, the caffeic acid sensor based on the Cu,Ni-BTC film showed a detection limit and sensitivity as low as 0.7 µM (1.5 times less than that of the unmodified electrode) and 0.924 µA µM−1 cm−2 (13.2 times higher than that of the unmodified electrode), respectively. The developed sensor also exhibited high reproducibility (RSD = 2.61%), good stability (RSD = 6.47%) after 7 days, and good selectivity towards CA. Real sample tests using apple juice showed good recovery (92.2–98.4%). Further studies will be conducted in the near future to better understand the growth mechanism of the MOF films, control their morphology and utilize them in other electronic applications.
1. Introduction
Caffeic acid (CA) (3,4-dihydro-cinnamic acid, C9H8O4) is a polyphenol antioxidant found in various plant species (apples and olives), foods (oils), beverages (beer, wine, and pear juice) and some medicines.1 The mechanism behind the antioxidant behavior of CA is mainly related to the interaction between this molecule and free radicals via hydrogen atom transfer and single-electron transfer.2 CA has shown antiproliferative effects against various cancer cells,3 bacteria and viruses4,5 and protective effects against brain-related diseases.6 For these reasons, CA has been used in skincare products, liver protection products, antioxidant supplements, herbal supplements, medicines,7 and foodstuffs. Hence, it is essential to develop tools for monitoring CA levels in foods (fresh fruits, beverages and other foods) and other kinds of products. Conventional methods used to detect CA, including high-performance liquid chromatography (HPLC),8 gas chromatography/mass spectrometry (GC/MS),9 and capillary electrophoresis,10 can provide high accuracy, but all of them are time-consuming and expensive and require highly skilled operators.Electrochemical sensors are a fast and on-site tool for monitoring the level of electroactive compounds such as caffeic acid. Different kinds of materials have been employed to modify the electrode surface in order to improve the electrochemical signals via the enhancement of its charge transfer, electrical conductivity or adsorption capacity for the target compound. Conducting polymers such as PEDOT11 and polydopamine12 have been utilized to improve the stability and conductivity of sensors. Metal nanoparticles (Au and Ag),13,14 bimetallic nanostructures (Pt–Ru15 and Pt–Ni16), and oxide particles17,18 have been widely utilized or combined with other materials to improve electron transfer kinetics and electrical conductivity and possibly introduce electrocatalytic activity. Carbon-based materials19 and two-dimensional materials20 have also been demonstrated to be quite effective in improving the performance of sensors for electrochemical CA detection. A common drawback of the above-given sensing materials is their limited adsorption of the target organic compound such as CA due to their rigid nature.
Metal–organic frameworks (MOFs) are crystalline porous materials assembled using metal ions and organic linkers. In recent years, MOFs have gained significant attention due to their high specific surface area, tunable porosity, and abundance of metal active sites,21,22 despite their low conductivity and stability. Specifically, bimetallic MOFs with mixed-metal centers have been demonstrated to leverage the synergy, thereby enhancing their electrocatalytic performance and stability.23 Jun et al. reported a Ni–Cu bimetallic MOF with high energy density and good stability for energy storage.24 Several works have demonstrated the use of bimetallic MOF-based sensors for electrochemically detecting phenolic compounds. For example, Gong et al. developed a sensing platform using 2D bimetallic Ni/Co MOFs and MWCNTs for dopamine, acetaminophen, and bisphenol A (BPA) detection.25 Nguyen et al. developed a sensing platform for BPA detection using a Cu,Zn-BTC/CNT composite.26 Huang et al. developed a novel bimetallic Ce–Ni-MOF with high sensitivity and a low LOD for BPA determination.27 Several studies have reported the use of MOFs (GR/CuO@Cu-BTC28 and NH2-MIL-101(Fe) nanoparticles29) for the electrochemical detection of CA.
Usually, the MOF materials are drop-casted onto the electrode surface, thus limiting their adhesion and increasing the electrode resistance, which are not very beneficial for the sensing performances of the developed electrochemical sensor. Several techniques, such as chemical vapor deposition,30 liquid-phase epitaxy,31 and gas ALD/MLD,32 have been developed to create MOF thin films. The disadvantages of these techniques are their time-consuming nature, high cost and low reproducibility due to complex fabrication processes and expensive instruments.33 However, MOF thin films fabricated via electrochemical methods have much shorter synthesis times and good controllability.34–36
In this work, we describe an electrochemical sensing platform with high sensitivity and good selectivity based on a bimetallic MOF (Cu,Ni-BTC) for CA detection. The Cu,Ni-BTC film was electrodeposited on a GCE by chronoamperometry. The presence of an alternative metal center (Ni2+) with good electrocatalytic activity, along with a copper center (Cu2+), might be beneficial for improving the electrical conductivity and electrochemical kinetics at the electrode/electrolyte interface. The porous structure of MOF-based materials should facilitate better adsorption of the target organic compounds onto the electrode surface, as usual, thus improving the recorded electrochemical signals. The morphology, structure and electrochemical behavior of the as-prepared film were investigated, and the correlation between these factors was analyzed to better understand why the electrochemical signals were improved. A new mechanism for the electrochemical oxidation of CA on the MOF-based film was proposed. The performances (calibration curve, repeatability, reproducibility, stability, interference and real sample tests) of the sensing platform were thoroughly tested. The aim of this work was to develop a highly sensitive sensor for in situ CA detection in fruits using an electrochemically grown bimetallic metal–organic framework film (Cu,Ni-BTC).
2. Experimental
2.1. Materials
Copper(II) chloride (CuCl2·2H2O), nickel chloride hexahydrate (NiCl2·6H2O), trimesic acid (H3BTC), N,N-dimethylformamide (DMF, 98%), potassium ferricyanide (K3[Fe(CN)6]) and potassium ferrocyanide (K4[Fe(CN)6]·3H2O) were purchased from Sigma-Aldrich and used without further purification. All aqueous solutions were prepared daily with double-distilled water at room temperature. Glassy carbon electrodes (GCEs) (diameter = 3 mm) were used and served as the working electrode for all experiments.2.2. Electrochemical growth of the Cu,Ni-BTC film on the GCE
Cu,Ni-BTC was synthesized using a cathodic electrodeposition method. The electrodeposition solution was prepared by dissolving 10 mM Et3N, 15 mM H3BTC, 9 mM CuCl2·2H2O and 1 mM NiCl2·6H2O in a DMF solvent under vigorous stirring for 3 minutes to ensure homogeneity. Before modifying the electrode surface, the GCE was polished with a 0.3-µm Al2O3 nanopowder, followed by rinsing thoroughly with ethanol and distilled water and drying at room temperature. The initial investigation of the electrochemical deposition of the Cu,Ni-BTC film was conducted using cyclic voltammetry (CV) in the potential range from −1.5 V to 0 V at a scan rate of 50 mV s−1 for 5 cycles. To obtain a more uniform film, chronoamperometry was employed at applied voltages of −0.8 V, −0.9 V, −1 V and −1.1 V (vs. Ag/AgCl) for 5 minutes under identical conditions, and the results were compared to identify the optimal growth conditions of the Cu,Ni-BTC film on the GCE, which were then selected for further experiments.2.3. Morphological and structural characterizations of the electrodeposited Cu,Ni-BTC film
The surface morphology of the samples was examined using field-emission scanning electron microscopy (FE-SEM, JEOL JMS-IT800) at an accelerating voltage of 5 kV. Energy-dispersive X-ray spectroscopy (EDX, JED-2300) was used to identify and quantify the elements present in the samples. X-ray photoelectron spectroscopy (XPS) measurements were performed using a PHI Versa Probe III XPS system employing a monochromatic Al-Kα source at 1486.6 eV. All electrochemical measurements were performed on an Autolab PGSTAT 302N controlled by NOVA 2.0 software equipped with a conventional three-electrode system. The system consisted of a platinum (Pt) auxiliary electrode, an Ag/AgCl/sat. KCl reference electrode, and a bare GCE or modified electrodes as the working electrodes.2.4. Characterization of the electrochemical behaviors of the electrodeposited Cu,Ni-BTC film
The electrochemical behavior of the bare electrode and modified electrodes was investigated using the cyclic voltammetry (CV) technique in a 0.1 M KCl solution and a 0.1 M KCl solution containing 5 mM Fe(CN)63−/4− (conducted separately). To estimate the charge transfer resistance, electrochemical impedance spectroscopy (EIS) measurements were performed in a 0.1 M KCl solution containing 5 mM Fe(CN)63−/4− in the frequency range from 10−1 to 105 Hz.2.5. Oxidation of caffeic acid on the electrodeposited Cu,Ni-BTC film
The reaction mechanism of CA oxidation on the bare and modified electrodes was investigated by scanning electrodes in a PBS solution (pH 3) containing 500 µM caffeic acid in the potential window between −400 mV and 1 V at a scan rate of 50 mV s−1. The effect of the scan rate and pH level on the redox behaviors of CA on the GCE and Cu,Ni-BTC/GCE was also evaluated by the CV technique.2.6. Sensing performances
3. Results and discussions
3.1. Electrochemical synthesis process of the Cu,Ni-BTC film
The Cu,Ni-BTC film is electrodeposited on a glassy carbon electrode using the cathodic deposition method. The formation of the Cu,Ni-BTC film begins with the deprotonation of BTC3− ions derived from the H3BTC organic ligand. This is followed by the coordination of BTC3− with Cu2+ and Ni2+ ions derived from CuCl2·2H2O and NiCl2·6H2O, respectively, to form Cu,Ni-BTC crystals. As observed in Fig. S1, a distinct peak corresponding to H+ reduction at −0.88 V is observed in the first scan cycle. This peak is associated with the deprotonation of the organic ligand via the following reaction: H3BTC + 3Et3N → BTC3− + 3Et3NH+.37 In the subsequent cycles, the intensity of this peak decreases due to a local decrease in the concentration of the precursor (H3BTC) near the electrode surface. Triethylamine (Et3N) is used as a probase to accelerate the deprotonation of H3BTC in solution.38 Other probases, like nitrate (NO3−), can be used in the MOF synthesis process; however, they generally demand high reduction overpotentials.38–40 Simultaneously, under negative potentials, water molecules from hydrated salts (CuCl2·2H2O and NiCl2·6H2O) undergo electrolysis (2H2O + 2e− → H2 + 2OH−), which contributes to the increase in the local pH near the electrode surface, thereby promoting the growth of MOF crystals.41 In addition, the peak observed at −0.24 V in the first and second cycles might originate from the oxidation of Cu(0), which exists on the electrode surface due to the partial reduction of the Cu2+ precursor at the beginning of the growth process.3.2. Survey of the structure and morphology of the Cu,Ni-BTC film
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni is 3.67, which is different from the initial precursor ratio of 9
Ni is 3.67, which is different from the initial precursor ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, probably due to the stronger affinity of Ni2+ ions for BTC ligands compared to Cu2+ ions.
1, probably due to the stronger affinity of Ni2+ ions for BTC ligands compared to Cu2+ ions.
The growth of metal–organic framework (MOF) thin films represents a critical advancement towards integrating MOFs into practical devices such as sensors and optoelectronic devices.43,44 MOF thin films can be fabricated through various methods, including solvothermal synthesis, layer-by-layer assembly, vapor-phase deposition, and electrochemical techniques.45,46 Among these, electrodeposition offers distinct benefits, such as mild synthesis conditions at room temperature, short growth times, high scalability, energy efficiency, precise control over the film thickness and homogeneity, and the ability to form uniform coatings on complex geometries without requiring high temperatures or pressures.47
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
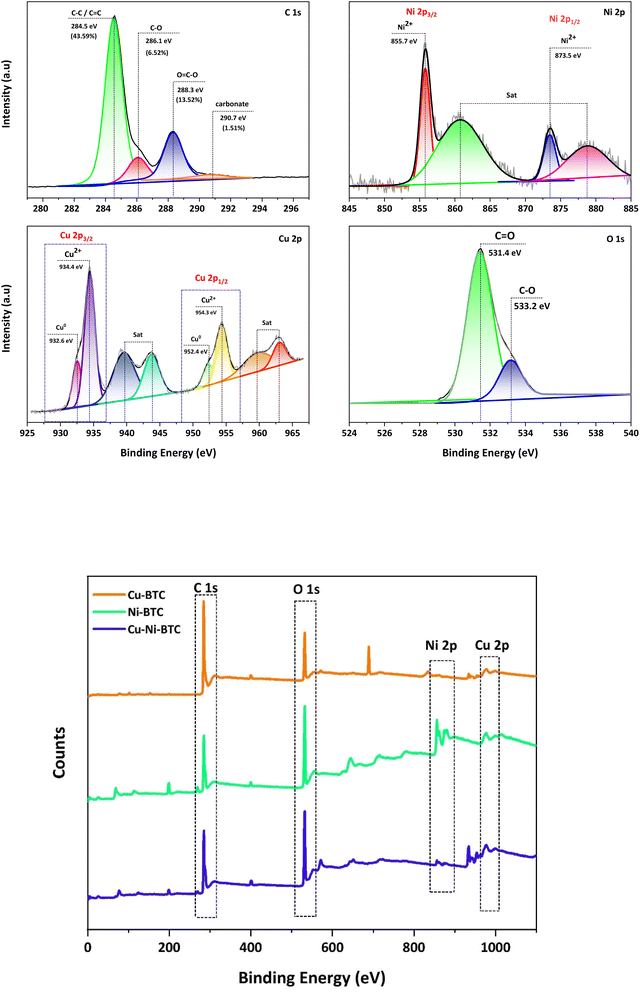
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni is close to 3.39, aligning with the EDX results discussed above. In the C 1s region, there is one main peak at 284.5 eV (C–C/C
Ni is close to 3.39, aligning with the EDX results discussed above. In the C 1s region, there is one main peak at 284.5 eV (C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and three other peaks at higher binding energies, which correspond to C–O (286.1 eV), O–C
C) and three other peaks at higher binding energies, which correspond to C–O (286.1 eV), O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.3 eV) and carbonate (290.7 eV). The O 1s spectrum shows two binding energies corresponding to C
O (288.3 eV) and carbonate (290.7 eV). The O 1s spectrum shows two binding energies corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (531.4 eV) and C–O (533.2 eV). The high-resolution Cu 2p and Ni 2p spectra show the chemical states of Cu2+ and Ni2+, respectively. In the Ni 2p region, the binding energies at 855.7 eV and 861 eV are assigned to Ni 2p3/2 and its satellite peaks, while the binding energies at 873.5 eV and 878 eV are attributed to Ni 2p1/2 and its satellite peaks, respectively.24 In the Cu 2p region, the characteristic Cu2+ binding energy peaks are observed at 934.4 eV (Cu 2p3/2) and 954.3 eV (Cu 2p1/2), whereas the binding energy peaks located at 932.6 eV (Cu 2p3/2) and 952.4 (Cu 2p1/2) are related to Cu0.48,49 This indicates that a portion of Cu2+ ions in the Cu,Ni-BTC structure is reduced to Cu0, leading to the coexistence of Cu2+ and Cu0 within the Cu,Ni-BTC structure.
O (531.4 eV) and C–O (533.2 eV). The high-resolution Cu 2p and Ni 2p spectra show the chemical states of Cu2+ and Ni2+, respectively. In the Ni 2p region, the binding energies at 855.7 eV and 861 eV are assigned to Ni 2p3/2 and its satellite peaks, while the binding energies at 873.5 eV and 878 eV are attributed to Ni 2p1/2 and its satellite peaks, respectively.24 In the Cu 2p region, the characteristic Cu2+ binding energy peaks are observed at 934.4 eV (Cu 2p3/2) and 954.3 eV (Cu 2p1/2), whereas the binding energy peaks located at 932.6 eV (Cu 2p3/2) and 952.4 (Cu 2p1/2) are related to Cu0.48,49 This indicates that a portion of Cu2+ ions in the Cu,Ni-BTC structure is reduced to Cu0, leading to the coexistence of Cu2+ and Cu0 within the Cu,Ni-BTC structure.
 | ||
| Fig. 2 XPS analysis of the electrodeposited Cu,Ni-BTC film. The survey spectrum and high-resolution C 1s, O 2p, Cu 2p, and Ni 2p spectra were recorded using monochromatic Al–K (1486.6 eV). | ||
To better understand the state of vacancies in the Cu,Ni-BTC film, we also measured the XPS spectra of Cu-BTC and Ni-BTC films grown via a similar approach (see more details in the SI). For the Cu-BTC film, the two peaks associated with Cu2+ are observed at 934.7 eV (Cu 2p3/2) and 954.1 eV (Cu 2p1/2) in the Cu 2p spectrum, whereas a peak located at 532.0 eV is observed in the O 1s spectrum. For the Ni-BTC film, the two peaks associated with Ni2+ are observed at 856.1 eV (Ni 2p3/2) and 873.7 eV (Ni 2p1/2) in the Ni 2p spectrum, whereas two peaks located at 531.5 eV and 532.9 eV are observed in the O 1s spectrum. The presence of the satellite peak at a higher binding energy (533.2 eV) in the O 1s spectrum of Cu,Ni-BTC might be due to the increase in the concentration of oxygen vacancies in MOF structures; meanwhile, the shift in the positions of peaks related to Cu2+ and Ni2+ is ignorable.50–52
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in benzene rings at 1006 cm−1, and the symmetric and asymmetric stretching of O–C–O at 1458 cm−1 and 1548 cm−1, respectively.53 In addition, the vibrational modes relevant to Cu2+ ions are observed in the 100–500 cm−1 region. Further, the bands at 185 cm−1 and 277 cm−1 correspond to Cu–O and Cu–Cu atomic vibrations, respectively, while the band at 500 cm−1 is linked to Cu–O stretching involving carboxylate bridging oxygen atoms.53,54 These results indicate the successful coordination of copper ions and nickel ions with BTC.
C in benzene rings at 1006 cm−1, and the symmetric and asymmetric stretching of O–C–O at 1458 cm−1 and 1548 cm−1, respectively.53 In addition, the vibrational modes relevant to Cu2+ ions are observed in the 100–500 cm−1 region. Further, the bands at 185 cm−1 and 277 cm−1 correspond to Cu–O and Cu–Cu atomic vibrations, respectively, while the band at 500 cm−1 is linked to Cu–O stretching involving carboxylate bridging oxygen atoms.53,54 These results indicate the successful coordination of copper ions and nickel ions with BTC.3.3. Survey of the electrochemical properties of the Cu,Ni-BTC film
3.4. Mechanism of the oxidation of caffeic acid on the Cu,Ni-BTC film
CV was employed to investigate the electrochemical behavior of CA on the bare GCE, Ni-BTC/GCE, Cu-BTC/GCE, and Cu,Ni-BTC/GCE in the presence of 500 µM CA in a PBS buffer with pH = 3 at a scan rate of 50 mV s−1 (Fig. 4). It can be seen that the peak separation was shortened (by 160 mV) and the peak current was increased (by 1.5 times) on the Cu,Ni-BTC-modified GCE compared with the bare electrode. As mentioned above, the good electrocatalytic activity of bimetallic centers and the effective adsorption of target molecules inside the porous structure of the Cu,Ni-BTC film are mainly responsible for such good kinetics of electrochemical reactions.
 | ||
| Fig. 4 Mechanism for the electrochemical oxidation and reduction of caffeic acid on the electrodeposited Cu,Ni-BTC film. (A) Cyclic voltammograms recorded on the Cu,Ni-BTC film, Cu-BTC, Ni-BTC and GCE in a PBS solution (pH 3) containing 500 µM caffeic acid and (B) the proposed redox mechanism of CA on the Cu,Ni-BTC film.52 | ||
The oxidation of CA on the bare GCE follows a different reaction pathway (Fig. S3). At anodic potentials, one hydroxyl group (–OH) on the catechol loses an electron (−e) to the electrode, generating a semiquinone radical (–O˙) on the aromatic ring. Simultaneously, one proton (H+) from the –OH group is released into the solution, and the resonance is stabilized across the aromatic ring. Then, the second hydroxyl group loses another electron and proton to form ortho-quinone.62 Because the ortho-quinone has a conjugated system with double bonds and a carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), it can more easily receive 2e− and 2H+ to regenerate CA.
O), it can more easily receive 2e− and 2H+ to regenerate CA.
The key difference in the oxidation mechanisms of CA on the bare and modified electrodes is related to the presence of metal centers. In the case of the bare electrode, the electrochemical oxidation of CA is simply governed by the charge exchange between the electrode surface and CA molecules; thus, the recorded signals are not very high, and the oxidation process is more reversible. Otherwise, there is a strong involvement of metal ions in the electrochemical oxidation of CA molecules on MOF-modified electrodes; then, the electrochemical signals are much improved, whereas the reaction is somehow quasi-reversible (as discussed in the next sections).
3.5. Sensing performances
| Electrode | Linear range (µM) | Regression equation | Sensitivity (µA µM−1 cm−2) | LOD (µM) | LOQ (µM) |
|---|---|---|---|---|---|
| Cu,Ni-BTC | 2–25 | I (µA) = 0.0647 × Ccaffeic acid (µM) − 0.0485 (R2 = 0.9987) | 0.924 | 0.70 | 2.33 |
| Cu-BTC | 2–25 | I (µA) = 0.0437 × Ccaffeic acid (µM) − 0.0576 (R2 = 0.9968) | 0.624 | 1.11 | 3.70 |
| Ni-BTC | 2–25 | I (µA) = 0.0319 × Ccaffeic acid (µM) + 0.2092 (R2 = 0.9946) | 0.456 | 1.44 | 4.80 |
| Bare GCE | 2–25 | I (µA) = 0.0049 × Ccaffeic acid (µM) + 0.0040 (R2 = 0.997) | 0.070 | 1.07 | 3.57 |
| Electrode | Matrix/sample | Linear range (µM) | Sensitivity (µA µM−1 cm−2) | LOD (µM) | References |
|---|---|---|---|---|---|
| a Note: DL-PAMMCNTPE, DL-phenylalanine (DL-PA)-modified multiwalled carbon nanotube paste electrode. | |||||
| CuO/GCE | Caffeinated drinks | 0.05–229.5 | 0.0722 | 0.04 | 18 |
| GR/CuO@Cu-BTC/GCE | Red wine | 0.02–10 | 1.873 | 0.007 | 28 |
| CuONPs/CPE | Honey | 5.0–50.0 | 0.9677 | 3.21 | 17 |
| DL-PAMMCNTPE | Apple juice and coffee powder | 20–600 | — | 0.28 | 64 |
| Co-NC/MWCNTs/GCE | Food samples | 0.5–50 | — | 0.162 | 65 |
| CaO/g-C3N4/PVA/GCE | Blood plasma samples | 0.01–70 | — | 0.0024 | 66 |
| PtNi/C/GCE | Wine samples | 0.75–591.783 | 0.0389 | 0.5 | 16 |
| 111.783–591.783 | 0.0107 | ||||
| CoFeSe2/f-CNF/GCE | Wine sample | 0.01–263.96 | 2.04 | 0.002 | 67 |
| MCO/GR/GCE | Food sample | 0.01–0.69 | 1.494 | 0.003 | 68 |
| Cu,Ni-BTC/GCE | Apple juice | 2–25 | 0.924 | 0.7 | This work |
 | ||
| Fig. 8 (A) Reproducibility, (B) stability and (C) interference tests. Values represent mean ± SD (n = 3). | ||
The stability study was carried out by synthesizing a Cu,Ni-BTC-modified GCE and performing daily DPV measurements in a PBS solution (pH = 3) containing 20 µM CA for seven consecutive days (Fig. 8B). It was found that the as-prepared sensor exhibited good stability for one week with RSD = 6.47% (Table S5) and then started to decay, and the RSD remained only 11.37% on the 11th day.
4. Conclusions
In this study, we successfully developed an electrochemical sensor for in situ CA determination using an electrochemically grown bimetallic MOF film (Cu,Ni-BTC). The results demonstrated that the Cu,Ni-BTC-modified electrode had higher conductivity and electron transfer ability than the homometallic Cu-BTC, Ni-BTC and bare electrode. The sensitivity and LOD of the as-prepared Cu,Ni-BTC/GCE for CA detection were 0.924 µA µM−1 cm−2 (improved by 13.2 times compared to the bare electrode) and 0.7 µM (improved by 1.5 times compared to the bare electrode), respectively. The developed sensor also exhibited high reproducibility (RSD = 2.61%), good stability (RSD = 6.47%), and good recovery in real samples (92.5–95%). These findings pave the way to developing highly uniform porous films based on metal–organic framework structures for further sensing applications. In the near future, our research team will focus more on the formation of uniform and thin MOF films that exhibit many interesting electronic behaviors (i.e., gating effect in transistors) for other sensing applications.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ra08073h.Acknowledgements
The authors would like to express our sincere thanks to the financial support from University of Science and Technology of Hanoi (USTH) for research group “Sensing systems based on Electronic NanoStructures (SENS)” (SENS) through the research program for promoting emerging and top-tier research groups at USTH.References
- A. Birková, Caffeic acid: a brief overview of its presence, metabolism, and bioactivity, Bioact. Compd. Health Dis., 2020, 3, 74 Search PubMed.
- K. M. M. Espíndola, et al., Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma, Front. Oncol., 2019, 9, 541 CrossRef.
- K. Celińska-Janowicz, et al., Constituents of Propolis: Chrysin, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid Induce PRODH/POX-Dependent Apoptosis in Human Tongue Squamous Cell Carcinoma Cell (CAL-27), Front. Pharmacol, 2018, 9, 336 CrossRef.
- E. Pinho, et al., Antibacterial Potential of Northeastern Portugal Wild Plant Extracts and Respective Phenolic Compounds, BioMed Res. Int., 2014, 2014, 1–8 Search PubMed.
- H. Utsunomiya, et al., Inhibition by caffeic acid of the influenza A virus multiplication in vitro, Int. J. Mol. Med., 2014, 34, 1020–1024 Search PubMed.
- D. Huang, et al., Evaluation on monoamine neurotransmitters changes in depression rats given with sertraline, meloxicam or/and caffeic acid, Genes Dis., 2019, 6, 167–175 Search PubMed.
- M. Yazar, M. Sevindik, I. Uysal and A. O. Polat, Effects of Caffeic Acid on Human Health: Pharmacological and Therapeutic Effects, Biological Activity and Toxicity, Pharm. Chem. J., 2025, 59, 49–55 Search PubMed.
- H. Wang, Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC, Food Chem., 2004, 87, 307–311 Search PubMed.
- O. J. Lara-Guzmán, R. Álvarez-Quintero, E. Osorio, M. Naranjo-Cano and K. Muñoz-Durango, GC/MS method to quantify bioavailable phenolic compounds and antioxidant capacity determination of plasma after acute coffee consumption in human volunteers, Food Res. Int., 2016, 89, 219–226 CrossRef PubMed.
- N. Z. Alzoman, et al., CE-DAD Determination of Scutellarein and Caffeic Acid in Abelia triflora Crude Extract, J. Chromatogr. Sci., 2018, 56, 746–752 CAS.
- V. Karabozhikova and V. Tsakova, Electroanalytical determination of caffeic acid – Factors controlling the oxidation reaction in the case of PEDOT-modified electrodes, Electrochim. Acta, 2019, 293, 439–446 CrossRef CAS.
- L. C. Almeida, et al., Electrochemical deposition of bio-inspired laccase-polydopamine films for phenolic sensors, Electrochim. Acta, 2019, 319, 462–471 CrossRef CAS.
- D. E. F. dos Santos, et al., A Sustainable Nanomaterial Based on Gold Nanoparticles and Graphene for Highly Sensitive Electrochemical Sensing of Caffeic Acid in Coffees, Food Anal. Methods, 2024, 17, 1348–1358 CrossRef.
- G. Kesavan, T. Subramaniam and H. V. Manemaran, Development of Promising Flower-like Ag/SrFeO3 Nanosheet Electrode Materials: An Efficient and Selective Electrocatalytic Detection of Caffeic Acid in Coffee and Green Tea, ACS Omega, 2023, 8, 46414–46424 CrossRef CAS.
- Y. Li, et al., An ultrasensitive dietary caffeic acid electrochemical sensor based on Pd-Ru bimetal catalyst doped nano sponge-like carbon, Food Chem., 2023, 425, 136484 CrossRef CAS PubMed.
- J. Wang, et al., Ultra-stable Electrochemical Sensor for Detection of Caffeic Acid Based on Platinum and Nickel Jagged-Like Nanowires, Nanoscale Res. Lett., 2019, 14, 11 CrossRef PubMed.
- M. G. Galugerdi, E. Mahmoodi-Khaledi and H.-A. Rafiee-Pour, Detection of caffeic acid in honey using carbon paste electrode modified by copper (II) oxide (CuO) nanoparticles, Sci. Rep., 2025, 15, 23397 CrossRef CAS PubMed.
- A. Venkadesh, J. Mathiyarasu and S. Radhakrishnan, MOF mediated synthesis of porous copper oxide and their electrochemical sensing of caffeic acid in caffeinated drinks, Inorg. Chem. Commun., 2021, 128, 108573 Search PubMed.
- B. Parasuraman, et al., Rapid detection of caffeic acid in food beverages using a non-enzymatic electrochemical sensor based on a Bi2S3/CNF nanocomposite, Sustainable Food Technol., 2024, 2, 717–728 RSC.
- Y. A. Kumar, et al., A review on in-plane ordered MXenes-based materials in addressing challenges faced by biosensor applications, J. Energy Storage, 2025, 121, 116507 Search PubMed.
- Y. Xu, Q. Li, H. Xue and H. Pang, Metal-organic frameworks for direct electrochemical applications, Coord. Chem. Rev., 2018, 376, 292–318 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, The Chemistry and Applications of Metal-Organic Frameworks, Science, 2013, 341, 1230444 CrossRef PubMed.
- L. Chen, H.-F. Wang, C. Li and Q. Xu, Bimetallic metal–organic frameworks and their derivatives, Chem. Sci., 2020, 11, 5369–5403 RSC.
- J. Wan, et al., Transition Bimetal Based MOF Nanosheets for Robust Aqueous Zn Battery, Front. Mater., 2020, 7, 194 CrossRef.
- X. Gong, et al., A highly sensitive sensing strategy based on 2D bimetallic MOFs and multi-walled carbon nanotubes, Microchem. J., 2024, 205, 111135 CrossRef CAS.
- N. N. Tien, et al., Cu,Zr-BTC/CNT composite for electrochemical detection of endocrine disruptor bisphenol A, J. Mater. Sci., 2023, 58, 16699–16713 CrossRef CAS.
- X. Huang, et al., Fabrication of novel electrochemical sensor based on bimetallic Ce-Ni-MOF for sensitive detection of bisphenol A, Anal. Bioanal. Chem., 2020, 412, 849–860 CrossRef CAS.
- X. Tu, et al., Self-template synthesis of flower-like hierarchical graphene/copper oxide@copper(II) metal-organic framework composite for the voltammetric determination of caffeic acid, Microchim. Acta, 2020, 187, 258 CrossRef CAS.
- L. Zheng, et al., In Situ NH2-MIL-101(Fe) Nanoparticles Modified Pencil Core Electrodes for Simultaneous Ratiometric Electrochemical Detection of Caffeic Acid and Acetaminophen, ACS Appl. Nano Mater., 2024, 7, 16295–16305 CrossRef CAS.
- I. Stassen, et al., Chemical vapour deposition of zeolitic imidazolate framework thin films, Nat. Mater., 2016, 15, 304–310 Search PubMed.
- O. Shekhah, et al., Controlling interpenetration in metal–organic frameworks by liquid-phase epitaxy, Nat. Mater., 2009, 8, 481–484 CrossRef CAS PubMed.
- K. B. Lausund and O. Nilsen, All-gas-phase synthesis of UiO-66 through modulated atomic layer deposition, Nat. Commun., 2016, 7, 13578 CrossRef CAS.
- X. Zhang, et al., Electrochemical deposition of metal–organic framework films and their applications, J. Mater. Chem. A, 2020, 8, 7569–7587 Search PubMed.
- M. S. Hosseini, S. Zeinali and M. H. Sheikhi, Fabrication of capacitive sensor based on Cu-BTC (MOF-199) nanoporous film for detection of ethanol and methanol vapors, Sens. Actuators, B, 2016, 230, 9–16 CrossRef CAS.
- J. Feng, T.-F. Liu and R. Cao, An Electrochromic Hydrogen-Bonded Organic Framework Film, Angew. Chem., Int. Ed., 2020, 59, 22392–22396 Search PubMed.
- J. Huang, et al., Electrochemical Exfoliation of Pillared-Layer Metal–Organic Framework to Boost the Oxygen Evolution Reaction, Angew. Chem., Int. Ed., 2018, 57, 4632–4636 CrossRef CAS.
- B. Zhang, et al., One-step controlled electrodeposition of iron-based binary metal organic nanocomposite, Appl. Surf. Sci., 2020, 504, 144504 CrossRef CAS.
- M. Li and M. Dincă, Selective formation of biphasic thin films of metal–organic frameworks by potential-controlled cathodic electrodeposition, Chem. Sci., 2014, 5, 107–111 RSC.
- M. Li and M. Dincă, On the Mechanism of MOF-5 Formation under Cathodic Bias, Chem. Mater., 2015, 27, 3203–3206 CrossRef CAS.
- V. Rosca, M. Duca, M. T. de Groot and M. T. M. Koper, Nitrogen Cycle Electrocatalysis, Chem. Rev., 2009, 109, 2209–2244 CrossRef CAS PubMed.
- N. Campagnol, et al., On the electrochemical deposition of metal–organic frameworks, J. Mater. Chem. A, 2016, 4, 3914–3925 RSC.
- N. Tien Dat, N. Ngoc Tien, N. T. T. Ngan and V. Thi Thu, Sensing interface based on electrodeposited Cu-BTC microporous film for electrochemical detection of the painkiller paracetamol, Analyst, 2023, 148, 1777–1785 RSC.
- M. M. Sabzehmeidani and M. Kazemzad, Recent advances in surface-mounted metal–organic framework thin film coatings for biomaterials and medical applications: a review, Biomater. Res., 2023, 27, 115 CrossRef PubMed.
- M. M. Sabzehmeidani, S. Gafari, S. jamali and M. Kazemzad, Concepts, fabrication and applications of MOF thin films in optoelectronics: A review, Appl. Mater. Today, 2024, 38, 102153 Search PubMed.
- L. Xue, G. Luo, X. Yang, Y. Qin and B. Zhang, Vapor-phase methods for synthesizing metal-organic framework thin films, Innovation Mater., 2024, 2, 100047 CrossRef CAS.
- A. Bétard and R. A. Fischer, Metal–Organic Framework Thin Films: From Fundamentals to Applications, Chem. Rev., 2012, 112, 1055–1083 Search PubMed.
- W.-J. Li, M. Tu, R. Cao and R. A. Fischer, Metal–organic framework thin films: electrochemical fabrication techniques and corresponding applications & perspectives, J. Mater. Chem. A, 2016, 4, 12356–12369 RSC.
- J. Zhang, T. Zhou and L. Wen, Selective Metallization Induced by Laser Activation: Fabricating Metallized Patterns on Polymer via Metal Oxide Composite, ACS Appl. Mater. Interfaces, 2017, 9, 8996–9005 CrossRef CAS.
- Y. Shao, et al., Degradation of chlortetracycline with simultaneous removal of copper (II) from aqueous solution using wheat straw-supported nanoscale zero-valent iron, Chem. Eng. J., 2020, 379, 122384 Search PubMed.
- L. Zhang, H. Yu, L. Han and K. Tao, Vacancy engineering of metal–organic framework derivatives for supercapacitors and electrochemical water splitting, Chem. Commun., 2025, 61, 8830–8842 RSC.
- C. Chen, X. Ji, Y. Xiong and J. Jiang, Ni/Ce co-doping metal–organic framework catalysts with oxygen vacancy for catalytic transfer hydrodeoxygenation of lignin derivatives vanillin, Chem. Eng. J., 2024, 481, 148555 CrossRef CAS.
- G. S. More, A. K. Kar and R. Srivastava, Cu–Ce Bimetallic Metal–Organic Framework-Derived, Oxygen Vacancy-Boosted Visible Light-Active Cu2O–CeO2/C Heterojunction: An Efficient Photocatalyst for the Sonogashira Coupling Reaction, Inorg. Chem., 2022, 61, 19010–19021 CrossRef CAS.
- R. Nivetha, et al., Cu based Metal Organic Framework (Cu-MOF) for electrocatalytic hydrogen evolution reaction, Mater. Res. Express, 2020, 7, 114001 CrossRef CAS.
- Z. Dong, et al., High pressure effects on hydrate Cu-BTC investigated by vibrational spectroscopy and synchrotron X-ray diffraction, RSC Adv., 2017, 7, 55504–55512 RSC.
- A. A. Talin, et al., Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices, Science, 2014, 343, 66–69 CrossRef CAS.
- S. S. Damasceno, B. B. Dantas, J. Ribeiro-Filho, M. Antônio, D. Araújo, M. Galberto and J. Da Costa, Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review, Curr. Pharm. Des., 2017, 23, 3015–3023 CrossRef CAS.
- F. Mohseni-Sardari, M. Mazloum-Ardakani, H. Mohammadian-Sarcheshmeh, Z. Alizadeh and S. Houshmand, Enzyme-free glutamate sensor using peony flower-like structure of nickel metal–organic framework/MWCNTs nanocomposite, Inorg. Chem. Commun., 2025, 179, 114734 CrossRef CAS.
- S. Ebadi, K. Ghanbari and M. Zahedi-Tabrizi, Development of an electrochemical sensor based on Ni-Bio-MOF and a molecular imprinted polymer for determination of diclofenac: electrochemical and DFT investigations, RSC Adv., 2025, 15, 16983–16998 RSC.
- B. Dey, et al., Highly sensitive and selective electrochemical sensor for carbendazim detection in fruit juice using novel bi-metallic metal organic framework anchored graphite rod electrode, Inorg. Chem. Commun., 2025, 178, 114643 CrossRef CAS.
- Y. Shi, H. Xu, Z. Gu, C. Wang and Y. Du, Sensitive detection of caffeic acid with trifurcate PtCu nanocrystals modified glassy carbon electrode, Colloids Surf., A, 2019, 567, 27–31 CrossRef CAS.
- T. Shoosri, et al., Bimetallic copper- and nickel-rich Cu–Ni phyllosilicate catalysts for the liquid phase selective hydrogenation of furfural to furfuryl alcohol, RSC Adv., 2024, 14, 38232–38244 RSC.
- R. Nehru, Y.-F. Hsu and S.-F. Wang, Electrochemical determination of caffeic acid in antioxidant beverages samples via a facile synthesis of carbon/iron-based active electrocatalyst, Anal. Chim. Acta, 2020, 1122, 76–88 CrossRef CAS PubMed.
- H. Hotta, H. Sakamoto, S. Nagano, T. Osakai and Y. Tsujino, Unusually large numbers of electrons for the oxidation of polyphenolic antioxidants, Biochim. Biophys. Acta, Gen. Subj., 2001, 1526, 159–167 CrossRef CAS PubMed.
- K. B, J. G. Manjunatha, S. M. Osman and N. Ataollahi, Electrochemical polymerized DL-phenylalanine modified carbon nanotube sensor for the selective and sensitive determination of caffeic acid with riboflavin, Sci. Rep., 2024, 14, 30950 CrossRef CAS.
- K. Yang, et al., Fabrication of cobalt single-atom catalyst supported on multiwalled carbon nanotubes for enhanced electrochemical sensing of caffeic acid in food samples, Microchem. J., 2025, 213, 113744 Search PubMed.
- A. Karthika, et al., Green synthesized CaO decorated ternary CaO/g-C3N4/PVA nanocomposite modified glassy carbon electrode for enhanced electrochemical detection of caffeic acid, Sci. Rep., 2024, 14, 28714 Search PubMed.
- M. Sakthivel, et al., Entrapment of bimetallic CoFeSe2 nanosphere on functionalized carbon nanofiber for selective and sensitive electrochemical detection of caffeic acid in wine samples, Anal. Chim. Acta, 2018, 1006, 22–32 CrossRef CAS.
- T. S. Priya, et al., Non-enzymatic electrochemical detection of caffeic acid in food samples with spinel magnesium cobalt oxide incorporated graphene nanohybrid electrocatalyst, J. Alloys Compd., 2024, 1002, 175335 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |





