Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlling structural and photophysical properties in sequentially methylated phenoxazines
Alexander Hubera,
Felix van der Vight a,
Viktoria Heisinga,
Christoph Wölperb,
Oleg Prymakb,
Hatem M. A. Aminb,
Kenneth E. Maly
a,
Viktoria Heisinga,
Christoph Wölperb,
Oleg Prymakb,
Hatem M. A. Aminb,
Kenneth E. Maly c and
Jens Voskuhl
c and
Jens Voskuhl *a
*a
aFaculty of Chemistry (Organic Chemistry), CENIDE and Center of Medical Biotechnology (ZMB), University of Duisburg-Essen, Universitätsstraße 7, 45141 Essen, Germany. E-mail: jens.voskuhl@uni-due.de
bFaculty of Chemistry (Inorganic Chemistry), University of Duisburg-Essen, Universitätsstraße 7, 45141 Essen, Germany
cDepartment of Chemistry and Biochemistry, Wilfrid Laurier University, 75 University Avenue W., Waterloo, ON N2L 3C5, Canada
First published on 9th December 2025
Abstract
The “magic methyl” effect is widely regarded as one of the most extraordinary features in small-molecule drug design and has recently been recognized as a subtle yet powerful tool for fine-tuning the photophysical properties of luminophores. In this context, we investigated how steric pressure influences the structural and photophysical attributes in a series of sequentially methylated phenoxazines. Structural elucidation using X-ray diffraction revealed that the steric strain of ortho-positioned methyl groups induces out-of-plane twisting of the N-arylated rings, which significantly alters the packing interactions by preventing dense π stacking. Complementary quantum chemical calculations indicate changes in the antiaromatic character due to partial rehybridization of the bridging nitrogen atom from sp2 to a more trigonal pyramidal geometry. Steady-state and time-resolved spectroscopy further highlighted the correlations between out-of-plane bending and emissive behavior, as characterized by distinctly pronounced changes in molar absorptivity and Stokes shifts. Finally, an effective strategy to overcome steric hindrance was demonstrated by extending the overall molecular conjugation, resulting in intense emission with high absolute photoluminescence quantum yields in both solution and the solid state.
Introduction
In recent years, the utilization of the methyl group, one of the simplest functional organic units in chemistry, has attracted tremendous interest in small-molecule drug design.1 Numerous reports mentioned that implementing a single methyl group into a drug candidate can lead to a significant increase in its pharmacological properties, often improving biological activity and potency through changes in solubility, selectivity, stability, conformation, and binding affinity.2,3 Although intra- and intermolecular interactions of methyl groups were largely overlooked in the past due to the relatively weak nature of London dispersion forces, C–H⋯π interactions were proven to be of paramount importance in molecular recognition.4 A prominent example is found in DNA, where the methyl substituent at the 5-position of the nucleotide base thymine aids in stabilizing the double helix.5 In contrast, less stable RNA has incorporated uracil units that lack methyl groups at this specific position. The seemingly inexplicable improvements in particular attributes resulting from the incorporation of methyl substituents have led to the phenomenon being colloquially referred to as the “magic methyl effect”.6 Beyond biological applications, methyl groups are utilized to improve energy storage properties in materials science,7 modulate the rotation of molecular motors in supramolecular chemistry,8 and are frequently incorporated into luminescent scaffolds for the precise tailoring of their photophysical properties.9,10Based on previous findings of steric demand caused by aromatic backbones in dihydrodibenzo[a,c]phenazines,11,12 Tian's group reported on the conformational changes and varying photophysical properties of ortho-methylated 5,10-dihydrophenazines.13 These compounds can undergo excited-state planarization from V-shaped geometries in their ground states, resulting in long-wavelength emission through vibration-induced emission (VIE).14,15 Specifically, the steric strain of the ortho-positioned methyl groups induced bent geometries in the electronic ground-states S0, causing hypsochromic shifts of the absorption maxima from 416 to 324 nm. Surprisingly, these distorted structures exhibited an even higher tendency for structural twisting in the excited state than the sterically unaffected parent compounds, leading to bathochromically shifted emission maxima and thus large Stokes shifts. Hua and coworkers16 leveraged these attributes for photodynamic therapy (PDT) under both normoxic and hypoxic conditions by employing compounds that exhibited aggregation-induced emission (AIE),17,18 which were capable of mitochondrial targeting and generating type I reactive oxygen species (ROS).
Bryce's group observed similar effects of steric pressure in phenoxazinyl-substituted dibenzothiophene-S,S-dioxides, which can adopt pyramidal or axial conformations, resulting in blue-shifted absorbances and enlarged Stokes shifts.19 Although the methylated congeners displayed less pronounced thermally activated delayed fluorescence (TADF), replacing oxygen-containing phenoxazine (POA) groups with sulfur-containing phenothiazine (PTA) groups enabled intense room temperature phosphorescence (RTP) in the presence of ortho-methyl groups.
These phenothiazine systems are known for their inherent ability to switch between quasi-equatorial and quasi-axial conformations based on the substitution pattern.20,21 Recently, Li and colleagues examined the influence of steric hindrance on the parent phenothiazine core, which allowed for gradual changes in folding angles from 180° to 90°.22 In particular, moving the ortho-methyl group from the PTA core to the N-arylated substituted ring inverted the preferred geometrical arrangement (from axial to equatorial and vice versa) upon photoexcitation.23
Maly's group reported several studies on N,N-diaryl diazadioxatetrahydropentacene luminophores derived from terephthalonitrile scaffolds, investigating the effect of methylated N-arylated substituents on the crystal structures and fundamental photophysical characteristics.24,25 Building on this work, we recently examined the effects of meticulously balancing the steric hindrance caused by ortho-positioned methyl groups on the photophysical properties of similar alkylated heteropentacyclic luminophores.26 Steric pressure not only enabled emission color tuning from red to green, but also served as a design principle for novel structures that demonstrate photoluminescence insensitive to the microenvironment. In general, this concept refers to the ability of a material to exhibit solution and solid-state emission (SSSE).27–29 This indicates that photoluminescence is the favored deexcitation pathway, whether in diluted monomeric conditions or in the solid state, including polycrystalline powders, single crystals, aggregates, thin films, or when embedded in materials.30,31 In the past, it was often reported that novel luminophores were effective emitters in either solution or the solid state, but not both. This limitation arises from issues such as aggregation-caused quenching (ACQ)32,33 or AIE, also known as solid-state luminescence enhancement (SLE).34 Consequently, achieving omnipotent emission is a highly desirable key attribute for organic luminescent materials, as it enables overcoming the challenges of quenching in applications such as organic light-emitting diodes (OLEDs),35 bioimaging,36,37 or photocatalysis.38,39
This study emphasizes steric strain as a fundamental design principle, motivating our investigation into how sequentially relocated methyl groups affect the structural and photophysical properties of functionalized phenoxazines. Molecular geometries and structural changes were examined using single-crystal X-ray diffraction (scXRD) and density functional theory (DFT), and correlated with the photophysical properties using steady-state and time-resolved spectroscopic techniques.
Results and discussion
Synthesis and design
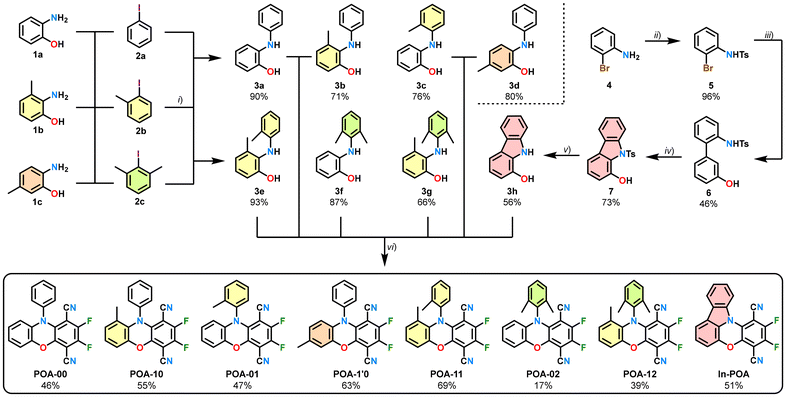
The structural and photophysical response to steric pressure was evaluated by comparing the properties of two primary N-phenyl substituted phenoxazine compounds POA-XX, differing in the absence (POA-00) or presence (POA-10) of an ortho-positioned methyl group next to the central oxazine core (Scheme 1). Additionally, the response of relocating the methyl group to alternative positions was assessed. In reference compound POA-1′0, the methyl group was set in the para position of the oxazine nitrogen atom, allowing for controlling purely electron donating effects. Further relocation was performed in the ortho position of the peripheral N-aryl ring (-X1), resulting in the tolyl-substituted derivative POA-01 and xylyl-functionalized POA-02. Penultimately, an attempt was made to surmount the steric hindrance of the ortho-positioned methyl group by combining the phenoxazine scaffold POA-1X with the N-tolyl (POA-11) or N-xylyl (POA-12) substituent. The series was completed by forcing a conjugation of both the terminal aminophenol-backbone and the N-aryl substituent through a covalent bond. This was realized by the design of the indolo-phenoxazine compound In-POA.The synthesis was carried out starting from either 2-aminophenol (1a), 3-methyl-2-aminophenol (1b), or 5-methyl-2-aminophenol (1c) for all POA-XX compounds (Scheme 1). The specific N-arylations were achieved in high yields (>65%) by using the respective iodobenzenes (2a–2c) as reagents and employing copper-catalyzed Ullmann–Goldberg conditions, following modified procedures from Li et al.40 These N-arylated aminophenol derivatives were subsequently converted into the target phenoxazines via nucleophilic aromatic substitution reactions with 2,3,5,6-tetrafluoroterephthalonitrile (TFTN). Under these reaction conditions, competitive formation of the corresponding heteropentacyclic side products was observed,26 resulting in a low yield (17%) for POA-02 and moderate yields (40–70%) for the other compounds. Although the purifications of all products were feasible using column chromatography, POA-10 co-eluted with its corresponding heteropentacene by-product as an inseparable mixture, necessitating recrystallization for complete purification.
The synthesis of the indolo-compound In-POA commenced with tosylation of o-bromoaniline (4), which gave N-tosylated aniline 5 in 96% yield. Compound 5 was reacted with 3-hydroxyphenylboronic acid to furnish biphenyl 6 in 46% yield, following procedures from Youn et al.41 Then, palladium-catalyzed C–H amidation was utilized for the formation of protected hydroxy–carbazole 7 (73%). Finally, deprotection of the N-tosyl group (56% yield) allowed the reaction of hydroxy-carbazole 3h with TFTN, giving In-POA in 51% yield (see the SI for detailed procedures). All final products were characterized using IR spectroscopy, high-resolution mass spectrometry, 1H, 13C, and 19F NMR spectroscopy (Fig. S2–S13), and the signals were assigned using 2D NMR techniques. High-performance liquid chromatography (Fig. S14) ensured high sample purity (>99%).
X-ray diffractometric analysis
Single crystals of seven compounds were grown and measured using X-ray diffractometry. Deposition numbers CCDC 2490301–2490307 contain the crystallographic data used for this study. Except for POA-02, which was crystallized by slow evaporation from a concentrated acetone solution, the crystals were grown by slow diffusion of overlayered solvent mixtures. For POA-10, a chloroform solution was overlayered with cyclohexane, whereas for the other compounds, dichloromethane was used as the base solvent and overlayered with cyclohexane (POA-00), butanone (POA-01), methanol (POA-1′0, In-POA), or ethyl acetate/chlorobenzene (POA-12). Despite several attempts, no suitable crystals of POA-11 were obtained due to poor sample scattering. However, by serendipitous discovery, the heteropentacyclic side product was crystallized and measured using X-ray diffractometry (deposition number CCDC 2490263, see Fig. S40).42 Fig. 1 displays the molecular crystal structures of selected compounds with perpendicular and in-plane views of the luminophore unit (see Fig. S39 for the remaining compounds). | ||
| Fig. 1 Molecular crystal structures of the compounds POA-00, -10, -12, and In-POA (perpendicular and in-plane views). The displacement ellipsoids are displayed at 50% probability levels. | ||
Compounds POA-00 and -10 crystallize in the monoclinic space group P21/c, albeit POA-10 crystallized with two molecules in the asymmetric unit, showing pseudo-translational symmetry along b (see Table S14 for more information). The methyl group at position C11 in POA-10 induces a discernible bending of the heteroaromatic core by pushing the N-aryl substituent out of plane. This is apparent from the measured angles αONC of the bridging oxygen (O1) and nitrogen atoms with the ipso-carbon atom of the aromatic substituent (C15), which reduces from almost in-plane (177°) in POA-00 to out-of-plane (120°) in POA-10 (Table 1). Additionally, the steric pressure induces a bending of the oxazine ring, resulting in a generally less planar structure. This is indicated by the increase in the fold angle θAP–TN (5° compared with 22°) as a planarity indicator, which was determined by defining mean planes for both the aminophenol and terephthalonitrile rings. Consequently, the adjacent nitrile group is less bent in POA-10 (αCCN = 176°) than in POA-00 (172°), because repulsive interactions are reduced.
Interestingly, the steric hindrance is countered in POA-12 by the bulky xylyl-substituent, which aids in maintaining a somewhat in-plane geometry (αONC = 176°), although it causes a distorted structure (θAP–TN = 11°). This is particularly surprising since the related xylyl-substituted compound POA-02 without the core-methyl group at position C11 displays a comparably higher out-of-plane twisting (αONC = 155°), similarly to Maly's reported heteropentacyclic congener.24 Both xylyl-substituted compounds, as well as POA-01 and POA-1′0, crystallized in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . In the latter two, relocating the methyl group away from position C11 eliminates the steric demand, resulting in similar structural parameters to those of POA-00. Implementing a covalent bond between the aminophenol ring and the N-aryl substituent forms the indolo-compound In-POA, which crystallized in the orthorhombic space group Pbca (see Table S15 for more information). Since the thin plate diffracted poorly, quantitative results should be interpreted with caution. Overall, all rings are forced into conjugation, but the repulsive interactions with the adjacent nitrile group induce a slight helical distortion in the molecular backbone.
. In the latter two, relocating the methyl group away from position C11 eliminates the steric demand, resulting in similar structural parameters to those of POA-00. Implementing a covalent bond between the aminophenol ring and the N-aryl substituent forms the indolo-compound In-POA, which crystallized in the orthorhombic space group Pbca (see Table S15 for more information). Since the thin plate diffracted poorly, quantitative results should be interpreted with caution. Overall, all rings are forced into conjugation, but the repulsive interactions with the adjacent nitrile group induce a slight helical distortion in the molecular backbone.
Additionally, the intermolecular contacts in crystal structures were analyzed to identify the most relevant interactions in the crystal packing, particularly the changes that occur when methyl groups are introduced. Generally, the compounds with planar geometries (POA-00, -01, -1′0) form columns dominated by inverted anti-co-facial oriented π⋯π interactions (see Fig. S41A and S43A–S44A, blue lines). The alternating orientation of the terminal rings enhances the electrostatic interactions between the electron-deficient terephthalonitrile ring and the electron-rich aminophenol ring. The π stacks are interconnected by either fluorine- or nitrile-driven contacts (see Fig. S41B and S43B–S44B, orange lines), forming sheets that extend into three-dimensional networks through hydrogen bonding (green and red lines). These interaction motifs are consistent with reported packing analyses of related phenoxazine-based derivatives.43 The introduction of a methyl group into tolyl-substituted phenoxazine POA-01 leads to a slightly larger π⋯π distance (3.71 Å) compared to that of POA-00 (3.57 Å, see Tables S16, S17, S20 and S21). To compensate for this increase, the methyl group is involved in additional nitrile-driven hydrogen bonds (2.87 Å, 2.88 Å, see Fig. S43 and Table S21). Compound POA-1′0 with the highest overall planarity also exhibits the closest π⋯π contacts (3.56 Å, Fig. 2A and Tables S22, S23).
Significant packing divergences emerge with increasing molecular distortion, as observed in the xylyl-functionalized molecules POA-02 and -12 (see Fig. S45 and S46). The lower in-plane angle αONC in POA-02 (155°) causes one methyl group to shield the core system from classic π⋯π stacking. Instead, CN⋯π stacking (3.49 Å, 3.55 Å, Tables S24 and S25) primarily characterizes dimer-like arrays interlocked by hydrogen bonds (purple line, see Fig. S45A). Similar CN⋯π stacking occurs in POA-12, but due to the higher core planarity, the contacts are closer (3.39 Å, 3.39 Å, see Fig. S46A, Tables S26 and S27). The dimers are interconnected by C–H⋯π (3.00 Å) and slipped π⋯π contacts (3.43 Å, 1.75 Å offset), forming a tilted staircase (purple lines). Although the methyl groups are involved in hydrogen bonding, the large spacing results in a significantly fewer intermolecular interactions. As a result, F⋯F interactions (2.85 Å) are present in the formation of sheets.
The highly conjugated structure In-POA shows the expected high degree of again inverted anti-co-facial oriented π⋯π stacking (3.44 Å, 3.59 Å, Tables S28 and S29), forming distorted honeycomb-like sheets through hydrogen bonding (see Fig. S47, blue lines).
The most discernible deviations in the crystal packing are again evident in POA-10. Due to the distorted central geometry, π stacked arrays are not formed. Instead, a brick-like pattern is present (see Fig. S42), comprising trimeric units with considerable slipped π⋯π (3.54 Å, 14° angle of mean planes) and C–H⋯π interactions (2.53 Å, blue lines, Fig. 2B and Tables S18, S19). The individual layers are interlinked by close hydrogen bonding originating from the bridging nitrogen atom of the oxazine core (2.58 Å, orange lines, Fig. 2B). This nitrogen atom exhibits a trigonal pyramidal geometry with partial sp3 hybridization (vide infra), which improves its ability to accept hydrogen bonds compared to the nitrogen atom in POA-00. Moreover, the layers expand to a three-dimensional network through nitrile-mediated interactions, including hydrogen bonding and π contacts (see purple lines, Fig. S42B). Notably, the individual interactions of the two independent molecules in the asymmetric unit differ only slightly and can be considered approximately equivalent. Energy decomposition analysis (EDA)44 calculations for trimers of compounds POA-00 (Fig. 2A) and POA-10 (Fig. 2B) confirmed that the total interaction energy of POA-10 is decreased due to reduced π stacking (see Table S35). The interaction energies are dominated by dispersion.
Powder X-ray diffractometry was used to quantify the crystallinity of the bulk powders (see Fig. S48). All data indicate similar crystalline phases, as observed in the single crystals. Although the PXRD pattern could not be simulated for POA-11, the experimental peak profile is similar to that of POA-01. For POA-01, -1′0, -12, and In-POA, additional minor phases were observed (marked with asterisks). All extracted crystallite sizes indicate the presence of microcrystalline domains (>100 nm).
Quantum chemical calculations
Density functional theory (DFT)45 calculations within the Gaussian 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 program were performed to elucidate structural and electronic effects induced by steric strain. All calculations employed the functional PBE047 with the TZVP48 basis set, which has proven reliable for calculating related phenoxazines.30 Dispersion correction (D3BJ)49 was considered for ground-state calculations, whereas excited states were evaluated using time-dependent density functional theory (TD-DFT).50 All optimized geometries were confirmed as energetic minima by the absence of imaginary frequencies. Crystal structure geometries were applied as initial guesses, and the obtained geometries after optimizing the structural parameters generally match those acquired from X-ray diffractometry (vide supra). In the case of POA-11, a bent starting geometry analogous to POA-10 was adopted, based on the structure of the aforementioned heteropentacyclic side product (Fig. S40).42 The optimized bending angle for POA-11 was 129°, indicating a preferred out-of-plane twisting, albeit less pronounced than for POA-10 (117°).
46 program were performed to elucidate structural and electronic effects induced by steric strain. All calculations employed the functional PBE047 with the TZVP48 basis set, which has proven reliable for calculating related phenoxazines.30 Dispersion correction (D3BJ)49 was considered for ground-state calculations, whereas excited states were evaluated using time-dependent density functional theory (TD-DFT).50 All optimized geometries were confirmed as energetic minima by the absence of imaginary frequencies. Crystal structure geometries were applied as initial guesses, and the obtained geometries after optimizing the structural parameters generally match those acquired from X-ray diffractometry (vide supra). In the case of POA-11, a bent starting geometry analogous to POA-10 was adopted, based on the structure of the aforementioned heteropentacyclic side product (Fig. S40).42 The optimized bending angle for POA-11 was 129°, indicating a preferred out-of-plane twisting, albeit less pronounced than for POA-10 (117°).
To quantify the steric effects of the ortho-methylated structures POA-10, -11, and -12, relaxed potential energy scans were performed for the out-of-plane bending of the N-arylated rings. The plane angle αONC of 178° in POA-00 was sequentially reduced in 5° increments over 20 steps. Methyl groups were attached to their respective positions, and the geometry was optimized at each step. The resulting energies are plotted in Fig. 3 as a function of the bending angle αONC, relative to the energy at the initial geometry.
In the absence of an ortho-methyl group, such as for POA-00, the out-of-plane bending leads to the expected energetic destabilization. In contrast, the out-of-plane bending in POA-10 stabilizes the structure significantly by 5.1 kcal mol−1 at an energetic minimum near αONC = 118°, similar to that for previously reported substituted heteropentacenes.26 Interestingly, POA-11 displays energetic minima at both respective angles of interest (129° and 178°). Due to steric pressure, the out-of-plane conformation is again preferred, with a stabilization energy of 2.5 kcal mol−1 at 129° compared with the conformer optimized at 178°. The parameters of both conformers were then optimized without restraints to estimate their relative populations at room temperature. The resulting Gibbs free energy difference of 1.0 kcal mol−1 corresponds to Boltzmann populations of 84% (αONC = 129°) and 16% (αONC = 178°), respectively, indicating the coexistence of both conformers. In the case of two methyl groups present at the N-aryl ring, as in POA-12, out-of-plane bending becomes disfavoured due to repulsive interactions, as demonstrated by the evident destabilization (Fig. 3, green curve). Hence, the N-xylyl substitution facilitates surmounting structural rearrangements induced by steric strain.
Since steric pressure affects the molecular geometry, a natural bond order (NBOs) analysis was conducted (Fig. 4). In POA-00, the lone pair of the bridging oxazine nitrogen atom exhibits almost pure p-character (>99%), consistent with sp2-hybridization of this atom. In contrast, while this lone pair still shows predominant p-character (90%) in POA-10, an additional s-character (10%) also contributes. Consequently, the nitrogen atom showcases partial sp2/sp3-like hybridization, which significantly perturbs the electronic properties of this molecule.
 | ||
| Fig. 4 Comparison of the lone pair character of the bridging oxazine nitrogen atom for POA-00 (left) and POA-10 (right) from the in-plane view, calculated using PBE0-D3BJ/TZVP. | ||
Fig. 5 illustrates the stabilizing out-of-plane bending in POA-10 by the significant decrease in energy of the highest occupied molecular orbital (HOMO, −6.7 eV), which is reduced by 0.6 eV compared with that of POA-00 (−6.1 eV, Table S32). In cyclic voltammetry experiments (the potential reported here is normalized vs. Fc/Fc+), POA-00 indeed exhibits a lower reversible redox potential (+0.803 V) than POA-10 (+0.934 V, Fig. S51), consistent with the qualitative theoretical HOMO ordering, see details in SI. In contrast, the energies of the lowest unoccupied molecular orbitals (LUMOs) are less influenced by the steric demand. This is because the LUMOs are localized at the terephthalonitrile side, which experiences less impact from the geometrical rearrangement due to steric effects, as displayed by the virtual natural transition orbitals (NTOs, see Fig. S49 and S50). In total, the HOMO–LUMO energy gap increases for POA-10, coinciding with a hypsochromic shift of the absorption maximum (from λab = 421 nm for POA-00 to λab = 399 nm for POA-10).
The sterically induced geometrical reorganization also affects the aromaticity of the oxazine core, as assessed by computing nucleus-independent chemical shifts (NICS) 1 Å above the ring centroids.51,52 The calculated NICS(1) value of −10.0 ppm of the model compound benzene using GIAO-PBEO/TZVP matched the literature value, signifying aromaticity.53 For POA-00, the central oxazine ring shows a NICS(1) value of 6.2 ppm, denoting an antiaromatic character as expected for phenoxazines. In contrast, a NICS(1) index of −0.2 ppm was obtained for POA-10, indicating a non-aromatic character. Analogous trends were obtained from using the out-of-plane component of the shielding tensors as indicated by the corresponding NICS(1)zz values (see Table S31).54 Although NICS indices quantitatively indicate (anti)aromaticity, their single scalar descriptors of magnetic responses must be interpreted cautiously in heteroaromatic systems with partial pyramidalization.55 Accordingly, the trends were corroborated by complementary bond length analysis using the harmonic oscillator model of aromaticity (HOMA, see eqn (S1), Table S30 for details).52,56
POA-10 showcases a disrupted conjugation compared with POA-00, which also results in a reduced oscillator strength fab (0.075 versus 0.158) for the monoelectronic excitation involving the HOMOs to the LUMOs (see Table S32). Notably, compound In-POA displays the most extended conjugation through the contribution of the terminal carbazole ring to the HOMO (Fig. 5), leading to the highest overall increased oscillator strength for absorption (fab = 0.196). In terms of theoretical absorption wavelengths, all calculated λab,calc values show good agreement with the experimentally determined absorption maxima in THF (see Table S33). Additionally, the geometrical parameters of the compounds were optimized in their respective excited singlet states S1. All POA-XX derivatives display rotations of the N-arylated rings in the S1 states, which correlate with large Stokes shifts. Indeed, the theoretical emission wavelengths λem,calc generally match the emission wavelengths measured for the THF solutions, except for POA-10 (Δλem,THF–calc = 0.421 eV). This deviation is attributed to the well-known underestimation of charge transfer excitation energies in the selected computational method, as a quinoid structure is formed (see Fig. S49). The deviation can be overcome by using the CC2/def2-TZVP method, which provides more accurate results (see Table S34).57,58
Investigation of the photophysical properties
Ultimately, the effect of steric pressure on the photophysical properties of the compounds was investigated, commencing with optically diluted solutions. To cover a range of different solvent polarities, cyclohexane (CH), tetrachloromethane (CCl4), dichloromethane (DCM), tetrahydrofuran (THF), acetonitrile (MeCN) and methanol (MeOH) were selected. UV/Vis absorption spectra were recorded in all solvents, exhibiting similar features with intense, strong, and sharp absorption bands around 235 nm along with broader, less intense absorption maxima at 390–450 nm (Fig. S15). Generally, the measured absorption maxima follow the trends predicted by the DFT calculations (vide supra). The λab values for the in-plane compounds, such as POA-00, are around 450 nm in DCM and shift towards 396 nm for POA-10 (Fig. 6A). Compound POA-11 displays an expected in-between behavior (λab = 427 nm, see Table S1).Additionally, molar absorption coefficients ε were determined for all compounds in THF solutions (Fig. S16 and S17). The out-of-plane bending leads to a considerable reduction in ε when comparing POA-00 with -10 (5940 versus 3380 L mol−1 cm−1). Noteworthy, In-POA demonstrates the highest overall ε with a value of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 L mol−1 cm−1. Hence, the molar absorption coefficients agree well with the calculated oscillator strengths.
040 L mol−1 cm−1. Hence, the molar absorption coefficients agree well with the calculated oscillator strengths.
Steady-state and time-resolved photoluminescence spectroscopy studies were employed to examine the excited-state properties. All lifetimes τav_amp are in the nanosecond range up to 10 ns, confirming fluorescence as the dominant radiative deactivation mechanism (Fig. S30–S37). All compounds demonstrate analogous solvatochromism, showing turquoise emission in cyclohexane, green in CCl4, yellow luminescence in DCM, and dark yellow in MeOH (see Fig. S18–S25). This color change in emission with increasing solvent polarity reflects the progressive stabilization of intramolecular charge-transfer (ICT) states.59 The structured emission bands observed in non-polar solvents correspond to vibronic progressions. In contrast, in polar solvents such as acetonitrile and methanol, the emission bands become broader and less structured, accompanied by reduced intensities, indicating that enhanced non-radiative relaxation processes occur in these environments. Most interestingly, POA-00 and -10 exhibit approximately identical emission wavelengths in all solvents (Table S2), although displaying distinct absorption differences (Fig. 6B). Consequently, POA-10 possesses Stokes shifts that are nearly twice as high as those of POA-00 (Table 2). The Stokes shifts were plotted as a function of the orientation polarization factors Δf using the Lippert–Mataga equation (Fig. S26 and Table S11).60 The slope and intercept values of POA-00 are around 4000 cm−1, increasing to approximately 6000 cm−1 for POA-10. On the one hand, the steeper slope of POA-10 indicates a more pronounced polarity enhancement upon excitation compared with POA-00, suggesting increased sensitivity to solvent polarity. On the other hand, the larger intercept of POA-10 reflects an intrinsically higher ground-state polarity, aligning with a larger inherent dipole moment change associated with the twisted geometry.61
| POA-00 | POA-10 | In-POA | ||
|---|---|---|---|---|
| Cyclohexane | λab [nm] | 436 | 388 | 441 |
| λem [nm] | 516 | 499 | 518 | |
| Δνab–em [cm−1] | 3556 | 5733 | 3371 | |
| ΦPL | 0.52 ± 0.03 | 0.31 ± 0.02 | 0.65 ± 0.03 | |
| kr(knr)[108 s−1] | 0.59 (0.54) | 0.35 (0.78) | 0.69 (0.37) | |
| τav_amp [ns] | 8.88 ± 0.01 | 8.88 ± 0.01 | 9.40 ± 0.01 | |
| DCM | λab [nm] | 449 | 396 | 454 |
| λem [nm] | 553 | 559 | 551 | |
| Δνab–em [cm−1] | 4189 | 7363 | 3878 | |
| ΦPL | 0.10 ± 0.02 | 0.05 ± 0.02 | 0.30 ± 0.02 | |
| kr(knr)[108 s−1] | 0.4 (3.6) | 0.2 (4.3) | 0.53 (1.24) | |
| τav_amp [ns] | 2.50 ± 0.01 | 2.22 ± 0.02 | 5.64 ± 0.01 | |
| Powder | λex [nm] | 512 | 462 | 533 |
| λem [nm] | 548 | 516 | 565 | |
| Δνex–em [cm−1] | 1283 | 2265 | 1063 | |
| ΦPL | 0.30 ± 0.02 | 0.38 ± 0.02 | 0.44 ± 0.02 | |
| kr (knr) [108 s−1] | 0.24 (0.56) | 0.41 (0.67) | 0.64 (0.81) | |
| τav_amp [ns] | 12.59 ± 0.05 | 9.21 ± 0.04 | 6.89 ± 0.02 |
The enhanced geometrical rearrangements of POA-10 result in generally lower absolute photoluminescence quantum yields ΦPL (see Fig. S38 for an exemplary report file) and reduced radiative deactivation rate constants kr compared with POA-00 (Table 2). In contrast, relatively high ΦPL values up to 0.65 (In-POA) were measured for the non-polar cyclohexane and CCl4 solutions, highlighting the strongly emissive nature of the compounds (see Tables S3–S10). Particularly, In-POA showcases the best overall ΦPL and kr values, even reaching a ΦPL value of 0.12 in acetonitrile (Table S10), which matches the calculated high oscillator strength fem (0.146). Because In-POA possesses a covalent bond between the phenoxazine stator unit and the rotor N-aryl ring, non-radiative dissipation through rotation is suppressed in solution, which is evident by the generally lower knr values (Table 2). Table 2 summarizes selected photophysical parameters of the compounds POA-00, POA-10, and In-POA.
Additionally, the photophysical properties of the powders were assessed (Table S12). It is worth noting that all compounds displayed strong solvent dependency in terms of their optical properties, resulting in various poly-microcrystalline habits. To ensure comparability, the compounds were measured after rapid evaporation of their DCM solutions. In general, all compounds show intense emission in the solid state (Fig. S27). As already observed in the crystal packings, the out-of-plane twisted ring of POA-10 averts dense π stacking, resulting in a higher ΦPL value for the powder of POA-10 (0.38) compared with -00 (0.30). Since geometric relaxations in the solid state are more restricted than in solution, the emission color of POA-10 appears green (λem = 516 nm) instead of yellow, as seen for POA-00 (λem = 548 nm). Compounds POA-11 and -01 both show photophysical properties similar to those of -00 as powders, although possessing higher knr values, which may be due to the presence of additional methyl groups in the rotor unit. Orange emission colors are displayed by POA-1′0, -02, and -12, whereas In-POA again exhibits the highest ΦPL (0.44) and kr values (0.64 × 108 s−1). Due to the pronounced absolute photoluminescence quantum yield values in most solvents and the solid state, In-POA demonstrates the best SSSE properties among the presented compounds (Fig. 6D).
This trend is further supported by comparing the emissive behavior in aggregation studies (at 15 µM) using binary THF/water mixtures (Fig. S28). While increasing water content (20–60%) initially causes polarity-induced quenching, aggregation at higher water contents restores or even enhances emission in most cases.62 However, because all measurements were conducted using a fixed excitation wavelength, the ΦPL values must be discussed for accurate comparison (Table S13). Here, all aggregates formed at THF/H2O 10/90 show significantly higher ΦPL values than the THF solutions, except for In-POA (0.22, 0.32, respectively; see Fig. S29 for the corresponding sizes at the 10/90 THF/H2O ratios using dynamic light scattering, DLS).
Conclusions
In conclusion, this study elucidated how sterically demanding ortho-positioned methyl groups influence the structural and photophysical properties of eight phenoxazine-derived luminophores. Single-crystal X-ray diffractometry revealed that the ortho-methyl groups induce significant geometrical distortions through out-of-plane twisting and disrupt π⋯π dominated interactions in the crystal packing. Complementary DFT calculations disclosed partial rehybridization of the oxazine nitrogen atom, transitioning from planar sp2 to a trigonal pyramidal sp2/sp3 geometry, accompanied by a loss of the antiaromatic character. While all compounds exhibit pronounced photoluminescence in various molecular environments, the geometric and electronic perturbations manifested in distinct photophysical responses. POA-10 exemplifies the most substantial impact of steric pressure through enlarged Stokes shifts. In contrast, the rigid derivative In-POA highlights the strongest and most balanced solution and solid-state emission behavior due to increased conjugation. Hence, this study comprises a modular approach to leverage the efficient steric strain of methyl groups as a strategy for fine-tuning structural and photophysical properties. Establishing steric pressure as a molecular design principle is anticipated to further advance the development of stimuli-responsive and microenvironment-insensitive materials in smart photonic systems and optoelectronic applications.Author contributions
A. Huber: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing – original draft, and writing – review and editing; F. v. d. Vight: conceptualization, formal analysis, and investigation; V. Heising: investigation and formal analysis; C. Wölper: data curation and validation; O. Prymak: data curation and formal analysis; H. M. A. Amin: data curation, electrochemical measurements, and visualization; K. E. Maly: validation and writing – review & editing; J. Voskuhl: conceptualization, resources, supervision, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: detailed synthetic procedures and additional analytical data regarding spectroscopic characterization, X-ray diffractometric analyses, quantum chemical calculations, and cyclic voltammetry. See DOI: https://doi.org/10.1039/d5qo01437a.CCDC 2490301–2490307 contain the supplementary crystallographic data for this paper.63a–g
Acknowledgements
We thank Finn Rethemeier for his assistance during synthesis and Lea Höfmann and Sophia Stadtfeld for their help with the analyses. Prof. Dr Gebhard Haberhauer is acknowledged for his support and fruitful discussions regarding quantum chemical calculations. Additionally, we thank Dr Constantin G. Daniliuc for determining the crystal structure of the heteropentacyclic side product of compound POA-11. Prof. Dr Georg Jansen is acknowledged for his financial support.References
- E. J. Barreiro, A. E. Kümmerle and C. A. M. Fraga, The Methylation Effect in Medicinal Chemistry, Chem. Rev., 2011, 111, 5215–5246 CrossRef CAS PubMed.
- P. de S. M. Pinheiro, L. S. Franco and C. A. M. Fraga, The Magic Methyl and Its Tricks in Drug Discovery and Development, Pharmaceuticals, 2023, 16, 1157 CrossRef CAS PubMed.
- C. S. Leung, S. S. F. Leung, J. Tirado-Rives and W. L. Jorgensen, Methyl Effects on Protein–Ligand Binding, J. Med. Chem., 2012, 55, 4489–4500 CrossRef CAS PubMed.
- M. Nishio, Y. Umezawa, M. Hirota and Y. Takeuchi, The CH/π interaction: Significance in molecular recognition, Tetrahedron, 1995, 51, 8665–8701 CrossRef CAS.
- Y. Umezawa and M. Nishio, Thymine-methyl/π interaction implicated in the sequence-dependent deformability of DNA, Nucleic Acids Res., 2002, 30, 2183–2192 CrossRef CAS PubMed.
- H. Schönherr and T. Cernak, Profound Methyl Effects in Drug Discovery and a Call for New C-H Methylation Reactions, Angew. Chem., Int. Ed., 2013, 52, 12256–12267 CrossRef PubMed.
- J. J. Lee, K. Jeong, S. Kwon, H. Yook, S. M. Kim, J. W. Han, J. Choi and J. H. Park, ‘Magic methyl effect’ in 2-benzylpyridine-based H2 storage materials: Enhanced H2 storage/release performances, Energy Storage Mater., 2024, 67, 103259 CrossRef.
- Y. Gisbert, M. Fellert, C. N. Stindt, A. Gerstner and B. L. Feringa, Molecular Motors’ Magic Methyl and Its Pivotal Influence on Rotation, J. Am. Chem. Soc., 2024, 146, 12609–12619 CrossRef CAS PubMed.
- Y. Li, R. W. Weerasinghe, Y. Hu, X. Tan, B. Cai, C. Adachi and C.-Y. Chan, The magic methyl effect of thermally activated delayed fluorescent emitters on blue organic light-emitting diodes, J. Mater. Chem. C, 2025, 13, 12691–12698 RSC.
- S. Bhuin, S. Bhattacharya and M. Chakravarty, Acceptor–donor–acceptor-linked triphenylamine and phenothiazine motifs as cousin molecules: the methyl effect on stimuli-responsiveness, crystallochromism, and dual-state emission, New J. Chem., 2021, 45, 21236–21247 RSC.
- Z. Chen, X. Jin, R. Shen, W. Li, L. Sun, J. Su, D.-H. Qu, Z. Zhang and H. Tian, Capturing the Progressive Conformational Evolutions of Sterically-Congested Dihydrophenazines via Crystallization, Angew. Chem., Int. Ed., 2025, 64, e202424597 CrossRef CAS PubMed.
- Z. Zhang, Y.-S. Wu, K.-C. Tang, C.-L. Chen, J.-W. Ho, J. Su, H. Tian and P.-T. Chou, Excited-State Conformational/Electronic Responses of Saddle-Shaped N,N′-Disubstituted-Dihydrodibenzo[a,c]phenazines: Wide-Tuning Emission from Red to Deep Blue and White Light Combination, J. Am. Chem. Soc., 2015, 137, 8509–8520 CrossRef CAS PubMed.
- X. Jin, S. Li, L. Guo, J. Hua, D.-H. Qu, J. Su, Z. Zhang and H. Tian, Interplay of Steric Effects and Aromaticity Reversals to Expand the Structural/Electronic Responses of Dihydrophenazines, J. Am. Chem. Soc., 2022, 144, 4883–4896 CrossRef CAS PubMed.
- Z. Zhang, W. Song, J. Su and H. Tian, Vibration–Induced Emission (VIE) of N,N′–Disubstituted–Dihydribenzo[a,c]phenazines: Fundamental Understanding and Emerging Applications, Adv. Funct. Mater., 2020, 30, 1902803 CrossRef CAS.
- X. Jin, S. Guo, X. Wang, M. Cong, J. Chen, Z. Zhang, J. Su, D. Qu and H. Tian, Sequential Multistep Excited–State Structural Transformations in N,N′–Diphenyl–dihydrodibenzo[a,c]phenazine Fluorophores, Angew. Chem., Int. Ed., 2023, 62, e202305572 CrossRef CAS PubMed.
- S. Li, X. Jin, Z. Zhang, J. Li and J. Hua, An AIE-active type I photosensitizer based on N,N′-diphenyl-dihydrophenazine for high-performance photodynamic therapy under hypoxia, Mater. Chem. Front., 2023, 7, 3738–3746 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun., 2001, 1740–1741 RSC.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: Together We Shine, United We Soar!, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- J. S. Ward, R. S. Nobuyasu, M. A. Fox, A. S. Batsanov, J. Santos, F. B. Dias and M. R. Bryce, Bond Rotations and Heteroatom Effects in Donor–Acceptor–Donor Molecules: Implications for Thermally Activated Delayed Fluorescence and Room Temperature Phosphorescence, J. Org. Chem., 2018, 83, 14431–14442 CrossRef CAS PubMed.
- Y. Wang, J. Yang, M. Fang, Y. Gong, J. Ren, L. Tu, B. Z. Tang and Z. Li, New Phenothiazine Derivatives That Exhibit Photoinduced Room–Temperature Phosphorescence, Adv. Funct. Mater., 2021, 31, 2101719 CrossRef CAS.
- L. Mayer, L. May and T. J. J. Müller, The interplay of conformations and electronic properties in N-aryl phenothiazines, Org. Chem. Front., 2020, 7, 1206–1217 RSC.
- Y. Gao, W. Yuan, Y. Li, A. Huang, Y. Fang, A. Li, K. Wang, B. Zou, Q. Li and Z. Li, Accurately adjusted phenothiazine conformations: reversible conformation transformation at room temperature and self-recoverable stimuli-responsive phosphorescence, Light: Sci. Appl., 2025, 14, 99 CrossRef CAS PubMed.
- M. Gao, R. Wu, Y. Zhang, Y. Meng, M. Fang, J. Yang and Z. Li, New Molecular Photoswitch Based on the Conformational Transition of Phenothiazine Derivatives and Corresponding Triplet Emission Properties, J. Am. Chem. Soc., 2025, 147, 2653–2663 CrossRef CAS PubMed.
- L. K. Hiscock, V. S. Patel, A. M. Raj, C. Amoah, A. J. Talreja, W. G. Skene and K. E. Maly, Luminescent N-aryl-heteroacene derivatives, Can. J. Chem., 2023, 101, 186–197 CrossRef CAS.
- L. K. Hiscock, C. Yao, W. G. Skene, L. N. Dawe and K. E. Maly, Synthesis of Emissive Heteroacene Derivatives via Nucleophilic Aromatic Substitution, J. Org. Chem., 2019, 84, 15530–15537 CrossRef CAS PubMed.
- A. Huber, T. Thiele, T. Rex, C. G. Daniliuc, C. Wölper, R. Y. Lorberg, L. Höfmann, C. A. Strassert, M. Giese and J. Voskuhl, Steric Pressure in Heteropentacenes Modulates the Photophysical Properties – A Molecular Design Strategy for Functional Materials, Chem. Sci., 2025, 16, 15723–15733 RSC.
- A. Huber, J. Dubbert, T. D. Scherz and J. Voskuhl, Design Concepts for Solution and Solid–State Emitters – A Modern Viewpoint on Classical and Non–Classical Approaches, Chem. – Eur. J., 2023, 29, e202202481 CrossRef CAS PubMed.
- J. L. Belmonte-Vázquez, Y. A. Amador-Sánchez, L. A. Rodríguez-Cortés and B. Rodríguez-Molina, Dual-State Emission (DSE) in Organic Fluorophores: Design and Applications, Chem. Mater., 2021, 33, 7160–7184 CrossRef.
- T. Stoerkler, T. Pariat, A. D. Laurent, D. Jacquemin, G. Ulrich and J. Massue, Excited-State Intramolecular Proton Transfer Dyes with Dual-State Emission Properties: Concept, Examples and Applications, Molecules, 2022, 27, 2443 CrossRef CAS PubMed.
- A. Huber, L. Schmidt, T. Gatz, J. Bublitz, T. Rex, S. T. N. Sailaja, E. Verheggen, L. Höfmann, C. Wölper, C. A. Strassert, S. K. Knauer and J. Voskuhl, Stepwise Modulation of Bridged Single–Benzene–Based Fluorophores for Materials Science, Chem. – Eur. J., 2025, 31, e202404263 CrossRef CAS PubMed.
- S. T. N. Sailaja, I. Maisuls, J. Kösters, A. Hepp, A. Faust, J. Voskuhl and C. A. Strassert, Naphthalonitriles featuring efficient emission in solution and in the solid state, Beilstein J. Org. Chem., 2020, 16, 2960–2970 CrossRef CAS PubMed.
- J. B. Birks, Photophysics of aromatic molecules, Wiley-Interscience, London, New York, 1970 Search PubMed.
- J. Gierschner, L. Lüer, B. Milián-Medina, D. Oelkrug and H.-J. Egelhaaf, Highly Emissive H-Aggregates or Aggregation-Induced Emission Quenching? The Photophysics of All-Trans para-Distyrylbenzene, J. Phys. Chem. Lett., 2013, 4, 2686–2697 CrossRef CAS.
- J. Gierschner, J. Shi, B. Milián-Medina, D. Roca-Sanjuán, S. Varghese and S. Park, Luminescence in Crystalline Organic Materials: From Molecules to Molecular Solids, Adv. Opt. Mater., 2021, 9, 2002251 CrossRef CAS.
- I. S. Park, S. Y. Lee, C. Adachi and T. Yasuda, Full–Color Delayed Fluorescence Materials Based on Wedge–Shaped Phthalonitriles and Dicyanopyrazines: Systematic Design, Tunable Photophysical Properties, and OLED Performance, Adv. Funct. Mater., 2016, 26, 1813–1821 CrossRef CAS.
- A. Huber, J. Koch, K. Rudolph, A. Höing, F. Rizzo, S. K. Knauer and J. Voskuhl, Deoxyestrone-based lipofection agents with solution- and solid-state emission properties, Org. Biomol. Chem., 2023, 21, 5762–5767 RSC.
- J. Dubbert, A. Höing, N. Graupner, Ľ. Rajter, M. Dunthorn, S. K. Knauer, A. Galstyan, F. Rizzo and J. Voskuhl, Cationic Solution and Solid-State Emitters – Robust Imaging Agents for Cells, Bacteria, and Protists, Chem. – Eur. J., 2023, 29, e202300334 CrossRef CAS PubMed.
- M. N. Khatun, S. Nandy, C. Srinivas, S. Kumar and P. K. Iyer, Molecularly Engineered Photosensitizers Based on Superoxide Generation, Solid State White Light Emission, Excimer-Driven Photosensitization, and Photoredox Catalysis, Adv. Opt. Mater., 2025, 13, e01052 CrossRef CAS.
- J.-M. Heo, J. Park, M. F. Flórez-Angarita, L. Wang, C. Yu, J. Choi, H. Woo, B. Milián-Medina, A. J. Matzger, M. S. Kwon, J. Gierschner and J. Kim, Elucidating the molecular structural origin of efficient emission across solid and solution phases of single benzene fluorophores, Nat. Commun., 2025, 16, 5560 CrossRef PubMed.
- Y. Li, H. Wang, L. Jiang, F. Sun, X. Fu and C. Duan, Copper–Catalyzed Direct Synthesis of Di– and Triphenylamines: A Dramatic Accelerating Effect of 2−Aminophenols, Eur. J. Org. Chem., 2010, 6967–6973 CrossRef CAS.
- S. W. Youn, Y. H. Kim and Y. H. Jo, Palladium-Catalyzed Regioselective Synthesis of 1-Hydroxycarbazoles Under Aerobic Conditions, Adv. Synth. Catal., 2019, 361, 462–468 CrossRef CAS.
- A. Huber, C. G. Daniliuc and J. Voskuhl, CCDC 2490263: Experimental Crystal Structure Determination, CSD Commun., DOI:10.5517/ccdc.csd.cc2plb2q.
- L. K. Hiscock, K. E. Maly and L. N. Dawe, Crystal Packing of a Series of 1,2,3,4-Substituted Phenoxazine and Dibenzodioxin Heterocycles, Cryst. Growth Des., 2019, 19, 7298–7307 CrossRef CAS.
- F. M. Bickelhaupt and E. J. Baerends, in Reviews in Computational Chemistry, John Wiley & Sons, Ltd, 2000, pp. 1–86 Search PubMed.
- M. Bursch, J. Mewes, A. Hansen and S. Grimme, Best–Practice DFT Protocols for Basic Molecular Computational Chemistry, Angew. Chem., Int. Ed., 2022, 61, e202205735 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- A. Schäfer, C. Huber and R. Ahlrichs, Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr, J. Chem. Phys., 1994, 100, 5829–5835 CrossRef.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- F. Furche and R. Ahlrichs, Adiabatic time-dependent density functional methods for excited state properties, J. Chem. Phys., 2002, 117, 7433–7447 CrossRef CAS.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. von R. Schleyer, Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS PubMed.
- R. Gleiter and G. Haberhauer, Aromaticity and other conjugation effects, Wiley, Weinheim, 2012 Search PubMed.
- R. Gleiter, G. Haberhauer and S. Woitschetzki, From Eight-Membered 10π Electron Sulfur–Nitrogen Cycles to Bicycles and Cages: A Theoretical Approach, Chem. – Eur. J., 2014, 20, 13801–13810 CrossRef CAS PubMed.
- R. Gershoni-Poranne and A. Stanger, Magnetic criteria of aromaticity, Chem. Soc. Rev., 2015, 44, 6597–6615 RSC.
- A. Stanger, NICS – Past and Present, Eur. J. Org. Chem., 2020, 3120–3127 CrossRef CAS.
- T. M. Krygowski and M. K. Cyrański, Structural Aspects of Aromaticity, Chem. Rev., 2001, 101, 1385–1420 CrossRef CAS PubMed.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- O. Christiansen, H. Koch and P. Jørgensen, The second-order approximate coupled cluster singles and doubles model CC2, Chem. Phys. Lett., 1995, 243, 409–418 CrossRef CAS.
- S. K. Behera, S. Y. Park and J. Gierschner, Dual Emission: Classes, Mechanisms, and Conditions, Angew. Chem., Int. Ed., 2021, 60, 22624–22638 CrossRef CAS PubMed.
- Z. N. Scheller, D. Liese, H. Siera, N. Semleit, M. Schmiedtchen, C. Wölper and G. Haberhauer, The Origin of Dual-Emission in PLICT Compounds – an Empirical Approach, Chem. – Eur. J., 2024, 30, e202304143 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
- J. Maillard, K. Klehs, C. Rumble, E. Vauthey, M. Heilemann and A. Fürstenberg, Universal quenching of common fluorescent probes by water and alcohols, Chem. Sci., 2021, 12, 1352–1362 RSC.
- (a) CCDC 2490301: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plc9z; (b) CCDC 2490302: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plcb0; (c) CCDC 2490303: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plcc1; (d) CCDC 2490304: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plcd2; (e) CCDC 2490305: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plcf3; (f) CCDC 2490306: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plcg4; (g) CCDC 2490307: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plch5.
| This journal is © the Partner Organisations 2026 |